Abstract
In Thailand, chronic kidney disease (CKD) screening was reported in 2009 with an overall prevalence of 17.5% and the highest at 22.2% in the northeastern region. This study aimed to find out CKD prevalence of the Kidney Disease Improving Global Outcomes criteria and their related risk factors in the rural community. A population-based study was conducted in the rural sub-districts of northeastern Thailand. Data of socio-demographic status, lifestyle, underlying diseases, blood pressure, and body mass index were recorded. Blood and urine analysis was conducted along with ultrasonography of kidneys. Specimen collection and analyses were repeated after 3 months, and the factors associated with CKD were studied by logistic regression analysis. A total of 2205 participants with a mean age of 57.8 ± 11.7 years and female predominance (66.7%) completed the study. The prevalence of CKD was 26.8%, i.e., stages 1 (7.3%); stage 2 (9.0%); stage 3a (6.0%); stage 3b (2.8%); stage 4 (1.4%); and stage 5 (0.3%). Hypertension, diabetes mellitus, and renal stones were the major underlying diseases. Only 3.5% of the participants were aware of having CKD. An increase in age, male, unemployment, current smoking, diabetes, hypertension, underweight, anemia, hyperuricemia, and leukocytosis were significantly associated factors with the disease. The study revealed that CKD has developed as a significant public health problem in rural northeastern Thailand and one out of every four people has CKD. Therefore, early interventions are essential for the proper management and prevention of CKD.
Subject terms: Diseases, Health care, Nephrology, Risk factors
Introduction
Chronic kidney disease (CKD) is causing a huge burden in terms of occurrence, treatment cost, morbidity, and mortality throughout the world each year1,2. Global estimated death and disability-adjusted life years from the disease in 2017 were 1.2 million and 35.8 million3. Diabetes mellitus (DM) and hypertension (HT) are the main causes of CKD4,5. Moreover, the association of factors such as race, sex, age, diet, socioeconomic status, family history, smoking, nephrotoxin, and others with CKD has made the situation more challenging6. A better knowledge of epidemiology is, therefore, inevitable to discourse on its multi-dimensional effects, especially in low and middle-income countries including Thailand.
Over the past two decades, epidemiological studies have revealed a soaring national prevalence of CKD ranging from 4.6 to 17.5% in Thai populations, more severely affecting the inhabitants of the rural and low economic northeastern region7–12. The observed discrepancy in the prevalence was due to CKD diagnostic criteria, estimated glomerular filtration rate (eGFR) equation, study design, and population. For example, a Thai Screening and Early Evaluation of Kidney Disease (SEEK) study reported 17.5% CKD occurrence among national adult populations with 22.2% prevalence in Northeast Thailand using criteria of reduced eGFR calculated by the modification of diet in renal disease (MDRD) formula, hematuria, and albuminuria10. On the other hand, a recently published study revealed a lower nationwide CKD frequency of 8.7% based on the eGFR from the Chronic Kidney Disease–Epidemiology Collaboration (CKD-EPI) equation and proteinuria, however, the highest prevalence of CKD was also observed among Northeastern adults7. As in the majority of population-based CKD screening, these findings were based on the measurement of one-time eGFR, hematuria, and albuminuria or proteinuria. The reports on CKD burden containing additional diagnosis criteria such as kidney ultrasonography as well as the confirmation of reduced eGFR and other kidney function markers after a subsequent period are scarce. Therefore, this study was designed to investigate CKD prevalence among rural northeastern Thai populations using the recent Kidney Disease Improving Global Outcomes (KDIGO) criteria including detection of chronic renal structural abnormalities by ultrasound, and to enumerate predisposing factors as some risk factors that could be modified and may prevent or retard the progression of CKD to end-stage kidney disease (ESKD).
Results
Characteristics of all participants
A total of 2789 participants from 24 villages participated in the study and 2205 completed the entire screening procedure, accounting for a 79% response rate. About 19% of the participants dropped out between the interview and the first round of sample collection. A comparison between the total participants, those who completed, and dropped out of the study is shown in Table S1 in which the distribution of proportions of age, sex, education, occupation, and income between the total participants and the 2205 participants completing follow-up were similar, however, the significant differences between the participants completing follow-up and the 584 drop-out participants were revealed in such parameters that the dropped subjects were younger (53.2 vs. 57.8 years), more males (42.1% vs. 33.3%), less in farming occupation (42.8% vs. 63.5%), and had more highly educated degree and income. An average age of the final enrolled participants was 57.8 ± 11.7 years with female predominance (66.7%). A majority of the participants were educated up to primary school (79.0%), agriculture was their main occupation (63.5%), and most (90.6%) had monthly incomes of less than 10,000 Baht. The participants were underweight (4.9%), normal weight (26.6%), overweight (18.7%) with class I obesity (37.1%), and class II obesity (12.7%). The prevalence of CKD (26.8%), DM (21.0%), and HT (31.3% in which 41.7% of them had accompanying DM) was rather high. Additionally, the proportions of hypercholesterolemia (45.5%), hyperuricemia (21.1%), and anemia (32.0%) were also unexpectedly excessive.
Usage of non-steroidal anti-inflammatory drugs (NSAIDs) and analgesic drugs was common (39.0%), and 46.9% of these subjects were currently taking some of the drugs. Nearly 70% of taken medication was self-accessed and illegally obtained from peddlers or at grocery shops in the villages. The amount of salt intake was two-fold the WHO recommendation. The current use of agricultural herbicides was 28.2%. The participants with current smoking and drinking habits were 12.2% and 25.0%.
Awareness of CKD and related diseases
Although CKD and its related diseases were discovered as a large burden in this rural region, awareness of these diseases was quite low. Only 21 of 592 CKD cases (3.5%) had perceived their renal dysfunction and structural abnormality. In other words, 96.5% of them were unaware of their CKD. Considerable percentages of unrecognized HT (23.5%), DM (22.7%), and renal stones (69.1%) were discovered.
