Abstract
Preliminary evidence indicates beneficial effects of omega-3 polyunsaturated fatty acids (PUFAs) in early psychosis. The present study investigates the molecular mechanism of omega-3 PUFA-associated therapeutic effects in clinical high-risk (CHR) participants. Plasma samples of 126 CHR psychosis participants at baseline and 6-months follow-up were included. Plasma protein levels were quantified using mass spectrometry and erythrocyte omega-3 PUFA levels were quantified using gas chromatography. We examined the relationship between change in polyunsaturated PUFAs (between baseline and 6-month follow-up) and follow-up plasma proteins. Using mediation analysis, we investigated whether plasma proteins mediated the relationship between change in omega-3 PUFAs and clinical outcomes. A 6-months change in omega-3 PUFAs was associated with 24 plasma proteins at follow-up. Pathway analysis revealed the complement and coagulation pathway as the main biological pathway to be associated with change in omega-3 PUFAs. Moreover, complement and coagulation pathway proteins significantly mediated the relationship between change in omega-3 PUFAs and clinical outcome at follow-up. The inflammatory protein complement C5 and protein S100A9 negatively mediated the relationship between change in omega-3 PUFAs and positive symptom severity, while C5 positively mediated the relationship between change in omega-3 and functional outcome. The relationship between change in omega-3 PUFAs and cognition was positively mediated through coagulation factor V and complement protein C1QB. Our findings provide evidence for a longitudinal association of omega-3 PUFAs with complement and coagulation protein changes in the blood. Further, the results suggest that an increase in omega-3 PUFAs decreases symptom severity and improves cognition in the CHR state through modulating effects of complement and coagulation proteins.
Subject terms: Schizophrenia, Molecular neuroscience
Introduction
The brain is a lipid-rich organ and 60% of its total membrane is composed of phospholipids [1]. Polyunsaturated Fatty acids (PUFAs) are a vital component of neuronal membrane phospholipids. Omega-3 and omega-6 fatty acids are two major classes of PUFAs present in the brain, among which omega-3 PUFAs have superior health benefits in humans [2–6]. Pre-clinical investigations have identified several biological mechanisms in which omega-3 PUFAs play an important role, such as the maintenance of cell membrane integrity [7, 8], release of specialized pro-resolving mediators [9–12], modification of gut microbiome [13] and regulation of synaptic pruning activity in the brain [14–16].
In psychotic disorder, existing literature has indicated an association of PUFA-related dietary factors such as insufficient consumption of omega-3 PUFAs [17–19] or abnormal fat metabolism [20–27] with the occurrence of psychotic symptoms. The membrane phospholipid hypothesis of schizophrenia proposes a possible link between PUFA abnormalities and psychosis and suggests a potential therapeutic role of omega-3 PUFAs in the treatment of schizophrenia and related disorders at an early stage [20, 28–40]. To date, the evidence for the therapeutic role of omega-3 PUFAs in the Clinical High Risk (CHR) population appears inconclusive. The first omega-3 fatty acid placebo-controlled randomized UHR trial the Vienna High Risk study found a large preventive effect on transition rate [41] while a consecutive multi-center replication study (the NEURAPRO trial) was not able to confirm these prior finding [42, 43]. The Vienna High Risk trial found a reduction in phospholipase A2 (PLA2) activity in relation to omega-3 PUFAs at 12 weeks follow-up [44]. PLA2 is involved in fatty acid metabolism which is found to be increased in schizophrenia patients [45]. Furthermore, omega-3 PUFA supplementation also indicated an increase in soluble intercellular adhesion molecule-1 (sICAM-1) in Vienna High Risk study participants [46]. In contrast, in the NEURAPRO study, an inverse relationship between omega-3 PUFAs and plasma cytokines was found, but this association did not indicate any clinical benefits on psychopathology of CHR participants [47]. Knowledge of the underlying mechanism of omega-3 PUFAs will provide an insight into the biological role of omega-3 PUFAs in the pathophysiology of psychosis and help in designing early intervention strategies [41, 48, 49].
The current study investigates the relationship of PUFAs (omega-3 and omega-6) with plasma proteomic pathways in a clinical CHR population. We performed mass spectrometry-based analysis on plasma samples at baseline and follow-up, to investigate the longitudinal association between PUFAs and the plasma proteome. Furthermore, we evaluated the proteomic pathways through which omega-3 PUFAs may influence psychopathology in CHR participants. Thus, through this study we addressed the following research questions:
-
I.
Are there any associations between changes in omega-3 PUFAs with plasma proteins at follow-up in CHR participants?
-
II.
Which biological pathways are most substantially influenced by change in PUFAs (both omega-3 and omega-6 PUFAs)?
-
III.
Do the identified plasma proteins mediate the relationship between change in omega-3 PUFAs and clinical outcomes?
Materials and methods
Study participants
The NEURAPRO study is a multicentre randomized placebo-controlled clinical trial registered with the Australian New Zealand Clinical Trial Registry as ACTRN 12608000475347. The study was performed abiding with the Declaration of Helsinki [50] and adhering to the National Health and Medical Research Council of the Australia National Statement on Human Research. The trial aimed to evaluate the therapeutic role of omega-3 PUFAs in preventing the development of psychosis in CHR patients. Informed consent was obtained from all the participants or from their parents/guardians if they were younger than 17 years. The inclusion and exclusion criteria of the participants of the study are provided in [51].
The participants received either omega-3 PUFAs (840 mg eicosapentaenoic acid [EPA] and 560 mg docosahexaenoic acid [DHA] per day) or placebo (an equivalent dose of paraffin oil) for 6 months [43]. The adherence to the study interventions was assessed monthly. At the end of 6 months, a low adherence of 43% to the omega-3 intervention and a 41% to the placebo was reported [52].
Measurement of omega-3 PUFAs
Fasting blood samples were collected at baseline and 6-month follow-up. The molecular percentage of the total sum of the omega-3 and omega-6 fatty acids in erythrocyte membrane rafts were measured based on the phosphatidyl-ethanolamine fraction using gas chromatography [53]. Total omega-3 PUFAs comprise of alpha linolenic acid (18:3), eicosapentaenoic acid (20:5), docosapentaenoic acid (22:5), and docosahexaenoic acid (22:6). Total omega-6 PUFAs include linoleic acid (18:2), gamma-linoleic acid (18:3), eicosadienoic acid (20:2), dihomo gamma-linoleic acid (20:3), arachidonic acid (20:4) and adrenic acid (22:4). Since a poor adherence to the study intervention was observed in both study arms, the erythrocyte membrane levels were used as objective measure of dietary intake of PUFAs (exposure variable) [54, 55].
Quantification of plasma proteome
Plasma samples of baseline and follow-up time points were used for discovery-based, data-dependant acquisition (DDA), mass spectrometry. For sample preparation steps and mass spectrometry protocols refer to Supplementary Methods.
Clinical outcome measures
The clinical outcomes of psychotic symptom severity (PSS), functional status, and cognitive status at 6-months follow-up were considered for the analyses. The PSS was assessed using the Comprehensive Assessment of At-Risk Mental State (CAARMS) scale [56]. The subscales of positive symptoms from the CAARMS assessment (unusual thought content, non-bizarre ideas, perceptual abnormalities, and disorganized speech) were used for the calculation of the PSS score. The summed scores of the product of global rating scale score (0–6) and frequency (0–6) of these subscales were calculated, as per previous research [57, 58]. Functional outcome was measured using the Social and Occupational Functional assessment Scale (SOFAS) and cognitive outcome using the Brief Assessment of Cognition in Schizophrenia (BACS), both at 6-months follow-up [59, 60].
Statistical analysis
Statistical analysis was performed using IBM® SPSS® statistics version 26 and STATA IC® version 16.
Analysis 1- Identification of proteins and pathways associated with change in PUFAs
Linear regression models were used to assess longitudinal associations between 6-month change in erythrocyte PUFAs (total omega-3 or total omega-6 PUFAs) and plasma proteins at follow-up. Models were adjusted for age and sex. Proteins that were significantly associated (p < 0.05) with change in total omega-3 and omega-6 PUFAs were then taken forward for pathway analysis. Pathway analysis was conducted using the Reactome Pathway Knowledgebase Enrichment Analysis and a probability factor (p-value) was generated for each pathway based on the protein representations [61]. A list of biological pathways based on the p-values after Benjamini-Hochberg correction for multiple tests (FDR 5%) was generated. The UNIPROT entities that were associated with total omega-3 PUFAs were considered for further analysis.
