Abstract
The lack of a theory capable of connecting the amino acid sequence of a light-absorbing protein with its fluorescence brightness is hampering the development of tools for understanding neuronal communications. Here we demonstrate that a theory can be established by constructing quantum chemical models of a set of Archaerhodopsin reporters in their electronically excited state. We found that the experimentally observed increase in fluorescence quantum yield is proportional to the computed decrease in energy difference between the fluorescent state and a nearby photoisomerization channel leading to an exotic diradical of the protein chromophore. This finding will ultimately support the development of technologies for searching novel fluorescent rhodopsin variants and unveil electrostatic changes that make light emission brighter and brighter.
Subject terms: Computational chemistry, Excited states, Quantum chemistry, Photobiology
Arch-3 rhodopsin variants are common fluorescent reporters of neuronal activity. Here, the authors show with quantum chemical modelling that a set of these proteins reveals a direct proportionality between their observed fluorescence intensity and the stability of an exotic excited-state diradical intermediate.
Introduction
The imaging of neural activity requires bright fluorescent probes localized in the neuron membrane. Rhodopsins are membrane proteins that can be expressed in neurons and used for triggering, silencing and reporting on neuronal action potentials1,2. The prototypical fluorescent reporter is Archaerhodopsin-3 (Arch3), an archaeal rhodopsin from Halorubrum sodomense3–5. However, the fluorescence of Arch3 is extremely dim; its quantum yield (FQY) of ca. 1.1·10−4 is almost four orders of magnitude lower than that of the green fluorescent protein (GFP)6–11. Furthermore, the fluorescence does not come from the dark-adapted state but rather from a photochemically produced photocycle intermediate that cannot deliver a prompt emission signal11. These facts not only make single neuron studies impossible with common microscopy techniques, but impair the investigation of neural activity at the population level as wide-field imaging techniques require improved spatial and temporal resolution12. In order to achieve better reporters, Arch3 has been engineered via directed evolutionary approaches and random mutagenesis, ultimately discovering variants such as the Archers13, the QuasArs12, the Archons14, Arch5 and Arch713. Recently, Hegemann and coworkers have investigated new Archon1 variants to elucidate the mechanism of fluorescence voltage sensitivity15. These variants feature a higher 10−3−10−2 FQYs enabling, among other applications, the imaging of neuronal activity in living mammals and invertebrates16–18. It has also been suggested that such enhanced fluorescence originates, in contrast to Arch3, from a one-photon electronic excitation and, therefore, must come from the protein dark-adapted state12,14,15. Here we report on a (mechanistic) theory that explains how one-photon FQY is enhanced in Arch3 variants. We demonstrate that, to be predictive, such theory requires the mapping of an isomerization path producing a twisted intramolecular diradical intermediate (TIDIR) located in proximity of a conical intersection (CoIn). The energy difference between TIDIR and the emissive planar fluorescent state (FS), or ΔETIDIR-FS, controls the magnitude and positions of the S1 photoisomerization barrier (EfS1) that, in turn, determines the FQY.
Based on the above result, we show that the increased FQY displayed by certain Arch3 variants is determined by inhibiting an electrostatic barrier suppression mechanism operating in the naturally occurring progenitor.
Results and discussion
Arch photoisomerization mechanism and S1 PES topography
The function of rhodopsins is triggered by the photoisomerization of their retinal chromophores (Fig. 1A) that also operate as fluorophores19. Consistently with previous studies on fluorescent proteins20, we hypothesize that the competition between isomerization and light emission determines the FQY value (see Supplementary Methods 3). This can be understood by examining a schematic representation of the chromophore excited state isomerization path (Fig. 1B) leading to a CoIn between the excited (S1) and the ground (S0) states where excited state decay occurs. From the scheme it is evident that the isomerization barrier (EfS1) located along the potential energy surface of the spectroscopically allowed S1 state is a critical quantity. The higher EfS1 is, the slower the isomerization is. Consequently, paths with high EfS1 will feature an approximately planar fluorescent state FS with long S1 lifetimes and, thus, high FQY. Below, we demonstrate a proportionality between computed EfS1 and observed FQY by constructing multi-configurational quantum-chemical based models (MCQC) of the dark-adapted state of a set of Arch3 variants called the Arch-set (Supplementary Methods 1–3 and Supplementary Figs. 1–3). We will also show that the proportionality between computed EfS1 and observed FQY remains valid when EfS1 is replaced by the isomerization energy ΔETIDIR-FS. Our MCQC models employ the well-established CASSCF21 zeroth-order wavefunction defined by the selection of a (12,12) active space including all the π-electrons and orbitals of the retinal chromophore. Although the trends in spectral properties discussed throughout the text are well reproduced at this level, we discuss the results obtained after multi-state (XMS-CASPT2)22,23 energy and geometrical corrections to the CASSCF geometries.
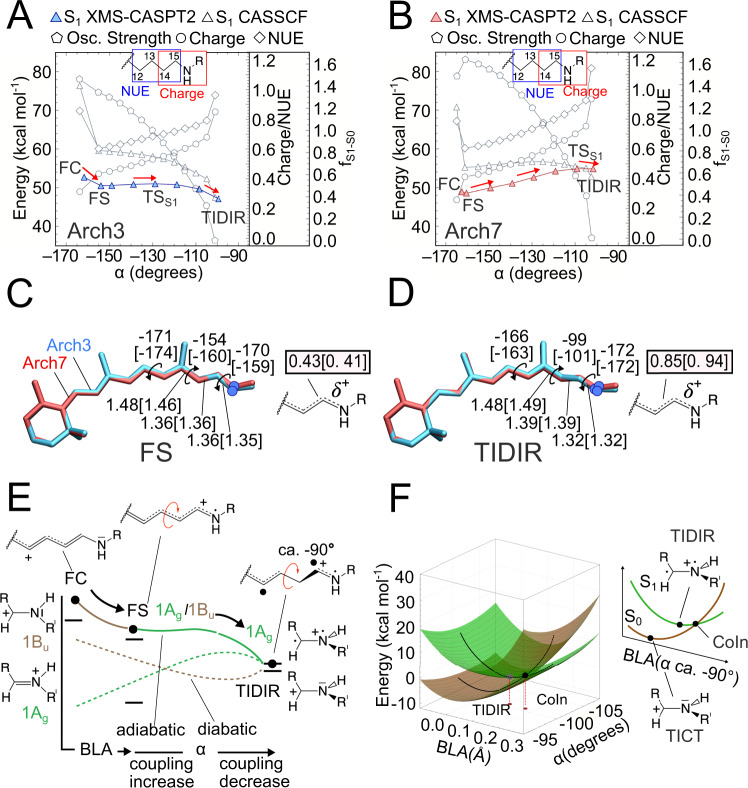
Fig. 1. Photoisomerization mechanism of Archaerhodopsins.
