Abstract
Angiogenesis is a characteristic of glioblastoma (GBM), the most fatal and therapeutic-resistant brain tumor. Highly expressed angiogenic cytokines and proliferated microvascular system made anti-angiogenesis treatments a thoroughly plausible approach for GBM treatment. Many trials have proved to be not only as a safe but also as an effective approach in GBM retardation in a certain time window as seen in radiographic response rates; however, they have failed to implement significant improvements in clinical manifestation whether alone or in combination with radio/chemotherapy. Bevasizumab, an anti-vascular endothelial growth factor-A (VEGF-A) antibody, is the only agent that exerts meaningful clinical influence by improving progression-free survival (PFS) and partially alleviate clinical symptoms, nevertheless, it could not prolong the overall survival (OS) in patients with GBM. The data generated from phase II trials clearly revealed a correlation between elevated reperfusion, subsequent to vascular normalization induction, and improved clinical outcomes which explicitly indicates anti-angiogenesis treatments are beneficial. In order to prolong these initial benefits observed in a certain period of time after anti-angiogenesis targeting, some aspects of the therapy should be tackled: recognition of other bypass angiogenesis pathways activated following anti- angiogenesis therapy, identification of probable pathways that induce insensitivity to shortage of blood supply, and classifying the patients by mapping their GBM-related gene profile as biomarkers to predict their responsiveness to therapy. Herein, the molecular basis of brain vasculature development in normal and tumoral conditions is briefly discussed and it is explained how "vascular normalization" concept opened a window to a better comprehension of some adverse effects observed in anti-angiogenesis therapy in clinical condition. Then, the most targeted angiogenesis pathways focused on ligand/receptor interactions in GBM clinical trials are reviewed. Lastly, different targeting strategies applied in anti-angiogenesis treatment are discussed.
Keywords: Angiogenesis Pathway, Cell Adhesion Molecules, Clinical Trial, Glioblastoma, Tyrosine-Kinase Receptors
Introduction
Compensatory angiogenesis has been long introduced as the fuel for the tumor progression engine as well as an appealing target to curb tumor propagation. Glioblastoma multiforme (GBM) is a highly vascularized tumor that has been vastly investigated in anti-angiogenesis therapy (1), partly because of its resistance to current standard therapies including surgical resection, radiation, and chemotherapy. Moreover, patients with GBM show a survival period of less than 15 months. Also, patients with recurrent GBM have minimal treatment options and the survival time is less than 6 months. Therefore, GBM remains as one of the deadliest malignancies. Recent studies have demonstrated promising results from new anti-angiogenesis approaches that raise hope treating several solid tumors including GBM. Among these treatments, several FDA (U.S. Food and Drug Administration)-approved anti-vascular endothelial growth factor (VEGF) agents have been demonstrated satisfactory results such as bevacizumab (Avastin) for GBM, advanced colorectal cancer, non-small cell lung cancer, and breast cancer, Sorafenib (Nexavar) for patients with advanced renal cell and hepatocellular carcinoma, and sunitinib (Sutent) for advanced renal cell carcinoma and progressive gastrointestinal stromal tumor (2). Also, it has been shown that a combination of Sorafenib and administration of mesenchymal stem cell (MSC) improve the anti-angiogenesis effect of the drug in hepatocellular carcinoma (HCC) (3). Such promising results support the need for additional investigations.
This review summarizes angiogenesis in tumor tissue with a focus on angiogenic pathways in GBM, their targeting, the approaches’ pros and cons, and related clinical trials.
Development of brain vasculature
Brain is a metabolically active organ in the body and its metabolism and physiochemical activity are absolutely oxygen dependent. The constant rate of oxygen consumption in brain tissue is 3.5 ml of O2 /100 g of brain tissue/minutes accounting for 20% of total oxygen consumption in the body’s rest state in a conscious young man. This oxygen is delivered and distributed precisely and unevenly throughout the brain based on different regions’ activity and metabolism. A highly developed architecture of the brain vasculature is responsible for such a delicate oxygenation and blood supply with different perfusion rates in different areas (4).
Many studies have suggested the formation of embryonic brain vasculature from a primary surrounding peri-neural vascular plexus (PNVP) at certain stages of development in which brain is invaded in specific stereotypical patterns (5). The cellular basis for development of neurovascular plexus seems to be the interactions between endothelial and neural cells that are also involved in developing the blood-brain barrier (BBB). Somatic angioblasts have been proven to be the source of ECs contributing to the ingression of vessel sprouts in the avian neural tube. Signals from the developing nervous system direct sprouting vessels into the distinct areas of the brain. Contribution of neurovascular unit composed of ECs, pericytes, neurons, and astrocytes results in the development of the BBB and regulation of cerebral blood flow (6).
Molecular basis of vascular development in the nervous system
Although numerous molecules have been identified as angiogenic factors so far, the number of these factors is still expanding. Fibroblast growth factor1 (FGF1) is one of the first pre-angiogenic factors reported to be involved in vasculogenesis in developing the brain. Besides, VEGF as another pre-angiogenic factor with known effect on PNVP formation and subsequent vessel sprouts ingression into the developing neural tube, induces migration and patterning of angioblasts migrating from somitic mesoderm (7). Also, the role of integrin signaling pathways in the maturation of neurovascular structure through enhanced interaction of ECs and pericytes as well as induction of BBB development has been reported in many studies.
The other signaling pathways contributing to neurovascular development are semaphorins/plexin receptors, slits/Robo receptors, neuropilins (co-receptors of both VEGF and plexin receptors), netrins receptor UNC/DCC, and canonical Wnt signaling pathways (8).
Angiogenesis in brain tumors
Judah Folkman suggested that solid tumors meet tumor cells’ need to excess nutrient and oxygen supply as well as their pervasiveness by provoking angiogenesis. Numerous studies have been conducted since then in an attempt to unveil the underlying mechanisms in malignancies and their differences form physiologic angiogenesis for more precisely modulate angiogenesis applying antiangiogenesis therapy (9).
There are some differences between angiogenesis in cancer and normal tissues in terms of vascular structure, interactions between vascular ECs and pericytes, rate of blood flow, vascular permeability, and the maturation level (10). Similar to other cancer tissues, pathological vessels in GBM are tangled, unorganized, highly permeable and leaky, significantly larger in diameter, and thick in the basement membrane. Besides, the loose interaction between ECs and pericytes, and low pericyte coverage led to the development of a leaky, hemorrhagic vascular network as well as an impaired BBB leaving tumor tissue hypoxic and necrotic. Other common features of the vasculature architecture in cancer tissues are their irregularity, heterogeneity in shape and tissue distribution, uneven vessels diameter, excessive branching with some dead-end vessels, tortuosity, as well as chaotic structure of vascular network at all levels of arterioles, venules and capillaries (11).
Furthermore, the tight balance between pro and antiangiogenesisfactors is one of the main regulators of physiologic vasculogenesis, whereas in GBM the balance heavily tips in favor of the expression of pro-angiogenic genes activated in response to tissue hypoxia, hypoxia-inducible factor1 (HIF-1) and acidosis. Highly active angiogenesis leads to glomeruloid tuft formation comprising perivascular and endothelial cells multilayered (12).
Some important molecules which are active in tumor vasculogenesis are VEGF, stromal-derived factor 1 (SDF1), platelet-derived growth factor (PDGF), granulocyte macrophage-colony stimulating factor (GM-CSF), insulinlike growth factor1 (IGF1), plasminogen activation factor 1 (PAI1), nitric oxide (NO), cyclooxygenase2 (COX2) and thrombospondin 2 (TSP2) (13).
It has been shown that VEGF increases vascular permeability through an increase in vascular wall pore size and number as well as a decrease in pericyte coverage. Besides, SDF1, as a very important modulator of brain tumor vasculogenesis, mediates its effect via the recruitment of intratumoral marrow-derived precursors into tumor endothelium. Vasculature maturation status is also different in tumor tissues; while most of the normal vessels are mature, stable and quiescent in normal tissue. They constantly proliferate and expand from the pre-existing vessels in tumor (14).
Vascular normalization theory
Since Folkman’s proposition in anti-angiogenesis therapy to entirely block vessel growth in tumor settings in 1970s, it has been shown that applying excessive anti-angiogenesis factors may result in aggravating hypoxia recurrence fueling tumor progression. To conquer the unfavorable outcomes, “vascular normalization” concept was suggested by Jain in 2014 aiming to normalize tumor vasculature by restoring the balance between pro- and anti-angiogenesis agents to improve blood supply and drug access in the tumor region rather than totally destroying abnormal vessels. The concept was successfully examined using bevacizumab in mouse models of neuroblastoma in which the treatment led to normal vasculature function and structure (11).
