Abstract
Appropriate attentional resource allocation could minimize exaggerated dual-task interference due to basal ganglia dysfunction in Parkinson’s disease (PD). Here, we assessed the electroencephalography (EEG) functional connectivity to investigate how task prioritization affected posture-motor dual-tasks in PD. Sixteen early-stage PD patients and 16 healthy controls maintained balance in narrow stance alone (single-posture task) or while separating two interlocking rings (postural dual-task). The participants applied a posture-focus or supraposture-focus strategy in the postural dual-task. Postural sway dynamics, ring-touching time, and scalp EEG were analyzed. Both groups exhibited smaller postural sway size, postural determinism, and ring-touching time with the supraposture-focus versus posture-focus strategy. PD patients exhibited higher mean inter-regional connectivity strength than control subjects in both single and dual-task postural conditions. To cope with dual-task interference, PD patients increased inter-regional connectivity (especially with the posture-focus strategy), while control subjects reduced inter-regional connectivity. The difference in mean connectivity strength between the dual-task condition with supraposture-focus and single-posture condition was negatively correlated to the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III total scores and hand-related sub-scores. Our findings suggest differential task prioritization effects on dual-task performance and cortical reorganization between early-stage PD and healthy individuals. Early-stage PD patients are advocated to use a supraposture-focus strategy during a postural dual-task. In addition, with a supraposture-focus strategy, PD patients with mild motor severity could increase compensatory inter-regional connectivity to cope with dual-task interference.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11357-022-00516-4.
Keywords: EEG, Functional connectivity, Task prioritization, Dual-task, Parkinson’s disease
Introduction
Postural instability is a critical motor dysfunction in patients with Parkinson’s disease (PD), especially moderate-to-advanced PD [1]. Falling risk is also a concern in patients with early-stage PD (i.e., Hoehn and Yahr (H&Y) stage 1–2) [2, 3], although postural instability at this stage can be masked by increasing cognitive effort toward postural control [4]. However, postural stability in early-stage PD is deteriorated by dual-task interference [5], as a suprapostural task competes with a postural task for limited attentional resources [6]. There is an increasing focus on the diagnostic value of dual-task postural stability in the preclinical or early-stage PD population prior to the development of cardinal balance problems [7, 8].
In daily life, people often must perform a task in an upright stance, which mechanically involves task prioritization and allocation of attentional resources. Patients with PD are usually suggested to prioritize the postural task for the sake of safety, presuming that a “posture-second” (or supraposture-focus) strategy will result in postural degradation [9, 10]. By adopting a “posture-first” (or posture-focus) strategy, patients with PD have been reported to increase their gait speed without a significant decline in suprapostural performance [11, 12]. However, a posture-focus strategy is not necessarily most favorable for postural stability [13]. At least among young individuals, healthy elderly individuals, and patients with early-stage PD, a posture-focus strategy impaired the automaticity of postural control, resulting in a greater sway size and regularity of postural trajectory [14–17]. It seems that optimal task prioritization for patients with PD depends on individual characteristics and clinical phases relating to functional reserve and residual resource capacity [13].
Disruption of fronto-parietal circuits of the dopamine network affects frontal executive function, which is related to the coordinated processing of concurrent tasks and task switching, especially in people having PD with freezing gait [18]. Irrespective of task prioritization, PD patients tend to rely on compensatory mechanisms to execute a postural dual-task due to limitations of executive function. Compared to healthy counterparts, brain imaging studies have revealed increased cognitive effort of PD patients, supporting their more pronounced and widespread cortical activities during a postural dual-task [18–20]. In patients with PD, the fronto-parietal networks are especially active [21, 22], implying sensory dependence and physiological arousal as compensation for exaggerated dual-task interference [23]. Compensatory cortical activities for diminished striatal control could contribute to loss of motor automaticity for a postural dual-task [18, 20], which is a potential neural marker of fall propensity in early-stage PD. There is presently no established association between neural compensatory mechanisms in postural dual-task and behavioral heterogeneity in early-stage PD, despite great research efforts made to examine the relationship between connectivity strength in a resting, relaxed, or single-task condition and clinical outcomes (e.g., the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score) [24–28].
In the present study, we aimed to differentiate changes in electroencephalography (EEG) functional connectivity for task prioritization (posture-focus strategy vs. supraposture-focus strategy) in a postural dual-task between patients with early-stage PD and healthy controls. We further aimed to assess the relationship between variations in EEG functional connectivity and behavioral assessments of motor severity in early-stage PD. The design is helpful to gain insight into neurological mechanisms of task prioritization for a postural dual-task, subservient to effective neural markers and dual-task training guidelines for early-stage PD. An increase in task demand of a postural dual-task is expected to challenge executive function and impair stance balance in PD more than their healthy counterparts [29, 30]. In reference to a single-posture task condition, we hypothesized that early-stage PD patients would show greater and more widespread functional connectivity in a postural dual-task than their healthy counterparts. For a larger dual-task cost [15, 16], greater modulation of functional connectivity between postural dual-task and single-posture task was expected when adopting a posture-focus strategy than a supraposture-focus strategy. Next, we hypothesized that motor severity in early-stage PD could associate with the strength of task-related functional connectivity for a postural dual-task, with either a posture-focus strategy or a supraposture-focus strategy, in comparison with the single-posture task.
Methods
Participants
For this study, we recruited 16 patients with early-stage PD (mean age: 62.4 ± 7.5 years) and 16 age-matched healthy control subjects (mean age: 61.8 ± 8.0 years). The inclusion criteria for patients were as follows: diagnosed with idiopathic PD, according to the United Kingdom PD Society Brain Bank clinical diagnostic criteria [31]; PD onset age of ≥ 40 years; and modified H&Y stage of 1–2. Patients were excluded if they had a history of brain surgery; history of other diseases and conditions that could influence balance ability, a Mini-Mental State Examination (MMSE) score < 26, or moderate or severe postural tremor or kinetic tremor in the hands, defined by items 3.15 (postural tremor) and 3.16 (kinetic tremor) of the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) [32]. The healthy controls all had an MMSE score of ≥ 26, had no previous neuromuscular or cardiopulmonary disorders, and were not taking any medication that might influence balance or cognition. Table 1 presents the participants’ demographic and clinical characteristics. All procedures in this study were approved by the National Taiwan University Hospital Research Ethics Committee (Clinical Trial Registration No.: NCT03298503), and all of the participants gave written informed consent.
Table 1.
