Abstract
Background: Autism spectrum disorder (ASD) is identified by developmental deficits that lead to repetitive/stereotypic patterns of behavior and impaired social interactions. Studies have been indicated that exercise can decrease stereotypic behaviors in animal models of ASD. This research was designed to discover the effects of different models of forced exercise on stereotypical behaviors in a rat model of ASD induced by thimerosal (THIM). Materials and Methods: Fifty-six male Wistar rats were divided into eight groups. The rats were received saline (1 ml/kg) or THIM (300 μg Hg/kg) by four intramuscular injections on 7, 9, 11, and 15 postnatal days. The rats were also treated by several protocols of treadmill exercise, including non-sedentary, sedentary, protocol 1, protocol 2, and a combination of protocols 1 and 2. Results: Our study showed that THIM decreased the grooming time compared to the control group. Moreover, protocol 2 exercise significantly decreased grooming time in stranger zone 2 compared to the THIM group. Conclusions: Our results showed that stereotypical behaviors exaggerated by THIM and moderate exercise could improve ASD-associated behaviors in the THIM-treated rats. Hence, moderate exercise may be a useful protocol for the treatment of ASD.
Keywords: Autism, Thimerosal, Grooming, Exercise
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder identified by social communication decrease and social interaction deficit, as well as respective behaviors [1] or stereotypic movements [2]. Evidence has shown that patients with ASD experience many mental disorders, indicating that 79% of ASD patients have at least one psychiatric disorder at some point in their life [3]. The neurodevelopmental hypothesis described the etiology of ASD as impaired prenatal development and many pathological processes that begin before the brain fully matures [4]. Thimerosal (THIM), known as sodium ethylmercurithiosalicylate, is an ethylmercury-containing pharmaceutical compound containing 49.6% mercury by weight and metabolizes into ethylmercury and thiosalicylate [5]. Since 1999, the issue of THIM as a reason for ASD and other important neurodevelopmental disorders has turned into a hot topic [6,7,8].Unfortunately, there has been no certain medical treatment for ASD so far. Lack of effective treatments or medications showed a need to decrease stereotypical behaviors without critical side effects from medications. Evidence has shown the positive effects of exercise on ASD symptoms [9,10]. It has been shown that customized physical education effectively decreases the stereotypical behaviors of a child with ASD and dramatically decreases anxiety in patients with ASD [11].Iardanis and Aptesislis performed physical education and exercise for children with ASD to determine the effects on socialization, communication, and behavior [12]. They also demonstrated positive behavior associated with increased physical activity [12]. Pan et al. [11] evaluated the impact of the swimming programs on behavioral and social skills in children with ASD. The results demonstrated that it had been associated with a reduction in antisocial behavior problems [11]. Previous studies have also evaluated the impact of physical activity and exercise interventions on individuals with ASD [13,14], resulting in positive outcomes.Thus, we examined the effects of different models of forced exercise on stereotypical behaviors in a rat model of ASD-induced by THIM.
Materials and Methods
1. Animals:
Male Wistar rats (weighing 200-250g) were obtained from the animal house Institute of cognitive sciences studies (ICSS; Tehran, Iran). All rats had ad libitum access to food and water and were housed in a room with a standard temperature (22±2°C) and a 12/12h light/dark cycle (lights on at 7). All tests were carried out between 9 h and 14 h, and each rat was tested only once.
2. Drugs:
THIM was purchased from Merck (Germany) and Acros (Acros organic, Thermo fisher scientific, USA) companies. THIM administration was done on postnatal days (PND) 7, 9, 11, and 15 at the dose of (300 μg Hg/ kg ((per kg) in a volume of 50 μl, intramuscular into the glutei maximi.
3. Study Groups:
The 56 newborn rats were randomly divided into eight groups, and each group consisted of some subgroups. The schedule was as follows [15,16]: Group 1. Saline (1 ml/kg) was injected without exercise. Group 2. Saline (1 ml/kg) was injected plus protocol 1 exercise (PND 21-50). Group 3. Saline (1 ml/kg) was injected plus protocol 2 exercise (PND 51-80). Group 4. Saline (1 ml/kg) was injected plus protocol 1 exercise (PND 21-50) and protocol 2 exercise (PND 51-80). Group 5. THIM (300 μg Hg/kg) was injected without exercise Group 6. THIM 300 μg Hg/kg) was injected per injection plus protocol 1 exercise (PND 21-50). Group 7. THIM (300 μg Hg/kg) was injected per injection plus protocol 2 exercise (PND 51-80). Group 8- THIM (300 μg Hg/kg) was injected per injection plus protocol 1 exercise (PND 21-50) and protocol 2 exercise (PND 51-80).