Angiogenesis pathways and anti-angiogenesis strategies
Many factors are known to affect ECs to promoting or eliminating angiogenesis. ECs receive these signals from the microenvironment either through their growth factor tyrosine kinase receptors (RTKs) such as VEGFR and epidermal growth factor receptor (EGFR), or via other growth factor receptors including TGF-β/TβRII and Wnt /Frizzled (Fzd) family receptor in addition to receptors involved in cell adhesion like integrins. Many studies have been conducted to target these signaling pathways for their potential effects in anti-angiogenesis therapy in GBM.
Growth factor tyrosine kinase receptors (RTKs)
The angiogenic effect of several growth factors including VEGFs, EGFs, FGFs, platelet-derived growth factors (PDGFs), angiopoietins (Ang-1 and Ang-2), hepatocyte growth factor (HGF) and ephrins is mediated through tyrosine kinase receptors. Ligand binding to the receptors leads to receptor homo- or hetero-dimerization and downstream signal activation. Some clinically targeted RTK are briefly discussed in the following section.
VEGF/VEGFR pathway
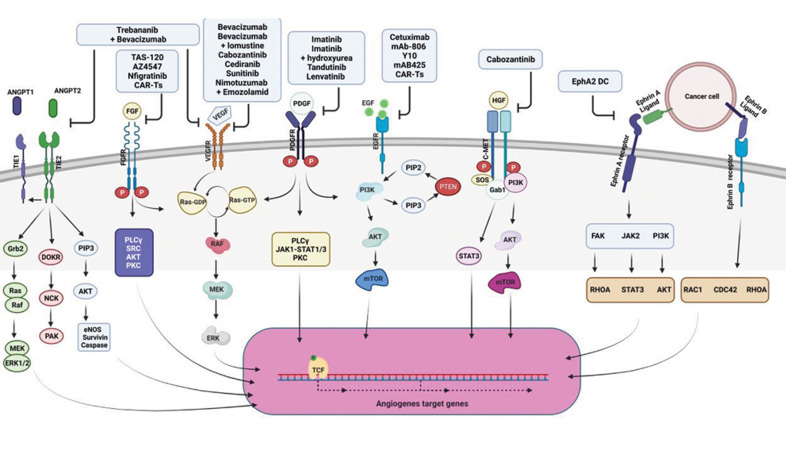
VEGFs are secreted by different cell types, including tumor cells, fibroblasts, and inflammatory cells, mostly in response to increasing tissue hypoxia in cancer. VEGF family including VEGFAs (VEGFA-165, -121, -145, -189 and -(xxx) b), VEGFB, VEGFC, VEGFD and placenta growth factor (PGF) bind to their receptors such as VEGFR1 (FLT-1), VEGFR2 (FLK1 or KDR) and VEGFR3 (FLT4) in addition to co-receptors proteoglycans and neurophilins in both physiologic and pathologic conditions. Depending on which VEGF/VEGFR is activated the outcome is different: VEGFAs (VEGF-165, 145-189)/VEGFR-1,-2 activation is predominantly in favor of angiogenesis while VEGF-12, – (xxx)b/VEGFR1, -2 activation is anti-angiogenic, VEGFB/ VEGFR1 activation is involved in fatty acid uptake in EC , VEGFC, VEGFD/VEGFR3, -2 induce lymphangiogenesis and PIGF/VEGFR1 recruits inflammatory cells. Besides, it has been demonstrated that VEGFAs/VEGFR2 activation develops immune privileged area in tumor site by suppression of T-cell function and dendritic cells (DCs) activation, as well as recruitment of regulatory T-cells (Tregs) and myeloidderived suppressor cells (MDSCs) (15). Hence, targeting this pathway especially the interaction between VEGF-A and VEGFR-2, the main intra-tumoral angiogenesis pathway, has been vastly studied in cancer. For instance, Atorvastatin and deforolimus have been successfully used to reduce VEGF concentration in both glioma spheroids (16) and endometrial stromal cells (17), respectively. Some of the agents with proven efficiency in anti-angiogenesis therapy in cancer preclinical and clinical trials are now being marketed and prescribed to patients with cancer. To date, four inhibitors have been approved by the FDA: sunitinib and pazopanib for metastatic renal-cell carcinoma and gastrointestinal stromal tumors, vandetanib for medullary thyroid cancer and sorafenib for unresectable hepatocellular carcinoma and metastatic renal cell carcinoma (RCC). Besides, bevacizumab (Avastin) (Table 1), an anti-VEGF aptamer, is being used as an off-label drug for wet age-related macular degeneration that has FDA full approval for the treatment of adult patients with GBM (Fig .1) (2).
Table 1.
Anti-angiogenesis clinical trials in GBM-targeting receptor tyrosine kinases (RTKs)
|
| |||||
|---|---|---|---|---|---|
| Target class | Treatments | Target | Clinical trial result | No. /phase | Trial reference No. |
|
| |||||
| VEGF/VEGFR | Bevacizumab (Avastin) | VEGF-A | rGBM | II/ 167 | NCT00345163 |
| ORR: %:28.2, PFS (months): - | |||||
| PFS6 (%):42.6, OS (months): 8.6 | |||||
| Bevacizumab (Avastin), Lomustine | VEGF-A | rGBM | III/ 592 | NCT01290939 | |
| Ongoing | |||||
| EGF/EGFR | Nimotuzumab (OSAG101) | L2 domain of EGFR | Cerebral GBM | II/ 39 | NCT03388372 |
| + Temozolamid, | ORR %: 72.2, PFS (months): 11.9 | ||||
| + standard | PFS6(%): -, OS (months): 24.5 | ||||
| treatment | |||||
| CAR-T cell | EGFRvIII | MG Recruiting | /I | NCT01454596 | |
| CAR-T cell | EGFRvIII | GBM Recruiting | /I | NCT02209376 | |
| CAR-T cell | IL13Rα2 | rMG Completed | 6/I | NCT01082926 | |
| FGF/FGFR | AZ4547 | FGFR1-3 | rGBM | I/II 14 | NCT02824133 |
| IGF1 R | Suspended | ||||
| KDR | |||||
| Infigratinib | FGFR1-3 | rGBM | II/ 26 | NCT01975701 | |
| (BGJ398) | ORR %: 7.7, PFS (months): 1.7 | ||||
| PFS6(%): -, OS (months): 6.7 | |||||
| TAS-120 | FGFR1-4 | Advanced solid tumor like GBMRecruiting patients | 386/ I/II | NCT02052778 | |
| PDGF/PDGFR | Lenvatinib | VEGFR | rGBM | II / 32 | - |
| (E7080) | FGFR | ORR %: -, PFS (months): 1.911.9 | |||
| PDGFR | PFS6(%):8.3, OS (months): 28 | ||||
| Imatinib | PDGFRα | rGBM | I/II/ 50 | - | |
| mesylate | PDGFRβ | PFS6%: 33 | |||
| (Gleevec/ST1571) | Bcr-Abl | ||||
| c-FMS | |||||
| HGF/c-MET | Cabozantinib | c-Kit | nGBM | ||
| MET | ORR (%): 17. 6, PFS (months): 3.7 | ||||
| VEGFR2 | PFS6(%): 22.3 OS (months): - | II/ 152 | NCT00704288 | ||
| Ephrins/Eph | α-type-1 | EphA2, IL-13 | MG | ||
| polarized DC | receptor-α2 | Proved safety, immunogenicity, and preliminary clinical activity of poly-ICLC-boosted αDC1-based vaccines. | |||
| vaccine loaded with | YKL-40 | I/II / 41 | NCT00766753 | ||
| IL-13, receptor-α2, YKL-40, and gp100 | gp100 | ||||
| Tandutinib | PDGFRβ | GBM | I/II /19,30 | ||
| FMS-like TK3 | Terminated due to unmet prespecified goal of SFP | ||||
| c-Kit | |||||
|
| |||||
VEGF; Vascular endothelial growth factor, PDGFR; Platelet-derived growth factor, FLT-3 TKI; Fms-like tyrosine kinase 3 tyrosine kinase inhibitor, EGF; Epidermal growth factor, EGFR; Epidermal growth factor receptor, FGFR; Fibroblast growth factor receptor, HER2; Human epidermal growth factor receptor, IGFR1R; Insulin-like growth factor type 1 receptor, KDR; Kinase insert domain receptor, PDGFR; Platelet-derived growth factor, ANG; Angiopoietin, Tie; Tyrosine kinase with immunoglobulin-like and EGF-like domains 1, HGF; Hepatocyte growth factor, c-MET; Tyrosineprotein kinase Met, Eph; Erythropoietin-producing human hepatocellular receptors, DC; Dendritic cell, YKL-40; Chitinase-3-like protein 1, gp100; Glycoprotein 100, EphA2; EPH receptor A2, Bcr-Abl; Breakpoint cluster region – Abelson murine leukemia virus, c-FMS; Feline McDonough sarcoma, FMS-like TK3; FMS-like tyrosine kinase 3, Met; Mesenchymal-epithelial transition factor, GBM; MG; Malignant glioma, Glioblastoma, rGBM; Recurrent GBM, ORR; Overall response rate, PFS; Progression free survival, PFS6; Progression-free survival at 6 months, OS; Overall survival, and mOS; Median survival rate.