Patient demographics and characteristics (N = 32)
| Participant details | PD group (n = 16) | Control group (n = 16) | p-value |
|---|---|---|---|
| Age, years | 62.4 ± 7.5 | 61.8 ± 8.0 | 0.82 |
| Age range, years | 50.5–73.8 | 50.0–75.1 | – |
| Sex, M/F | 9/7 | 8/8 | – |
| Disease duration, years | 4.8 ± 1.4 | – | – |
| Modified H&Y stage, off state | 1.7 ± 0.3 | – | – |
| MMSE, off state | 28.8 ± 1.1 | 29.3 ± 0.9 | 0.17 |
| MDS-UPDRS motor scores, off state | 27.1 ± 5.6 | – | – |
| Hand-related sub-scores | 12.7 ± 4.2 | – | – |
| Posture and gait sub-scores | 3.4 ± 1.0 | – | – |
Data are presented as mean ± standard deviation unless otherwise indicated
PD, Parkinson’s disease; Modified H&Y stage, Modified Hoehn and Yahr stage; MMSE, Mini-Mental Status Examination; MDS-UPDRS, Movement Disorder Society Unified Parkinson’s Disease Rating Scale
The sample size was calculated based on data from a previous study [16], which indicated that 28 participants (14 participants per group) would be sufficient to detect the effects of task prioritization on dual-task performance (Cohen’s d = 0.56, power = 0.8, α = 0.05).
Experimental apparatus and data recording
The participants were instructed to stand with their feet together on a force plate (9260AA6, Kistler, Switzerland) and to concurrently control a pair of interlocking rings (suprapostural task). The participants kept their elbows in 90° flexion and held two sticks. Attached to the end of each stick was a metal ring (diameter: 4 cm), and the two rings were interlocked. The participants were asked to prevent the two rings from touching each other while remaining in a steady stance. When the two rings touched each other, the computer received a contact signal from an A/D card (USB-6221, National Instruments, USA).
Cortical activities were recorded using a NuAmps amplifier (NeuroScan Inc., USA) with 30 different EEG channels (Fp1/2, Fz, F3/4, F7/8, FT7/8, FCz, FC3/4, Cz, C3/4, CPz, CP3/4, Pz, P3/4, T3/4, T5/6, TP7/8, Oz, and O1/2). The electrodes were placed based on the 10–20 electrode system of the International Federation. The ground electrode was placed along the midline, ahead of Fz. To monitor vertical and horizontal eye movements and blinks, electrodes were placed above the left eyebrow, below the left eye, and horizontally on the outer canthi of both eyes. The impedances of all electrodes were below 5 kΩ, and all electrodes were referenced to linked mastoids of both sides. EEG data were band-pass filtered at 0.1–100 Hz. All behavioral data and EEG data were synchronized with a sampling rate of 1 kHz.
Experimental conditions
All clinical assessments and dual-task examinations were performed in the morning, at least 12 h after the most recent administration of anti-parkinsonian medications (medication-off test). After assessment of MDS-UPDRS part III (only for the PD group), all participants participated in the following four test conditions in a random sequence (Supplementary Fig. 1): controlled ring task while sitting, single-posture task (Single), dual-task with posture-focus instruction (Dual_PF), and dual-task with supraposture-focus instruction (Dual_SF). In the controlled ring task, the participants kept the two rings apart in a sitting position. In the single-posture condition, the participants were asked to maintain balance while standing with their feet together. In the Dual_PF condition, the participants were instructed to pay the most attention to maintain their posture as stable as possible (primary task) and to perform their best at preventing the two rings from touching (secondary task). In the Dual_SF condition, the participants were instructed to pay the most attention to prevent the two rings from touching (primary task) and to perform their best at maintaining a stable posture (secondary task). A practice trial was conducted before six 30-s testing trials in each experimental condition. Participants were asked to look straight ahead and not to look at their feet or the rings in all experimental conditions. Immediately following each dual-task trial, participants were asked to rate the percentage of their attention that they felt had been directed toward “keeping posture stable” or “preventing the two rings from touching,” using an analog scale (0–100%).
Data analysis
For the suprapostural task, performance was evaluated based on the total time that the rings touched in each trial (ring-touching time) (Fig. 1a). To assess the size of postural sway, the center-of-pressure (CoP) trajectory was calculated after the CoP data was low-pass filtered at 6 Hz. Recurrence quantification analysis (RQA) was performed to quantify the complexity of the CoP trajectory [33–35]. The embedding dimension for RQA was set as four according to the false nearest neighbors algorithm [36], and the time delay parameter was set as one based on a previous CoP study of postural dual-task [37]. In the RQA, determinism (%DET) was used to quantify the predictability of the postural dynamic system. A greater %DET of the CoP data indicates that postural dynamics is more regular, implying that postural control is less automatic [34, 35, 38]. For more detailed information on RQA, please refer to Online resource 2.
Fig. 1.
(a) Quantifications of postural regularity and supraposutral task accuracy using recurrence quantification analysis (RQA) and ring-touch time, respectively. Darkened points indicate points that are recurrent in time. %DET is the percentage of recurrent points that fall on upward diagonal line segments. %DET indexes the degree of determinism of posture sway. Higher %DET reflects higher postural regularity. A larger ring-touch time reflects poorer supraposutral task accuracy. (b) The pooled adjacent matrix of the phase-lag index (PLI) of artifact-free EEG epochs for two task configurations: Dual_SF and Single. Mean PLI was obtained by averaging the cell values of the PLI adjacent matrixes, excluding the diagonal values. A contrasting wiring diagram on the scalp shows the topological distributions of suprathreshold connectivity by contrasting the mean PLI of electrode pairs between two task configurations: Dual_SF vs. Single (> 2.629, p < 0.005). Red line: connectivity strength of Dual_SF > connectivity strength of Single, p < 0.005; blue line: connectivity strength of Dual_SF < connectivity strength of Single, p < 0.005. Dual_SF, dual-task with supraposture-focus; Single, single-posture task
The EEG data were conditioned with a zero-phase finite impulse response (FIR) band-pass filter (cut-off frequencies: 1 Hz and 60 Hz, 60 db/octave). With the NeuroScan software program (NeuroScan Inc., EI Paso, TX, USA), the blinks were automatically detected and corrected through the creation of bipolar vertical EOG channels by subtracting activity in the infraorbitally placed electrode from the supraorbitally placed electrode, and the creation of bipolar horizontal EOG channels by subtracting activity between the two electrodes placed on the outer canthi of both eyes. The conditioned EEG data were segmented in 2-s epochs. After that, the researchers visually inspected for survival eye-blink artifacts which were not corrected by the NeuroScan software program according to experiences. Less than 8% of the 2-s epochs (7 epochs per experimental condition) with suspected eye-blink were rejected. To characterize the strength of inter-regional connectivity, a phase-lag index (PLI) was applied to each artifact-free 2-s epoch EEG time series for all 30 electrode pairs. On an individual basis, inter-regional PLIs were computed from all not-excluded 2-s epochs. PLI matrixes of all EEG electrode pairs were averaged to get mean PLI (m-PLI) for the subject in a given condition. PLI was selected because this index is insensitive to common sources [39, 40]. Based on the Hilbert transformation, the PLI features the distribution asymmetry of phase differences of the instantaneous phases between a given pair of EEG epochs. If is the phase difference, the PLI is defined as follows:, where sgn is a function that extracts the sign of a real number. The PLI functional connectivity was calculated using the HERMES function in Matlab [41]. Calculation of PLI across all pairs of EEG channels resulted in the 30 × 30 PLI adjacent matrix. Within the 30 × 30 PLI adjacent matrix, the paired t-test was used to examine significant differences in the PLI of an electrode pair between any of the two task conditions, e.g., Dual_SF vs. Single (Fig. 1b). The 2-D t-matrix represented task-dependent PLI differences for all electrode pairs. Suprathreshold connections with high values (> 2.947, p < 0.005) were highlighted to visualize topological differences in connectivity strength between any of the two task conditions. The results of paired t-test for PLI are fairly robust to departures from normality [42]. A set of task-specific suprathreshold connections were mapped on the scalp for the PD and control groups. In addition, we examined task-dependent variations in the network-based suprathreshold connectivity. A permutation test was performed 5000 times to examine variations in the null distribution of the suprathreshold connectivity between any of the task conditions for the PD and control groups. Methodological details of corrected network-based statistics were documented in the work of Zalesky et al. [43].
