Summary
The establishment of centromere-specific CENP-A chromatin is influenced by epigenetic and genetic processes. Central domain sequences from fission yeast centromeres are preferred substrates for CENP-ACnp1 incorporation, but their use is context dependent, requiring adjacent heterochromatin. CENP-ACnp1 overexpression bypasses heterochromatin dependency, suggesting that heterochromatin ensures exposure to conditions or locations permissive for CENP-ACnp1 assembly. Centromeres cluster around spindle-pole bodies (SPBs). We show that heterochromatin-bearing minichromosomes localize close to SPBs, consistent with this location promoting CENP-ACnp1 incorporation. We demonstrate that heterochromatin-independent de novo CENP-ACnp1 chromatin assembly occurs when central domain DNA is placed near, but not far from, endogenous centromeres or neocentromeres. Moreover, direct tethering of central domain DNA at SPBs permits CENP-ACnp1 assembly, suggesting that the nuclear compartment surrounding SPBs is permissive for CENP-ACnp1 incorporation because target sequences are exposed to high levels of CENP-ACnp1 and associated assembly factors. Thus, nuclear spatial organization is a key epigenetic factor that influences centromere identity.
Keywords: centromere identity, CENP-A, spindle-pole body, heterochromatin, spatial organization, fission yeast, S. pombe
Graphical abstract
Highlights
-
•
Episomes bearing heterochromatin localize near fission yeast SPB-centromere clusters
-
•
Only centromere DNA inserted near active centromeres assembles CENP-A chromatin
-
•
Centromere DNA directly tethered to the SPB assembles CENP-A chromatin
-
•
Nuclear position is an epigenetic factor that influences centromere identity
Wu et al. show that heterochromatin tends to associate with fission yeast spindle-pole bodies (SPBs) where centromeres cluster. The requirement for heterochromatin in CENP-A/kinetochore establishment on centromeric DNA is bypassed by placing or tethering centromeric DNA near SPB-centromere clusters. Thus, nuclear positioning influences centromere identity.
Introduction
Centromeres are specialized chromosomal sites where multiprotein complexes known as kinetochores are assembled. Kinetochores attach chromosomes to spindle microtubules to mediate accurate mitotic and meiotic chromosome segregation. The assembly of kinetochores in many eukaryotes, including yeasts and humans, relies on specialized centromeric chromatin in which canonical histone H3 is replaced by the CENP-A/cenH3 histone H3 variant (Cnp1 in fission yeast, Schizosaccharomyces pombe). CENP-A-containing chromatin provides the underlying epigenetic mark that specifies the chromosomal site at which kinetochores assemble. CENP-A is required to establish and maintain centromere identity and thus indicates active centromeres.1,2
In organisms with monocentric chromosomes, centromeres are confined to a single locus on each chromosome. Such centromeres are often composed of long tandem arrays of repetitive sequences, such as α-satellite repeats on human chromosomes.3 These repeats provide a substrate for the de novo establishment of CENP-A chromatin and the assembly of functional kinetochores when introduced into human cells. Thus, α-satellite repeats trigger centromere formation. Acentric chromosomes lacking centromeres are unable to attach to spindle microtubules and are lost during cell division. However, following centromere ablation through centromere inactivation or deletion of centromere DNA, neocentromeres can arise spontaneously at unusual locations that lack sequence similarity to normal centromere DNA but allow stable segregation of such acentric chromosomes.3,4 Thus, centromeric DNAs are not the only sequences that can trigger the assembly of functional kinetochores. Once assembled at a particular location, including neocentromeres or sites that do not usually incorporate CENP-A, CENP-A chromatin is stably propagated at that site though cell division using intrinsic maintenance mechanisms.5,6 Consequently, prior CENP-A assembly can mark a chromosomal locus for continued persistence of CENP-A chromatin on one homolog, whereas the same locus remains devoid of CENP-A on the other.3
The fission yeast genome is carried on three monocentric chromosomes with regional centromeres of 40–110 kb comprising two distinct domains (Figure S1): CENP-ACnp1 chromatin assembles across the central domain consisting of central core (cc) and flanking innermost repeat (imr) DNA, which are surrounded by outer repeats (otr-dg/dh) assembled in Clr4 histone H3 lysine 9 methyl-(H3K9me)-transferase-dependent heterochromatin.7,8 The central core of centromere 2 (cc2) is unique, but the central cores of cen1 and cen3 share the same sequence. imr elements are unique to each centromere and mark the transition between CENP-ACnp1 chromatin and the heterochromatic otr-dg/dh repeats, which are conserved in sequence, but not in arrangement, between the three centromeres.9,10 tRNA genes that reside in each imr element demarcate these distinct centromeric domains and prevent heterochromatin from encroaching into the central CENP-ACnp1 chromatin domain.11,12 Two divergent Schizosaccharomyces species (S. octosporus and S. cryophilus) share a similar centromere domain organization.13
Like human α-satellite centromeric DNA, fission yeast central domain DNA is a preferred substrate for CENP-ACnp1 and kinetochore assembly. This preferred status is underscored by the observation that, in contrast to other sequences, naive central domain DNA borne on minichromosomes readily assembles and maintains CENP-ACnp1 chromatin following transient CENP-ACnp1 overexpression, bypassing the usual requirement for adjacent heterochromatin.14,15 Interestingly, despite having no sequence homology with S. pombe centromeres, central domains from S. octosporus and S. cryophilus are competent to assemble CENP-ACnp1 chromatin and functional centromeres in S. pombe, indicating that fission yeast central domains possess conserved instructive features.13 S. pombe central domain sequences are transcribed by RNAPII and exhibit high rates of histone H3 turnover, which may contribute to the replacement of S-phase-deposited placeholder H3 with CENP-ACnp1 during the subsequent G2.16,17 H3 is evicted from central domain chromatin even in the absence of CENP-A and kinetochore proteins.16 The Mis18 complex acts in concert with the CENP-A chaperone, HJURP, to recognize pre-existing CENP-A nucleosomes and ensure their persistence at particular locations by mediating H3 replacement with CENP-A in new H3-containing nucleosomes assembled during the preceding S phase.5,6,18 Thus, fission yeast central domain DNA possesses innate sequence-driven properties that program H3 eviction, making it a favored substrate for CENP-ACnp1 chromatin assembly, which, once assembled, is rendered heritable though an intricate read-write mechanism.
Centromeres are tightly clustered around spindle-pole bodies (SPBs; centrosome equivalents; Figure 1A) during interphase in both fission (S. pombe) and budding (Saccharomyces cerevisiae) yeast.19, 20, 21 In S. cerevisiae, SPB-to-centromere microtubules persist in G1 and mediate SPB-centromere clustering.21, 22, 23 Proper centromere clustering around S. pombe SPBs is dependent on the functions of SPB component Sad1 (LINC complex SUN domain protein) and Lem2 (LEM domain inner nuclear membrane protein), which is distributed around the entire nuclear envelope (NE) but is concentrated at SPBs.24,25 Csi1 that resides at the kinetochore-SPB interface is required for Lem2 accumulation around SPBs, and it acts with Lem2 to maintain SPB-centromere associations.24,26,27 The CENP-A assembly factors Scm3HJURP, Mis16RbAP46/48, Mis18, and Eic1/Mis19 are concentrated at centromeres clustered close to SPBs from late anaphase to prophase, including during G2 when new CENP-ACnp1 is incorporated.16,28, 29, 30, 31
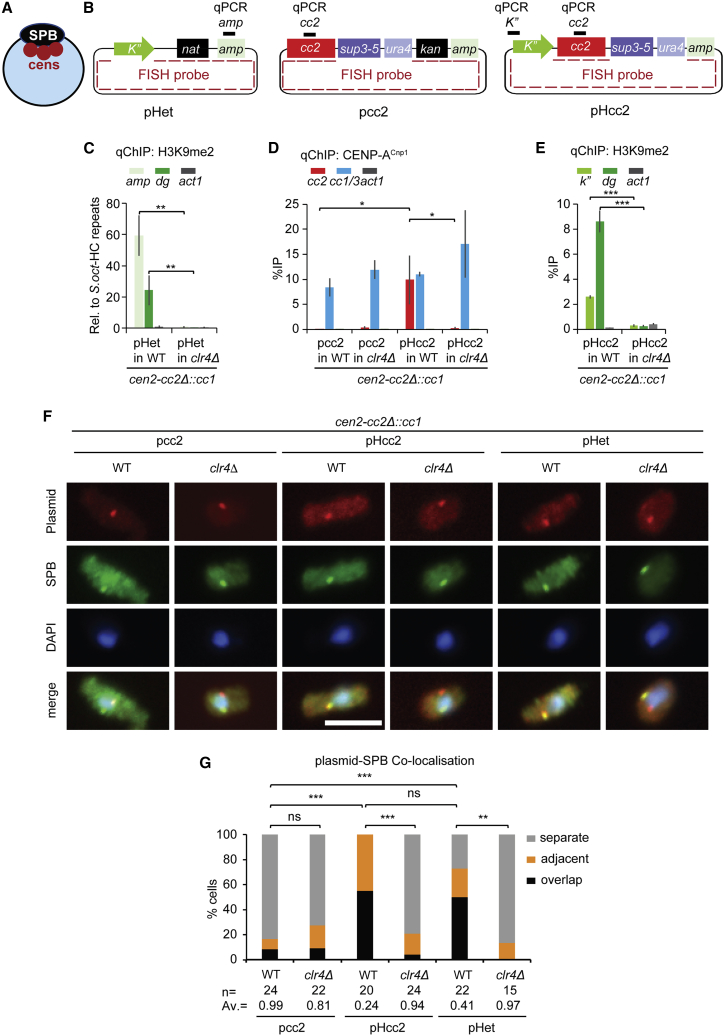
Figure 1.
Centromeric heterochromatin colocalizes with the SPB-centromere cluster
(A) Carton showing clustering of three endogenous centromeres (red circles) at the SPB (black oval) during interphase.
(B) Diagram of pHet, pcc2, and pHcc2 minichromosomes. Black bars above each plasmid map represent qChIP primer sites on ampicillin gene (amp), cc2, and K″ repeats of plasmids, respectively. Dashed red line in plasmids indicates position of FISH probe.
(C–E) qChIP analyses for H3K9me2 levels (C and E) on amp gene of pHet (C); K″ repeats of pHcc2 (E); dg repeats of centromeric HC and act1 gene; CENP-ACnp1 levels (D) on cc2, cc1/3 (indicates sequences common to cc1 and cc3), and act1 in WT and clr4Δ cells containing cc2Δ::cc1 at cen2 transformed with pHet (C), pcc2 (D), or pHcc2 (D and E). %IP levels in S. pombe were normalized to %IP of cen3 HC repeats from spiked-in S. octosporus chromatin in (C). qChIP results in (D) and (E) are reported as %IP. Data are mean ± SD (error bars) (n = 3–4 experimental replicates). ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005 (unpaired t test).
(F) Representative images of plasmid DNA FISH (red; probe as indicated in A), SPB location (green; anti-Cdc11), and DNA staining (blue, DAPI) in WT and clr4Δ cells transformed with pcc2, pHcc2, or pHet. Images were scaled relative to the maximum values of histogram. Scale bars, 5 μm.
(G) Cells were classified into three groups according to the 3D distances between plasmid and SPB (Cdc11): overlap (≤0.3 μm), adjacent (0.3–0.5 μm), or separate (0.5–3 μm).
Percentage of interphase cells (n, number analyzed from 3 independent experiments) in each category. AV, average distance; ns, no significance; ∗∗p < 0.001, ∗∗∗p < 0.0001 (Mann-Whitney U test) (see also STAR Methods and Figures S1–S3).
Although fission yeast centromeric central domains are the preferred substrate for CENP-ACnp1 assembly, the establishment of CENP-ACnp1 chromatin is subject to epigenetic regulation. The de novo assembly of CENP-ACnp1 chromatin and functional centromeres on central domain sequences is dependent on the presence of adjacent outer repeat heterochromatin (Figure S2).32,33 Direct transformation of naked minichromosome DNA into cells lacking heterochromatin compared with crossing minichromosomes preassembled in chromatin from wild-type (WT) cells results in a different fate: in the former, the central domain is assembled in H3 chromatin; in the latter, it is assembled in CENP-ACnp1 chromatin (Figures S2B and S2C).32 These observations indicate that both context and prior history are important for determining chromatin state. Synthetic heterochromatin, assembled by tethering the Clr4 H3K9-methyltransferase, substitutes for outer repeats in promoting CENP-ACnp1 assembly on minichromosomes when placed next to central domain DNA (Figure S2F).34 Thus, the properties of adjacent heterochromatin itself, rather than other features of outer repeat elements, are critical for de novo CENP-ACnp1 assembly. Heterochromatin could promote the establishment of CENP-ACnp1 chromatin by the recruitment of chromatin modifiers that influence turnover or other properties of histone H3 chromatin on the adjacent central domain to favor CENP-ACnp1 deposition.15,16 Alternatively, because CENP-ACnp1 overexpression circumvents the need for flanking heterochromatin in such CENP-ACnp1 chromatin establishment assays15 (Figure S2), and endogenous heterochromatin domains are located at the nuclear periphery,19,35,36 it is possible that centromeric heterochromatin places such minichromosomes at a nuclear location that encourages de novo CENP-ACnp1 chromatin assembly. The centromere clusters at S. pombe SPBs would be expected to provide a compartment naturally enriched with CENP-ACnp1 and its loading factors.