Fig 1.
Tyrosine kinase receptor-dependent angiogenesis pathways and their targeting in clinical trials on glioblastoma. Different types of inhibitors (shown in upper blue boxes) targeting RTK-dependent angiogenesis pathways (RTKs are shown on the cell membrane) have been investigated in clinical trials on GBM to modulate angiogenesis. RTK; Tyrosine kinase receptor, ANGPT; Angiopoietin, FGF; Fibroblast growth factor, VEGF; Vascular endothelial growth factor, PDGF; Platelet-derived growth factor, EGF; Epidermal growth factor, HGF; Hepatocyte growth factor, and TCF; Transcription factor.
Despite examining a wide range of agents to target VEGF/VEGFR in clinical trials, almost all trials have failed to improve overall survival (OS) except Bevasizumab. Although the radiographic responses, progression-free survival (PFS) and brain edema improve in many cases for a period of time, GBM relapse following anti-VEGF/ anti-VEGFR treatments has been reported. Hence several problems should be considered to enhance the benefits observed from a cumulative number of studies on VEGF/ VEGFR targeting: alternative pathways activated after anti-VEGF/anti-VEGFR treatments should be explored, some biomarkers should be defined to better classify and recognize those patients most benefit from the treatments and an optimum dosing and timing should be defined.
High affinity and low affinity ligands /EGFR
EGFR, also designated as HER1 (human EGFR) or ErbB1 (named after its homology to a viral oncogene called erythroblastic leukemia viral oncogene), is one of the four members of the ErbB family; HER2/neu (ErbB2), HER3 (ErbB-3), and HER4 (ErbB-4). The receptor activation triggers manifold responses depending on the type of ligands binding to the receptor. More than 40 ligands have been recognized that can be classified into high-affinity ligands, such as EGF, transforming growth factor alpha (TGF-α), and heparin-binding EGF-like growth factor (HB-EGF); and low-affinity ligands, such as amphiregulin (AREG), epiregulin (EREG), and epigen (EPGN). There is evidence showing the alteration of gene expression in the receptor in different types of cancers like GBM in which EGFR amplification induces GBM proliferation, invasion and drug resistance. Also, EGFR alterations are applied as a prognostic marker for GBM. There have been around 20 clinical trials to target EGFR using agents such as Cetuximab, Afatinib, Erlotinib, Gefitinib, Lapatinib, Nimotuzumab, to name a few. Most of the drugs have produced poor results in terms of clinical outcomes, because of EGFR heterogeneity, drug low specificity and low BBB penetration (Table 1). Besides, it has been interestingly proposed that clinical successes achieved applying tyrosine kinase inhibitors (TKIs) against EGFRSs are associated with particular mutations in EGFR rather than the type of tumor as the same non-respondent mutations in GBM do not respond to treatments in lung adenocarcinoma (LUAD) as well. More importantly, it has been demonstrated that the drugs with the same target have clinically varied outcomes. Therefore, this pathway response to treatment is highly specific and any improvements in the field depend on the recognition and classification of mutations in EGFRs as well as precisely identification of target sites for drugs (18).
FGF/FGFR pathway
FGFs family are secreted mostly from fibroblasts and deposited close to the ECs’ basement membrane. There are 23 members in the family, FGF1 through FGF23, which all bind to FGFR1, -4 except FGF11 through FGF-14 that play role in intracellular processes other than FGFs related functions. Accumulating data propose that the interaction of these factors with their corresponding transmembrane tyrosine kinase receptors affect tumor angiogenesis both in early (basal lamin degradation, migration and proliferation) and in late phase (morphogenesis and vessel maturation). For instance, FGF-2/FGFR-2 increases the expression of matrix metalloproteinase 9 (MMP9) and enhances vascular smooth muscle cell (VSMC) migration.
Furthermore, there is a growing number of reports showing the relationship between upregulation of FGFs or FGFRs and the occurrence of different cancers. For example, FGF7 and FGF10 are present in stroma in prostate, FGFR3 is overexpressed in t (4;14) multiple myeloma, FGF-1, FGF-2 and FGF-5 are overexpressed in pancreatic ductal adenocarcinoma (PDAC), FGF-1 induces chemoresistance and highly expressed FGFR-1 is a hall mark for tumor progression (19, 20). There are some small molecule inhibitors that target FGFR1–3 like PD173074, AZ4547, BGJ398 and JNJ-493 which are not specific for either FGFR subtypes or isoforms. Amongst them, AZ4547, dovitinib, PD173074 and ponatinib effectively reduced pediatric glioma cell growth in vitro in comparison to the chemotherapeutic agent Temozolomide. AZ4547and BGJ398 (Table 1) were investigated in a clinical phase I/II and a phase II trial, respectively in patients with recurrent isocitrate dehydrogenase 1 (IDH) wild-type gliomas. The trials are suspended due to some side effects in the patients. Furthermore, the results of a clinical trial on BGJ398 (Table 1) in malignant glioma patients have not yet been published (21). Besides, a phase I/II trial of TAS-120 (Table 1), an irreversible FGFR inhibitor, is registering those suffering from advanced solid tumors, including brain tumors such as GBM (Fig .1) (22).
Among all FGFs/FGFRRs, FGF2/FGFR axis seems the most important axis for target therapy in GBM for the determinative role of FGF2 in glioma vascularization, GSC self-renewal and tumor growth. Although targeting tyrosine kinase domain of FGFRs by available inhibitors can block a prominent step in signal transduction, their lack of specificity provokes off-target effects and/or other complications. Therefore, it is critical to design more specific FGFRs inhibitors for highly sensitive and more precise target therapy.
PDGF/PDGFR pathway
Platelet-derived growth factors (PDGFs) are secreted by a variety of cells like tumor cells, ECs, macrophages, and transformed fibroblasts. The growth factors consist of PDGF-A, -D peptides which are in interaction with their receptors, PDGFR-a and PDGFR-b, evoke signalings comparable with those in VEGF/VEGFR signaling especially in recruiting pericytes to neo-vessels and secretion of a broad range of proangiogenic cytokines (23). Therefore, PDGF/ PDGFR pathway has been a promising target in cancer therapy and the results of numerous investigations were so encouraging that several agents could get approval as anti-PDGF/PDGFR drugs in several solid tumors, such as Sorafenib (Nexavar) for metastatic RCC and unresectable HCC, Sunitinib (Sutent) for gastrointestinal stromal tumor (GIST) and unresectable pancreatic neuroendocrine tumors, Pazopanib (Votrient) for metastatic RCC and advanced soft tissue sarcoma, and Axitinib (Inlyta) for metastatic RCC (24).
Although improvements in other solid tumors were impressive, anti-angiogenesis therapy has still remained ineffective in GBM. Preclinical trials have demonstrated that imatinib (Table 1), a PDGF receptor kinase inhibitor augments the radiotoxicity; however, the effect was minor in Phase II clinical trials in GBM patients (25). Moreover, in Phase II and Phase III of a clinical trial in which a combination of imatinib and hydroxyurea (Table 1) was investigated, no beneficial effect for recurrent GBM (rGBM) treatment was observed (Fig .1) (26).
There is solid evidence that high expression of PDGF and PDGFR can be detected in most GBMs. Also, induction of PDGF and PDGFR overexpression, mostly PDGFB, induces GBM in laboratory animals. These data suggest that there is definitely a positive correlation between GBM and PDGF/ PDGFR mutation and the lack of exact PDGF/PDGFR mutations profile is the key factor in clinical trials failure. Also, PDGFR profiling could hopefully improve clinical indices through early detection of the responsive subgroups of patients.
ANG/TIE pathway
ANGs are a family of growth factors (ANG-1, 4) secreted by endothelial and parenchymal cells that can bind to tyrosine kinase receptors (TIE-1 and TIE-2) on ECs. The abnormal activity of ANG-1, -2/TIE2 signaling mediates EC sprouting, pericytes recruitment, vascular remodeling and tumor plasticity. Although targeting each of these two ligands or both has improved clinical pre-targeted outcome, concomitant hindrance of both VEGF (using bevacizumab) and ANG2 (using Trebananib) (Table 1) results in both vasculature normalization and the survival benefit in comparison to inhibition of either pathway alone in GBM (Fig .1) (2).