Statistical analysis
On account of a potential violation of normality and homogeneity of variance for a small data set, the Friedman test was used to examine the task effect (Single vs. Dual_PF vs. Dual_SF) on postural variables (postural sway and postural %DET), suprapostural performance (ring-touching time), and inter-regional EEG connectivity (m-PLI) for the PD and healthy control groups. Post-hoc comparisons were performed by Wilcoxon signed-rank test. On the other hand, the group differences in postural sway, postural %DET, ring-touching time, and m-PLI between the PD and healthy control groups in the Single (or controlled ring), Dual_PF, and Dual_SF conditions were examined with Mann–Whitney U test. Additionally, Spearman correlation was used to examine the correlation between m-PLI in the single-posture/dual-task conditions (Single, Dual_PF, Dual_SF, Dual_PF − Single, Dual_SF − Single, and Dual_PF − Dual_SF) and the scores of MDS-UPRDS part III (sum score of MDS-UPRDS part III, hand-related sub-scores of MDS-UPDRS part III, and posture and gait sub-scores of MDS-UPDRS part III). The m-PLI for the Dual_PF − Single and Dual_SF − Single were calculated by subtracting m-PLI in the single-posture condition from m-PLI in the Dual_PF and Dual_SF conditions. The m-PLI for the Dual_PF − Dual_SF was the m-PLI difference between the two postural dual-task conditions. For the hand-related sub-scores of MDS-UPDRS part III, we summed the scores of items 3.4, 3.5, 3.6, 3.15, 3.16, 3.17, and 3.18 of MDS-UPDRS. For the posture and gait sub-scores of MDS-UPDRS part III, we summed the scores of items 3.9, 3.10, 3.11, 3.12, and 3.13 of MDS-UPDRS. The level of significance was set at p < 0.05. Signal processing and statistical analyses were completed using MATLAB v. R2018a (MathWorks, USA) and SPSS v. 21 (SPSS Inc., USA). All data are presented as the mean ± standard error.
Results
All participants reported that they paid major attention to the postural task in each trial of the Dual_PF condition (control group: 72.46 ± 2.58%; PD group: 70.63 ± 2.41%) and paid major attention to the suprapostural task in each trial of the Dual_SF condition (control group: 79.36 ± 2.30%; PD group: 75.09 ± 2.03%).
Behavior performance
The Friedman test revealed that the size of postural sway was affected by the task condition for the both PD (χr2(2) = 17.43, p < 0.001) and control (χr2(2) = 23.63, p < 0.001) groups (Fig. 2a). Post-hoc analyses showed that the PD group had the largest postural sway in the Dual_PF condition, compared to that in the Single (p = 0.001) and Dual_SF (p = 0.001) conditions. For the control group, postural sway was largest in the Single condition and was smallest in the Dual_SF condition (Single > Dual_PF: p = 0.005; Single > Dual_SF: p = 0.001; Dual_PF > Dual_SF: p < 0.001). The group difference in the size of postural sway was significant, with greater postural sway for the PD group under the Dual_SF condition (p = 0.047). There was a marginally significant group difference in the size of postural sway under the Dual_PF condition (p = 0.056). No significant group difference in the size of postural sway under the Single condition (p = 0.616).
Fig. 2.

Comparison of (a) CoP trajectory and (b) %DET of CoP trajectory in the Single, Dual_PF, and Dual_SF conditions and (c) ring-touching time in the Sitting, Dual-PF, and Dual-SF conditions, between the PD and control groups. Dual_SF, dual-task with supraposture-focus; Dual_PF, dual-task with posture-focus; Single, single-posture task; Sitting, controlled ring task in sitting position
The Friedman test revealed that postural %DET varied with the task condition for the both PD (χr2(2) = 14.63, p = 0.001) and control (χr2(2) = 30.13, p < 0.001) groups (Fig. 2b). For the PD group, post-hoc analyses showed that postural %DET was greatest in the Dual_PF condition than that in the Single (p = 0.007) and Dual_SF (p = 0.001) conditions. For the control group, postural %DET was largest and smallest in the Single condition and the Dual_SF condition, respectively (Single > Dual_PF: p = 0.004; Single > Dual_SF: p < 0.001; Dual_PF > Dual_SF: p < 0.001). The group difference in postural %DET was significant for the both dual-task conditions (Single: p = 0.616; Dual_PF: p = 0.001; Dual_SF: p = 0.001). The PD group exhibited greater postural %DET than the control group.
The Friedman test revealed that the ring-touching time was tuned to the task condition in both the PD (χr2(2) = 11.38, p = 0.003) and control (χr2(2) = 13.50, p = 0.001) groups (Fig. 2c). Post-hoc analyses indicated that the ring-touching time was longer in the Dual_PF condition than the controlled ring (Sitting) (p = 0.006) and Dual_SF (p = 0.001) conditions for the PD group. For the control group, the ring-touching time was shortest in the Dual_SF condition (Dual_SF < Sitting, p = 0.002; Dual_SF < Dual_PF, p = 0.003). The ring-touching time was longer in the PD group than the control group under the dual-task conditions (Sitting: p = 0.838; Dual_PF: p = 0.035; Dual_SF: p = 0.014).
