Abstract
The historical association between respiratory infections and neuropsychiatric symptoms dates back centuries, with more recent literature highlighting a link between viral infections and schizophrenia. Maternal influenza infection during pregnancy has been associated with the development of schizophrenia in offspring. Viral infections in neonates, children, and adolescents have also been associated with later development of schizophrenia. Neuroinvasive and/or systemic infections are thought to increase risk for psychopathology via inflammatory mechanisms, particularly when exposure occurs during critical neurodevelopmental windows. Several human coronaviruses (HCoVs) have been associated with psychotic disorders and increasing reports of the neuropsychiatric manifestations of COVID-19 suggest it has neuroinvasive properties similar to those of other HCoVs. These properties, in conjunction with its ability to generate a massive inflammatory response, suggest that COVID-19 may also contribute to future psychopathology. This review will summarize the psychopathogenic mechanisms of viral infections and discuss the neuroinvasive and inflammatory properties of COVID-19 that could contribute to the development of psychotic disorders, with a focus on in utero, neonatal, and childhood exposure.
Keywords: Coronavirus, COVID-19, Inflammation, Influenza, Maternal infection, Neurodevelopment, Pregnancy, Psychosis, SARS-CoV-2, Schizophrenia, Viral infections
1. Introduction
Infectious theories of mental illness were introduced in the 19th century and have been frequently revisited (Yolken and Torrey, 1995). Observations of neuropsychiatric symptoms in respiratory illness date back even further, to the 1385 German flu pandemic (Menninger, 1919). Literature from subsequent pandemics characterize the flu not as a respiratory illness, but a multisystem disease with inherent neuropsychiatric effects including depression, insomnia, and psychosis (Honigsbaum, 2013). Psychotic symptoms reported in this earlier literature were largely self-limited and most famously described in Karl Menninger’s 1919 case series on psychosis following the Spanish flu, noting that most psychotic symptoms had resolved by the five-year follow-up (Menninger, 1919, 1926). It was not until the 1990s that epidemiological observations revealing a correlation between late winter/early spring births and adult schizophrenia generated greater interest in the notion that viral respiratory infections could be a risk factor for persistent mental illness, specifically schizophrenia (Yolken and Torrey, 1995). Since then, several extensively reviewed birth cohort studies have linked maternal influenza infection during pregnancy to development of schizophrenia in offspring (Boksa, 2008; Brown, 2006; Kępińska et al., 2020; Selten and Termorshuizen, 2017). Childhood and peripubertal infections have also been characterized as risk factors for adult-onset psychosis (Dalman et al., 2008; Hickie and Banati, 2009; Khandaker et al., 2012). The relevance of these findings is of mounting importance, particularly as a number of immunologic disturbances have been described in adults with schizophrenia (Howes, 2017; Müller, 2018).
Now, over a century after the Spanish flu, we face an equally alarming threat in the COVID-19 pandemic. Though primarily thought of as a respiratory virus, it too has been shown to damage multiple organ systems, including the nervous system (Wu et al., 2020). Rising reports of neuropsychiatric manifestations of COVID-19 have surfaced, including delirium (Beach et al., 2020), encephalitis (Li et al., 2020d), strokes (Morassi et al., 2020), and smell and taste abnormalities (Machado and Gutierrez, 2020). Others have already drawn attention to the possibility of long-term neuropsychiatric sequelae from COVID-19 (Cowan, 2020; Taquet et al., 2021; Troyer et al., 2020), including psychosis, which was linked to different forms of coronaviruses in one retrospective study (Severance et al., 2011). This review will expand on the link between viral respiratory illness and psychosis, with a discussion of the inflammatory model of schizophrenia, psychopathogenic mechanisms of viral infections, and COVID-19 literature suggestive of psychiatric sequelae. Given the evidence that risk for future psychopathology is mediated by timing of exposure (Ratnayake et al., 2013), we will focus on the impact of individual infection during neurodevelopmentally vulnerable periods and of maternal infection on neonates.
2. Inflammatory model of schizophrenia
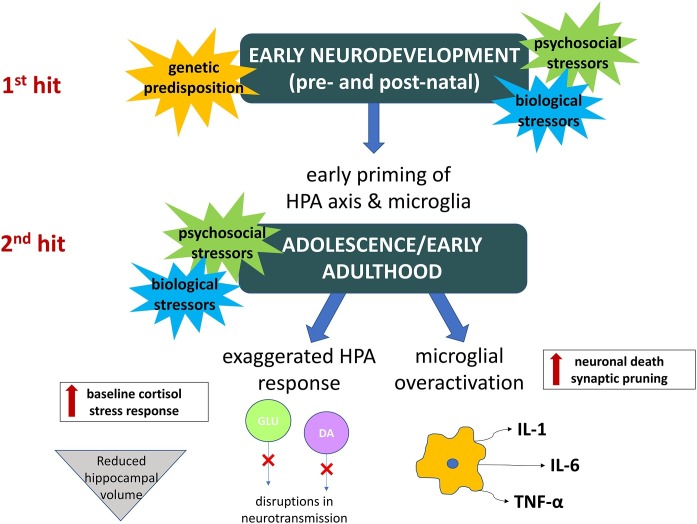
Genetic predisposition, psychosocial stress, and biological insults are all thought to play a synergistic role in the pathophysiology of schizophrenia (Walker and Diforio, 1997). Stress during both pre- and postnatal neurodevelopment is particularly impactful and primes individuals for a pathological response to a second “hit,” typically during adolescence or early adulthood (Maynard et al., 2001). For example, exposure to prenatal maternal infection followed by psychological trauma in adolescence has been associated with a higher risk of developing schizophrenia than either insult alone (Debost et al., 2017). Both psychosocial and biological stressors, the latter including infection and immune activation, cause hypothalamic-pituitary-adrenal (HPA) axis dysregulation, which has been linked to abnormalities observed in schizophrenia. These include elevated baseline cortisol, reduced hippocampal volume, and disturbances in dopamine and glutamate transmission (Popoli et al., 2012; Pruessner, 2017). Immune activation also has more direct implications in schizophrenia, illustrated by shared genetic risk factors with autoimmune disease (Pouget et al., 2019), presence of inflammatory biomarkers, and evidence of central nervous system (CNS) inflammation in response to both local and systemic infection (Müller, 2018). Microglia, the brain’s innate immune cells, release inflammatory cytokines in response to stress or infection and, like the HPA axis, can be primed by early stressors to have an exaggerated response to later insults. Microglial overactivation is also implicated in schizophrenia and may result in aberrant synaptic pruning and altered neurotransmitter metabolism secondary to excess cytokine release (Howes, 2017; Raison et al., 2017). Fig. 1 is a pictorial representation of how constitutional (e.g., biological and genetic) and environmental factors intersect in promoting an inflammatory substrate for the development of psychotic illness.
Fig. 1.
Inflammatory model of psychotic illness.
Perinatal factors (constitutional and environmental) may prime the individual to be sensitized (1st hit) to the effects of future stressors (2nd hit), which may inform long-lasting changes predisposing to psychopathology. Relevant changes include maintenance of stress response impacting neurodevelopment (e.g., reduced hippocampal volume), alterations in circulating neurotransmitter levels (e.g., dopamine and glutamate), and heightened immune activation, with overexpression of cytokines and a sustained inflammatory substrate that may lead to aberrant neuronal pruning and cell death. The combination of these factors underlies a possible inflammatory basis for development of psychotic illness.
Key: DA = dopamine, GLU = glutamate, HPA = hypothalamic-pituitary-adrenal, IL = interleukin, TNF = tumor necrosis factor.
3. Viral infections and schizophrenia
Viruses damage the CNS via both direct invasion and immune activation. This can lead to acute neuropsychiatric effects from processes such as encephalitis and meningitis (Somand and Meurer, 2009), with the potential for long-term psychopathology.
3.1. Viral neuroinvasion
Viruses have developed several tactics to access the CNS. They infect leukocytes that carry them across the blood-brain barrier (BBB), hijack retrograde axonal transport machinery from peripheral nerves, bind CNS endothelial proteins to gain entry, and invade the olfactory nerve (McGavern and Kang, 2011). Neurotropic viruses linked to schizophrenia include influenza, herpesviruses, enteroviruses, and retroviruses, among others (Fatemi, 2009). In patients with schizophrenia, antigens from such viruses have been found in post-mortem brain tissue (Stevens et al., 1983) and antibody titers against them have been shown to be higher than in healthy controls (Krause et al., 2010). There is also evidence that viruses alter dopamine metabolism (Jang et al., 2009; Li et al., 2006) and affect glutamate transmission via molecular mimicry at N-methyl-d-aspartate (NMDA) receptors (Kannan et al., 2017; Kępińska et al., 2020), leading to changes that may inform psychotic symptoms.
3.2. Immune activation
The sheer number of viruses implicated in schizophrenia indicates that the causative link is likely not pathogen-specific, but immune-mediated (Müller, 2010). Because CNS infection is so threatening to an organism, the local immune response tends to be robust. Local CNS response is also triggered by systemic infection, which causes microglial activation and cytokine release in the brain (Raison et al., 2017). Proinflammatory cytokines are elevated in both the blood and cerebrospinal fluid (CSF) of patients with schizophrenia, with serum interleukin (IL) 6 notably linked to both presence and severity of disease (Müller, 2018, 2010). Complement, which plays a role in synaptic pruning, has also been found to be elevated in first-episode psychosis patients with poor treatment response (Druart and Le Magueresse, 2019; Mondelli et al., 2020).
3.3. Critical periods
Effects on the CNS appear to be greatest when infections occur in critical neurodevelopmental windows such as childhood and adolescence (Blomstrom et al., 2014; Hickie and Banati, 2009). A large meta-analysis showed a nearly two-fold increased risk of adult nonaffective psychosis associated with childhood CNS infection, particularly viral infection (Khandaker et al., 2012). Cohort studies of children hospitalized for any type of infection showed highest risk of later psychosis in those infected under age three or in preadolescence (Blomstrom et al., 2014; Liang and Chikritzhs, 2012). Elevated childhood IL-6 correlates with later psychosis risk in a dose-dependent manner (Khandaker et al., 2014) and both childhood and adolescent elevations in the inflammatory marker C-reactive protein (CRP) are associated with adult schizophrenia (Metcalf et al., 2017; Müller, 2018).