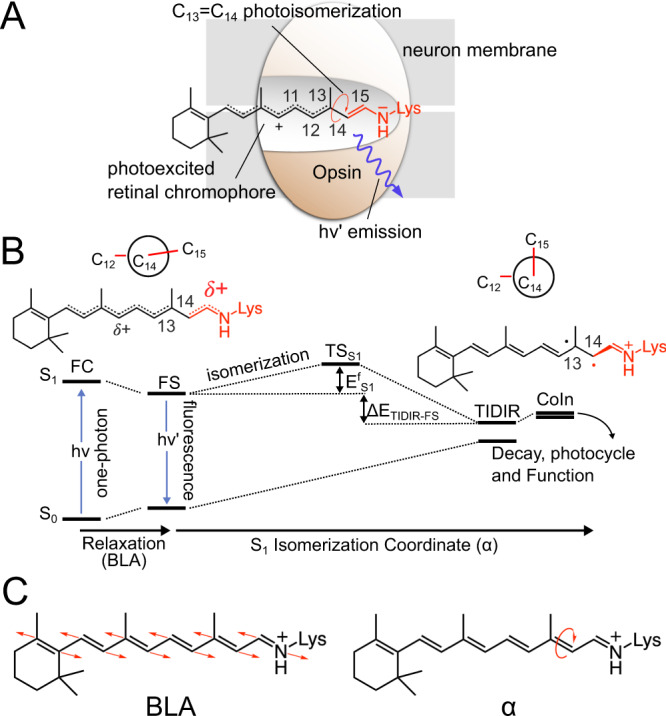
A Lewis formula representing the initial S1 chromophore structure. B Representation of the chromophore isomerization path. FS corresponds to the fluorescent state. TIDIR represents the photoisomerization channel located near CoIn. FS and TIDIR are represented by Lewis formulas displaying distinct degrees of double bond twisting and charge transfer. C Main components of the reaction coordinate. BLA is numerically defined as the difference between the average single-bond length minus the average double-bond length along the C5 to N conjugated chain (for convenience, below we consider the BLA of the framed moiety exclusively). α is defined by the dihedral angle C12-C13-C14-C15.
Before focusing on the relationship between EfS1 and FQY, it is useful to describe the changes of the all-trans retinal chromophore of Archaearhodopsins24 along the isomerization path computed using the constructed Arch3 model. It is established that at least two geometrical coordinates are implicated in rhodopsin S1 isomerization coordinates (Fig. 1C)25–27. The first describes the chromophore initial relaxation from the Franck-Condon (FC) point and corresponds to a bond length alternation (BLA) stretch. The second is the twisting (α) of the reactive trans C13 = C14 bond ultimately leading to the 13-cis configuration. As shown in Fig. 2A and consistently with the scheme of Fig. 1B, such relaxation leads to the potentially emissive FS intermediate. This process is followed by progression along a flat potential energy region characterized by a monotonic decrease in α and connecting FS to a TIDIR intermediate located close to a sloped28 CoIn. As it will be explained below, this previously unreported intermediate differs, in terms of electronic structure and topography, from the locally excited (LE) intermediate identified in a ring-locked derivative of bovine rhodopsin by Laricheva et al.29.
Fig. 2. Comparison between computed Arch3 and Arch7 S1 isomerization paths.
A Variations in potential energy (relative to the S0 equilibrium structure), charge distribution, free valence and oscillator strength along the Arch3 path. The energy profile in color is given after MS correction. Red arrows indicate progression along the isomerization coordinate. B Same data for Arch7. C Main geometrical chromophore parameters for the FS fluorescent intermediates of Arch3 and Arch7 (values in square brackets). The S1 positive charge fraction on the C-C-N moiety are also given. D Same data for the photoisomerization channel TIDIR. E Schematic “decomposition” of the Arch3 adiabatic energy profile of panel B in terms of diabatic states associated to Lewis formulas of the CH2 = NH2(+) minimal model. The progression of the electronic and geometrical structure of the relevant moiety of the full chromophore is given at the top. F Schematic representation of the CoIn region of Arch3 including the twisted diradical TIDIR along the relevant components of the reaction coordinate. The same coordinate also spans the CoIn branching plane. The potential energy scale is relative to the CoIn.
Electronic character of the S1 PES
In Fig. 2B we show that such a progression is replicated by the MCQC model of the top fluorescent variant Arch7. However, the comparison between the Arch3 and Arch7 S1 energy profiles shows a significantly increased EfS1 in the latter. Such an increase is seen in both the zeroth-order wavefunction calculation and in the more quantitative profiles obtained after applying geometrical and multi-state second-order perturbative correction (Supplementary Methods 4 and 5, Supplementary Figs. 4–7). Due to the high computational cost of QM/MM analytical Hessians, the TSs discussed throughout the text are approximated by the energy maxima along the relaxed scan connecting FS and TIDIR. These TSs must be considered approximate as it has not been possible to carry out a geometry optimization starting from a computed Hessian matrix as well as to compute a Hessian matrix at the end of the TS search. Furthermore, despite the increased FQY of the investigated Arch variants, recent measurements of the excited state lifetime (ESL) of some of the variants were found to be in the time range of picoseconds30,31. For this reason, we don’t account in our calculation for T1/S1 intersystem crossing (ISC) as a viable competitive process to S1 emission also considering that T1 is a π-π* state with orbitals parallel (non-orthogonal) to those characterizing the S1 state. Therefore, the singlet to triplet transition would be “forbidden” by the El-Sayed rule.
