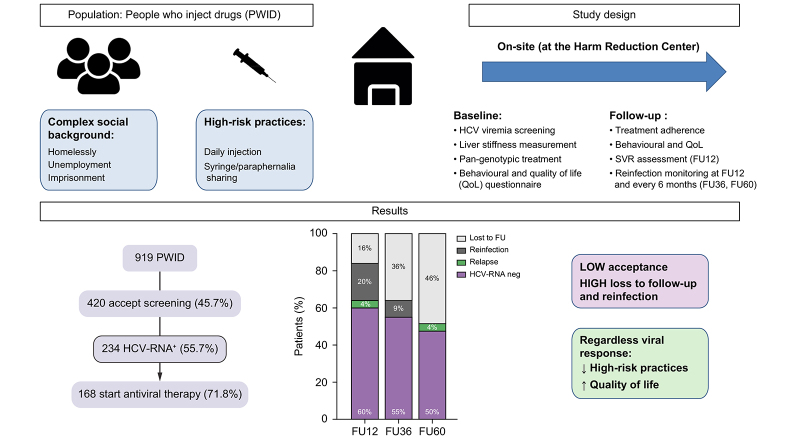
Abstract
Background & Aims
Significant scale-up of treatment among people who inject drugs (PWID) is crucial to achieve WHO HCV elimination targets. We explored the impact of on-site HCV diagnosis and treatment on PWID in an externalised hepatology clinic at the biggest harm reduction centre (HRC) in Barcelona attending to a marginalised PWID population with ongoing high-risk practices.
Methods
On-site HCV point-of-care testing was performed for diagnosis and treatment delivery. HCV-RNA was assessed at SVR12 (sustained virologic response at 12 weeks) and every 6 months. The programme included behavioural questionnaires at baseline and after treatment.
Results
Between 2018 and 2020, 919 individuals were prospectively enrolled. Of these, only 46% accepted HCV screening. HCV-RNA+ prevalence was 55.7% (n = 234). Of the 168 (72%) individuals starting treatment, 48% were foreigners, 32% homeless, 73% unemployed, and 62% had a history of incarceration. At enrolment, 70% injected drugs daily and 30% reported sharing needles or paraphernalia. Intention-to-treat SVR12 was 60%; only 4% were virological failures, the remaining were either early reinfections (20%) or losses to follow-up (16%). The overall reinfection rate during follow-up was 31/100 persons/year. HIV coinfection and daily injection were associated with a higher risk of reinfection. Nonetheless, beyond viral clearance, antiviral therapy was associated with a significant reduction in injection frequency, risk practices, and homelessness.
Conclusions
HCV treatment can be successfully delivered to active PWID with high-risk practices and has a significant benefit beyond HCV elimination. However, approaching this difficult spectrum of the PWID population implies significant barriers such as low rate of screening acceptance and high dropout and reinfection rates.
Lay summary
People who inject drugs attending harm reduction centres represent the most difficult population to treat for hepatitis C. We show that hepatitis C treatment has a significant benefit beyond viral cure, including improving quality of life, and decreasing injection frequency and risk practices. However, intrinsic barriers and the high reinfection rates hamper the achievement of viral microelimination in this setting.
Keywords: Hepatitis C, Drug users, Antiviral therapy, Dried blood spot testing, High-risk practices
Abbreviations: BP, bodily pain; DAA, direct-acting antivirals; DBS, dried blood spot; DDIs, drug–drug interactions; FU12, 12 weeks of follow-up; GH, general health; HRC, harm reduction centre; LSM, liver stiffness measurement; MCS, mental component summary; MH, mental health; NSPs, needle and syringe programmes; OR, odds ratio; OST, opioid substitution therapy; PCS, physical component summary; PF, physical functioning; PoCT, point-of-care testing; PP, per protocol; PWID, people who inject drugs; RP, role physical; RE, role emotional; SF, social functioning; SVR, sustained virologic response; SVR12, sustained virologic response at 12 weeks; TARGA, antiretroviral therapy; VT, vitality
Graphical abstract
Highlights
-
•
HCV treatment can be successfully delivered to active PWID with high-risk practices.
-
•
HCV treatment has benefits beyond sustained virological response.
-
•
PWID reported lower injection frequency and risk practices after engaging in the HCV programme.
-
•
However, linkage-to-care for PWID attending harm reduction centres is challenging.
-
•
The high dropout and reinfection rates hamper HCV microelimination in this population.
Introduction
Current treatment with highly effective direct-acting antivirals (DAA) offers a unique opportunity to reduce HCV-related liver disease burden and improve epidemic control. However, in Spain and many other countries, viraemic HCV infection is generally diagnosed at specialised health centres and HCV therapy can only be prescribed in hospital settings. Because people who inject drugs (PWID) often do not have access to primary care services or get lost in referrals to hospitals, hepatitis C diagnosis in these individuals remains inadequate.1 Linkage-to-care is also dependent on drug use, psychiatric comorbidities, and social and economic barriers. In addition, PWIDs may face stigma and discrimination within the conventional health care system, posing additional barriers to HCV elimination.2 Thus, despite the high prevalence of HCV infection among PWID (30–70%) and the evidence of excellent DAA treatment outcomes in terms of efficacy and safety, many individuals remain unscreened, and furthermore, most of those who are diagnosed remain untreated.3
In addition to the considerable burden of HCV infection among PWIDs, transmission continues as a result of ongoing risk behaviours.4 Thus, a relevant and unique characteristic of this high-risk group is the possibility of HCV reinfection after successful antiviral therapy as a result of recurrent HCV exposures. PWID reporting ongoing injection drug use after cure have higher HCV reinfection incidence, ranging between 4 and 30/100 persons/year.[5], [6], [7] These differences in reinfection rates reflect the heterogenicity of PWID cohorts which often include former or sporadic injectors attending addiction treatment centres.