EEG functional connectivity
Although the Friedman test showed that m-PLI was not task-dependent for the PD (χr2(2) = 0.88, p = 0.646) and control (χr2(2) = 1.500, p = 0.472) groups, the m-PLI was generally greater in the PD group than in the control group (Single: p = 0.023; Dual_PF: p = 0.003; Dual_SF: p = 0.006) (Fig. 3). In terms of PLI, the population means of inter-regional functional connectivity for each experimental condition in the control and PD groups are presented in Supplementary Fig. 2. To visualize topological differences in inter-regional connectivity due to variations in task configuration, we generated a task-specific wiring diagram of EEG suprathreshold connectivity (p < 0.005) on the scalp for both the control and PD groups (Fig. 4). The results of corrected network-based statistics indicated significant task modulation effects on suprathreshold connectivity for the control and PD groups (p ≤ 0.001, corrected), except for the insignificant difference in network suprathreshold connectivity between the Dual_SF and Dual_PF conditions in the PD group (p = 0.119, corrected). In the PD group, suprathreshold connectivity markedly increased in both the Dual_PF and Dual_SF conditions compared to the single-posture condition (Fig. 4a,b, right plots). Compared to a supraposture-focus strategy, a posture-focus strategy added more to the connectivity strength in bilateral hemispheres. In contrast, in the control group, the connectivity strength significantly decreased in the dual-task conditions, regardless of posture-focus or supraposture-focus strategy. Moreover, the control group demonstrated focus-dependent connectivity modulation, showing a pronounced decrease of connectivity strength in the frontal-parietal area of the cortex under the Dual_SF condition (Fig. 4c, left plot). On the other hand, focus-dependent modulation of inter-regional connectivity was much less evident in the PD group (Fig. 4c, right plot).
Fig. 3.
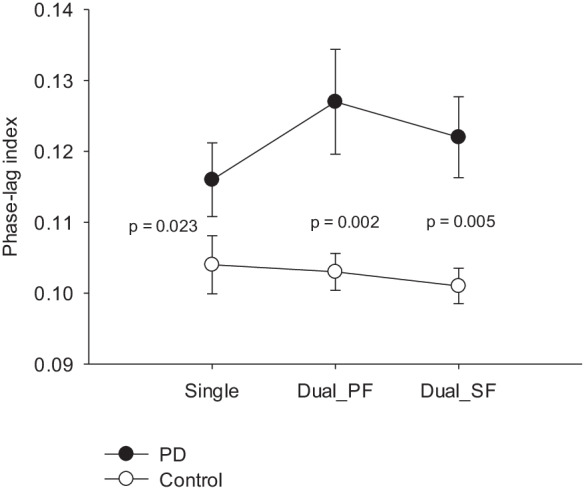
Comparison of the mean phase-lag index in the Single, Dual_PF, and Dual_SF conditions between the PD and control groups. Dual_SF, dual-task with supraposture-focus; Dual_PF, dual-task with posture-focus; Single, single-posture task
Fig. 4.
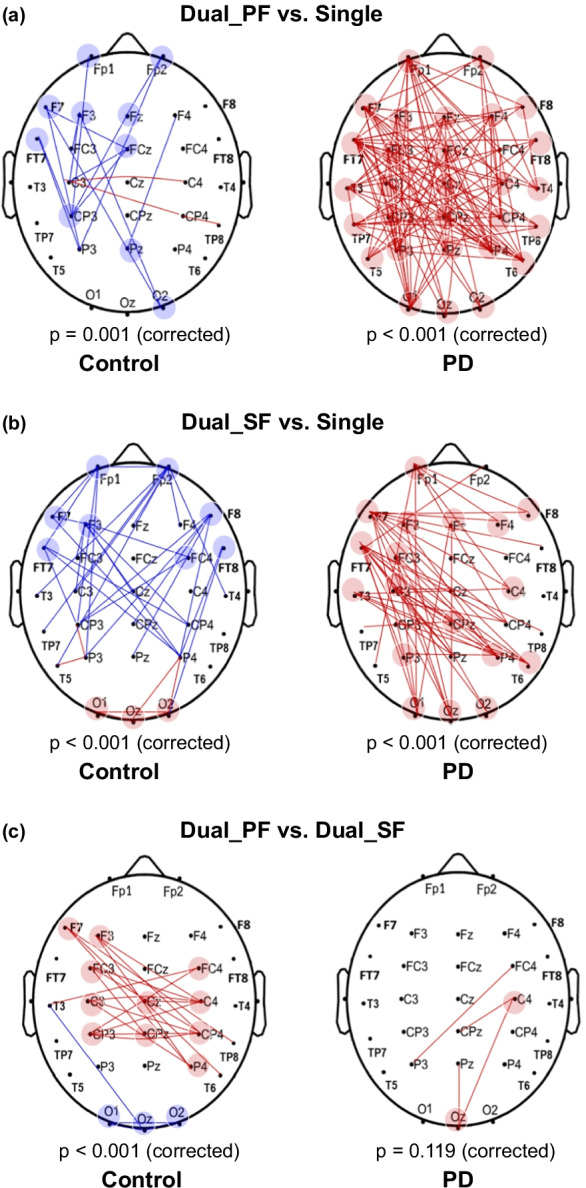
Contrasting wiring diagram due to variations in task configuration for the control and PD groups. (a) Red line: connectivity strength of Dual_PF > connectivity strength of Single (p < 0.005); blue line: connectivity strength of Dual_PF < connectivity strength of Single (p < 0.005). (b) Red line: connectivity strength of Dual_SF > connectivity strength of Single (p < 0.005); blue line: connectivity strength of Dual_SF < connectivity strength of Single (p < 0.005). (c) Red line: connectivity strength of Dual_PF > connectivity strength of Dual_SF (p < 0.005); blue line: connectivity strength of Dual_PF < connectivity strength of Dual_SF (p < 0.005). Dual_SF, dual-task with supraposture-focus; Dual_PF, dual-task with posture-focus; Single, single-posture task
Table 2a–c presents the correlation results between MDS-UPDRS part III and m-PLI. The total scores of MDS-UPDRS part III were not significantly correlated with m-PLI in the Single, Dual_PF, or Dual_SF conditions (p > 0.05) (Table 2a). Interestingly, the total score of MDS-UPDRS part III was negatively correlated with m-PLI in the Dual_SF condition after subtracting baseline m-PLI of the single-posture condition. (Dual_SF − Single: r = − 0.638, p = 0.008). Additionally, the hand-related sub-scores of MDS-UPDRS part III showed a strong negative correlation with m-PLI under the Dual_SF condition after subtracting baseline m-PLI of the single-posture condition (Dual_SF − Single: r = − 0.826, p < 0.001) (Table 2b). However, there was no significant correlation between m-PLI and the posture and gait sub-scores of MDS-UPDRS part III (p > 0.05) (Table 2c).
Table 2.
Spearman correlation between Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III and mean phase-lag index (m-PLI) of functional connectivity for the PD group in different task configurations (N = 16)
| Single | Dual_PF | Dual_SF | Dual_PF − Single | Dual_SF − Single | Dual_PF − Dual_SF |
|---|---|---|---|---|---|
| (a) MDS-UPDRS part III | |||||
| r = 0.066 | r = − 0.360 | r = − 0.378 | r = − 0.319 | r = − 0.638 | r = 0.092 |
| p = 0.807 | p = 0.171 | p = 0.149 | p = 0.229 | p = 0.008 | p = 0.736 |
| (b) MDS-UPDRS part III (hand) | |||||
| r = 0.145 | r = − 0.272 | r = − 0.427 | r = − 0.402 | r = − 0.826 | r = 0.292 |
| p = 0.593 | p = 0.309 | p = 0.099 | p = 0.123 | p < 0.001 | p = 0.272 |
| (c) MDS-UPDRS part III (posture and gait) | |||||
| r = − 0.022 | r = − 0.143 | r = − 0.388 | r = − 0.093 | r = − 0.295 | r = 0.155 |
| p = 0.936 | p = 0.598 | p = 0.138 | p = 0.732 | p = 0.268 | p = 0.566 |
Dual_SF, dual-task with supraposture-focus; Dual_PF, dual-task with posture-focus; Single, single-posture task.