Comparisons between the CKD- and non-CKD groups
Baseline characteristics of the participants are described in Table 1, which shows that 592 cases (26.8%) were identified to have CKD. Education, occupation, and monthly income were statistically different between the participants with and without CKD. A higher percentage of participants with primary school, unemployed status, and less than 10,000 Baht per month income was revealed in the CKD group. The participants with CKD were older and male predominance, had greater proportions of;—underweight, DM, HT, renal stones, gout, cardiovascular disease (CVD) and higher total WBC count, uric acid levels and lower hemoglobin and LDL cholesterol levels compared to non-CKD participants.
Table 1.
Baseline characteristics of CKD and non-CKD patients.
| Variables | CKD (n = 592) |
Non-CKD (n = 1,613) |
P value |
|---|---|---|---|
| Age, years (mean ± SD) | 64.0 ± 11.0 | 55.5 ± 11.1 | < 0.001 |
| Sex: Male (n, %) | 234 (39.5) | 501 (31.1) | < 0.001 |
| Weight, kg (mean ± SD) | 60.4 ± 11.9 | 61.7 ± 11.0 | 0.014 |
| Height, cm (mean ± SD) | 155.6 ± 7.52 | 156.4 ± 7.34 | 0.016 |
| Body mass index, kg/m2 (mean ± SD) | 24.9 ± 4.4 | 25.2 ± 4.3 | 0.123 |
| UW/NW/OW/Obesity, (%) | 7.3/26.0/18.1/48.6 | 4.1/26.8/18.9/50.2 | 0.047 |
| Education, n (%) | < 0.001 | ||
| Did not study | 4 (0.6) | 8 (0.5) | |
| Primary school | 515 (87.0) | 1,226 (76.0) | |
| Middle school | 27 (4.6) | 148 (9.2) | |
| High school/vocational | 35 (5.9) | 157 (9.7) | |
| Diploma/high vocational | 5 (0.8) | 31 (1.9) | |
| Bachelor’s degree | 6 (1.0) | 38 (2.3) | |
| Postgraduate | 0 (0.0) | 5 (0.3) | |
| Occupation, n (%) | < 0.001 | ||
| Farmer | 347 (58.6) | 1,054 (65.3) | |
| Trader | 20 (3.4) | 69 (4.3) | |
| Jobs in factories, companies | 3 (0.5) | 38 (2.4) | |
| Government/enterprise | 6 (1.0) | 27 (1.7) | |
| Student | 0 (0.0) | 8 (0.5) | |
| Other | 49 (8.3) | 199 (12.3) | |
| Unemployed | 167 (28.2) | 218 (13.5) | |
| Monthly income (Baht), n (%) | 0.001 | ||
| < 10,000 | 556 (93.9) | 1,442 (89.4) | |
| > 10,000 | 36 (6.1) | 171 (10.6) | |
|
Underlying diseases HT (overall), n (%) |
307 (51.9) | 383 (23.7) | < 0.001 |
| HT (without DM), n (%) | 147 (24.8) | 255 (15.8) | < 0.001 |
| HT (with DM), n (%) | 160 (27.0) | 128 (7.9) | < 0.001 |
| DM, n (%) | 212 (35.8) | 250 (15.5) | < 0.001 |
| Renal stone, n (%) | 128 (21.6) | 21 (1.3) | < 0.001 |
| Gout, n (%) | 24 (4.1) | 31 (1.9) | 0.004 |
| CVD, n (%) | 15 (2.5) | 16 (1.0) | 0.006 |
| SLE, n (%) | 4 (0.7) | 12 (0.7) | 1.000 |
| Serum creatinine (mg/dL) (mean ± SD) | 1.1 ± 0.7 | 0.8 ± 0.2 | < 0.001 |
| eGFR, ml/min/1.73 m2 (mean ± SD) | 70.4 ± 25.4 | 91.9 ± 14.5 | < 0.001 |
| UACR, mg/g [median (IQR)] | 26.7 (6.5–109.6) | 5.8 (3.6–10.4) | < 0.001 |
| Hemoglobin, g/dL (mean ± SD) | 12.3 ± 1.7 | 12.9 ± 1.3 | < 0.001 |
| Anemia, n (%) | 278 (47.0) | 426 (26.4) | < 0.001 |
| Female | 184/358 (51.4) | 306/1,112 (27.5) | < 0.001 |
| Male | 94/234 (40.2) | 120/501 (24.0) | < 0.001 |
| Leukocytosis, n (%) | 90 (15.2) | 157 (9.7) | < 0.001 |
| Serum WBC, cells/ml (mean ± SD) | 7,987 ± 2,189 | 7,369 ± 1,886 | < 0.001 |
| Hyperuricemia, n (%) | 230 (38.9) | 236 (14.6) | < 0.001 |
| Uric acid, mg/dL (mean ± SD) | 6.1 ± 1.7 | 5.2 ± 1.3 | < 0.001 |
| Hyperlipidemia, n (%) | 226 (38.2) | 778 (48.2) | < 0.001 |
| LDL cholesterol, mg/dL (mean ± SD) | 122.0 ± 39.6 | 129.9 ± 37.9 | < 0.001 |
CKD chronic kidney disease; UW underweight; NW normal weight; OW overweight; HT hypertension; DM diabetes mellitus; CVD cardiovascular disease; SLE systemic lupus erythematosus; eGFR estimated glomerular filtration rate; UACR urine albumin creatinine ratio; WBC white blood cell; LDL low-density lipoprotein; SD standard deviation.
Regarding the association of CKD with behaviors, smoking habits, NSAID and analgesic use, and alcohol consumption were significantly different between the CKD and non-CKD groups. Daily water intake was significantly lower in the participants with CKD than without CKD. No significant differences were found for the variables such as usage of herbicide, herbs, and daily salt intake between the two groups (Table 2).
Table 2.
