Abstract
Objective
Evidence suggests the contributory role of oxidative stress (OS) to sperm DNA damage and eventually, male infertility. Antioxidant supplementation has exhibited favorable results regarding seminal OS, sperm DNA damage, and chromatin integrity. We aimed to evaluate the effect of alpha-lipoic acid (ALA) supplementation on semen analysis, sperm DNA damage, chromatin integrity, and seminal/intracellular OS in infertile men with high sperm DNA damage.
Materials and Methods
In this randomized triple-blind placebo-controlled clinical trial study, we opted for a triple-blind controlled clinical trial design. Considering the study’s inclusion criteria for the level of sperm DNA fragmentation (higher than the threshold of 30 and 15%), 70% of participants were selected for this clinical research study. Subjects were divided into case and control groups receiving oral ALA (600 mg/day) and placebo for eighty days, respectively. Sperm parameters and functional tests were examined and compared before and after treatment. The final sample size was 34 and 29 for ALA and placebo receivers, respectively.
Results
No significant differences were observed about anthropometrics and baseline measures of semen analysis, DNA damage, OS, and chromatin integrity between the two groups. Conventional semen parameters were enhanced insignificantly in both groups (P>0.05). DNA damage decreased significantly in the ALA group, as per sperm chromatin structure assay (SCSA, P<0.001). Moreover, chromomycin A3 (CMA3) staining results indicated a decrease in nuclear protamine deficiency post-ALA therapy (P=0.004). Lipid peroxidation decreased significantly after treatment with ALA (P=0.003). Further, seminal antioxidant capacity/activity did not differ significantly in either of the groups (registration number: IRCT20190406043177N1).
Conclusion
An 80-day course of oral ALA supplementation (600 mg/day) alleviates sperm OS, DNA damage, and chromatin integrity in men with high sperm DNA damage.
Keywords: Alpha-Lipoic Acid, DNA Damage, Male Infertility, Sperm
Introduction
Infertility is defined as the failure to conceive despite the performance of regular unprotected coitus for a minimum of a year affecting 15-24% of couples (1). Of all infertility cases, approximately 50% are due to male factors and male suboptimal sperm parameters (2). The quality of sperm plays an indisputable leading role in male fertility. However, the criteria by which a spermatozoon is considered ‘qualified’ to induce pregnancy still need to be determined. Sperm concentration, motility, and morphology have classically been known as sperm quality representatives, although it is now believed that as many as 26% of the male population with ‘normal’ semen parameters could face infertility (i.e., unexplained infertility) (3, 4). Further, despite advances in human knowledge upon reproduction, the exact etiology behind 30-40% of impaired semen analyses remains unknown, nomenclature as idiopathic male infertility. The emerging evidence has drawn attention to sperm DNA damage as a potential underlying etiology (5, 6). On the other hand, it has been indicated that oxidative stress (OS) majorly contributes to infertility of unknown origin (in 30-80% of infertile men) (7). Similarly, men with unexplained infertility tend to exhibit higher reactive oxygen species (ROS) levels in their seminal fluid compared to fertile normozoospermic individuals (8).
ROS represents a vast number of free and non-free radical chemical compounds produced by oxidative metabolism. ROS generated at the levels of sperm mitochondria and plasma membrane and acts as signaling molecules that mediate capacitation, acrosome reaction, hyperactivation, and sperm-zona pellucida fusion (9). OS portrays a state in which the homeostatic equilibrium between the oxidizing and reductant molecules shifts in favor of the former. The OS occurs as a result of ROS overproduction in the seminal plasma by either morphologically abnormal sperms and/or leukocytes, or due to the lack of antioxidants. The sperm plasma membrane rich in polyunsaturated fatty acids and the simultaneous lack of compensatory mechanisms expose the cell to oxidative damage. Finally, OS is supposedly the main contributor to sperm DNA damage (10, 11).
Evidence shows that there is many studies have exhibited a meaningful association between the increase in sperm DNA damage and prolonged conception period, decreased fertilization rate, impairments in embryo cleavage, higher rates of miscarriage and pregnancy loss, and birth defects in the offspring (12). OS-alleviating role of oral antioxidant supplementation has been also widely explored in the context of male infertility and many studies have detected favorable outcomes regarding motility, OSinduced damage, and DNA fragmentation in sperm (13).
Alpha-lipoic acid (ALA), thiotic acid, is a natural Krebs cycle co-enzyme, reputed for its potent antioxidant characteristics. ALA and its reduced form, dihydrolipoic acid (DHLA), act in both aqueous and lipid phases and exert intense antioxidant properties. In addition to in vitro-confirmed direct ROS scavenging characteristics, DHLA regenerates non-enzymatic endogenous antioxidants, namely vitamin C and E, and augments enzymatic antioxidants such as glutathione and superoxide dismutase. Both ALA and DHLA serve as metal chelating agents by forming stable compounds with elements Cu, Mn, Zn, Ars, Cd, and Hg (14). Interestingly, DHLA does not turn into a free radical in the process of neutralizing these agents (14-16).
Many studies have underlined the beneficial impacts of ALA supplementation on OS-derived conditions, namely diabetic neuropathy, glaucoma, cataracts, and alcoholic liver disease (14). Also, the effects of ALA on male fertility have been the subject of several animal models and a few controlled clinical trials. As evidenced, ALA administration ameliorates enzymatic/non-enzymatic antioxidant features, mitigates OS-mediated damages to the testicular structure, and maintains sex-hormone balance; all of which may lead to the improvement in semen parameters and the consequent fertility efficacy (16-18). However, human studies are confined to two exclusive clinical trials: Through a clinical trial on asthenoteratospermic men, Haghighian et al. (18) indicated that ALA supplementation could enhance semen quality in terms of conventional semen parameters. Further, a recent controlled randomized trial has shown that ALA administration after microsurgical varicocelectomy could significantly improve sperm motility and DNA fragmentation in the patients (19). Therefore, both studies have underlined the need for further trials on the subject.