A growing number of studies are revealing that ANG2 inhibition not only improve vascular normalization but also induces anti-tumor immunity which suggests ANG2 targeting as a favorable approach in combination therapy of treatment- resistant tumors.
HGF/c-MET pathway
The cellular mesenchymal-epithelial transition protein (c-MET), a transmembrane tyrosine kinase family, is activated by binding to the pleiotropic HGF and triggers proliferation, survival and motility of both normal and tumor-associated cells and ECs. It has been evidenced that MET expression is elevated in tumor cells, blood vessels, and peri-necrotic areas of glioma sample (27). Moreover, HGF secretion from vasculature and neurons accelerates glioma invasion and proliferation in MET-positive cells (28). Due to the crucial role of HGF/c-MET pathway in GBM development and possibly in anti-VEGF therapy resistance induction, its inhibition has been considerably studied in numerous preclinical and clinical studies (29). Some of the monoclonal antibodies used for this purpose in clinical trials are as follow: Rilotumumab (AMG102), a neutralizing antibody against HGF (30) Onartuzumab, a humanized monovalent monoclonal anti-MET antibody (31), Crizotinib, an available ATP competitive selective inhibitor for MET inhibition (32) and Volitinib, a highly selective small molecule and ATP competitive MET kinase inhibitor (33). None of these mAbs showed positive clinical outcomes for their toxicity as Rilotumumab and for GBM stem cells heterogenicity as Crizotinib. Besides, many of the clinical studies were terminated without any published data such as those on Volitinib and small molecule inhibitor, SGX523. Amongst these mAbs, only cabozantinib (Table 1) treatment, a MET and VEGFR2 inhibitor, showed only modest clinical benefits in patients with GBM (Fig .1) (34).
Notwithstanding the fact that a wide range of agents from small-molecule inhibitors to mAbs have been long investigated on the pathway, there has been no phase III trial so far. A fact that reinforces an urgent need for a comprehensive review of the past trials to build up a solid conclusion on HGF/c-MET mutations and their responsiveness to therapy to more effectively select the patients who most benefit from the targeted therapies.
Ephrins/Eph pathway
Erythropoietin-producing human hepatocellular receptors (Ephs) are grouped into two subgroups EphAs (EphA1-8 and EphA10 in human) and EphBs (EphB1-4 and EphB6 in human). The receptor interacts with Eph receptor-interacting proteins (Ephrins)and subsequently exerts its effects on angiogenesis and stem cell differentiation. Recent studies in GBM have proven that these proteins play a role in both tumorigenesis and tumor progression in adult brain tumors, which proposes them as valuable therapeutic targets (35). Encouraging results from targeting Eph in pre-clinical GBM models have led to several vaccine trials targeting Ephrins. Amongst them EphA2 vaccine (Table 1) constructed by loading four of the glioma-associated antigen epitopes (EphA2, interleukin (IL)-13 receptor-α2, YKL-40, and gp100) on α-type 1 polarized dendritic cells has been proven to be safe in men with GBM (Fig .1) (36).
Since Ephs exert both tumorigenesis and anti-tumor effect depending on the tumor stage and the type of the receptor, it is crucial to specify the drugs for specific tumor stage and tumor subgroup. Besides, it has been shown that the expression pattern of Ephrins/Eph is different among patients which strongly suggests personalized therapy for a particular subgroup of patients with specific gene expression pattern.
RTK-independent angiogenesis signaling
Those signalings not classified as RTKs are discussed under this category here such as serin/tyrosine kinase TGF-β/ TβRII and cell adhesion molecules such as integrins.
TGF-β/TβRII
TGF superfamily, secreted from immune cells, stromal cells and tumor cells, comprises more than 30 growth factors including TGF-βs (TGF-β1, β2-and, β3), growth and differentiation factors (GDFs), activin, bone morphogenetic proteins (BMPs), nodal, and anti-mullerian hormone (AMH). Among these factors, TGF-βs are correlated with angiogenesis and modulate cell proliferation, differentiation, and tissue homeostasis following binding to their receptors, TGF-βRII and its disturbed function has been reported in fibrosis and tumorigenesis. It has been shown that TGF-βs, especially TGF-β1and -β2, have a crucial role in angiogenesis mediating up-regulation and induction of different angiogenic factors including VEGF, FGF and PAI-1 in GBM (37). Hence, TGF- β has been targeted in numerous studies in preclinical and clinical studies in a varied range of diseases and cancers such as sclerosis, pancreatic cancer (PAC), HCC, RCC and GBM.
Although both preclinical and clinical trials strongly propose TGF- β signaling, particularly TGF-β/TβRII, targeting as an effective treatment for metastatic tumors, serious adverse effects have been repetitively reported after long-term hindrance of the signaling in several animal studies. For example, hemorrhage and inflammation in ulcers of heart valves have been observed following TβRI blockade. Also, some cases of chronic inflammation resulting in precancerous conditions have been detected after TGF-β signaling hindrance. Therefore, targeting of the pathway should be applied with caution considering the pleiotropic function of the pathway both in normal and pathological condition.
There are currently several clinical trials on glioma to inhibit TGF-β signaling via different approaches:
Antisense oligonucleotide (AON): in a Phase IIb and III clinical trials the efficiency of an AON called AP12009 (Table 2) to block TGF-β2 mRNA translation has been studied (38).
Soluble receptors or their ectodomain constructs to sequester the ligand: in a Phase I trial i LY2157299 (Table 2), a Kinase inhibitor targeted for TGFβR1 (ALK-5), is going to be examined on volunteers (39).
Table 2.
Anti-angiogenesis clinical trials in GBM – targeting RTK-independent angiogenesis pathways
|
| |||||
|---|---|---|---|---|---|
| Target class | Treatments | Target | Clinical trial result | No. /phase | Trial reference No. |
|
| |||||
| TGFβ/TGF- βR | Galunisertib | TGFβR1 | MG | 65/ I, I/II | NCT01682187 |
| (LY2157299) | Recruiting | ||||
| Fresolimumab | TGF-β | Glioma | 12/ II | - | |
| (GC1008) | No significant clinical benefits | ||||
| Trabedersen | Refractory anaplastic astrocytoma or secondary glioblastoma | 27/ III | NCT00761280 | ||
| (AP12009) | Terminated for recruitment issues | ||||
| The survival rate with 10 μM | |||||
| OS:(months):35.7 for AP12009; | |||||
| OS:(months): (23.1) for AP12009 + chemotherapy | |||||
| IFN/IFNR | Cationic liposome-mediated IFN-β gene transfer | IFN-β | High grade glioma 50% reduction in disease severity and fewer CD34− immunoreactive vessels | 5/ I | - |
| Conventional radiation therapy followed by recombinant human IFN-β, | IFN-β | GBM survival benefit in patients who remained stable after radiation therapy | 109/ II | - | |
| Nimustine (ACNU) | IFN-β | rGlioma | 97/ II | 11A0232177 | |
| +Vincristin | Improved survival in patients with GBM | ||||
| +Carboplatin, | |||||
| + IFN-β | |||||
| + Radiotherapy | |||||
| IFN/IFNR | Peginterferon α-2b | IFN α-2b | rGBM | 7/ II | NCT00047879 |
| (PEG-Intron) | Confirmed safety and mild efficacy | ||||
| TMZ, | rGBM | 34 on IFN and 29 on PEG/ II | - | ||
| +IFN α-2b | Confirmed safety and mild efficacy | ||||
| and/or | PFS6: 31% for patients on IFN | ||||
| TMZ, | PFS6: 38% for patients on PEG | ||||
| +Long-acting IFN α-2b(PEG) | |||||
| TMZ | High grade glioma | -/ III | NCT01765088 | ||
| and/or | Recruiting | ||||
| TMZ | |||||
| + IFN-α | |||||
| Notch/NotchR | R4733 | rGBM | /I, II | NCT01189240 | |
| (RO4929097) | Terminated, no results available | NCT01131234 | |||
| NCT01269411 | |||||
| NCT01122901 | |||||
| TNF/TNFR | VB-111 | VB-111 | rGBM | 256/III | NCT02511405 |
| +Bevacizumab | ORR %: -, PFS (months): - | ||||
| PFS6(%): -, mOS:(months): 6.8 | |||||
|
| |||||
TGF-β; Transforming growth factor beta, VB-111; Adenoviral vector expressing PPE & Fas-TNFR1 chimera, TNFR; Tumor necrosis factor receptor, IFNR; Interferon receptor, IFNR; Interferon receptor, Integrin L; Integrin ligand, TMZ; Temozolomide, MG; Malignant Glioma, rGlioma; Recurrent Glioma, PFS; Progression free survival, ORR; Overall response rate, PFS; Progression free survival, PFS6; Progression-free survival at 6 months, OS; Overall survival, and PEG; Polyethylene glycol.