Here, we test whether the positioning of centromeric DNA relative to existing centromeres and/or SPBs influences de novo CENP-ACnp1 chromatin assembly and the recruitment of kinetochore proteins. Heterochromatin-bearing plasmids localize close to SPBs, suggesting that heterochromatin may play a positioning role in promoting the establishment of CENP-ACnp1 chromatin. We demonstrate that potentially functional centromeric central domain DNA does not assemble CENP-ACnp1 or kinetochore proteins unless inserted close to an already functional native centromere or neocentromere. Thus, proximity to an existing centromere in cis on the same chromosome promotes CENP-ACnp1 and kinetochore assembly. Direct tethering of naive minichromosome-borne central domain DNA to SPB-associated proteins in the absence of flanking heterochromatin revealed that proximity in trans to SPB-centromere clusters is also sufficient to trigger the assembly of CENP-ACnp1 chromatin and recruitment of kinetochore components. Thus, we define a key role for spatial genome organization, in particular centromere clustering, and the resulting nuclear compartmentalization in determining centromere identity. Our findings reveal that centromeric heterochromatin functions to position centromeres within a nuclear compartment that ensures de novo CENP-ACnp1 chromatin assembly.
Results
Centromeric heterochromatin mediates localization near the SPB-centromere cluster
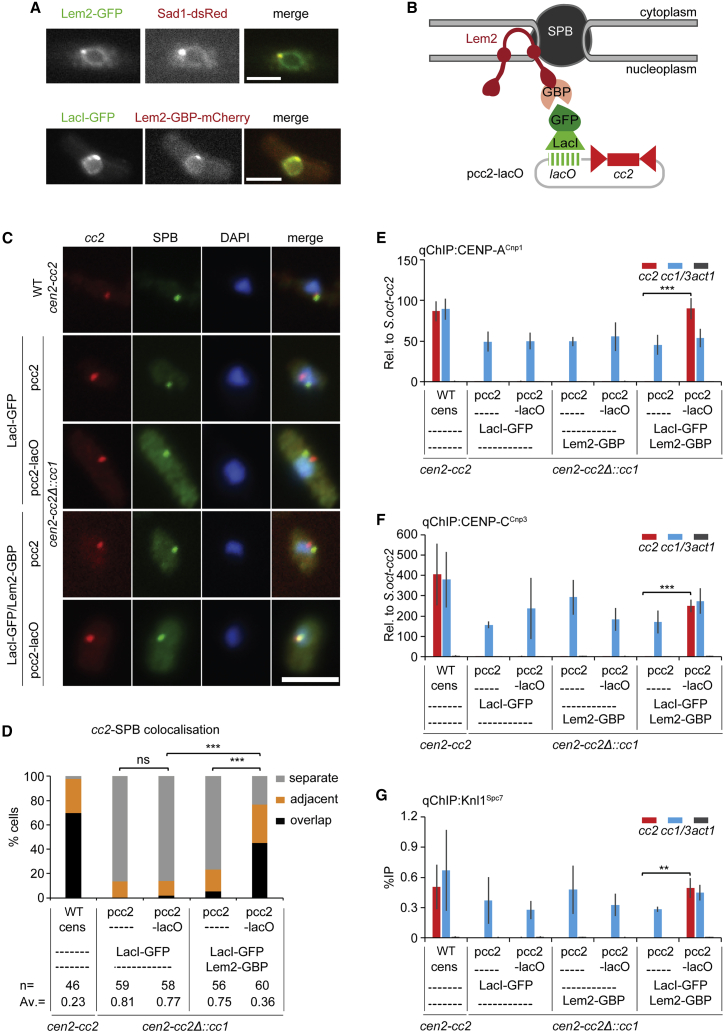
Endogenous fission yeast centromeres are clustered together at the SPB during interphase (Figure 1A).19,37 The de novo assembly of CENP-ACnp1 chromatin on naive centromeric central domain DNA that is freshly introduced into fission yeast as DNA by transformation on plasmid-based minichromosomes requires H3K9me-dependent heterochromatin formation on the flanking outer dg/dh (K/L) centromere repeat DNA (Figures S2A, S2B, and S2D).32 Heterochromatin may influence CENP-ACnp1 chromatin establishment through nuclear positioning cues. To test whether centromeric heterochromatin promotes localization close to SPBs, we utilized autonomously replicating minichromosomes, which are less constrained than endogenous chromosomal regions with respect to their positioning within nuclei. In all strains used, 6 kb of endogenous cc2 was replaced with 5.5 kb of cen1 central domain DNA (cc2Δ::cc1; Figure S1B). Thus, cc2 DNA carried by minichromosomes are unique sequences in these strains, allowing their specific analysis by quantitative chromatin immunoprecipitation (qChIP). As a consequence of this manipulation, sequences common to WT cc1 and cc3 are present at all three endogenous centromeres in cc2Δ::cc1 cells and provide a positive control comparator for CENP-ACnp1 and kinetochore protein association. The establishment of centromere function (i.e., mitotic segregation ability) is assayed by replica plating fresh transformants to indicator plates (STAR Methods; Figure S2). Both centromeric heterochromatin (which ensures sister-centromere cohesion and hence biorientation of centromeres on the spindle)38,39 and CENP-ACnp1 chromatin (which recruits the kinetochore) are required for centromere function.32 The establishment of CENP-ACnp1 chromatin (and kinetochore protein recruitment) and the establishment of heterochromatin are assayed by qChIP on cultures grown from randomly picked transformants, as various plasmid/strain combinations are not capable of establishing full centromere function (Figures S2 and S3). The pHet minichromosome carries outer repeat DNA (K″, 2 kb) that is sufficient to trigger Clr4-dependent de novo heterochromatin formation when transformed into WT, but not clr4Δ, cells (Figures 1B and 1C; Table S2).32,34 pcc2 carries 8.6 kb of cen2 central domain DNA but lacks outer repeat DNA heterochromatin (Figure 1B; Table S2) and thus cannot assemble CENP-ACnp1 chromatin or kinetochores (Figures 1D and S2D).15,32 However, pHcc2, carrying both outer repeat and cc2 DNA (Figure 1B; Table S2) forms heterochromatin, which permits CENP-ACnp1 chromatin (Figures 1D and 1E), kinetochores, and functional centromeres to be frequently established de novo in WT cells following transformation (Figures S2A and S2G–S2I).15,32
Fluorescence in situ hybridization (FISH) to the backbone plasmid and/or cc2 sequences (Figure 1B) allowed pHet, pcc2, or pHcc2 minichromosome localization in WT cells relative to SPBs (Cdc11, SPB-specific centriolin ortholog). pcc2 was found at, or in close proximity to, SPBs in 17% of cells; however, the presence of a heterochromatic repeat on pHcc2 with resulting CENP-ACnp1 and kinetochore assembly increased SPB colocalization to 100% (Figures 1F, 1G, and S3A). Consistent with a requirement for heterochromatin for SPB association, only low levels of pHcc2-SPB colocalization were detected in clr4Δ cells where heterochromatin and CENP-ACnp1/kinetochores are unable to assemble (Figures 1D–1G, S2G, and S3A).32 Moreover, pHet, which only assembles heterochromatin, localized close to SPBs in 73% of WT cells but only 13% of clr4Δ cells (Figures 1C, 1F, 1G, and S3A). The assembly of synthetic heterochromatin via TetR-Clr440 increased colocalization of a ptetO plasmid with the SPB 3.5-fold (29% colocalization in TetR-Clr4 cells versus 8% in control cells), suggesting that heterochromatin assembled independently of outer repeat (K) sequences can also mediate localization with SPBs (Figures S3B–S3E).
Together, these data indicate that centromeric outer-repeat-induced heterochromatin is sufficient to mediate frequent colocalization with SPBs where centromeres and CENP-ACnp1 assembly factors are concentrated. Thus, we propose that centromeric heterochromatin promotes exposure of adjacent cc2 centromere DNA to this CENP-ACnp1 assembly-factor-rich nuclear compartment, thereby ensuring the assembly of CENP-ACnp1 chromatin and kinetochores.
Centromeric central domain DNA assembles CENP-ACnp1 chromatin when inserted close to native centromeres
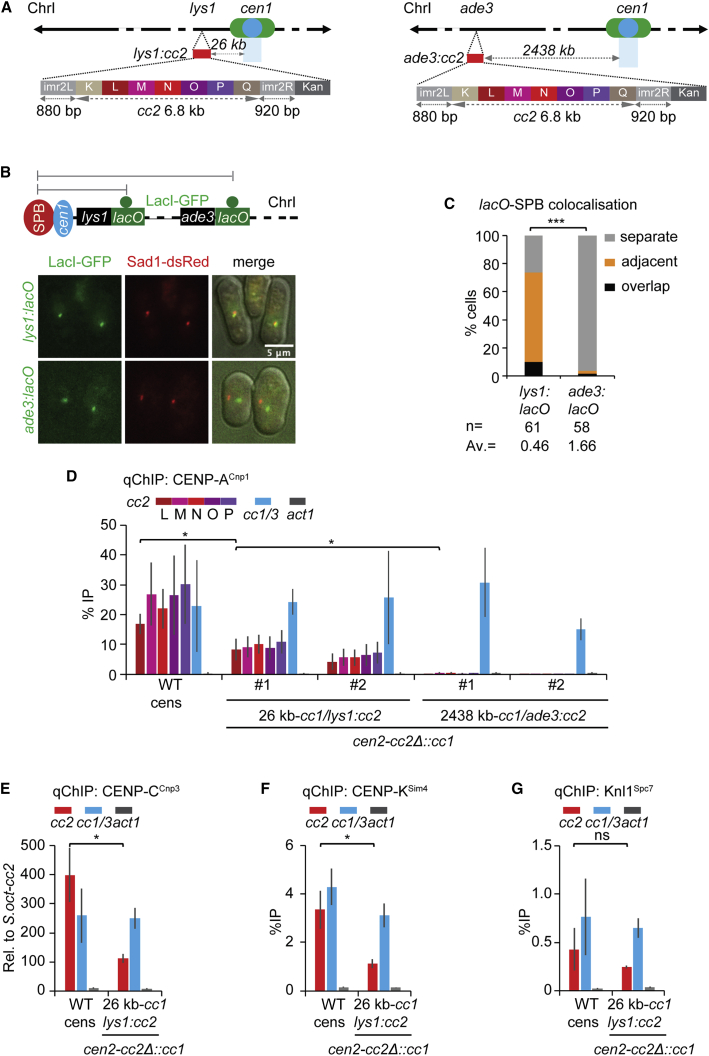
To test whether a nuclear compartment formed by SPB-centromere clustering might stimulate de novo CENP-ACnp1 chromatin assembly, we inserted 8.6 kb of cc2 DNA near or far from cen1 and assayed for the presence of CENP-ACnp1 chromatin. In all strains used, endogenous cc2 had been replaced with cc1 so that regions L-to-Q of the resulting 8.6-kb cc2 insertions are unique (cc2Δ::cc1; Figure S1B). The lys1 locus resides just 26 kb from cc1 and 11.3 kb from the left otr1 heterochromatin repeat, while ade3 is a distant 2,438 kb from cc1 (Figure 2A). Microscopy measurements demonstrated that lys1 and ade3 decorated with LacI-GFP on lacO-array insertions41 are positioned in close proximity to or distant from SPBs, respectively, in three-dimensional nuclear space (Figures 2B and 2C). qChIP analysis showed that CENP-ACnp1 was uniformly incorporated onto regions L-P across cc2 following insertion at lys1 (lys1:cc2). In contrast, no CENP-ACnp1 enrichment was observed on cc2 inserted at ade3 (ade3:cc2) (Figure 2D). In addition, kinetochore proteins CENP-CCnp3, CENP-KSim4, and Knl1Spc7 were also recruited to lys1:cc2 (Figures 2E–2G), indicating that CENP-ACnp1 deposition on lys1:cc2 results in recruitment of both inner and outer kinetochore proteins. CENP-ACnp1 was also incorporated on cc2 inserted at sdh1, 24 kb to the right of cen1-cc1, or at a location we named itg10, 27 kb from the right side of cen2-cc2Δ::cc1 (itg10:cc2; Figures S4A and S4B). Insertion of cc2 at locations 41 kb (vps29:cc2) and 47 kb (bud6:cc2) that is further away on the left side of cen1-cc1 resulted in progressively less CENP-ACnp1 incorporation, suggesting that the level incorporated on inserted cc2 DNA is dependent on its proximity in cis to functional cen1 (Figure S4C). Thus, either proximity to an endogenous centromere in cis on the same chromosome, or exposure to a distinct nuclear compartment formed by SPB-centromere clusters, effectively mediates de novo CENP-ACnp1 assembly and kinetochore protein recruitment on naive central domain DNA. cc2 DNA inserted close to cen1 might acquire CENP-ACnp1 chromatin as a result of it spreading from cen1 into lys1:cc2. However, little or no CENP-ACnp1 enrichment was detected at three positions (i–iii) between cen1 and lys1:cc2 (Figure S4D). Thus, CENP-ACnp1 does not uniformly spread along the chromosome from its normal location at cen1-cc1 into the lys1:cc2 insert.
Figure 2.
CENP-ACnp1 chromatin is established on the centromere-adjacent lys1:cc2 central domain
(A) Ectopic cc2, carrying 880 bp imr2L, 6.8 kb cc2 (subdivided into K-to-Q regions; 6 kb is unique), and 920 bp imr2R DNA, was inserted at lys1 (lys1:cc2; 26 kb from cc1) or ade3 (ade3:cc2; 2438 kb from cc1) on ChrI in cc2Δ::cc1 strain.