Discussion
Postural responses for the control and early-stage PD groups were differently modulated with the addition of manual task and focus strategy. In the control group, performing the postural dual-task with the supraposture-focus strategy was associated with the smallest postural sway and postural %DET. In the early-stage PD group, performing the postural dual-task with the posture-focus strategy was associated with the largest postural sway and postural %DET (Fig. 2a,b). Changes in suprapostural performance from sitting to standing were also group-dependent. The control group showed the smallest ring-touching time (or the highest suprapostural task precision) when performing the postural dual-task with the supraposture-focus strategy, whereas the early-stage PD group exhibited the largest ring-touching time when performing the postural dual-task with the posture-focus strategy (Fig. 2c). At the cortical level, mean connectivity strength (m-PLI) did not significantly vary with task variations (Fig. 3), but the early-stage PD group demonstrated greater m-PLI than the control group. Relative to the single-posture condition, a concurrent manual task reduced suprathreshold connectivity in the control group, but conversely, increased suprathreshold connectivity in the early-stage PD group (Fig. 4). With the supraposture-focus strategy, the increased m-PLI due to dual-task interference was negatively correlated with the total scores and hand-related sub-scores of MDS-UPDRS part III in the PD group (Table 2a,b).
Differences in cortical connectivity modulation due to dual-task interference between early-stage PD and healthy individuals
When performing a ring-touching task under a narrow stance with either the posture-focus or supraposture-focus strategy, healthy controls consistently led to reduced postural sway, postural %DET, and ring-touching time, compared to those in the single-posture and controlled ring conditions. The dual-task interference effect on sway minimization, postural automaticity, and suprapostural accuracy corresponded with the concept of the facilitatory control of adaptive resource sharing hypothesis [44, 45]. The facilitatory effects on posture and supraposture were conceptually ascribed to the integration of posture and suprapostural tasks into a perception–action unit by effective processes to reorganize brain resources. The hypothesis of adaptive resource sharing would be supported by the wiring diagrams of each EEG electrode pair in the present study. Our novel evidence revealed that the dual-task conditions reduced suprathreshold connectivity in healthy controls, compared to the single-posture condition (Fig. 4a,b, left plots). This scenario provides a good explanation of the mutual facilitation of postural and suprapostural tasks under the framework of the adaptive resource sharing hypothesis [45, 46]. In the dual-task, cognitive control over the postural component was reduced, in parallel with increased posture automaticity (smaller postural %DET) and reduced sway size (Fig. 2a,b). Functional magnetic resonance imaging (fMRI) revealed decreased cortical activation of the fronto-parietal network and increased basal ganglia activation when healthy adults performed movements more automatically [21, 47]. In terms of suprathreshold connectivity in this study, the decrease in the connectivity strength for the dual-task conditions speaks for a release of cortical resources in favor of increasing resource availability to improve the suprapostural ring-touching task (Fig. 2c). During a postural dual-task, the healthy controls could tacitly minimize their consumption of common limited resources at the cortical level, possibly by shifting the task-load to the subcortical areas (e.g., basal ganglia or cerebellum), which have reciprocal connections with the brainstem and cerebral cortex [47, 49]. Nevertheless, in PD, the processing of a postural dual-task with multiple sources would be impaired, as basal ganglia could modulate the feedforward information from the cerebral cortex and real-time sensory feedback via GABA-ergic projections to the cerebral cortex [50, 51].
Unlike the control group, the early-stage PD group showed mutual interference in the postural dual-task, especially in the Dual_PF condition. The concurrent manual task did not add suprapostural task accuracy or reduce postural sway and postural %DET (Fig. 2). In the early-stage PD group, regardless of the attentional focus strategies, the additional suprapostural task increased the suprathreshold connectivity in the dual-task conditions, compared to the single-posture condition (Fig. 4a,b). Moreover, m-PLI was greater for the early-stage PD group than for the healthy controls (Fig. 3). As executive dysfunction can develop in the early stages of PD [52, 53], the increased cortical connectivity was to compensate for executive deficits that impair the ability to rapidly switch between two response sets [54]. Additionally, flexible resource allocation to subcortical structures was ineffective in the early-stage PD group, which might partly relate to improper dopamine function in the basal ganglia and decreased thickness of the perirhinal cortex [55, 56]. Functionally, the suprapostural motor task increased reliance on central resources at the cortical level for the early-stage PD group. This inflexibility of resource allocation makes early-stage PD patients more prone to fall under the postural dual-task conditions. In the PD group, the increased cortico-cortical connections (suprathreshold connectivity) when adding a suprapostural task were widespread across the whole cortex (Fig. 4a,b, right plots); thus, the compensatory mechanism is hypothesized to involve the use of sensory dependence and physiological arousal to deal with dual-task interference [23]. Our preliminary findings are parallel to the findings of Vervoort et al. [18], who used fMRI to demonstrate that PD patients exhibited more pronounced hyper-connectivity in the sensorimotor cortex during dual-task walking, compared to that in the resting state. It has also been reported that hyper-connectivity among the frontal area, motor cortical areas, and the inferior parietal lobule, during motor execution or in resting state, is a compensatory mechanism for motor impairment in patients with PD [57–60]. However, although the PD group might compensate for basal ganglia dysfunction by increasing cortical connectivity, they still had larger postural sway and longer ring-touching time under the dual-task conditions compared to the control group (Fig. 2a,c). It indicates that a posture-motor dual-task is optimized with flexible resource sharing rather than compensational use of cortical resource [61].
Reorganization of cortical connectivity for postural dual-task with attentional focus
In both the early-stage PD and control groups, the supraposture-focus strategy was superior to the posture-focus strategy in terms of smaller postural sway, less postural %DET, and less ring-touching time (Fig. 2). These results replicated the prioritization effect on posture-motor dual-task previously reported in healthy adults and early-stage PD patients [15–17]. Although mean connectivity strength (m-PLI) did not significantly differ between the two postural dual-task conditions (Dual_PF and Dual_SF) (Fig. 3), the posture-focus strategy resulted in greater suprathreshold connectivity than the supraposture-focus strategy in both the control and early-stage PD groups (Fig. 4c). These scenarios support the idea that the posture-focus strategy utilized more cortical resources than the supraposture-focus strategy. In the healthy controls, the posture-focus strategy led to greater connectivity strength in the frontal-central-parietal area, which is crucially involved in monitoring balance and recognizing postural instability [62]. In addition, according to the differences of suprathreshold connectivity between the Dual_PF and Dual_SF conditions (Fig. 4c), we observed that the greater suprathreshold connectivity resulted from the posture-focus strategy was more prominent in the control group than the early-stage PD group. The findings of m-PLI and suprathreshold connectivity did not support the hypothesis that the early-stage PD participants would have greater functional connectivity modulation between the single-posture and dual-task conditions when they adopted a posture-focus strategy. It was likely that dual-task interference had already taxed the majority of limited central capacity in patients with PD [5] that left no room for connectivity modulation with task prioritization effect. Despite relatively smaller attentional focus-related differences in suprathreshold connectivity (Fig. 4c), dual-task performances were very different between the two attentional focus strategies within the PD group. At the behavioral level, the early-stage PD group still exhibited superior postural and suprapostural performance with the supraposture-focus strategy (Fig. 2). Compared to the supraposture-focus strategy, the posture-focus strategy led to a stronger circuit loop in the central-parietal-occipital area. The specific roles of inter-regional connectivity relate to the direction and maintenance of visuospatial attention and postural awareness [63, 64], which partly explains the larger postural sway and higher postural regularity for weighting on the postural goal.