Possible behaviors related to chronic kidney disease.
| Variables | CKD (n = 592) |
Non-CKD (n = 1613) |
P value |
|---|---|---|---|
| Smoking habits | 0.011 | ||
| No | 427 (72.1) | 1263 (78.3) | |
| Quit | 78 (13.2) | 168 (10.4) | |
| Till now | 87 (14.7) | 182 (11.3) | |
| Total DOC and Total NSAID use, n (%) | 0.020 | ||
| Never used | 389 (65.7) | 957 (59.3) | |
| Used to take | 112 (18.9) | 344 (21.3) | |
| Still taking | 91 (15.4) | 312 (19.3) | |
| Herbal use, n (%) | 99 (16.7) | 296 (18.4) | 0.377 |
| Drinking water, mL/day (mean ± SD) | 1933 ± 932 | 2054 ± 964 | 0.009 |
| Salt intake, g/day (mean ± SD) | 4.1 ± 4.2 | 4.2 ± 4.6 | 0.709 |
| Fish sauce intake, ml/day (mean ± SD) | 9.5 ± 7.0 | 9.2 ± 8.0 | 0.425 |
|
MSG intake, g/day (mean ± SD) Fermented fish sauce intake, ml/day (mean ± SD) |
4.8 ± 9.7 9.5 ± 10.5 |
5.0 ± 5.4 8.6 ± 9.5 |
0.646 0.070 |
| Alcohol consumption, n (%) | 0.010 | ||
| Do not drink | 387 (65.4) | 1028 (63.7) | |
| Used to drink | 79 (13.3) | 160 (9.9) | |
| Continued drinking | 126 (21.3) | 425 (26.4) | |
| Exercise, n (%) | 327 (55.2) | 905 (56.1) | 0.743 |
| Frequency, times/week [median (IQR)] | 6 (3–7) | 5 (3–7) | 0.361 |
| Use of herbicide | 0.638 | ||
| Never | 381 (64.4) | 990 (61.4) | |
| Have used | 50 (8.5) | 163 (10.1) | |
| Still using | 161 (27.2) | 460 (28.5) |
CKD chronic kidney disease; DOC drug over the counter; NSAIDs non-steroidal anti-inflammatory drugs; MSG monosodium glutamate; IQR interquartile range; SD standard deviation.
Characteristics of CKD in the rural community
The distribution of eGFR and CKD cases according to sex and age group are shown in Table S2 and Figs. S1, S2. About one third of age group 60–69 years and a half of age ≥ 70 years participants had CKD and males revealed higher CKD prevalence than females for all age groups. The causes of CKD were identified in 454 of 592 cases, when DM, HT, and renal stones were the top three etiologies; 23.3% of CKD subjects (6.3% of all participants in the study) were of unknown causes. Table S3 summarizes the total cases investigated in rounds 1 and 2 of examinations and numbers of CKD cases defined by various criteria of KDIGO. Proportions of CKD according to criteria of persistent eGFR < 60 mL/min/1.73 m2 were 39.2%, albuminuria 49.0%, hematuria 17.1%, and abnormal renal ultrasound 56.3% in which parenchymatous changes and renal stones were the main findings (Table S4). Notably, 123 cases accounting for 20.8% of those CKD subjects, or 5.6% of total cases, were classified as CKD by abnormal ultrasound findings alone without any other CKD criteria.
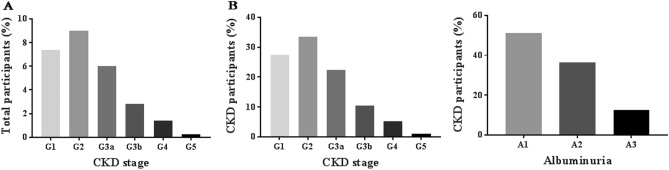
As shown in Fig. 1, the prevalence of each CKD stage according to eGFR levels assessed by CKD-EPI formula were G1; 7.3% (162 cases), G2; 9.0% (198 cases), G3a; 6.0% (133 cases), G3b; 2.8% (62 cases), G4; 1.4% (31cases), and G5; 0.3% (6 cases). Proportions of CKD subjects classified by albuminuria category were A1; 13.7% (302 cases), A2; 9.8% (215 cases) and A3; 3.4% (75 cases). Additionally, proportions of albuminuria categories in each CKD stage are shown in Fig. S3 and distributions of CKD cases in each GFR and albuminuria categories in which CKD was defined by hematuria and ultrasound criteria are illustrated in Fig. S4.
Figure 1.
(A) Prevalence of CKD classified as GFR category (G1-G5) in total participants, and (B) Distribution of CKD stages (G1-G5) and albuminuria category (A1-A3) in the CKD population. Note that the majority of participants were in the early stages of CKD.
Prevalence of CKD increased to 27.8% with stages 1 (122 cases, 5.5%); stage 2 (224 cases, 10.2%); stage 3a (139, 6.3%); stage 3b (93, 4.2%); stage 4 (30, 1.4%); and stage 5 (6 cases, 0.3%) if CKD was defined and classified by eGFR levels assessed with the MDRD formula. A comparison of CKD prevalence in the population according to eGFR levels calculated by CKD-EPI, MDRD and the reported Thai Eqs. 13 is shown in Table S5.
Factors associated with CKD
The univariate logistic regression analysis of the risk factors associated with CKD is shown in Table 3. Notably, the odds of having CKD were higher in combined HT and DM as compared to either DM or HT. Multivariate analyses to represent the adjusted ORs of risks were applied for two models namely Model 1 and Model 2. The first model used continuous variables:—SBP, BMI, plasma glucose, hemoglobin, serum uric acid, WBC count, and LDL-cholesterol, whereas the second model used categorical variables, i.e., presence or absence of being underweight, DM, hypertension, anemia, hyperuricemia, leukocytosis, and hyperlipidemia in the analyses. The variables having a significant positive association in both the models were age (OR 1.05 for every 1-year increase), DM (OR 2.18 for presence and OR 1.008 for every 1 mg/dL FPG increase), HT (OR 1.80 for appearance and OR 1.009 for every 1 mmHg SBP increase), anemia (OR 1.65 for existence and OR 0.79 for every 1 g/dL hemoglobin increase), leukocytosis (OR 1.40 for presence and OR 1.14 for every 1000-cells/mL WBC increase), and hyperuricemia (OR 2.68 for presence and OR 1.42 for every 1 mg/dL uric acid increase). Furthermore, unemployed status (OR 1.38), current smoking (OR 1.56 compared to no smoking), had significant positive associations with CKD in Model 1, and male gender (OR 1.73) and underweight (OR 1.64) in Model 2. The analysis of CKD risk factors excluding anemia and hemoglobin as variables (Table S6), and the factors associated with CKD stage 3–5 (with and without anemia and hemoglobin as variables) in the models are shown in Table S7 and Table S8. The prominent risk factors related with CKD G3-5 were age, male sex, and presence of;—DM, HT, hyperuricemia, and anemia.