4. Maternal infection and schizophrenia
Fetal brain development begins during the third week of gestation, with the most rapid period of myelination occurring in the first two postnatal years (Cordeiro et al., 2015). Thus, maternal infection, inflammation, and stress during the prenatal and neonatal periods carry the greatest risk for the fetus/infant. Importantly, maternal infection is the most robustly supported infectious risk factor for schizophrenia (Kępińska et al., 2020). Some viruses have the potential to directly infect the fetus in utero. The TORCH pathogens (Toxoplasmosis, Other, Rubella, Cytomegalovirus, and Herpes), for example, are linked to schizophrenia and implicated in fetal brain anomalies and neurodevelopmental disorders (al-Haddad et al., 2019; Brown et al., 2005; Brown, 2006; Fuglewicz et al., 2017). Influenza, on the other hand, has only rarely been reported to cross the placenta (Brown et al., 2004; Lieberman et al., 2011; Yawn et al., 1971), yet there is a large body of literature associating maternal influenza with schizophrenia in offspring (Kępińska et al., 2020). The association of maternal respiratory infection and schizophrenia in offspring has been demonstrated primarily in the first two trimesters, with some conflicting evidence as to whether first- or second-trimester infection confers greater risk (Brown et al., 2004, 2000; Mednick, 1988).
4.1. Maternal immune activation
As with childhood and later infection, the role that maternal infection plays in the risk of offspring developing schizophrenia is thought to be more related to inflammation than to any one pathogen (Brown, 2006). Elevation in inflammatory cytokines following infection in pregnancy is termed maternal immune activation (Boulanger-Bertolus et al., 2018). Many of these cytokines are implicated in neurodevelopmental regulation (Fatemi, 2009), and indeed maternal immune activation has been associated with abnormalities in dopaminergic and GABAergic neurons, microglia, and Schwann cells in offspring (Kępińska et al., 2020). Increased levels of maternal IL-8 and CRP in humans are related to development of schizophrenia in offspring (Brown et al., 2004; Canetta et al., 2014). Maternal fever, which supports immune response, may play a role as well. Hyperthermia poses greatest risk to the CNS in early development (Edwards, 2007) when high temperatures can disrupt neuronal migration (Hinoue et al., 2001). The placenta, which serves to transfer oxygen, nutrients, and other needed substances from mother to fetus, can also transport inflammatory mediators and pathogenic antibodies generated during infection (al-Haddad et al., 2019; Donnelly and Campling, 2019; Kępińska et al., 2020).
5. Human coronaviruses and nervous system involvement
Since its identification in December 2019, the SARS-CoV-2 virus responsible for COVID-19 has infected over 130 million people worldwide (World Health Organization, 2021). It is the newest member of the human coronavirus (HCoV) group, identified in the 1960s (Mahase, 2020). The most common HCoV infections present as simple colds (Kahn, 2006), but prior to COVID-19 there were two HCoVs that caused outbreaks of severe respiratory disease. Severe Acute Respiratory Syndrome (SARS) infected about 8000 people from 2002 to 2004 and Middle East Respiratory Syndrome (MERS) infected roughly 2500 people between 2012 and 2018 (National Institute of Allergy and Infectious Diseases, 2020). COVID-19 has greatly surpassed these outbreaks in both incidence and infectivity, bringing unprecedented attention to HCoVs. Increasing attention has been paid to its neuropsychiatric complications, which can range from mild to severe, and include headaches and dizziness (Mao et al., 2020; Zhu et al., 2020), delirium (Beach et al., 2020), meningitis, encephalitis (Li et al., 2020d; Steardo et al., 2020), seizures (Mao et al., 2020), and strokes (Morassi et al., 2020). A substantial portion of those affected have reported fatigue, impaired cognition, anxiety, and depression, with symptoms often persisting for months beyond initial infection (Graham et al., 2021; Huang et al., 2021a; Mazza et al., 2021; Taquet et al., 2021). These reports have understandably alarmed both the medical community and public. As described with influenza, HCoVs can significantly impact the nervous system.
5.1. Neuropsychiatric complications of HCoVs
Although SARS and MERS were known as respiratory infections, both had the ability to infect the nervous system (Li et al., 2016; Netland et al., 2008). Complications included peripheral neuropathies (Tsai et al., 2004), encephalopathies, motor deficits, paralysis, and coma (Arabi et al., 2015; Wu et al., 2020). Psychiatric manifestations included depression, anxiety, mania, and psychosis, though the former two were thought to be related to the stress of having a life-threatening and stigmatizing illness, while the latter two were nearly always associated with steroid use (Cheng et al., 2004; Rogers et al., 2020). The most compelling data linking HCoVs to psychosis are from Severance et al. (2011), who found that individuals with psychotic symptoms and a diagnosed mood or psychotic disorder had significantly higher seropositivity rates for the endemic HCoVs. Given that these HCoVs typically produce mild illness and that inflammation is the unifying link between viral infections and psychosis, it is suspected that COVID-19, a more severe infection capable of inducing a massive inflammatory response (Steardo et al., 2020), is even more likely to contribute to future psychotic symptoms. Though it is unclear if there are data linking SARS to later-onset or chronic psychosis, those most at risk for this outcome (i.e., neonates and children) would have only recently entered the period of late adolescence or early adulthood, when psychotic disorders often emerge. In the next section we will outline the neuroinvasive and inflammatory features of COVID-19 most relevant to the pathogenesis of schizophrenia and discuss their impact in the populations most likely to be affected.
6. Neuroinvasion and inflammation in COVID-19
SARS-CoV-2 is thought to have similar neurotropism to SARS-CoV and likely enters the CNS via the hematogenous route (Baig, 2020) or via peripheral neurons, using retrograde axonal transport to reach the brain (Wu et al., 2020). The two share 79 % genomic homogeneity (Kolifarhood et al., 2020) and the same cell entry receptor, angiotensin-converting enzyme 2 (ACE2). ACE2 is expressed in both neurons and glia (Steardo et al., 2020) and has a ten- to twenty-fold higher binding affinity for SARS-CoV-2 than SARS-CoV (Wrapp et al., 2020).
6.1. Role of ACE2
While overall ACE2 expression in the CNS is low (Li et al., 2020c), there are several areas of relatively higher expression. These include dopaminergic and serotonergic nuclei, glutamatergic neurons, and the lateral ventricles. In the substantia nigra, the percent expression of ACE2 approaches that of the lungs (Chen et al., 2021). These areas are neurochemically and structurally implicated in schizophrenia (DeLisi et al., 2006; Stępnicki et al., 2018; Walker et al., 2018), and while damage to them is not necessarily causative, their relative vulnerability to infection could play a role in shaping future psychopathology. ACE2 also shares significant genetic co-expression with dopamine decarboxylase, an enzyme involved in dopamine and serotonin synthesis. SARS-CoV was shown to downregulate ACE2, and it has been suggested that the ability of SARS-CoV-2 to do the same could result in altered neurotransmitter levels (Nataf, 2020; Verdecchia, 2020).
6.2. Demyelination
Increasing numbers of case reports and series describe COVID-19 patients with associated demyelinating disease (Maury et al., 2021). Both peripheral and central nervous system pathologies have been described, including Guillain-Barré syndrome and acute demyelinating encephalomyelitis (Brun et al., 2020; Mackenzie et al., 2021; Palaiodimou et al., 2021; Toscano et al., 2020; Zuberbühler et al., 2021). Several demyelinating diseases are associated with psychotic symptoms. Likewise, demyelination is thought to play a role in the pathophysiology of schizophrenia, in which CNS white matter, composed largely of myelin, is reduced (Mighdoll et al., 2015). The effects of demyelination may be especially damaging during adolescence and early adulthood, when myelination is critical to the development of cognitive function (Xu and Li, 2011).
6.3. Olfactory dysfunction
Anosmia (complete loss of sense of smell) and hyposmia (decreased sense of smell) are widely associated with COVID-19, even in mild and minimally symptomatic cases (Lechien et al., 2020; Soler et al., 2020). Prevalence of olfactory dysfunction appears greater in COVID-19 compared to other respiratory viruses (Printza and Constantinidis, 2020) and is likely underestimated according to studies using objective chemosensory testing in anosmic/hyposmic COVID-19 patients (Moein et al., 2020; Vaira et al., 2020). The olfactory system’s direct connection to the CNS can provide a pathway for neurotropic agents (Machado and Gutierrez, 2020) and olfactory dysfunction is associated with both neurodegenerative diseases and schizophrenia (Doty, 2017; Moberg, 1999). Importantly, the spontaneous improvement of anosmia and hyposmia in upwards of 80–90% of COVID-19 patients suggests that the virus damages olfactory epithelium, not olfactory neurons (which do not express ACE2) (Machado and Gutierrez, 2020; Parente-Arias et al., 2021; Sayin and Yazici, 2020; Soler et al., 2020; Yan et al., 2020). However, olfactory dysfunction in COVID-19 may still be a harbinger of neuroinflammation. It has been suggested that olfactory ensheathing glial cells found in the olfactory nerve and bulb (Barnett, 2004) could serve as an intermediary between ACE2-expressing olfactory epithelial cells and olfactory neurons, inciting a local immune response (Moein et al., 2020). Because olfactory bulb microglia are in a constitutively primed state (Doty, 2017), their activation could trigger a cytokine cascade and more widespread neuroinflammation (Majde, 2010).
6.4. Systemic inflammatory response
The ability of COVID-19 to generate a massive systemic inflammatory response known as a cytokine storm is arguably its most destructive feature. This immune response results in multiorgan damage and, in many cases, death (Ahmadpoor and Rostaing, 2020). Even in milder disease, proinflammatory cytokines appear to be upregulated (Sarzi-Puttini et al., 2020) with multiple studies showing elevations in serum IL-2, IL-6, IL-8, IL-1β, tumor necrosis factor (TNF) α, and interferon (IFN) γ (Cao, 2020; Capecchi et al., 2020; Wan et al., 2020). Both infection itself and hypoxia stimulate cytokine release, which can increase BBB permeability (Mukandala et al., 2016; Pan et al., 2011). COVID-19 is also associated with complement activation (Cao, 2020) and increased CRP, erythrocyte sedimentation rate (ESR), and ferritin (Chen et al., 2020b; Liu et al., 2020a; Zhu et al., 2020), all of which indicate systemic inflammation. As discussed in Section 2, systemic inflammatory markers are elevated in schizophrenia, with the potential for a neuroinflammatory response (Müller, 2018).