The progression along α is documented in Fig. 2C, D where we report the values of the relevant geometrical parameters of FS and TIDIR as well as the charge residing on the Schiff base moiety C14-C15-N. In fact, the geometrical changes are accompanied by variations of the chromophore electronic character. These can be conveniently followed by computing the fraction of positive charge and free valence (NUE)32 residing on C14-C15-N (Supplementary Methods 6 and Supplementary Figs. 4, 7, 9 and 10). Following a recent report on the variants of DRONPA220, a soluble GFP-like protein, we interpret the results in Fig. 2A, B in terms of mixing of two diabatic states describing the covalent (1Ag) and charge-transfer (1Bu) characters of polyenes33. The computed C14-C15-N charge and NUE progression point to an S1 state initially dominated by the 1Bu charge transfer character as already reported for other rhodopsins. However, such character decreases when relaxing to an FS that features a larger 1Ag weight, then constantly increasing all along the isomerization path. In summary, the electronic character evolves from mixed charge-transfer/covalent 1Ag/1Bu characters at FC and FS, to a substantially pure 1Ag character at TIDIR that features an α value of ca. −90°, two spin-paired, but non-interacting, radical centers located on two orthogonal π-systems and a positive charge fully confined on C14-C15-N. This process is accompanied by a change in the S1-S0 oscillator strength (fS1-S0) along α, from values typical of allowed electronic transitions to a forbidden transition at TIDIR.
The evolution going from a mixed 1Ag/1Bu character at FS to a 1Ag covalent/diradical character at TIDIR, provides information on the origin of the EfS1 barrier. As illustrated in Fig. 2E the electronic coupling between the 1Ag and 1Bu diabatic states (represented, for simplicity, by the Lewis formula of a methylimine cation model) would initially increase due to the decrease in their energy gap. However, when α approaches orthogonality, the coupling decreases rapidly and becomes negligible at TIDIR. The negligible coupling at TIDIR is related to the vicinity of a CoIn (see Fig. 2F) where the 1Ag/1Bu coupling is zero. A CoIn deformation along the negative BLA direction lifts the degeneracy and intercepts TIDIR (see Supplementary Methods 7 and Supplementary Figs. 11 and 12). Notice that the same deformation along the S0 potential energy surface achieves the twisted intramolecular charge transfer TICT structure34 corresponding to the transition state driving the chromophore thermal isomerization also documented for bovine rhodopsin33. In conclusion, we associate the variation in the magnitude and position of EfS1 (i.e., of TSS1 in Fig. 1B) with the variation along α of the 1Ag and 1Bu diabatic energies and their 1Ag/1Bu coupling.
S1 isomerization barrier determines an increased FQY in the Arch variants
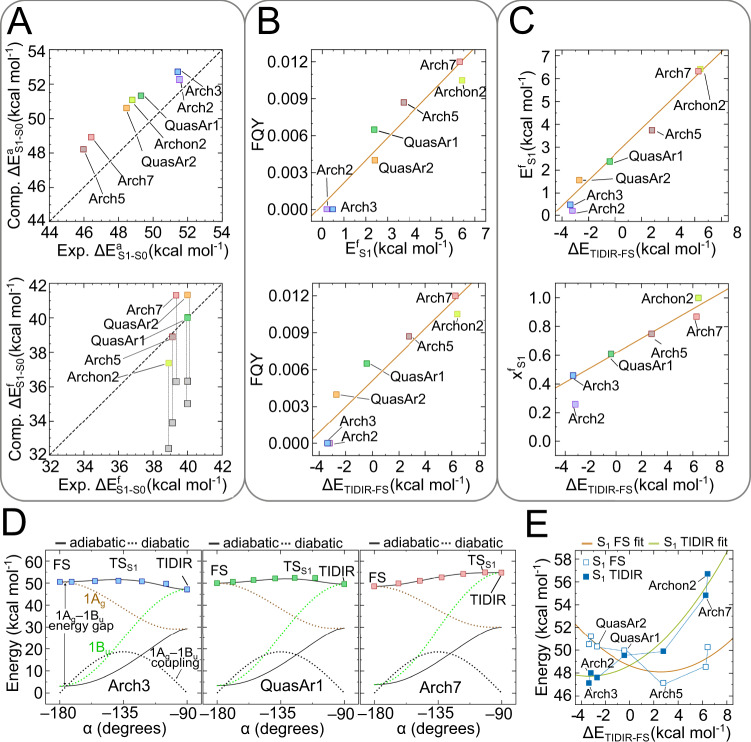
We now show that the isomerization mechanism above provides the basis for understanding the FQY variations along the Arch-set. To do so, we first demonstrate the existence of a correlation between computed EfS1 and observed FQY values and then rationalize it based on FS and TIDIR charge distributions (or 1Ag and 1Bu character) calculated by constructing the MCQM models of all variants and using them to map the progression along α. The model accuracy is documented in Fig. 3A where we compare, after multi-state perturbative correction, the computed and observed trends of absorption (ΔEaS1-S0, top) and emission (ΔEfS1-S0, bottom) vertical excitation energies. The analysis of the results demonstrates that, in all variants, FS and TIDIR feature the same distinct charge distributions (Supplementary Figs. 4 and 7).
Fig. 3. Relationship between computed S1 isomerization properties and observed FQY along the Arch-set.
A Comparison between computed and observed excitation energies associated with light absorption at the ground state equilibrium structure (top) and light emission from the fluorescent state (bottom). Vertical excitation energy associated to emission features a correction of 4.5 kcal mol−1 to account for kinetic energy derived from ref. 24. Gray and colored squares represent the emissions before and after correction, respectively. B Relationship between S1 isomerization barrier and FQY (top) and between S1 isomerization energy and FQY (bottom). Linear fits are given as orange lines. C Relationship between S1 isomerization barrier and isomerization energy (top) and between S1 isomerization transition state position and isomerization energy for three Arch3 variants. Linear fits are given as orange lines. D One-dimensional model for the relationship between barrier and isomerization energy. E Non-linear relationship between the FS and TIDIR S1 excitation energies with respect to S0 FC and the isomerization energy. Source data are provided in the Source Data file (d).