To fill the mentioned gap, we aimed to conduct a randomized, triple-blind placebo-controlled clinical trial to evaluate the effect of oral ALA administration on sperm DNA integrity, chromatin integrity, and sperm parameters as well as seminal OS markers in infertile human subjects with high sperm DNA damage.
Materials and Methods
Study design
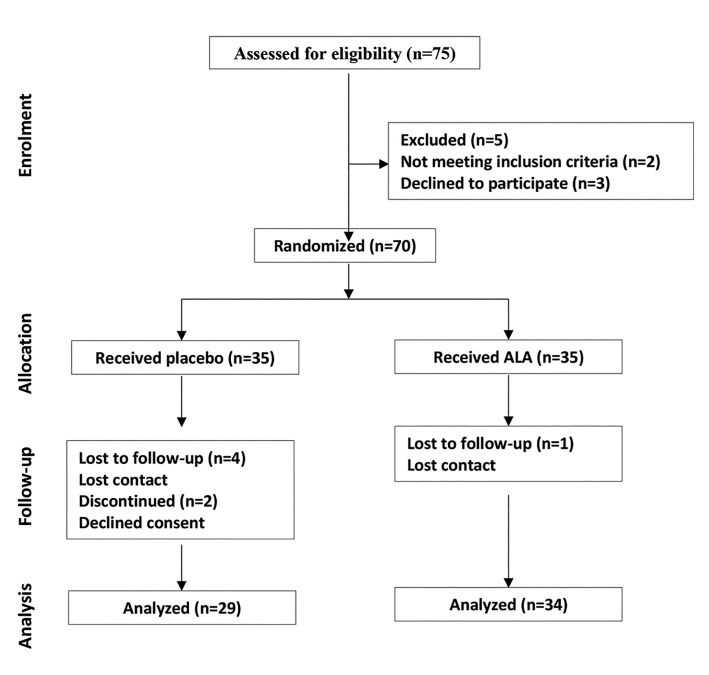
The present randomized triple-blind placebo-controlled clinical trial was held in Royan Institute (Tehran, Iran) between July 2018 and June 2020, we randomized infertile men with sperm DNA damage over the threshold to evaluate ALA medication efficacy versus placebo regarding conventional sperm parameters, seminal/ intracellular OS, and DNA damage alleviation capabilities (Fig .1). All the sperm analysis and sperm functional tests were conducted in native semen samples. The study protocol was approved by Royan Institute Ethics Committee for Research Involving Human Subjects (IR.ACECR.ROYAN.REC.1397.108) and the Iranian Registry for Clinical Trials (IRCT20190406043177N1).
Fig 1.
Study design according to CONSORT.
Patient recruitment
Men -referred to the Royan Institute clinic- with or without impaired semen analysis with high sperm DNA damage were eligible for our study. According to WHO guidelines, impaired semen analysis was defined as the presence of at least one of the following conditions: sperm concentration < 15 million/ml, total motility < 40%, and normal morphology < 4%, otherwise semen analysis was considered to be normal (3). For all the couples, the extent of sperm DNA damage was defined with either sperm chromatin structure assay (SCSA) or terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) method, and individuals with values higher than the threshold of 30% and 15% were considered eligible, respectively (6, 20).
Medical conditions potentially affecting the fertility status were ruled out by available routine investigations for the participants and their female partners. We excluded the subjects with recent/ongoing history of varicocele, leukocytospermia, chemo-radiation, cytotoxic medication, and malignancies. Female partners were considered normal in the presence of normal menstrual cycles, vaginal ultrasounds, and hysterosalpingographies. Couples with female-related infertility, including polycystic ovary syndrome, endometriosis, and tubal factor, were excluded from the study.
Individuals were thoroughly educated on the aim and the rationale behind the study design and the interventional groups, randomization, sample collection, and delivery to the designated laboratory. Age, anthropometrics, and medical and medication/supplementation history were provided from each of the participants. After assuring the couples of not incurring any cost and their right to acquire the obtained results, a signed written consent form was obtained from each.
Interventions
Applying a computer-mediated random digits table and simple randomization method, we allocated the subjects to drug and control groups. Patients in the case group received a cumulative daily dose of 600 mg of ALA (Raha company, Isfahan, Iran), while controls were given the placebo (600 mg, made of starch) with identical appearance and taste, both daily for 80 consecutive days (18). There are no reports of adverse drug reactions for the oral intake of ALA in the literature and it is considered safe to consume (18, 19).
Two semen samples were obtained from every participant: one before the medication course initiation and the other promptly after its termination. Samples were given by masturbation following 2-7 days of sexual abstinence (3). Once delivered to the laboratory, samples were weighed, let to liquefy at room temperature, and subsequently evaluated in terms of viscosity and liquefaction by applying a wide-bore pipette.
Randomization
To randomly allocate the subjects to medication and control groups, we applied permuted blocks. According to our sample size, all the possible permutations of tenunit blocks were obtained, and subsequently, a sequence of seven blocks was designated by applying a random number table. Randomization was carried out by persons unrelated to the study operations. ALA and placebo were packed identically and were indiscernible. The randomization sequence was not disclosed before the termination of statistical analysis, and patients, drug prescribers, data collectors, and statistical analysts were kept blinded to it.
Primary outcomes
Conventional semen analysis
Concentration
Samples were diluted by applying 1% formalin in sodium bicarbonate solution (1:10) and placed in the sperm counting chamber (Sperm meter, sperm processor, Garkheda, Aurangabad, India). Applying an optical microscope (LABOMED CxL; 20×), a trained laboratory technician counted the number of sperm (a minimum of 200 sperm per sample) and recorded the observations as million sperms/milliliter (3).