6.5. Neuropsychiatric symptoms in “long-haulers”
A subset of COVID-19 patients known as “long-haulers” have reported persisting neuropsychiatric symptoms for months following acute infection. These include fatigue, “brain fog,” anxiety, depression, and psychosis (Graham et al., 2021; Huang et al., 2021a; Lopez-Leon et al., 2021). This phenomenon is estimated to affect over 80 % of hospitalized patients (Graham et al., 2021) and is similar to post-viral syndromes observed in prior flu pandemics (Honigsbaum and Krishnan, 2020). Remarkably, long-term neuropsychiatric symptoms of COVID-19 appear to occur even in mildly symptomatic individuals, with an estimated 10 % of all patients experiencing lasting symptoms (Huang et al., 2021b; Kingstone et al., 2020; Rubin, 2020).
While new-onset psychopathology associated with COVID-19 could be driven largely by the psychological toll of being ill, the severity and prevalence of post-viral neuropsychiatric symptoms suggest that the virus may trigger a protracted immune response (Baig, 2020). Indeed, Mazza et al. (2021) found that systemic inflammation predicted depressive symptomatology and cognitive impairment three months following acute COVID-19 infection. The risk of psychiatric sequelae also appears uniquely elevated in COVID-19, as demonstrated in a large, matched cohort study in which risk of a new psychiatric diagnosis in the six months following COVID-19 infection was greater than that following other respiratory infections (Taquet et al., 2021).
6.6. Multisystem inflammatory syndrome in children
Severe COVID-19 infection was initially thought to spare children, the majority of whom have presented with milder symptoms and less inflammatory marker elevation compared to adults (Dong et al., 2020b; Leung, 2020; Ludvigsson, 2020). However, in April of 2020 the first case of COVID-19-related Kawasaki disease, an inflammatory illness that typically affects young children, was reported in a six-month-old girl (Jones et al., 2020). Since then, more reports of atypical Kawasaki-like inflammatory syndromes in children with COVID-19 have surfaced and have been termed Multisystem Inflammatory Syndrome in Children (MIS-C) (Centers for Disease Control and Prevention, 2020). Many of these cases are associated with elevated inflammatory markers including procalcitonin, ferritin, and CRP (Deza Leon et al., 2020; Licciardi et al., 2020; Riphagen et al., 2020). Although MIS-C is not associated with the majority of childhood COVID-19 infections, the acuity of the inflammatory response in those affected is worrisome given the associations between childhood infection, inflammation, and later-onset psychosis (Blomstrom et al., 2014; Khandaker et al., 2014; Liang and Chikritzhs, 2012). A large study of children and adolescents hospitalized for COVID-19 or MISC-C also showed that 22 % had neurologic involvement, raising concern for neuroinvasion and locally incited neuroinflammation (LaRovere et al., 2021). Finally, the emergence of the highly transmissible SARS-CoV-2 B117 strain has generated growing concern that pediatric infection rates will rise, particularly as schools continue to re-open (Ratmann et al., 2021).
7. Maternal COVID-19 infection
Most current data on maternal and neonatal outcomes in COVID-19 infection are limited to third trimester findings (Ashraf et al., 2020; Islam et al., 2020; Karimi et al., 2021; Liu et al., 2020b; Wang et al., 2020a). While previous literature suggests that maternal infection in earlier trimesters confers greater risk of schizophrenia in offspring (Brown et al., 2004, 2000), the literature on maternal COVID-19 infection still highlights a number of concerning features.
7.1. Vertical transmission and neonatal infection
As with influenza, HCoVs are not strongly suspected to cross the placenta. SARS and MERS showed no evidence of vertical transmission (Di Mascio et al., 2020) and the majority of case series and studies of maternal COVID-19 infection have failed to demonstrate infection in neonates, placentae, amniotic fluid, or cord blood (Baergen and Heller, 2020; Chi et al., 2021; Edlow et al., 2020; Karimi-Zarchi et al., 2020; Khan et al., 2020; Liu et al., 2020c; Muhidin et al., 2020; Pereira et al., 2020; Schwartz, 2020; Yang et al., 2020). However, there are rare reports of SARS-CoV-2 genome detection in umbilical cord blood, placentae, vaginal mucosa, breastmilk, and amniotic fluid, supporting the possibility of vertical transmission (Fenizia et al., 2020; Halici-Ozturk et al., 2021; Penfield et al., 2020; Sukhikh et al., 2021). In most reported neonatal infections, SARS-CoV-2 reverse transcriptase polymerase chain reaction (RT-PCR) testing was not done early enough to confirm vertical transmission (Ghema et al., 2021; Savasi et al., 2020; Wang et al., 2020b; Zeng et al., 2020b), though some have highlighted the possibility of false negatives in early infection (Robaina-Castellanos, 2021). There have also been reports of RT-PCR-negative infants with elevated SARS-CoV-2 IgM, an antibody too large to cross the placenta and therefore thought to signal possible in utero infection (Bwire et al., 2021; Dong et al., 2020a; Zeng et al., 2020a). This is controversial, however, as IgM has a high false positive rate in diagnosing congenital infections (Kimberlin and Stagno, 2020).
Most documented deliveries in women with COVID-19 occurred by Cesarean section (C-section) due to the debated belief that it can reduce vertical transmission risk (American College of Obstetrics and Gynecology, 2021; Ashraf et al., 2020; Chi et al., 2021; Fenizia et al., 2020; Ghema et al., 2021; Zaigham and Andersson, 2020). However, there are multiple reports of vaginal births with no detection of neonatal infection as well (Chen et al., 2020d; Cribiù et al., 2021; Muhidin et al., 2020). While vertical transmission of SARS-CoV-2 is thought to be unlikely or at least rare (Duran et al., 2020; Edlow et al., 2020; Islam et al., 2020; Makvandi et al., 2021), infants born to COVID-19-positive mothers have been shown to have elevated IL-6, CRP, and ESR, even when they themselves test negative for the infection (Dong et al., 2020a; Yu et al., 2020; Zeng et al., 2020a). Whether these abnormalities stem from undetected neonatal infection or maternal immune activation, early biological stressors may prime neonates for an increased risk of developing psychotic illness after exposure to later stressors (Howes, 2017).
7.2. Maternal immune activation in COVID-19
The physiologic and immune changes that occur during pregnancy can result in an increased severity of viral infections, as noted during the SARS and MERS pandemics (Dashraath et al., 2020). COVID-19 infection has not appeared to be more severe in pregnant women than in the general population (Savasi et al., 2020; Islam et al., 2020; de Vasconcelos Gaspar and Santos Silva, 2021). However, COVID-19-positive pregnant women still commonly present with fever and elevated CRP (Breslin et al., 2020; Muhidin et al., 2020; Pereira et al., 2020; Zaigham and Andersson, 2020). First- and third-trimester gestational periods are also characterized as proinflammatory states, which could potentiate the impact of cytokine elevations seen in even mild infections (Uc, 2020). IL-6 elevation has been repeatedly observed in COVID-19 infection (Capecchi et al., 2020; Liu et al., 2020a; Shi et al., 2020), including during pregnancy (Yin et al., 2020), and serves as a marker of maternal immune activation that has been implicated in the pathogenesis of schizophrenia in offspring (Aguilar-Valles et al., 2020; Martins-Filho et al., 2020; Müller, 2018; Smith et al., 2007).
7.3. Placental pathology
While several studies have demonstrated the presence SARS-CoV-2 in placental tissue (Cribiù et al., 2021; Fenizia et al., 2020; Robaina-Castellanos, 2021), the majority of studies have had negative findings in this regard (Blasco Santana et al., 2021; Chi et al., 2021; Edlow et al., 2020; Li et al., 2020b; Mirbeyk et al., 2021). It was initially thought that placental ACE2 expression might facilitate maternal-to-fetal viral transmission through the placenta (Valdés et al., 2006; Wang et al., 2020a), but this has not been clearly validated by clinical reports. For instance, Cribiù et al. (2021) demonstrated that none of the neonates of ten COVID-19-positive women with infected placental tissue contracted the virus. While ACE2 is widely expressed in the placenta, the transmembrane serine protease 2 (TMPRSS2) that activates SARS-CoV-2 is not similarly expressed (Golden and Simmons, 2020). Furthermore, ACE2 and TMPRSS2 have very little co-localization in the placenta throughout pregnancy, which may protect against fetal infection (Edlow et al., 2020; Kotlyar, 2021). Placental infection does appear to trigger expression of genes involved in the inflammatory response, which could threaten the fetus via placental damage (Cribiù et al., 2021; Fenizia et al., 2020). In COVID-positive women without known placental infection, there have been numerous reports of placental abnormalities such as fetal and maternal vascular malperfusions, thrombi, and infarctions, all of which indicate oxygenation abnormalities that can adversely impact the fetus. There have also been reports of inflammatory placental pathology such as villitis and umbilical arteritis (Baergen and Heller, 2020; Edlow et al., 2020; Shanes et al., 2020).
7.4. Breastfeeding
The majority of studies on postpartum women with COVID-19 did not detect SARS-CoV-2 in subjects’ breastmilk and risk of vertical transmission from breastfeeding is generally considered low (Chi et al., 2021; Duran et al., 2020; Kim, 2021; Li et al., 2020a; Mirbeyk et al., 2021; Williams et al., 2020). While there have been reports of SARS-CoV-2 RNA found in the breastmilk of infected mothers (Centeno‐Tablante et al., 2021; Fenizia et al., 2020; Groß et al., 2020; Jafari et al., 2021), one study found viral RNA present on breast skin before but not after washing, suggesting that breastmilk could be contaminated by virus present on skin or in respiratory droplets. The same study found high concentrations of SARS-CoV-2 specific IgA and IgG in breastmilk, with the majority of milk samples demonstrating ability to neutralize the virus in vitro (Pace et al., 2021). In addition to the known benefits of breastfeeding on infant immunity and development, there may be an increased risk of schizophrenia with shorter duration of breastfeeding (McCreadie, 1997; Sorensen et al., 2005). This is significant given early recommendations discouraging breastfeeding in COVID-19-positive mothers (Schmid et al., 2020). However, revised guidelines encourage breastfeeding with proper viral precautions (Mimouni et al., 2020; Pountoukidou et al., 2021; Schwartz, 2020).