Table 3.
Factors associated with chronic kidney disease.
| Factors | Crude OR (95% CI) | P value | Model 1 | Model 2 | ||
|---|---|---|---|---|---|---|
| Adjusted OR | P value | Adjusted OR | P value | |||
| Age (every 1- year increase) | 1.07 (1.06–1.09) | < 0.001 | 1.05 (1.03–1.06) | < 0.001 | 1.04 (1.03–1.06) | < 0.001 |
| Male | 1.45 (1.19–1.76) | < 0.001 | 1.26 (0.86–1.84) | 0.241 | 1.71 (1.20–2.45) | 0.003 |
| Low monthly income (< 10,000 Baht) | 1.83 (1.26–2.66) | 0.001 | 0.89 (0.58–1.38) | 0.612 | 0.88 (0.57–1.36) | 0.575 |
| Less education (none plus primary school) | 2.19 (1.67–2.86) | < 0.001 | 0.96 (0.69–1.34) | 0.804 | 0.98 (0.70–1.37) | 0.919 |
| Unemployed status | 2.51 (2.00–3.16) | < 0.001 | 1.38 (1.03–1.85) | 0.029 | 1.33 (0.999–1.78) | 0.051 |
| Smoking habits | 0.013 | |||||
| no | 1 | 1 | 1 | |||
| quit | 1.38 (1.03–1.85) | 0.030 | 0.86 (0.55–1.36) | 0.523 | 0.80 (0.51–1.26) | 0.331 |
| continues now | 1.40 (1.06–1.85) | 0.019 | 1.58 (1.02–2.44) | 0.040 | 1.45 (0.95–2.23) | 0.087 |
| NSAID use | 0.019 | |||||
| Never used | 1 | 1 | 1 | |||
| Used to take | 0.80 (0.63–1.02) | 0.074 | 0.95 (0.71–1.27) | 0.744 | 0.89 (0.67–1.18) | 0.418 |
| Still taking | 0.72 (0.55–0.93) | 0.013 | 0.80 (0.59–1.08) | 0.141 | 0.80 (0.59–1.08) | 0.143 |
| Alcohol consumption | 0.010 | |||||
| Do not drink | 1 | 1 | 1 | |||
| Used to drink | 1.31 (0.98–1.76) | 0.069 | 0.85 (0.56–1.30) | 0.463 | 0.82 (0.54–1.25) | 0.355 |
| Continuous drinking | 0.79 (0.63–0.99) | 0.044 | 0.78 (0.57–1.07) | 0.123 | 0.78 (0.57–1.08) | 0.133 |
| Drinking water (every increase of 500 ml a day) | 0.93 (0.86–0.98) | 0.009 | 0.99 (0.93–1.05) | 0.644 | 0.98 (0.92–1.04) | 0.510 |
| Underweight (BMI < 18.5 kg/m2) | 1.85 (1.24–2.76) | 0.003 | 1.64 (1.03–2.63) | 0.038 | ||
| BMI (every 1 kg/m2 increase) | 0.98 (0.96–1.005) | 0.123 | 0.99 (0.96–1.02) | 0.450 | ||
| DM | 3.04 (2.45–3.77) | < 0.001 | 2.20 (1.69–2.86) | < 0.001 | ||
| Fasting plasma glucose (every 1-mg/dL increase) | 1.007 (1.006–1.009) | < 0.001 | 1.008 (1.005–1.01) | < 0.001 | ||
| HT without DM | 2.49 (1.96–3.16) | < 0.001 | 1.80 (1.42–2.29) | < 0.001 | ||
| HT with DM | 5.39 (4.14–7.04) | < 0.001 | ||||
| SBP (every 1-mmHg increase) | 1.024 (1.019–1.030) | < 0.001 | 1.010 (1.003–1.016) | 0.004 | ||
| Anemia | 2.46 (2.02–2.99) | < 0.001 | 1.64 (1.30–2.07) | < 0.001 | ||
| Hemoglobin (every 1-g/dL increase) | 0.78 (0.73–0.83) | < 0.001 | 0.79 (0.73–0.87) | < 0.001 | ||
| Hyperuricemia | 3.71 (2.99–4.60) | < 0.001 | 2.69 (2.10–3.44) | < 0.001 | ||
| Serum uric acid (every 1-mg/dL increase) | 1.52 (1.42–1.63) | < 0.001 | 1.42 (1.30–1.54) | < 0.001 | ||
| Leukocytosis | 1.66 (1.26–2.20) | < 0.001 | 1.40 (1.001–1.96) | 0.049 | ||
| WBC count (every 1000-cells/mL increase) | 1.16 (1.11–1.22) | < 0.001 | 1.14 (1.08–1.21) | < 0.001 | ||
| Hyperlipidemia | 0.66 (0.55–0.80) | < 0.001 | 0.81 (0.65–1.01) | 0.061 | ||
| LDL-cholesterol (every 1-mg/dL increase) | 0.994 (0.992–0.997) | < 0.001 | 0.998 (0.995–1.000) | 0.097 | ||
Model 1 used continuous variables of SBP, BMI, plasma glucose, hemoglobin, serum uric acid, serum white blood cells, and LDL cholesterol. Model 2 used categorical variables as presence of underweight, DM, HT, anemia, hyperuricemia, leukocytosis, and hyperlipidemia.
NSAID non-steroidal anti-inflammatory drug; BMI body mass index; DM diabetes mellitus; HT hypertension; SBP systolic blood pressure; WBC white blood cell; LDL low-density lipoprotein; OR odds ratio; CI confidence interval.
Discussion
The current study is one of few population-based studies and the first in northeastern Thailand applying the KDIGO criteria to screen for CKD. We confirmed albuminuria, hematuria, and reduced eGFR after subsequent time, and performed ultrasonography of kidneys to obtain precise prevalence and risk factors of CKD in the study population. The study found that 26.8% of a representative cross-section population in study regions had CKD and the major etiologies were DM, HT, and renal stones. Moreover, age, male sex, unemployment, smoking, underweight, anemia, hyperuricemia, and leukocytosis were the significant risk factors for the CKD.