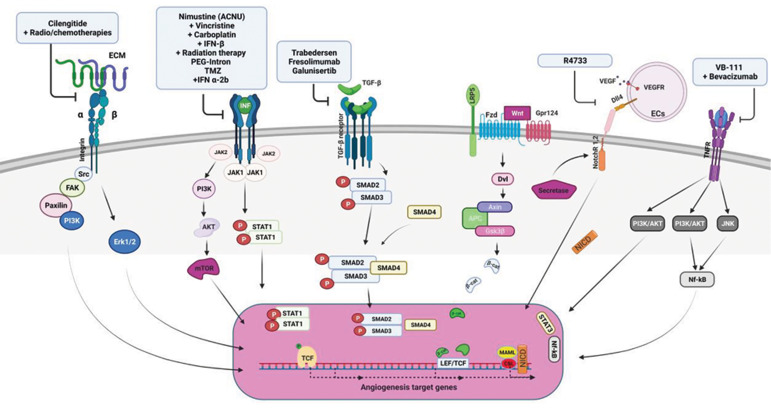
Antibodies: in a Phase I/II trial GC1008 (Table 2), an antibody against TGF-β, has been tested to suppress TGF-β receptor kinase activity (Fig .2) (40).
Fig 2.
RTK-independent angiogenesis pathways alongside their clinical targeting in glioblastoma. Different clinical inhibitors (upper blue boxes) targeting signalings involved in GBM angiogenesis through RTK-independent angiogenesis pathways (growth factor receptors: TGF-β/TβR2, TNFα/TNFR1 and TNFR2, Interferons/IFNsR, Wnt/Fzd receptor) and cell adhesion molecules (Notch and Integrins) are shown here. ECM; Extracellular matrix, INF; Interferon, TGF; Transforming growth factor, LRP5; Low-density lipoprotein receptor-related protein 5, FZD; Frizzled, Gpr124; G-protein coupled receptor 124, Dll4; Delta-like 4, TNF; Tumor necrosis factor, ECs; Endothelial cells, MAML; Mastermind-like proteins, TCF; Transcription factor, NICD; Notch intracellular domain, and Dvl; Dishevelled limits.
There is solid evidence that the pathway can be a promising target in spite of documented adverse side effects and many pharmaceutical companies that are still interested in producing drugs to target TGF-β/TβRII. To reduce the intricacy of the results and guarantee the safety of the drugs, designing agents that can specifically target either particular subgroups of the receptors or the molecules in downstream of TGF-β signaling is critical (41).
TNFα/TNFR1 and TNFR2
Tumor necrosis factor alpha (TNFα, TNF, cachectin), an inflammatory cytokine, belongs to TNF superfamily and is mostly secreted by immune system cells. It is indicated that the cytokine possesses a pro-angiogenic effect which may be either directly by induction of ECs differentiation or indirectly through triggering the secretion of other angiogenic factors. Also, it is shown that expression of TNFR2 is elevated in six cancer types including GBM.
Although TNF blockers have been successfully administered for autoimmune diseases and are among the best seller biologics, they have failed to meet the pre-targeted clinical expectations as anti-tumor drugs for their severe side effects. Different experiments have suggested α as a potential target in glioma, however, it couldn’t be clinically translated due to its high systemic toxicity at therapeutic doses (Fig .2) (42). To achieve more satisfactory results and reduce off-target effects localized therapies such as isolated organ perfusion in soft tissue sarcoma and melanoma has been developed. Yet, the problem remains unsolved for many cancers, particularly for highly metastatic ones (43).
Interferon-α/β/interferon-α/β receptor (IFNAR) and interferon-gamma/interferon-gamma receptor (IFNGR)
Interferons (IFN), initially discovered as antiviral responsiveness mediators, are currently recognized as multifunctional proteins involved in inflammation, proliferation, and differentiation and fall into 3 categories; IFN type I (IFN-α, IFN-β, IFN-ε, IFN-κ and IFN-ω), IFN type II (IFN-γ in humans) and IFN type III. They trigger a cascade of responses following binding to their glycoprotein receptors. It has been demonstrated that IFN-α and -β possess anti-angiogenesisproperties mediated by angiogenic factors inhibition (44). 50% reduction in disease severity and less CD34− immunoreactive vessels have been shown in 2 patients in a clinical trial phase II in which IFN-β gene therapy (Table 2) was applied for 5 patients with high-grade glioma (45). Also, in a Phase II clinical trial, recombinant human IFN-β (Table 2) was administered after radiation therapy and the results showed little survival benefit and high tolerance in GBM patients (46). Furthermore, a Phase II clinical trial proved to be safe that showed mild survival benefit of applying a combination of nimustine (ACNU), vincristine, carboplatin, and IFN-β (Table 2) in addition to radiotherapy in GBM patients (Fig .2) (47).
The efficacy of peginterferon α-2b (Table 2), an antiangiogenesisfactor, alone and in combination with thalidomide, an anti-angiogenesisdrug, in patients with glioma is being assessed in a phase II clinical trial (48). In another phase II trial, patients with GBM were treated with standard therapy using TMZ (Table 2), in combination with either short-acting IFNα-2b (nonpegylated) or long-acting IFNα-2b (pegylated). Safety and mild efficacy were observed in both regimens (49). In a phase III trial on cytokine therapy in patients with newly diagnosed GBM, TMZ was utilized with or without IFN-α (Table 2) (50) and results published. Some other trials are under investigation and attempt to find the best combination therapies (Fig .2) (51).
Despite encouraging results in preclinical trials on gliomas, clinical outcomes are puzzling: the results from IFN-γ targeting were dissatisfying, IFN-β targeting produced perplexing results and IFN-β targeting is still under investigation both alone and in combination with other approaches (52).
Wnt/Frizzled (Fzd) family receptor
Wnt signaling as a complex signaling pathway is implicated in proliferation, differentiation and migration of cells particularly stem cells in embryonic and adult tissues. There are three signaling pathways of Wnt signaling: canonical Wnt pathway, noncanonical planar cell polarity pathway, and noncanonical Wnt/ calcium pathway which regulate gene transcription, cell cytoskeleton and cell inner calcium, respectively. There are convincing reports on the positive correlation between altered expressions of different members of Wnt, GBM genesis and progression which are proposed as a factor to discriminate between normal and malignant cells in the human brain. Abnormalities in canonical Wnt signaling are associated with GBM stem cells (GSCs) formation, chemo- and radiotherapy resistance, and poor prognosis while non-canonical Wnt dysfunction is more related to the invasiveness of GBM. Therefore, a variety of agents targeting Wnt have been examined such as non-steroidal anti-inflammatory drugs (NSAIDs) and Resveratrol which may be opening windows to clinical trials (Fig .2) (53).
Notch signaling pathway
Notch signaling is activated when cell interaction is mediated by membrane bound Notch receptors (Notch-1, 4) and related membrane-bound ligands (Jagged-1, Jagged-2; Delta-like 1, 3, and 4). All the receptors and ligands are expressed on the cell surface of ECs membrane except Notch-3 and Dll-3 and mediate tip-to-tip contact between blood vessel sprouts and endothelial cells (54). It has been demonstrated that Notch activity is correlated with hypoxia and some cancer-related molecular pathways like ERK/MAPK and PI3K/AKT/mTOR which amplify malignancy in GBM and its targeting reduces malignancy characteristics. For instance, high Notch-1 expressing GBM surgical samples affect stem cell and angiogenesis pathways. Notch inhibition using treatment with DAPT (a chemical compound and γ-secretase inhibitor) showed reduction in Nestin and elaboration in Ki-67 (a proliferation marker). In another study, it has been shown that a combination of radiation therapy and Notch inhibition hinders self-renewal and propagation in tumor explants. There have been several clinical trials using RO4929097 (a g-secretase inhibitor) (Table 2) to unravel the correlation between Notch pathway and GBM patients’ survival. All the trials were discounted due to the drug suppliers’ decision not manufacturing the drug anymore (Fig .2) (55).
The importance of Notch signaling not only in the induction of tumor but also in the development of therapy resistance highlights exploring the best combination therapy to hinder tumor progression as well as tumor recurrence after applying standard cytotoxic therapies.
Integrins
Integrins (ITG) are transmembrane proteins comprised of two non-covalently associated α (18) and β (with 8) subunits that form 24 heterodimers responsible for crosstalk between a cell and another cell or ECM. As Intrgrin’s expression pattern is different in normal and GBM tissues, they may serve both as prognosis biomarkers (some of them like αvβ5 are assumed to be expressed specifically on GBM cells) and as radio/chemotherapy specificity (αvβ3 and α3β1 high expression were associated with poor prognosis in patients with GBM). Also, it has been evidenced that αvβ3, αvβ5 and αvβ8 are related to angiogenesis in GBM and their targeting is vastly studied in anti-angiogenesis therapy: αvβ3/αvβ5 were targeted by Cilengitide (Table 2) in phase I/II clinical trials in GBM, in combination with radio/chemotherapies, both in recurrent and in newly diagnosed GBM. The results showed clinical benefit on progression free survival (PFS) and good tolerance in overall survival (OS) (Fig .2).