Analysis 2- Relationship of total omega-3 associated proteins and clinical outcome
The relationship of total omega-3 PUFAs associated proteins (from analysis 1) with clinical outcomes at 6-month follow-up were assessed using a linear regression model. The PSS, SOFAS, and BACS scores were used for the analysis. The models were adjusted for age, sex, and corresponding baseline protein levels.
Analysis 3: Univariate mediation model
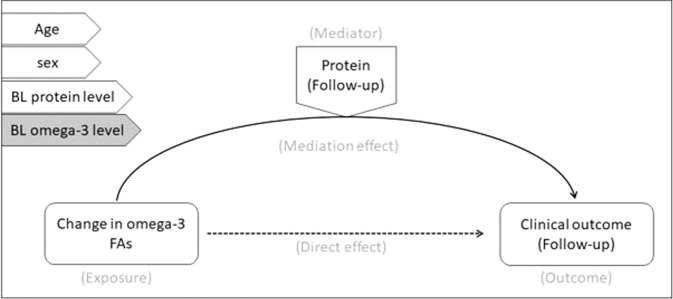
Mediation analysis was performed to evaluate the potential mediating role of plasma proteins in the relationship between total omega-3 PUFAs and clinical outcomes [62]. Regression-based mediation analysis was performed in IBM® SPSS® using the PROCESS platform. Regression beta coefficients were constructed using a conventional mediation analysis model with a bootstrap sample size of 5000 and a 95% confidence interval. In the mediation model, the change in total omega-3 levels were used as exposure variable, the protein measures at follow-up were used as mediators and the clinical and neurocognitive outcomes (PSS/SOFAS and BACS) at follow-up were used as the outcome measures. The mediation analysis was adjusted for age, sex, and corresponding baseline plasma protein levels (Fig. 1). The role of baseline total omega-3 PUFAs on the mediation model was then assessed by repeating the model with baseline total omega-3 PUFAs levels as an additional covariate.
Fig. 1. Mediation model.
The general structure of the mediation model mentioning the exposure (change in omega-3 levels), mediator (protein levels) and outcome variables (clinical outcome). The model was adjusted for the covariates (age, sex, baseline protein and omega-3 levels) mentioned on the left side of the picture.
Results
From 285 CHR participants in the NEURAPRO trial, 146 participants provided plasma samples at both time-points, baseline, and 6-month follow-up. Out of these, 128 participants had erythrocyte omega-3 PUFA levels and proteomic measurements at both time-points. These 128 participants were considered for the statistical analysis and the baseline characteristics of these participants are given in Table 1. A total of 165 proteins from the discovery proteomics approach that passed quality control were eligible for analysis.
Table 1.
Participants’ demographic, anthropometric, PUFA, and clinical characteristics at baseline and follow-up.
| Baseline | 6-month follow-up | ||
|---|---|---|---|
| Demographic details | |||
| Age in years, mean ± SD | 18 ± 4 | — | |
| BMI in kg/m2, mean ± SD | 24.20 ± 5.43 | — | |
| Gender, n (%) | Female | 81 (63%) | — |
| Male | 47 (37%) | — | |
| Biological and clinical measures | |||
| Erythrocyte membrane fatty acid levels in %, mean ± SD | Total omega-3 fatty acids | 11.94 ± 1.68 | 13.34 ± 4.40 |
| Total omega-6 fatty acids | 35.56 ± 1.73 | 31.47 ± 4.12 | |
| Positive symptom severity (PSS) score, mean ± SD | 25 ± 22 | 14 ± 15 | |
| Social and Occupational Functional Assessment Scale score, mean ± SD | 55 ± 10 | 67 ± 15 | |
| Brief Assessment of Cognition in Schizophrenia- composite score, mean ± SD | 25 ± 22 | 51 ± 13 | |
SD Standard deviation.
Results from analysis 1: The longitudinal association between change in PUFAs and plasma proteins
In a linear regression model, a 0 to 6-month change in total omega-3 PUFAs was associated with 24 plasma proteins at follow-up after adjusting for age, sex, and baseline total omega-3 levels. Using pathway analysis, these 24 proteins represented three major biological pathways, (i) the immune system, (ii) hemostasis (coagulation), and (iii) vesicle mediated transport. The complement system and sub-pathways were the top pathways denoted by the change in total omega-3 PUFA associated proteins (Table 2 and Supplementary Table 1). In the coagulation cascade pathway, the plasma proteins associated with change in omega-3 PUFAs (hereafter omega-3 related proteins) associated with platelet activation and clotting cascade-related mechanisms (Table 2 and Supplementary Table 1). In contrast to omega-3 fatty acids, the plasma proteins associated with a change in omega-6 PUFAs did not indicate any major biological pathways (Supplementary Tables 2 and 3).
Table 2.
Pathways significantly associated with 6-month change in total omega-3 PUFAs.
| Pathway name | #Entities found | #Reactions found | #Interactors found | Entities FDR |
|---|---|---|---|---|
| Regulation of complement cascade | 10 | 31 | 6 | <0.001 |
| Complement cascade | 10 | 49 | 6 | <0.001 |
| Initial triggering of complement | 6 | 11 | 0 | <0.001 |
| Classical antibody-mediated complement activation | 5 | 2 | 0 | <0.001 |
| Creation of C4 and C2 activators | 5 | 2 | 0 | <0.001 |
| Platelet degranulation | 5 | 2 | 0 | <0.001 |
| Innate immune system | 14 | 114 | 8 | <0.001 |
| Binding and Uptake of Ligands by Scavenger Receptors | 5 | 9 | 1 | <0.001 |
| Post-translational protein phosphorylation | 4 | 1 | 0 | <0.001 |
| Response to elevated platelet cytosolic Ca2+ | 5 | 2 | 0 | <0.001 |
| Scavenging of heme from plasma | 4 | 6 | 1 | <0.001 |
| FCGR activation | 4 | 6 | 0 | <0.001 |
| Terminal pathway of complement | 2 | 5 | 1 | 0.002 |
| Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) | 4 | 1 | 0 | 0.009 |
| Role of phospholipids in phagocytosis | 4 | 6 | 1 | 0.012 |
| Plasma lipoprotein assembly | 3 | 6 | 1 | 0.013 |
| Transport of gamma-carboxylated protein precursors from the endoplasmic reticulum to the Golgi apparatus | 2 | 2 | 0 | 0.013 |
| FCGR3A-mediated IL10 synthesis | 4 | 10 | 1 | 0.013 |
| Parasite infection | 4 | 14 | 1 | 0.013 |
| FCGR3A-mediated phagocytosis | 4 | 14 | 1 | 0.013 |
| Leishmania phagocytosis | 4 | 14 | 1 | 0.013 |
| Gamma-carboxylation of protein precursors | 2 | 2 | 0 | 0.016 |
| Formation of Fibrin Clot (Clotting Cascade) | 3 | 14 | 0 | 0.016 |
| Removal of aminoterminal propeptides from gamma-carboxylated proteins | 2 | 2 | 0 | 0.017 |
| Defective F9 secretion | 1 | 1 | 0 | 0.021 |
| Activation of C3 and C5 | 2 | 2 | 1 | 0.021 |
| Gamma-carboxylation, transport, and amino-terminal cleavage of proteins | 2 | 6 | 0 | 0.021 |
| Regulation of actin dynamics for phagocytic cup formation | 4 | 7 | 1 | 0.025 |
| Chylomicron assembly | 2 | 2 | 1 | 0.039 |
| Intrinsic Pathway of Fibrin Clot Formation | 2 | 5 | 0 | 0.039 |
| Neutrophil degranulation | 4 | 4 | 0 | 0.044 |
| Leishmania parasite growth and survival | 4 | 10 | 1 | 0.044 |
| Anti-inflammatory response favouring Leishmania parasite infection | 4 | 10 | 1 | 0.044 |
| Fcgamma receptor (FCGR) dependent phagocytosis | 4 | 19 | 1 | 0.048 |
The table shows the lists of pathways that were significantly represented by total omega-3 associated plasma proteins. The names of pathways are given in the order of p-values from low to high.