(B) Representative images of live cells expressing Sad1-dsRed (SPB marker) and LacI-GFP bound to lys1:lacO or ade3:lacO.41 Images were scaled relative to the maximum intensity in the set of images. Scale bars, 5 μm.
(C) 3D distances between lys1:lacO or ade3:lacO and SPBs (Sad1). Percentage of G2 cells (n, number analyzed from 3 independent experiments) in each category, classified as in Figure 1. AV, average distance. ∗∗∗p < 0.0001 (Mann-Whitney U test) (see also STAR Methods).
(D) qChIP for CENP-ACnp1 at regions L-P of cc2, cc1/3 and act1 in WT cens strain carrying endogenous cen2-cc2 or cen2-cc2Δ::cc1 strain with lys1:cc2 or ade3:cc2 insertions. # number indicates individual isolates.
(E–G) qChIP analyses for CENP-CCnp3 (E), CENP-KSim4 (F), and Knl1Spc7 (G) levels at cc2, cc1/3, and act1 genes in WT cens strain carrying endogenous cen2-cc2 or cen2-cc2Δ::cc1 strain with lys1:cc2. %IP levels in S. pombe were normalized to %IP of S. octosporus central core from spiked-in chromatin in (E). qChIP results in (D), (F), and (G) were reported as %IP. Data are mean ± SD (n = 3). ns, no significance; ∗p < 0.05 (unpaired t test) (see also Figures S1, S4, and S5).
To assess the impact of cc2 insertions on cell viability, strains were grown on media containing the vital dye, phloxine B. Regardless of cc2 location (cen2-cc2, lys1:cc2, or ade3:cc2), colonies were pale pink, indicative of normal growth (Figure S4E). In strains overexpressing nmt41-CENP-ACnp1 (hi-CENP-ACnp1), the ade3:cc2 centromere-distal-insertion strain was darker pink than cen2-cc2 and centromere-proximal (lys1:cc2) strains, indicating decreased viability. CENP-ACnp1 was detectable on ade3:cc2 by qChIP only upon hi-CENP-ACnp1, indicating that arm-located cc2 is competent for CENP-ACnp1 incorporation under certain conditions (Figure S4F). These observations are consistent with CENP-ACnp1-overexpression-induced dicentric formation (cen1 and centromere-proximal ade3:cc2) and associated reduced viability. The fact that lys1:cc2 strains exhibit normal viability despite the incorporation of CENP-ACnp1 at even endogenous levels of expression, suggests that the chromosome-bearing lys1:cc2 is functionally monocentric due to the proximity of lys1:cc2 to cen1.
These analyses demonstrate that cc2 DNA, a known substrate for fission yeast CENP-ACnp1 and kinetochore assembly, incorporates CENP-ACnp1 when inserted in cis close to native centromeres. The finding that the levels of CENP-ACnp1 incorporated decrease with increasing distance from a centromere suggests that proximity to native centromeres provides an environment that is more favorable for CENP-ACnp1 and kinetochore assembly on naive centromere DNA.
Proximity to functional centromeres, not locus-specific context, promotes CENP-ACnp1 chromatin establishment
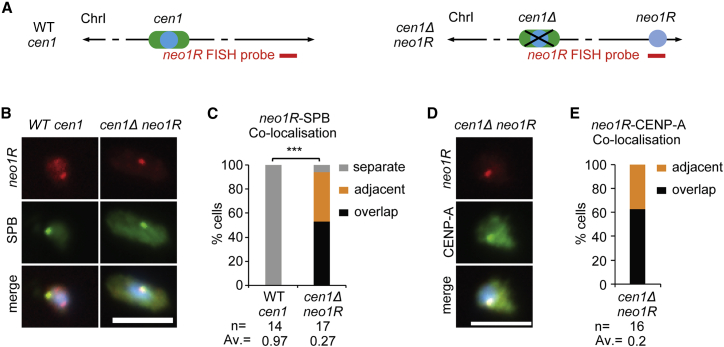
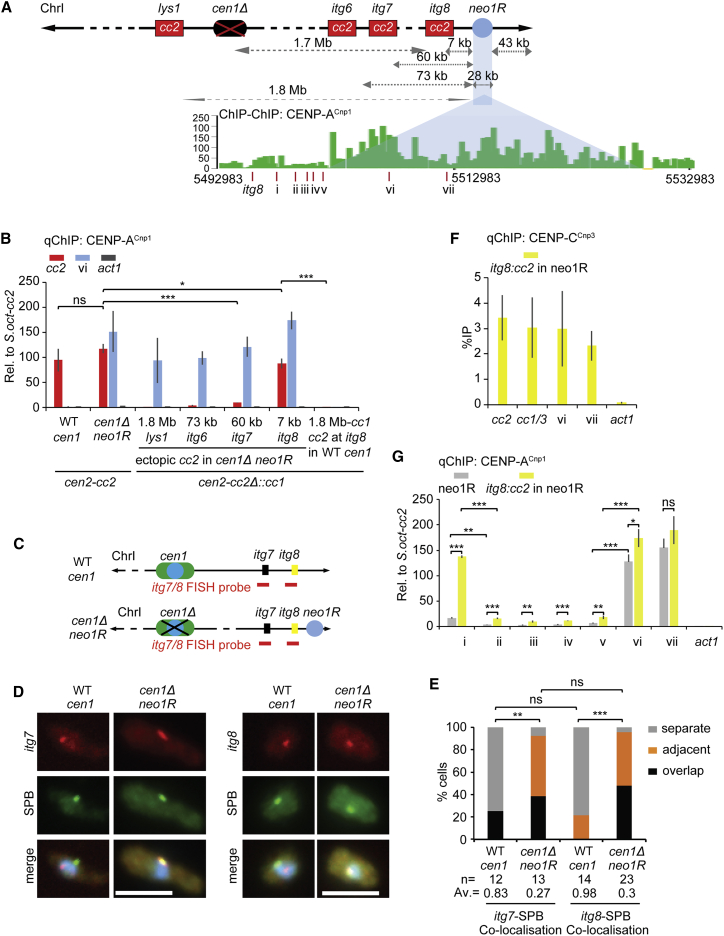
Neocentromeres form near fission yeast telomeres when an endogenous centromere is deleted (Figure 3A).4 Deletion of cen1 (cen1Δ) results in neocentromeres being formed over the left (neo1L; cd39) or right (neo1R; cd60) subtelomeric regions on chromosome I.4 FISH demonstrates that prior to neocentromere formation the subtelomeric neo1R locus is not located near SPBs, whereas upon CENP-ACnp1 assembly and neocentromere formation, neo1R joins the interphase SPB-centromere cluster in 94% of cells, where CENP-A is concentrated (Figures 3A–3E). Unlike when cc2 was inserted at lys1 in cells with a nearby functional cen1 (Figure 2), insertion of cc2 at lys1 in cen1Δ cells with the neo1R neocentromere 1.8-Mb away failed to incorporate CENP-ACnp1 (Figures 4A and 4B). This finding suggests that CENP-ACnp1 fails to be incorporated at lys1:cc2 upon insertion in cells with this neocentromere because lys1 is displaced from the centromere cluster. Thus, a prediction is that insertion of cc2 close to a region where neocentromeres can form will only result in CENP-ACnp1 incorporation when an active neocentromere is present. We therefore inserted cc2 at locations 73 (itg6), 60 (itg7), and 7 kb (itg8) from the neo1R region in cells with a WT cen1 (no subtelomeric neocentromere) or with an active neocentromere neo1R (WT cen1 deleted) (Figure 4A).
Figure 3.
neo1R neocentromere clusters with endogenous centromeres at the SPB during interphase
(A) Diagram represents strains with cen1 or lacking cen1 but carrying neo1R neocentromere (cen1Δ neo1R). Red line indicates position of neo1R DNA FISH probe (ChrI: 5,513,871–5,530,124).
(B and D) Representative images of neo1R DNA FISH (red; probe as indicated in A), SPB location (green; anti-Cdc11; B) or centromere clusters (green; anti-CENPCnp1; D), and DNA staining (blue, DAPI) in WT cen1 (B) and cen1Δ neo1R cells. Images were scaled as in Figure 1. Scale bars, 5 μm.
(C and E) 3D distances between neo1R DNA and SPBs (Cdc11; C) or centromere clusters (CENP-ACnp1; E).
Percentage of interphase cells (n, number analyzed) in each distance category, classified as in Figure 1. AV, average distance. ∗∗∗p < 0.0001 (Mann-Whitney U test) (see also Figure S1).
Figure 4.
CENP-ACnp1 chromatin can establish on central domain DNA inserted close to the neocentromere
(A) Ectopic cc2 inserted at lys1, itg6 (ChrI: 5,435,010–5,435,237), itg7 (ChrI: 5,447,816–5,448,235), and itg8 (ChrI: 5,501,647–5,502,134), 1.8 Mb, 73, 60, and 7 kb from neo1R CENP-ACnp1 domain, respectively. ChIP-CHIP analysis for CENP-ACnp1 in cen1Δ neo1R (cd60) strain was obtained from Ishii et al.4 Red lines indicate itg8 and 7 qChIP primer sites (i–vii).
(B) qChIP analyses of CENP-ACnp1 levels at cc2, cc1/3, and act1 in WT cen1 or cen1Δ neo1R strain with lys1:cc2, itg6:cc2, itg7:cc2, or itg8:cc2 insertions (genome positions as indicated in A).
(C) Diagram represents WT-cen1 or cen1Δ neo1R strains. Red line indicates position of itg7 or itg8 DNA FISH probe (ChrI 5,438,081–5,453,142; ChrI 5,495,975–5,508,459), respectively.
(D) Representative images of itg7 or itg8 DNA FISH (red; probe as indicated in C), SPB location (green, anti-Cdc11), and DNA staining (blue, DAPI) in WT cen1 and cen1Δ neo1R cells. Images scaled as in Figure 1. Scale bars, 5 μm.
(E) 3D distances between itg7 or itg8 and SPBs (Cdc11), percentage of interphase cells (n, number analyzed) in each category, classified as in Figure 1. AV, average distance. ns, no significance; ∗∗p < 0.001, ∗∗∗p < 0.0001 (Mann-Whitney U test).
(F) qChIP analyses for CENP-CCnp3 levels at cc1/3, cc2, act1, and site i and ii within neo1R in cen1Δ neo1R strain with itg8:cc2 insertion. qChIP results were reported as %IP.
(G) qChIP analyses for CENP-ACnp1 levels at 7 loci (i–vii, positions as indicated in A) and act1 in cen1Δ neo1R strain, with or without itg8:cc2 insertion.
ns, no significance; ∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005 (unpaired t test). %IP levels in S. pombe were normalized to %IP of S. octosporus central core from spiked-in chromatin (B and G). Data are mean ± SD (n = 3) (see also Figure S1).
Unlike WT cells where itg7 and itg8 were spatially distant from SPBs, both itg7 and itg8 were positioned close to SPB-centromere clusters in 92% and 96% of neo1R cells, respectively (Figures 4C–4E). CENP-ACnp1 was enriched on lys1:cc2, but not itg7:cc2 or itg8:cc2, in cells with WT cen1 (Figure 4B). However, in cells with cen1Δ neo1R, the pattern was reversed: no CENP-ACnp1 incorporation occurred on lys1:cc2, whereas low or high levels of CENP-ACnp1 were detected on itg7:cc2 and itg8:cc2 that are located 60 and 8 kb from the active neo1R neocentromere, respectively. In addition, kinetochore protein CENP-CCnp3 was recruited to itg8:cc2 at levels comparable to sites vi and vii within neo1R and endogenous centromeres (Figure 4F). Little or no CENP-ACnp1 was detected on the itg6:cc2 and itg7:cc2 insertions at greater distances from this neocentromere (Figure 4B).
In cen1Δ neo1R cells, with or without cc2 inserted at itg8, we tested for CENP-ACnp1 enrichment at five positions (sites i–v) between itg8 and the active neo1R centromere and two positions (sites vi and vii) within neo1R (Figure 4A). As expected, high levels of CENP-ACnp1 were detected at sites vi and vii within the characterized neo1R neocentromere4 in cells with or without cc2 inserted at itg8. However, substantial CENP-ACnp1 incorporation was only observed 5.2 kb from neo1R (site i; 1.6 kb from itg8, yellow bar; Figure 4G) if cc2 was inserted at itg8, whereas little or no CENP-ACnp1 enrichment was detected at sites i–v between itg8 and neo1R in cells lacking cc2 at itg8 (gray bars; Figure 4G).
These analyses demonstrate that the deletion of native cen1 prevents de novo CENP-ACnp1 incorporation on cc2 subsequently inserted at lys1 but permits CENP-ACnp1 assembly on cc2 when inserted close to a resulting neocentromere. The fact that CENP-ACnp1 is not detected at most positions between the neo1R centromere and itg8:cc2 indicates that as at native cen1 (Figure S4D), CENP-ACnp1 chromatin does not spread uniformly from the pre-existing neocentromere to the nearby inserted cc2 DNA. We conclude that it is the proximity of lys1 or itg8 to functional centromeres, rather than properties of sequences immediately flanking these loci, that allows the naturally CENP-ACnp1-permissive cc2 DNA substrate to assemble CENP-ACnp1 when inserted at these locations.