Integrins are definitely promising molecules in GBM therapy either as targets or as diagnostic tools. Future investigation should discover a correlation between different subtypes of GBM and expression pattern of integrins for both better prediction of radio/chemo-resistant patients and more personalized targeting of the tumors (56).
All in all, the initial achievements from these preclinical and clinical trials encourage more discoveries in laboratories to pave the way from bench to bedside where a combination of approaches could more effectively curb tumor growth. Proper dosing and targeting can meaningfully affect the outcomes achieved by selecting a perfectly designed vehicle for the desired drug to guarantee both the drug and the recipient’s safety. In the following section the most prominent targeting strategies in GBM therapy is discussed.
Targeting strategies
Monoclonal antibodies (mAbs)
Monoclonal antibodies can specifically target a mutated ligand or its receptors. Bevacizumab (Table 2), an antiVEGF-A, is the first FDA-approved anti-angiogenesis monoclonal antibody as the first-line treatment for recurrent GBM patients (2). While, the outcome was favorable in both phase I and II; the side effects were overwhelming in phase III. Yet there are hopes for Bevacizumab to be used as an adjuvant in addition to traditional therapy. Although some anti-EGFR antibodies such as Cetuximab, mAb-806 and Y10 (Table 2) have shown promising preclinical results, that the clinical findings did not meet the expectations (Fig .1).
There have been numerous clinical trials examining monoclonal antibodies on different tumors like GBM, yet the most important concern is their side effects which stem from on/off-target toxicities, leading to an autoimmune reaction in both normal and tumor tissues. Hence, clinicians should take the assessment of risk-benefit ratio between anti-tumor efficacy and related side effects into account when determining optimal therapies (Fig .2) (57).
MicroRNAs (miRNAs)
A network of different miRNAs is involved in GBM angiogenesis; while some are angiogenesis promotors like miR‑296, miR‑21, and miR‑210‑3p, others are angiogenesis inhibitors like miR-15b and miR-299. Some miRNAs that promote glioma invasion are vastly studied: miR-10b, miR-21, miR-221/222 which are upregulated and miR-124, miR-34a, miR-181, miR-451, miR-146, miR-218, miR-326 which are downregulated in GBM.
miR-10b inactivation using antagomiR in a mouse GBM model resulted in a dramatic decrease in tumor growth. Several miRNA-targeted therapeutics have reached clinical trials including a mimic of the tumor suppressor miRNAs: miR-34 in phase I clinical trials for cancer and anti-miR122 in phase II trials for hepatitis. Also, there are currently two miRNA-based Phase I trials on cancer, there are no trials on GBM as of yet (58).
Aptamers
Aptamers, high affinity single-stranded DNA/RNA molecules, have been proved to be safe and effective as both diagnostic and therapeutic tools. They efficiently discriminate not only between normal and tumor tissues but also between different tumor types.
There is an anti-VEGF aptamer called pegaptanib approved by FDA for the treatment of wet age-related macular degeneration. Other aptamers have been investigated in clinical trials for a range of diseases including coronary artery disease, renal cell carcinoma, type 2 diabetes mellitus and albuminuria, chronic lymphocytic leukemia, hemophilia, and anemia from chronic inflammation. Although there is no clinical trial on GBM, a magnetic nanocrystal-conjugated VEGF receptor 2-specific aptamer was successfully evaluated using MRI in GBM-bearing mice for GBM diagnosis (59).
Gene therapy
Gene delivery using either viral or non-viral carriers in combination with current standard-of-care treatments delivers suicide genes, immunomodulatory genes, tumor-suppressor genes and oncolytic viruses to the target region. A number of gene therapy-based anti-angiogenesis clinical trials are now active on GBM: in a clinical trial HSV-1 expressing IL-12 (M032) (NCT02062827) is being investigated and in another trial VB-111 (Table 2) and bevacizumab are being used simultaneously. Although limited therapeutic benefits have been reported to date, a number of clinical trials verified gene therapy using various methods as a safe method (60).
Small molecules
There has been a growing interest in using antiangiogenesis small molecules targeting various components of angiogenic pathways to overcome the BBB obstacles. For instance, AZD2171 (Cediranib) (Table 1), a VEGFR inhibitor, increased therapy response rate by 30% in patients with recurrent GBM in a non-randomized phase II trial (61). In a phase II study, tivozanib and pazopanib exerted a slight anti-tumour activity with no prolonged PFS in patients with recurrent GBM (62). Imatinib (Glivec-Novartis), one of the most widely used anti-PDGF receptors and its downstream pathway, has been well tolerated with almost no beneficial effect on high-grade glioma in adults (63). Tandutinib a multikinase inhibitor has been investigated in phase II studies in combination with bevacizumab. Although the approach was effective, it was more toxic than bevacizumab monotherapy (64). Also, Sunitinib (Table 2) proved to be feasible and safe with promising antitumor activity in phase I/II in patients with GBM has entered the phase II/III (65). The majority of clinical trials on small molecules for GBM therapy have fallen lack of clinical benefits in brain (i.e., gefitinib and erlotinib) because tumor cells develop new mutations after exposure to drugs and become drug resistant. Additionally, low efficacy of small molecules for heterogeneity of GBM cell population leads to target therapy inefficiency. Nevertheless, there still are hopes for development of combination therapy using these molecules (66).
Angiostatin and endostatin
Angiogenesis is orchestrated by a fine-tuned balance between angiogenesis promotors and inhibitors in adult normal tissues. Angiostatin and endostatin are endogenous angiogenesis inhibitors whose overexpression is of particular interest in many diseases. Viral gene transfer of these two proteins is currently being investigated in phase I and II clinical trials on advanced head/neck carcinoma and macular degeneration. Even though it proved to be beneficial in GBM animal models, no clinical trials have been done yet (67).
Cell therapy using chimeric antigen receptor T-cells (CAR-T cells)
One particularly encouraging area in cancer therapy is chimeric antigen receptors (CARs) in which T-cells are genetically engineered to produce the desired T-cell receptor to target a specific protein. Since the approach has been successful in treating some of the hematological malignancies, they are currently under investigation for a range of solid tumors including GBM. Among several CAR-Ts against six GBM-associated antigens, four are currently being tested in clinical trials to target EGFRvIII, IL13Rα2, HER2, and EphA2 (Table 1). Although the outcomes were not as satisfactory as the results obtained from treatment of acute lymphoblastic leukemia (ALL) and chronic lymphoblastic leukemia (CLL) therapy, the strategy is being improved to overcome the intrinsic obstacles of GBM immune therapy such as GBM ability to evade immune surveillance, to suppress the local immune response and to induce T-cell apoptosis as well as GBM’s antigens heterogeneity (68).
Resistance to anti-angiogenesis treatment
Although mono anti-angiogenesis therapy raised enthusiasm at first, no or unsustainable beneficial effects set the scene for combination therapy to increase therapy effectiveness. It is proposed that adaptive and intrinsic resistance mediate the unresponsiveness in anti-angiogenesis therapy. Adaptive resistance include: activation of alternative angiogenesis pathways to restore angiogenesis in response to shortage of blood supply and oxygen, shifts in tumor cell metabolism, invasion of tumor cells, activation of autophagy, trans-differentiation of GBM cells to ECs and increase in glioblastoma stem cell (GSC) self-renewal. In intrinsic resistance, patients or tumors are naturally insensitive to that particular anti-angiogenesis therapy as tumor cells in pancreatic ductal adenocarcinoma grow in a hypoxic environment with no or little vascularity (69). Despite the mechanisms mentioned above as the inducers of the resistance, further investigation is still required to more effectively curb angiogenesis.
Conclusion
Longitudinal studies on anti-angiogenesis therapy have led to successful management of some abnormal conditions such as inflammatory and autoimmune diseases. However; the effect of the strategy seems incongruous when it comes to GBM therapy; while some of the patients show normalized vasculature in a particular time frame following anti-angiogenesis therapy, others present no beneficial and/or severe adverse effects. This partly reflects the fact that highly progressive tumor like GBM may survive in the absence of blood circulation. Besides, conceptually, anti-angiogenesis treatment could totally block angiogenesis that the invasive single tumor cells still can spread through the brain.