Results from analysis 2: The association between omega-3 related plasma proteins and clinical outcome at 6-month follow-up
Association with positive symptom severity
In linear regression models, three plasma proteins at follow-up associated cross-sectionally with the PSS score at follow-up: Complement component 5 (C5), and protein S100A-9 showed a positive association (β coef = 3.54, CI 95%ile: 0.79 to 6.30, p-value = 0.01* & 3.40, CI 95%ile:0.27 to 6.52; p-value = 0.03*, respectively), while Immunoglobulin heavy constant gamma chain-4 (IGHG-4) showed an inverse association with the PSS score (β coef = −3.13, CI95%ile: −5.79 to −0.47 & p-value = 0.02*) (Table 3).
Table 3.
Results of linear regression model II, showing a relationship between plasma proteins (which were showing an association with change in omega-3 FAs) with clinical outcomes at 6 months follow-up.
| Clinical outcomes | PSS | SOFAS | BACS | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein names | Coef. | P value | 95% Conf. interval | Coef. | P value | 95% Conf. interval | Coef. | P value | 95% Conf. interval | |
| Alpha-1-antitrypsin | SERPINA1 | 0.41 | 0.78 | −2.44 to 3.27 | 0.02 | 0.99 | −2.73 to 2.77 | −0.77 | 0.55 | −3.32 to 1.77 |
| Alpha-1B-glycoprotein | A1BG | 0.79 | 0.57 | −1.94 to 3.53 | 0.05 | 0.97 | −2.59 to 2.69 | −0.50 | 0.7 | −3.08 to 2.08 |
| Apolipoprotein C-I | APOC1 | 1.03 | 0.47 | −1.80 to 3.86 | −1.22 | 0.37 | −3.94 to 1.49 | −1.16 | 0.36 | −3.68 to 1.35 |
| Apolipoprotein C-III | APOC3 | −0.98 | 0.5 | −3.82 to 1.87 | 1.30 | 0.34 | −1.40 to 4.01 | 2.85 | 0.03* | 0.36 to 5.34 |
| Apolipoprotein D | APOD | 1.17 | 0.41 | −1.62 to 3.96 | 2.77 | 0.04* | 0.13 to 5.41 | 3.87 | 0.00* | 1.25 to 6.50 |
| Apolipoprotein E | APOE | −2.15 | 0.12 | −4.87 to 0.58 | 1.37 | 0.3 | −1.24 to 3.97 | 3.09 | 0.01* | 0.71 to 5.48 |
| Apolipoprotein L1 | APOL1 | 1.23 | 0.38 | −1.52 to 3.99 | 1.13 | 0.4 | −1.51 to 3.78 | 1.96 | 0.12 | −0.49 to 4.42 |
| Caspase-14 | CASP14 | 1.84 | 0.19 | −0.95 to 4.62 | 1.08 | 0.43 | −1.60 to 3.75 | −1.29 | 0.31 | −3.81 to 1.24 |
| Coagulation factor V | F5 | −0.05 | 0.97 | −2.86 to 2.76 | 1.48 | 0.28 | −1.20 to 4.16 | 3.67 | 0.00* | 1.22 to 6.11 |
| Complement C1q subcomponent subunit B | C1QB | −0.01 | 0.99 | −2.83 to 2.80 | 0.95 | 0.49 | −1.75 to 3.64 | 3.93 | 0.00* | 1.58 to 6.28 |
| Complement C5 | C5 | 3.54 | 0.01* | 0.79 to 6.30 | −3.23 | 0.02* | −5.87 to −0.59 | −0.88 | 0.49 | −3.38 to 1.63 |
| Complement component C7 | C7 | 0.16 | 0.91 | −2.66 to 2.98 | 0.06 | 0.97 | −2.62 to 2.74 | 2.06 | 0.1 | −0.39 to 4.51 |
| Complement factor B | CFB | 0.78 | 0.58 | −2.01 to 3.58 | −1.98 | 0.15 | −4.66 to 0.71 | −3.18 | 0.02* | −5.72 to −0.63 |
| Complement factor I | CFI IF | 2.49 | 0.07 | −0.22 to 5.21 | −2.36 | 0.08 | −4.97 to 0.25 | −0.30 | 0.81 | −2.77 to 2.16 |
| Filamin A-interacting protein 1-like protein | FILIP1L | 0.18 | 0.9 | −2.56 to 2.92 | 0.84 | 0.54 | −1.84 to 3.53 | 1.20 | 0.34 | −1.28 to 3.67 |
| Galectin-3-binding protein | LGALS3BP | 0.24 | 0.87 | −2.67 to 3.16 | −0.89 | 0.53 | −3.68 to 1.91 | 0.27 | 0.85 | −2.46 to 2.99 |
| Haptoglobin | HP | 1.07 | 0.45 | −1.72 to 3.85 | −1.34 | 0.32 | −4.01 to 1.33 | −0.58 | 0.65 | −3.11 to 1.95 |
| Immunoglobulin heavy constant gamma 2 | IGHG2 | −1.83 | 0.19 | −4.56 to 0.89 | 0.81 | 0.54 | −1.80 to 3.42 | −0.85 | 0.5 | −3.32 to 1.62 |
| Immunoglobulin heavy constant gamma 4 | IGHG4 | −3.13 | 0.02* | −5.79 to −0.47 | −0.22 | 0.87 | −2.80 to 2.36 | 1.98 | 0.11 | −0.46 to 4.42 |
| Immunoglobulin heavy variable 1–18 | IGHV1-18 | 0.2 | 0.89 | −2.59 to 3.00 | −0.05 | 0.97 | −2.74 to 2.65 | 0.75 | 0.56 | −1.79 to 3.29 |
| Immunoglobulin heavy variable 3–7 | IGHV3-7 | −0.29 | 0.84 | −3.05 to 2.48 | 0.27 | 0.84 | −2.38 to 2.91 | 1.57 | 0.21 | −0.87 to 4.01 |
| Immunoglobulin kappa variable 3–20 | IGKV3-20 | −0.25 | 0.86 | −3.08 to 2.57 | 0.28 | 0.84 | −2.41 to 2.97 | 2.27 | 0.08 | −0.24 to 4.79 |
| Protein S100-A9 | S100A9 | 3.4 | 0.03* | 0.27 to 6.52 | −1.48 | 0.34 | −4.56 to 1.60 | −0.63 | 0.66 | −3.50 to 2.23 |
| Talin-1 | TLN1 | 1.02 | 0.47 | −1.74 to 3.78 | −1.95 | 0.14 | −4.58 to 0.68 | 0.40 | 0.75 | −2.04 to 2.83 |
The table shows the results of linear regression models between plasma proteins with clinical outcomes at follow-up. The models were adjusted for age, sex, and corresponding baseline protein levels. PSS Positive Symptom Severity score (based on the CAARMS assessment), SOFAS Social and Occupational Functional Assessment Scale, BACS composite score of Brief Assessment of Cognitive Function & *significant findings.
Association with functional outcome
Complement C5 associated inversely with the SOFAS score at follow-up (β coef = −3.23, CI95%ile = −5.87 to −0.59 & p-value = 0.02*). Apolipoprotein D (Apo D) at follow-up revealed a positive association with SOFAS score with a β coefficient of 2.77 (CI 95%ile: 0.13 to 5.41, p-value = 0.04*) (Table 3).
Association with cognition
In linear regression models, six proteins that are involved with the complement-coagulation cascade and lipid transport pathways associated with cognition. Among these, Complement Factor B (CFB) inversely associated with BACS score at follow-up (β coef = −3.18, CI 95%ile: 5.72 to −0.63 & p-value = 0.02), whereas Complement C1q subcomponent-B (C1QB) and coagulation factor V (F5) were positively associated with BACS score at follow-up (β coef = 3.93, CI95%ile: 1.58 to 6.28, p-value = 0.001* & β coef = 3.67, CI95%ile = 1.22 to 6.11; p-value = 0.004*). From the proteins involved in lipid transport mechanism, Apolipoprotein E, C-III and D were positively associated with BACS score (β coef = 3.09, 2.85, and 3.87; CI95%ile = (0.71 to 5.48), (0.36 to 5.34), and (1.25 to 6.50), p-value = 0.01*, 0.03*, and 0.004*, respectively) (Table 3).