The REDAN La Mina is the largest harm reduction centre (HRC) in Catalonia.8 The centre covers basic needs and facilitates access to needle and syringe programmes (NSPs), but has a limited infrastructure and lacks blood extraction facilities. In addition, the centre attends to a marginalised high-risk PWID population with a significant proportion of migrants, particularly from endemic areas for HCV, such as Eastern Europe.9 A recent study in a cohort of 100 individuals in this centre reported a prevalence of viraemic HCV infection of 63% with only 36% of participants with a previous HCV diagnosis having received antiviral treatment before participating in the study and thus, confirming the presence of significant gaps in the HCV continuum as a result of health system, provider, and patient barriers.10,11
As the most effective programmes at ensuring a successful HCV care cascade among PWID are models built on already existing infrastructures for drug users,1,12 our study aimed at evaluating whether active PWID attending this HRC could be successfully recruited and treated through an externalised hepatology outpatient clinic. The specific endpoints of our study were: (1) to evaluate a pilot HCV screening and direct linkage-to-care programme addressed to active PWID to guarantee prompt global access to antiviral therapy at the HRC; (2) to evaluate the impact of a HCV screening and treatment programme on drug injection habits, use of social resources, and behavioural practices; and (3) to assess the rate of HCV reinfection and associated risk factors by monitoring HCV-RNA by point-of-care testing (PoCT) and HCV genotype in dried blood spot (DBS) samples during follow-up.
Patients and methods
Study design and population
This is a prospective study that piloted an externalised hepatology outpatient clinic embedded in an urban HRC in Barcelona, Spain between November 2018 and November 2020. Eligible patients included those ≥18 years old and willing to participate in the study by written informed consent. Exclusion criteria included decompensated liver disease, pregnancy, concomitant uncontrolled mental health disorder, or any contraindications to receive all-oral antiviral therapy with a pan-genotypic regimen. The study was approved by the institutional Ethics Board at Hospital Clínic, Barcelona, Spain (HCB/2018/0755 and HCB/2019/0783).
On-site strategy for screening and linkage-to-care
At recruitment, on-site screening with PoCT for HCV-IgG antibody and HCV-RNA (fingerstick capillary blood) were offered to all HRC users. HCV-IgG PoCT was offered only when the self-reported HCV status was unknown, whereas in the remaining cases HCV-RNA assessment was the first option for testing. When the HCV-RNA result was positive, a DBS sample was collected and patients underwent liver stiffness measurement (LSM) and a comprehensive bio-behavioural questionnaire the same day. The questionnaire included sociodemographic information, history and frequency of current drug use, patterns of injection (including high-risk practices), sexual risk behaviours as well as use of opioid substitution therapy (OST).
The patient’s clinical history and concomitant medication was telematically evaluated by the hepatologist to prescribe antiviral therapy. Patients initiated antiviral therapy within 1 week after diagnosis. Choice of antiviral therapy was based on the physician’s criteria considering fibrosis stage and drug–drug interactions (DDIs). Treatment duration was 12 weeks for sofosbuvir/velpatasvir and 8 weeks for glecaprevir/pibretasvir. Treatment was delivered at the HRC and adherence was assessed by daily or weekly visits according to the individual needs of the patient. HCV-RNA testing (GeneXpert®, Cepheid, Sunnyvale, CA, USA) and DBS collection as well as the bio-behavioural questionnaire were performed at the HRC at 12 weeks of follow-up after the end of treatment (FU12) and every 6 months thereafter (FU36, FU60) to assess sustained virological response (SVR), reinfection, and impact of antiviral therapy on drug use behaviour and quality of life (Short Form-12 [SF-12] Health Survey). For individuals with positive HCV-RNA at any point after end of therapy, a DBS sample was analysed to differentiate relapse vs. reinfection by sequencing of an NS5B fragment in comparison with the baseline DBS sample, and re-treatment was proposed.
Individuals with compensated advanced liver disease assessed by portable LSM ≥9.5 kPa were referred to a specialised hepatology clinic, although treatment was initiated at the HRC. For patients imprisoned during treatment, antiviral therapy was delivered at the prison from the HRC and vice versa in case of release. HCV-RNA blood test analyses were performed while in prison if needed, and bi-directional communication with the hepatologist allowed continued adherence monitoring or evaluation of SVR/reinfection.
HCV-RNA testing by GeneXpert®
Capillary whole-blood (100 μl) was collected by fingerstick according to the manufacturer's instructions and immediately tested with the Xpert FS assay on a 4-module GeneXpert® System operated by the study nurse (lower limit of detection [LoD], 35 IU/ml; lower limit of quantification [LoQ], 100 IU/ml). Test results were validated remotely from the reference laboratory using TeamViewer software (TeamViewer, Göppingen, Germany).
HCV-RNA testing and genotyping in DBSs
Fingerprick capillary blood samples were obtained from all viraemic participants as DBSs and were subjected to automated nucleic acids extraction and qualitative testing for HCV-RNA as previously described.13,14 Briefly, DBS cards were dried at room temperature, kept in individual zipped plastic bags with desiccant, and stored at room temperature. Samples were sent to the laboratory for testing. Baseline DBS samples were stored at –80 °C until processing if HCV-RNA was positive at any of the follow-up points. For HCV genotyping, RNA previously extracted from DBS samples was subjected to reverse-transcription. NS5B amplification was performed by heminested-PCR, followed by Sanger sequencing and phylogenetic analysis along with reference sequences were performed as previously described.15 When this methodology failed to provide a valid sequence as a result of low viral loads, the Abbott HCV Genotype II assay Abbott Laboratories (Abbott Park, IL, USA) was used from the RNA previously extracted from DBS samples.
Statistical analysis
Continuous variables are reported as median and IQR and categorical variables are reported as absolute and relative frequencies. Groups were compared using the t test, Mann-Whitney U test, or Wilcoxon test for continuous variables and the Χ2 test or Fisher’s exact test for categorical variables, as appropriate. Significance was established as a 2-sided p value of <0.05. Multivariate logistic regression was used to analyse the association between patient characteristics and reinfection. We used the forward stepwise selection method and included only variables with statistical significance (p <0.05) at the univariate analysis after adjustment and discarding interactions and without forcing the entry of variables into the model. Analyses were performed using IBM SPSS version 26 (IBM Corp, Armonk, NY, USA).