7.5. Other considerations
Clinical guidelines surrounding delivery and postnatal care in confirmed and suspected maternal COVID-19 cases have employed an abundance of caution to avoid infecting neonates. However, from a neurodevelopmental standpoint this approach may have unintended consequences. Many of these guidelines create delivery conditions likely to increase maternal and neonatal stress (Maynard et al., 2001). Many COVID-19-positive women underwent C-sections (Zaigham and Andersson, 2020), which may affect white matter development and functional connectivity, as well as negatively impact offspring’s immune system and cognitive function (Deoni et al., 2019; Polidano et al., 2017). Visitors are strictly limited, often to one, meaning that mothers undergo childbirth in relative isolation (Arora et al., 2020). Perhaps most dishearteningly, babies born to COVID-19-positive mothers cannot have skin-to-skin contact after delivery (Dashraath et al., 2020; Duran et al., 2020; Pountoukidou et al., 2021), are separated from their mothers immediately following birth, and can be isolated from them for up to several weeks (Chen et al., 2020a; Schmid et al., 2020; Schwartz, 2020). This is concerning, as the number of days infants are separated from their COVID-19-positive mothers was found to be negatively correlated with infant development in several domains (Wang et al., 2020c). Even brief maternal separation is known to increase infant cortisol (Larson et al., 1991) and there is evidence for increased microglial density in response to maternal separation (Howes, 2017).
8. Limitations
COVID-19 is a new disease and our understanding of its pathogenesis is still evolving. The body of literature on the virus is limited and conducting research during a pandemic poses unique challenges that can compromise quality (Padala et al., 2020). The emergence of new and highly contagious strains of the virus may outpace research and make existing literature less generalizable (Korber et al., 2020; Sah et al., 2021). At this time, it is not clear whether patients with an established psychiatric diagnosis are at higher risk for neuropsychiatric sequelae, though they are at increased risk of COVID-19-associated morbidity and mortality (Wang et al., 2021). Vaccination strategies in psychiatric patients will be an important area for future research, given the potential for neuropsychiatric side effects, interactions with psychotropic medications, and differing immune responses to vaccinations in this population (De Hert et al., 2021; Mazereel et al., 2021). The mechanisms by which inflammation promotes psychopathology are also not fully understood, nor are they limited to schizophrenia. Immune system activation and cytokine elevations are associated with other psychiatric disorders, including depressive and anxiety disorders, which are beyond the scope of this review (Costello et al., 2019.; Dantzer et al., 2008).
9. Conclusion
The sheer prevalence of COVID-19 portends that if even a small portion of those affected develop later psychotic disorders, the psychiatric burden could be enormous. The elements linking prior respiratory viral pandemics to psychosis – neuroinvasion (Fatemi, 2009), inflammation (Müller, 2010), childhood infection (Blomstrom et al., 2014), and maternal infection (Kępińska et al., 2020) – are all represented in COVID-19. Although its acute neurological manifestations have generated the most speculation about long-term neuropsychiatric sequelae, its inflammatory profile may be more relevant to later development of schizophrenia (Müller, 2018), particularly for neonates and children. While sobering, the scope of COVID-19 provides a unique opportunity to research the pathogenesis of psychotic disorders, identify populations at-risk, and develop tailored interventions. For example, identifying more specific inflammatory biomarkers could lead to the use of targeted immune therapies for schizophrenia (Kroken et al., 2019). A number of anti-inflammatory treatments shown to be beneficial in schizophrenia (Hong and Bang, 2020; Müller, 2019, 2018) could be studied for prophylactic use in vulnerable cohorts affected by COVID-19. Healthcare providers also have the opportunity to avoid some of the pitfalls of maternal influenza research by prospectively banking serological samples from pregnant women to ensure that timing of infection is accurately recorded (Selten and Termorshuizen, 2017). Our understanding of the interconnectedness of viruses and psychosis has broadened immensely through the study of pandemics and we are hopeful that future research will elucidate how this applies to COVID-19.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Aguilar-Valles A., Rodrigue B., Matta-Camacho E. Maternal immune activation and the development of dopaminergic neurotransmission of the offspring: relevance for schizophrenia and other psychoses. Front. Psychiatry. 2020;11:852. doi: 10.3389/fpsyt.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadpoor P., Rostaing L. Why the immune system fails to mount an adaptive immune response to a Covid-19 infection. Transpl. Int. 2020;33(7):824–825. doi: 10.1111/tri.13611. [DOI] [PubMed] [Google Scholar]
- al-Haddad B.J.S., Oler E., Armistead B., Elsayed N.A., Weinberger D.R., Bernier R., Burd I., Kapur R., Jacobsson B., Wang C., Mysorekar I., Rajagopal L., Adams Waldorf K.M. The fetal origins of mental illness. Am. J. Obstet. Gynecol. 2019;221:549–562. doi: 10.1016/j.ajog.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American College of Obstetrics and Gynecology . 2021. FAQs for Obstetrician-Gynecologists. [WWW Document]. URL https://www.acog.org/clinical-information/physician-faqs/covid-19-faqs-for-ob-gyns-obstetrics (Accessed 5.2.2021. [DOI] [PubMed] [Google Scholar]
- Arabi Y.M., Harthi A., Hussein J., Bouchama A., Johani S., Hajeer A.H., Saeed B.T., Wahbi A., Saedy A., AlDabbagh T., Okaili R., Sadat M., Balkhy H. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K.S., Mauch J.T., Gibson K.S. Labor and delivery visitor policies during the COVID-19 pandemic: balancing risks and benefits. JAMA. 2020;323(24):2468–2469. doi: 10.1001/jama.2020.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M.A., Keshavarz P., Hosseinpour P., Erfani A., Roshanshad A., Pourdast A., Nowrouzi-sohrabi P., Chaichian S., Poordast T. Vol. 21. 2020. p. 12. (Coronavirus Disease 2019 (COVID-19): A Systematic Review of Pregnancy and the Possibility of Vertical Transmission). 3. [PMC free article] [PubMed] [Google Scholar]
- Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A.M. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci. Ther. 2020;26:499–501. doi: 10.1111/cns.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S.C. Olfactory ensheathing cells: unique glial cell types? J. Neurotrauma. 2004;21:375–382. doi: 10.1089/089771504323004520. [DOI] [PubMed] [Google Scholar]
- Beach S.R., Praschan N.C., Hogan C., Dotson S., Merideth F., Kontos N., Fricchione G.L., Smith F.A. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen. Hosp. Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasco Santana L., Miraval Wong E., Álvarez-Troncoso J., Sánchez García L., Bartha J.L., Regojo‐Zapata R.M. Maternal and perinatal outcomes and placental pathologic examination of 29 SARS‐CoV ‐2 infected patients in the third trimester of gestation. J. Obstet. Gynaecol. Res. 2021 doi: 10.1111/jog.14784. jog.14784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomstrom A., Karlsson H., Svensson A., Frisell T., Lee B.K., Dal H., Magnusson C., Dalman C. Hospital admission with infection during childhood and risk for psychotic illness—a population-based cohort study. Schizophr. Bull. 2014;40:1518–1525. doi: 10.1093/schbul/sbt195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Maternal infection during pregnancy and schizophrenia. J. Psychiatry Neurosci. 2008;33:183–185. [PMC free article] [PubMed] [Google Scholar]
- Boulanger-Bertolus J., Pancaro C., Mashour G.A. Increasing role of maternal immune activation in neurodevelopmental disorders. Front. Behav. Neurosci. 2018;12:230. doi: 10.3389/fnbeh.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K., Ring L., Landau R., Purisch S., Friedman A.M., Fuchs K., Sutton D., Andrikopoulou M., Rupley D., Sheen J.-J., Aubey J., Zork N., Moroz L., Mourad M., Wapner R., Simpson L.L., D’Alton M.E., Goffman D. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am. J. Obstet. Gynecol. MFM. 2020;2:100118. doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S. Prenatal infection as a risk factor for schizophrenia. Schizophr. Bull. 2006;32:200–202. doi: 10.1093/schbul/sbj052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A.S., Schaefer C.A., Wyatt R.J., Goetz R., Begg M.D., Gorman J.M., Susser E.S. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophr. Bull. 2000;26:287–295. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- Brown A.S., Begg M.D., Gravenstein S., Schaefer C.A., Wyatt R.J., Bresnahan M., Babulas V.P., Susser E.S. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch. Gen. Psychiatry. 2004;61:7. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown A., Schaefer C.A., Quesenberry C.P., Liu L., Babulas V.P., Susser E.S. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Brun G., Hak J.-F., Coze S., Kaphan E., Carvelli J., Girard N., Stellmann J.-P. COVID-19—white matter and globus pallidum lesions. Neurol. Neuroimmunol. Neuroinflamm. 2020;7 doi: 10.1212/NXI.0000000000000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwire G.M., Njiro B.J., Mwakawanga D.L., Sabas D., Sunguya B.F. Possible vertical transmission and antibodies against SARS‐CoV‐2 among infants born to mothers with COVID‐19: a living systematic review. J. Med. Virol. 2021;93(3):1361–1369. doi: 10.1002/jmv.26622. [DOI] [PubMed] [Google Scholar]
- Canetta S., Sourander A., Surcel H.-M., Hinkka-Yli-Salomäki S., Leiviskä J., Kellendonk C., McKeague I.W., Brown A.S. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am. J. Psychiatry. 2014;171:960–968. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi P.L., Lazzerini P.E., Volterrani L., Mazzei M.A., Rossetti B., Zanelli G., Bennett D., Bargagli E., Franchi F., Cameli M., Valente S., Cantarini L., Frediani B. Antirheumatic agents in covid-19: is IL-6 the right target? Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217523. annrheumdis-2020-217523. [DOI] [PubMed] [Google Scholar]