Results of analysis 3: Univariate mediation analysis
Positive symptom severity
In a univariate mediation model of C5, IGHG-4, and S100A49, total omega-3 did not exert any direct or total effect on PSS score at follow-up. However, C5 and S100A49 exerted a significant negative, indirect effect (mediation effect) on the relationship between change in total omega-3 PUFAs and PSS score at follow-up [β coef = −0.21 and −0.18; 95%ile CI = (−0.46 to −0.03) and (−0.42 to −0.01)] (Table 4 and Fig. 1).
Table 4.
Results of univariate mediation analysis of omega-3 fatty acid associated plasma proteins on clinical outcome at follow-up.
| Outcome | Mediator | Mediation effect β coef (95%ile CI) | Direct effect β coef (95%ile CI) | Total effect β coef (95%ile CI) |
|---|---|---|---|---|
| PSS | Complement C5 | −0.21* (−0.46 to −0.03) | −0.06 (0.85 to −0.70) | −0.27 (0.39 to −0.90) |
| Protein S100-A9 | −0.18* (−0.42 to −0.01) | −0.09 (−0.78 to 0.73) | −0.27 (0.38 to −0.90) | |
| Immunoglobulin heavy constant gamma 4 | −0.17 (−0.46 to 0.029) | −0.09 (−0.72 to 0.54) | −0.26 (−0.88 to 0.37) | |
| SOFAS | Complement C5 | 0.19* (0.006 to 0.42) | 0.11 (−0.51 to 0.73) | 0.30 (−0.30 to 0.90) |
| Apolipoprotein D | 0.15 (−0.004 to 0.34) | 0.20 (−0.42 to 0.82) | 0.34 (−0.25 to 0.95) | |
| BACS | Complement factor B | 0.11 (−0.02 to 0.31) | 0.54 (−0.04 to 1.13) | 0.65* (−0.07 to 1.24) |
| Complement C1q subcomponent subunit B | 0.24* (0.05 to 0.54) | 0.38 (−0.19 to 0.95) | 0.62* (0.06 to 1.18) | |
| Coagulation factor V | 0.18* (0.02 to 0.38) | 0.47 (−0.10 to 1.05) | 0.66* (0.09 to 1.23) | |
| Apolipoprotein E | 0.16 (−0.01 to 0.43) | 0.46 (−0.11 to 1.03) | 0.62* (0.06 to 1.18) | |
| Apolipoprotein C-III | 0.14 (−0.02 to 0.39) | 0.52 (−0.06 to 1.10) | 0.66 (−0.10 to 1.23) | |
| Apolipoprotein D | 0.15 (−0.02 to 0.33) | 0.51 (0.08 to −0.06) | 0.67 (0.10 to 1.24) |
The table shows the results of mediation analysis using change in omega-3 PUFAs, plasma proteins and clinical outcomes as exposure, mediator and outcome variables, respectively. The model is adjusted for age, sex and baseline total omega-3 levels. CI confidence interval, PSS Positive Symptom Severity score, SOFAS Social and Occupational Functional Assessment scale, BACS Brief Assessment of Cognitive Function & *significant findings.
The bold values in the table represent the β coefficient and confidence intervals of significant proteins.
Functional outcome
For SOFAS score at follow-up, no direct or total effect was observed for total omega-3 PUFAs. However, Complement C5 showed a significant positive mediation effect on the relationship of change in total omega-3 PUFAs on the SOFAS score at follow-up [β coef = 0.19; 95%CI = (0.01 to 0.42)] (Table 4).
Cognitive outcome
Univariate mediation analysis was developed for six plasma proteins (FB, C1QB, F5, Apo E, Apo CIII & Apo D). A significant positive total effect was observed for total omega-3 PUFAs associated with cognitive outcome. C1QB and F5 exerted a significant positive mediation effect on total omega-3 PUFAs related cognitive improvement [β coef = 0.24 & 0.18; 95%CI = (0.05 to 0.54) & (0.02 to 0.38)] (Table 4). This mediation effect of C1QB and F5 was found to be 39 and 27% of the total effect of total omega-3 PUFAs on cognition, respectively.
Role of baseline total omega-3 PUFAs on the mediation effect
In this model, no total effect was observed for change in total omega-3 PUFAs on any of the clinical outcomes. However, the mediation effect of complement and coagulation proteins on total omega-3 associated clinical outcome remained significant after adjusting the models for baseline total omega-3 PUFAs (Supplementary Table 4).
Discussion
The current study investigated both the biological and clinical effect of PUFAs in a clinical high-risk population for a first psychotic episode. We have previously shown in this sample that supplementation with fish oil can have beneficial clinical effects in CHR individuals [63]. Our findings now provide evidence for a plausible mechanism of action of omega-3 PUFAs in early psychosis patients. The mass-spectrometry-based exploration of the plasma proteome at baseline and follow-up time points enabled us to study the longitudinal relationship of total omega-3 PUFAs on various biological mechanisms associated with psychopathology of CHR participants. First, change in total omega-3 PUFAs was associated with plasma proteins that represent inflammation, clotting and vesicle mediated transport mechanisms in CHR participants (Table 3). Secondly, among the omega-3 PUFA associated proteins, those participating in immune pathways of the complement system (C5, CFB, C1QB, and S100A9), the coagulation pathway (F5) and lipid transport pathways (Apo E, Apo CIII, and Apo D) were significantly associated with clinical outcomes. Thirdly, the results of the mediation analysis demonstrated that omega-3 PUFAs might exert a beneficial clinical response through immune pathway proteins (mainly the complement and coagulation cascade). There was evidence that C5 and S100A9 mediated the association of change in total omega-3 PUFAs with reduction in positive symptom severity and improvement in functioning. Furthermore, the proteins F5 and C1QB mediated the association between change in total omega-3 PUFAs and cognitive improvement at follow-up.
The current study is the first to observe that the complement cascade as the top biological pathway is related with change in omega-3 PUFAs in a CHR population. These observations provide vital evidence in omega-3-based treatment response in psychosis for the following reasons: (i) Genetic studies have reported evidence of a potentially causal relationship between increased long chain PUFA concentrations and lowered risk of psychosis [64, 65]; (ii) Complement related immune activity has been found to be involved in the pathophysiology of schizophrenia [66–70]; and (iii) in rodents, Madore et al. found a link between maternal omega-3 PUFA and microglia associated synaptic pruning through complement protein activity in off-springs [15]. However several studies have observed the beneficial effects of omega-3 PUFAs in various inflammatory and metabolic conditions [14, 15, 71–74], one recent study by Manousopoulou et al., explored the influence of dietary intake of omega-3 PUFAs on the plasma proteome of patients with fatty liver disease. The study reported that the coagulation pathway was highly influenced by the intake of marine omega-3 PUFAs in patients compared with healthy controls [75]. Whereas in the current study the inter-individual heterogeneity was taken into account by quantifying the proteins in each sample. Moreover, in the present study, erythrocyte membrane omega-3 level was used, which not only reflects the dietary intake of omega-3 PUFAs but also indicates the neuronal membrane omega-3 PUFAs [54, 55, 76, 77].
In line with our findings, existing evidence indicates consistent associations of complement dysregulation with psychotic symptoms in early psychosis although this has not be seen in established schizophrenia [78–84]. Complement and coagulation pathway proteins (that were associated with omega-3 PUFAs) indicated a relationship with psychotic symptoms (PSS), functioning status (SOFAS), and cognitive symptoms (BACS), whereas lipoprotein assembly proteins associated with cognition and functional outcome. The mediation analysis revealed a potential molecular mechanism through which total omega-3 PUFAs could influence clinical outcomes (as measured by PSS, a positive symptom score calculated based on CAARMS score), SOFAS (functioning), and BACS scales (cognition) (Fig. 2). Complement protein C5 mediated the association between change in total omega-3 PUFAs with reduction in positive symptoms and improvement in functional outcome in CHR. S100A9 which can regulate the expression of C3 [85, 86] mediated the omega-3 PUFA-associated reduction in PSS. In the mediation models, no direct association (no direct effect) was obtained for change in total omega-3 PUFAs (exposure) with clinical outcomes. However, a significant indirect association (indirect effect) was observed for change-in omega-3 PUFAs with clinical outcomes through the mediators (C5 and S100A9 for PSS; C5 for SOFAS score). Such type of mediation without direct effect is called as indirect-only mediation [87]. In mediation models of cognitive outcome, a significant direct and mediating effect was observed. Importantly, an increase in omega-3 PUFAs significantly associated with a high cognitive score at follow-up in which complement and coagulation proteins (C1QB and F5) exerted a partial mediation effect. Such mediation is termed ‘complementary mediation’, where the mediators (plasma proteins) complement the association of omega-3 PUFAs on cognitive outcome [87]. This partial mediation of C1QB and F5 contributed approximately 33 and 27% of the total effect of change in total omega-3 PUFAs on cognition. The latter mediation effect did not change even when adjusting the model for baseline omega-3 FAs. Previous investigations in the same cohort have reported significant cross-sectional and longitudinal associations of omega-3 PUFAs (EPA and DHA) on plasma immune markers, although these associations did not indicate any clinical significance [47]. Hence, our results from the mediation analysis suggest that omega-3 PUFAs associated changes in complement and coagulation proteins (F5, C1QB, C5, and S100A9) partially mediate the clinical response in a CHR state.
