Abstract
Objective
The most common mutation in cystic fibrosis (CF), (ΔF508-CFTR), results in impaired protein maturation, folding and transportation to the surface of the cell. As a consequence of impaired protein maturation and/or transport from the extracellular matrix to the cell, different systems are influenced, including gastrointestinal system and glandular system, reproductive system and respiratory systems. CF models are essential tools to provide further knowledge of CF pathophysiology. With this aim, we designed a transgenic CF model based on the homologous recombination (HR) system.
Materials and Methods
In this experimental study, a specifically designed construct containing the CFTR gene with F508del was cloned into a PTZ57R cloning vector and then the construct was transformed into the male pronucleus by microinjection after in vitro fertilization (IVF). Then the rates of blastocyst formation and embryonic development at 72 hours after IVF, were evaluated using the inverted microscope and the insertion of the construct was approved by polymerase chain reaction (PCR) method.
Results
The CFTR gene was successfully cloned into the PTZ57R cloning vector and overall, from 22 injected cells, 5 blastocysts were observed after pronuclear injection of the CFTR gene construct. PCR verification of the blastocyst with CFTR-specific primers represented complete recombination of CFTR into the mouse genome.
Conclusion
For the first time we designed a unique genome construction that can be detected using a simple PCR method. The pronuclear injection was performed for the transformation of the genome construct into the male pronuclei using microinjection and the development of zygote to the blastocyst stage has been observed following transgenesis.
Keywords: Animal Model, Cystic Fibrosis, Homologous Recombination, Polymerase Chain Reaction
Introduction
The cystic fibrosis transmembrane conductance regulator (CFTR) gene contains about 189.36 kilo base pairs and produces a 1,480 amino acids protein. Over 2,000 distinct variants of CFTR have been identified as a cause of CF (1). These different mutations are classified based on the mechanism of CFTR dysfunction, including impaired protein synthesis, protein instability, channel regulation and electrolyte imbalance (2). The F508del variation is the most prevalent variation and is observed in 70% of patients. The deletion of three nucleotides results in a phenylalanine acid amine deletion. This change in the 508th position of the protein structure causes impaired protein folding, maturation, and/or electrolyte transportation between cells and extracellular matrix (3).
Apart from the earlier description, late 1930’s century, CF is a major challenge. There isn't any significant treatment for patients suffering from CF disease, and most of treatment strategies are focused on aleiviation of symptoms and increase the life expance of patients up to 40 years (4). Therefore, designing an animal model that accurately mimics the disease pattern and counterpoises various microenvironments can efficiently produce good insights into CF disease (5). The recent revolution in producing animal models brought up some genome editing techniques such as transcription activator-like effector nucleases (TALENs); zinc-finger nuclease (ZFNs); and clustered regularly interspaced short palindromic repeats Cas9 (CRISPRCas9); as well as homologous recombination (HR) system for CF research (6).
HR system accounts as a simple system that the desired gene can be integrated into the specific site through either embryonic stem cells or somatic cell nuclear transfer, as well as pronucleus transfer (PNT) (7). In order to increase the porportion of integration rate, the genomic content is flanked into homology arms. These homology arms consist of long genomic regions with a thousand base pairs that are a complement of the desired region of the genome. It is called site specific recombination that generates a line of a transgenic animal harboring the desired gene inserted into the genome. Using homologous-directed repair (HDR), a double-strand DNA breaks down and a donor DNA with a 200 to 800 bp homology arm is inserted into the site of genomic DNA breakage. To increase the efficiency of homologous recombination, the use of longer homology arms and the Crispr/cas9 system for genome modification is recommended (8).
In the present study, to produce a transgenic model, we designed an artificial genetic construct of the CFTR gene containing F508del by performing some necessary modifications. Then desired construct had synthesized and cloned into the bacterial vector. There are different CF animal models, including pig (9), ferret (10, 11), rabbit (12), and rat (13). But, according to a systematic review study performed in 2020, the majority of studies in the generation of transgenic CF models were performed on different mouse models using either pronuclear injection or other recombination systems (14). In order to test our novel construct, the bacterial vector harboring the CFTR construct was injected into the male pronucleus of mature mouse zygotebased on an HR system. The embryo development up to a mature blastocyst formation was monitored, and the presence of an injected gene was detected using a simple polymerase chain rection (PCR) method.