Although this CKD prevalence was unable to be directly compared with the earlier CKD screening studies in the Thai population7,10 because of the differences of study design, enrolled population, standard criteria used to define CKD along with the different equations for eGFR, the observed frequency of CKD in the villagers who really lived in the rural regions was very high and one explanation might be the older participants in this study (57.8 years vs. 45.2 years in Thai SEEK10 and 47 years in Aekplakorn, et al. study7). A comparison with global data in similar age subgroups and GFR values evaluated by the CKD-EPI Eq. 14, however, discovered that the CKD prevalence was quite high in that 17.9% of the participants were identified with CKD based on eGFR and/or microalbuminuria but the number reached 19.2% when the criterion of hematuria was applied in the study. About one-fifth of the CKD cases were identified by ultrasound alone in which renal stones and parenchymatous changes were the main findings (Table S4). Although uncommonly performed in an epidemiological study and its operator dependent, ultrasound is effective in early detection of CKD among the general population15.
The other fact that strengthens these results is proving the chronicity of the initially decreased eGFR (< 60 ml/min/1.73 m2). It was discovered that overdiagnosis of CKD was as low as 1.6% on subsequent analysis of eGFR after 3 months (Table S3). Contrarily, the discrepancies between the initial and repeated prevalence of albuminuria (4.7%) and hematuria (5.8%) were rather high (Table S3). The majority of CKD participants were identified in the early phase (stage 1–3). This is crucial especially when the progression of early-stage CKD to ESKD can be slowed down by avoiding the associated risk factors and proper care and management. Consequently, the analysis of risk factors was also attempted in the study.
One of the significant risk factors associated with CKD in the study population was age. The prevalence of CKD was substantially increased in the age groups of 60–69 and ≥ 70 years as in previous Thai and global reports7,10,14,16. Older age is known as a vulnerable risk for renal injury, however, this also means they have aging kidneys which may be not kidney damage and amending the CKD definition to include age specific thresholds for GFR was suggested16–19. In addition, although CKD prevalence is higher in women than men in most geographical regions, but variations exist around the globe as in the current study17. This could possibly be linked to an unhealthier lifestyle and a higher chance to contact agents related to CKD from an agricultural environment, particularly among men in this study. Another positively associated factor with CKD in the population was underweight, which may be due to the loss of muscle mass and function (sarcopenia) as reported in both non-dialysis and dialysis CKD patients but more in the latter 3 stages and aged individuals20,21. Supporting these current findings, underweight Chinese subjects were revealed to have a high risk for kidney failure22.
Current smokers had a risk for CKD compared with non-smokers as reported in previous studies23,24. Proposed mechanisms of renal damage by smoking were endothelial cell injury, mesangial proliferation, insulin resistance, and increases of oxidative stress and advanced glycation end products25. As expected, CKD was significantly associated with its traditional risk factors i.e., HT, DM, renal stones, and gout in the study population. These factors are the major challenges for CKD prevention and control in the region, especially when a swift increase in the incidence of HT and DM in Thailand had been witnessed in the past 2–3 decades26. This study also discovered a sizable number of unrecognized DM, HT and renal stones in rural communities. Likewise, a report has shown that more than 80% of people in Thailand are unaware of having DM, and HT27. Therefore, policies to screen and control DM and HT and nephrolithiasis are vital in CKD prevention.
In the present study, the magnitudes and severity of anemia were higher than an expectation from CKD complications. Although anemia is a well-known complication of CKD, some previous studies have stated that anemia by itself may be a risk factor or co-incident with CKD from other causes28,29. The current study revealed that hyperuricemia is associated with CKD which previous postulated mechanisms of renal injury were augmentation of systemic hypertension, afferent arteriopathy and activation of oxidative stress30.
There was a positive association between increasing total WBC count and risk for CKD similar to what has been reported in US adults31. This finding hints at the possibility of systemic inflammation as the risk factor for CKD. It was found that polypharmacy was common in the study population. A group of steroids and painkillers such as NSAIDs, analgesic drugs, and muscle relaxants in a plastic pouch were easily available from peddlers or at groceries (Fig. 2). There was, however, no significant association between the use of over-the-counter drugs and NSAID and CKD in the study. This may be due to the avoidance of these drugs in the high-risk group and known CKD patients as advised by their primary care physicians or nephrologists.
Figure 2.
The representative images of steroids, painkillers, and NSAIDs are illegally available. Polypharmacy is common in the Northeast region of Thailand.
The present study has some limitations. First, the frequency of an aged population was comparatively higher than in previous studies in Thailand and the study focused on rural areas of one province, therefore, the results may not be generalized nationwide. The population was to be analyzed as a study to confirm that they represented the entire northeast population of Thailand, however, selection bias occurred from the migration of villagers for jobs and study or refusal to participate in the study that could not be controlled. Secondly, a cross-sectional design of the study interpreted an association but not a causal effect between kidney damage markers and concomitant factors. The strength of this study was to demonstrate how a high CKD prevalence exists, the importance of screening, and the urgency to prevent and slow CKD progression in rural regions.
In conclusion, the results showed that CKD has developed as a significant public health problem in rural areas of Thailand such as that one out of every four has CKD. These important findings are consistent with previous Thai studies and higher due to an older age group of population and the use of different criteria for CKD screening. It is therefore recommended to exercise potential interventions to reduce the related risk factors established in this study.