Despite the failure of anti-angiogenesis therapy to improve clinical courses in patients with GBM in the majority of clinical trials, the concept is strengthening with the continuing discovery of angiogenic factors. These findings can be applied in either multi-target therapy or combination therapy to more rigorously hinder tumor progression as multi-targeted RTKI and decoy receptors that are recently of particular interest in anti-angiogenesis therapy. Furthermore, in the absence of a profile of biomarkers associated with GBM, any improvement in this field of therapy is impossible. Therefore, a very essential step to move forward in angiogenesis targeting is to define GBM-related biomarkers to fulfil these requirements: to predict the therapy-responsive subpopulation of patients, determine the proper molecular target in individual patient and provide a tracking way for following up with the efficiency of therapy. These biomarkers can be the expression pattern of GBM-related genes and/or circulating biomarkers. Last but not the least, issues regarding possible toxicity of the treatments, drug dosing and timing, and the drug delivery approaches should be addressed.
Acknowledgements
We are grateful to Dr. Mohammad Vasei for all his help and support and Mr. Behrad Rastgar for writing and editing assistance. This study was supported by a grant from Tehran University of Medical Sciences (project number: 9121607002). The authors declare no conflict of interest.
Authors’ Contributions
F.D.; Participated in explicit literature search and classification as well as summarizing papers and writing the manuscript. Sh.A.; Contributed to writing the manuscript and preparation of the figures. Z.B.; Contributed in manuscript writing. S.E.-B., J.V., M.S.; Contributed in manuscript revision. J.A.; Supervised the study. All authors read and approved the final manuscript.
References
- 1.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10(6):417–427. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 2.Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hajighasemlou S, Nikbakht M, Pakzad S, Muhammadnejad S, Gharibzadeh S, Mirmoghtadaei M, et al. Sorafenib and mesenchymal stem cell therapy: a promising approach for treatment of HCC. Evid Based Complement Alternat Med. 2020;9602728 doi: 10.1155/2020/9602728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rink C, Khanna S. Significance of brain tissue oxygenation and the arachidonic acid cascade in stroke. Antioxid Redox Signal. 2011;14(10):1889–1903. doi: 10.1089/ars.2010.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feeney Jr JF, Watterson RL. The development of the vascular pattern within the walls of the central nervous system of the chick embryo. J Morphol. 1946;78(2):231–303. doi: 10.1002/jmor.1050780205. [DOI] [PubMed] [Google Scholar]
- 6.Lok J, Gupta P, Guo S, Kim WJ, Whalen MJ, van Leyen K, et al. Cell-cell signaling in the neurovascular unit. Neurochem Res. 2007;32(12):2032–2045. doi: 10.1007/s11064-007-9342-9. [DOI] [PubMed] [Google Scholar]
- 7.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131(7):1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- 8.Bautch VL, James JM. Neurovascular development: the beginning of a beautiful friendship. Cell Adh Migr. 2009;3(2):199–204. doi: 10.4161/cam.3.2.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bairey D, Blickstein D, Shaklai M. Tumor angiogenesis--prognostic and therapeutic implications. Harefuah. 1997;132(2):117–120. [PubMed] [Google Scholar]
- 10.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3(6):401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 11.Wu JB, Tang YL, Liang XH. Targeting VEGF pathway to normalize the vasculature: an emerging insight in cancer therapy. Onco Targets Ther. 2018;11:6901–6909. doi: 10.2147/OTT.S172042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61(3):215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 13.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 14.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978–978. doi: 10.3389/fimmu.2018.00978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bayat N, Izadpanah R, Ebrahimi-Barough S, Norouzi Javidan A, Ai A, Mokhtari Ardakan MM, et al. The anti-angiogenic effect of atorvastatin in glioblastoma spheroids tumor cultured in fibrin gel: in 3D in vitro model. Asian Pac J Cancer Prev. 2018;19(9):2553–2560. doi: 10.22034/APJCP.2018.19.9.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ai J, Ebrahimi S, Ai A, Karimi R, Bahrami N. Effect of deforolimus and VEGF on angiogenesis in endometrial stromal cells following three-dimensional culture. Stem Cell Discov. 2013;3(1):7–12. [Google Scholar]
- 18.Oprita A, Baloi SC, Staicu GA, Alexandru O, Tache DE, Danoiu S, et al. Updated insights on EGFR signaling pathways in glioma. Int J Mol Sci. 2021;22(2):2–3. doi: 10.3390/ijms22020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korc M, Friesel RE. The role of fibroblast growth factors in tumor growth. Curr Cancer Drug Targets. 2009;9(5):639–651. doi: 10.2174/156800909789057006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khalid EB, Ayman EE, Rahman H, Abdelkarim G, Najda A. Natural products against cancer angiogenesis. Tumour Biol. 2016;37(11):14513–14536. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- 21.Dieci MV, Arnedos M, Andre F, Soria JC. Fibroblast growth factor receptor inhibitors as a cancer treatment: from a biologic rationale to medical perspectives. Cancer Discov. 2013;3(3):264–279. doi: 10.1158/2159-8290.CD-12-0362. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez-Pascual A, Siebzehnrubl FA. Fibroblast growth factor receptor functions in glioblastoma. Cells. 2019;8(7):715–715. doi: 10.3390/cells8070715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, Adjei AA. Targeting angiogenesis in cancer therapy: moving beyond vascular endothelial growth factor. Oncologist. 2015;20(6):660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manzat Saplacan RM, Balacescu L, Gherman C, Chira RI, Craiu A, Mircea PA, et al. The role of PDGFs and PDGFRs in colorectal cancer. Mediators Inflamm. 2017;2017:4708076–4708076. doi: 10.1155/2017/4708076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holdhoff M, Kreuzer KA, Appelt C, Scholz R, Na IK, Hildebrandt B, et al. Imatinib mesylate radiosensitizes human glioblastoma cells through inhibition of platelet-derived growth factor receptor. Blood Cells Mol Dis. 2005;34:181–185. doi: 10.1016/j.bcmd.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Dresemann G, Weller M, Rosenthal MA, Wedding U, Wagner W, Engel E, et al. Imatinib in combination with hydroxyurea versus hydroxyurea alone as oral therapy in patients with progressive pretreated glioblastoma resistant to standard dose temozolomide. J Neurooncol. 2010;96:393–402. doi: 10.1007/s11060-009-9976-3. [DOI] [PubMed] [Google Scholar]
- 27.Al-Abd AM, Alamoudi AJ, Abdel-Naim AB, Neamatallah TA, Ashour OM. Anti-angiogenic agents for the treatment of solid tumors: Potential pathways, therapy and current strategies - a review. J Adv Res. 2017;8(6):591–605. doi: 10.1016/j.jare.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunkel P, Muller S, Schirmacher P, Stavrou D, Fillbrandt R, Westphal M, et al. Expression and localization of scatter factor/hepatocyte growth factor in human astrocytomas. Neuro Oncol. 2001;3(2):82–88. doi: 10.1093/neuonc/3.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng F, Guo D. MET in glioma: signaling pathways and targeted therapies. J Exp Clin Cancer Res. 2019;38(1):270–270. doi: 10.1186/s13046-019-1269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Affronti ML, Jackman JG, McSherry F, Herndon JE 2nd, Massey EC Jr, Lipp E, et al. Phase II study to evaluate the efficacy and safety of rilotumumab and bevacizumab in subjects with recurrent malignant glioma. Oncologist. 2018;23(8):889–e898. doi: 10.1634/theoncologist.2018-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cloughesy T, Finocchiaro G, Belda-Iniesta C, Recht L, Brandes AA, Pineda E, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of onartuzumab plus bevacizumab versus placebo plus bevacizumab in patients with recurrent glioblastoma: efficacy, safety, and hepatocyte growth factor and O(6)- methylguanine-DNA methyltransferase biomarker analyses. J Clin Oncol. 2017;35(3):343–351. doi: 10.1200/JCO.2015.64.7685. [DOI] [PubMed] [Google Scholar]
- 32.Junca A, Villalva C, Tachon G, Rivet P, Cortes U, Guilloteau K, et al. Crizotinib targets in glioblastoma stem cells. Cancer Med. 2017;6(11):2625–2634. doi: 10.1002/cam4.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia H, Dai G, Weng J, Zhang Z, Wang Q, Zhou F, et al. Discovery of (S)-1-(1-(Imidazo[1,2-a]pyridin-6-yl)ethyl)-6-(1-methyl-1Hpyrazol- 4-yl)-1H-[1,2,3]triazolo[4,5-b]pyrazine (volitinib) as a highly potent and selective mesenchymal-epithelial transition factor (c- Met) inhibitor in clinical development for treatment of cancer. J Med Chem. 2014;57(18):7577–7589. doi: 10.1021/jm500510f. [DOI] [PubMed] [Google Scholar]