4. Exercise Protocols:
4.1. Postnatal Teatment (Exercise Protocol 1):
The day of delivery was considered as PND. In PND groups, animals were put on the stationary treadmill (sedentary), pups from dams prenatally ran from PND 21 to PDN 50 (protocol 1). At first, the rat pups of the exercise group were familiarized with the treadmill with a speed of 2m/min for 10 min/day on days 1 and 2 of exercise. Next, the animals were forced to run on the treadmill for 30 min once a day for 18 days. The intensity of exercise applied for the exercise group was running at the speed of 2m/min for the first 5 min, 5m/min for the next 5 min, and the speed of 8 m/min for the last 20 min at an eight inclination. Control animals were put on stationary treadmills for the same period [17].
4.2 Exercise Protocol 2:
For this protocol, 51-80 days male Wistar rats were used at the beginning of all experiments. Rats ran as forced exercise on the treadmill at 0° inclination Sunday to Thursday during the light phase (9:00 am to 2:30 pm) for up to four weeks. The treadmills were equipped with grids that induce a slight electric shock (average intensity of 0-0.05 mA with an inter-pulse interval of 1-2 seconds) to force the animals to run constantly [18].
4.3. Exercise Protocol 1 + 2:
Protocol 1 was achieved on PND 21-50, and the juvenile rat did running exercise with protocol 2 on PND 51-80 (Figure-1).
Figure 1.
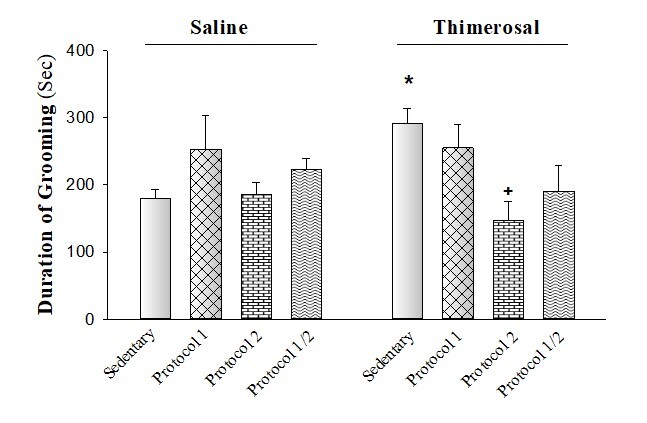
The effect of exercise protocols treatment on stereotypical behaviors (grooming) in the THIM-exposed rat. The rats received several kinds of exercise protocols in the presence or absence of THIM. The duration of grooming is an index of ASDlike behaviors. Seven rats were used in each group. The data are shown as mean±S.E.M. *P<0.05 as compared to the sedentary/saline group as the control group. +P<0.05 as compared to the sedentary/THIM group as a control group.
5. Grooming Behavior in Three-Chambered Social Interaction Room:
The duration of this stage was 10 minutes. The time of direct interactions between the subject rat and the containment lodging (stranger 1) or not lodging (stranger 2) rat individually and the duration of other behaviors such as selfgrooming was recorded and monitored.
6. Open Field Test (OFT):
The size of the open field cage in this study was 40 cm × 60 cm × 50 cm, which was prepared of white plastic. The cage floor was divided into 12 equal squares that were observable by the experimenter. A video camera was located on top of the apparatus to record the tests. Each rat was located in the center of the cage. The number of locomotor activities in the apparatus was counted for 5 min. The apparatus was cleaned with ethanol 70% after each test [19].
7. Ethical Issues:
Experiments were performed on Male Wistar rats (weighting 290-300 gr). The rats had free access to food and water and housed in room with an ambient temperature of 22±2°C and 12:12 hr light/dark cycle (lights on at 7.00 h). The animals were only handled for weighing, drug administration and cage cleaning (Co number: IR.IAU.LIAU.REC.1401.001). All procedures were approved by the Research and ethics committee of Rasht Branch, Islamic Azad University, Rasht, Iran (dissertation code: 117214423962001).