Fig. 2.
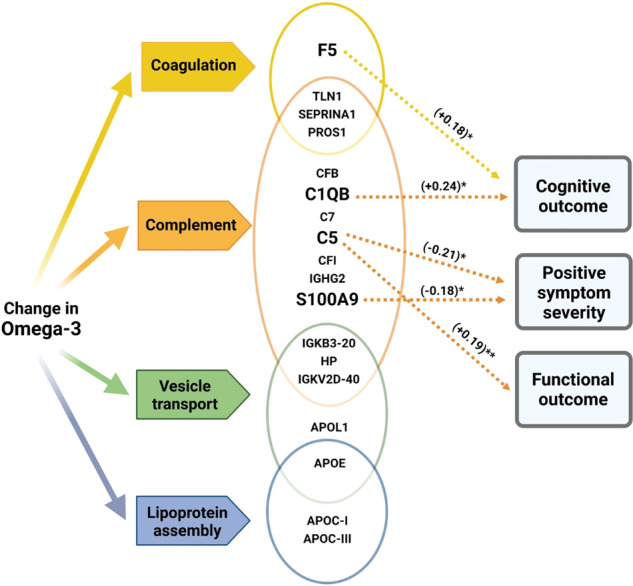
Schematic representation of key results depicting the relationship of total omega-3 PUFAs, key plasma proteins/pathways, and clinical outcome in a clinically high-risk population.
The key proteins that indicated a mediation effect were previously found to be involved with neuronal development and functioning [88, 89]. The activated products of C5, namely C5a, and S100A9, are pro-inflammatory in nature and play crucial roles in neuronal progenitor cell proliferation [90–92]. In our study, the mediation analysis suggests that an increase in total omega-3 PUFAs leads to symptomatic improvement by reducing the potentially pro-inflammatory components (C5 & S100A9). Similarly, the mediation analysis suggested that total omega-3 PUFAs improve cognition by increasing proteins (C1QB) that are involved in the synaptic pruning process [93, 94]. The animal study by Madore et al. provided a similar relationship of omega-3 PUFAs-C1Q-cognition axis. Madore et al.’s study reported that C1Q-receptor level was reduced in omega-3 deficient animals resulting in cognitive impairment [15]. Such symptom-specific complement alterations in a psychiatric population unfolds novel therapeutic opportunities to consider complement-targeted medicine in the early intervention of psychosis.
Our study has several strengths: (i) a state-of-the-art discovery proteomic approach allowed us to investigate a wide range of molecular mechanisms from plasma samples, (ii) the availability of biological and clinical data of the NEURAPRO clinical trial enabled us to look at both the biological and clinical relationship of omega-3 PUFAs at the same time, (iii) the exposure variable “erythrocyte membrane total omega-3 PUFA levels” provided a reliable measure of dietary omega-3 PUFAs and neuronal membrane omega-3 PUFAs [95], (iv) we used a unique study population (CHR) with mild psychotic symptoms, functional decline and cognitive impairment with no exposure to anti-psychotic medication [51], (v) the availability of both erythrocyte total omega-3 PUFAs and plasma proteome data at two time points provided the possibility of analyzing the longitudinal biological effects in the study population, and (vi) the findings have important clinical implications to early intervention strategies in psychosis.
Our study is not without limitations. Firstly, in the statistical analysis, the results were not adjusted for multiple corrections mainly due to the exploratory nature of the analysis and the nature of the mass spectrometry, which is a DDA based discovery approach. Secondly, in the statistical analysis, we adjusted the models for age and sex. The association of other covariates such as BMI and exposure to anti-depressants on both biological and clinical variables is not clearly understood and hence was not considered in the analysis. Finally, the absence of a direct effect in the mediation analysis limited us from understanding the percentage contribution of mediation in the overall effect [87, 96].
In conclusion, our findings provide novel insights into omega-3 PUFA-related protein mechanisms in the psychopathology of CHR participants. Pathway analysis indicated that the complement cascade showed the strongest association with change in omega-3 PUFAs. Furthermore, current findings suggest that the impact of omega-3 PUFAs on clinical symptoms in psychosis is mediated, at least in part, through complement and coagulation pathway proteins. For positive symptoms and functional outcome, the complement cascade proteins C5 and S100A9 exerted an ‘indirect-only mediation’ effect. Whereas for cognitive outcome, complement and coagulation pathway proteins (C1QB and F5) expressed a ‘complementary mediation’ effect. We speculate that omega-3 PUFAs may improve PSS and functional status through anti-inflammatory properties and enhance cognition by modifying C1Q-mediated synaptic pruning. Our study opens future opportunities to investigate the immune-associated intervention strategies in psychosis mainly targeting complement pathway proteins. Omega-3 PUFA are safe and can easily be used even in primary care settings. Since no biological treatment has yet been firmly established in CHR patients [97], the current findings also have potential implications for early intervention and treatment guidelines.
Supplementary information
Acknowledgements
The NEURAPRO clinical trial (anzctr.org.au Identifier 12608000475347) was supported by the Stanley Medical Research Institute (Grant No. 07TGF-1102 [to PDM, GPA, and BN]), National Health and Medical Research Council (NHMRC) Australia (Grant No. 566529 [to PDM, IBH, ARY, and GPA], NHMRC Senior Research Fellowship Grant No. 1080963 [to GPA], Career Development Fellowship Grant No. 1027532 [to BN], Senior Research Fellowship Grant No. 566593 [to ARY], and Senior Principal Research Fellowship Grant No. 1060996 and Colonial Foundation [to PDM]). This work was supported by an Irish Health Research Board research grant to DC (HRB ILP POR 2019-005), Wellcome Trust Innovation Flagship Award (220438Z/20/Z). This publication has emanated from research supported by Health Research Board (HRB) [to DC, MF] under grant number HRB/HRA/PHR/2015-1293. The research was funded by a research grant from Science Foundation Ireland (SFI) under Grant Number 16/RC/3948 and co-funded under the European Regional Development Fund and by FutureNeuro industry partners.
Author contributions
Conceptualization: SR, DC, and PA. Methodology: SR, MF, and DC. NEURAPRO clinical trial- study group: CM, MS, MB, NM, MS, SS, IH, GB, EC, LDH, DHMN, MN, AR, SV, RS, AT, ARY, BN, PM, and PA. Mass spectrometry analysis: SR, MF, KW, and GC. Formal Analysis: SR, CH, and DM. Data curation: SR, MF, and KW. Writing-original draft: SR. Writing- review and editing: CH, DM, MH, JB, MC, MF, CM, MS, MB, NM, MS, SS, GB, MN, MN, AR, SV, RS, AT, ARY, BN, PM, PA, and DC.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: G. Paul Amminger, David Cotter.
Contributor Information
Subash Raj Susai, Email: subashrajsusai@rcsi.ie.
David Cotter, Email: drcotter@rcsi.ie.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-02217-0.