Motility
Semen (10 µl) was placed on a pre-warmed sperm counting chamber and was covered with a coverslip with a depth of 20 µm. The motility was assessed utilizing computer-assisted sperm analysis (CASA) and LABOMED CxL optical microscope in a minimum of five different microscopic fields (≥200 sperm evaluated per field). Four distinct sperm motions were determined: rapid progressive, slowly progressive, non-progressive, and immotile. Ultimately the percentage of total sperm motility and progressive sperm motility were reported (3).
Abnormal sperm morphology
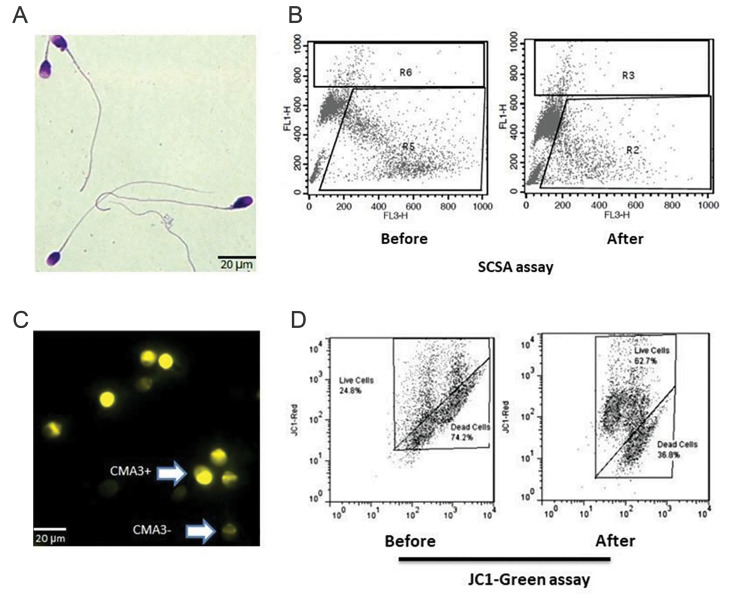
According to Tygerberg’s criteria, two smears were obtained and fixed by methanol-dissolved triarylmethane dye per sample. The smears were later stained by eosinophilic xanthene and basophilic thiazine solutions (Diff-Quick staining) (Fig .2A). The smears were evaluated regarding head, neck, and tail abnormalities under high microscopic magnification (×1000). At last, the percentage of sperm with abnormal morphology was expressed (3).
Fig 2.
Assessment of sperm functional tests. A. Assessment of sperm morphology with Diff- Quick staining, B. Representative histograms of sperm chromatin structure assay (SCSA) test before and after ALA therapy in an infertile man, C. Chromomycin A3 (CMA3) staining for assessment of sperm protamine deficiency; CMA3 negative or the sperm with normal protamine content and CMA3 positive or protamine deficient sperm, and D. Representative histograms of sperm JC1 staining for assessment of mitochondrial membrane potential before and after alpha-lipoic acid (ALA) therapy in an infertile man (20 μm=100 magnification).
Viability
In short, 1 g of eosin Y (color index: 45380) and 10 g of nigrosine (color index: 50420) dyes were separately solved in 100 ml of distilled water. The semen sample was then mixed with eosin (one drop each), and after 30 seconds, three drops of the suspended nigrosine were added to the mixture. One minute later, a thin smear was obtained from the well-blended mixture and was left to dry up for five additional minutes. Subsequently, the slides were monitored using a bright-field optic microscope with ×100 magnification (LABOMED CxL). A minimum of 200 sperm was evaluated per replicate (3).
Sperm DNA integrity/damage evaluation
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)
A commercial detection kit was used for TUNEL (Apoptosis Detection System Fluorescein, Promega, Mannheim, Germany). Briefly, a semen aliquot containing 3×106 sperm was centrifuged (800 g, 5 minutes, room temperature). After removing the seminal plasma, phosphate-buffered saline (PBS, Sama Tashkhis, Iran) was added to the pellet. Next, each sample was equally divided into negative/positive control and the test tubes. The tube’s pellet was then fixed in 4% paraformaldehyde (methanol-free) for 30 minutes (pH=7.4). Next, the samples were diluted with PBS and permeabilized with 0.2% Triton X-100 in PBS for 5 minutes followed by PBS -wash and were resuspension in 50 µl of the staining solution for one hour (37°C, dark room).
For negative control, the TdT enzyme from the kit was not added to the tube, and for the positive control, the samples were incubated with DNase I (40 IU/ml for 10 minutes) before the fixation. Finally, we analyzed the data by flow cytometry (FACScan BD FACS Calibur, Becton– Dickinson, San Jose, CA, USA). A minimum of 10,000 sperm was examined per assay (21).
Sperm chromatin structure assay
Two million sperms were separated from each sample, and container volume was raised to 1 ml using TNE buffer (Tris–HCl+sodium chloride+Ethylenediaminetetra acetic acid [EDTA]) buffer. Following the addition of 400 μl acid-detergent solution to 200 μl of the diluted semen sample, the mixture was stained with 1200 μl of acridine orange (Sigma, St. Louis, USA) staining solution (Fig .2B). Almost 10,000 sperm per sample were monitored/ analyzed with a flow cytometer (FACSCalibur Becton Dickinson, San Jose, CA, USA), and the percentage of DNA fragmentation was calculated accordingly (22).
Aniline blue staining
Two smears were obtained and washed from each sample. Then, the slides were fixed and stained by glutaraldehyde (2.5%) and 5% aniline blue (AB, aqueous) in 4 % acetic acid, respectively. Afterward, the slides were dried out using consecutive ethanol baths (70%, 96%, and 100%) and embedded in xylol (5 minutes). Finally, the smears were coated with Entellan rapid mounting medium. Randomly, a minimum of 200 sperm was counted by an instructed individual applying an optical microscope (bluish sperm implied nuclear immaturity) (23).