The bottom panel of Fig. 3B shows that computed EfS1 and observed FQY variations are directly proportional. If one assumes that the Hammond-Leffler postulate35,36 is valid for an S1 isomerization, EfS1 and ΔETIDIR-FS (i.e., the reaction endothermicity or, simply, isomerization energy in Fig. 1B) must also correlate. This leads to the conjecture that ΔETIDIR-FS is proportional to FQY. The bottom panel of Fig. 3B demonstrates that such proportionality exists. In other words, a progressive stabilization of the “reactant” FS and/or destabilization of the “product” TIDIR, must lead to higher FQYs. The relationship between the computed EfS1 and ΔETIDIR-FS for the entire Arch-set can be modeled by using a basic two-state one-mode Hamiltonian that fits the computed S1 and S0 energy profiles along mode α in terms of 1Ag and 1Bu diabatic energies and a harmonic 1Ag/1Bu coupling function (Supplementary Methods 8 and Supplementary Fig. 12). The maximum along the resulting S1 energy profile represents TSS1 and, as demonstrated in Fig. 3D for Arch3, QuasAr1 and Arch7, replicates the computed MCQC S1 energy profiles. As shown in Fig. 3C the model Hamiltonian supports the existence of a proportionality between EfS1 (top panel) and the TSS1 position XfS1 (bottom panel) and ΔETIDIR-FS. We stress that this mechanistic model assumes no “a priori” relationship between diabatics energy difference and diabatic coupling, but is a simple valence-bond type description of our adiabatic S1 PES, assuming two pure resonance formulas (1Ag and 1Bu), whose weights are associated to the documented variation in positive charge distribution along the reaction path.
The ΔETIDIR-FS increases along the Arch-set may originate from either FS stabilization or a TIDIR destabilization effects (or from both effects). In Fig. 3E we show that the first effect (i.e., substantially the 0-0 excitation energy) shows a decrease until Arch5 that has the lowest excitation energy and then increase up to Archon2. In contrast, the TIDIR destabilization with respect to the same reference (i.e., the destabilization of the photoisomerization channel) increases almost monotonically indicating a higher sensitivity of FQY to mutations changing ΔETIDIR-FS rather than the difference in energy between FC and FS. The described ΔETIDIR-FS changes are confirmed by probing the light-induced dynamics of the Arch-set. To do so we employed the variants MCQC models to propagate a quantum-classical trajectory released from the FC point with zero initial nuclear velocities (Supplementary Methods 9 and Supplementary Figs. 14 and 15). As discussed in the literature, these trajectories mimic the evolution of the center of the S1 population and are useful to detect barrierless or nearly barrierless isomerization paths and validate the accuracy of computed torsional scans27,37.
Consistently with the trend in EfS1 values, the Arch3 trajectory reaches the CoIn region and decays to S0 on a sub-500 fs timescale via a C13 = C14 isomerization pointing to species that do not display significant fluorescence. In contrast, the QuasAr1 and Arch7 trajectories representing the remaining variants, orbit in the FS region for the entire 450 fs simulation time.
Molecular determinants of the isomerization barrier
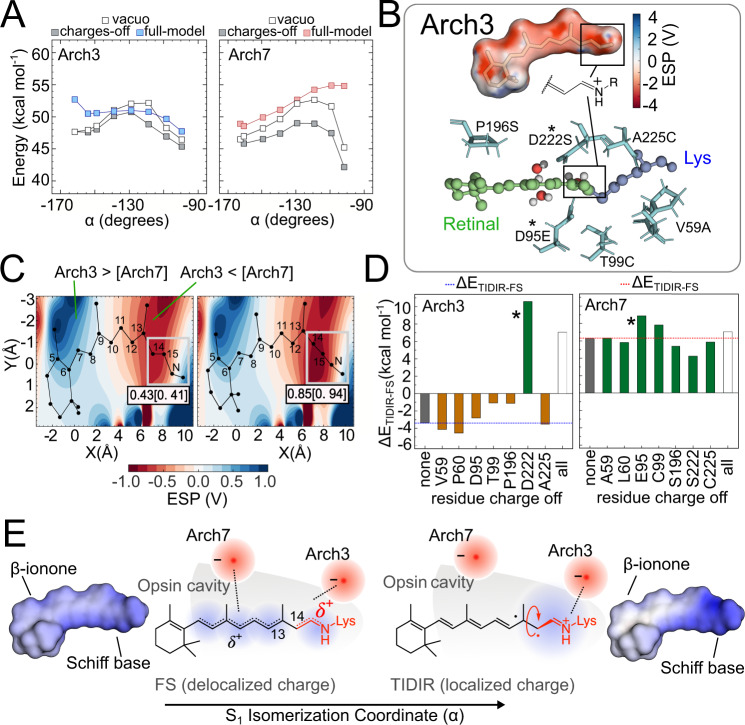
Above we have demonstrated that our MCQM models produce a ΔETIDIR-FS trend proportional to the observed FQYs for the entire Arch-set. We now focus on the limiting cases of Arch3 and Arch7 to show that the proportionality between ΔETIDIR-FS and FQY is linked to the variations in the protein (opsin) sequence. This is possible because, as seen in Fig. 2C, D, the FS and TIDIR retinal chromophores have distinct charge distributions and, therefore, ΔETIDIR-FS must be sensitive to the protein electrostatics. In other words, an opsin electrostatic potential (ESPopsin) stabilizing FS or/and destabilizing TIDIR would produce a larger ΔETIDIR-FS value and, in turn, enhance FQY. Such effect has been investigated by recomputing the S1 energy profiles along α after setting to zero the opsin charges of the MCQC models (Supplementary Methods 10 and Supplementary Figs. 15–17). It is apparent from inspection of the energy profiles in Fig. 4A, B that, in the absence of the protein electrostatics, both Arch3 and Arch7 display a sizable energy barrier along α. However, the left panel of Fig. 4A demonstrates (compare the full model with the charges-off energy profiles) that, in Arch3, the opsin charges stabilize TIDIR with respect to FS yielding a low ΔETIDIR-FS value and, consequently, a negligible EfS1. In contrast, the right panel of Fig. 4A shows that, in Arch7, the opsin charges have the opposite effect. We can conclude that in the absence of the protein electrostatics, the geometrical deformation imposed by the opsin cavity on the chromophore backbone leads to a sizable EfS1 value. Notice that the geometrical deformation is the result of both cavity steric and electrostatic effects on the chromophore isomerization coordinate and that these effects are common to Arch3 and Arch7, as well as to all members of the Arch-set (Supplementary Methods 10). This has been confirmed by recomputing the same energy profile in vacuo (i.e., in absence of the VdW interaction between protein cavity and chromophore) that still show a barrier in all cases.