Centromeric heterochromatin is not required for de novo CENP-ACnp1 incorporation on centromere DNA placed close to an existing centromere
In minichromosome-based establishment assays, H3K9me-dependent heterochromatin is needed to allow de novo CENP-ACnp1 incorporation on adjacent cc2 central domain DNA (Figure S2).32 If the nuclear environment formed by SPB-centromere clustering is sufficient to promote de novo CENP-ACnp1 assembly, the prediction is that centromeric heterochromatin would not be required when central domain DNA is inserted close to endogenous centromeres. The lys1:cc2 insertion is positioned only 11.3 kb from endogenous cen1 heterochromatic dh/otr1 repeats (Figure S5A). To determine whether centromeric heterochromatin influences CENP-ACnp1 chromatin establishment at lys1, we inserted cc2 DNA at this locus in either WT or heterochromatin-deficient clr4Δ cells (lack Clr4 H3K9 methyltransferase). FISH confirmed that the lys1 locus and lys1:cc2 insertion remain near SPBs in cells lacking Clr4 (Figures S5B–S5E). qChIP demonstrated that CENP-ACnp1 was established on lys1:cc2 insertions made in either WT or clr4Δ cells and that both CENP-CCnp3 and Knl1Spc7 kinetochore proteins were recruited (Figures S5F–S5H). Thus, the de novo assembly of CENP-ACnp1 and kinetochore proteins at lys1:cc2 occurs independently of nearby centromeric heterochromatin.
We conclude that centromeric heterochromatin is not required to assemble CENP-ACnp1 and kinetochore proteins on freshly introduced centromeric DNA if that DNA is positioned in cis close to an existing centromere, which clusters with other centromeres and associated CENP-ACnp1 plus its assembly factors, around SPBs. The placement of centromeric central domain DNA close to active centromeres bypasses the requirement for heterochromatin. This lack of a need for centromeric heterochromatin is consistent with heterochromatin normally influencing the establishment of CENP-ACnp1 chromatin by sequestering freshly introduced centromeric DNA at SPBs.
Direct tethering of centromeric DNA to SPBs mediates establishment of CENP-ACnp1 chromatin
Insertion of central domain cc2 DNA near endogenous centromeres indicates that proximity in cis to SPB-centromere clusters enhances CENP-ACnp1 chromatin establishment. If the SPB-centromere cluster creates a nuclear compartment that promotes CENP-ACnp1 assembly, then positioning centromeric cc2 DNA in trans near SPBs might also lead to CENP-ACnp1 and kinetochore assembly. To directly test whether the SPB-centromere compartment influences CENP-ACnp1 chromatin establishment on centromeric DNA, we artificially tethered episomal minichromosomes to SPBs. The inner nuclear membrane (INM) protein Lem2 localizes around the NE and also exhibits strong colocalization with SPBs (Figure 5A).24 Lem2 is also specifically enriched across the central domain of fission yeast centromeres.26,42 Arrays of lacO sites (2.8 kb; ∼90 lacO sites) were placed in pcc2, generating pcc2-lacO (Table S2), which was then transformed into cells constitutively expressing a LacI-GFP fusion protein (binds pcc2-lacO) and Lem2 fused to both GFP-binding protein (GBP) and mCherry (Lem2-GBP-mCherry; Figures 5A and 5B). Therefore, cells expressing both Lem2-GBP-mCherry and LacI-GFP should tether pcc2-lacO to SPBs. Indeed, Lem2-mediated tethering resulted in the pcc2-lacO FISH signal being in close proximity to SPBs in 77% of cells, whereas in the absence of tethering components it was located away from SPBs in >77% of cells examined (Figures 5C and 5D). Crucially, this Lem2-mediated tethering of pcc2-LacO near SPBs resulted in CENP-ACnp1 incorporation at cc2 on SPB-adjacent pcc2-lacO, whereas CENP-ACnp1 was not detected on untethered pcc2-lacO or pcc2 itself (Figure 5E). In addition to CENP-ACnp1, the inner kinetochore protein, CENP-CCnp3, and outer kinetochore protein, Knl1Spc7, were also assembled on the cc2 central domain of pcc2-lacO, but only when it was tethered at SPBs (Figures 5F and 5G).
Figure 5.
Tethering cc2 DNA to Lem2 allows CENP-ACnp1 incorporation and kinetochore protein recruitment
(A) Representative images of live cells expressing Lem2-GFP and Sad1-dsRed or LacI-GFP and Lem2-GBP-mCherry. Images were scaled as in Figure 2. Scale bars, 5 μm.
(B) Schematic representation of the tethering system used to force pcc2-lacO association with Lem2-GBP-mCherry at the NE and SPB. pcc2-lacO is bound by LacI-GFP and ultimately tethered to Lem2-GBP-mCherry via GFP/GBP interaction.
(C) Representative images of cc2 DNA FISH (red), SPB location (green, anti-Cdc11), and DNA staining (blue, DAPI) in WT cens strain carrying endogenous cen2-cc2 or cen2-cc2Δ::cc1 strain expressing LacI-GFP or both LacI-GFP and Lem2-GBP-mCherry transformed with pcc2 or pcc2-lacO. Fluorescence of LacI-GFP and Lem2-GBP-mCherry was dissipated by the immunofluorescence/DNA FISH procedure and did not contribute punctate signal. Images were scaled as in Figure 1. Scale bars, 5 μm.
(D) 3D distances between cc2 and SPBs (Cdc11), percentage of interphase cells (n, number analyzed) in each category, classified as in Figure 1. AV, average distance; ns, no significance; ∗∗∗p < 0.0001 (Mann-Whitney U test).
(E–G) qChIP analyses for CENP-ACnp1 (E), CENP-CCnp3 (F), and Knl1Spc7 (G) levels at cc2, cc1/3, and act1 in WT cens strain carrying endogenous cen2-cc2 or cen2-cc2Δ::cc1 strain expressing LacI-GFP or Lem2-GBP-mCherry, or both of them transformed with pcc2 or pcc2-lacO. %IP levels in S. pombe were normalized to %IP of S. octosporus central core from spiked-in chromatin in (E) and (F). qChIP results in (G) reported as %IP. Data are mean ± SD (n = 3). ∗∗p < 0.005, ∗∗∗p < 0.0005 (unpaired t test) (see also Figures S1, S6, and S7).
These analyses demonstrate that direct tethering of cc2 DNA to SPBs enables CENP-ACnp1 chromatin to be established without the need for adjacent heterochromatin. However, Lem2 is not an SPB-specific protein and thus Lem2-mediated pcc2-lacO tethering does not rule out the possibility that the non-SPB fraction of Lem2, localized around the NE (Figure 5A), somehow contributes to CENP-ACnp1 and kinetochore protein enrichment. The Alp4 and Alp6 proteins are components of the SPB-associated γ-tubulin complex, and a proportion of both proteins localize on the nucleoplasmic side of SPBs in interphase.43 Cells expressing Alp4-GBP-mCherry or Alp6-GBP-mCherry fusion proteins and LacI-GFP, transformed with pcc2-LacO, were therefore generated (Figures S6A and S6B). Both Alp4-GBP- and Alp6-GBP-mediated tethering resulted in pcc2-lacO being located close to SPBs in 82%–90% of cells, whereas in >79% of cells lacking tethering components, pcc2-lacO was located distant from SPBs (Figures S6C–S6E). Importantly, SPB tethering via Alp4-GBP or Alp6-GBP resulted in CENP-ACnp1 incorporation on the cc2 region of pcc2-lacO (Figures S6F and S6G). Thus, the direct tethering of cc2 DNA to SPBs via SPB-specific components enables CENP-ACnp1 chromatin establishment. The establishment of CENP-ACnp1 chromatin on pcc2-lacO transformed into Lem2-GBP/LacI-GFP-expressing cells was unaffected by the absence of Clr4-dependent heterochromatin (clr4Δ; Figure S7).
Together, these manipulations reveal that in the absence of adjacent heterochromatin, the forced localization of centromeric central domain DNA, the native substrate for fission yeast CENP-ACnp1 assembly, to SPBs is sufficient to trigger CENP-ACnp1 chromatin and kinetochore assembly.
Loss of centromere-SPB association prevents CENP-ACnp1 chromatin establishment
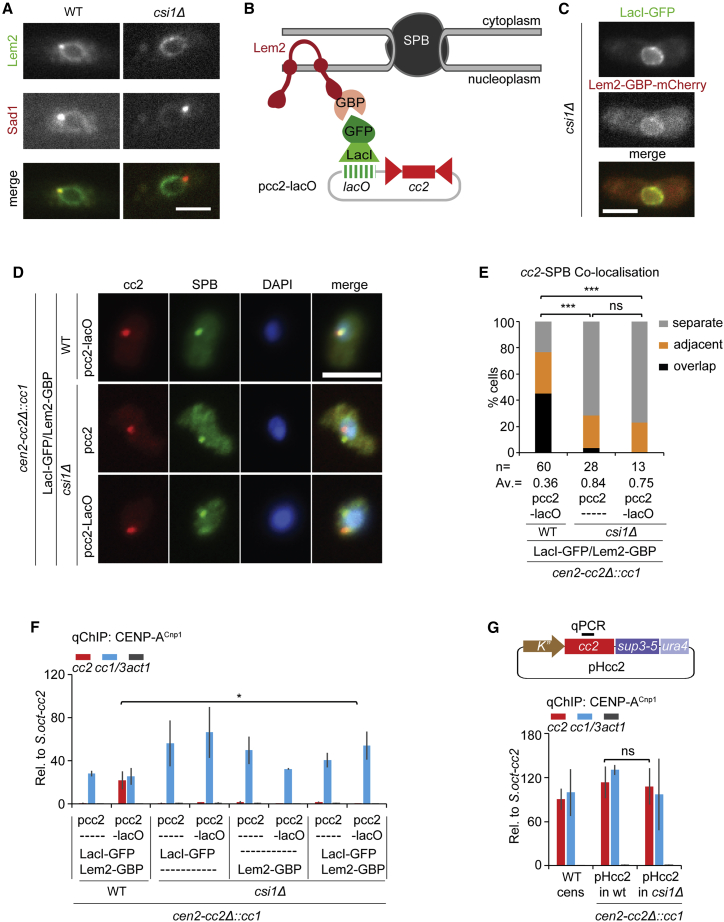
If the de novo establishment of CENP-ACnp1 chromatin on centromeric DNA tethered near SPBs depends on the surrounding nuclear compartment, then loss of centromere-SPB association would be expected to hinder CENP-ACnp1 incorporation. The accumulation of Lem2 at SPBs requires the Csi1 protein; in cells lacking Csi1 (csi1Δ) Lem2, Lem2-GBP-mCherry and associated LacI-GFP are mainly localized around the nuclear periphery (Figures 6A and 6C).24 We therefore used csi1Δ cells to test whether the loss of the SPB-associated Lem2 pool affects Lem2-mediated tethering of pcc2-lacO at SPBs (Figures 6A and 6B). Indeed, pcc2-lacO was located near SPBs in only 23% of csi1Δ cells compared with 77% of WT cells expressing Lem2-GBP-mCherry and LacI-GFP (Figures 6D and 6E). Furthermore, csi1Δ cells were unable to establish CENP-ACnp1 chromatin on Lem2-tethered pcc2-lacO (Figure 6F). However, CENP-ACnp1 can assemble de novo on cc2 of pHcc2 transformed into csi1Δ cells (Figure 6G), indicating that Csi1 itself is not required for CENP-ACnp1 establishment. Thus, Lem2 needs to be concentrated at SPBs in order to induce CENP-ACnp1 incorporation on tethered centromeric DNA.
Figure 6.
Loss of Csi1 prevents CENP-ACnp1 chromatin establishment on Lem2-tethered pcc2-lacO
(A and C) Representative images of live WT and csi1Δ cells expressing Lem2-GFP and Sad1-dsRed (A) or LacI-GFP and Lem2-GBP-mCherry (C). Images were scaled as in Figure 2. Scale bars, 5 μm.
(B) Forced association of pcc2-lacO with Lem2-GBP-mCherry at NE in csi1Δ using same tethering system as in Figure 5. In csi1Δ, pcc2-lacO is expected to detach from the SPB due to loss of Lem2 from SPB.
(D) Representative images of cc2 DNA FISH (red), SPB location (green, anti-Cdc11), and DNA staining (blue, DAPI) WT or csi1Δ strains expressing both LacI-GFP and Lem2-GBP-mCherry transformed with pcc2 or pcc2-lacO. Images were scaled as in Figure 1. Scale bars, 5 μm.
(E) Percentage of interphase cells (n, number analyzed) displaying distinct degrees of cc2 DNA colocalization with SPBs (Cdc11). Cells were classified into three groups as in Figure 1. AV, average distance. ns, no significance; ∗∗∗p < 0.0001 (Mann-Whitney U test).
(F and G) qChIP analyses for CENP-ACnp1 at cc2, cc1/3, and act1 in indicated strains transform with pcc2 or pcc2-lacO (F) or pHcc2 (G). qChIP primer site on pHcc2-borne cc2 is indicated as black bar above plasmid map (G). %IP levels in S. pombe were normalized to %IP of S. octosporus central core from spiked-in chromatin. Data are mean ± SD (n = 3). ns, no significance; ∗p < 0.05 (unpaired t test) (see also Figure S1).