Materials and Methods
This experimental study was approved by the Research and Ethics Committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1395.753). All animal treatments were carried out in accordance with US NIH guidelines for the care and use of laboratory animals (15).
Animals
Approximately 210 oocytes were obtained from five healthy female B6D2F1 mice (C57BL/6×DBA2). These 4-8 weeks old mice (weighing 11.9 ± 0.9 g) were purchased from the Royan Institute, Tehran, Iran. Hybrid mice such as B6D2F1 have a high fertility rate, as well as a high quality and the number of releasing oocytes which were suitable for reproductive research. Spermatozoa were obtained from five healthy male B6D2F1 mice (C57BL/6×DBA2) at 8 weeks of age.
All animals were housed at a constant temperature and humidity (22-28°C, 55 ± 5%), under a 12-hour light/dark cycle, and their food and water were provided according to the libitum standard laboratory diet. The animals were adapted to laboratory conditions for 10 days before the experiment. In order to have a minimum animal suffering and applying for our transgenesis method evaluation, the study was conducted on a small group of mice.
Construct designing
The human CFTR gene sequence was obtained from the UniProt database (www.uniprot.org) and compared with the mouse CFTR gene sequence using the Clustal Omega tool (plugged in UniProt) to identify the rate of similarity between human CFTR and mouse CFTR gene sequences. Then, the entire CFTR gene sequence was screened and the intron regions across the length of the gene were omitted and all exon sequences were merged respectively. Some necessary modifications had been performed, including the insertion of a promotor site along with modification in the initial and terminal sequences of the promotor to have a restriction site for SacI and NdeI restriction enzymes (RE), respectively. After insertion into the mouse genome the mouse promotor would render the transcription procedure. Also, to digest the construct genome from the vector, a modification was performed at the end of the genome sequence to have a restriction site for the BglII RE. To have a frameshift mutation in the CFTR sequence, a stuffer sequence containing 200 nucleotides was inserted at the position of 508th nucleotide, the most common CF mutations. Following, the codon optimization was performed to make a BamHI restriction site to remove the stuffer for further evaluation and gene therapy approaches. Finally, codon optimization was performed in an amino acid codon to have a unique sequence. This alternation aimed to determine the inserted sequence in the mouse genome using a simple PCR method. The designed CFTR gene sequence, then was incorporated within the PCC1 cloning vector (GenCArt Bioneer, Korea). The schematic model of the designed construct is illustrated in Figure 1.
Fig 1.
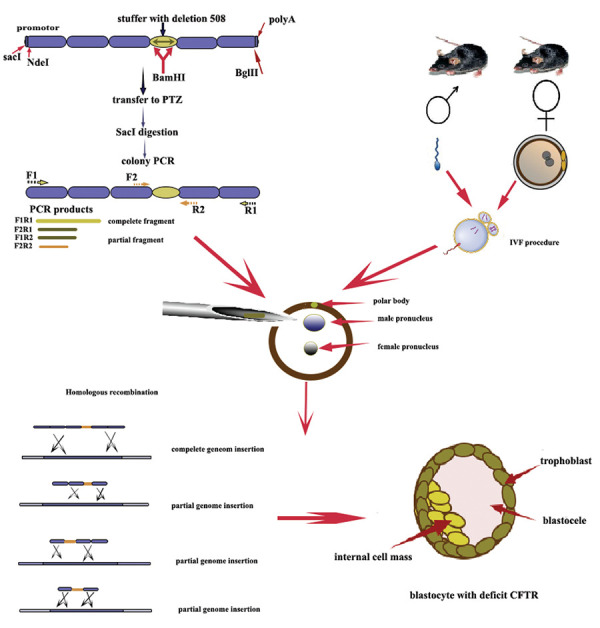
Graphical abstract of the study.