Chromomycin A3
Briefly, from each sample, two smears of sperm were washed and later fixed with Carnoy’s solution. Afterward, smears were stained with 200 μl of Chromomycin A3 (CMA3) staining solution (0.25 mg/ml). After washing with 1x-PBS (×3). By use of an epifluorescence microscope (Olympus, Japan) with suitable filters (460- 470 nm, 100× magnified), a minimum of 200 sperm was evaluated: Sperm with insufficient protamine content ~ light yellow; sperm with adequate protamine content ~ dark yellow (Fig .2C) (24).
Reactive oxygen species generation
Mitochondrial membrane potential evaluation: JC1 staining assay (JC1-MMP)
Briefly, the samples were diluted with PBS to an approximate concentration of 3-5 million sperms/ ml. JC-1 dye (preserved at-20°C) was thawed at 37°C and was added to the attenuated samples (1 µl to 1 ml) followed by incubation for 15 minutes at 37°C. Subsequently, the samples were centrifuged at 3000 RPM for five minutes. The resultant supernatant was removed, and the cell pellet was suspended again with 1 ml of PBS. For each sample, the level of JC-1 stainability was evaluated using a flow cytometer (FACSCalibur Becton Dickinson, San Jose, CA, USA). Red and green fluorescence implied normal and abnormal mitochondrial membrane potentials, respectively (Fig .2D). The results were eventually expressed as the percentage of green cells reflecting the sperm percentage possessing abnormal mitochondrial membrane potentials (25).
Lipid peroxidation
To assess the extent of seminal lipid peroxidation, we evaluated malondialdehyde (MDA) by utilizing a commercial colorimetric MDA assay kit (ZB-MDA96A, ZellBio GmbH, Ulm, Germany). Briefly, 100 µL of seminal samples were mixed with 100 µL and 200 µL of R4 reagent and Chromogenic solution, respectively. The mixture then was heated using a boiling water bath for one hour. Next, the tube was cooled in an ice bath and centrifuged for 10 minutes (10,000 rpm). 200 µL of the supernatant was pipetted into a microplate and read at 535 nm.
Total antioxidant capacity
We used a commercial kit (ZB-TAC-96A, ZellBio GmbH, Germany) to measure total antioxidant capacity (TAC) in the seminal plasma. As per manufacturer instructions, seminal plasma was initially centrifuged at 600 g for 10 minutes. Then, 10 µL of the sample was added to 190 µL working chromogen reagent on the microplates followed by covering and 2 minutes of incubation at room temperature. Lastly, the samples were read at the wavelength of 490 nm (26).
Superoxide dismutase activity
Superoxide dismutase (SOD) was also measured with the use of a ZellBio kit (ZB-SOD-96A, Zellbio GmbH, Ulm, Germany). Briefly, the sample was mixed with EDTA and centrifuged for 10 minutes (2000-3000 rpm). Then, the supernatant was added to the wells and blended with the reagents for homogenization. Afterward, applying an enzyme-linked immuno-absorbent assay (ELISA) microplate reader, the absorbance (412 nm) was then measured at 0 and 2 minutes.
Secondary outcome
We opted for pregnancy rate as the secondary outcome. To do so, patients were followed up to determine pregnancy occurrence for approximately six months after the medication’s termination. The percentage of couples with clinically approved (confirmation by ultrasound) pregnancies in each interventional group was calculated and reported as the pregnancy rate. Recurrent pregnancy loss was defined and measured as the occurrence of two or more failed pregnancies (27).
Sample size and statistical analysis
Our estimate indicated that a total of 75 infertile men with damaged sperm DNA would correctly endorse a relative effect size of 22% in the composite outcome measure (µ0 =67; µ1 =0.87) with the power and one-tailed level of significance of 0.9 and 0.05, respectively (1- β=0.9; α=0.05) (18).
The analysis was performed utilizing IBM SPSS Statistics for Windows (version 26). As confirmed by the Kolmogorov-Smirnov test, the variables were normally distributed. Consequently, the independentsample t-test was used to underlie the dispersion of the variables between ALA and placebo groups, while intra-group differences were evaluated applying the paired-sample t test. The data were expressed as mean ± standard deviation, and the P values calculated lower than 0.05 were considered significant. Flow cytometry data were analyzed using WIN MDI 2.9 software (The Scripps Institute, Flow Cytometry Core Facility, USA).
Results
In the current study, 35 individuals to each of the interventional groups were assigned by use of permuted blocks. One and six were lost in ALA and placebo groups due to refusal to continue therapy or not showing up for the endpoint sampling; consequently, the sample size was confined to the final 34 ALA and 29 ALA placebo receivers (Fig .1).
No statistically significant difference was observed between ALA and placebo receivers regarding mean anthropometric values namely age, height, weight, and body mass index. Likewise, the difference between baseline measures of conventional semen parameters, sperm DNA/chromatin indexes, and indicators of ROS generation showed no differences between two groups (P>0.05, Table 1). The abortion rate was higher in the ALA group in comparison with the placebo (97.06% vs. 70.37%, P=0.004). Moreover, a significantly higher prevalence of recurrent pregnancy loss was detected in the ALA group compared to the placebo receivers (85.29% vs. 55.56%, P=0.01).
Table 1.