8. Statistical Analysis:
At first, data normality of distribution and homogeneity of variance were evaluated. To assess stereotype behaviors in the threechamber paradigm test and locomotor activity on the open field test, two-way ANOVA was applied. All statistical tests were directed using SPSS (SPSS Inc., Chicago,IL). P<0.05 was considered to show statistical significance.
Results
Effect of Exercise Protocols Treatment On Stereotyped Behaviors (Grooming) in the THIM-Exposed Rats
Two-way ANOVA followed by posthoc analysis revealed that administration of THIM increased the duration of grooming while only protocol 2 restored this response (THIM effect F [1, 48]=0.1, P<0.05; protocols effect F [3, 48]=3.25, P< 0.05; and THIM-protocol interaction effect F [3, 48]=3.69, P<0.05; Figure-1).
Effect of Exercise Protocols Treatment On Locomotor Activity in the THIM-Exposed Rat
Two-way ANOVA followed by posthoc analysis displayed that injection of THIM had no significant effect on locomotor activity (THIM effect F [1, 48]=2.85, P>0.05, protocols effect F [3, 48]=1.28, P>0.05; and THIM-protocol interaction effect F [3, 48]=1.14, P>0.05; Figure-2).
Figure 2.
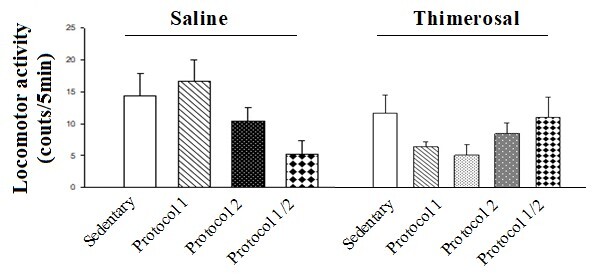
The effect of exercise protocols treatment on locomotor activity in the THIM-exposed rat. The animals received some kinds of exercise protocols in the presence or absence of THIM. Seven animals were used in each group. The data are exhibited as mean±S.E.M.
Discussion
This research evaluated the effects of durationintensity of exercise on stereotyped behaviors in autistic rats. According to studies, many physiological changes in THIM-treated animals are similar to the symptoms of ASD. ASD is identified according to the characteristic neurobehavioral patterns such as stereotyped behaviors and movement impairment [20,21], an increase in anxiety [20]. Thus in the present research, we measured these similar behaviors in a rat model of ASD. One of these behaviors is grooming. As we know, grooming is the main activity during the wakefulness phase of the rats [21]. Grooming usually manifests itself in response to unexpected stimuli, conflict situations, or frustration. This stress and conflict-related grooming are described as a displacement activity. Note that both novel and stressful stimuli can significantly augment the pituitaryadrenal system. This relationship between the novel and/or stressful stimuli and grooming as displacement activity is so important due to the potency of adrenocorticotropic hormone (ACTH) and other pituitary peptides (ACTH, melanocyte-stimulating hormone [MSH], and β-MSH) to induce excessive grooming through intraventricular administration[22]. In this study, we showed that THIM administration could alleviate the ASD-like behavioral time (grooming) compared to the control group. Previous studies have also shown that THIM increased repetitive and stereotyped behaviors, the core symptoms of ASD [23,24]. In these studies [23,24], high-intensity aerobic activity can worsen stereotypic behaviors in children with ASD, while mild- and moderate-intensity aerobic activities induce great reductions in these behaviors. Previous studies have revealed that physical activity can improve stereotyped behaviors [9,24]. It has been suggested that exercise-induced short-term decrease of stereotypic behaviors in individuals with ASD [14]. It has also been reported that a mere 3-min bout of running improves stereotypic behaviors in an autistic patient [25]. Furthermore, water-based exercise along with social skills training can dramatically improve the behaviors of patients with ASD [26]. Additionally, a previous study has reported that short- and low-moderate-intensity exercise in patients with ASD can significantly reduce self-stimulatory behaviors [26]. The therapeutic effect of exercise in ASD has been shown in many studies [27,28,29]. Unlike the present research, many previous studies have suggested that high-intensity exercise