Cloning of the CFTR gene in the PTZ57R cloning vector
For cloning the CFTR construct into the PTZ57R vector, the CFTR gene construct was amplified using specific primer sets designed for the initial and end sites of the CFTR construct. We used NCBI BLAST and Oligo7 software (Molecular Biology Insights, Inc., Cascade Co.) to design and validate primer sets. The sequence of the primers is presented in the Table 1. The amplification was performed using the PCR Qiagen kit (201445., Qiagen, Hilden, Germany) under the following program: initial denaturation at 94°C (5 minutes), followed by 29 cycles with denaturation at 94°C (45 seconds), annealing at 57°C (45 seconds), extension at 72°C (120 seconds), with the final extension step at 72°C (10 minutes). The PCR products were electrophoresed and the results were observed on the 1% agarose gel (A9539, Sigma-Aldrich, Germany). The 1 µl of PCR product was ligated with a 10 µl PTZ57R vector using T4 DNA ligase (L6030- W-L, Qiagen, Hilden, Germany), and after 3 hours incubation at 37° C, the ligation was confirmed by gel electrophoresis. Then, the CaCl2 transformation method (16) was employed to transform the ligated CFTR and PTZ57R vector into the E.coli Top10 as a competent host. The colonies were cultured on a LB (Luria-Bertani) agar plate containing 100 μg/mL ampicillin. The assessment of the transformation was performed using colony PCR and RE digestion with the SacI RE (1078A, Takara, Dalian, China) because due to the ligation, the orientation of the gene was changed, and double digestion was performed with SacI RE.
Table 1.
Sequence of primers used for cloning and polymerase chain reaction
|
| |
|---|---|
| Primer | Primer sequencing (5´-3´) |
|
| |
| CFTR | F1: GAG CTC GGA TCC AGG AAC CCA GG |
| R1: AGA TCT AAG CCT TGT ATC TTG CAC C | |
| F2: CCT AAC TGA GAC CTT ACA CCG TTT | |
| R2: AAA CGG TGT AAG GTC TCA GTT AGG | |
|
| |
In vitro fertilization procedure
IVF procedurewas performed on8- week-old female mice by superovulation with 10 IU Pregnant male serum Gonadotropin (PMSG, C0434, Sigma-Aldrich, Germany), following injection of 10 IU human choronic gonadotropin (hCG, 9002-61-3, Sigma Aldric, Germany) after 50 hours. Cumulus-oocyte complexes (COCs) were separated from the ampulla of the oviduct 14 hours after a single dose injection hCG. For sperm collection, the 8 to10-week-old male mice were euthanized by cervical dislocation. Then spermatozoa were isolated from the cauda epididymis as well as the vasa deferentia. The sperm suspension was collected and cultured in the human tubal fluid (HTF) medium, containing 4 mg/mL bovine serum albumin (BSA, MR-070-D, Sigma Aldric, Germany) and incubated at 37°C with 5% CO2 for 45 minutes. Then, COCs were inseminated with approximately 1×106 sperm/ mL in 100 μl of HTF medium and then incubated for 6 hours. The pronuclear injection was applied on mature zygote with two pronuclei following about6 hours incubation at the incubator and rested on an ice plate for 30 minutes, they were placed in a potassium simplex optimized (KSOM) medium (MR-107-D, Sigma-Aldrich, Germany) that riches in essential amino acids supplemented with a 4% BSA. The rates of blastocyst formation and embryo development was evaluated at 24 and 96 hours after IVF, respectively (17).
Pronuclear plasmid microinjection
The concentration of the purified plasmids was measured using Nanodrop (ND2000, NanoDrop Technologies, USA). The amount of 4 µg/µl was needed for the injection of PCR product to the male pronucleus. Thus, 1 µl of purified PCR product was diluted with a sterile Tris/Editium (TE) buffer. The diluted PCR product was then inserted into an injection needle with a Pasteur pipette. Approximately 210 mature oocytes were obtained from female mice, resulting in the development of 170 MII stage zygotes with two pronuclei which were selected for pronuclear injection.
The magnitude of the microscope (Nikon TE2000, Narishige, USA) was adjusted at 60X and then the mature zygote with two distinct pronuclei was transferred in Flushing Holding medium (FHM) media and fixed with a holder needle; then injection was performed by pushing the diluted PCR product with a TE buffer (1:50) into the male pronucleus. All procedures were performed under the cold chain to keep the integrity of the cell membrane of the zygote. After injection, they were placed on an ice plate to heal the zygote’s cell wall and then transferred into the KSOM buffer. The presence of mature blastocyst was observed after 24 and 96 days of the micro-injection. Approximately 210 mature oocytes were obtained from female mice, resulting in the development of 170 MII stage zygotes with two pronuclei which were selected for pronuclear injection. Mature 2PN zygotes were then transferred to the 5ml FHM media. Each FHM media consisted of about 5 to 10 zygotes. The concentration of PCR products was measured after purifying with drop dialysis using Nanodrop for calculation of the amount of DNA for injection.