Together, these data indicate that pericentromeric heterochromatin is sufficient to mediate frequent colocalization with SPBs where centromeres and CENP-ACnp1 assembly factors are concentrated. We conclude that heterochromatin promotes the exposure of adjacent cc2 centromere DNA to this CENP-ACnp1 assembly-factor-rich nuclear sub-compartment, thus ensuring the assembly of CENP-A chromatin and kinetochores (Figure 7).
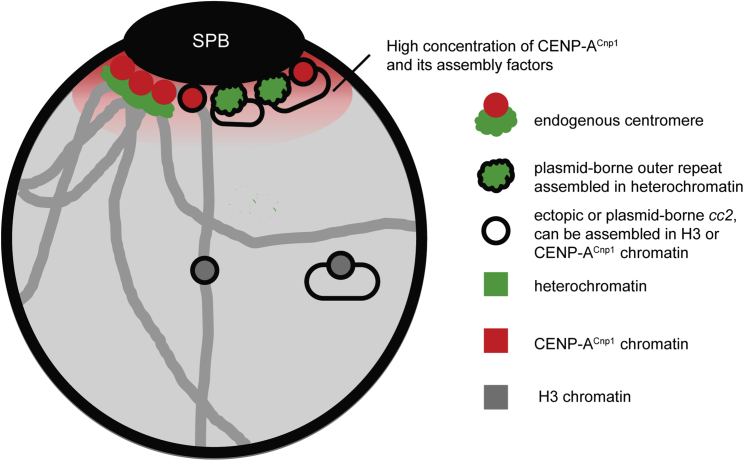
Figure 7.
Model: Centromere identity is influenced by nuclear spatial organization
Due to clustering of endogenous centromeres (CENP-ACnp1-assembled central domains, red circles; heterochromatic outer repeats, green) at SPBs and incorporation of CENP-ACnp1 at centromeres in G2, the zone around SPBs forms a nuclear sub-compartment rich in CENP-ACnp1 and its assembly factors (red-shaded cloud). Ectopic central domain (outlined circles) inserted at centromere-proximal sites exposed the high-CENP-ACnp1 SPB/centromere sub-compartment, promoting de novo incorporation of CENP-ACnp1, unlike centromere-distal locations. Similarly, only minichromosomes bearing heterochromatin, which mediates localization close to the SPB, exposes the adjacent central domain to the high-CENP-ACnp1 SPB/centromere sub-compartment, resulting in CENP-ACnp1 incorporation. Heterochromatin, green; CENP-ACnp1, red; neutral H3 chromatin, gray (see also Figure S1).
Discussion
Assembly of CENP-A chromatin is epigenetically regulated. Here, we demonstrate that in addition to the impact of chromatin context and prior CENP-A history, spatial location within the nucleus is an epigenetic influence on the chromatin fate of centromeric DNA. We show that heterochromatin causes minichromosomes to localize near SPBs, providing a likely explanation for the role of heterochromatin in promoting CENP-ACnp1 chromatin establishment on adjacent centromeric sequences. By placing a CENP-ACnp1 assembly competent sequence (cc2) in various spatial contexts, we demonstrate that being in the vicinity of centromere clusters at SPBs triggers de novo CENP-ACnp1 chromatin establishment.
Despite epigenetic factors being important in the establishment of CENP-A chromatin, certain sequences are preferred, including human α-satellite arrays and fission yeast central domains. Rather than the precise sequence being critical, evidence suggests that innate properties of central domain regions, such as their unusual transcriptional landscape and high rates of histone H3 turnover, are permissive for CENP-ACnp1 incorporation into chromatin.15, 16, 17 Although central domain sequences are the preferred substrate for CENP-ACnp1 assembly in fission yeast, de novo assembly of CENP-ACnp1 chromatin is context dependent. Outer-repeat-directed or synthetic heterochromatin promotes CENP-ACnp1 chromatin establishment on the adjacent central domain DNA.32,34 CENP-ACnp1 overexpression induces de novo CENP-ACnp1 chromatin establishment on plasmid-based minichromosomes devoid of heterochromatin and carrying only central domain sequences.15
We have previously suggested two models to explain these observations.15,32,44 In the “modifier” model, heterochromatin performs a chromatin-directed role such as recruitment of histone-modifying enzymes or remodelers that influence histone dynamics to favor CENP-ACnp1 incorporation on adjacent central domain regions. In this scenario, CENP-ACnp1 overexpression would shift the equilibrium away from transcription-dependent histone H3 recycling and toward CENP-ACnp1 deposition. In the “positioning” model, the role of heterochromatin, due to its own localization, would place central domain DNA at a nuclear location permissive for CENP-ACnp1 deposition, such as a compartment exhibiting high levels of CENP-ACnp1 and associated assembly factors. In this model, overexpression of CENP-ACnp1 would bypass heterochromatin’s function by making a greater proportion of nuclear space permissive for CENP-ACnp1 assembly.
Here, we have utilized FISH to demonstrate that minichromosome-borne heterochromatin preferentially locates close to SPBs. We hypothesize that any sequence positioned at this location will be exposed to high concentrations of CENP-ACnp1 and its assembly factors because centromeres are clustered at SPBs for most of the cell cycle. However, only sequences such as centromeric central domain DNA, with embedded properties that drive transcription-coupled H3 replacement with CENP-ACnp1, actually incorporate CENP-ACnp1.16
Support for the hypothesis that a key role for heterochromatin in CENP-ACnp1 establishment is to position the central domain within the SPB-centromere cluster compartment of nuclei is provided by our finding that centromeric cc2 DNA inserted close to endogenous or neocentromeres assembled CENP-ACnp1 chromatin, whereas cc2 inserted at locations far away from centromeres did not. The positioning of lys1 and itg8 close to SPBs in WT and neocentromere-containing cells, respectively, correlates with the incorporation of CENP-ACnp1 on cc2 when inserted at these sites. Although the failure of CENP-ACnp1 to assemble on centromere-distal sites such as ade3 could be attributed to selection against deleterious dicentric formation on this endogenous chromosome, a strain bearing ade3:cc2 does not show decreased viability compared with strains with cc2 at cen2 or cen1-proximal lys1:cc2 (Figure S7). Centromere-distal cc2 is not refractory to CENP-ACnp1 incorporation, as combining CENP-ACnp1 overexpresssion with ade3:cc2 results in the incorporation of CENP-ACnp1 and reduced viability, consistent with dicentric chromosome formation. In addition, we have previously shown that cc2 present on the arm of a 530-kb non-essential linear minichromosome also does not normally assemble CENP-ACnp1. However, that minichromosome is capable of dicentric formation because overexpressed CENP-ACnp1 incorporates into cc2 and causes missegregation.15 Thus, placing central domain DNA near centromeres in cis results in CENP-ACnp1 incorporation. Moreover, direct tethering of minichromosome-borne central domain DNA in trans to SPB-associated proteins also triggered the de novo assembly of CENP-ACnp1 chromatin, bypassing the requirement for heterochromatin. Thus, when susceptible sequences are positioned in the vicinity of SPBs, the establishment of CENP-ACnp1 chromatin is uncoupled from the presence of heterochromatin. These observations indicate that nuclear positioning is an epigenetic factor that is important for establishing centromere function, and the function that heterochromatin provides is positioning information.
Our finding that centromeric central domain cc2 DNA inserted close to an endogenous natural centromere or neocentromere results in gain of CENP-ACnp1 chromatin is consistent with the centromere-SPB cluster providing a favorable microenvironment for de novo CENP-ACnp1 and kinetochore assembly. Interestingly, it has previously been proposed that during a brief period in meiotic prophase when centromeres and telomeres colocalize at the SPB, telomeres contribute to a SPB-focused microenvironment, which promotes the incorporation of GFP-CENP-ACnp1 and reinforces centromere identity in meiosis.45
Although we do not detect CENP-ACnp1 enrichment at sites between endogenous cc1 or neo1R and cc2 inserted at lys1 or itg8, respectively, it is possible that CENP-ACnp1 spreads in cis along intervening chromatin, but properties of these sequences provoke its loss. CENP-ACnp1 spreading may be favored or hindered by innate sequence features such as their transcriptional activity. Low levels of transcription, open chromatin, or nucleosome turnover may promote CENP-ACnp1 spreading. Moreover, the topology of the intervening chromatin may place a region physically closer or further away, depending on its level of compaction and/or looping in three-dimensional space.
Once assembled, CENP-ACnp1 chromatin and thus kinetochores are self-propagating.5,6,18 However, de novo establishment may be required if catastrophic events result in complete CENP-ACnp1 loss. For example, a double-strand break and resection in the CENP-ACnp1 chromatin domain of a centromere could result in disassembly of all CENP-ACnp1 chromatin at that centromere. In such circumstances, continued association of the damaged centromere with the SPB via flanking heterochromatin could ensure the re-establishment of CENP-ACnp1 chromatin and kinetochores following repair of central domain DNA.
Fission yeast neocentromeres arise most frequently in subtelomeric regions, and immature neocentromeres near rDNA can be stabilized by relocation to subtelomeric regions or upon acquisition of adjacent heterochromatin.4,46 When overexpressed, CENP-ACnp1 is incorporated at moderate levels over subtelomeric regions.14 Therefore, subtelomeric regions represent favored, but secondary, sites for CENP-ACnp1 and kinetochore assembly. H3K9me-dependent heterochromatin is normally assembled adjacent to telomeres.47 During interphase, fission yeast telomeres are attached to the NE via INM proteins Bqt3 and Bqt4.48 Although Hi-C analysis does not detect frequent contacts between telomere and centromere regions,49 we suggest that as a consequence of their association with the nuclear periphery, subtelomeric regions are highly constrained in their nuclear explorations, essentially being confined to the NE’s inner surface rather than having access to the entire nuclear volume. This constraint on spatial exploration would make telomeres more likely than arm sites to meet SPB-centromere clusters, thereby exposing them to the immediate nuclear compartment containing high levels of CENP-ACnp1 and its assembly factors. Thus, nuclear-envelope association offers an attractive explanation for the subtelomeric location of most fission yeast neocentromeres. As neocentromeres arise rarely at telomeres, even in the absence of telomeric heterochromatin,4 it is possible that telomere anchoring at the NE contributes to their role as secondary CENP-ACnp1 assembly sites.
In fission yeast, centromeres cluster at the SPB throughout the cell cycle, except during mitosis, after which they return to the SPB in anaphase. CENP-ACnp1 and several CENP-ACnp1 assembly factors and chaperones, such as Scm3HJURP, Mis16RbAP46/48, Mis18, and Eic1/Mis19, are concentrated on centromeres around the SPB during interphase.28, 29, 30, 31 Mammalian centromeres are not localized close to centrosomes (SPB equivalent) during most of the cell cycle. However, after mitotic chromosome segregation, mammalian centromeres transiently cluster at spindle poles in late anaphase/telophase, subsequently dispersing during G1.50 Centromere clustering is also pronounced in plants that exhibit an overt “Rabl” configuration, where centromeres and telomeres are clustered at opposite sides of interphase nuclei.51 Intriguingly, the Mis18 CENP-A assembly complex is normally recruited to human centromeres in late anaphase/telophase prior to arrival of the HJURP CENP-A chaperone and new CENP-A incorporation in early G1.18 Therefore, centromeres of complex eukaryotes are briefly clustered together at precisely the time when assembly factors are recruited to centromeres. This spatiotemporal coordination may maximize the local concentration of CENP-A and its assembly factors to ensure the efficient removal of H3 placeholder nucleosomes and the replenishment of CENP-A nucleosomes in centromeric chromatin.52 However, we note that loss of CDK (cyclin-dependent kinase) regulation in mammalian cells allows premature CENP-A deposition in G2 cells.53 Moreover, mammalian CENP-A can be loaded at centromeres exiting a manipulated mitosis (in the absence of microtubules and BubR1) without chromosome segregation/movement to the spindle poles.54 The possible influence of centromere clustering on CENP-A assembly during normal mammalian cell cycles will require more direct investigation.
Once CENP-ACnp1 chromatin and kinetochores are assembled at fission yeast centromeres, it is clear that heterochromatin-independent connections with SPBs are established. Centromeres remain clustered at SPBs in the absence of pericentromeric H3K9me-dependent heterochromatin, but SPB-centromere clustering is disrupted when essential kinetochore components such as Mis6 are defective.37 Thus, once assembled, an intact interphase kinetochore structure, rather than pericentromeric heterochromatin, appears to provide the main physical link between functional centromeres and SPBs. Interestingly, cells defective in the essential kinetochore component Mis6 display both SPB-centromere declustering37 and reduced CENP-ACnp1 levels at centromeres,55 suggesting that clustering might impact CENP-ACnp1 maintenance at centromeres.
The tendency for heterochromatin to concentrate at SPBs may be mediated by interactions between heterochromatin proteins and SPB components. Indeed, proteomic analyses show that several SPB proteins are enriched with Swi6HP1 heterochromatin.42 A plasmid-based minichromosome (ptetO) bearing completely synthetic TetR-Clr4-driven heterochromatin also colocalizes with SPBs, albeit less frequently than pHet bearing K-repeat-directed heterochromatin (Figures 1 and S5). Thus, in addition to heterochromatin itself, other unknown factors bound to K-repeat, but not synthetic heterochromatin, may contribute to robust SPB association. Although such factors may influence minichromosome-SPB association, it is clear that artificially tethering central domain DNA to SPBs bypasses the need for heterochromatin for establishing high levels of CENP-ACnp1 chromatin in cells lacking Clr4-dependent heterochromatin (Figure S7).