References
- 1.Hsu MC, Huang YS, Ouyang WC. Beneficial effects of omega-3 fatty acid supplementation in schizophrenia: Possible mechanisms. Lipids Health Dis. 2020;19:159. doi: 10.1186/s12944-020-01337-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyall SC. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA, DPA, and DHA. Front Aging Neurosci. 2015;7:52. doi: 10.3389/fnagi.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denis I, Potier B, Heberden C, Vancassel S. Omega-3 polyunsaturated fatty acids and brain aging. Curr Opin Clin Nutr Metab Care. 2015;18:139–46. doi: 10.1097/MCO.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 4.Anderson BM, Ma DW. Are all n-3 polyunsaturated fatty acids created equal? Lipids Health Dis. 2009;8:33. doi: 10.1186/1476-511X-8-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: Are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614s–625s. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pusceddu MM, Kelly P, Stanton C, Cryan JF, Dinan TG. N-3 Polyunsaturated fatty acids through the lifespan: Implication for psychopathology. Int J Neuropsychopharmacol. 2016;19:pyw078. [DOI] [PMC free article] [PubMed]
- 7.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45:559–79. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 8.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Bannenberg G, Serhan CN. Specialized pro-resolving lipid mediators in the inflammatory response: An update. Biochim Biophys Acta. 2010;1801:1260–73. doi: 10.1016/j.bbalip.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gregory MK, Gibson RA, Cook-Johnson RJ, Cleland LG, James MJ. Elongase reactions as control points in long-chain polyunsaturated fatty acid synthesis. PLoS One. 2011;6:e29662. doi: 10.1371/journal.pone.0029662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Groeger AL, Cipollina C, Cole MP, Woodcock SR, Bonacci G, Rudolph TK, et al. Cyclooxygenase-2 generates anti-inflammatory mediators from omega-3 fatty acids. Nat Chem Biol. 2010;6:433–41. doi: 10.1038/nchembio.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cipollina C, Salvatore SR, Muldoon MF, Freeman BA, Schopfer FJ. Generation and dietary modulation of anti-inflammatory electrophilic omega-3 fatty acid derivatives. PLoS One. 2014;9:e94836. doi: 10.1371/journal.pone.0094836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, et al. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain, Behav, Immun. 2017;59:21–37. doi: 10.1016/j.bbi.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 14.Leyrolle Q, Madore C, Joffre C, Gressens P, Layé S, Nadjar A. Early-life PUFAs modulate shaping of neuronal circuits by microglia. GLIA. 2017;65:E272. [Google Scholar]
- 15.Madore C, Leyrolle Q, Morel L, Rossitto M, Greenhalgh AD, Delpech JC, et al. Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat Commun. 2020;11:6133. doi: 10.1038/s41467-020-19861-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Presumey J, Bialas AR, Carroll MC. Complement system in neural synapse elimination in development and disease. Adv Immunol. 2017;135:53–79. doi: 10.1016/bs.ai.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Pawelczyk T, Grancow-Grabka M, Kotlicka-Antczak M, Trafalska E, Pawelczyk A. A randomized controlled study of the efficacy of six-month supplementation with concentrated fish oil rich in omega-3 polyunsaturated fatty acids in first episode schizophrenia. J Psychiatr Res. 2016;73:34–44. doi: 10.1016/j.jpsychires.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: A systematic review. J Psychiatr Res. 2013;47:197–207. doi: 10.1016/j.jpsychires.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Jakobsen AS, Speyer H, Nørgaard HCB, Karlsen M, Hjorthøj C, Krogh J, et al. Dietary patterns and physical activity in people with schizophrenia and increased waist circumference. Schizophrenia Res. 2018;199:109–15. doi: 10.1016/j.schres.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Bentsen H, Solberg DK, Refsum H, Gran JM, Bøhmer T, Torjesen PA, et al. Bimodal distribution of polyunsaturated fatty acids in schizophrenia suggests two endophenotypes of the disorder. Biol Psychiatry. 2011;70:97–105. doi: 10.1016/j.biopsych.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Smesny S, Milleit B, Nenadic I, Preul C, Kinder D, Lasch J, et al. Phospholipase A2 activity is associated with structural brain changes in schizophrenia. Neuroimage. 2010;52:1314–27. doi: 10.1016/j.neuroimage.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Šakić M, Karlović D, Vidrih B, Peitl V, Crnković D, Vrkić N. Increased calcium-independent lipoprotein phospholipase A2 but not protein S100 in patients with schizophrenia. Psychiatr Danubina. 2016;28:45–50. [PubMed] [Google Scholar]
- 23.Sun GY, Shelat PB, Jensen MB, He Y, Sun AY, Simonyi A. Phospholipases A2 and inflammatory responses in the central nervous system. Neuromolecular Med. 2010;12:133–48. doi: 10.1007/s12017-009-8092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potvin S, Stip E, Sepehry AA, Gendron A, Bah R, Kouassi E. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Pae CU, Yu HS, Lee KU, Kim JJ, Lee CU, Lee SJ, et al. BanI polymorphism of the cytosolic phospholipase A2 gene may confer susceptibility to the development of schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2004;28:739–41. doi: 10.1016/j.pnpbp.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Barbosa NR, Junqueira RM, Vallada HP, Gattaz WF. Association between BanI genotype and increased phospholipase A2 activity in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2007;257:340–3. doi: 10.1007/s00406-007-0736-0. [DOI] [PubMed] [Google Scholar]
- 27.Yang G, Xu H, Zhang H, Yu Q, Wu Y, Shi J, et al. Association between PLA2G12A polymorphisms and schizophrenia in a Han Chinese population from Northeast China. PLoS One. 2016;11:e0159584. doi: 10.1371/journal.pone.0159584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horrobin DF. The membrane phospholipid hypothesis as a biochemical basis for the neurodevelopmental concept of schizophrenia. Schizophrenia Res. 1998;30:193–208. doi: 10.1016/s0920-9964(97)00151-5. [DOI] [PubMed] [Google Scholar]
- 29.Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophrenia Res. 2000;42:7–17. doi: 10.1016/s0920-9964(99)00095-x. [DOI] [PubMed] [Google Scholar]
- 30.Assies J, Lieverse R, Vreken P, Wanders RJA, Dingemans PMJA, Linszen DH. Significantly reduced docosahexaenoic and docosapentaenoic acid concentrations in erythrocyte membranes from schizophrenic patients compared with a carefully matched control group. Biol Psychiatry. 2001;49:510–22. doi: 10.1016/s0006-3223(00)00986-0. [DOI] [PubMed] [Google Scholar]
- 31.Khan MM, Evans DR, Gunna V, Scheffer RE, Parikh VV, Mahadik SP. Reduced erythrocyte membrane essential fatty acids and increased lipid peroxides in schizophrenia at the never-medicated first-episode of psychosis and after years of treatment with antipsychotics. Schizophrenia Res. 2002;58:1–10. doi: 10.1016/s0920-9964(01)00334-6. [DOI] [PubMed] [Google Scholar]
- 32.Arvindakshan M, Ghate M, Ranjekar PK, Evans DR, Mahadik SP. Supplementation with a combination of ω-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophrenia Res. 2003;62:195–204. doi: 10.1016/s0920-9964(02)00284-0. [DOI] [PubMed] [Google Scholar]
- 33.Peet M, Shah S, Selvam K, Ramchand CN. Polyunsaturated fatty acid levels in red cell membranes of unmedicated schizophrenic patients. World J Biol Psychiatry. 2004;5:92–99. doi: 10.1080/15622970410029917. [DOI] [PubMed] [Google Scholar]