The concentration of the PCR products applied for pronuclear injection was about 520 µl/ml. Around 4 ng/ml was needed to inject into the male pronucleus. Therefore, 1 µl of PCR products were diluted with a 99 μl sterile TE buffer. The diluted PCR product was transferred to the injection needle and whilst the orientation of the polar body was at top of the cell, the construct was injected into male pronuclei. Then the injection dish was kept in the cold plate to repair the zygote’s cell wall and finally transferred to the KSMO culture plate.
construction insertion confirmation
The PCR method was employed to confirm the presence of corresponding CFTR gene sequences in mature blastocytes and morulae. The PCR was performed using all primer sets including F1R1, F1R2, F2R2, and F2R1 primers for identification of either complete or partial insertion of the CFTR gene construct (The PCR program was mentioned in the cloning section). Primers, applied for amplification of CFTR, were specific for the designed construct that just attached to the genome while the CFTR gene construct was inserted. The F1R1 primers amplified both the initial and end of the CFTR gene construct, while F2R2 attached to the middle of the construct contained a 200 bp fragment inserted at the 508th position of the CFTR protein. F1R2 and F2R1 amplified the partial sequence of the genome within the inserted stuffer site. Therefore, even in the case of partial insertion of the CFTR gene, the function of the whole genome would knock down the entire genome.
Results
Gene construction
The sequence of the human CFTR gene was compared with the mouse CFTR gene. Using the Clustal Omega tool (plugged in UniProt), 78.5% identity between human CFTR and mouse CFTR based on reference gene was observed. To design the construct, the CFTR gene was screened and all the intron regions were excluded while exon regions connected to each other. The CFTR gene exons were listed in Table 2.
Table 2.
The list of CFTR gene exons
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Region | Start | End | Region length | Phase at end | Region | Start | End | Region length | Phase at end |
|
| |||||||||
| UTR | 117,120,016 | 117,120,148 | Exon | 117,234,984 | 117,235,112 | 129 | 0 | ||
| Exon | 117,120,149 | 117,120,201 | 53 | 2 | Exon | 117,242,880 | 117,242,917 | 38 | 2 |
| Exon | 117,144,307 | 117,144,417 | 111 | 2 | Exon | 117,243,586 | 117,243,836 | 251 | 1 |
| Exon | 117,149,088 | 117,149,196 | 109 | 0 | Exon | 117,246,728 | 117,246,807 | 80 | 0 |
| Exon | 117,170,953 | 117,171,168 | 216 | 0 | Exon | 117,250,573 | 117,250,723 | 151 | 1 |
| Exon | 117,174,330 | 117,174,419 | 90 | 0 | Exon | 117,251,635 | 117,251,862 | 228 | 1 |
| Exon | 117,175,302 | 117,175,465 | 164 | 2 | Exon | 117,254,667 | 117,254,767 | 101 | 0 |
| Exon | 117,176,602 | 117,176,727 | 126 | 2 | Exon | 117,267,576 | 117,267,824 | 249 | 0 |
| Exon | 117,180,154 | 117,180,400 | 247 | 0 | Exon | 117,282,492 | 117,282,647 | 156 | 0 |
| Exon | 117,182,070 | 117,182,162 | 93 | 0 | Exon | 117,292,896 | 117,292,985 | 90 | 0 |
| Exon | 117,188,695 | 117,188,877 | 183 | 0 | Exon | 117,304,742 | 117,304,914 | 173 | 2 |
| Exon | 117,199,518 | 117,199,709 | 192 | 0 | Exon | 117,305,513 | 117,305,618 | 106 | 0 |
| Exon | 117,227,793 | 117,227,887 | 95 | 2 | Exon | 117,306,962 | 117,307,162 | 201 | 0 |
| Exon | 117,230,407 | 117,230,493 | 87 | 2 | UTR | 117,307,163 | 117,308,718 | ||
| Exon | 117,231,988 | 117,232,711 | 724 | 0 | Chromosome: chr7, Genbank ID: NM_000492 , Length coding sequence : 4440 nucleotides | ||||
|
| |||||||||
Cloning and confirmation of DNA construct
The CFTR gene was successfully cloned into the PTZ57R cloning vector and the insertion of gene construct was confirmed on colonies observed after transformation using colony PCR method with the F1R1 primers. PCR results indicated the presence of 4,600 bp related fragment on the 1% gel agarose. Also, after ligation of the CFTR construct in to PTZ57R cloning vector, the vector was digested using the SacI RE confirmed the insertion of the CFTR gene into the PTZ57R cloning vector. The results of PTZ+ CFTR gene cloning into E. coli TOP10 cells are shown in Figure 2.