Comparison of the measures of conventional semen analysis and sperm DNA damage assays (SCSA and TUNEL) before and eighty days after initiation of the designated intervention for each experimental group: Alpha-lipoic acid (600 mg/day) versus placebo
|
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Placebo | ALA | P value | |||||
| a. Before | b. After | c. Before | d. After | a vs. b1 | c vs. d1 | a vs. c2 | b vs. d2 | |
|
| ||||||||
| Semen volume (ml) | 3.43 ± 1.34 | 3.31 ± 1.22 | 4.24 ± 1.86 | 3.89 ± 1.72 | 0.379 | 0.132 | 0.069 | 0.161 |
| Sperm concentration (106/ml) | 63.54 ± 64.47 | 54.56 ± 35.24 | 50.09 ± 47.64 | 59.8 ± 43.49 | 0.335 | 0.181 | 0.353 | 0.614 |
| Total motility (%) | 47.16 ± 27.27 | 41.52 ± 22.56 | 45.64 ± 25.02 | 47.27 ± 26.43 | 0.144 | 0.653 | 0.819 | 0.364 |
| Progressive motility (%) | 34.73 ± 19.31 | 25.49 ± 15.88 | 28.64 ± 18.28 | 28.07 ± 22.55 | 0.997 | 0.849 | 0.239 | 0.631 |
| Viability (%) | 84.44 ± 13.54 | 80.63 ± 20.52 | 80.58 ± 18.1 | 83.87 ± 13.65 | 0.407 | 0.409 | 0.386 | 0.493 |
| Normal morphology (%) | 2.19 ± 2.27 | 1.67 ± 1.33 | 1.64 ± 1.52 | 1.48 ± 1.09 | 0.104 | 0.377 | 0.268 | 0.563 |
| SCSA (%) | 30.08 ± 14.24 | 32.41 ± 14.62 | 36.98 ± 15.67 | 25.38 ± 14.68 | 0.319 | 0.0005 | 0.085 | 0.072 |
| TUNEL (%) | 16.23 ± 9.2 | 20.54 ± 10.44 | 18.2 ± 12.64 | 13.7 ± 8.58 | 0.029 | 0.107 | 0.514 | 0.514 |
|
| ||||||||
Data are presented as mean ± SD . 1 ; Paired t test, 2 ; Two-sample t test, SCSA; Sperm chromatin structure assay, and TUNEL; Terminal deoxynucleotidyl transferase dUTP nick end labeling.
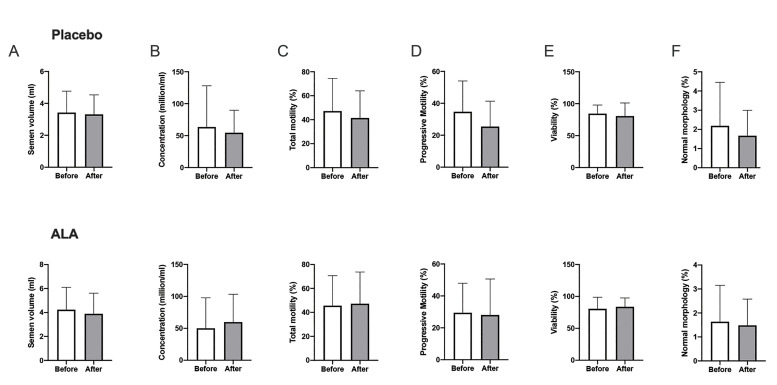
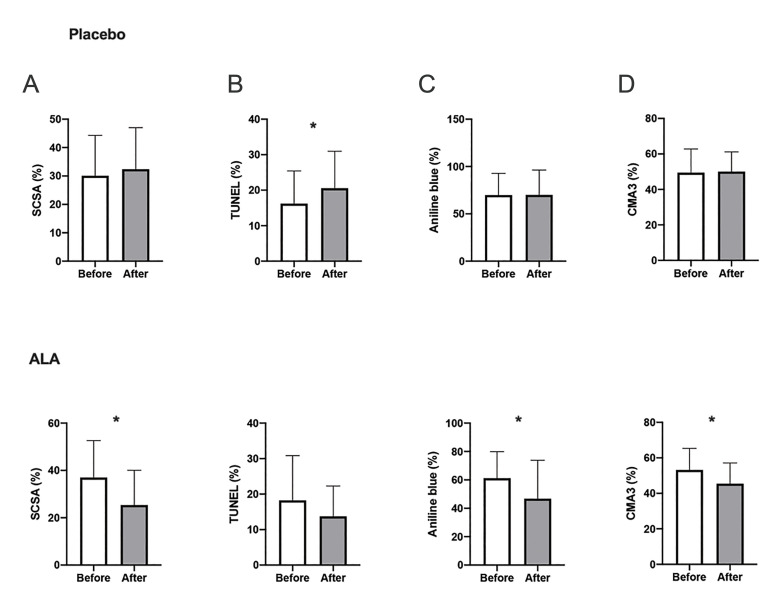
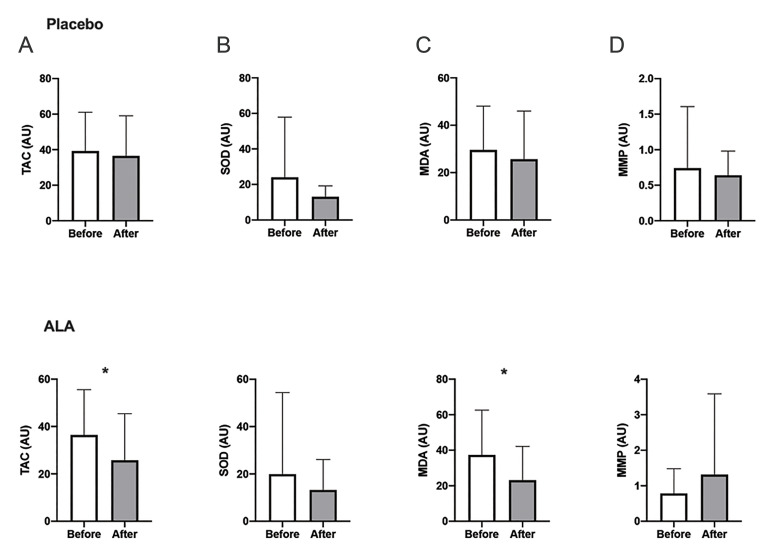
The results of conventional semen analysis showed significant intra-group differences after the termination of treatment in neither of the interventional groups (Fig .3). We witnessed improvement in sperm chromatin represented by AB (remnant histones) and CMA3 staining (protamine deficiency) after ALA therapy (P=0.03 and P<0.005, respectively). In the ALA group, our analysis revealed a significant decrease in sperm DNA damage by the SCSA test (P<0.005) while the TUNEL results did not differ significantly (P=0.11). Conversely, the mean sperm DNA fragmentation increased significantly among placebo receivers (TUNEL, P=0.03, Fig .4). The extent of lipid peroxidation (i.e., MDA generation) and TAC decreased significantly after ALA medication (both P<0.005) while post-intervention levels of SOD did not differ significantly in either of the groups (P>0.05, Fig .5). Finally, no significant change in mean mitochondrial membrane potential was seen in the ALA (P=0.60) and placebo (P=0.24) groups.