Functional correlation between cortical connectivity and motor severity
We found a significantly negative correlation between the MDS-UPDRS part III scores and m-PLI for the addition of the ring-touching task with a supraposture-focus strategy (Dual_SF − Single) (Table 2a,b). Interestingly, the larger increase in cortical connectivity strength for the addition of the suprapostural motor task was associated with less overall motor impairment (i.e., total scores of MDS-UPDRS part III) and less hand motor severity (i.e., hand-related sub-scores of MDS-UPDRS part III). However, the connectivity strengths for the single-posture task and postural dual-task, or the connectivity differences between the postural dual-task and single-posture task, were independent of posture and gait-related impairments (Table 2c). The connectivity strength significantly correlated with hand impairment but not with postural and gait impairment may because the difference of involved tasks in the Single condition and the Dual_SF condition was the ring-touching task in our present study. This finding functionally underscores the importance of compensatory increases in EEG connectivity. Furthermore, the association between severity of hand movement and connectivity strength is particularly significant when a supraposture-focus strategy was adopted. With a supraposture-focus strategy, participants had to devote more attentional resource to the ring-touching task. The association between greater functional connectivity strength and less motor severity might indicate that the patients in early-stage PD with better motor (or hand) function could better shift attention and increase cognitive processes to cope with the suprapostural task in compensation for functional deficits in basal ganglia [65]. This argument of the functionally importance of greater brain network connectivity is also supported by several previous fMRI-based studies. As evaluated with MDS-UPDRS part III, lower postural instability and gait difficulty-dominant (PIGD) scores are associated with compensatory increases in connectivity between the putamen and sensorimotor cortex or within the calcarine area in patients with early-stage PD [66, 67]. Likewise, improvement of motor symptoms, as assessed with UPDRS part III, is linked to increased functional cerebellar connectivity after administration of levodopa [68]. For a postural dual-task, the significant correlation between the change of cortical connectivity strength and motor severity in this study implies that the organizations of scalp EEG connectivity could be used to characterize variant brain resource processes in PD with different motor severities.
Methodological concerns
Notably, this study only targeted patients with early-stage PD (modified H&Y stage: 1–2) and investigated a relatively small group. The compensatory mechanisms at the behavioral and cortical levels could differ with disease progression [17, 24]. Additionally, this study could not provide direct neural evidence of subcortical regulation and network interaction between the cortical and subcortical areas. Due to remarkable movement-related artifact, the use of fMRI to explore activation patterns of subcortical structures is limited in a real postural dual-task paradigm. However, scalp EEG can detect cortical responses in a postural dual-task setting, which enables us to speculate some possible roles of subcortical structures that have a dense connection with the cortical motor loop.
Conclusions
For the healthy control group, the supraposture-focus strategy was an optimal task prioritization strategy in terms of sway reduction and facilitation of postural automaticity during a postural dual-task. For patients with early-stage PD, the posture-focus strategy was not favorable for the postural dual-task, resulting in the worst suprapostural task performance and stance destabilization with a propensity to fall under postural dual-task conditions. Regardless of attentional focus, both the PD and control groups exhibited distinct neural mechanisms for the postural dual-task. While healthy individuals reduced their reliance on cortical engagement for the concurrent suprapostural task, patients with early-stage PD tended to tax cortical resources, especially with the posture-focus strategy. Collectively, we suggest that an early posture-manual dual-task rehabilitative intervention with a supraposture-focus strategy could be administered with the aim of either compensatory use of cortical resources (like in high-function early-stage PD) or adaptive resource sharing with subcortical structures (like in healthy controls) to cope with dual-task interference.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
C.Y.H., R.M.W., and I.S.H. designed the experiments. C.Y.H., R.M.W., and L.C.C. conducted the experiments. C.Y.H., L.C.C., and I.S.H. analyzed the data. C.Y.H. and I.S.H. wrote the paper. C.Y.H acquired funding.
Funding
This work was supported by a grant from the Ministry of Science and Technology, R.O.C. Taiwan (grant no. MOST 109–2314-B-002–115-MY3).
Data availability
The data sets generated and analyzed in this study are available from the corresponding author upon reasonable request.
Code availability
The scripts for analysis are available from the corresponding author upon reasonable request.
Declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the Helsinki declaration and approved by the National Taiwan University Hospital Research Ethics Committee.
Consent for publication
For Supplementary Fig. 1, the participant has provided consent for medical photography and consented for the photographs to be used in the publication.