Methods
Study design and subjects
This study was a part of a sub-project under Chronic Kidney Disease Prevention in the Northeast of Thailand (CKDNET) Project32 in which the population-based study was conducted in two rural sub-districts namely Don Chang and Khok Samran of Khon Kaen province, located in the Northeast Thailand from 2017 to 2019. The residents in 24 villages (of these sub-districts), willing to participate and meeting the inclusion criteria: aged ≥ 18 years and without psychopathic disorders were enrolled in the study. The two sub-districts were selected for the research because their population and socio-economic levels were representatives of northeastern Thailand. This was confirmed by analyzing the existing censuses of the Northeast Thailand, Khon Kaen province, Khok Samran, and Don Chang districts before starting the study (https://hdcservice.moph.go.th/hdc/reports/page.php?cat_id=ac4eed1bddb23d6130746d62d2538fd0)). The distributions of age and sex of population in these areas were quite similar with about 48% males and the highest proportion in age 40–49 years (Table S9). Also, the average personal monthly income in 2015 was below 10,000 Baht in both Northeast Thailand and Khon Kaen province (6790 vs. 7813 Baht) (https://www.nesdc.go.th/ewt_w3c/ewt_dl_link.php?nid=7787). Stratified cluster sampling was performed to enroll the participants as distributed in the censuses. In reality, however, the younger and early middle-aged villagers, especially the males, were out of their villages to study and/or work. Furthermore, they were less interested to participate in the study resulting in a selection bias with a higher proportion of females (65%) and the higher age group of 50–59 years as shown in Table S9.
Sample size estimation
The sample size calculation was based on the objectives to evaluate prevalence and factors associated with CKD by stratified-cluster sampling and performing multiple logistic regression analyses. HT was used as the main risk factor of CKD and data from the Thai SEEK study were used as a reference in which the proportion of HT was 27.5% with the event rate of CKD in HT was 34.4% and in non-HT was 11.9%10. The multiple correlation coefficient between HT and the remaining covariates in multiple logistic regression was set as 0.8 and a design effect for stratified-cluster sampling was set as two times higher than simple random sampling. The sample size of 1700 subjects were required using the formula suggested by Hsieh, Block, and Larsen (1998) for a power of 90% and a targeted significance level of 0.0533. The dropout was set at 25%, therefore, the least total sample size was 2268 cases.
Procedure and measurements
An outline of the study procedures is illustrated in Fig. 3. All on-site screening was performed by the work-specific research team including trained post-graduate students, nurses, technicians, physicians, faculty members, and health volunteers. Data were obtained by interviewing the participants in their homes a month before the initial sample collection step with a pre-designed questionnaire on socio-demographic status that included age, sex, education level, monthly income, history of personal and family health, lifestyle (smoking, alcohol consumption, physical exercise, use of NSAIDs), dietary patterns and farming practices (use of insecticides, herbicides, etc.), underlying diseases and medications, and symptoms related to kidney diseases.
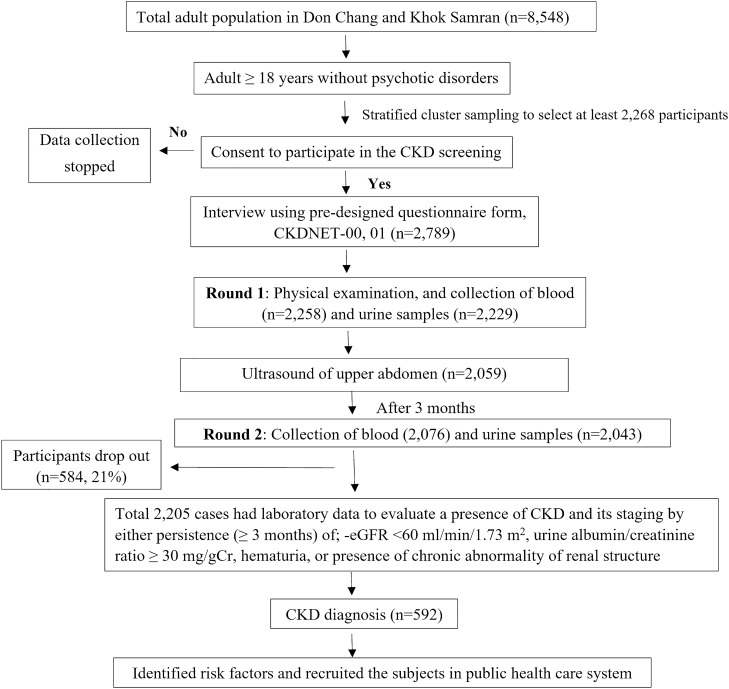
Figure 3.
An outline of the CKD screening procedure. The participants were surveyed using a pre-designed questionnaire on their socio-economic status, lifestyle, and underlying diseases. Physical examinations and sample collections were performed twice following the KDIGO criteria. Upon being diagnosed with CKD, the participants were enrolled in the national public health care system.
The first round of blood and urine sample collection was performed with prior notice to the participants to maintain 8–10 h of overnight fasting. First, arterial blood pressure was measured after resting for 5–10 min in a sitting position by a TERUMO digital blood pressure monitor ES-W100 (Terumo Corporation, Japan). The measurements were repeated in case of a hypertensive reading, and the average value of two measurements was used. Then, weight and height were measured and recorded in the examination form. Body composition analysis for fat, muscle, and water contents was also conducted using a bio-impedance machine (Fresenius Medical Care, Germany). Finally, blood was collected in vials with and without anticoagulants, as well as fresh morning spot urine was received in a sterile plastic container. Samples were then stored and transported at the controlled temperature of 4–10 °C to the central laboratory, Srinagarind Hospital, KKU within 6 h.
Only the participants who completed the socio-economic survey and got through the first round of blood and urine collections were continued to the next steps. Upper abdominal ultrasonography was performed after a month of sample collections, by three radiologists to investigate structural abnormalities in the kidneys. Any questionable findings were double-checked by another investigator. The participants with abnormalities in the first ultrasound but without other criteria of CKD were repeated after 12 weeks for confirmation. The inter-observer agreement (Cohen’s kappa) among these three operators performing in 102 participants was 0.83 (small size of kidneys), 0.88 (renal stones), 0.79 (hydronephrosis), and 0.78 (parenchymatous changes).