- 34.Reddy RD, Keshavan MS, Yao JK. Reduced red blood cell membrane essential polyunsaturated fatty acids in first episode schizophrenia at neuroleptic-naive baseline. Schizophrenia Bull. 2004;30:901–11. doi: 10.1093/oxfordjournals.schbul.a007140. [DOI] [PubMed] [Google Scholar]
- 35.Kale A, Naphade N, Sapkale S, Kamaraju M, Pillai A, Joshi S, et al. Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never-medicated schizophrenia patients: implications for altered one-carbon metabolism. Psychiatry Res. 2010;175:47–53. doi: 10.1016/j.psychres.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 36.Van der Kemp WJM, Klomp DWJ, Kahn RS, Luijten PR, Pol HEH. A meta-analysis of the polyunsaturated fatty acid composition of erythrocyte membranes in schizophrenia. Schizophrenia Res. 2012;141:153–61. doi: 10.1016/j.schres.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 37.McEvoy J, Baillie RA, Zhu H, Buckley P, Keshavan MS, Nasrallah HA, et al. Lipidomics reveals early metabolic changes in subjects with schizophrenia: effects of atypical antipsychotics. PLoS One. 2013;8:e68717. doi: 10.1371/journal.pone.0068717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNamara RK. Deciphering the role of docosahexaenoic acid in brain maturation and pathology with magnetic resonance imaging. Prostaglandins, Leukotrienes Essent Fat Acids. 2013;88:33–42. doi: 10.1016/j.plefa.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rice SM, Schafer MR, Klier C, Mossaheb N, Vijayakumar N, Amminger GP. Erythrocyte polyunsaturated fatty acid levels in young people at ultra-high risk for psychotic disorder and healthy adolescent controls. Psychiatry Res. 2015;228:174–6. doi: 10.1016/j.psychres.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Hoen WP, Lijmer JG, Duran M, Wanders RJA, van Beveren NJM, de Haan L. Red blood cell polyunsaturated fatty acids measured in red blood cells and schizophrenia: a meta-analysis. Psychiatry Res. 2013;207:1–12. doi: 10.1016/j.psychres.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 41.Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67:146–54. doi: 10.1001/archgenpsychiatry.2009.192. [DOI] [PubMed] [Google Scholar]
- 42.Markulev C, McGorry PD, Nelson B, Yuen HP, Schaefer M, Yung AR, et al. NEURAPRO‐E study protocol: A multicentre randomized controlled trial of omega‐3 fatty acids and cognitive‐behavioural case management for patients at ultra high risk of schizophrenia and other psychotic disorders. Early Intervention Psychiatry. 2017;11:418–28. doi: 10.1111/eip.12260. [DOI] [PubMed] [Google Scholar]
- 43.McGorry PD, Nelson B, Markulev C, Yuen HP, Schäfer MR, Mossaheb N, et al. Effect of ω-3 polyunsaturated fatty acids in young people at ultrahigh risk for psychotic disorders: The NEURAPRO randomized clinical trial. JAMA Psychiatry. 2017;74:19–27. doi: 10.1001/jamapsychiatry.2016.2902. [DOI] [PubMed] [Google Scholar]
- 44.Smesny S, Milleit B, Hipler UC, Milleit C, Schafer MR, Klier CM, et al. Omega-3 fatty acid supplementation changes intracellular phospholipase A2 activity and membrane fatty acid profiles in individuals at ultra-high risk for psychosis. Mol Psychiatry. 2014;19:317–24. doi: 10.1038/mp.2013.7. [DOI] [PubMed] [Google Scholar]
- 45.Law MH, Cotton RGH, Berger GE. The role of phospholipases A2 in schizophrenia. Mol Psychiatry. 2006;11:547–56. doi: 10.1038/sj.mp.4001819. [DOI] [PubMed] [Google Scholar]
- 46.Smesny S, Milleit B, Schaefer MR, Hesse J, Schlogelhofer M, Langbein K, et al. Effects of omega-3 PUFA on immune markers in adolescent individuals at ultra-high risk for psychosis—Results of the randomized controlled Vienna omega-3 study. Schizophrenia Res. 2017;188:110–7. doi: 10.1016/j.schres.2017.01.026. [DOI] [PubMed] [Google Scholar]
- 47.Susai SR, Mongan D, Healy C, Cannon M, Nelson B, Markulev C, et al. The association of plasma inflammatory markers with omega-3 fatty acids and their mediating role in psychotic symptoms and functioning: An analysis of the NEURAPRO clinical trial. Brain, Behav, Immun. 2021;99:147–56. doi: 10.1016/j.bbi.2021.09.018. [DOI] [PubMed] [Google Scholar]
- 48.Amminger GP, Schafer MR, Schlogelhofer M, Klier CM, McGorry PD. Longer-term outcome in the prevention of psychotic disorders by the Vienna omega-3 study. Nat Commun. 2015;6:7934. [DOI] [PMC free article] [PubMed]
- 49.Amminger GP, Mechelli A, Rice S, Kim SW, Klier CM, McNamara RK, et al. Predictors of treatment response in young people at ultra-high risk for psychosis who received long-chain omega-3 fatty acids. Transl Psychiatry. 2015;5:e495. doi: 10.1038/tp.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. [DOI] [PubMed]
- 51.Markulev C, McGorry PD, Nelson B, Yuen HP, Schaefer M, Yung AR, et al. NEURAPRO-E study protocol: A multicentre randomized controlled trial of omega-3 fatty acids and cognitive-behavioural case management for patients at ultra high risk of schizophrenia and other psychotic disorders. Early Intervention Psychiatry. 2017;11:418–28. doi: 10.1111/eip.12260. [DOI] [PubMed] [Google Scholar]
- 52.Schlögelhofer M, McGorry PD, Nelson B, Markulev C, Yuen HP, Schåfer M, et al. The neurapro study: Adherence to study medication. Schizophrenia Bull. 2018;44:S132–S133. [Google Scholar]
- 53.McLaverty A, Allott KA, Berger M, Hester R, McGorry PD, Nelson B, et al. Omega‐3 fatty acids and neurocognitive ability in young people at ultra‐high risk for psychosis. Early Intervention Psychiatry. 2021;15:874–81. doi: 10.1111/eip.13025. [DOI] [PubMed] [Google Scholar]
- 54.Pierigè F, Serafini S, Rossi L, Magnani M. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60:286–95. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 55.Owen AJ, Peter‐Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish oil dose‐and time‐response effects on cardiac phospholipid fatty acid composition. Lipids. 2004;39:955. doi: 10.1007/s11745-004-1317-0. [DOI] [PubMed] [Google Scholar]
- 56.Yung AR, Yuen HP, McGorry PD, Phillips LJ, Kelly D, Dell’Olio M, et al. Mapping the onset of psychosis: The Comprehensive Assessment of At-Risk Mental States. Aust NZ J Psychiatry. 2005;39:964–71. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 57.Morrison AP, French P, Stewart SL, Birchwood M, Fowler D, Gumley AI, et al. Early detection and intervention evaluation for people at risk of psychosis: Multisite randomised controlled trial. BMJ (Clin Res ed) 2012;344:e2233. doi: 10.1136/bmj.e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morrison AP, Pyle M, Maughan D, Johns L, Freeman D, Broome MR, et al. Antipsychotic medication versus psychological intervention versus a combination of both in adolescents with first-episode psychosis (MAPS): A multicentre, three-arm, randomised controlled pilot and feasibility study. Lancet Psychiatry. 2020;7:788–800. doi: 10.1016/S2215-0366(20)30248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keefe RS, Goldberg TE, Harvey PD, Gold JM, Poe MP, Coughenour L. The brief assessment of cognition in schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Res. 2004;68:283–97. doi: 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 60.Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scandinavica. 2000;101:323–9. [PubMed] [Google Scholar]
- 61.Jassal B, Matthews L, Viteri G, Gong C, Lorente P, Fabregat A, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2020;48:D498–D503. doi: 10.1093/nar/gkz1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hayes AF. Introduction to mediation, moderation, and conditional process analysis second edition. A regression-based approach. Guilford Press, New York, 2017.