Fig. 4. Origin of the S1 isomerization barrier in Arch3 and Arch7.
A Effect of the opsin charges on the isomerization energy profile for Arch3 (left) and Arch 7 (right). B Top. Arch3 opsin electrostatic potential projected on the chromophore solvent accessible surface. Bottom. The cavity amino acids distinguishing Arch3 from Arch7. The * symbol indicates the Arch3 (D222) and Arch7 (E95) chromophore counterions. C Two-dimensional plots showing the Arch3-Arch7 electrostatic potential difference at FS (left) and TIDIR (right). The total charge of the framed Schiff-base moiety is also given. D Variation in the Arch3 (left) and Arch7 (right) isomerization energy value after zeroing the residue charge of the cavity residues in panel B bottom. Reference values are shown as gray bars. Negative and positive isomerization energies are shown in orange and green, respectively. Finally, the effect of zeroing the charges of all residues simultaneously is also given as an open bar. E Point charge model for the delocalization-confinement mechanism. The Arch3-Arch7 ESP difference of C can be modeled in terms of a re-locating effective negative charge from the Schiff base region to a cavity region displaced towards the β-ionone ring of the chromophore. The increase in distance between the effective charge and the confined positive charge of TIDIR explains its electrostatic destabilization in Arch7 with respect to Arch3. The computed chromophore electrostatic potential projected on the chromophore solvent accessible surface is given at the left and right of the figure to support the schematic representation of a delocalized and confined charge given at the center. Source data are provided in the Source Data file (a, c, d).
The described electrostatic stabilization of TIDIR relative to FS in Arch3, and the consequent disappearance of the barrier, has been rationalized by mapping the ESPopsin on the chromophore solvent accessible surface (Fig. 4B, top). The map shows a prevalent negative ESPopsin value in the area surrounding the Schiff base moiety whose effect along the isomerization coordinate is described by plotting the difference (ΔESPopsin) between the Arch3 and Arch7 electrostatics. In fact, the ΔESPopsin mapped along a cross-section roughly parallel to the FS (Fig. 4C, left) and TIDIR (Fig. 4C, right) chromophore backbones, demonstrates that the opsin of Arch3 preferentially stabilizes the positive charge fully confined on the Schiff base moiety of TIDIR while the opsin of Arch7 better stabilizes the delocalized charge spread towards the β-ionone of FS. These effects can be also discussed using the model Hamiltonian presented above where the 1Bu diabatic dominates the FS while the 1Ag diabatic dominates the TIDIR. Since a Schiff base confined charge is a characteristic of 1Ag, the Arch3 electrostatics must stabilize the covalent/diradical 1Ag character at TIDIR while the Arch7 electrostatics destabilizes it consistently with the diabatic model of Fig. 3D. It is possible to demonstrate that this effect remains valid when one uses a more realistic two-state two-mode Hamiltonian model generating two-dimensional adiabatic energy surfaces function along both BLA and α (Supplementary Methods 8).
Role of the main counterion in the barrier generation or suppression
At this point we propose an atomistic mechanism for the Arch3 barrier suppression. The differences in the ESPopsin of Arch3 and Arch7 is a product of the protein sequence variation and, more specifically, from the seven residue replacements displayed at the bottom of Fig. 4B. In our MCQC models, the ESPopsin is produced by the point charges centered on the atoms of each protein residue (Supplementary Methods 2). Therefore, the ΔESPopsin of Fig. 4C must reflect the difference in point charges before and after the residue replacements. The effect of such difference has been investigated by recomputing ΔETIDIR-FS after setting the point charges of residues 59, 60, 95, 99, 196, 222, 225 as a group or individually. The results in Fig. 4D show that when setting to zero the charges of all residues, the ΔETIDIR-FS of Arch3 and Arch7 become similar. More specifically and consistently with the results in Fig. 4A, B, the Arch3 point charges must cause a ca. 9 kcal mol−1 decrease in ΔETIDIR-FS while the corresponding Arch7 residues do not seem to affect the original ca. 6 kcal mol−1 ΔETIDIR-FS value. From the analysis of the individual residues, it is apparent that the counterion at position 222 dominates the Arch3 electrostatics. Indeed, during the Arch3 to Arch7 transition the counterion is moved from position 222 to position 95 while the position 222 is taken by an uncharged cysteine. In conclusion, the counterion relocation appears to be the main mechanism for the ΔETIDIR-FS modulation. This is not a general mechanism. In fact, other members of the Arch-set all have the counterion in position 222 (Supplementary Methods 1) but display a regularly increasing ΔETIDIR-FS value. In these cases, the effect of the residue replacements must cause a ESPopsin change that partially screen the counterion effect found in Arch3, which may be interpreted in terms of a “virtual relocation” of the counterion as explained below.
We propose that this relocation and “diffusion” of the negatively charged counterion (a virtual counterion), coupled with a delocalization-then-confinement mechanism of the positive charge of the chromophore, explains the regular change in isomerization barriers. In brighter Arch-3 variants (Arch-5 and Arch-7) the virtual counterion is increasingly distant and more diffuse from the Schiff base moiety. In these variants, at α = 0° the chromophore positive charge is delocalized, and its centroid is close to the counterion, leading to a stabilization of FS. As soon as α progresses and approaches a 90° twist, the confinement of the chromophore charge on the Schiff base moiety gradually increases, causing its centroid to drift away from the virtual counterion, inevitably determining a de-stabilization of TIDIR.