Blood and urine specimen collections were repeated after 3 months for the round 2. Urinary albumin to creatinine ratio (UACR, mg/g) and eGFR was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) Eq. 34. Also, the presence of red blood cells in urine was investigated under a microscope. The participants were then categorized as CKD if matched with either of the following criteria for 3 months or longer: 1) eGFR < 60 ml/min/1.73 m2, or 2) presence of any kidney damage; UACR ≥ 30 mg/g in two consecutive samples; ultrasonography examination indicating at least one of the following chronic kidney conditions and structural abnormalities: parenchymatous change of kidney, renal stones, complex cysts and polycystic disease (excluding simple cysts), small kidney size (< 8.5 cm.) with parenchymal change, presence of hydronephrosis, tumor in the kidneys, unilateral kidney (post nephrectomy, renal agenesis or renal atrophy), and presence of urine red blood cells (RBC) ≥ 3–5 cells/high power field (HPF) in two consecutive samples.
Variability of interest and definitions
CKD was defined based on the KDIGO criteria as the presence of either one or more parameters of renal damage or reduced estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2 for 3 months or longer35. The classification of CKD was based on eGFR levels as stage 1: ≥ 90, stage 2: 60–89, stage 3a: 45–59, stage 3b: 30–44, stage 4: 15–29, and stage 5: < 15 ml/min/1.73 m2. UACR levels were divided into 3 categories as follows: A1 < 30, A2 30–300 and A3 > 300 mg/g, when UACR ≥ 30 mg/g was considered as albuminuria. Hyperuricemia was defined as serum acid uric > 7 mg/dL for males and > 6 mg/dL for females. DM was defined by diagnosed DM from physicians and being on medication, or levels of fasting plasma glucose (FPG) ≥ 126 mg/dL or hemoglobin A1c ≥ 6.5% for two times. HT was considered when subjects had a persistent systolic blood pressure (SBP) ≥ 140 mm Hg, and diastolic blood pressure (DBP) ≥ 90 mm Hg or were diagnosed as hypertensive by physicians and being on medication. Anemia was diagnosed if the participants had hemoglobin levels < 13 g/dL in males and < 12 g/dL in females and hypercholesterolemia was defined as low-density lipoprotein (LDL)-cholesterol ≥ 130 mg/dL or on cholesterol-lowering medicines. The participants were considered to have awareness of HT, DM, CKD, and other related conditions/diseases if they had an earlier diagnosis by physicians.
Participants’ education was categorized into 7 levels: no schooling, primary school, middle school, high school/vocational certificate, diploma/high or vocational school, bachelor’s degree, and postgraduate. Economic conditions were grouped into 2 levels based on monthly income: ≤ 10,000 Baht and ≥ 10,000 Baht. Physical exercise was measured in terms of frequency/week and exercise duration. The body mass index (BMI) was calculated as weight (kg)/ height (m2). Then, the BMI classifications for Asian adults from the Regional Office for the Western Pacific (WPRO) of the World Health Organization (WHO) were used, and subsequently, the subjects were categorized into underweight (< 18.5), normal (18.5–22.9), overweight (23–24.9), class I obesity (25–29.9), and class II obesity (≥ 30)36.
Laboratory assessment
Blood was analyzed for red blood cell count, hematocrit, platelets (DC sheath flow method), white blood cells (WBC), WBC differentiation (fluorescent flow cytometry), and hemoglobin (cyanide-free SLS method) using an XS-800i hematology analyzer (Sysmex Corporation, Japan) while serums were investigated for alanine transferase (IFCC method without pyridoxal phosphate), uric acid (enzymatic colorimetric method), fasting blood sugar (kinetic reaction: HK/G6PDH), creatinine (enzymatic method standardized against Isotope Dilution Mass Spectrometry, IDMS) and LDL-Cholesterol (direct assay) by Cobas 6000 analyzer (Roche Diagnostics, USA). Urine was assessed for creatinine (enzymatic method) and microalbumin (immunoturbidimetric method) with the Cobas 8000 analyzer series (502 and 702 modules, Roche Diagnostics, USA) as well as routine urinalysis performed using flow cell digital imaging in the iQ200 urine microscopy analyzer (Beckman Coulter, Inc., USA).
Ethics consideration
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki that was approved by the Ethics Committee for Human Research, Faculty of Medicine, Khon Kaen University (KKU), Thailand (HE 601,035). All the participants provided written informed consent before the study.
Statistical analyses
Statistical analyses were performed using STATA version 17.0 and a P-value < 0.05 was considered statistical significance. Data were presented as mean ± SD, median (interquartile range; IQR), or percentage. Inter-group comparisons were performed by the Chi-square test or Fisher’s Exact test for categorical variables and Student’s t-test, Mann–Whitney U-test for continuous variables, as appropriate. A logistic regression analysis was applied to predict an association between CKD and relevant covariates, and Odds Ratios (ORs) were reported with 95% confidential intervals (CIs). The ORs that accounted for the effects in univariable analysis with a P-value < 0.05 were entered into a multivariate logistic regression model and the interaction effects were evaluated. In the current study, the magnitude and severities of anemia were higher than expectation from CKD complications and some dependent variables were affected by anemia that needed adjustment for the demonstration of the real effect on those variables. Therefore, anemia as one of the independent variables was added. Data, however, were also analyzed by excluding anemia from multivariable analysis. Additionally, two models were investigated; Model 1 is better in terms of biostatistics analysis because data of the dependent variables were input as continuous data therefore, resulting in a greater amount and diversity of data compared to the Model 2, in which data were input as categorical data, i.e., DM, HT, Anemia, Hyperuricemia, leucocytosis, and hyperlipidemia. Some studies, however, focused on these categorical variables as the risk factors rather than detailing with them as individual data, therefore 2 models for benefit in comparison with the other studies were performed.
A priori target was set for an acceptable level of missing data as < 5% and used the multiple imputation method for handling the missing data. These missing data were replaced with a set of predicted values imputed from the other variables of existing data which contained the natural variability and uncertainty of the right values; then multiple imputed data sets were combined and repeatedly analyzed to produce the final single overall analysis result.
Supplementary Information
Acknowledgements
The authors would like to thank all the provincial health officers, health personnel, and participants for their active participation in the project. We also would like to acknowledge Professor James A. Will for editing the manuscript via Publication Clinic KKU, Thailand.
Author contributions
S.A. K.T., and U.C. conceived and designed the research project. U.C., S.B., N.S., P.T., P.P., W.S., A.T., A.S., P.C., and S.A. carried out the screening and investigation. SA interpreted the results and performed the statistics. U.C., A.S. and S.A. drafted the manuscript. K.T. and S.A. provided critical feedback and commented on the manuscript. All the authors agreed to the final version of the manuscript for publication.