greatly decreased stereotyped behavior, and its therapeutic effect was much more than low-intensity exercise [26,30,31,32,33]. On the other hand, some research did not reveal a therapeutic effect of intensity exercise on stereotyped behavior in individuals who do a running/jogging protocol for up to fifteen minutes. This result may be associated with the intensity of the exercise [31]. Moreover, researchers have declared that disruptions in neurotransmitter systems such as serotonin and dopamine have a main role in the induction of stereotypical behaviors in ASD [34]. Evidence links stereotypical behaviors to the function and level of dopamine and serotonin. It has been revealed that hyperserotonaemia is interconnected with declarative abilities and self-injuries behaviors in patients with ASD [35]. Research indicates that physical activities significantly change dopaminergic and serotoninergic systems and decrease stereotypical behaviors in ASD [36]. Several other factors play a strong role in decreasing grooming behavior in autistic rats. It has been shown that N-methyl-D-aspartate (NMDA) gene can induce long-lasting changes in cognition and behaviors [37]. NMDA can improve ASD-like behaviors via balancing excitatory-inhibitory circuits [38]. Exercise can be mediated through Ca2+ influx via NMDA receptor, whose expression is enhanced following exercise. This process activates the mitogen-activated protein kinase (MAPK) cascade through Ca2+/ calmodulin-dependent protein kinase [39,40]. The activated MAPK affects many important factors, such as the cAMP response elementbinding protein that its expression is enhanced following exercise [41].
Conclusions
Treatment with THIM increased the stereotypical behaviors. Also, stereotypical behaviors were reversed by moderate exercise. Hence, moderate exercise may be a beneficial method for the treatment of ASD.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Association AP. USA: American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- Brugha T, Cooper SA, McManus S, Purdon S, Smith J, Scott F, et al. UK: The NHS Informaiton Centre; 2012. [Google Scholar]
- Lever AG, Geurts HM. Psychiatric co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. J Autism Dev Disord. 2016;6(46):1916–30. doi: 10.1007/s10803-016-2722-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petschenka G, Agrawal AA. How herbivores coopt plant defenses: natural selection, specialization, and sequestration. Curr Opin Insect Sci. 2016;(14):17–24. doi: 10.1016/j.cois.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Geier DA, Kern JK, King PG, Sykes LK, Geier MR. A case-control study evaluating the relationship between Thimerosal-containing Haemophilus influenzae Type b vaccine administration and the risk for a pervasive developmental disorder diagnosis in the United States. Biol Trace Elem Res. 2015;1-2(163):28–38. doi: 10.1007/s12011-014-0169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson P, Auty D, Ayres D, Backhouse C, Barr G, Betancourt M, et al. Improved search for muon-neutrino to electron-neutrino oscillations in MINOS. Phys Rev Lett. 2011;18(107):181802–181802. doi: 10.1103/PhysRevLett.107.181802. [DOI] [PubMed] [Google Scholar]
- Gallagher C, Goodman M. Hepatitis B triple series vaccine and developmental disability in US children aged 1–9 years. Toxicological and Environmental Chemistry. 2008;5(90):997–1008. [Google Scholar]
- Young HA, Geier DA, Geier MR. Thimerosal exposure in infants and neurodevelopmental disorders: an assessment of computerized medical records in the Vaccine Safety Datalink. J Neurol Sci. 2008;1-2(271):110–8. doi: 10.1016/j.jns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Movahedi A, Marandi SM, Abedi A. Kata techniques training consistently decreases stereotypy in children with autism spectrum disorder. Res Dev Disabil. 2012;4(33):1183–93. doi: 10.1016/j.ridd.2012.01.018. [DOI] [PubMed] [Google Scholar]