Here, we have demonstrated that the specific location of centromere sequences within nuclei (i.e., their spatial context) exerts an epigenetic influence on the eventual CENP-A chromatin state attained by specific DNA sequences. Our analyses demonstrate that the SPB-centromere cluster forms a sub-compartment within the nucleus that promotes CENP-A and kinetochore assembly on DNA sequences, presenting the required features to facilitate CENP-A chromatin assembly in place of canonical H3 chromatin. Thus, spatial positioning in the nucleus is a hitherto unrecognized epigenetic determinant of centromere identity
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse monoclonal anti-H3K9me2 | Gift from Takeshi Urano, mAb5.1.1 | N/A |
| Sheep polyclonal anti-CENP-ACnp1 | In-house preparation15 | N/A |
| Sheep polyclonal anti-CENP-CCnp3 | In-house preparation15 | N/A |
| Sheep polyclonal anti-CENP-KSim4 | In-house preparation15 | N/A |
| Sheep polyclonal anti-KNL1Spc7 | Gift from Kevin Hardwick | N/A |
| Sheep anti-Cdc11 | Gift from Ken Sawin | N/A |
| Donkey anti-Sheep IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat#A-11015; RRID: AB_2534082 |
| Sheep polyclonal Anti-Digoxigenin-Rhodamine, Fab fragments | Roche | Cat#11207750910; RRID: AB_514501 |
| Bacterial and virus strains | ||
| NEB 5-alpha Competent E. coli (High Efficiency) | New England Biolabs | Cat#C2987H |
| Chemicals, peptides, and recombinant proteins | ||
| Nourseothricin (cloNAT) | Werner BioAgents | CAS#96736-11-7 |
| Hygromycin B (Hyg) | Duchefa Biochemie | CAS#31282-04-9 |
| Geneticin Selective Antibiotic (G418 Sulfate) | Gibco Life Technologies | CAS#10131027 |
| Carbenicillin Disodium Salt | Invitrogen | CAS#10177012 |
| Formaldehyde, 37% | MERCK | CAS#F8775 |
| Glycine | MERCK | G8790 |
| Glutaraldehyde solution, 50% | MERCK | CAS#111-30-8 |
| Zymolyase-100T | MP Biomedicals | Cat#08320932 |
| Lallzyme | Litmus Wines | Lallzyme-MMX |
| Protein G Dynabeads (Life Technologies) | Thermo Fisher Scientific | Cat#10009D |
| Protein G-Agarose | Roche | Cat#11243233001 |
| Critical commercial assays | ||
| QIAquick PCR Purification Kit | QIAGEN | Cat#28104 |
| Monarch Plasmid Miniprep Kit | New England Biolabs | Cat#T1010L |
| FastStart Taq DNA Polymerase | Roche | Cat#12032953001 |
| Light Cycler 480 SybrGreen Master Mix | Roche | Cat#04887352001 |
| DIG-Nick Translation Mix | Roche | Cat#11745816910 |
| NEB Golden Gate Assembly Kit (BsaI-HF v2) | New England Biolabs | Cat# E1601S |
| Experimental models: Organisms/strains | ||
| S. pombe strains, see Table S1 | This study | N/A |
| S. octosporus | From Nick Rhind56 | yFS286 |
| Oligonucleotides | ||
| Primers, see Table S3 | This study | N/A |
| Recombinant DNA | ||
| Plasmid pLSB-Kan, see Table S2 | 57 | Addgene#166700; RRID: Addgene_166700 |
| Plasmid clr4-pLSB-Kan, see Table S2 | This study | N/A |
| Plasmid pMC52, see Table S2 | This study | N/A |
| Plasmid pFA6a-GBP-mCherry-hygMX6, see Table S2 | Gift from Julia Promisel Cooper25 | N/A |
| Plasmid pFA6a-GFP-NatMX6, see Table S2 | This study | N/A |
| Plasmid pMC2 (pcc2), see Table S2 | This study | N/A |
| Plasmid pMC12 (pcc2-lacO), see Table S2 | This study | N/A |
| Plasmid pHcc2, see Table S2 | This study | N/A |
| Plasmid pMC183 (pHET), see Table S2 | This study | N/A |
| Plasmid pMC1, see Table S2 | This study | N/A |
| Software and algorithms | ||
| Roche LightCycler software version 1.5.1.62 | Roche | N/A |
| Nikon NIS Elements software version 5.21.03 | Nikon | RRID: SCR_014329 |
| Fiji | ImageJ, http://fiji.sc | RRID: SCR_002285 |
| Fiji-based bespoke in-house 3D analysis code | This study; https://doi.org/10.5281/zenodo.5657360 | N/A |
| SnapGene 5.2.5 | GSL Biotech LLC | RRID: SCR_015052 |
| Prism Version 9.1.0 | GraphPad | RRID: SCR_002798 |
| pombase | https://www.pombase.org/ | RRID: SCR_006586 |
| Other | ||
| Glusulase | NEN | NEE-154 |
| KpnI-HF | New England Biolabs | Cat#R3142S |
| XhoI | New England Biolabs | Cat# R0146S |
| SacI-HF | New England Biolabs | Cat# R3156S |
| MscI | New England Biolabs | Cat# R0534S |
| T4 DNA Ligase | New England Biolabs | Cat# M0202S |
| PMSF | MERCK | CAS#329-98-6 |
| Yeast Protease Inhibitor Cocktail | MERCK | P8215 |
| IGEPAL CA-630 NP40 | MERCK | Cat# 56741 |
| Chelex 100 Chelating Resin | Bio-Rad | Cat#1421253 |
| VECTASHIELD Antifade Mounting Medium | Vector Laboratories | Cat# H-1000-10 |
| L-Lysine hydrochloride | MERCK | Cat# 657-27-2 |
| Bovine Serum Albumin (BSA) | MERCK | Cat# A0281 |
| RNase A | Qiagen | Cat#19101 |
| Dextran sulfate sodium salt | MERCK | D8906 |
| deionized formamide | MERCK | S4117 |
| Denhardt′s Solution 50x | MERCK | D2532 |
| Gelatin from cold water fish skin | MERCK | Cat#G7765 |
| Ambion DNase I (RNase-free) | Thermo Fisher Scientific | Cat#AM2222 |
Resource availability
Lead contact
Requests concerning resources or material should be directed to and will be fulfilled by the lead contact Robin Allshire (robin.allshire@ed.ac.uk).
Materials availability
All plasmids and Schizosaccharomyces pombe (fission yeast) strains generated or used for this study are available form the lead contact without restriction.
Experimental model and subject details
Yeast strains
Yeast strains used in this study and their genotypes are listed in Table S1.
Standard genetic and molecular methods were used as described.58 All ectopic cc2 insertions were made in cc2Δ::cc1 strains15 by integrating linear cen2 central domain constructs (∼880 bp imr2L, -6.8 kb cc2 and ∼920 bp imr2R, abbreviated as cc2) by homologous recombination (HR). pMC52 (Table S2), bearing 8.6 kb of cc2 and kanMX6 selection cassette, was used as a starting plasmid for linear cc2 constructs. Two flanking DNA fragments of the desired target locus for cc2 insertions were amplified using primers listed in Table S3 by PCR. Restriction enzyme KpnI/XhoI-digested first fragment was cloned into KpnI/XhoI-digested pMC52, which were then digested by SacI/MscI and ligated with SacI/MscI-digested second PCR fragment by T4 DNA ligase (M0202S; NEB). Linear cc2 constructs were obtained by SacI/KpnI digestion of the resulting plasmids and transformed into desired strain for cc2 insertion.
For the construction of Lem2/Alp4/Alp6-GBP-mCherry and Lem2-GFP, the GBP-mCherry-hygMX6 and GFP-natMX6 cassette in plasmid pFA6a-GBP-mCherry-hygMX625 and pFA6a-GFP-NatMX6 were amplified by PCR and integrated into genome by HR.59
clr4Δ mutant was created by CRISPR/Cas9 method as described previously.57 Briefly, clr4 gene-specific sgRNA was cloned into Cas9 containing pLSB-KAN plasmid by Golden Gate Assembly kit (E1601S, NEB). The resulting plasmid clr4-pLSB-KAN and clr4 HR template obtained by annealing primer pair WW748/WW749 (Table S3) were co-transformed into S. pombe by sorbitol-electroporation method.
Transformants were grown on appropriate selection plates and screened for correct integration or clr4Δ mutant by yeast colony PCR using primers listed in Table S3. All plasmids and primers used in this study are listed in Tables S2 and S3 respectively.
Yeast growth medium and conditions
All strains were grown at 32°C in YES (Yeast Extract with Supplements) rich medium or PMG (Pombe Minimal Glutamate) minimal medium, as appropriate. Selection for dominant markers was performed on YES medium supplemented with 100 μg/ml clonNAT (96736-11-7, Werner BioAgents), 100 μg/ml G418 (10131027, Gibco), or 123 μg/ml HygMX6 (31282-04-9, Duchefa Biochemie). clr4Δ transformants were selected on YES supplemented with G418 plate and re-streaked to non-selective YES medium to allow loss of plasmid clr4-pLSB-KAN. Transformants with cc2 insertions were selected on YES supplemented G418. Plasmids pcc2 (pMC2; carrying 8.6 kb of cc2) and pcc2-LacO (pMC12; carrying 8.6 kb of cc2 and 2.8 kb of lacO) were selected on YES containing 100 μg/ml G418 in wt strains or on PMG-uracil in csi1Δ (csi1Δ::ura4) strain. Strains carrying plasmid pHet (pMC183; carrying 2 kb of K” repeats), ptetO (pMC171; bearing 4 tet operators embedded in 2 kb of randomized AT-rich sequence) were selected on YES supplemented with clonNAT or whereas pHcc2 (H denotes 5.6 kb of K” repeats, cc2 denotes 8.6 kb of cc2) were selected on PMG-adenine-uracil medium, respectively.
Bacteria
DH5α E. coli strains (C2987H, NEB) were grown in LB medium at 37°C. E. coli competent cells carrying plasmids were selected on LB agar plates supplemented with 100 μg/ml of ampicillin or LB liquid supplemented with 50 μg/ml Carbenicillin (10177012; Invitrogen).
Method details
Yeast genetic crosses
To obtain desired genotypes, two strains with opposite mating type (h+/h-) were mixed and grown on the nitrogen starved ME plate for sporulation at 32°C for 2 days. Asci was digested in glusulase (NEE-154, NEN) to release spores that were then plated on appropriate selective medium and grown at 32°C.
Yeast colony PCR
Yeast strains were suspended in SPZ buffer (1.2 M sorbitol, 100 mM sodium phosphate and 2.5 mg/ml Zymolyase-100T (08320932, MP Biomedicals)) and incubated at 37°C for 30 min. The resulted mixtures were used as PCR template for strain genotyping by Roche FastStart Taq polymerase PCR kit (12032953001, Roche) supplemented with primers.
Yeast transformation
Yeast cells were transformed using the sorbitol-electroporation method. Log phase cultures were harvested and resuspended in pre-transformation buffer (25 mM DTT, 0.6 M sorbitol and 20 mM HEPES, pH7.6) and incubated at 32°C with 180 rpm shaking for 10 min. Cells were washed three times in ice-cold 1.2 M sorbitol, mixed in an ice-cold cuvette with 200 ng of plasmid DNA or purified DNA fragments obtained by QIAquick PCR Purification Kit (28104, QIAGEN) and then pulsed by an electroporator (Bio-Rad Gene Pulser II) at a setting of 2.25kV, 200Ω and 25μF. Cells were either directly plated on medium with prototrophic selection directly or grown overnight in non-selective liquid before selection for antibiotic resistance (G418/cloNAT/HygMX6). Single colonies were isolated from selective medium.
Serial dilution spotting assays
Equal amounts of starting cells for each strain were serially diluted 5-fold and then spotted onto PMG plate complemented with 2.5 ug/ml vital dye Phloxine B. Colonies with a higher proportion of dead cells stain darker pink. Cells were grown at 25 °C or 32 °C for 3-5 days and then photographed.
Centromere establishment assay on minichromosome pHcc2
Fresh transformant colonies carrying circular plasmid-based minichromosome pHcc2 were replica-plated from PMG -adenine -uracil to PMG low-adenine plates (10 μg/ml adenine) and incubated at 32°C for 2 days to determine initial frequency of establishment of functional centromeres. Plasmid pHcc2 contains the sup3e tRNA selection marker that suppresses the ade6-704 mutation within strains, thus colony color on these PMG low-adenine plates will indicate minichromosome loss (red colonies) or retention (white/pale pink colonies). In the absence of centromere establishment, minichromosomes behave as episomes that are rapidly lost. Minichromosomes that established functional centromere (need both heterochromatin and CENP-ACnp1 chromatin) segregate efficiently during mitosis. Minichromosomes which occasionally integrate at genome will give a false-positive white phenotype. To assess the frequency of such integration events and to confirm establishment of centromere segregation function, colonies providing a the white/pale-pink phenotype upon replica plating were re-streaked to single colonies on PMG-low-adenine plates. Red/white sectored colonies are indicative of centromere function with low levels of minichromosome loss, whereas pure white colonies are indicative of integration into endogenous chromosomes. Therefore, the number of sectored colonies divided by the number of total colonies (minus pure white colonies) was used to calculate the centromere establishment frequency (%) on minichromosome pHcc2.