Fig 3.
Comparison between the mean measures of seminal analysis parameters before and after intervention (paired t test). A. Semen volume, and sperm B. Concentration, C. Total motility, D. Progressive motility, E. Viability, and F. Normal morphology. ALA; Alpha lipoic acid.
Fig 4.
Intragroup comparison between the mean values of sperm DNA damage before and after intervention (paired t test). Examined by A. SCSA and B. TUNEL assays, and the level of sperm chromatin compaction/protamination as indicated by the results of C. Aniline blue and D. CMA3 staining techniques. *; Denotes P<0.05, ALA; Alpha lipoic-acid, SCSA; Sperm chromatin structure assay, TUNEL; Terminal deoxynucleotidyl transferase dUTP nick end labeling, and CMA3; Chromomycin A3.
Fig 5.
Comparison between levels of seminal oxidative determinants before and after intervention. A. Total antioxidant capacity, B. Superoxide dismutase, C. Malondialdehyde, and D. Mitochondrial membrane potential. *; Denotes P<0.05, ALA; Alpha lipoic-acid, TAC; Total antioxidant capacity, SOD; Superoxide dismutase, MDA; Malondialdehyde, MMP; Mitochondrial membrane potential, and AU; Arbitrary unit.
Patients were further followed up for the occurrence of pregnancy: In the ALA group, eight patients achieved natural pregnancy, while the number for the control group was three (pregnancy rates 23.53% and 10.34%, respectively). Pearson’s chi-square test revealed no statistically significant relationship between the type of treatment and the incidence of pregnancy (P=0.169).
Discussion
As of today, the etiology behind a notable proportion of male infertility cases remains unknown. A recent study has unveiled that as much as 80% of infertile men exhibit some extent of OS in their seminal fluid, which is a potentially reversible condition (28). As mentioned, seminal enzymatic or non-enzymatic antioxidants provide the seminal fluid with oxidative homeostasis primarily through scavenging mechanisms, which ultimately leads to a reduction in ROS content to a satisfactory level. Lately, empirical antioxidant supplementation for infertility has drawn considerable attention. Despite enhancing sperm motility and attenuating seminal OS, however, most of the studied antioxidants confer minimal or no effect on the sperm DNA (29).
Opting triple-blind controlled clinical trial design, the present study is the first to evaluate the effects of ALA supplementation on sperm DNA in human subjects. Recruiting men with infertility of unknown origin, we aimed to focus on ROS overproduction as the principal contributor to sperm DNA damage and consequently to infertility. Generally, our results indicated that infertile men with over-threshold DNA damage could benefit from an 80-day course of ALA supplementation with the daily dosage of 600 mg: chromatin integrity and DNA damage status showed significant improvement postmedication, as assessed by SCSA, AB, and CMA3 assays. However, TUNEL results decreased insignificantly in the case group, while in contrast, placebo receivers gained a statistically significant increase in the TUNEL-assessed DNA damage. Also, both SCSA and TUNEL assays showed lower mean measures of sperm DNA damage in the ALA group compared to the placebo group after treatment.
The DNA integrity status outlines the degree of chromatin compaction, which in sperm is obtained by several mechanisms, most notably through exchanging histone nucleoproteins for protamines (30). Protamine provides more compacted sperm DNA compared to histones primarily through forming intra-/inter-DNA disulfide bonds. Compacted DNA is less vulnerable to damage, namely nicks and fragmentations (31, 32). Consistent with this, in the ALA group we observed a simultaneous decrease and increase in residual histone and sperm nuclear protamine content, respectively. In this regard, ALA has been shown to uphold intracellular cysteine concentration presumably through actively reducing cystine to cysteine or by increasing cellular cysteine uptake; all of which enhance one-carbon metabolism providing methyl groups mandatory for disulfide tight bonds needed for the optimum compaction of sperm DNA (33).
Moreover, in the ALA group, our analysis indicated a significant decrease in the mean sperm DNA damage according to the SCSA assay. Conversely, TUNEL results did not show a meaningful alleviation in sperm DNA fragmentation among ALA receivers while underlining a corresponding significant elevation in the placebo group. These findings are potentially attributable to the nature of damages that TUNEL and SCSA assays could detect. In short, the TUNEL assay measures the pre-existing double-strand DNA breaks (i.e., fragmentations) while SCSA evaluates the DNA’s susceptibility to single-strand nicks (34). Meanwhile, the persistence of the pre-existing fertility-deteriorating condition through the course of the study (80 days) coupled with receiving a placebo could be explanatory for the observed increase of TUNEL-assessed sperm DNA damage in the control group.