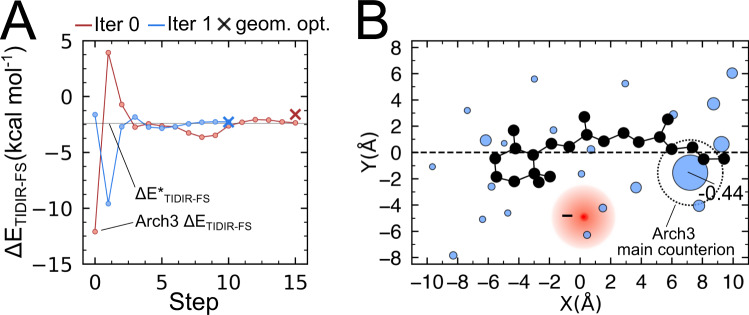
To support this conclusion, we developed a basic model that allows to compute (i.e., optimize) the protein charge distribution inducing a specific ΔETIDIR-FS value. This is done by allowing the relocation and fragmentation (i.e., diffusion) of the negative charge of the main counterion to other cavity residue positions, to mimic what observed in Fig. 4E. This can be achieved by defining a scalar function of the cavity residue charge vector (q) which returns ΔETIDIR-FS. Such ΔETIDIR-FS(q) function is differentiated numerically to study how ΔETIDIR-FS responds to q. As detailed in Supplementary Methods 11, the problem of determining q can be formulated as a constrained optimization. In Fig. 5, we show how the optimization modifies the charge distribution of Arch3 to reproduce the ΔETIDIR-FS value computed for Arch7 at the zeroth-order level. The resulting q shows that, for Arch3 to reproduce Arch7 excited state properties, a relocation of ca. 50% of D222 negative charge to other cavity residues is necessary, thus supporting the conclusion that a relocation and diffusion of the counterion is indeed a determinant of the TIDIR destabilization in the Arch-set.
Fig. 5. Predicted change of counterion charge distribution of Arch3 QM/MM model to yield the Arch7 ΔETIDIR-FS value.
A ΔETIDIR-FS (SA2-CASSCF/AMBER level) change along the optimization steps leading from the Arch3 to the Arch7 value. The optimization is driven by the target ΔETIDIR-FS values (ΔE*TIDIR-FS, indicated by a thin horizontal line) and corresponds to the minimization of the square of the scalar function ΔETIDIR-FS(q) − ΔE*TIDIR-FS (i.e., ΔΔETIDIR-FS(q)) as a function of the cavity residue charge vector q. Each iteration of the algorithm (two full iterations are reported in the panel) is divided in two parts; (i) first q is optimized at fixed FS and TIDIR geometries via conjugated-gradient optimization such that ΔETIDIR-FS(q) = ΔE*TIDIR-FS and then (ii) the FS and TIDIR QM/MM model geometries are relaxed, and ΔETIDIR-FS is recomputed. If the difference between the ΔETIDIR-FS calculated after part ii and ΔE*TIDIR-FS is above a selected threshold, i-ii are repeated. The circles indicate the steps of part i, and the crosses indicate the results of geometrical relaxation of part ii. B Final charge distribution obtained after convergence of the optimization above. Two-dimensional representation of the fraction of negative charges residing in the cavity residues is proportional to the radius of the blue circles (the main counterion D222 final charge is indicated). The barycenter of the corresponding negative charge is shown as a red circle. The original localized Arch3 negative charge is indicated by the large open circle centered at the original counterion position Source data are provided in the Source Data file (a, b).
Conclusions
The engineering of efficient, rhodopsin-based action-potential reporters (also called Genetically Encodable Voltage Indicators or GEVIs in the literature) is a challenge currently addressed by experimentally investigating the fluorescence and voltage sensitivity mechanisms15 and by tuning the protein fluorescence via several rounds of random mutagenesis and/or directed evolution. Above we have investigated the increase in fluorescence intensity in a set of Arch3 variants using MCQC models with second order perturbative corrections. The key results are: (i) the FQY is primarily determined by the competition between S1 emission and isomerization extending the mechanism reported for DRONPA220 to retinal proteins, (ii) the isomerization rate is governed by the stability of the rather exotic TIDIR intermediate with respect to the fluorescent state, (iii) the TIDIR stability is modulated by the electrostatics of the protein that increasingly offset the S1 isomerization energy barrier when going from Arch7 to Arch3.
The atomistic mechanism for the TIDIR stabilization appears to be a consequence of the cavity electrostatics on the distinct charge distributions of the FS and TIDIR chromophores. As schematically (top schemes) and computationally (bottom plots) displayed in Fig. 4E, the chromophore positive charge is largely delocalized in the FS but confined on the C14-C15-N fragment in the TIDIR. In this situation the described change in electrostatics from Arch3 to Arch7 leads to a reduced stabilization of the Schiff base confined with respect to the delocalized charge. In simple terms, the effect of the amino acid (and atomic point charges) replacement resulting in the Arch3 to Arch7 progressive TIDIR destabilizing along the Arch-set, can be interpreted as the gradual relocation of a negative charge from the Schiff base region to a region closer to the β-ionone ring.
The presented mechanism has both methodological and biological implications. The first is related to the fact that TIDIR and FS, being energy minima on the S1 potential energy surface, are computationally fast to locate. It is thus possible to envision the development of a tool for the in silico selection of highly fluorescent Arch3 variants. Such tool would be based on the automated38 construction of MCQC models and the calculation of the corresponding ΔETIDIR-FS value to be maximized via in silico mutational experiments. The biological implication is instead related to the hypothesis that the changes in sequence, and thus electrostatics, leading to a negligible Arch3 fluorescence could have occurred also in nature through a natural selection process aimed at increasing the protein photoisomerization rate and improve the protein function. We cannot exclude that the same mechanism plays a role in other microbial rhodopsin evolution. On the other hand, an inverse natural selection process leading to the suppression of the photoisomerization, and maximization of the fluorescence output may have generated the recently discovered Neorhodopsin from Rhizoclosmatium globosum39 that displays an intense fluorescence with a ca. 0.2 FQY and therefore much closer to that of DRONPA2 and other optimized green fluorescent protein variants.
Methods
The three-dimensional structures of the Arch-set proteins were obtained either from the corresponding crystallographic structures deposited in the Protein Data Bank (Arch2 and Arch3 with PDB ID:3WQJ and 6GUX, respectively) or via the comparative model (the Arch3 variants) approach described in Supplementary Methods 1. The corresponding QM/MM models were constructed from the obtained three-dimensional structures according to the a-ARM based protocol38 discussed in Supplementary Methods 2 with the QM and MM subsystems interacting via an electrostatic embedding scheme. The retinal chromophore atoms, the chromophore-bound lysine side chain atoms starting from the N-terminal to the Cε and the hydrogen link atom (HLA) are included in the QM subsystem and treated at the multiconfigurational (CASSCF) level, while the rest of the atoms of the model are treated at the MM level using the AMBER94 force field40.