Results
Characteristics of the study population
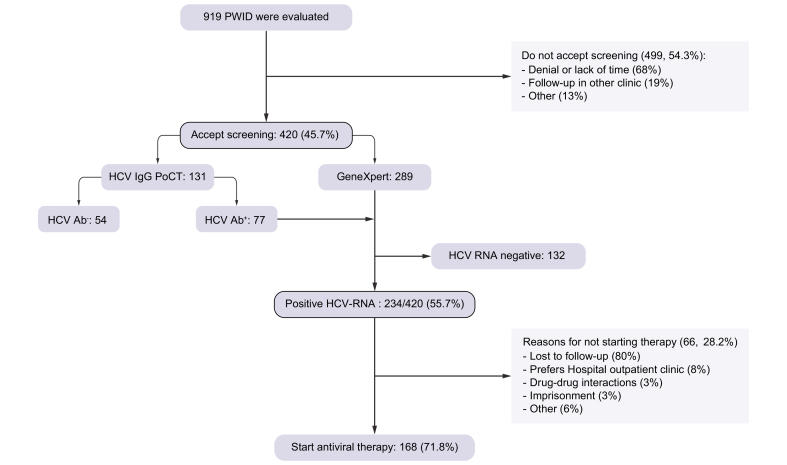
The study flow-chart is shown in Fig. 1. During the study period, 919 active PWID attending the HRC were evaluated. Of these, 420 accepted screening (46%). The main reason for non-acceptance of HCV screening was denial or lack of time (68%). When evaluating the differences among those accepting screening or not, both cohorts were similar regarding gender, age, and foreign nationality. However, those accepting screening were more frequently homeless and daily injectors (Table S1). Among those accepting screening, the prevalence of active HCV infection was 55.7% (234/420) and of these, 72% of patients initiated antiviral therapy. The main reason for not starting therapy (n = 66) was loss to follow-up. As shown in Table 1, these patients had complex social backgrounds and severe drug injection patterns with high-risk practices. Importantly, there were no major differences between patients initiating antiviral therapy or not.
Fig. 1.
Study flowchart.
PWID, people who inject drugs.
Table 1.
Baseline characteristics of PWID population with positive HCV-RNA according to HCV antiviral treatment status.
| Variables | Total HCV-RNA+ n = 234 | Treated n = 168 | Untreated n = 66 |
|---|---|---|---|
| Age (years) | 41 (34–47) | 41 (35–48) | 38 (32–45) |
| Male | 207 (88) | 147 (88) | 60 (90) |
| Foreign nationality | 116 (49) | 80 (48) | 36 (54) |
| Homeless | 82 (35) | 54 (32) | 28 (42) |
| Family support | 124 (53) | 98 (58) | 26 (39) |
| Unemployment | 151 (65) | 123 (73) | 28 (44) |
| Previously incarcerated∗ | 123 (60) | 104 (63) | 19 (50) |
| Educational level∗: | |||
| None | 4 (2) | 4 (2) | 0 (0) |
| Primary education | 91 (44) | 71 (43) | 20 (53) |
| Secondary education | 49 (24) | 43 (26) | 6 (16) |
| Highschool | 15 (7) | 13 (8) | 2 (5) |
| University degree | 25 (12) | 22 (13) | 3 (8) |
| Vocational training | 13 (6) | 12 (7) | 1 (3) |
| Healthcare system attendance: | |||
| Primary care | 66 (28) | 56 (33) | 10 (15) |
| Hospital | 43 (18) | 30 (18) | 13 (20) |
| Drug injection (previous 6 months): | |||
| >Once/day | 132 (56) | 99 (59) | 33 (51) |
| Once/day | 25 (11) | 19 (11) | 6 (10) |
| Weekly | 30 (13) | 22 (13) | 8 (12) |
| <Weekly | 41 (17) | 23 (14) | 18 (27) |
| None | 6 (3) | 5 (3) | 1 (2) |
| Drug consumption (previous 6 months): | |||
| Cocaine | 207 (88) | 145 (86) | 62 (95) |
| Heroin | 208 (89) | 145 (86) | 63 (95) |
| Cocaine and heroin | 174 (79) | 125 (74) | 49 (74) |
| Cannabis | 113 (48) | 93 (55) | 20 (32) |
| Speedball | 171 (73) | 116 (69) | 55 (83) |
| Syringe sharing (previous 6 months)∗ | 36 (18) | 29 (17) | 7 (18) |
| Paraphernalia sharing (previous 6 months)∗ | 77 (38) | 59 (36) | 18 (45) |
| Risky sexual relationships (previous 6 months)† | 77 (44) | 63 (41) | 14 (56) |
| Alcohol consumption (previous 6 months) | 72 (31) | 59 (35) | 13 (19) |
| >28 units/week | 27 (12) | 22 (13) | 5 (8) |
| Opioid substitution therapy (OST) | 104 (44) | 82 (49) | 22 (33) |
| Concomitant psychiatric medication∗ | |||
| Benzodiazepines | 80 (39) | 65 (39) | 25 (63) |
| Antidepressants | 34 (17) | 28 (17) | 6 (15) |
| Antipsychotics | 23 (11) | 19 (11) | 4 (10) |
| HIV+ | 47 (20) | 33 (20) | 14 (21) |
| TARGA therapy among HIV+ | 27/47 (57) | 19/33 (58) | 8/14 (57) |
| Previous HCV+ diagnosis | 152 (65) | 123 (73) | 29 (44) |
| Previous HCV antiviral therapy | 36 (15) | 29 (17) | 7 (11) |
| HCV-RNA (IU/ml) | 538,000 (91,300–1,875,000) | 538,000 (74,725–1,665,000) | 511,000 (113,500–2,680,000) |
| Baseline FibroScan® (kPa) | n = 199 | n = 168 | n = 31 |
| Fibrosis stage | 6 (4.9–7.5) | 6 (4.8–7.6) | 6 (4.9–7.5) |
| F0–1 | 150 (75) | 126 (75) | 24 (77) |
| F2 | 27 (14) | 23 (14) | 4 (13) |
| F3 | 12 (6) | 11 (6) | 1 (3) |
| F4 | 10 (5) | 8 (5) | 2 (6) |
| Advanced fibrosis (LSM ≥9.5 kPa) | 22 (11) | 19 (11) | 3 (10) |
Qualitative variables are expressed as n (%) and quantitative variables median (P25–P75). LSM, liver stiffness measurement; PWID, people who inject drugs; TARGA, antiretroviral therapy.
Available in n = 205, 165, and 40 individuals in each group.