Funding
This work was supported by Khon Kaen University through the Chronic Kidney Disease Northeast Thailand research project (CKDNET 2017, 2018, 2019). SA received research funds from Thailand Science Research and Innovation (TSRI), through Program Management Unit for Competitiveness (PMUC), contract number C10F630030, and the National Research Council of Thailand (NRCT, 2020). The funders have no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Data availability
The datasets generated in the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-22538-w.
References
- 1.Jha V, et al. Chronic kidney disease: Global dimension and perspectives. Lancet. 2013;382:260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2.Xie Y, et al. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCD Risk Factor Collaboration Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NCD Risk Factor Collaboration Worldwide trends in blood pressure from 1975 to 2015: A pooled analysis of 1479 population-based measurement studies with 19.1 million participants. Lancet. 2017;389:37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kazancioglu R. Risk factors for chronic kidney disease: An update. Kidney Int Suppl. 2013;2011(3):368–371. doi: 10.1038/kisup.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aekplakorn W, et al. Women and other risk factors for chronic kidney disease of unknown etiology in Thailand: National health examination V survey. Sci. Rep. 2021;11:21366. doi: 10.1038/s41598-021-00694-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anutrakulchai S, et al. Mortality and treatment costs of hospitalized chronic kidney disease patients between the three major health insurance schemes in Thailand. BMC Health Serv. Res. 2016;16:528. doi: 10.1186/s12913-016-1792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chittinandana A, Chailimpamontree W, Chaloeiphap P. Prevalence of chronic kidney disease in Thai adult population. J. Med. Assoc. Thai. 2006;89(Suppl 2):S112–120. [PubMed] [Google Scholar]
- 10.Ingsathit A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol. Dial. Transplant. 2010;25:1567–1575. doi: 10.1093/ndt/gfp669. [DOI] [PubMed] [Google Scholar]
- 11.Ong-Ajyooth L, Vareesangthip K, Khonputsa P, Aekplakorn W. Prevalence of chronic kidney disease in Thai adults: A national health survey. BMC Nephrol. 2009;10:35. doi: 10.1186/1471-2369-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkovic V, et al. High prevalence of chronic kidney disease in Thailand. Kidney Int. 2008;73:473–479. doi: 10.1038/sj.ki.5002701. [DOI] [PubMed] [Google Scholar]
- 13.Praditpornsilpa K, et al. The need for robust validation for MDRD-based glomerular filtration rate estimation in various CKD populations. Nephrol. Dial. Transplant. 2011;26:2780–2785. doi: 10.1093/ndt/gfq815. [DOI] [PubMed] [Google Scholar]
- 14.Hill NR, et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. Plos One. 2016;11:e0158765. doi: 10.1371/journal.pone.0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krol E, et al. Early detection of chronic kidney disease: Results of the PolNef study. Am. J. Nephrol. 2009;29:264–273. doi: 10.1159/000158526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delanaye P, et al. CKD: A call for an age-adapted definition. J. Am. Soc. Nephrol. 2019;30:1785–1805. doi: 10.1681/ASN.2019030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third national health and nutrition examination survey. Am. J. Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 18.Davies DF, Shock NW. Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J. Clin. Invest. 1950;29:496–507. doi: 10.1172/JCI102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. Prevalence of chronic kidney disease in China: A cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 20.An JN, et al. Late stage 3 chronic kidney disease is an independent risk factor for sarcopenia, but not proteinuria. Sci. Rep. 2021;11:18472. doi: 10.1038/s41598-021-97952-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mori K, et al. Impact of diabetes on sarcopenia and mortality in patients undergoing hemodialysis. BMC Nephrol. 2019;20:105. doi: 10.1186/s12882-019-1271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reynolds K, et al. Body mass index and risk of ESRD in China. Am. J. Kidney Dis. 2007;50:754–764. doi: 10.1053/j.ajkd.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Provenzano M, et al. Smoking habit as a risk amplifier in chronic kidney disease patients. Sci. Rep. 2021;11:14778. doi: 10.1038/s41598-021-94270-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J, et al. Cigarette smoking and chronic kidney disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Nephrol. Dial. Transplant. 2017;32:475–487. doi: 10.1093/ndt/gfw452. [DOI] [PubMed] [Google Scholar]
- 25.Yacoub R, et al. Association between smoking and chronic kidney disease: A case control study. BMC Public Health. 2010;10:731. doi: 10.1186/1471-2458-10-731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Tiptaradol S, Aekplakorn W. Prevalence, awareness, treatment and control of coexistence of diabetes and hypertension in thai population. Int. J. Hypertens. 2012;2012:386453. doi: 10.1155/2012/386453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iseki K, Kohagura K. Anemia as a risk factor for chronic kidney disease. Kidney Int. Suppl. 2007 doi: 10.1038/sj.ki.5002481. [DOI] [PubMed] [Google Scholar]
- 29.Stauffer ME, Fan T. Prevalence of anemia in chronic kidney disease in the United States. Plos One. 2014;9:e84943. doi: 10.1371/journal.pone.0084943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu P, Liu Y, Han L, Xu G, Ran JM. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: A meta-analysis of 15 cohort studies. Plos One. 2014;9:e100801. doi: 10.1371/journal.pone.0100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erlinger TP, et al. Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am. J. Kidney Dis. 2003;42:256–263. doi: 10.1016/s0272-6386(03)00650-4. [DOI] [PubMed] [Google Scholar]
- 32.Cha'on U, et al. CKDNET, a quality improvement project for prevention and reduction of chronic kidney disease in the Northeast Thailand. BMC Public Health. 2020;20:1299. doi: 10.1186/s12889-020-09387-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat. Med. 1998;17:1623–1634. doi: 10.1002/(sici)1097-0258(19980730)17:14<1623::aid-sim871>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.KDIGO CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 36.World Health Organization. The Asia-Pacific perspective: Redefining obesity and its treatment. https://apps.who.int/iris/handle/10665/206936 (2000).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated in the current study are available from the corresponding author on reasonable request.