- 34.Cloughesy TF, Drappatz J, de Groot J, Prados MD, Reardon DA, Schiff D, et al. Phase II study of cabozantinib in patients with progressive glioblastoma: subset analysis of patients with prior antiangiogenic therapy. Neuro Oncol. 2018;20(2):259–267. doi: 10.1093/neuonc/nox151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Genander M, Frisen J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010;22(5):611–616. doi: 10.1016/j.ceb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–336. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han J, Alvarez-Breckenridge CA, Wang QE, Yu J. TGF-beta signaling and its targeting for glioma treatment. Am J Cancer Res. 2015;5(3):945–955. [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahapatra AK, Suri A, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodon J, Carducci MA, Sepulveda-Sanchez JM, Azaro A, Calvo E, Seoane J, et al. First-in-human dose study of the novel transforming growth factor-beta receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res. 2015;21(3):553–560. doi: 10.1158/1078-0432.CCR-14-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.den Hollander MW, Bensch F, Glaudemans AWJM, Enting RH, Bunskoek S, Munnink THO, et al. zr-GC1008 PET imaging and GC1008 treatment of recurrent glioma patients. J Clin Oncol. 2013;31(15):1–2. [Google Scholar]
- 41.Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-beta pathway. J Hematol Oncol. 2021;14(1):55–55. doi: 10.1186/s13045-021-01053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burton ER, Libutti SK. Targeting TNF-alpha for cancer therapy. J Biol. 2009;8(9):85–85. doi: 10.1186/jbiol189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer R, Kontermann RE, Pfizenmaier K. Selective targeting of TNF receptors as a novel therapeutic approach. Front Cell Dev Biol. 2020;8:401–401. doi: 10.3389/fcell.2020.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakka SS, Rao JS. Antiangiogenic therapy in brain tumors. Expert Rev Neurother. 2008;8(10):1457–1473. doi: 10.1586/14737175.8.10.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakabayashi T, Natsume A, Hashizume Y, Fujii M, Mizuno M, Yoshida J. A phase I clinical trial of interferon-beta gene therapy for high-grade glioma: novel findings from gene expression profiling and autopsy. J Gene Med. 2008;10(4):329–339. doi: 10.1002/jgm.1160. [DOI] [PubMed] [Google Scholar]
- 46.Colman H, Berkey BA, Maor MH, Groves MD, Schultz CJ, Vermeulen S, et al. Phase II radiation therapy oncology group trial of conventional radiation therapy followed by treatment with recombinant interferon-beta for supratentorial glioblastoma: results of RTOG 9710. Int J Radiat Oncol Biol Phys. 2006;66(3):818–824. doi: 10.1016/j.ijrobp.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 47.Aoki T, Takahashi JA, Ueba T, Oya N, Hiraoka M, Matsui K, et al. Phase II study of nimustine, carboplatin, vincristine, and interferonbeta with radiotherapy for glioblastoma multiforme: experience of the Kyoto Neuro-Oncology Group. J Neurosurg. 2006;105(3):385–391. doi: 10.3171/jns.2006.105.3.385. [DOI] [PubMed] [Google Scholar]
- 48.National Institutes of Health Clinical Center (CC) Phase II trial of peginterferon alpha-2b and thalidomide in adults with recurrent gliomas. 2002. Available from: https://clinicaltrialsgov/ct2/show/record/ NCT00047879. (31 Jul 2021)
- 49.Groves MD, Puduvalli VK, Gilbert MR, Levin VA, Conrad CA, Liu VH, et al. Two phase II trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br J Cancer. 2009;101(4):615–620. doi: 10.1038/sj.bjc.6605189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z. A phase III trial on adjuvant temozolomide with or without interferon-alpha in newly diagnosed high-grade gliomas. 2013 Available from: https://clinicaltrialsgov/ct2/show/record/ NCT01765088. (31 Jul 2021) [Google Scholar]
- 51.Mooney J, Bernstock JD, Ilyas A, Ibrahim A, Yamashita D, Markert JM, et al. Current approaches and challenges in the molecular therapeutic targeting of glioblastoma. World Neurosurg. 2019;129:90–100. doi: 10.1016/j.wneu.2019.05.205. [DOI] [PubMed] [Google Scholar]
- 52.Jackson C, Ruzevick J, Phallen J, Belcaid Z, Lim M. Challenges in immunotherapy presented by the glioblastoma multiforme microenvironment. Clin Dev Immunol. 2011;2011:732413–732413. doi: 10.1155/2011/732413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zuccarini M, Giuliani P, Ziberi S, Carluccio M, Iorio PD, Caciagli F, et al. The role of wnt signal in glioblastoma development and progression: a possible new pharmacological target for the therapy of this tumor. Genes (Basel) 2018;9(2):105–105. doi: 10.3390/genes9020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kofler NM, Shawber CJ, Kangsamaksin T, Reed HO, Galatioto J, Kitajewski J. Notch signaling in developmental and tumor angiogenesis. Genes Cancer. 2011;2(12):1106–1116. doi: 10.1177/1947601911423030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gersey Z, Osiason AD, Bloom L, Shah S, Thompson JW, Bregy A, et al. Therapeutic targeting of the Notch pathway in glioblastoma multiforme. World Neurosurg. 2019;131:252–263. doi: 10.1016/j.wneu.2019.07.180. [DOI] [PubMed] [Google Scholar]
- 56.Malric L, Monferran S, Gilhodes J, Boyrie S, Dahan P, Skuli N, et al. Interest of integrins targeting in glioblastoma according to tumor heterogeneity and cancer stem cell paradigm: an update. Oncotarget. 2017;8(49):86947–86968. doi: 10.18632/oncotarget.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farber SH, Elsamadicy AA, Atik AF, Suryadevara CM, Chongsathidkiet P, Fecci PE, et al. The safety of available immunotherapy for the treatment of glioblastoma. Expert Opin Drug Saf. 2017;16(3):277–287. doi: 10.1080/14740338.2017.1273898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beyer S, Fleming J, Meng W, Singh R, Haque SJ, Chakravarti A. The role of miRNAs in angiogenesis, invasion and metabolism and their therapeutic implications in gliomas. Cancers (Basel) 2017;9(7):85–85. doi: 10.3390/cancers9070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Catuogno S, Esposito CL. Aptamer cell-based selection: overview and advances. Biomedicines. 2017;5(3):49–49. doi: 10.3390/biomedicines5030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kane JR, Miska J, Young JS, Kanojia D, Kim JW, Lesniak MS. Sui generis: gene therapy and delivery systems for the treatment of glioblastoma. Neuro Oncol. 2015;17(Suppl 2):ii24–ii36. doi: 10.1093/neuonc/nou355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Batchelor TT, Duda DG, di Tomaso E, Ancukiewicz M, Plotkin SR, Gerstner E, et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J Clin Oncol. 2010;28(17):2817–2823. doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalpathy-Cramer J, Chandra V, Da X, Ou Y, Emblem KE, Muzikansky A, et al. Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J Neurooncol. 2017;131(3):603–610. doi: 10.1007/s11060-016-2332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, et al. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European organisation for research and treatment of cancer brain tumor group study. J Clin Oncol. 2008;26:4659–4565. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Odia Y, Sul J, Shih JH, Kreisl TN, Butman JA, Iwamoto FM, et al. A phase II trial of tandutinib (MLN 518) in combination with bevacizumab for patients with recurrent glioblastoma. CNS Oncol. 2016;5(2):59–67. doi: 10.2217/cns-2015-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brahm CG, van Linde ME, Labots M, Kouwenhoven MC, Aliaga ES, Enting RH, et al. A phase II/III trial of high-dose, intermittent sunitinib in patients with recurrent glioblastoma: the STELLAR study. Ann Oncol. 2019;30:v157–8. [Google Scholar]
- 66.de Vries NA, Buckle T, Zhao J, Beijnen JH, Schellens JH, van Tellingen O. Restricted brain penetration of the tyrosine kinase inhibitor erlotinib due to the drug transporters P-gp and BCRP. Invest New Drugs. 2012;30:443–449. doi: 10.1007/s10637-010-9569-1. [DOI] [PubMed] [Google Scholar]
- 67.Clavreul A, Pourbaghi-Masouleh M, Roger E, Menei P. Nanocarriers and nonviral methods for delivering antiangiogenic factors for glioblastoma therapy: the story so far. Int J Nanomedicine. 2019;14:2497–2513. doi: 10.2147/IJN.S194858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mao G, Sampath P, Sengupta S. Updates on chimeric antigen receptor-mediated glioblastoma immunotherapy. R I Med J (2013) 2017;100(6):39–42. [PubMed] [Google Scholar]
- 69.Sofuni A, Iijima H, Moriyasu F, Nakayama D, Shimizu M, Nakamura K, et al. Differential diagnosis of pancreatic tumors using ultrasound contrast imaging. J Gastroenterol. 2005;40(5):518–525. doi: 10.1007/s00535-005-1578-z. [DOI] [PubMed] [Google Scholar]