Available in n = 179, 154, and 25 individuals in each group. The remaining individuals declined to answer or referred ‘unknown’.
Focusing on patients starting antiviral therapy (n = 168), most were male (88%) with median age 41 years old, 48% were foreigners, 32% homeless, and 58% had no family support (Table 1). The rate of unemployment was high (73%) and only 30% had studies beyond secondary education. In addition, more than two-thirds had been previously incarcerated. With regard to drug use, most (70%) were daily injectors, with 59% more than once a day and the most common injected drugs were cocaine, heroin, and speedball. In addition, a significant proportion of patients reported high-risk practices such as syringe or paraphernalia sharing (17% and 35%, respectively), or sexual risk behaviours (37%). HIV coinfection was present in 20% but only 19/33 were under antiretroviral therapy. Regarding HCV infection, a significant proportion of patients (73%) had a previous diagnosis but only 24% had received antiviral therapy. Only 11% had advanced fibrosis (F3–4). All patients received a pan-genotypic regimen. Patients receiving a 12-week treatment duration (58%) were more frequently HIV+ (30% vs. 7%), had received previous HCV antiviral therapy (24% vs. 8%) and had advanced fibrosis (16% vs. 4%), all p <0.05. There were no other differences regarding baseline characteristics among both groups (Table S2).
HCV-care cascade
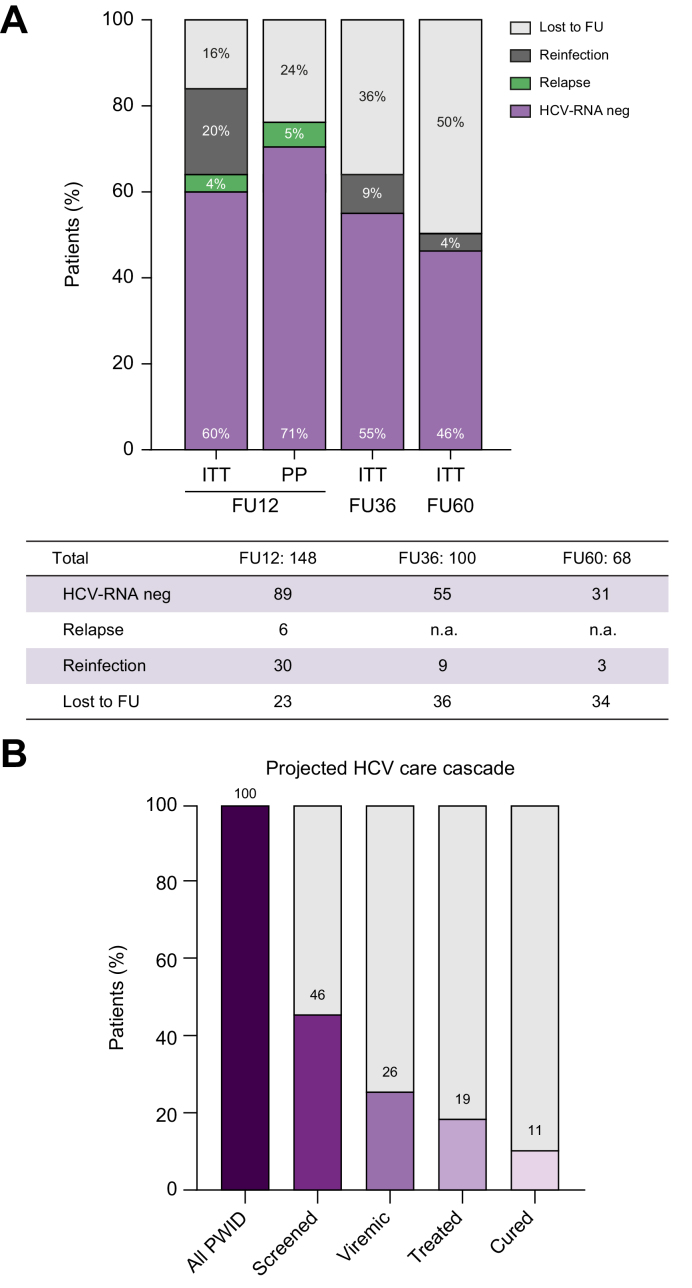
Outcomes after treatment are depicted in Fig. 2. Out of the 148 patients who reached 12 weeks of follow-up (FU12), undetectable HCV-RNA by intention-to-treat (ITT) was 60% and per protocol (PP) 71%. Importantly, among those with follow-up data, the reasons for HCV-RNA positivity per ITT were relapse in 6 patients (4%) and reinfection in 30 (15%). All individuals who relapsed had prematurely discontinued antiviral therapy and had completed less than 50% of the total treatment. During follow-up, reinfection was diagnosed in 42 individuals, with reinfection rates diminishing during subsequent follow-up being 9% and 4.5% at FU36 and FU60; respectively. On the contrary, losses to follow-up increased from 16% at FU12 to 36% at FU36 and 46% at FU60. Regarding safety only 9 patients reported adverse events (headache and gastrointestinal symptoms) and all were mild. Among those lost to follow-up, there were 4 deaths unrelated to the study, (2 as a result of drug overdose, 1 to an underlying cardiac condition, and 1 to suicide); none occurred while on treatment.
Fig. 2.
HCV care cascade.
(A) Treatment outcomes at FU12, FU36, FU60 by ITT or, in the case of FU12 also PP excluding losses to follow-up. (B) Projected HCV care cascade considering screening and treatment acceptance at the harm reduction centre. FU, follow-up; ITT, intention-to-treat; n.a., not applicable; PP, per protocol; PWID, people who inject drugs.
Fig. 2B projects the HCV care cascade at the HRC extrapolating the results of our study in terms of screening acceptance rate (46%), HCV-RNA+ prevalence (56%), treatment initiation rate (72%) and percentage of HCV-RNA undetectable at FU12 per ITT (60%).
Adherence during antiviral therapy
Considering all patients initiating antiviral therapy, 134 (80%) patients completed ≥80% of total prescribed therapy but only 60% did so within the expected treatment duration (8 or 12 weeks ± 3 days). Gaps of treatment discontinuation, even if longer than 7 days, did not affect SVR rates (Fig. S1). Thus, adherence did not significantly affect overall SVR rates in this study. Only premature treatment discontinuation (median treatment duration 3 [2–4] weeks) was associated to high relapse rates in 6 of 10 with FU12 assessment.