Fig 2.

Cloning of PTZ+CFTR gene into E. coli TOP10 cells. AMD; Ampliciline supplimented dish.
Pronuclear injection
Following injection of CFTR gene construct Approximately 170 MII stage zygotes with two pronuclei were developed, and the developmental rate was about 80.9%.
The presence of mature blastocysts was examined at 24 and 96 hours after micro-injection in which about 22 blastocysts along with 10 morulae were developed after being injected. The overall rate of development of either morula and blastocytes cells in transgenic zygotes was 18.8%, while the rate of blastocyte development was 12.9%. The developed blastocysts and morulae are illustrated in Figure 3.
Confirmation of mutant CFTR gene insertion
All mature blastocysts along with morula stages of injected cells lysed with lysis buffer and the PCR technique was conducted using either F1R1, F2R1, F1R2, and F2R2 primers. PCR results were shown in Figure 4. In accordance with PCR results, 5 blastocysts out of 22 mature injected blastocytes and 2 morulae out of 10 injected morulae showed a positive PCR for the presence of CFTR either in a partial or complete insertion.
Fig 3.

Pronuclear injection of CFTR in the mouse zygote. Embryo numbers 1, 3, 5, 6, 7; Blastocysts stage and Embryo numbers 2, 4; Morulae stage, and the rest didn’t develop.
Fig 4.

PCR products of the blastocyst with CFTR primers. Line numbers 2, 4, and 6 represent complete recombination of CFTR into the mouse genome, while sample number 1 represents a partial insertion of the construct as the F2R1 primers could amplify this region. The last band is the controlling band and the PCR result of the plasmid alone. 1 F2R1 (about 2600 bp); 2, 4, 6 F1R1 primer (4600 bp), number 8 plasmid as a positive control, and number 9, 5 kb DNA ladder.
Discussion
The first transgenic mouse model of CF was created in 1994 based on the HR system, that contained 78% similarity in amino acid sequences with human CFTR protein (18). Other mouse models were developed using replacement and/or insertional strategies in which this procedure resulted in the knockout of the gene, whilst insertional procedure produces less than 10% of normal CFTR mRNA. Inducing mutation at the site of CFTR gene construct insertion by double-strand HR, ‘hit and run’, various mouse models of CF would be produced. It is worth noting that the mutated mice are less fertile than normal mice. Also, the CF mutant mice breeding is much laborious work and needs a hard attempt. Recently the G542X CF mouse model was generated using a CRISPR/ Cas9 genome editing system that leading to 40.9% of mice expressing the G542X mutation (19). HR was a predominant way for the alteration of genetic information, hence, it contributed to the important process of genetic replication with a double-strand break, formation of the replication fork, and horizontal gene transfer through meiosis, leading to the genome integrity and diversity (20-22).
Our study was aimed to use an HR system by introducing the foreign DNA into the male pronucleus. This was through the pronuclear injection method to produce a CFTR mutant model. We use codon optimization to design a unique construct whilst inserted into the genome, that made it easily detectable. We used a simple PCR technique without genome sequencing after insertion.
There is a variety of strategies to insert the exogenous DNA into the host genome. The majority applicable method is microinjection that the DNA is injected into the male pronucleus which has been shown to have a 100% efficiency. Although, the birth rate of offspring is about 4-8% (23). The pronuclear injection technology has been used to study the molecular and cellular functions of many genes (24). In this technology, the foreign gene, artificially introduced into cells, contributed to rearrangements of nucleotides and render the mutations. Thus, different copies of the gene were arranged randomly in a case of cytoplasmic insertion of DNA or direct injection into the nuclei. The integration of foreign DNA fragments into a genome may occur either by heterologous or HR with different mechanisms and frequencies. The heterologous recombination accounts as a most frequent recombination leading to random integration (25). In this current study following injection of CFTR gene construct, the mutated CFTR was randomly integrated into the host genome and development of zygotes to blastocyte stage was observed three days after the pronuclear injection and found that 22 blastocysts along with 10 morulae were developed after injection indicating the development of zygotes after CFTR gene construct insertion. The overall rate of development in transgenic zygotes was 18.8%, whilst the rate of blastocyst development was 12.9%. Hence, the birth rate of offspring after microinjection of DNA into the male pronucleus was very low (~ 4-8%) (23) which we also observed a low rate of blastocyte in our current study.