Quantitative chromatin immunoprecipitation (qChIP)
For ChIP of cells containing plasmid minichromosomes, three independent transformant colonies were randomly picked from PMG-ade-ura or YES+antibiotic plates and grown to 50-100 ml cultures in appropriate selective media (the centromere-establishment status of colonies (if relevant) was not determined prior to picking). Log phase cultures were fixed in 1% formaldehyde (F8775, MERCK) for 15 min followed by quenching in 125 mM Glycine (G8790, MERCK) at room temperature. ChIP was performed as previously described.60 2.5x108 cells were used for each ChIP. Briefly, cells were lysed by bead beating (Biospec) in 350 μl Lysis Buffer (50 mM Hepes-KOH pH 7.5, 140 mM NaCl, 1 mM EDTA, 1% (v/v) Triton X-100 and 0.1% (w/v) sodium deoxycholate) supplemented with 3.5 μl of 100 mM PMSF (329-98-6, MERCK) and 3.5 μl of 100 mM yeast protease inhibitor (P8215, MERCK). Where indicated, ∼5x107 fixed, lysed S. octosporus cells56 were added to each initial crude cell lysates as a spike-in control. Crude cell lysates were sonicated using a Bioruptor (Diagenode) at 4°C on high voltage for 20 min (20 cycles of 30 s ON/OFF), followed by centrifugation at 13000 rpm for 10 min to pellet cell debris. The resulting supernatant was used for following steps.
For H3K9me2 ChIP, 10 μl lysate was retained as crude ‘input’ sample, whereas 300 μl of the remaining lysates were incubated overnight with 20 μl of washed protein G Dynabeads (10009D, Thermo Fisher Scientific) and 1 μl of mouse anti-H3K9me2 (mAb5.1.1, gift from Takeshi Urano).
For CENP-ACnp1/CENP-CCnp3/KnlSpc7 ChIP, lysates were precleared for 1 h with 25 μl of washed protein-G agarose beads (11243233001, Roche) and 10 μl of precleared lysate was retained as crude ‘input’ sample. 300 μl of the remaining pre-cleared lysates were incubated overnight with appropriate amount of antibody (10 μl of sheep CENP-ACnp1, CENP-CCnp3, CENP-KSim4 serum15 (in-house preparation), 3 μl of affinity-purified sheep anti-Spc7 (a gift from Kevin Hardwick) and 25 μl of protein-G agarose beads.
After immunoprecipitation, the crude “IP” samples on beads were washed in Lysis Buffer, Lysis Buffer supplemented with 500 mM NaCl, Wash Buffer (10 mM Tris-HCl pH 8, 250 mM LiCl, 0.5% IGEPAL NP40 (56741, MERCK) 0.5% (w/v) sodium deoxycholate and 1 mM EDTA) and TE Buffer (10mM Tris-HCl pH 8, 1 mM EDTA). DNA was recovered from input and IP samples using Chelex resin (1421253, BioRad). Quantitative PCR reactions (qPCR) were performed using a LightCycler 480 SybrGreen Master Mix (04887352001, Roche) and analyzed using Roche LightCycler software (version 1.5.1.62). Primers used for qPCR are listed in Table S3. ChIP enrichments on regions of interest were calculated as the ratio of “IP” sample to the corresponding “input” sample using the ΔCT method and represented as %IP. Where indicated, for spike-in qChIPs, %IP levels in S. pombe were normalized to %IP from spiked-in S. octosporus chromatin (specified in the figure legends).
Fluorescence microscopy
Live fission yeast cells were mounted on a 2% agarose pad formed on 1 mm SuperFrost slides (Thermo Scientific) whereas fixed cells (immunofluorescence and DNA FISH) were mounted in VECTASHIELD Mounting Medium (H-1000-10, Vector Laboratories) on 1 mm Polysine slides (Thermo Scientific). Microscopy was performed with Nikon Ti2 inverted microscope equipped with a ×100 1.49 NA CFI Plan Apochromat TIRF objective, Lumencor Spectra X light source (Lumencor, Beaverton, OR USA) and a Photometrics Prime 95B camera (Teledyne Photometrics, Birmingham, UK), all controlled by Nikon NIS Elements software version 5.21.03 (RRID: SCR_014329). Filter sets from Semrock (Semrock, Rochester, New York, USA) were used to image Lem2-GFP, LacI-GFP, Alexa Fluor 488 (A-11015, Invitrogen) at excitation 488 nm, emission 535 nm, Sad1-dsRed, Rhodamine at excitation 554 nm, emission 590 nm, Lem2/Alp4/Alp6-mCherry at excitation 578 nm, emission 630 nm and DAPI, excitation 378 nm, emission 460 nm. A Mad City nano drive (Mad City Labs, Madison, WI, USA) was used to produce whole cell 3 dimensional (3D) images with a step size of 0.3 μm. All images were processed by Fiji software (RRID: SCR_002285). Live cell images were scaled relative to the maximum intensity in the set of images to allow comparison between images, but fixed cell images were scaled relative to the maximum value of histogram (specified in figure legends).
Localization and fluorescence in situ hybridization (FISH)
For Immunofluorescence/DNA FISH, cells were initially subjected to a similar Immunofluorescence protocol as described previously with some modifications60 and subsequent FISH process. Briefly, log phase yeast cultures were fixed with 3.7% formaldehyde for 7 min at room temperature, washed by PEM buffer (100 mM PIPES pH 7, 1 mM EDTA, 1 mM MgCl2) and PEMS buffer (100 mM PIPES pH 7, 1 mM EDTA, 1 mM MgCl2, 1.2 M Sorbitol), followed by cell-wall digestion in PEMS buffer supplemented with 1 mg/ml Zymolyase-100T and 1 mg/ml Lallzyme (Lallzyme-MMX, Litmus Wines) at 37°C for 90 min. After permeabilization in PEMS containing 1% Triton X-100 for 5 min at room temperature, cells were washed, blocked in PEMBAL (PEM containing 1% BSA (A0281, MERCK), 0.1% sodium azide, 100 mM lysine hydrochloride (657-27-2, MERCK)) for 1 h. Cells were then incubated overnight at 4°C with 1:500 anti-Cdc1160 (a SPB protein; gift from Ken Sawin) or 1:500 anti-CENP-ACnp1 (in-house preparation) in 500 μl of PEMBAL. Cells were then washed three times with PEMBAL and incubated overnight with 1:500 Alexa-488-coupled donkey anti-sheep secondary antibody (A-11015, Invitrogen) in 500 μl of PEMBAL. Cells were then washed in PEMBAL and PEM buffer and re-fixed in 3.7% formaldehyde and 0.25% glutaraldehyde (111-30-8, MERCK) for 15 min, washed with PEM buffer and treated with 1 mg/ml sodium borohydride in PEM buffer. After incubation with 2 μl of 10 mg/ml RNase A (19101, Qiagen) in 100 μl of PEMBAL at 37°C for 2h, cells were denatured in 100 μl of freshly prepared 0.1 M NaOH for 1 min and hybridized with 2 μl of DNA FISH probe in 100 μl hybridization buffer (10% Dextran sulphate (D8906, MERCK), 50% deionized formamide (S4117, MERCK), 2XSSC, 5X Denhardts (D2532, MERCK), 0.5 mg/ml denatured salmon sperm DNA) at 37°C overnight.
For lys1, itg7, itg8 and neo1R FISH probe, a ∼12.5 kb region (ChrI: 3,727,604-3,737,389 and ChrI: 3,739,857-3,742,327) spanning lys1 gene, ∼15 kb region (ChrI 5,438,081-5,453,142) spraining itg7 locus (ChrI 5,447,817-5,448,235), ∼12.5 kb region (ChrI: 5,495,975-5,508,459) spanning itg8 locus (ChrI: 5,500,986-5,502,881) and ∼16.3 kb region (ChrI: 5,513,871-5,530,124) within neo1R CENP-ACnp1 domain were amplified by PCR using primers listed in Table S3 respectively. ptetO plasmid was used to make FISH probe for ptetO. Plasmid pMC52, pMC1 was used to make cc2 and plasmid backbone DNA FISH probes, respectively. cc2 DNA FISH probe was used to locate cc2 at endogenous cen2, lys1 and plasmid pcc2 and pHcc2, while plasmid backbone probe was used to locate pHet. FISH probes were obtained by DIG labeling 500 ng DNA (PCR products or plasmids) using DIG-Nick Translation Mix (11745816910, Roche) supplemented with 1 μl of 1:50 diluted DNase I (AM2222, Ambion).
After hybridization with DNA FISH probe, cells were washed with 2XSSC containing 0.1% sodium azide and incubated with 1:100 sheep anti-DIG-Rhodamine (11207750910; Roche) in 100 μl of PBS-BAG (PBS buffer supplemented with 1% BSA (A0281, MERCK), 0.1% sodium azide and 0.5% cold water fish gelatin (G7765, MERCK)) at room temperature overnight. Cells were finally stained with 4’,6-diamidino-2-phenylindole (DAPI), mounted in VECTASHIELD Mounting Medium on Polylysine slides and imaged using Nikon NIS Elements software (version 5.21.03) on a Nikon Ti2 inverted microscope as indicated above. All images are scaled relative to the maximum value of histogram.
3D distance measurements
3D distances between spots in two channels (green and red): Cdc11/CENP-ACnp1 (green) and DNA FISH (red) or lys1:lacO (ade3:lacO)/LacI-GFP (green) and Sad1-dsred (red), were measured by Fiji using in-house script (https://zenodo.org/record/5657360#.Yn7YZBPMLUJ). Briefly, the center of spot in each channel were determined in X-Y using the Fiji “Find Maxima…” function with same threshold (Prominence>500), applied to a Z-projection. The Z-positions of each spot were then determined as the slice with the maximum pixel intensity at each X-Y position. The distance to the nearest red spot for each green spot was reported if within 3 μm representing the diameter of the fission yeast nucleus. If no red spot was detected within 3 μm then that green spot was not included in the analysis. Distances between the resulting spots in each channel were measured by equation:
Live mono-nuclear cells 8-12 μm in length and only one SPB (Sad1-dsRed) nucleus-associated dot were recognized as G2 cells and subjected to distance measurements between LacI-GFP (binds to lys1:lacO or ade3:lacO) and Sad1-dsRed. For immunofluorescence/DNA FISH, mononuclear cells with nuclear green-red spot pairs and only one SPB (Cdc11) or centromere cluster (CENP-ACnp1) spot were recognized as interphase cells and retained for distance measurement between DNA FISH locus (red) and protein Cdc11 or CENP-ACnp1 (green).
Quantification and Statistical Analysis
All quantification and statistical details of experiments are described in the figure legends or in the methods section. The qChIP results are obtained from more than 3 independent experimental replicates (n ≥ 3) and represented as mean ± SD (standard deviation, error bars). Significance of the differences in qChIP results was evaluated using Unpaired t test with a p value threshold < 0.05, by Prism Version 9.1.0 software (RRID: SCR_002798). 3D distance measurement results were obtained by analyzing n number of interphase cells from 3 independent experimental replicates. Average distance for each strain were calculated and indicated as “AV.” (specified in figure legends). Cells were classified into three groups according to the distance: overlap (≤0.3 μm), adjacent (0.3-0.5 μm) or separate (0.5-3 μm). The results were reported as percentage of cells (% cells) in each group. For statistical significance analysis of distance data, Mann-Whitney U test with a p value threshold <0.01 was performed by Prism Version 9.1.0 software (RRID: SCR_002798) and the detailed results were showed in Data S1.
Acknowledgments
We are grateful to the members of Allshire lab, especially Manu Shukla, Nitobe London, and Dominik Hoelper, for helpful suggestions and comments on the manuscript and Sharon White for organizational support. Takeshi Urano is thanked for the 5.1.1 (H3K9me) antibody, Ken Sawin for the Cdc11 antibody, Kevin Hardwick for the Spc7 antibody, Julie Cooper for the plasmid pF6a-GBP-mCherry-Hyg, and Nick Rhind for the S. octosporus strain. W.W. is supported by the Darwin Trust of Edinburgh. R.C.A. is a Wellcome Principal Research Fellow (095021 and 200885); the Wellcome Centre for Cell Biology is supported by core funding from Wellcome (203149). The Allshire lab dedicates this study to the memory of our dear colleague and friend Sasha Kagansky, whose research and insights were an inspiration for this project.
Author contributions
Conceptualization, W.W., A.L.P., and R.C.A.; methodology, W.W., T.M., D.A.K., and A.L.P.; investigation, W.W.; visualization, W.W.; writing – original draft, W.W.; writing – review & editing, W.W., A.L.P., and R.C.A.; funding acquisition, R.C.A.; supervision, A.L.P. and R.C.A.
Declaration of interests
The authors declare no competing interests.
Published: July 12, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.cub.2022.06.048.
Supplemental information
Data and code availability
-
•
All original microscopy images, qChIP and 3D distance measurements data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at GitHub (https://zenodo.org/record/5657360#.Yn7YZBPMLUJ) and is publicly available as of the date of publications. DOIs is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.