Determinants and outcomes of reinfection
As previously mentioned, 42 patients had a reinfection after the first treatment with most cases occurring as early as FU12 (71%). We aimed to analyse the determinants of reinfection during follow-up to identify those individuals at higher risk (Table 2). Socioeconomic factors and drug-use behaviours were associated to a higher risk of reinfection; specifically, being homeless, living with HIV+, and having high-risk practices such as daily drug injection and syringe, or paraphernalia sharing. In addition, we found that imprisonment either before or during treatment were more frequent among those experiencing reinfection. When performing multivariate regression analysis, only HIV+ (adjusted odds ratio [OR]: 5.6; 95% CI 1.9–15.9); p = 0.001) and daily injection habit (OR 2.8; 95% CI (1.1–7.2); p = 0.03) remained as independent predictors of reinfection. Outcomes after reinfection are depicted in Fig. S2.
Table 2.
Differences between PWID presenting with reinfection or not during follow-up.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variables | No reinfection n = 83 | Reinfection n = 42 | p | OR (95% CI); p (adjusted) |
| Baseline | ||||
| Age (years) | 42 (35–49) | 41 (34–47) | 0.40 | |
| Male | 72 (87) | 37 (88) | 0.77 | |
| Foreign nationality | 30 (36) | 18 (43) | 0.56 | |
| Homeless | 23 (28) | 21 (50) | 0.009 | |
| Unemployment | 59 (71) | 34 (81) | 0.13 | |
| Secondary education or lower | 58 (69) | 27 (64) | 0.59 | |
| Previously incarcerated | 45 (54) | 32 (76) | 0.039 | |
| Daily drug injection | 55 (66) | 34 (81) | 0.09 | |
| Cocaine | 67 (81) | 42 (100) | 0.01 | |
| Heroin | 72 (87) | 34 (81) | 0.65 | |
| Syringe sharing | 11 (13) | 12 (29) | 0.049 | |
| Paraphernalia sharing | 29 (35) | 16 (38) | 0.69 | |
| Risky sexual relationships∗ | 36 (43) | 16 (38) | 0.84 | |
| Alcohol consumption | 36 (43) | 10 (24) | 0.10 | |
| >28 units/week | 13 (16) | 3 (7) | 0.25 | |
| Opioid substitution therapy | 42 (51) | 20 (48) | 0.85 | |
| HIV+ | 11 (13) | 17 (40) | 0.002 | 5.6 (1.9–15.9); p = 0.001 |
| Previous HCV+ diagnosis | 67(81) | 34 (81) | 0.99 | |
| Previous HCV antiviral therapy | 13 (16) | 11 (26) | 0.22 | |
| Advanced fibrosis |
9 (11) |
6 (14) |
0.99 |
|
| During antiviral therapy | ||||
| 8 weeks treatment duration | 36 (43) | 20 (48) | 0.70 | |
| Treatment intake | ||||
| <80% | 10 (12) | 5 (12) | 0.99 | |
| <50% | 7 (8) | 3 (7) | 0.99 | |
| Imprisonment during treatment | 3 (4) | 7 (17) | 0.03 | |
| Daily drug injection | 23 (28) | 22 (52) | 0.009 | 2.8 (1.1–7.2); p = 0.03 |
Qualitative variables are expressed as n (%) and quantitative variables median (P25–P75). Univariate and multivariate competing risk regression analysis for reinfection including homelessness, syringe sharing, HIV, and daily injection. Values in bold denote statistical significance.
n = 119.
Positive impact of enrolment in a HCV programme on risk practices, social resources, and quality of life
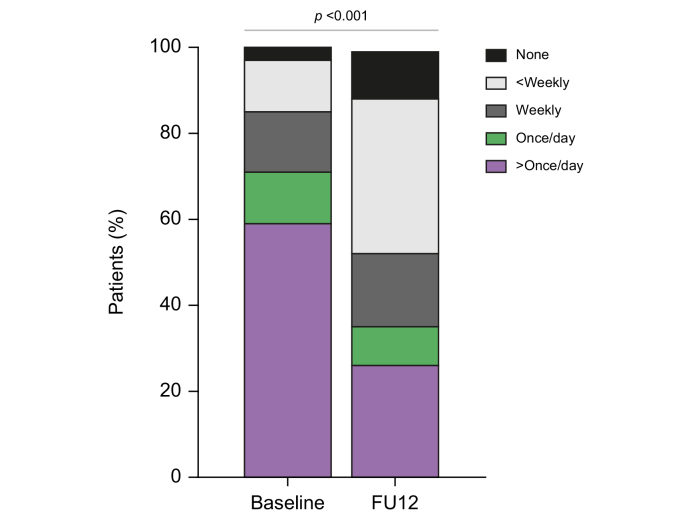
Finally, we were interested in assessing how enrolment in an HCV testing and linkage-to-care programme could impact on risk practices and social networks among the 125 PWID completing the bio-behavioural questionnaire at baseline and FU12. As shown in Table 3, the inclusion in the programme for HCV antiviral therapy had a positive impact on PWID with high risk practices regardless of treatment outcome. The most striking effect was an overall decrease of injection frequency in 40% of patients, with a marked decrease in the proportion of daily injectors compared with baseline (71% vs. 36%, p <0.05) (Fig. 3). Self-reported risk practices such as syringe and paraphernalia sharing, as well as high-risk sexual relationships, were also markedly reduced. In addition, the number of PWID linked to an OST programme also increased. Importantly, other relevant social characteristics such as homelessness improved after being engaged in the programme. Achieving or not SVR did not appear to influence the observed improvements on risk practices and social network (Table S3). Longer follow-up (FU36) was associated with a greater improvement in homelessly, injection frequency, harm practices, and OST engagement (Table S4).
Table 3.