- Centeno‐Tablante E., Medina‐Rivera M., Finkelstein J.L., Rayco‐Solon P., Garcia‐Casal M.N., Rogers L., Ghezzi‐Kopel K., Ridwan P., Peña‐Rosas J.P., Mehta S. Transmission of SARS‐CoV‐2 through breast milk and breastfeeding: a living systematic review. Ann. N. Y. Acad. Sci. 2021;1484(1):32–54. doi: 10.1111/nyas.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Multisystem Inflammatory Syndrome in Children (MIS-C) [WWW Document] URL https://www.cdc.gov/mis-c/index.html (accessed 6.11.20) [Google Scholar]
- Chen D., Yang H., Cao Y., Cheng W., Duan T., Fan C., Fan S., Feng L., Gao Y., He F., He J., Hu Y., Jiang Y., Li Y., Li J., Li Xiaotian, Li Xuelan, Lin K., Liu C., Liu J., Liu X., Pan X., Pang Q., Pu M., Qi H., Shi C., Sun Y., Sun J., Wang X., Wang Y., Wang Zilian, Wang Zhijian, Wang C., Wu S., Xin H., Yan J., Zhao Y., Zheng J., Zhou Y., Zou L., Zeng Y., Zhang Y., Guan X. Expert consensus for managing pregnant women and neonates born to mothers with suspected or confirmed novel coronavirus (COVID ‐19) infection. Int. J. Gynecol. Obstet. 2020;149(2):130–136. doi: 10.1002/ijgo.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H., Wang T., Zhang Xiaoyun, Chen H., Yu H., Zhang Xiaoping, Zhang M., Wu S., Song J., Chen T., Han M., Li S., Luo X., Zhao J., Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J. Med. Virol. 2020 doi: 10.1002/jmv.25789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Wang K., Yu J., Howard D., French L., Chen Z., Wen C., Xu Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021;11:1860. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S.K.-W., Tsang J.S.-K., Ku K.-H., Wong C.-W., Ng Y.-K. Psychiatric complications in patients with severe acute respiratory syndrome (SARS) during the acute treatment phase: a series of 10 cases. Br. J. Psychiatry. 2004;184:359–360. doi: 10.1192/bjp.184.4.359. [DOI] [PubMed] [Google Scholar]
- Chi J., Gong W., Gao Q. Clinical characteristics and outcomes of pregnant women with COVID-19 and the risk of vertical transmission: a systematic review. Arch. Gynecol. Obstet. 2021;303(2):337–345. doi: 10.1007/s00404-020-05889-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro C.N., Tsimis M., Burd I. Infections and brain development. Obstet. Gynecol. Surv. 2015;70:644–655. doi: 10.1097/OGX.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello H., Gould R.L., Abrol E., Howard R. Systematic review and meta-analysis of the association between peripheral inflammatory cytokines and generalized anxiety disorder. BMJ Open. 2019;9(7) doi: 10.1136/bmjopen-2018-027925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan H.R. Is schizophrenia research relevant during the COVID-19 pandemic? Schizophr. Res. 2020 doi: 10.1016/j.schres.2020.04.002. S0920996420302097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribiù F.M., Erra R., Pugni L., Rubio-Perez C., Alonso L., Simonetti S., Croci G.A., Serna G., Ronchi A., Pietrasanta C., Lunghi G., Fagnani A.M., Piñana M., Matter M., Tzankov A., Terracciano L., Anton A., Ferrazzi E., Ferrero S., et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J. Clin. Invest. 2021;131(6):e145427. doi: 10.1172/JCI145427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalman C., Allebeck P., Gunnell D., Harrison G., Kristensson K., Lewis G., Lofving S., Rasmussen F., Wicks S., Karlsson H. Infections in the CNS during childhood and the risk of subsequent psychotic illness: a cohort study of more than one million Swedish subjects. Am. J. Psychiatry. 2008;165:59–65. doi: 10.1176/appi.ajp.2007.07050740. [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’Connor J.C., Freund G.G., Johnson R.W., Kelley K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashraath P., Jing Lin Jeslyn W., Mei Xian Karen L., Li Min L., Sarah L., Biswas A., Arjandas Choolani M., Mattar C., Lin S.L. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.03.021. S0002937820303434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Hert M., Mazereel V., Detraux J., Van Assche K. Prioritizing COVID ‐19 vaccination for people with severe mental illness. World Psychiatry. 2021;20(1):54–55. doi: 10.1002/wps.20826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcelos Gaspar A., Santos Silva I. SARS-CoV-2 in pregnancy—the first wave. Medicina. 2021;57(3):241. doi: 10.3390/medicina57030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debost J.-C.P.G., Larsen J.T., Munk-Olsen T., Mortensen P.B., Meyer U., Petersen L. Joint effects of exposure to prenatal infection and peripubertal psychological trauma in schizophrenia. Schizophr. Bull. 2017;43:171–179. doi: 10.1093/schbul/sbw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisi L.E., Szulc K.U., Bertisch H.C., Majcher M., Brown K. Understanding structural brain changes in schizophrenia. Clin. Res. 2006;8:8. doi: 10.31887/DCNS.2006.8.1/ldelisi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni S.C., Adams S.H., Li X., Badger T.M., Pivik R.T., Glasier C.M., Ramakrishnaiah R.H., Rowell A.C., Ou X. Cesarean delivery impacts infant brain development. Am. J. Neuroradiol. 2019;40(1):169–177. doi: 10.3174/ajnr.A5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deza Leon M.P., Redzepi A., McGrath E., Abdel-Haq N., Shawaqfeh A., Sethuraman U., Tilford B., Chopra T., Arora H., Ang J., Asmar B. COVID-19–associated pediatric multisystem inflammatory syndrome. J. Pediatr. Infect. Dis. Soc. 2020 doi: 10.1093/jpids/piaa061. piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mascio D., Khalil A., Saccone G., Rizzo G., Buca D., Liberati M., Vecchiet J., Nappi L., Scambia G., Berghella V., D’Antonio F. Outcome of Coronavirus spectrum infections (SARS, MERS, COVID 1-19) during pregnancy: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajogmf.2020.100107. MFM 100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Tian J., He S., Zhu C., Wang J., Liu C., Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020:e20200702. doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- Donnelly L., Campling G. Functions of the placenta. Anaesth. Intensive Care Med. 2019;20:392–396. doi: 10.1016/j.mpaic.2019.04.004. [DOI] [Google Scholar]
- Doty R.L. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 2017;16:478–488. doi: 10.1016/S1474-4422(17)30123-0. [DOI] [PubMed] [Google Scholar]
- Druart M., Le Magueresse C. Emerging roles of complement in psychiatric disorders. Front. Psychiatry. 2019;10:573. doi: 10.3389/fpsyt.2019.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran P., Berman S., Niermeyer S., Jaenisch T., Forster T., Gomez Ponce de Leon R., De Mucio B., Serruya S. COVID-19 and newborn health: systematic review. Rev. Panam. Salud Pãºblica. 2020;44:1. doi: 10.26633/RPSP.2020.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow A.G., Li J.Z., Collier A.Y., Atyeo C., James K.E., Boatin A.A., Gray K.J., Bordt E.A., Shook L.L., Yonker L.M., Fasano A., Diouf K., Croul N., Devane S., Yockey L.J., Lima R., Shui J., Matute J.D., Lerou P.H., et al. Vol. 2. 2020. p. 25. (Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic). 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.J. Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit. Anom. 2007;47:84–89. doi: 10.1111/j.1741-4520.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- Fatemi S.H. Potential microbial origins of schizophrenia and their treatments. Drugs Today. 2009;45:305. doi: 10.1358/dot.2009.045.004.1353924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenizia C., Biasin M., Cetin I., Vergani P., Mileto D., Spinillo A., Gismondo M.R., Perotti F., Callegari C., Mancon A., Cammarata S., Beretta I., Nebuloni M., Trabattoni D., Clerici M., Savasi V. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuglewicz A., Piotrowski P., Stodolak A. Relationship between toxoplasmosis and schizophrenia: a review. Adv. Clin. Exp. Med. 2017;26:1033–1038. doi: 10.17219/acem/61435. [DOI] [PubMed] [Google Scholar]
- Ghema K., Lehlimi M., Toumi H., Badre A., Chemsi M., Habzi A., Benomar S. Outcomes of newborns to mothers with COVID-19. Infect. Dis. Now. 2021 doi: 10.1016/j.idnow.2021.03.003. S2666991921000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden T.N., Simmons R.A. Maternal and neonatal response to COVID-19. Am. J. Physiol.-Endocrinol. Metab. 2020;319(2):E315–E319. doi: 10.1152/ajpendo.00287.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.L., Clark J.R., Orban Z.S., Lim P.H., Szymanski A.L., Taylor C., DiBiase R.M., Jia D.T., Balabanov R., Ho S.U., Batra A., Liotta E.M., Koralnik I.J. Persistent neurologic symptoms and cognitive dysfunction in non‐hospitalized Covid‐19 “long haulers”. Ann. Clin. Transl. Neurol. 2021 doi: 10.1002/acn3.51350. acn3.51350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groß R., Conzelmann C., Müller J.A., Stenger S., Steinhart K., Kirchhoff F., Münch J. Detection of SARS-CoV-2 in human breastmilk. Lancet. 2020;395:1757–1758. doi: 10.1016/S0140-6736(20)31181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halici-Ozturk F., Ocal F.D., Aydin S., Tanacan A., Ayhan S.G., Altinboga O., Dinc B., Moraloglu Ö.T., Sahin D. Investigating the risk of maternal-fetal transmission of SARS-CoV-2 in early pregnancy. Placenta. 2021;106:25–29. doi: 10.1016/j.placenta.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickie I.B., Banati R. Are common childhood or adolescent infections risk factors for schizophrenia and other psychotic disorders? Med. J. Aust. 2009;190:S17–21. doi: 10.5694/j.1326-5377.2009.tb02652.x. [DOI] [PubMed] [Google Scholar]
- Hinoue A., Fushiki S., Nishimura Y., Shiota K. In utero exposure to brief hyperthermia interferes with the production and migration of neocortical neurons and induces apoptotic neuronal death in the fetal mouse brain. Dev. Brain Res. 2001;132:59–67. doi: 10.1016/S0165-3806(01)00295-4. [DOI] [PubMed] [Google Scholar]
- Hong J., Bang M. Anti-inflammatory strategies for schizophrenia: a review of evidence for therapeutic applications and drug repurposing. Clin. Psychopharmacol. Neurosci. 2020;18:10–24. doi: 10.9758/cpn.2020.18.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigsbaum M. “An inexpressible dread”: psychoses of influenza at fin-de-siècle. Lancet. 2013;381:988–989. doi: 10.1016/S0140-6736(13)60701-1. [DOI] [PubMed] [Google Scholar]