Consent to participate
Written informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim SD, Allen NE, Canning CG, Fung VS. Postural instability in patients with Parkinson’s disease Epidemiology pathophysiology and management. CNS Drugs. 2013;27(2):97–112. doi: 10.1007/s40263-012-0012-3. [DOI] [PubMed] [Google Scholar]
- 2.Chastan N, Debono B, Maltête D, Weber J. Discordance between measured postural instability and absence of clinical symptoms in Parkinson’s disease patients in the early stages of the disease. Mov Disord. 2008;23(3):366–372. doi: 10.1002/mds.21840. [DOI] [PubMed] [Google Scholar]
- 3.Halmi Z, Dinya E, Málly J. Destroyed non-dopaminergic pathways in the early stage of Parkinson’s disease assessed by posturography. Brain Res Bull. 2019;152:45–51. doi: 10.1016/j.brainresbull.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Brachman A, Marszałek W, Kamieniarz A, Michalska J, Pawłowski M, Juras G. Detection of postural control in early Parkinson’s disease: clinical testing vs modulation of center of pressure. PLoS One. 2021;16(1):e0245353. doi: 10.1371/journal.pone.0245353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu T, Hallett M. Dual task interference in Parkinson’s disease. Eur Neurol Rev. 2009;4(2):34–7. doi: 10.17925/ENR.2009.04.02.34. [DOI] [Google Scholar]
- 6.Boisgontier MP, Beets IA, Duysens J, Nieuwboer A, Krampe RT, Swinnen SP. Age-related differences in attentional cost associated with postural dual tasks: increased recruitment of generic cognitive resources in older adults. Neurosci Biobehav Rev. 2013;37(8):1824–1837. doi: 10.1016/j.neubiorev.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 7.McIsaac TL, Fritz NE, Quinn L, Muratori LM. Cognitive-motor interference in neurodegenerative disease: a narrative review and implications for clinical management. Front Psychol. 2018;9:2061. doi: 10.3389/fpsyg.2018.02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chastan N, Decker LM. Posturo-locomotor markers of preclinical Parkinson’s disease. Neurophysiol Clin. 2019;49(2):173–180. doi: 10.1016/j.neucli.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Bloem BR, Grimbergen YA, van Dijk JG, Munneke M. The, “posture second” strategy: a review of wrong priorities in Parkinson’s disease. J Neurol Sci. 2006;248(1–2):196–204. doi: 10.1016/j.jns.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 10.Peterson DS, Phan V, Richmond SB, Lee H. Effects of dual-tasking on time-to-boundary during stance in people with PD: a preliminary study. Clin Biomech (Bristol, Avon) 2021;88:105420. doi: 10.1016/j.clinbiomech.2021.105420. [DOI] [PubMed] [Google Scholar]
- 11.Canning CG. The effect of directing attention during walking under dual-task conditions in Parkinson’s disease. Parkinsonism Relat Disord. 2005;11(2):95–99. doi: 10.1016/j.parkreldis.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Yogev-Seligmann G, Rotem-Galili Y, Dickstein R, Giladi N, Hausdorff JM. Effects of explicit prioritization on dual task walking in patients with Parkinson’s disease. Gait Posture. 2012;35(4):641–646. doi: 10.1016/j.gaitpost.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–770. doi: 10.1002/mds.24963. [DOI] [PubMed] [Google Scholar]
- 14.Jehu DA, Desponts A, Paquet N, Lajoie Y. Prioritizing attention on a reaction time task improves postural control and reaction time. Int J Neurosci. 2015;125(2):100–106. doi: 10.3109/00207454.2014.907573. [DOI] [PubMed] [Google Scholar]
- 15.Yu SH, Huang CY. Improving posture-motor dual-task with a supraposture-focus strategy in young and elderly adults. PLoS ONE. 2017;12(2):e0170687. doi: 10.1371/journal.pone.0170687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang CY, Chen YA, Hwang IS, Wu RM. Improving dual-task control with a posture-second strategy in early-stage Parkinson disease. Arch Phys Med Rehabil. 2018;99:1540–1546. doi: 10.1016/j.apmr.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 17.Hung YT, Chen LC, Wu RM, Huang CY. The effects of task prioritization on dual-tasking postural control in patients with Parkinson disease who have different postural impairments. Arch Phys Med Rehabil. 2020;101(7):1212–1219. doi: 10.1016/j.apmr.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Vervoort G, Heremans E, Bengevoord A, Strouwen C, Nackaerts E, Vandenberghe W, et al. Dual-task-related neural connectivity changes in patients with Parkinson’ disease. Neuroscience. 2016;317:36–46. doi: 10.1016/j.neuroscience.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 19.Peterson DS, Fling BW, Mancini M, Cohen RG, Nutt JG, Horak FB. Dual-task interference and brain structural connectivity in people with Parkinson’s disease who freeze. J Neurol Neurosurg Psychiatry. 2015;86(7):786–792. doi: 10.1136/jnnp-2014-308840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lench DH, Embry A, Hydar A, Hanlon CA, Revuelta G. Increased on-state cortico-mesencephalic functional connectivity in Parkinson disease with freezing of gait. Parkinsonism Relat Disord. 2020;72:31–36. doi: 10.1016/j.parkreldis.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128(Pt 10):2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- 22.Onu M, Badea L, Roceanu A, Tivarus M, Bajenaru O. Increased connectivity between sensorimotor and attentional areas in Parkinson’s disease. Neuroradiology. 2015;57(9):957–968. doi: 10.1007/s00234-015-1556-y. [DOI] [PubMed] [Google Scholar]
- 23.Azulay JP, Mesure S, Blin O. Influence of visual cues on gait in Parkinson’s disease: contribution to attention or sensory dependence? J Neurol Sci. 2006;248(1–2):192–195. doi: 10.1016/j.jns.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Berendse HW, Stam CJ. Stage-dependent patterns of disturbed neural synchrony in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S440–S445. doi: 10.1016/S1353-8020(08)70046-4. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Criaud M, Cho SS, Díez-Cirarda M, Mihaescu A, Coakeley S, et al. Abnormal intrinsic brain functional network dynamics in Parkinson’s disease. Brain. 2017;140(11):2955–2967. doi: 10.1093/brain/awx233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wen MC, Heng HSE, Hsu JL, Xu Z, Liew GM, Au WL, et al. Structural connectome alterations in prodromal and de novo Parkinson’s disease patients. Parkinsonism Relat Disord. 2017;45:21–27. doi: 10.1016/j.parkreldis.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Xiong Y, Liu S, Zhou R, Hu Z, Tong Y, et al. Predicting the post-therapy severity level (UPDRS-III) of patients with Parkinson’s disease after drug therapy by using the dynamic connectivity efficiency of fMRI. Front Neurol. 2019;10:668. doi: 10.3389/fneur.2019.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbasi N, Fereshtehnejad SM, Zeighami Y, Larcher KM, Postuma RB, Dagher A. Predicting severity and prognosis in Parkinson’s disease from brain microstructure and connectivity. Neuroimage Clin. 2020;25:102111. doi: 10.1016/j.nicl.2019.102111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–1256. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 30.Brauer SG, Morris ME. Can people with Parkinson’s disease improve dual tasking when walking? Gait Posture. 2010;31(2):229–233. doi: 10.1016/j.gaitpost.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129–70. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 33.Riley MA, Balasubramaniam R, Turvey MT. Recurrence quantification analysis of postural fluctuations. Gait Posture. 1999;9(1):65–78. doi: 10.1016/s0966-6362(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 34.Schmit JM, Riley MA, Dalvi A, Sahay A, Shear PK, Shockley KD, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson’s disease. Exp Brain Res. 2006;168:357–367. doi: 10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 35.Negahban H, Sanjari MA, Karimi M, Parnianpour M. Complexity and variability of the center of pressure time series during quiet standing in patients with knee osteoarthritis. Clin Biomech (Bristol Avon) 2016;32:280–5. doi: 10.1016/j.clinbiomech.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Kennel MB, Brown R, Abarbanel HD. Determining embedding dimension for phase-space reconstruction using a geometrical construction. Phys Rev A. 1992;45(6):3403–3411. doi: 10.1103/physreva.45.3403. [DOI] [PubMed] [Google Scholar]