Impact of antiviral therapy on injection patterns, high-risk practices and social networks among those with paired analysis at baseline and FU12 (n = 125).
| Variables | Baseline | FU12 | p |
|---|---|---|---|
| Homeless | 54 (43) | 28 (22) | 0.001 |
| Family support | 80 (64) | 77 (62) | 0.99 |
| Unemployment | 93 (74) | 78 (62) | 0.64 |
| Daily vs. non-daily drug injection | 89 (71) | 45 (36) | <0.001 |
| Drug injection frequency | |||
| >Once/day | 74 (59) | 33 (26) | <0.001 |
| Once/day | 15 (12) | 12 (10) | |
| Weekly | 17 (14) | 21 (17) | |
| <Weekly | 15 (12) | 45 (36) | |
| None | 4 (3) | 14 (11) | |
| Syringe sharing | 23 (18) | 7 (6) | 0.009 |
| Paraphernalia sharing | 45 (36) | 22 (18) | 0.012 |
| Risky sexual relationships∗ | 52 (44) | 22 (18) | 0.001 |
| Alcohol consumption | 46 (37) | 29 (23) | 0.09 |
| >28 units/week | 16 (13) | 8 (6) | 0.14 |
| Opioid substitution therapy | 62 (49) | 67 (54) | 0.045 |
Qualitative variables are expressed as n (%) and quantitative variables median (P25–P75). Paired t test or non-parametric paired sample tests were used where appropriate. Values in bold denote statistical significance.
n = 119. FU12, 12 weeks follow-up.
Fig. 3.
Improvement in injection pattern at baseline and FU12 after treatment (p <0.001 by paired analysis).
FU12, 12 weeks follow-up.
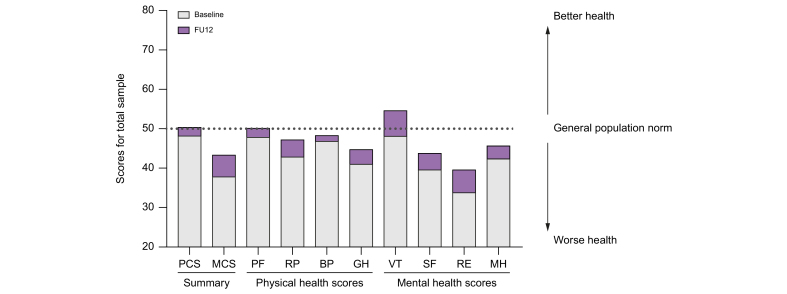
In addition, all components of quality of life evaluated using the SF-12 Health Survey improved at FU12 compared with baseline. Physical functioning and vitality reached levels comparable to the general population (Fig. 4). These data suggest a positive impact of enrolling in a microelimination programme beyond virological outcomes.
Fig. 4.
Quality of Life assessment at baseline and FU12 (p <0.05 by paired analysis).
BP, bodily pain; GH, general health; MCS, mental component summary; MH, mental health; PCS, physical component summary; PF, physical functioning; RP, role physical; RE, role emotional; SF, social functioning; VT, vitality.
Discussion
Most studies on HCV microelimination programmes in PWID claim very good results in terms of HCV elimination but might not offer a realistic perspective. In addition, there are few data on how enrolment in such programmes impacts injection habits and social networks and resources. The results of our study in a high-risk group of PWID attending a HRC are novel in the field as they stress the barriers to achieve microelimination, but also the benefits of implementing on-site all the steps of an HCV care cascade. Indeed, the target population of this study included a marginalised PWID population with high rates of unemployment, homelessness, and incarceration and a significant proportion of migrants with low economic resources and family support. In addition, this is a PWID population with high-risk practices in terms of daily injection, syringe or paraphernalia sharing, and sexual risk practices. Thus, this externalised liver disease clinic is positioned in the most difficult spectrum of the PWID population.
The first barrier we encountered was engaging the PWID population into the programme as only 46% accepted HCV screening despite repeated proposals and flexible schedule offered by the study team. Social and economic background may limit engagement in this type of programme because of other priorities above HCV diagnosis and treatment. In addition, the main purpose of a HRC is to offer assistance to proceed to safe injection thus, HCV screening was offered either before or after drug consumption and both would be not optimal moments to engage these individuals. Not unexpectedly, the second barrier was the not negligible rate of loss to follow-up, either to initiate antiviral treatment or fulfil any of the follow-up visits (18% and 29%, respectively). Because of the intrinsic characteristics of this population, most lacked mobile phones or other means of contact.
Despite the abovementioned barriers, this patient-centred circuit demonstrates that HCV treatment can be successfully delivered also to active PWID with high-risk practices. In terms of screening, almost one-third of those tested was a new diagnosis and were not aware of their status. Even among those with previous HCV diagnosis in our study, only 24% had received a previous course of antiviral therapy, reflecting the relevant barriers in linkage-to-care and treatment access within conventional health care circuits.14 Overall, among those individuals who accepted HCV screening, more than 70% of viraemic patients initiated antiviral treatment. In addition, and despite the complex background, most patients completed more than 80% of the total prescribed treatment, highlighting that prompt initiation of antiviral therapy after diagnosis and on-site monitoring is effective to engage this population in a real-life setting. Similarly to previous studies, our results also support the notion that missed doses while on treatment did not impact on outcomes if treatment uptake was >80%.16,17 Despite other risk factors for liver disease progression such as alcohol use and HIV coinfection,18 only 11% had advanced liver disease. This low rate of advanced disease is reassuring for those microelimination programmes that do not have the capability to assess liver disease stage.
A relevant finding of our study is the positive impact beyond HCV viral clearance in those PWID who engaged in the externalised HCV model. Although more than 89% were still active PWID after treatment, injection frequency was reduced from a daily to a non-daily basis in >30%. Being engaged in a healthcare programme focused on HCV also had a positive impact in risk practices such as syringe or paraphernalia sharing and sexual relationships. Furthermore, the risk behaviours further decreased with longer follow-up such as 36 weeks after therapy.
In addition, the psychological impact of living with hepatitis C is well documented with many people experiencing depression and anxiety, symptoms that are even more common among PWID.19 Importantly, in our study, participants reported improvements in all the components of the quality of life survey questionnaire after being engaged in the programme.