- Honigsbaum M., Krishnan L. Taking pandemic sequelae seriously: from the Russian influenza to COVID-19 long-haulers. Lancet. 2020;396(10260):1389–1391. doi: 10.1016/S0140-6736(20)32134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O.D. Inflammation and the neural diathesis-stress hypothesis of schizophrenia: a reconceptualization. Transl. Psychiatry. 2017;7:e1024. doi: 10.1038/tp.2016.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Pinto M.D., Borelli J.L., Mehrabadi M.A., Abrihim H., Dutt N., Lambert N., Nurmi E.L., Chakraborty R., Rahmani A.M., Downs C.A. COVID symptoms, symptom clusters, and predictors for becoming a long-hauler: looking for clarity in the haze of the pandemic [Preprint] Infect. Dis. (except HIV/AIDS) 2021 doi: 10.1101/2021.03.03.21252086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam Md.M., Poly T.N., Walther B.A., Yang H.C., Wang C.-W., Hsieh W.-S., Atique S., Salmani H., Alsinglawi B., Lin M.C., Jian W.S., Jack Li Y.-C. Clinical characteristics and neonatal outcomes of pregnant patients with COVID-19: a systematic review. Front. Med. 2020;7:573468. doi: 10.3389/fmed.2020.573468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafari M., Pormohammad A., Sheikh Neshin S.A., Ghorbani S., Bose D., Alimohammadi S., Basirjafari S., Mohammadi M., Rasmussen‐Ivey C., Razizadeh M.H., Nouri‐Vaskeh M., Zarei M. Clinical characteristics and outcomes of pregnant women with COVID‐19 and comparison with control patients: A systematic review and meta‐analysis. Rev. Med. Virol. 2021:e2208. doi: 10.1002/rmv.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang H., Boltz D., Sturm-Ramirez K., Shepherd K.R., Jiao Y., Webster R., Smeyne R.J. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc. Natl. Acad. Sci. 2009;106:14063–14068. doi: 10.1073/pnas.0900096106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., Nguyen E.L., Barsh G.R., Maskatia S., Mathew R. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- Kahn J.S. The widening scope of coronaviruses. Curr. Opin. Pediatr. 2006;18:42–47. doi: 10.1097/01.mop.0000192520.48411.fa. [DOI] [PubMed] [Google Scholar]
- Kannan G., Gressitt K.L., Yang S., Stallings C.R., Katsafanas E., Schweinfurth L.A., Savage C.L.G., Adamos M.B., Sweeney K.M., Origoni A.E., Khushalani S., Bahn S., Leweke F.M., Dickerson F.B., Yolken R.H., Pletnikov M.V., Severance E.G. Pathogen-mediated NMDA receptor autoimmunity and cellular barrier dysfunction in schizophrenia. Transl. Psychiatry. 2017;7:e1186. doi: 10.1038/tp.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi L., Vahedian-Azimi A., Makvandi S., Sahebkar A. In: Guest P.C., editor. Vol. 1321. Springer International Publishing; 2021. A systematic review of 571 pregnancies affected by COVID-19; pp. 287–298. (Clinical, Biological and Molecular Aspects of COVID-19). [DOI] [PubMed] [Google Scholar]
- Karimi-Zarchi M., Neamatzadeh H., Dastgheib S.A., Abbasi H., Mirjalili S.R., Behforouz A., Ferdosian F., Bahrami R. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr. Pathol. 2020:1–5. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kępińska A.P., Iyegbe C.O., Vernon A.C., Yolken R., Murray R.M., Pollak T.A. Schizophrenia and influenza at the centenary of the 1918-1919 Spanish influenza pandemic: mechanisms of psychosis risk. Front. Psychiatry. 2020;11:72. doi: 10.3389/fpsyt.2020.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Peng L., Siddique R., Nabi G., Nawsherwan Xue M., Liu J., Han G. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect. Control Hosp. Epidemiol. 2020:1–3. doi: 10.1017/ice.2020.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Zimbron J., Dalman C., Lewis G., Jones P.B. Childhood infection and adult schizophrenia: a meta-analysis of population-based studies. Schizophr. Res. 2012;139:161–168. doi: 10.1016/j.schres.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandaker G.M., Pearson R.M., Zammit S., Lewis G., Jones P.B. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry. 2014;71:1121. doi: 10.1001/jamapsychiatry.2014.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-H. Clinical implications of coronavirus disease 2019 in neonates. Clin. Exp. Pediatrics. 2021;64(4):157–164. doi: 10.3345/cep.2020.01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberlin D.W., Stagno S. Can SARS-CoV-2 infection be acquired in utero? More definitive evidence is needed. JAMA. 2020;323(18):1788–1789. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- Kingstone T., Taylor A.K., O’Donnell C.A., Atherton H., Blane D.N., Chew-Graham C.A. Finding the “right” GP: a qualitative study of the experiences of people with long-COVID. BJGP Open. 2020;4(5) doi: 10.3399/bjgpopen20X101143. bjgpopen20X101143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolifarhood G., Aghaali M., Saadati H.M., Taherpour N., Izadi N., Nazari S.S.H. Epidemiological and clinical aspects of COVID-19; a narrative review. Arch. Acad. Emerg. Med. 2020;8 [PMC free article] [PubMed] [Google Scholar]
- Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., Hastie K.M., Parker M.D., Partridge D.G., Evans C.M., Freeman T.M., de Silva T.I., Angyal A., Brown R.L., Carrilero L., et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182(4):812–827.e19. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotlyar A.M. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Syst. Rev. 2021:22. doi: 10.1016/j.ajog.2020.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D., Matz J., Weidinger E., Wagner J., Wildenauer A., Obermeier M., Riedel M., Müller N. The association of infectious agents and schizophrenia. World J. Biol. Psychiatry. 2010;11:739–743. doi: 10.3109/15622971003653246. [DOI] [PubMed] [Google Scholar]
- Kroken R.A., Sommer I.E., Steen V.M., Dieset I., Johnsen E. Constructing the immune signature of schizophrenia for clinical use and research; an integrative review translating descriptives into diagnostics. Front. Psychiatry. 2019;9:753. doi: 10.3389/fpsyt.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRovere K.L., Riggs B.J., Poussaint T.Y., Young C.C., Newhams M.M., Maamari M., Walker T.C., Singh A.R., Dapul H., Hobbs C.V., McLaughlin G.E., Son M.B.F., Maddux A.B., Clouser K.N., Rowan C.M., McGuire J.K., Fitzgerald J.C., Gertz S.J., Shein S.L., et al. 2021. Neurologic Involvement in Children and Adolescents Hospitalized in the United States for COVID-19 or Multisystem Inflammatory Syndrome; p. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson M., Gunnar M., Hertsgaard L. The effects of morning naps, car trips, and maternal separation on adrenocorticcal activity in human infants. Child Dev. 1991;62:362–372. [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A., Dequanter D., Blecic S., El Afia F., Distinguin L., Chekkoury-Idrissi Y., Hans S., Delgado I.L., Calvo-Henriquez C., Lavigne P., Falanga C., Barillari M.R., Cammaroto G., Khalife M., Leich P., Souchay C., Rossi C., Journe F., Hsieh J., Edjlali M., Carlier R., Ris L., Lovato A., De Filippis C., Coppee F., Fakhry N., Ayad T., Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung C. Clinical characteristics of COVID‐19 in children: are they similar to those of SARS? Pediatr. Pulmonol. 2020;55:1592–1597. doi: 10.1002/ppul.24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Okada T., Kodera M., Nara Y., Takino N., Muramatsu C., Ikeguchi K., Urano F., Ichinose H., Metzger D., Chambon P., Nakano I., Ozawa K., Muramatsu S. Viral-mediated temporally controlled dopamine production in a rat model of Parkinson disease. Mol. Ther. 2006;13:160–166. doi: 10.1016/j.ymthe.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Li K., Wohlford-Lenane C., Perlman S., Zhao J., Jewell A.K., Reznikov L.R., Gibson-Corley K.N., Meyerholz D.K., McCray P.B. Middle east respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J. Infect. Dis. 2016;213:712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Yang, Zhao R., Zheng S., Chen X., Wang J., Sheng X., Zhou J., Cai H., Fang Q., Yu F., Fan J., Xu K., Chen Y., Sheng J. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerging Infect. Dis. 2020;26(6):1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Chen L., Zhang J., Xiong C., Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One. 2020;15:e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Bai W., Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J. Med. Virol. 2020;92:552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-C., Bai W.-Z., Hashikawa T. Response to Commentary on “The neuroinvasive potential of SARS-CoV-2 may play a role in the respiratory failure of COVID-19 patients”. J. Med. Virol. 2020;92:707–709. doi: 10.1002/jmv.25824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang W., Chikritzhs T. Early childhood infections and risk of schizophrenia. Psychiatry Res. 2012;200:214–217. doi: 10.1016/j.psychres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Licciardi F., Pruccoli G., Denina M., Parodi E., Taglietto M., Rosati S., Montin D. SARS-CoV-2-induced kawasaki-like hyperinflammatory syndrome: a novel COVID phenotype in children. Pediatrics. 2020:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- Lieberman R.W., Bagdasarian N., Thomas D., Van De Ven C. Seasonal influenza a (H1N1) infection in early pregnancy and second trimester fetal demise. Emerg. Infect. Dis. 2011;17:107–109. doi: 10.3201/eid1701.091895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Li L., Xu M., Wu J., Luo D., Zhu Y., Li B., Song X., Zhou X. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Wang L.-L., Zhao S.-J., Kwak-Kim J., Mor G., Liao A.-H. Why are pregnant women susceptible to COVID-19? An immunological viewpoint. J. Reprod. Immunol. 2020;139 doi: 10.1016/j.jri.2020.103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Wang J., Li W., Zhou Z., Liu S., Rong Z. Clinical characteristics of 19 neonates born to mothers with COVID-19. Front. Med. 2020;14:193–198. doi: 10.1007/s11684-020-0772-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Leon S., Wegman-Ostrosky T., Perelman C., Sepulveda R., Rebolledo P., Cuapio A., Villapol S. 2021. More Than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis [Preprint]. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J.F. Systematic review of COVID‐19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado C., Gutierrez J.V. 2020. Anosmia and Ageusia as Initial or Unique Symptoms After SARS-COV-2 Virus Infection (preprint). Medicine & Pharmacology. [DOI] [Google Scholar]