- Bahrami F, Movahedi A, Marandi SM, Sorensen C. The effect of karate techniques training on communication deficit of children with autism spectrum disorders. J Autism Dev Disord. 2016;3(46):978–86. doi: 10.1007/s10803-015-2643-y. [DOI] [PubMed] [Google Scholar]
- Pan C-Y, Tsai C-L, Hsieh K-W. Physical activity correlates for children with autism spectrum disorders in middle school physical education. Res Q Exerc Sport. 2011;3(82):491–8. doi: 10.1080/02701367.2011.10599782. [DOI] [PubMed] [Google Scholar]
- Iliadis I, Apteslis N. The role of physical education and exercise for children with Autism Spectrum Disorder and the effects on socialization, communication, behavior, fitness, and quality of life. Dialogues in Clinical Neuroscience & Mental Health. 2020;1(3):71–81. [Google Scholar]
- Sorensen C, Zarrett N. Benefits of physical activity for adolescents with autism spectrum disorders: A comprehensive review. Review Journal of Autism and Developmental Disorders. 2014;4(1):344–53. [Google Scholar]
- Petrus C, Adamson SR, Block L, Einarson SJ, Sharifnejad M, Harris SR. Effects of exercise interventions on stereotypic behaviours in children with autism spectrum disorder. Physiother Can. 2008;2(60):134–45. doi: 10.3138/physio.60.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro MFH, Souza JMO, Grotto D, Batista BL, de Oliveira, Barbosa Jr. A systematic study of the disposition and metabolism of mercury species in mice after exposure to low levels of thimerosal (ethylmercury) Environ Res. 2014;(134):218–27. doi: 10.1016/j.envres.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Hornig M, Chian D, Lipkin WI. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry. 2004;9(9):833–45. doi: 10.1038/sj.mp.4001529. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Ko I-G, Kim B-K, Kim T-W, Kim S-E, Shin M-S, et al. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol Behav. 2010;5(101):660–5. doi: 10.1016/j.physbeh.2010.09.021. [DOI] [PubMed] [Google Scholar]
- Zagaar M, Alhaider I, Dao A, Levine A, Alkarawi A, Alzubaidy M, et al. The beneficial effects of regular exercise on cognition in REM sleep deprivation: behavioral, electrophysiological and molecular evidence. Neurobiol Dis. 2012;3(45):1153–62. doi: 10.1016/j.nbd.2011.12.039. [DOI] [PubMed] [Google Scholar]
- Ebrahimi-Ghiri M, Shahini F, Khakpai F, Zarrindast MR. Antinociceptive and antidepressive efficacies of the combined ineffective doses of S-ketamine and URB597. Naunyn Schmiedebergs Arch Pharmacol. 2019;11(392):1393–400. doi: 10.1007/s00210-019-01676-5. [DOI] [PubMed] [Google Scholar]
- Gjevik E, Eldevik S, Fjæran-Granum T, Sponheim E. Kiddie-SADS reveals high rates of DSM-IV disorders in children and adolescents with autism spectrum disorders. J Autism Dev Disord. 2011;6(41):761–9. doi: 10.1007/s10803-010-1095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples KL, Reid G. Fundamental movement skills and autism spectrum disorders. J Autism Dev Disord. 2010;2(40):209–17. doi: 10.1007/s10803-009-0854-9. [DOI] [PubMed] [Google Scholar]
- Dodonova S, Bobyntsev I, Belykh A, Telegina I, Muzaleva YA, Andreeva L, et al. Effects of peptides ACTH6–9 PGP and ACTH4–7-PGP on anxiety levels in rats in punished and unpunished behavior. Neuroscience and Behavioral Physiology. 2020;9(50):1203–8. [Google Scholar]
- Olczak M, Duszczyk M, Mierzejewski P, Meyza K, Majewska MD. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav Brain Res. 2011;1(223):107–18. doi: 10.1016/j.bbr.2011.04.026. [DOI] [PubMed] [Google Scholar]
- Schmitz SO, McFadden BA, Golem DL, Pellegrino JK, Walker AJ, Sanders DJ, et al. The Effects of Exercise Dose on Stereotypical Behavior in Children with Autism. Med Sci Sports Exerc. 2017;5(49):983–90. doi: 10.1249/MSS.0000000000001197. [DOI] [PubMed] [Google Scholar]
- Gordon R, Handleman JS, Harris SL. The effects of contingent versus non-contingent running on the out-of-seat behavior of an autistic boy. Child & family behavior therapy. 1986;3(8):37–44. [Google Scholar]