No significant difference was observed in mean MMP after medication. High MMP has been linked to optimum mitochondrial function, high viability, and motility in sperm, while low MMP is a sign of early cell death and apoptosis and is associated with sperm DNA damage (35, 36). On the other hand, hydrogen peroxide and superoxide radicals are byproducts of normal mitochondrial activities (37). Therefore, these observations further verify that ALA may benefit independent of mitochondrial function.
Our analysis unveiled a significant decrease in the level of MDA post-ALA treatment. MDA is the ultimate product of lipid peroxidation (38). The disturbance in membrane structure induced by lipid peroxidation affects vital cellular functions namely signal transduction and maintenance of ion and metabolite gradient necessary for optimal sperm function. The peroxides are generally associated with decreased sperm function and viability, DNA damage, and the ultimate fertility decrease (39). ALA has been proposed to quip the sperm with an extracellular shield and prevent lipid peroxidation, which may account for the observed reduced MDA (18). Concurrently, no significant difference was detected between the baseline and final measures SOD activity in the case group. However, TAC content showed a significant decrease in the ALA receivers, hypothetically imputable to the presence of a steady ROS scavenging process. Despite the findings mentioned above, no statistically significant changes were observed in favor of ALA supplementation efficacy regarding conventional semen parameters despite noticed alleviation in mean sperm DNA damage.
Finally, patients were monitored regarding the occurrence of pregnancy for a minimum of six months. As addressed in the result sections, we did not notice a significant association between the type of treatment (ALA or placebo) and the occurrence of natural. Considering the significant predominance in the case group regarding the abortion rate, the observed higher pregnancy rate might imply that ALA supplementation leads to enhanced pregnancy results in the infertile men with eminent DNA damage, possibly as a consequence of the lowered DNA damage enhancing the fertilizing capability of sperm, as well as a decrease in chromatin alterations incompatible with fetus viability which needs to be further investigated. Nevertheless, the statistical insignificance of improved pregnancy rate presumably stems from insufficiency in the sample size, proposing room for further clinical trials with larger sample sizes.
Conclusion
An 80-day course of ALA supplementation (600 mg/day) diminishes sperm DNA damage in men with high DNA damage. As indicated by our analysis, ALA medication ameliorates OS-derived lipid peroxidation leading to the consequent alleviation in DNA’s damage susceptibility and endorsement of DNA integrity by means of maintaining the optimal nuclear protamine content. However, further investigation could unveil the clinical aspects of such an association.
Acknowledgements
The authors would like to express their gratitude to Raha company (Iran, Isfahan) for providing ALA and placebo packs. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest.
Authors’ Contributions
M.H.N.-E., M.T.; Contributed to the conception, design, and coordination of the study, data analysis, revised the manuscript, and performed the final scientific manuscript revision. M.H., Z.F.Z., V.E.; Were in charge of patient recruitment and drug prescription, executed the laboratory analysis, and analyzed the data. B.A.; Analyzed the data, performed literature research, drafted the manuscript, revised the manuscript, and the final scientific manuscript revision. A.Sh., M.A.S.G.; Were in charge of patient recruitment and drug prescription. All authors read and approved the final manuscript.
References
- 1.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril. 2017;108(3):393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37–37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 6th ed.Geneva. World Health Organization; 2021. [Google Scholar]
- 4.Moghissi K, Wallach E. Unexplained infertility. Fertil Steril. 1983;39(1):5–21. doi: 10.1016/s0015-0282(16)46750-6. [DOI] [PubMed] [Google Scholar]
- 5.Haddock L, Gordon S, Lewis SEM, Larsen P, Shehata A, Shehata H. Sperm DNA fragmentation is a novel biomarker for early pregnancy loss. Reprod Biomed Online. 2021;42(1):175–184. doi: 10.1016/j.rbmo.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Aitken RJ, Bakos HW. Should we be measuring DNA damage in human spermatozoa?. New light on an old question. Hum Reprod. 2021;36(5):1175–1185. doi: 10.1093/humrep/deab004. [DOI] [PubMed] [Google Scholar]
- 7.Wagner H, Cheng JW, Ko EY. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J Urol. 2017;16(1):35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasqualotto FF, Sharma RK, Kobayashi H, Nelson DR, Thomas AJ, Agarwal A. Oxidative stress in normospermic men undergoing infertility evaluation. J Androl. 2001;22(2):316–322. [PubMed] [Google Scholar]
- 9.du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J Assist Reprod Genet. 2015;32(4):509–520. doi: 10.1007/s10815-014-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashki Ghaleno L, Alizadeh A, Drevet JR, Shahverdi A, Valojerdi MR. Oxidation of sperm DNA and male infertility. Antioxidants (Basel) 2021;10(1):97–97. doi: 10.3390/antiox10010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gualtieri R, Kalthur G, Barbato V, Longobardi S, di Rella F, Adiga SK, et al. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants. 2021;10(7):1025–1025. doi: 10.3390/antiox10071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis SEM, Simon L. Clinical implications of sperm DNA damage. Hum Fertil (Camb) 2010;13(4):201–207. doi: 10.3109/14647273.2010.528823. [DOI] [PubMed] [Google Scholar]
- 13.Duca Y, Calogero AE, Cannarella R, Condorelli RA, la Vignera S. Current and emerging medical therapeutic agents for idiopathic male infertility. Expert Opin Pharmacother. 2019;20(1):55–67. doi: 10.1080/14656566.2018.1543405. [DOI] [PubMed] [Google Scholar]