References
- 1.Allshire R.C., Karpen G.H. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat. Rev. Genet. 2008;9:923–937. doi: 10.1038/nrg2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mellone B.G., Fachinetti D. Diverse mechanisms of centromere specification. Curr. Biol. 2021;31:R1491–R1504. doi: 10.1016/j.cub.2021.09.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeBose-Scarlett E.M., Sullivan B.A. Genomic and epigenetic foundations of neocentromere formation. Annu. Rev. Genet. 2021;55:331–348. doi: 10.1146/annurev-genet-071719-020924. [DOI] [PubMed] [Google Scholar]
- 4.Ishii K., Ogiyama Y., Chikashige Y., Soejima S., Masuda F., Kakuma T., Hiraoka Y., Takahashi K. Heterochromatin integrity affects chromosome reorganization after centromere dysfunction. Science. 2008;321:1088–1091. doi: 10.1126/science.1158699. [DOI] [PubMed] [Google Scholar]
- 5.Mitra S., Srinivasan B., Jansen L.E.T. Stable inheritance of CENP-A chromatin: inner strength versus dynamic control. J. Cell Biol. 2020;219:e202005099. doi: 10.1083/jcb.202005099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westhorpe F.G., Straight A.F. The centromere: epigenetic control of chromosome segregation during mitosis. Cold Spring Harb. Perspect. Biol. 2014;7:a015818. doi: 10.1101/cshperspect.a015818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allshire R.C., Ekwall K. Epigenetic regulation of chromatin states in Schizosaccharomyces pombe. Cold Spring Harb. Perspect. Biol. 2015;7:a018770. doi: 10.1101/cshperspect.a018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martienssen R., Moazed D. RNAi and heterochromatin assembly. Cold Spring Harb. Perspect. Biol. 2015;7:a019323. doi: 10.1101/cshperspect.a019323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke L., Baum M., Marschall L.G., Ngan V.K., Steiner N.C. Structure and function of Schizosaccharomyces pombe centromeres. Cold Spring Harb. Symp. Quant. Biol. 1993;58:687–695. doi: 10.1101/sqb.1993.058.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi K., Murakami S., Chikashige Y., Funabiki H., Niwa O., Yanagida M. A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell. 1992;3:819–835. doi: 10.1091/mbc.3.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noma K., Cam H.P., Maraia R.J., Grewal S.I. A role for TFIIIC transcription factor complex in genome organization. Cell. 2006;125:859–872. doi: 10.1016/j.cell.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Scott K.C., Merrett S.L., Willard H.F. A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr. Biol. 2006;16:119–129. doi: 10.1016/j.cub.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 13.Tong P., Pidoux A.L., Toda N.R.T., Ard R., Berger H., Shukla M., et al. Interspecies conservation of organisation and function between nonhomologous regional centromeres. Nat. Commun. 2019;10:2343. doi: 10.1038/s41467-019-09824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo A.G., Pidoux A.L., Catania S., Durand-Dubief M., Choi E.S., Hamilton G., et al. Telomeric repeats facilitate CENP-A(Cnp1) incorporation via telomere binding proteins. PLoS One. 2013;8:e69673. doi: 10.1371/journal.pone.0069673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catania S., Pidoux A.L., Allshire R.C. Sequence features and transcriptional stalling within centromere DNA promote establishment of CENP-A chromatin. PLoS Genet. 2015;11:e1004986. doi: 10.1371/journal.pgen.1004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shukla M., Tong P., White S.A., Singh P.P., Reid A.M., Catania S., et al. Centromere DNA destabilizes H3 nucleosomes to promote CENP-A deposition during the cell cycle. Curr. Biol. 2018;28:3924–3936.e4. doi: 10.1016/j.cub.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh P.P., Shukla M., White S.A., Lafos M., Tong P., Auchynnikava T., Spanos C., Rappsilber J., Pidoux A.L., Allshire R.C. Hap2-Ino80-facilitated transcription promotes de novo establishment of CENP-A chromatin. Genes Dev. 2020;34:226–238. doi: 10.1101/gad.332536.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zasadzińska E., Foltz D.R. Orchestrating the specific assembly of centromeric nucleosomes. Prog. Mol. Subcell. Biol. 2017;56:165–192. doi: 10.1007/978-3-319-58592-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funabiki H., Hagan I., Uzawa S., Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller H., Gil J., Jr., Drinnenberg I.A. The impact of centromeres on spatial genome architecture. Trends Genet. 2019;35:565–578. doi: 10.1016/j.tig.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Winey M., O'Toole E.T. The spindle cycle in budding yeast. Nat. Cell Biol. 2001;3:E23–E27. doi: 10.1038/35050663. [DOI] [PubMed] [Google Scholar]
- 22.Guacci V., Hogan E., Koshland D. Centromere position in budding yeast: evidence for anaphase A. Mol. Biol. Cell. 1997;8:957–972. doi: 10.1091/mbc.8.6.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaspersen S.L. Anatomy of the fungal microtubule organizing center, the spindle pole body. Curr. Opin. Struct. Biol. 2021;66:22–31. doi: 10.1016/j.sbi.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebrahimi H., Masuda H., Jain D., Cooper J.P. Distinct ‘safe zones’ at the nuclear envelope ensure robust replication of heterochromatic chromosome regions. eLife. 2018;7:e32911. doi: 10.7554/eLife.32911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernández-Álvarez A., Bez C., O’Toole E.T., Morphew M., Cooper J.P. Mitotic nuclear envelope breakdown and spindle nucleation are controlled by interphase contacts between centromeres and the nuclear envelope. Dev. Cell. 2016;39:544–559. doi: 10.1016/j.devcel.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrales R.R., Forn M., Georgescu P.R., Sarkadi Z., Braun S. Control of heterochromatin localization and silencing by the nuclear membrane protein Lem2. Genes Dev. 2016;30:133–148. doi: 10.1101/gad.271288.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hou H., Zhou Z., Wang Y., Wang J., Kallgren S.P., Kurchuk T., Miller E.A., Chang F., Jia S. Csi1 links centromeres to the nuclear envelope for centromere clustering. J. Cell Biol. 2012;199:735–744. doi: 10.1083/jcb.201208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi T., Fujita Y., Iwasaki O., Adachi Y., Takahashi K., Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118:715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 29.Pidoux A.L., Choi E.S., Abbott J.K., Liu X., Kagansky A., Castillo A.G., Hamilton G.L., Richardson W., Rappsilber J., He X., Allshire R.C. Fission yeast Scm3: a CENP-A receptor required for integrity of subkinetochore chromatin. Mol. Cell. 2009;33:299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subramanian L., Toda N.R., Rappsilber J., Allshire R.C. Eic1 links Mis18 with the CCAN/Mis6/Ctf19 complex to promote CENP-A assembly. Open Biol. 2014;4:140043. doi: 10.1098/rsob.140043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams J.S., Hayashi T., Yanagida M., Russell P. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol. Cell. 2009;33:287–298. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Folco H.D., Pidoux A.L., Urano T., Allshire R.C. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science. 2008;319:94–97. doi: 10.1126/science.1150944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steiner N.C., Clarke L. A novel epigenetic effect can alter centromere function in fission yeast. Cell. 1994;79:865–874. doi: 10.1016/0092-8674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 34.Kagansky A., Folco H.D., Almeida R., Pidoux A.L., Boukaba A., Simmer F., Urano T., Hamilton G.L., Allshire R.C. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science. 2009;324:1716–1719. doi: 10.1126/science.1172026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfredsson-Timmins J., Henningson F., Bjerling P. The Clr4 methyltransferase determines the subnuclear localization of the mating-type region in fission yeast. J. Cell Sci. 2007;120:1935–1943. doi: 10.1242/jcs.03457. [DOI] [PubMed] [Google Scholar]
- 36.Pichugina T., Sugawara T., Kaykov A., Schierding W., Masuda K., Uewaki J., et al. A diffusion model for the coordination of DNA replication in Schizosaccharomyces pombe. Sci. Rep. 2016;6:18757. doi: 10.1038/srep18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saitoh S., Takahashi K., Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- 38.Bernard P., Maure J.F., Partridge J.F., Genier S., Javerzat J.P., Allshire R.C. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. doi: 10.1126/science.1064027. [DOI] [PubMed] [Google Scholar]
- 39.Nonaka N., Kitajima T., Yokobayashi S., Xiao G., Yamamoto M., Grewal S.I., Watanabe Y. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 2002;4:89–93. doi: 10.1038/ncb739. [DOI] [PubMed] [Google Scholar]
- 40.Audergon P.N., Catania S., Kagansky A., Tong P., Shukla M., Pidoux A.L., Allshire R.C. Epigenetics. Restricted epigenetic inheritance of H3K9 methylation. Science. 2015;348:132–135. doi: 10.1126/science.1260638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding D.Q., Hiraoka Y. Visualization of a specific genome locus by the lacO/LacI-GFP system. Cold Spring Harb. Protoc. 2017;2017 doi: 10.1101/pdb.prot091934. pdb.prot091934. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias N., Paulo J.A., Tatarakis A., Wang X., Edwards A.L., Bhanu N.V., et al. Native chromatin proteomics reveals a role for specific nucleoporins in heterochromatin organization and maintenance. Mol. Cell. 2020;77:51–66.e8. doi: 10.1016/j.molcel.2019.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bestul A.J., Yu Z., Unruh J.R., Jaspersen S.L. Molecular model of fission yeast centrosome assembly determined by superresolution imaging. J. Cell Biol. 2017;216:2409–2424. doi: 10.1083/jcb.201701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi E.S., Strålfors A., Catania S., Castillo A.G., Svensson J.P., Pidoux A.L., et al. Factors that promote H3 chromatin integrity during transcription prevent promiscuous deposition of CENP-A(Cnp1) in fission yeast. PLoS Genet. 2012;8:e1002985. doi: 10.1371/journal.pgen.1002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klutstein M., Fennell A., Fernández-Álvarez A., Cooper J.P. The telomere bouquet regulates meiotic centromere assembly. Nat. Cell Biol. 2015;17:458–469. doi: 10.1038/ncb3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogiyama Y., Ohno Y., Kubota Y., Ishii K. Epigenetically induced paucity of histone H2A.Z stabilizes fission-yeast ectopic centromeres. Nat. Struct. Mol. Biol. 2013;20:1397–1406. doi: 10.1038/nsmb.2697. [DOI] [PubMed] [Google Scholar]
- 47.Kanoh J., Sadaie M., Urano T., Ishikawa F. Telomere binding protein Taz1 establishes Swi6 heterochromatin independently of RNAi at telomeres. Curr. Biol. 2005;15:1808–1819. doi: 10.1016/j.cub.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 48.Chikashige Y., Yamane M., Okamasa K., Tsutsumi C., Kojidani T., Sato M., Haraguchi T., Hiraoka Y. Membrane proteins Bqt3 and -4 anchor telomeres to the nuclear envelope to ensure chromosomal bouquet formation. J. Cell Biol. 2009;187:413–427. doi: 10.1083/jcb.200902122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuguchi T., Fudenberg G., Mehta S., Belton J.M., Taneja N., Folco H.D., FitzGerald P., Dekker J., Mirny L., Barrowman J., Grewal S. Cohesin-dependent globules and heterochromatin shape 3D genome architecture in S. pombe. Nature. 2014;516:432–435. doi: 10.1038/nature13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerlich D., Beaudouin J., Kalbfuss B., Daigle N., Eils R., Ellenberg J. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell. 2003;112:751–764. doi: 10.1016/s0092-8674(03)00189-2. [DOI] [PubMed] [Google Scholar]
- 51.Oko Y., Ito N., Sakamoto T. The mechanisms and significance of the positional control of centromeres and telomeres in plants. J. Plant Res. 2020;133:471–478. doi: 10.1007/s10265-020-01202-2. [DOI] [PubMed] [Google Scholar]
- 52.Dunleavy E.M., Almouzni G., Karpen G.H. H3.3 is deposited at centromeres in S phase as a placeholder for newly assembled CENP-A in G1 phase. Nucleus. 2011;2:146–157. doi: 10.4161/nucl.2.2.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stankovic A., Guo L.Y., Mata J.F., Bodor D.L., Cao X.J., Bailey A.O., Shabanowitz J., Hunt D.F., Garcia B.A., Black B.E., Jansen L. A dual inhibitory mechanism sufficient to maintain cell-cycle-restricted CENP-A assembly. Mol. Cell. 2017;65:231–246. doi: 10.1016/j.molcel.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jansen L.E., Black B.E., Foltz D.R., Cleveland D.W. Propagation of centromeric chromatin requires exit from mitosis. J. Cell Biol. 2007;176:795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takahashi K., Chen E.S., Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288:2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- 56.Rhind N., Chen Z., Yassour M., Thompson D.A., Haas B.J., Habib N., Wapinski I., Roy S., Lin M.F., Heiman D.I., et al. Comparative functional genomics of the fission yeasts. Science. 2011;332:930–936. doi: 10.1126/science.1203357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torres-Garcia S., Di Pompeo L., Eivers L., Gaborieau B., White S.A., Pidoux A.L., et al. SpEDIT: a fast and efficient CRISPR/Cas9 method for fission yeast. Wellcome Open Res. 2020;5:274. doi: 10.12688/wellcomeopenres.16405.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreno S., Klar A., Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 59.Bähler J., Wu J.Q., Longtine M.S., Shah N.G., McKenzie A., 3rd, Steever A.B., Wach A., Philippsen P., Pringle J.R. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 60.Castillo A.G., Mellone B.G., Partridge J.F., Richardson W., Hamilton G.L., Allshire R.C., et al. Plasticity of fission yeast CENP-A chromatin driven by relative levels of histone H3 and H4. PLoS Genet. 2007;3:e121. doi: 10.1371/journal.pgen.0030121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All original microscopy images, qChIP and 3D distance measurements data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at GitHub (https://zenodo.org/record/5657360#.Yn7YZBPMLUJ) and is publicly available as of the date of publications. DOIs is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the Lead Contact upon request.