- Kern L, Koegel RL, Dunlap G. The influence of vigorous versus mild exercise on autistic stereotyped behaviors. Journal of autism and developmental disorders. 1984;1(14):57–67. doi: 10.1007/BF02408555. [DOI] [PubMed] [Google Scholar]
- Brand S, Jossen S, Holsboer-Trachsler E, Puehse U, Gerber M. Impact of aerobic exercise on sleep and motor skills in children with autism spectrum disorders–a pilot study. Neuropsychiatr Dis Treat. 2015;(11):1911–1911. doi: 10.2147/NDT.S85650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer E, Lloyd M. School-based fundamental-motor-skill intervention for children with autism-like characteristics: an exploratory study. Adapt Phys Activ Q. 2016;1(33):66–88. doi: 10.1123/APAQ.2015-0009. [DOI] [PubMed] [Google Scholar]
- Ketcheson L, Hauck J, Ulrich D. The effects of an early motor skill intervention on motor skills, levels of physical activity, and socialization in young children with autism spectrum disorder: A pilot study. Autism. 2017;4(21):481–92. doi: 10.1177/1362361316650611. [DOI] [PubMed] [Google Scholar]
- Lang R, Koegel LK, Ashbaugh K, Regester A, Ence W, Smith W. Physical exercise and individuals with autism spectrum disorders: A systematic review. Research in Autism Spectrum Disorders. 2010;4(4):565–76. [Google Scholar]
- Levinson LJ, Reid G. The effects of exercise intensity on the stereotypic behaviors of individuals with autism. Adapted Physical Activity Quarterly. 1993;3(10):255–68. [Google Scholar]
- Celiberti DA, Bobo HE, Kelly KS, Harris SL, Handleman JS. The differential and temporal effects of antecedent exercise on the self-stimulatory behavior of a child with autism. Res Dev Disabil. 1997;2(18):139–50. doi: 10.1016/s0891-4222(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Elliott RO, Dobbin AR, Rose GD, Soper HV. Vigorous, aerobic exercise versus general motor training activities: Effects on maladaptive and stereotypic behaviors of adults with both autism and mental retardation. J Autism Dev Disord. 1994;5(24):565–76. doi: 10.1007/BF02172138. [DOI] [PubMed] [Google Scholar]
- Schønecker B, Heller KE. The involvement of dopamine (DA) and serotonin (5-HT) in stress-induced stereotypies in bank voles (Clethrionomys glareolus) Appl Anim Behav Sci. 2001;4(73):311–9. doi: 10.1016/s0168-1591(01)00143-5. [DOI] [PubMed] [Google Scholar]
- Lanovaz MJ. Towards a comprehensive model of stereotypy: Integrating operant and neurobiological interpretations. Res Dev Disabil. 2011;2(32):447–55. doi: 10.1016/j.ridd.2010.12.026. [DOI] [PubMed] [Google Scholar]
- Meeusen R, De Meirleir. Exercise and brain neurotransmission. Sports Med. 1995;3(20):160–88. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- Lim AL, Taylor DA, Malone DT. Consequences of early life MK-801 administration: long-term behavioural effects and relevance to schizophrenia research. Behav Brain Res. 2012;1(227):276–86. doi: 10.1016/j.bbr.2011.10.052. [DOI] [PubMed] [Google Scholar]
- Mohammadi S, Asadi-Shekaari M, Basiri M, Parvan M, Shabani M, Nozari M. Improvement of autistic-like behaviors in adult rats prenatally exposed to valproic acid through early suppression of NMDA receptor function. Psychopharmacology (Berl) 2020;1(237):199–208. doi: 10.1007/s00213-019-05357-2. [DOI] [PubMed] [Google Scholar]
- Soderling TR. CaM-kinases: modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;3(10):375–80. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- Argiolas A, Melis MR, Murgia S, Schiöth HB. ACTH-and α-MSH-induced grooming, stretching, yawning and penile erection in male rats: site of action in the brain and role of melanocortin receptors. Brain Res Bull. 2000;5(51):425–31. doi: 10.1016/s0361-9230(99)00270-1. [DOI] [PubMed] [Google Scholar]
- Molteni R, Ying Z, Gómez‐Pinilla F. Differential effects of acute and chronic exercise on plasticity‐related genes in the rat hippocampus revealed by microarray. Eur J Neurosci. 2002;6(16):1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]