HR accounts as a common way of foreign DNA insertion into the host genome (26). The exogenic DNA integrated during the DNA replication mechanism of the host cell following breakage in double-strand DNA; as the breakage in DNA is random, the incorporation of exogenous DNA into the host genome would be random and consequently disrupt or prevent the expression of the host gene (27). But, the efficiency of integration is very low, hence HR is mostly followed by a double-strand break using TALEN, ZFNs, and CRISPR-cas9 technique that the CRISPR technique has been reported to have off-target effects (5). To successfully insert the gene in the animal model of diseases, it was necessary to include expression elements located at 5' and 3' ends of the gene sequence (28). To obtain a unique genome construct, some modification should be performed later verification test. This alternation included placement of RE sites to remove the designed gene construct and inserts it into the other vector, as well as identification of an inserting gene into the interest site within the animal genome (29). Therefore, after the introduction of the gene construct, the verification could be performed using RE and PCR products.
In the current study, we designed a specific CFTR gene construct and used an HR system without using breakage enzymes. The integration occurred during normal genome replication where two-strand DNA separated through the replication fork, and randomly the targeted exogenous gene incorporated with a homologs site in the genome. So, for a first time we succeed in insertion of gene construct and determine the insertion using simple PCR method.
Conclusion
We successfully designed and produced a transgenic model of CF based on a HR system. This was via transferring the specific designed artificial human CFTR gene into a male mouse pronucleus without the requirement of TALEN, ZFNs, or CRISPR-cas9 techniques to increase the efficiency of integration; and, we could then verify CFTR construct integration into the genome using simple PCR. The study can only be further developed after the blastocyst formation and implantation of the blastocyst passes through the embryonic development stages and gives birth to the CF fetuses. Hence, the production of the CF mouse model by this specifically designed CFTR construct is recommended for further studies.
Acknowledgements
The authors are thankful of the Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran Iran, for their cooperation. There is no financial support and conflict of interest in this study
Authors’ Contributions
B.K., M.S.; Contributed to conception and design. H.R.; Contributed to all experimental work, data gathering, statistical analysis, and data interpretation. B.K., M.S., S.H.; Experimental work. Kh.A.; Clinical consultation and overall supervision. H.R., S.K.; Drafted the manuscript and bioinformatic analysis. M.B.; Manuscript revision and cloning and experimental work. B.K., S.H.; Contributed in IVF procedure. All authors read and approved the final manuscript.
References
- 1.Lopes-Pacheco M. CFTR modulators: the changing face of cystic fibrosis in the era of precision medicine.Front Pharmacol. Front Pharmacol; 2020. pp. 101662–101662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Y, Wrennall JA, Cai Z, Li H, Sheppard DN. Understanding how cystic fibrosis mutations disrupt CFTR function: from single molecules to animal models. Int J Biochem Cell Biol. 2014;52:47–57. doi: 10.1016/j.biocel.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 3.McCarron A, Donnelley M, Parsons D. Airway disease phenotypes in animal models of cystic fibrosis. Respir Res. 2018;19(1):1–12. doi: 10.1186/s12931-018-0750-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodson ME, Simmonds NJ, Warwick WJ, Tullis E, Castellani C, Assael B, et al. An international/multicentre report on patients with cystic fibrosis (CF) over the age of 40 years. J Cyst Fibros. 2008;7(6):537–542. doi: 10.1016/j.jcf.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Rosen BH, Chanson M, Gawenis LR, Liu J, Sofoluwe A, Zoso A, et al. Animal and model systems for studying cystic fibrosis. J Cyst Fibros. 2018;17(2):S28–S34. doi: 10.1016/j.jcf.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu L, Batara J, Lu B. Application of genome editing technology to microrna research in mammalians.Kormann MSD, editor.Modern tools for genetic engineering.London.IntechOpen. IntechOpen; 2016. [Google Scholar]