The ground state optimized geometries are obtained via geometry optimization at the single-state CASSCF(12,12)/6-31G*/AMBER level of theory. Further refinement was performed introducing a perturbative geometrical correction to produce XMS-CASPT2/SA3-CASSCF(12,12)/ANO-L-vDZP/AMBER geometries (Supplementary Methods 4 and 5). Both the CASSCF and XMS-CASPT2 models were then used to compute reaction paths along the isomerization coordinate via a relaxed scan taking, as the reacting coordinate, the twisting of the reactive double bond. The CASSCF geometries were also employed to probe the excited state dynamics of three models via non adiabatic deterministic surface-hop trajectories propagated from the Franck-Condon (FC) point on S1 with zero initial velocities (FC trajectories) at the SA2-CASSCF(12,12)/6-31G*/AMBER level using the Tully algorithm41. All the QM/MM calculations were performed using the [Open]Molcas v19.11/TINKER and MOLCAS v8.1/TINKER packages42–44.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
The research has been partially supported by the following grants: NSF CHE-CLP-1710191, NIH GM126627-01, USIAS 2015, the Ohio Supercomputer Center, the MIUR (Ministero dell’Istruzione, dell’Università e della Ricerca) for a “Dipartimento di Eccellenza 2018-2022” and the Fondazione Banca d’Italia to M.O. The MIUR is also acknowledged for a Rita Levi Montalcini grant to D.P. N.F. and M.H.-R. acknowledge the financial support by Agence Nationale de la Recherche (project ULTRArchea, grant ANR-21-CE11-0029-03). L.B. and M.O. acknowledge partial support from European Union, Next Generation EU, MIUR Italia Domani Progetto mRNA Spoke 6 del “National Center for Gene Therapy and Drugs based on RNA Technology”. CUP di progetto B63C22000610006. The authors are grateful to Xuchun Yang and María del Carmen Marín for fruitful discussion.
Source data
Author contributions
Conceptualization and Project administration: M.O., Methodology: L.P.G., E.M., L.B., D.P., L.D.V., N.F., M.H.R., M.F., D.K., A.B.G., Investigation and Visualization: L.B., E.M., M.O., D.K., Funding acquisition: M.O., D.P., Supervision: D.P., L.D.V., M.O., Writing and Reviewing: M.O., L.B.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Cartesian coordinates of the FC, FS, CoIn and TIDIR geometries of the QM/MM models calculated at the SA2-CASSCF(12,12)/6-31 G*/AMBER level of theory for the models of the Arch set are provided as Supplementary Data 1.
Cartesian coordinates of the FC, FS and TIDIR geometries of the QM/MM models calculated at the XMS-CASPT2/SA3-CASSCF(12,12)/ANO-L-vDZP/AMBER level of theory for the models of the Arch set are provided as Supplementary Data 2. Source data is provided with this paper. Source data are provided with this paper.
Code availability
All the calculations discussed in this work are based on the MOLCAS/TINKER interface.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-33993-4.
References
- 1.Xu Y, Zou P, Cohen AE. Voltage imaging with genetically encoded indicators. Curr. Opin. Chem. Biol. 2017;39:1–10. doi: 10.1016/j.cbpa.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl Acad. Sci. 2005;102:17816–17821. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow BY, et al. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saint Clair EC, saint, Ogren JI, Mamaev S, Kralj JM, Rothschild KJ. Conformational changes in the archaerhodopsin-3 proton pump: detection of conserved strongly hydrogen bonded water networks. J. Biol. Phys. 2012;38:153–168. doi: 10.1007/s10867-011-9246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saint Clair EC, et al. Near-IR resonance Raman spectroscopy of archaerhodopsin 3: effects of transmembrane potential. J. Phys. Chem. B. 2012;116:14592–14601. doi: 10.1021/jp309996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patterson GH, Knobel SM, Sharif WD, Kain SR, Piston DW. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997;73:2782–2790. doi: 10.1016/S0006-3495(97)78307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim R, Tsien RY. Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr. Biol. 1996;6:178–182. doi: 10.1016/s0960-9822(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Hochbaum D, Harrison DJ, Cohen AE, Campbell RE. A comprehensive live cell screening approach for developing improved microbial rhodopsin-based voltage biosensors. Biophys. J. 2014;106:415a. [Google Scholar]
- 9.Flytzanis NC, et al. Archaerhodopsin variants with enhanced voltage-sensitive fluorescence in mammalian and Caenorhabditis elegans neurons. Nat. Commun. 2014;5:1–9. doi: 10.1038/ncomms5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kralj JM, Douglass AD, Hochbaum DR, Maclaurin D, Cohen AE. Optical recording of action potentials in mammalian neurons using a microbial rhodopsin. Nat. Methods. 2012;9:90–95. doi: 10.1038/nmeth.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maclaurin D, Venkatachalam V, Lee H, Cohen AE. Mechanism of voltage-sensitive fluorescence in a microbial rhodopsin. Proc. Natl Acad. Sci. 2013;110:5939–5944. doi: 10.1073/pnas.1215595110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hochbaum DR, et al. All-optical electrophysiology in mammalian neurons using engineered microbial rhodopsins. Nat. Methods. 2014;11:825–833. doi: 10.1038/nmeth.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McIsaac RS, et al. Directed evolution of a far-red fluorescent rhodopsin. Proc. Natl Acad. Sci. 2014;111:13034–13039. doi: 10.1073/pnas.1413987111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piatkevich KD, et al. A robotic multidimensional directed evolution approach applied to fluorescent voltage reporters. Nat. Chem. Biol. 2018;14:352–360. doi: 10.1038/s41589-018-0004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silapetere A, et al. QuasAr Odyssey: the origin of fluorescence and its voltage sensitivity in microbial rhodopsins. Nat. Commun. 2022;13:1–20. doi: 10.1038/s41467-022-33084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dempsey GT, et al. Cardiotoxicity screening with simultaneous optogenetic pacing, voltage imaging and calcium imaging. J. Pharm. Toxicol. Methods. 