We also found extremely relevant the reduction in homelessness observed after treatment, probably reflecting increased health awareness after HCV therapy. Unstable housing often co-occurs with illicit drug use and is an important risk factor for mortality independent of known risk factors including HIV infection and patterns of drug use.20 Furthermore, homelessness has also been linked with subsequent increases in drug use, injection-related risk behaviour, and relapse in those who have stopped injecting drugs.21
However, our results also provide relevant (and worrisome) data regarding reinfection, as other studies have been limited by their retrospective nature, or lack of systematic testing of persons at risk for HCV reinfection at regular and/or short intervals.[22], [23], [24] The high rates of reinfection (31/100 persons/year) also reflect that this study approached a PWID population with high-risk practices. In this cohort, HIV coinfection and regular daily injection after treatment were the only independent factors associated with a higher risk or reinfection. Current and daily injection drug use has been associated with higher rates of reinfection in other studies.25 The role of HIV infection in increasing the risk of HCV reinfection is not completely clear, but has been reported both among PWID and among men who have sex with men. HIV infection is associated with an approximately 3-fold reduction in rates of spontaneous recovery from an acute HCV infection.26 As such, one may postulate that HIV-infected persons have similar rates of HCV reinfection to HIV-uninfected persons but may be less likely to spontaneously clear these reinfections owing to a weaker immune response and are thus more likely to have reinfections detected.
It is important to highlight that more than 70% of cases of reinfection occurred within the first 12 weeks after antiviral therapy. In several studies reinfection has been defined as a positive HCV-PCR after documented initial HCV clearance which may have underestimated the true reinfection rates.6 The decrease in reinfection rate over time observed in our study may imply a development of specific protective behaviours and routines and these pragmatic strategies can be also used as models for other PWID to minimise reinfection.24 In addition, because of the high HCV prevalence at baseline, an ongoing test-and-treat strategy over time with the consequent decrease in HCV prevalence would be necessary before observing a reduction in HCV reinfection. Although the detection of reinfection in a treated population should not be interpreted to be a programme failure, it highlights the relevance of implementing educational strategies to minimise reinfections. If we are not able to combine easy access to treatments along with harm reduction and behavioural interventions, microelimination programmes will be unable to successfully eliminate hepatitis C.
Finally, our study has some limitations. First, the not negligible loss to follow-up rate precludes complete evaluation of treatment outcomes. Thus, we cannot exclude that individuals compliant with follow-up visits were those with more favourable outcomes. Second, because of the increasing rate of losses to follow-up we cannot discard an underestimation of the reinfection rate. However, we cannot discard that the highest risk individuals were infected within the early follow-up period whereas the remainder belonged to a lower-risk population. Finally, information on drug-use risk behaviours was self-reported and might be prone to response bias and socially desirable responses. However, previous studies also have shown that self-reported information on drug use has been shown to be reliable and valid.27
In summary, this patient-centred circuit demonstrates that HCV treatment can be successfully delivered to active PWID with high-risk practices and has a significant benefit in injection patterns and use of social resources. However, intrinsic barriers in this population such as low rate of screening acceptance and high loss to follow-up as well as significant high reinfection rates hamper a successful elimination of hepatitis C in PWID. Studies in the field should stress the difficulties rather than the achievements to promote specific interventions such as incentives and educational and harm reduction measures.
Financial support
The study was funded by the program Conquering Hepatitis Via Microelimination (CHIME) by Gilead Sciences (IN-ES-987-5349). The founder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. SL has received grants from Asociación Española para el Estudio del Hígado (AEEH) and Societat Catalana de Digestologia (SCD). XF has received support from Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (grant 2017_SGR_1753) and CERCA Programme/Generalitat de Catalunya and from the Spanish Health Ministry, Plan Nacional de Drogas (MSA_PNSD2020, Project 2020I018) P. SRT was supported by the Rio Hortega program (fellowship CM17/00015) of the ISCIII, co-funded by the European Social Fund (ESF). The CERCA Programme/Generalitat de Catalunya also provided support to the Germans Trias i Pujol Research Institute.
Authors’ contributions
Contributed to the study concept and design: SL, XF, JC, EM, NG, XM, JRU. Obtained funding: SL. Collected data: MG, AM. Responsible for GeneXpert results validation and DBS testing: EM, VS. Interpreted data: SL. Drafted the manuscript: SL and XF. Contributed to critical revision and approved the final version of the manuscript: all authors. Guarantor of the article: SL.
Data availability statement
Individual data is not public but will available upon reasonable request.
Conflicts of interest
SL received lecture and advisory fees from Gilead and Abbvie, and research grants from Gilead. XF has acted as advisor for Gilead and Abbvie. ZM received speaker fees and consultancy for Gilead, Abbvie, Alexion, Orphalan, Deep genomics. SRT has received lecture fees from Gilead and Abbvie. EM received lecture fees and research grants from Abbott GmbH & Co. K.G, Gilead Sciences, Cepheid, and Abbvie. VS has received travel sponsorship to attend scientific meetings from Gilead Sciences and Cepheid.
Please refer to the accompanying ICMJE disclosure forms for further details.
Acknowledgements
We would like to acknowledge Xavi Corral for developing the software that allowed registering adherence data. We would also like to thank Sara González-Gómez and Anna Not for their help in processing the DBS specimens, and the Genomics Unit at the Germans Trias i Pujol Research Institute.