- 37.Huang CY, Hwang IS. Behavioral data and neural correlates for postural prioritization and flexible resource allocation in concurrent postural and motor tasks. Hum Brain Mapp. 2013;34(3):635–650. doi: 10.1002/hbm.21460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu SH, Wu RM, Huang CY. Attentional resource associated with visual feedback on a postural dual task in Parkinson’s disease. Neurorehabil Neural Repair. 2020;34(10):891–903. doi: 10.1177/1545968320948071. [DOI] [PubMed] [Google Scholar]
- 39.Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. 2007;28(11):1178–1193. doi: 10.1002/hbm.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tewarie P, Hillebrand A, Schoonheim MM, van Dijk BW, Geurts JJ, Barkhof F, et al. Functional brain network analysis using minimum spanning trees in multiple sclerosis: an MEG source-space study. Neuroimage. 2014;88:308–318. doi: 10.1016/j.neuroimage.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 41.Niso G, Bruña R, Pereda E, Gutiérrez R, Bajo R, Maestú F, et al. HERMES: towards an integrated toolbox to characterize functional and effective brain connectivity. Neuroinformatics. 2013;11(4):405–434. doi: 10.1007/s12021-013-9186-1. [DOI] [PubMed] [Google Scholar]
- 42.Fradette K, Keselman HJ, Lix L, Algina J, Wilcox RR. Conventional and robust paired and independent-samples t tests: type I error and power rates. J Mod Appl Stat Methods. 2003;2:481–96. doi: 10.22237/jmasm/1067646120. [DOI] [Google Scholar]
- 43.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53(4):1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 44.Stoffregen TA, Smart LJ, Bardy BG, Pagulayan RJ. Postural stabilization of looking. J Exp Psychol Hum Percept Perform. 1999;25(6):1641–1658. doi: 10.1037/0096-1523.25.6.1641. [DOI] [Google Scholar]
- 45.Mitra S, Fraizer EV. Effects of explicit sway-minimization on postural–suprapostural dual-task performance. Hum Mov Sci. 2004;23(1):1–20. doi: 10.1016/j.humov.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Plummer P, Eskes G. Measuring treatment effects on dual-task performance: a framework for research and clinical practice. Front Hum Neurosci. 2015;9:225. doi: 10.3389/fnhum.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauvage C, De Greef N, Manto M, Jissendi P, Nioche C, Habas C. Reorganization of large-scale cognitive networks during automation of imagination of a complex sequential movement. J Neuroradiol. 2015;42(2):115–125. doi: 10.1016/j.neurad.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs JV, Horak FB. Cortical control of postural responses. J Neural Transm (Vienna) 2007;114(10):1339–1348. doi: 10.1007/s00702-007-0657-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57(1):192–198. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 50.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 51.Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10(1):1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 53.Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7(2):193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- 54.Wu T, Chan P, Hallett M. Effective connectivity of neural networks in automatic movements in Parkinson’s disease. Neuroimage. 2010;49(3):2581–2587. doi: 10.1016/j.neuroimage.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berry AS, Shah VD, Baker SL, Vogel JW, O'Neil JP, Janabi M, et al. Aging affects dopaminergic neural mechanisms of cognitive flexibility. J Neurosci. 2016;36(50):12559–12569. doi: 10.1523/JNEUROSCI.0626-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stav AL, Johansen KK, Auning E, Kalheim LF, Selnes P, Bjørnerud A, et al. Hippocampal subfield atrophy in relation to cerebrospinal fluid biomarkers and cognition in early Parkinson’s disease: a cross-sectional study. NPJ Parkinsons Dis. 2016;2:15030. doi: 10.1038/npjparkd.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mallol R, Barrós-Loscertales A, López M, Belloch V, Parcet MA, Avila C. Compensatory cortical mechanisms in Parkinson’s disease evidenced with fMRI during the performance of pre-learned sequential movements. Brain Res. 2007;1147:265–71. doi: 10.1016/j.brainres.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 58.Wu T, Wang L, Hallett M, Chen Y, Li K, Chan P. Effective connectivity of brain networks during self-initiated movement in Parkinson’s disease. Neuroimage. 2011;55(1):204–215. doi: 10.1016/j.neuroimage.2010.11.074. [DOI] [PubMed] [Google Scholar]
- 59.Göttlich M, Münte TF, Heldmann M, Kasten M, Hagenah J, Krämer UM. Altered resting state brain networks in Parkinson’s disease. PLoS ONE. 2013;8(10):e77336. doi: 10.1371/journal.pone.0077336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schönberger AR, Hagelweide K, Pelzer EA, Fink GR, Schubotz RI. Motor loop dysfunction causes impaired cognitive sequencing in patients suffering from Parkinson's disease. Neuropsychologia. 2015;77:409–420. doi: 10.1016/j.neuropsychologia.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 61.Morcom AM, Henson RNA. Increased prefrontal activity with aging reflects nonspecific neural responses rather than compensation. J Neurosci. 2018;38(33):7303–7313. doi: 10.1523/JNEUROSCI.1701-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hülsdünker T, Mierau A, Neeb C, Kleinöder H, Strüder HK. Cortical processes associated with continuous balance control as revealed by EEG spectral power. Neurosci Lett. 2015;592:1–5. doi: 10.1016/j.neulet.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 63.Ghosh P, Roy D, Banerjee A. Organization of directed functional connectivity among nodes of ventral attention network reveals the common network mechanisms underlying saliency processing across distinct spatial and spatio-temporal scales. Neuroimage. 2021;231:117869. doi: 10.1016/j.neuroimage.2021.117869. [DOI] [PubMed] [Google Scholar]
- 64.Malhotra P, Coulthard E, Husain M. Hemispatial neglect, balance and eye-movement control. Curr Opin Neurol. 2006;19(1):14–20. doi: 10.1097/01.wco.0000198101.87670.7e. [DOI] [PubMed] [Google Scholar]
- 65.Mentis MJ, Dhawan V, Feigin A, Delalot D, Zgaljardic D, Edwards C, et al. Early stage Parkinson’s disease patients and normal volunteers: comparative mechanisms of sequence learning. Hum Brain Mapp. 2003;20(4):246–258. doi: 10.1002/hbm.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen B, Pan Y, Jiang X, Wu Z, Zhu J, Dong J, et al. Altered putamen and cerebellum connectivity among different subtypes of Parkinson’s disease. CNS Neurosci Ther. 2016;26(2):207–214. doi: 10.1111/cns.13259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou Y, Ou R, Yang J, Song W, Gong Q, Shang H. Patterns of striatal and cerebellar functional connectivity in early-stage drug-naïve patients with Parkinson's disease subtypes. Neuroradiology. 2018;60(12):1323–1333. doi: 10.1007/s00234-018-2101-6. [DOI] [PubMed] [Google Scholar]
- 68.Mueller K, Jech R, Ballarini T, Holiga Š, Růžička F, Piecha FA, et al. Modulatory effects of Levodopa on cerebellar connectivity in Parkinson’s disease. Cerebellum. 2019;18(2):212–224. doi: 10.1007/s12311-018-0981-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated and analyzed in this study are available from the corresponding author upon reasonable request.
The scripts for analysis are available from the corresponding author upon reasonable request.