2016;81:240–250. doi: 10.1016/j.vascn.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Azimi Hashemi N, et al. Rhodopsin-based voltage imaging tools for use in muscles and neurons of Caenorhabditis elegans. Proc. Natl Acad. Sci. 2019;116:17051–17060. doi: 10.1073/pnas.1902443116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou S, et al. Genetically targeted all-optical electrophysiology with a transgenic cre-dependent optopatch mouse. J. Neurosci. 2016;36:11059–11073. doi: 10.1523/JNEUROSCI.1582-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ernst OP, et al. Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem. Rev. 2014;114:126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romei MG, Lin C-Y, Mathews II, Boxer SG. Electrostatic control of photoisomerization pathways in proteins. Science. 2020;367:76–79. doi: 10.1126/science.aax1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granovsky AA. Extended multi-configuration quasi-degenerate perturbation theory: The new approach to multi-state multi-reference perturbation theory. J. Chem. Phys. 2011;134:214113. doi: 10.1063/1.3596699. [DOI] [PubMed] [Google Scholar]
- 22.Roos BO, Taylor PR, Sigbahn PEM. A complete active space SCF method (CASSCF) using a density matrix formulated super-CI approach. Chem. Phys. 1980;48:157–173. [Google Scholar]
- 23.Battaglia S, Lindh R. Extended Dynamically Weighted CASPT2: The Best of Two Worlds. J. Chem. Theory Comput. 2020;16:1555–1567. doi: 10.1021/acs.jctc.9b01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.del Carmen Marín M, et al. Fluorescence enhancement of a microbial rhodopsin via electronic reprogramming. J. Am. Chem. Soc. 2018;141:262–271. doi: 10.1021/jacs.8b09311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schapiro I, et al. The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects. J. Am. Chem. Soc. 2011;133:3354–3364. doi: 10.1021/ja1056196. [DOI] [PubMed] [Google Scholar]
- 26.Schnedermann C, et al. Evidence for a vibrational phase-dependent isotope effect on the photochemistry of vision. Nat. Chem. 2018;10:449–455. doi: 10.1038/s41557-018-0014-y. [DOI] [PubMed] [Google Scholar]
- 27.Frutos LM, Andruniów T, Santoro F, Ferré N, Olivucci M. Tracking the excited-state time evolution of the visual pigment with multiconfigurational quantum chemistry. Proc. Natl Acad. Sci. 2007;104:7764–7769. doi: 10.1073/pnas.0701732104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atchity GJ, Xantheas SS, Ruedenberg K. Potential energy surfaces near intersections. J. Chem. Phys. 1991;95:1862–1876. [Google Scholar]
- 29.Laricheva EN, et al. Origin of fluorescence in 11-cis locked bovine rhodopsin. J. Chem. Theory Comput. 2012;8:2559–2563. doi: 10.1021/ct3002514. [DOI] [PubMed] [Google Scholar]
- 30.Penzkofer A, Silapetere A, Hegemann P. Photocycle dynamics of the Archaerhodopsin 3 based fluorescent voltage sensor Archon2. J. Photochem Photobio. B. 2021;225:112331. doi: 10.1016/j.jphotobiol.2021.112331. [DOI] [PubMed] [Google Scholar]
- 31.Penzkofer A, Silapetere A, Hegemann P. Photocycle dynamics of the Archaerhodopsin 3 based fluorescent voltage sensor QuasAr1. Int J. Mol. Sci. 2019;21:160. doi: 10.3390/ijms21010160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer I. Bond orders and valences from ab initio wave functions. Int J. Quantum Chem. 1986;29:477–483. [Google Scholar]
- 33.Gozem S, Schapiro I, Ferré N, Olivucci M. The molecular mechanism of thermal noise in rod photoreceptors. Science. 2012;337:1225–1228. doi: 10.1126/science.1220461. [DOI] [PubMed] [Google Scholar]
- 34.Bonačić-Koutecký, V., Koutecký, J. & Michl, J. Neutral and charged biradicals, zwitterions, funnels in S1, and proton translocation: their role in photochemistry, photophysics, and vision. Angew. Chem. Int. Ed. Engl.26, 170–189 (1987).
- 35.Hammond GS. A correlation of reaction rates. J. Am. Chem. Soc. 1955;77:334–338. [Google Scholar]
- 36.Leffler JE. Parameters for the description of transition states. Science (1979) 1953;117:340–341. doi: 10.1126/science.117.3039.340. [DOI] [PubMed] [Google Scholar]
- 37.Manathunga M, et al. Probing the photodynamics of rhodopsins with reduced retinal chromophores. J. Chem. Theory Comput. 2016;12:839–850. doi: 10.1021/acs.jctc.5b00945. [DOI] [PubMed] [Google Scholar]
- 38.Pedraza-González L, De Vico L, del Carmen Marín M, Fanelli F, Olivucci M. a-ARM: automatic rhodopsin modeling with chromophore cavity generation, ionization state selection, and external counterion placement. J. Chem. Theory Comput. 2019;15:3134–3152. doi: 10.1021/acs.jctc.9b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broser M, et al. NeoR, a near-infrared absorbing rhodopsin. Nat. Commun. 2020;11:1–10. doi: 10.1038/s41467-020-19375-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Case DA, et al. The Amber biomolecular simulation programs. J. Comput Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tully JC. Molecular dynamics with electronic transitions. J. Chem. Phys. 1990;93:1061–1071. [Google Scholar]
- 42.Fdez. Galván I, et al. OpenMolcas: from source code to insight. J. Chem. Theory Comput. 2019;15:5925–5964. doi: 10.1021/acs.jctc.9b00532. [DOI] [PubMed] [Google Scholar]
- 43.Aquilante, F. et al. Molcas 8: New capabilities for multiconfigurational quantum chemical calculations across the periodic table. J Comput Chem. 37, 506–41 (2016). [DOI] [PubMed]
- 44.Ponder, J. W. et al. TINKER: Software tools for molecular design, vol. 3 (Washington University School of Medicine, Saint Louis, MO, 2004).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Cartesian coordinates of the FC, FS, CoIn and TIDIR geometries of the QM/MM models calculated at the SA2-CASSCF(12,12)/6-31 G*/AMBER level of theory for the models of the Arch set are provided as Supplementary Data 1.
Cartesian coordinates of the FC, FS and TIDIR geometries of the QM/MM models calculated at the XMS-CASPT2/SA3-CASSCF(12,12)/ANO-L-vDZP/AMBER level of theory for the models of the Arch set are provided as Supplementary Data 2. Source data is provided with this paper. Source data are provided with this paper.
All the calculations discussed in this work are based on the MOLCAS/TINKER interface.