Footnotes
Author names in bold designate shared co-first authorship
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100580.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Grebely J., Dore G.J., Morin S., Rockstroh J.K., Klein M.B. Elimination of HCV as a public health concern among people who inject drugs by 2030 – what will it take to get there? J Int AIDS Soc. 2017;20:1–8. doi: 10.7448/IAS.20.1.22146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahern J., Stuber J., Galea S. Stigma, discrimination and the health of illicit drug users. Drug Alcohol Depend. 2007;88:188–196. doi: 10.1016/j.drugalcdep.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Fraser H., Martin N.K., Brummer-Korvenkontio H., Carrieri P., Dalgard O., Dillon J., et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2018;68:402–411. doi: 10.1016/j.jhep.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Report of the WHO global partners’ meeting on hepatitis elimination. 2020. https://www.who.int/publications/i/item/report-of-the-who-global-partners-meeting-on-hepatitis-elimination [Google Scholar]
- 5.Midgard H., Weir A., Palmateer N., Lo Re V., Pineda J.A., Macías J., et al. HCV epidemiology in high-risk groups and the risk of reinfection. J Hepatol. 2016;65:S33–S45. doi: 10.1016/j.jhep.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Falade-Nwulia O., Sulkowski M.S., Merkow A., Latkin C., Mehta S.H. Understanding and addressing hepatitis C reinfection in the oral direct-acting antiviral era. J Viral Hepat. 2018;25:220–227. doi: 10.1111/jvh.12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sacks-Davis R., Aitken C.K., Higgs P., Spelman T., Pedrana A.E., Bowden S., et al. High rates of hepatitis C virus reinfection and spontaneous clearance of reinfection in people who inject drugs: a prospective cohort study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Catalunya Generalitat. Sistema d’Informació sobre Drogodependències a Catalunya. 2020. https://drogues.gencat.cat/web/.content/minisite/drogues/professionals/epidemiologia/docs/INFORME-SIDC-2019_ok.pdf
- 9.Fernandez-Lopez L., Folch C., Majo X., Gasulla L., Casabona J. Implementation of rapid HIV and HCV testing within harm reduction programmes for people who inject drugs: a pilot study. AIDS Care. 2016;28:712–716. doi: 10.1080/09540121.2016.1164290. [DOI] [PubMed] [Google Scholar]
- 10.Saludes V., Antuori A., Lazarus J.V., Folch C., González-Gómez S., González N., et al. Evaluation of the Xpert HCV VL Fingerstick point-of-care assay and dried blood spot HCV-RNA testing as simplified diagnostic strategies among people who inject drugs in Catalonia, Spain. Int J Drug Pol. 2020;80 doi: 10.1016/j.drugpo.2020.102734. [DOI] [PubMed] [Google Scholar]
- 11.Folch C., Saludes V., Reyes-Ureña J., Antuori A., Ibáñez N., Majó X., et al. The hepatitis C care cascade among people who inject drugs accessing harm reduction services in Catalonia: major gaps for migrants. Int J Drug Pol. 2021;90 doi: 10.1016/j.drugpo.2020.103057. [DOI] [PubMed] [Google Scholar]
- 12.Degenhardt L., Peacock A., Colledge S., Leung J., Grebely J., Vickerman P., et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob Heal. 2017;5:e1192–e1207. doi: 10.1016/S2214-109X(17)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saludes V., Folch C., Morales-Carmona A., Ferrer L., Fernàndez-López L., Muñoz R., et al. Community-based screening of hepatitis C with a one-step RNA detection algorithm from dried-blood spots: analysis of key populations in Barcelona, Spain. J Viral Hepat. 2018;25:236–244. doi: 10.1111/jvh.12809. [DOI] [PubMed] [Google Scholar]
- 14.Saludes V., Antuori A., Folch C., González N., Ibáñez N., Majó X., et al. Utility of a one-step screening and diagnosis strategy for viremic HCV infection among people who inject drugs in Catalonia. Int J Drug Pol. 2019;74:236–245. doi: 10.1016/j.drugpo.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Saludes V., Antuori A., Reinhardt B., Viciana I., Clavijo E., Schreiber L., et al. Reliable resolution of ambiguous hepatitis C virus genotype 1 results with the Abbott HCV Genotype Plus RUO assay. Sci Rep. 2019;9:1–10. doi: 10.1038/s41598-019-40099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grebely J., Dalgard O., Conway B., Cunningham E.B., Bruggmann P., Hajarizadeh B., et al. Sofosbuvir and velpatasvir for hepatitis C virus infection in people with recent injection drug use (SIMPLIFY): an open-label, single-arm, phase 4, multicentre trial. Lancet Gastroenterol Hepatol. 2018;1253:1–9. doi: 10.1016/S2468-1253(17)30404-1. [DOI] [PubMed] [Google Scholar]
- 17.Norton B.L., Akiyama M.J., Arnsten J.H., Agyemang L., Heo M., Litwin A.H. High HCV cure rates among people who inject drugs and have suboptimal adherence: a patient-centered approach to HCV models of care. Int J Drug Pol. 2021;93 doi: 10.1016/j.drugpo.2021.103135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas D.L., Seeff L.B. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383–398. doi: 10.1016/j.cld.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Torrens M., Soyemi T., Bowman D., Schatz E. Beyond clinical outcomes: the social and healthcare system implications of hepatitis C treatment. BMC Infect Dis. 2020;20:702. doi: 10.1186/s12879-020-05426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zivanovic R., Milloy M.J., Hayashi K., Dong H., Sutherland C., Kerr T., et al. Impact of unstable housing on all-cause mortality among persons who inject drugs. Health behavior, health promotion and society. BMC Public Health. 2015;15:1–7. doi: 10.1186/s12889-015-1479-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linton S.L., Celentano D.D., Kirk G.D., Mehta S.H. The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug Alcohol Depend. 2013;132:457–465. doi: 10.1016/j.drugalcdep.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi C., Butt Z.A., Wong S., Buxton J.A., Islam N., Yu A., et al. Hepatitis C virus reinfection after successful treatment with direct-acting antiviral therapy in a large population-based cohort. J Hepatol. 2018;69:1007–1014. doi: 10.1016/j.jhep.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 23.Akiyama M.J., Lipsey D., Heo M., Agyemang L., Norton B.L., Hidalgo J., et al. Low hepatitis C reinfection following direct-acting antiviral therapy among people who inject drugs on opioid agonist therapy. Clin Infect Dis. 2020;70:2695–2702. doi: 10.1093/cid/ciz693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grebely J., Hajarizadeh B., Dore G.J. Direct-acting antiviral agents for HCV infection affecting people who inject drugs. Nat Rev Gastroenterol Hepatol. 2017;14:641–651. doi: 10.1038/nrgastro.2017.106. [DOI] [PubMed] [Google Scholar]
- 25.Young J., Rossi C., Gill J., Walmsley S., Cooper C., Cox J., et al. Risk factors for hepatitis C virus reinfection after sustained virologic response in patients coinfected with HIV. Clin Infect Dis. 2017;64:1154–1162. doi: 10.1093/cid/cix126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta S.H., Cox A., Hoover D.R., Wang X.H., Mao Q., Ray S., et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 27.Darke S. Self-report among injecting drug users: a review. Drug Alcohol Depend. 1998;51:253–263. doi: 10.1016/s0376-8716(98)00028-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual data is not public but will available upon reasonable request.