- Mackenzie N., Lopez-Coronel E., Dau A., Maloof D., Mattar S., Garcia J.T., Fontecha B., Lanata C.M., Guillen-Burgos H.F. Concomitant Guillain-Barre syndrome with COVID-19: a case report. BMC Neurol. 2021;21(1):135. doi: 10.1186/s12883-021-02162-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. Covid-19: first coronavirus was described in the BMJ in 1965. BMJ. 2020:m1547. doi: 10.1136/bmj.m1547. [DOI] [PubMed] [Google Scholar]
- Majde J.A. Neuroinflammation resulting from covert brain invasion by common viruses – a potential role in local and global neurodegeneration. Med. Hypotheses. 2010;75:204–213. doi: 10.1016/j.mehy.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makvandi S., Mahdavian M., Kazemi-Nia G., Vahedian-Azimi A., Guest P.C., Karimi L., Sahebkar A. In: Guest P.C., editor. Vol. 1321. Springer International Publishing; 2021. The 2019 novel coronavirus disease in pregnancy: a systematic review; pp. 299–307. (Clinical, Biological and Molecular Aspects of COVID-19). [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., Miao X., Li Y., Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Filho P.R., Tanajura D.M., Santos H.P., Santos V.S. COVID-19 during pregnancy: potential risk for neurodevelopmental disorders in neonates? Eur. J. Obstet. Gynecol. Reprod. Biol. 2020 doi: 10.1016/j.ejogrb.2020.05.015. S0301211520302645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury A., Lyoubi A., Peiffer-Smadja N., de Broucker T., Meppiel E. Neurological manifestations associated with SARS-CoV-2 and other coronaviruses: a narrative review for clinicians. Rev. Neurol. 2021;177(1–2):51–64. doi: 10.1016/j.neurol.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard T.M., Sikich L., Lieberman J.A., LaMantia A.-S. Neural development, cell-cell signaling, and the “two-hit” hypothesis of schizophrenia. Schizophr. Bull. 2001;27:457–476. doi: 10.1093/oxfordjournals.schbul.a006887. [DOI] [PubMed] [Google Scholar]
- Mazereel V., Van Assche K., Detraux J., De Hert M. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444–450. doi: 10.1016/S2215-0366(20)30564-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. Brain Behav. Immun. 2021 doi: 10.1016/j.bbi.2021.02.021. S0889159121000866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCreadie R. The nithsdale schizophrenia surveys. 16. Breast-feeding and schizophrenia: preliminary results and hypotheses. Br. J. Psychiatry. 1997;170:334–337. doi: 10.1192/bjp.170.4.334. [DOI] [PubMed] [Google Scholar]
- McGavern D.B., Kang S.S. Illuminating viral infections in the nervous system. Nat. Rev. Immunol. 2011;11:318–329. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mednick S.A. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry. 1988;45:189. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Menninger K.A. Psychoses associated with influenza. JAMA. 1919;72:235–241. doi: 10.1001/jama.1919.02610040001001. [DOI] [Google Scholar]
- Menninger K.A. Influenza and schizophrenia. An analysis of post-influenzal “dementia precox,” as of 1918, and five years later further studies of the psychiatry aspects of influenza. Am. J. Psychiatry. 1926;82:469–529. doi: 10.1176/ajp.151.6.182. [DOI] [PubMed] [Google Scholar]
- Metcalf S.A., Jones P.B., Nordstrom T., Timonen M., Mäki P., Miettunen J., Jääskeläinen E., Järvelin M.-R., Stochl J., Murray G.K., Veijola J., Khandaker G.M. Serum C-reactive protein in adolescence and risk of schizophrenia in adulthood: a prospective birth cohort study. Brain Behav. Immun. 2017;59:253–259. doi: 10.1016/j.bbi.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mighdoll M.I., Tao R., Kleinman J.E., Hyde T.M. Myelin, myelin-related disorders, and psychosis. Schizophr. Res. 2015;161:85–93. doi: 10.1016/j.schres.2014.09.040. [DOI] [PubMed] [Google Scholar]
- Mimouni F., Lakshminrusimha S., Pearlman S.A., Raju T., Gallagher P.G., Mendlovic J. Perinatal aspects on the covid-19 pandemic: a practical resource for perinatal–neonatal specialists. J. Perinatol. 2020 doi: 10.1038/s41372-020-0665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbeyk M., Saghazadeh A., Rezaei N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021 doi: 10.1007/s00404-021-06049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberg P. Olfactory dysfunction in schizophrenia a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram‐Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID‐19. Int. Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22587. alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondelli V., Di Forti M., Morgan B.P., Murray R.M., Pariante C.M., Dazzan P. Baseline high levels of complement component 4 predict worse clinical outcome at 1-year follow-up in first-episode psychosis. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.01.014. S0889159119310827. [DOI] [PubMed] [Google Scholar]
- Morassi M., Bagatto D., Cobelli M., D’Agostini S., Gigli G.L., Bnà C., Vogrig A. Stroke in patients with SARS-CoV-2 infection: case series. J. Neurol. 2020 doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhidin S., Moghadam Z.B., Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019-nCoV; a systematic review. Arch Acad Emerg Med. 2020;15:e49. [PMC free article] [PubMed] [Google Scholar]
- Mukandala G., Tynan R., Lanigan S., O’Connor J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016;6:6. doi: 10.3390/brainsci6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N. Immune system and schizophrenia. Curr. Immunol. Rev. 2010;6:213–220. doi: 10.2174/157339510791823673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N. Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophr. Bull. 2018;44:973–982. doi: 10.1093/schbul/sby024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N. COX-2 inhibitors, aspirin, and other potential anti-inflammatory treatments for psychiatric disorders. Front. Psychiatry. 2019;10:375. doi: 10.3389/fpsyt.2019.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataf S. An alteration of the dopamine synthetic pathway is possibly involved in the pathophysiology of COVID-19. J. Med. Virol. 2020 doi: 10.1002/jmv.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Diseases . 2020. COVID-19, MERS & SARS [WWW Document] URL https://www.niaid.nih.gov/diseases-conditions/covid-19 (accessed 6.11.20) [Google Scholar]
- Netland J., Meyerholz D.K., Moore S., Cassell M., Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008;82:12. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace R.M., Williams J.E., Järvinen K.M., Belfort M.B., Pace C.D.W., Lackey K.A., Gogel A.C., Nguyen-Contant P., Kanagaiah P., Fitzgerald T., Ferri R., Young B., Rosen-Carole C., Diaz N., Meehan C.L., Caffé B., Sangster M.Y., Topham D., McGuire M.A., et al. Vol. 12. 2021. p. 11. (Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padala P.R., Jendro A.M., Padala K.P. Conducting clinical research during the COVID-19 pandemic: investigator and participant perspectives. JMIR Public Health Surveill. 2020;6:e18887. doi: 10.2196/18887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaiodimou L., Stefanou M.I., Katsanos A.H., Fragkou P.C., Papadopoulou M., Moschovos C., Michopoulos I., Kokotis P., Bakirtzis C., Naska A., Vassilakopoulos T.I., Chroni E., Tsiodras S., Tsivgoulis G. Prevalence, clinical characteristics and outcomes of Guillain-Barré syndrome spectrum associated with COVID-19: a systematic review and meta-analysis. Eur. J. Neurol. 2021 doi: 10.1111/ene.14860. Apr 9. Epub ahead of print. PMID: 33837630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., P. Stone K., Hsuchou H., K. Manda V., Zhang Y., J. Kastin A. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011;17:3729–3740. doi: 10.2174/138161211798220918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parente-Arias P., Barreira-Fernandez P., Quintana-Sanjuas A., Patino-Castineira B. Recovery rate and factors associated with smell and taste disruption in patients with coronavirus disease 2019. Am. J. Otolaryngol. 2021:6. doi: 10.1016/j.amjoto.2020.102648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield C.A., Brubaker S.G., Limaye M.A., Lighter J., Ratner A.J., Thomas K.M., Meyer J.A., Roman A.S. Detection of severe acute respiratory syndrome coronavirus 2 in placental and fetal membrane samples. Am. J. Obstetr. Gynecol. MFM. 2020;2(3):100133. doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A., Cruz‐Melguizo S., Adrien M., Fuentes L., Marin E., Perez‐Medina T. Clinical course of Coronavirus Disease‐2019 (COVID‐19) in pregnancy. Acta Obstet. Gynecol. Scand. 2020 doi: 10.1111/aogs.13921. aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polidano C., Zhu A., Bornstein J.C. The relation between cesarean birth and child cognitive development. Sci. Rep. 2017;7(1):11483. doi: 10.1038/s41598-017-10831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popoli M., Yan Z., McEwen B.S., Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget J.G., Ollila H.M., Barker J., Spain S., Dand N., Trembath R., Martin J., Mayes M.D., Bossini-Castillo L., López-Isac E., Jin Y., Santorico S.A., Spritz R.A., Hakonarson H., Polychronakos C., Raychaudhuri S., Knight J. Cross-disorder analysis of schizophrenia and 19 immune-mediated diseases identifies shared genetic risk. Hum. Mol. Genet. 2019;28:3498–3513. doi: 10.1093/hmg/ddz145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pountoukidou A., Potamiti-Komi M., Sarri V., Papapanou M., Routsi E., Tsiatsiani A.M., Vlahos N., Siristatidis C. Management and prevention of COVID-19 in pregnancy and pandemic obstetric care: a review of current practices. Healthcare. 2021;9(4):467. doi: 10.3390/healthcare9040467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Printza A., Constantinidis J. The role of self-reported smell and taste disorders in suspected COVID‑19. Eur. Arch. Otorhinolaryngol. 2020;277:2625–2630. doi: 10.1007/s00405-020-06069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner M. The neural diathesis-stress model of schizophrenia revisited: an update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci. Biobehav. Rev. 2017;73:191–218. doi: 10.1016/j.neubiorev.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Raison C., Reed R., Rook G., Chir B., Miller A. Kaplan & Sadock’s Comprehensive Textbook of Psychiatry. Wolters Kluwer; 2017. Immune system and central nervous system interactions; pp. 178–200. [Google Scholar]
- Ratmann O., Bhatt S., Flaxman S. Implications of a highly transmissible variant of SARS-CoV-2 for children. Arch. Dis. Child. 2021 doi: 10.1136/archdischild-2021-321903. archdischild-2021-321903. [DOI] [PubMed] [Google Scholar]