- 63.Amminger GP, Nelson B, Markulev C, Yuen HP, Schäfer MR, Berger M, et al. The NEURAPRO biomarker analysis: Long-chain omega-3 fatty acids improve 6-month and 12-month outcomes in youths at ultra-high risk for psychosis. Biol Psychiatry. 2020;87:243–52. doi: 10.1016/j.biopsych.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 64.Jones HJ, Borges MC, Carnegie R, Mongan D, Rogers PJ, Lewis SJ, et al. Associations between plasma fatty acid concentrations and schizophrenia: a two-sample Mendelian randomisation study. Lancet Psychiatry. 2021;8:1062–70. doi: 10.1016/S2215-0366(21)00286-8. [DOI] [PubMed] [Google Scholar]
- 65.Coltell O, Sorlí JV, Asensio EM, Barragán R, González JI, Giménez-Alba IM et al. Genome-wide association study for serum omega-3 and omega-6 polyunsaturated fatty acids: Exploratory analysis of the sex-specific effects and dietary modulation in Mediterranean subjects with metabolic syndrome. Nutrients. 2020;12:310. [DOI] [PMC free article] [PubMed]
- 66.Prasad KM, Chowdari KV, D’Aiuto LA, Iyengar S, Stanley JA, Nimgaonkar VL. Neuropil contraction in relation to Complement C4 gene copy numbers in independent cohorts of adolescent-onset and young adult-onset schizophrenia patients-a pilot study. Transl Psychiatry. 2018;8:134. doi: 10.1038/s41398-018-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. Schizophrenia risk from complex variation of complement component 4. Nature. 2016;530:177–83. doi: 10.1038/nature16549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Whalley K. Psychiatric disorders: Linking genetic risk to pruning. Nat Rev Neurosci. 2016;17:199–199. doi: 10.1038/nrn.2016.20. [DOI] [PubMed] [Google Scholar]
- 69.Yilmaz M, Yalcin E, Presumey J, Aw E, Ma M, Whelan CW, et al. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat Neurosci. 2021;24:214–24. doi: 10.1038/s41593-020-00763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Purves-Tyson TD, Robinson K, Brown AM, Boerrigter D, Cai HQ, Weissleder C, et al. Increased macrophages and C1qA, C3, C4 transcripts in the midbrain of people with schizophrenia. Front Immunol. 2020;11:2002. doi: 10.3389/fimmu.2020.02002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mason RP, Libby P, Bhatt DL. Emerging mechanisms of cardiovascular protection for the omega-3 fatty acid eicosapentaenoic acid. Arteriosclerosis, Thrombosis, Vasc Biol. 2020;40:1135–47. doi: 10.1161/ATVBAHA.119.313286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brennan E, Kantharidis P, Cooper ME, Godson C. Pro-resolving lipid mediators: Regulators of inflammation, metabolism, and kidney function. Nat Rev Nephrol. 2021;17:725–39. doi: 10.1038/s41581-021-00454-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Amatruda M, Ippolito G, Vizzuso S, Vizzari G, Banderali G, Verduci E. Epigenetic effects of n-3 LCPUFAs: A role in pediatric metabolic syndrome. Int. J. Mol. Sci. 2019;20:2118. [DOI] [PMC free article] [PubMed]
- 74.Tajuddin N, Shaikh A, Hassan A. Prescription omega-3 fatty acid products: Considerations for patients with diabetes mellitus. Diabetes Metab Syndr Obes. 2016;9:109–18. doi: 10.2147/DMSO.S97036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Manousopoulou A, Scorletti E, Smith DE, Teng J, Fotopoulos M, Roumeliotis TI, et al. Marine omega-3 fatty acid supplementation in non-alcoholic fatty liver disease: Plasma proteomics in the randomized WELCOME* trial. Clin Nutr. 2019;38:1952–5. doi: 10.1016/j.clnu.2018.07.037. [DOI] [PubMed] [Google Scholar]
- 76.Harris WS. Omega-3 fatty acids and cardiovascular disease: A case for omega-3 index as a new risk factor. Pharmacol Res. 2007;55:217–23. doi: 10.1016/j.phrs.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, O’Brien D, et al. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011;65:808–17. doi: 10.1038/ejcn.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.English JA, Lopez LM, O’Gorman A, Föcking M, Hryniewiecka M, Scaife C, et al. Blood-based protein changes in childhood are associated with increased risk for later psychotic disorder: Evidence from a nested case-control study of the ALSPAC longitudinal birth cohort. Schizophr Bull. 2018;44:297–306. doi: 10.1093/schbul/sbx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Madrid-Gambin F, Föcking M, Sabherwal S, Heurich M, English JA, O’Gorman A, et al. Integrated lipidomics and proteomics point to early blood-based changes in childhood preceding later development of psychotic experiences: Evidence from the Avon longitudinal study of parents and children. Biol Psychiatry. 2019;86:25–34. doi: 10.1016/j.biopsych.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mongan D, Föcking M, Healy C, Susai SR, Heurich M, Wynne K, et al. Development of proteomic prediction models for transition to psychotic disorder in the clinical high-risk state and psychotic experiences in adolescence. JAMA Psychiatry. 2021;78:77–90. doi: 10.1001/jamapsychiatry.2020.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Föcking M, Sabherwal S, Cates HM, Scaife C, Dicker P, Hryniewiecka M, et al. Complement pathway changes at age 12 are associated with psychotic experiences at age 18 in a longitudinal population-based study: Evidence for a role of stress. Mol Psychiatry. 2021;26:524–33. doi: 10.1038/s41380-018-0306-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sabherwal S, Föcking M, English JA, Fitzsimons S, Hryniewiecka M, Wynne K, et al. ApoE elevation is associated with the persistence of psychotic experiences from age 12 to age 18: Evidence from the ALSPAC birth cohort. Schizophr Res. 2019;209:141–7. doi: 10.1016/j.schres.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Mongan D, Sabherwal S, Susai SR, Föcking M, Cannon M, Cotter DR. Peripheral complement proteins in schizophrenia: A systematic review and meta-analysis of serological studies. Schizophr Res. 2020;222:58–72. doi: 10.1016/j.schres.2020.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.González-Castro TB, Tovilla-Zárate CA, Hernández-Díaz Y, Fresán A, Juárez-Rojop IE, Ble-Castillo JL, et al. No association between ApoE and schizophrenia: Evidence of systematic review and updated meta-analysis. Schizophrenia Res. 2015;169:355–68. doi: 10.1016/j.schres.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 85.Wang S, Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in inflammation. Front Immunol. 2018;9:1298. doi: 10.3389/fimmu.2018.01298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schonthaler Helia B, Guinea-Viniegra J, Wculek Stefanie K, Ruppen I, Ximénez-Embún P, Guío-Carrión A, et al. S100A8-S100A9 protein complex mediates psoriasis by regulating the expression of complement factor C3. Immunity. 2013;39:1171–81. doi: 10.1016/j.immuni.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 87.Zhao X, Lynch J, Chen Q. Reconsidering Baron and Kenny: Myths and truths about Mediation analysis. J Consum Res. 2010;37:197–206. [Google Scholar]
- 88.Coulthard LG, Hawksworth OA, Woodruff TM. Complement: The emerging architect of the developing brain. Trends Neurosci. 2018;41:373–84. doi: 10.1016/j.tins.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 89.Heurich M, Föcking M, Mongan D, Cagney G, Cotter DR. Dysregulation of complement and coagulation pathways: Emerging mechanisms in the development of psychosis. Mol Psychiatry. 2021;27:1–14. [DOI] [PMC free article] [PubMed]
- 90.Coulthard LG, Hawksworth OA, Li R, Balachandran A, Lee JD, Sepehrband F, et al. Complement C5aR1 signaling promotes polarization and proliferation of embryonic neural progenitor cells through PKCzeta. J Neurosci: Off J Soc Neurosci. 2017;37:5395–407. doi: 10.1523/JNEUROSCI.0525-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tian Y, Cao R, Che B, Sun D, Tang Y, Jiang L, et al. Proinflammatory S100A9 regulates differentiation and aggregation of neural stem cells. ACS Chem Neurosci. 2020;11:3549–56. doi: 10.1021/acschemneuro.0c00365. [DOI] [PubMed] [Google Scholar]
- 92.Wang C, Iashchishyn IA, Kara J, Foderà V, Vetri V, Sancataldo G, et al. Proinflammatory and amyloidogenic S100A9 induced by traumatic brain injury in mouse model. Neurosci Lett. 2019;699:199–205. doi: 10.1016/j.neulet.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 93.Schafer D, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Greenberg ME, et al. Microglia and the complement cascade: Shaping neural circuits in the developing brain. Schizophrenia Res. 2012;136:S11–S12. [Google Scholar]
- 94.Yuzaki M. Synapse formation and maintenance by C1q family proteins: A new class of secreted synapse organizers. Eur J Neurosci. 2010;32:191–7. doi: 10.1111/j.1460-9568.2010.07346.x. [DOI] [PubMed] [Google Scholar]
- 95.Serra-Majem L, Nissensohn M, Øverby NC, Fekete K. Dietary methods and biomarkers of omega 3 fatty acids: A systematic review. Br J Nutr. 2012;107(Suppl 2):S64–76. doi: 10.1017/S000711451200147X. [DOI] [PubMed] [Google Scholar]
- 96.Sidhu A, Bhalla P, Zafar S. Mediating effect and review of its statistical measures. Empir Econ Lett. 2021;20:29–40. [Google Scholar]
- 97.Amminger GP, Lin A, Kerr M, Weller A, Spark J, Pugh C, et al. Cannabidiol for at risk for psychosis youth: A randomized controlled trial. Early Inter Psychiatry. 2022;16:419–32. doi: 10.1111/eip.13182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.