- 14.Salehi B, Berkay Yılmaz Y, Antika G, Boyunegmez Tumer T, Fawzi Mahomoodally M, Lobine D, et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules. 2019;9(8):356–356. doi: 10.3390/biom9080356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prathima P, Pavani R, Sukeerthi S, Sainath SB. α-Lipoic acid inhibits testicular and epididymal oxidative damage and improves fertility efficacy in arsenic-intoxicated rats. J Biochem Mol Toxicol. 2018;32(2) doi: 10.1002/jbt.22016. [DOI] [PubMed] [Google Scholar]
- 17.Truong T, Gardner DK. Antioxidants improve IVF outcome and subsequent embryo development in the mouse. Hum Reprod. 2017;32(12):2404–2413. doi: 10.1093/humrep/dex330. [DOI] [PubMed] [Google Scholar]
- 18.Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, triple-blind, placebo-controlled clinical trial examining the effects of alpha-lipoic acid supplement on the spermatogram and seminal oxidative stress in infertile men. Fertil Steril. 2015;104(2):318–324. doi: 10.1016/j.fertnstert.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Abbasi B, Molavi N, Tavalaee M, Abbasi H, Nasr-Esfahani MH. Alpha-lipoic acid improves sperm motility in infertile men after varicocelectomy: a triple-blind randomized controlled trial. Reprod Biomed Online. 2020;41(6):1084–1091. doi: 10.1016/j.rbmo.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12(4):466–472. doi: 10.1016/s1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 21.Henkel R, Hajimohammad M, Stalf T, Hoogendijk C, Mehnert C, Menkveld R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil Steril. 2004;81(4):965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Evenson DP. Sperm chromatin structure assay (SCSA ® ) Methods Mol Biol. 2013;927:147–164. doi: 10.1007/978-1-62703-038-0_14. [DOI] [PubMed] [Google Scholar]
- 23.Auger J, Mesbah M, Huber C, Dadoune JP. Aniline blue staining as a marker of sperm chromatin defects associated with different semen characteristics discriminates between proven fertile and suspected infertile men. Int J Androl. 1990;13(6):452–462. doi: 10.1111/j.1365-2605.1990.tb01052.x. [DOI] [PubMed] [Google Scholar]
- 24.Iranpour FG, Nasr-Esfahani MH, Valojerdi MR, Taki Al-Taraihi TM. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J Assist Reprod Genet. 2000;17(1):60–66. doi: 10.1023/A:1009406231811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivandzade F, Bhalerao A, Cucullo L. Analysis of the mitochondrial membrane potential using the cationic JC-1 dye as a sensitive fluorescent probe. Bio Protoc. 2019;9(1):e3128–e3128. doi: 10.21769/BioProtoc.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Varghese AC, Sharma RK. Markers of oxidative stress and sperm chromatin integrity. Methods Mol Biol. 2009;590:377–402. doi: 10.1007/978-1-60327-378-7_24. [DOI] [PubMed] [Google Scholar]
- 27.Bender Atik R, Christiansen OB, Elson J, Kolte AM, Lewis S, Middeldorp S, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):hoy004–hoy004. doi: 10.1093/hropen/hoy004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal A, Parekh N, Panner Selvam MK, Henkel R, Shah R, Homa ST, et al. Male oxidative stress infertility (MOSI): Proposed terminology and clinical practice guidelines for management of idiopathic male infertility. World J Mens Health. 2019;37(3):296–312. doi: 10.5534/wjmh.190055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arafa M, Agarwal A, Majzoub A, Panner Selvam MK, Baskaran S, Henkel R, et al. Efficacy of antioxidant supplementation on conventional and advanced sperm function tests in patients with idiopathic male infertility. Antioxidants. 2020;9(3):219–219. doi: 10.3390/antiox9030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hekmatdoost A, Lakpour N, Sadeghi MR. Sperm chromatin integrity: etiologies and mechanisms of abnormality, assays, clinical importance, preventing and repairing damage. Avicenna J Med Biotechnol. 2009;1(3):147–160. [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki VW, Moskovtsev SI, Willis J, Liu L, Mullen JBM, Carrell DT. DNA integrity is compromised in protamine-deficient human sperm. J Androl. 2005;26(6):741–748. doi: 10.2164/jandrol.05063. [DOI] [PubMed] [Google Scholar]
- 32.Manochantr S, Chiamchanya C, Sobhon P. Relationship between chromatin condensation, DNA integrity and quality of ejaculated spermatozoa from infertile men. Andrologia. 2012;44(3):187–199. doi: 10.1111/j.1439-0272.2010.01128.x. [DOI] [PubMed] [Google Scholar]
- 33.Cronan JE. Biotin and lipoic acid: synthesis, attachment, and regulation. EcoSal Plus. 2014;6(1):10–10. doi: 10.1128/ecosalplus.ESP-0001-2012. 1128/ecosalplusESP-0001- 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henkel R, Hoogendijk CF, Bouic PJD, Kruger TF. TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia. 2010;42(5):305–313. doi: 10.1111/j.1439-0272.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 35.Gallon F, Marchetti C, Jouy N, Marchetti P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil Steril. 2006;86(5):1526–1530. doi: 10.1016/j.fertnstert.2006.03.055. [DOI] [PubMed] [Google Scholar]
- 36.Agnihotri SK, Agrawal AK, Hakim BA, Vishwakarma AL, Narender T, Sachan R, et al. Mitochondrial membrane potential (MMP) regulates sperm motility. In Vitro Cell Dev Biol Anim. 2016;52(9):953–960. doi: 10.1007/s11626-016-0061-x. [DOI] [PubMed] [Google Scholar]
- 37.Mahfouz RZ, du Plessis SS, Aziz N, Sharma R, Sabanegh E, Agarwal A. Sperm viability, apoptosis, and intracellular reactive oxygen species levels in human spermatozoa before and after induction of oxidative stress. Fertil Steril. 2010;93(3):814–821. doi: 10.1016/j.fertnstert.2008.10.068. [DOI] [PubMed] [Google Scholar]
- 38.Dutta S, Majzoub A, Agarwal A. Oxidative stress and sperm function: a systematic review on evaluation and management. Arab J Urol. 2019;17(2):87–97. doi: 10.1080/2090598X.2019.1599624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. World J Mens Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]