- Ratnayake U., Quinn T., Walker D.W., Dickinson H. Cytokines and the neurodevelopmental basis of mental illness. Front. Neurosci. 2013;7 doi: 10.3389/fnins.2013.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riphagen S., Gomez X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395:1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaina-Castellanos G.R. Congenital and intrapartum SARS-CoV-2 infection in neonates: hypotheses, evidence and perspectives. MEDICC Rev. 2021;23(1):12. doi: 10.37757/MR2021.V23.N1.13. [DOI] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30203-0. S2215036620302030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin R. As their numbers grow, COVID-19 “long haulers” stump experts. JAMA. 2020;324(14):1381. doi: 10.1001/jama.2020.17709. [DOI] [PubMed] [Google Scholar]
- Sah P., Vilches T.N., Moghadas S.M., Fitzpatrick M.C., Singer B.H., Hotez P.J., Galvani A.P. Accelerated vaccine rollout is imperative to mitigate highly transmissible COVID-19 variants. EClinicalMedicine. 2021;35:100865. doi: 10.1016/j.eclinm.2021.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- Savasi V.M., Parisi F., Patanè L., Ferrazzi E., Frigerio L., Pellegrino A., Spinillo A., Tateo S., Ottoboni M., Veronese P., Petraglia F., Vergani P., Facchinetti F., Spazzini D., Cetin I. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (COVID-19) Obstet. Gynecol. 2020 doi: 10.1097/AOG.0000000000003979. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Sayin I., Yazici Z.M. Taste and smell impairment in SARS-CoV-2 recovers early and spontaneously: experimental data strongly linked to clinical data. ACS Chem. Neurosci. 2020;11(14):2031–2033. doi: 10.1021/acschemneuro.0c00296. [DOI] [PubMed] [Google Scholar]
- Schmid M.B., Fontijn J., Ochsenbein-Kölble N., Berger C., Bassler D. COVID-19 in pregnant women. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30175-4. S1473309920301754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz D.A. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch. Pathol. Lab. Med. 2020 doi: 10.5858/arpa.2020-0901-SA. arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- Selten J.-P., Termorshuizen F. The serological evidence for maternal influenza as risk factor for psychosis in offspring is insufficient: critical review and meta-analysis. Schizophr. Res. 2017;183:2–9. doi: 10.1016/j.schres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Severance E.G., Dickerson F.B., Viscidi R.P., Bossis I., Stallings C.R., Origoni A.E., Sullens A., Yolken R.H. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr. Bull. 2011;37:101–107. doi: 10.1093/schbul/sbp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Tan M., Chen X., Liu Y., Huang J., Ou J., Deng X. Infectious Diseases (except HIV/AIDS) 2020. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.E.P., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through Interleukin-6. J. Neurosci. 2007;27(40):10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler Z.M., Patel Z.M., Turner J.H., Holbrook E.H. A primer on viral-associated olfactory loss in the era of COVID-19. Int. Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somand D., Meurer W. Central nervous system infections. Emerg. Med. Clin. North Am. 2009;27:89–100. doi: 10.1016/j.emc.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Sorensen H.J., Mortensen E.L., Reinisch J.M., Mednick S.A. Breastfeeding and risk of schizophrenia in the Copenhagen perinatal cohort. Acta Psychiatr. Scand. 2005;112:26–29. doi: 10.1111/j.1600-0447.2005.00548.x. [DOI] [PubMed] [Google Scholar]
- Steardo L., Zorec R., Verkhratsky A. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. 2020:e13473. doi: 10.1111/apha.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stępnicki P., Kondej M., Kaczor A.A. Current concepts and treatments of schizophrenia. Molecules. 2018;23:2087. doi: 10.3390/molecules23082087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.R., Albrecht P., Lesta G., Krauthammer E. Viral antigen in the brain of schizophrenic patients? A preliminary report. Adv. Biol. Psychiatry. 1983;12:76–96. doi: 10.1159/000408318. [DOI] [Google Scholar]
- Sukhikh G., Petrova U., Prikhodko A., Starodubtseva N., Chingin K., Chen H., Bugrova A., Kononikhin A., Bourmenskaya O., Brzhozovskiy A., Polushkina E., Kulikova G., Shchegolev A., Trofimov D., Frankevich V., Nikolaev E., Shmakov R.G. Vertical transmission of SARS-CoV-2 in second trimester associated with severe neonatal pathology. Viruses. 2021;13(3):447. doi: 10.3390/v13030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021 doi: 10.1016/S2215-0366(21)00084-5. S2215036621000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2009191. NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.027. S088915912030489X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai L.-K., Hsieh S.-T., Chao C.-C., Chen Y.-C., Lin Y.-H., Chang S.-C., Chang Y.-C. Neuromuscular Disorders in Severe Acute Respiratory Syndrome. Arch. Neurol. 2004;61:1669–1673. doi: 10.1001/archneur.61.11.1669. https://doi.org/10.1001.archneur.61.11.1669. [DOI] [PubMed] [Google Scholar]
- Uc G. SARS-CoV-2 during pregnancy and associated cytokine-storm. Crit. Care Obstet. Gynecol. 2020;6 doi: 10.36648/2471-9803.6.2.14. [DOI] [Google Scholar]
- Vaira L.A., Hopkins C., Salzano G., Petrocelli M., Melis A., Cucurullo M., Ferrari M., Gagliardini L., Pipolo C., Deiana G., Fiore V., De Vito A., Turra N., Canu S., Maglio A., Serra A., Bussu F., Madeddu G., Babudieri S., Giuseppe Fois A., Pirina P., Salzano F.A., De Riu P., Biglioli F., De Riu G. Olfactory and gustatory function impairment in COVID ‐19 patients: Italian objective multicenter‐study. Head Neck. 2020 doi: 10.1002/hed.26269. hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés G., Neves L.A.A., Anton L., Corthorn J., Chacón C., Germain A.M., Merrill D.C., Ferrario C.M., Sarao R., Penninger J., Brosnihan K.B. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta. 2006;27:200–207. doi: 10.1016/j.placenta.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Verdecchia P. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020:7. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.F., Diforio D. Schizophrenia: a neural diathesis-stress model. Psychol. Rev. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walker C.K., Roche J.K., Sinha V., Roberts R.C. Substantia nigra ultrastructural pathology in schizophrenia. Schizophr. Res. 2018;197:209–218. doi: 10.1016/j.schres.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 doi: 10.1101/2020.02.10.20021832. 2020.02.10.20021832. [DOI] [Google Scholar]
- Wang C., Zhou Y.-H., Yang H.-X., Poon L.C. Intrauterine vertical transmission of SARS-CoV-2: what we know so far: vertical transmission of SARS-CoV-2. Ultrasound Obstet. Gynecol. 2020 doi: 10.1002/uog.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Guo L., Chen L., Liu W., Cao Y., Zhang J. A case report of neonatal COVID-19 infection in China. Clin. Infect. Dis. 2020;71(15):853–857. doi: 10.1093/cid/ciaa225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin. Infect. Dis. 2020;71(15):844–846. doi: 10.1093/cid/ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Xu R., Volkow N.D. Increased risk of COVID ‐19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124–130. doi: 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Namazova-Baranova L., Weber M., Vural M., Mestrovic J., Carrasco-Sanz A., Breda J., Berdzuli N., Pettoello-Mantovani M. The importance of continuing breastfeeding during COVID-19: in support to the WHO statement on breastfeeding during the pandemic. J. Pediatr. 2020 doi: 10.1016/j.jpeds.2020.05.009. S0022347620305837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organ; 2021. WHO Coronavirus Disease (COVID-19) Dashboard [WWW Document] URL https://covid19.who.int/ (Accessed 4.11.21) [Google Scholar]
- Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.-L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Xu X., Chen Z., Duan J., Hashimoto K., Yang L., Liu C., Yang C. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.03.031. S0889159120303573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Li X.-M. White matter abnormalities and animal models examining a putative role of altered white matter in schizophrenia. Schizophr. Res. Treat. 2011;2011:1–16. doi: 10.1155/2011/826976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P., Wang X., Liu P., Wei C., He B., Zheng J., Zhao D. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J. Clin. Virol. 2020;127:104356. doi: 10.1016/j.jcv.2020.104356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawn D., Pyeatte J., Joseph J., Eichler S., Garcia-Bunuel R. Transplacental transfer of influenza virus. JAMA. 1971;216:2. [PubMed] [Google Scholar]
- Yin M.-Z., Zhang L., Deng G.-T., Han C.-F., Shen M.-X., Sun H.-Y., Zeng F.-R., Zhang W., Chen L., Luo Q.-Q., Yao D.-J., Wu M., Yu S.-H., Chen H., Baud D., Chen X. Severe acute respiratory syndrome coronavirus 2 (sars-cov -2) infection during pregnancy in China: a retrospective cohort study. MedRxiv. 2020 doi: 10.1101/2020.04.07.20053744. 2020.04.07.20053744. [DOI] [Google Scholar]
- Yolken R.H., Torrey E.F. Viruses, Schizophrenia, and Bipolar Disorder. Clin. Microbiol. Rev. 1995;8:131–145. doi: 10.1128/cmr.8.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X., Liu Y., Xiao J., Liu H., Deng D., Chen S., Zeng W., Feng L., Wu J. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30176-6. S1473309920301766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020 doi: 10.1111/aogs.13867. aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Xu C., Fan J., Tang Y., Deng Q., Zhang W., Long X. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323(18):1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J., Zhou W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174(7):722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Ji P., Pang J., Zhong Z., Li H., He C., Zhang J., Zhao C. Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J. Med. Virol. 2020 doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbühler P., Conti M.E., León-Cejas L., Maximiliano-González F., Bonardo P., Miquelini A., Halfon J., Martínez J., Gutiérrez M.V., Reisin R. Guillain-Barre syndrome associated to COVID-19 infection: a review of published case reports. Rev. Neurol. 2021;72(March (6)):203–212. doi: 10.33588/rn.7206.2020487. English, Spanish PMID: 33710610. [DOI] [PubMed] [Google Scholar]