- 7.Leavitt AD, Hamlett I. Homologous recombination in human embryonic stem cells: a tool for advancing cell therapy and understanding and treating human disease. Clin Transl Sci. 2011;4(4):298–305. doi: 10.1111/j.1752-8062.2011.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Wang X, Zhu L, He Z, Liu G, Xu X, et al. Establishment of a rapid and scalable gene expression system in livestock by sitespecific integration. Gene. 2013;515(2):367–371. doi: 10.1016/j.gene.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, et al. Production of CFTR-null and CFTR-ΔF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118(4):1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, et al. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet. 1992;2(3):240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120(9):3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Livraghi-Butrico A, Hou X, Rajagopalan C, Zhang J, Song J, et al. Phenotypes of CF rabbits generated by CRISPR/Cas9- mediated disruption of the CFTR gene. JCI Insight. 2021;6(1):e139813–e139813. doi: 10.1172/jci.insight.139813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dreano E, Bacchetta M, Simonin J, Galmiche L, Usal C, Slimani L, et al. Characterization of two rat models of cystic fibrosis—KO and F508del CFTR—Generated by Crispr-Cas9. Animal Model Exp Med. 2019;2(4):297–311. doi: 10.1002/ame2.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leenaars CH, De Vries RB, Heming A, Visser D, Holthaus D, Reijmer J, et al. Animal models for cystic fibrosis: a systematic search and mapping review of the literature-Part 1: genetic models. Lab Anim. 2020;54(4):330–340. doi: 10.1177/0023677219868502. [DOI] [PubMed] [Google Scholar]
- 15.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 16.Zeng F, Hao Z, Li P, Meng Y, Dong J, Lin Y. A restriction-free method for gene reconstitution using two single-primer PCRs in parallel to generate compatible cohesive ends. BMC Biotechnol. 2017;17(1):32–32. doi: 10.1186/s12896-017-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vahdat-Lasemi M, Hosseini S, Jajarmi V, Kazemi B, Salehi M. Intraovarian injection of miR-224 as a marker of polycystic ovarian syndrome declines oocyte competency and embryo development. J Cell Physiol. 2019;234(8):13858–13866. doi: 10.1002/jcp.28067. [DOI] [PubMed] [Google Scholar]
- 18.Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, et al. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nat Genet. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- 19.Scholte BJ, Davidson DJ, Wilke M, De Jonge HR. Animal models of cystic fibrosis. J Cyst Fibros. 2004;3(Suppl 2):183–190. doi: 10.1016/j.jcf.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 20.Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26(4):447–450. doi: 10.1038/82586. [DOI] [PubMed] [Google Scholar]
- 21.Jasin M, Rothstein R. Repair of strand breaks by homologous recombination. Cold Spring Harb Perspect Biol. 2013;5(11):a012740–a012740. doi: 10.1101/cshperspect.a012740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Symington LS, Rothstein R, Lisby M. Mechanisms and regulation of mitotic recombination in Saccharomyces cerevisiae. Genetics. 2014;198(3):795–835. doi: 10.1534/genetics.114.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Popova E, Krivokharchenko A, Ganten D, Bader M. Efficiency of transgenic rat production is independent of transgene-construct and overnight embryo culture. Theriogenology. 2004;61(7-8):1441–1453. doi: 10.1016/j.theriogenology.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Sosa MAG, De Gasperi R, Elder GA. Animal transgenesis: an overview. Brain Struct Funct. 2010;214(2-3):91–109. doi: 10.1007/s00429-009-0230-8. [DOI] [PubMed] [Google Scholar]
- 25.Meyer M, de Angelis MH, Wurst W, Kühn R. Gene targeting by homologous recombination in mouse zygotes mediated by zinc-finger nucleases. Proc Natl Acad Sci USA. 2010;107(34):15022–15026. doi: 10.1073/pnas.1009424107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan BW, Zhao YF, Cao WG, Li N, Gou KM. Mechanism of random integration of foreign DNA in transgenic mice. Transgenic Res. 2013;22(5):983–992. doi: 10.1007/s11248-013-9701-z. [DOI] [PubMed] [Google Scholar]
- 27.Bouabe H, Okkenhaug K. Gene targeting in mice: a review. Methods Mol Biol. 2013;1064:315–336. doi: 10.1007/978-1-62703-601-6_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall B, Limaye A, Kulkarni AB. Overview: generation of gene knockout mice.Curr Protoc Cell Biol. Curr Protoc Cell Biol; 2009. Chapter 19. Unit 19; pp. 12.19.12.1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaghobi Moghaddam MA, Dehghan Esmatabadi MJ. A review of artificial genetic constructs and their applications as positive controls. J Human Gen Genom. 2019;3(1):e99853–e99853. [Google Scholar]


