Abstract
Tracking moment-to-moment change in input and detecting change sufficient to require altering behavior is crucial to survival. Here, we discuss how the brain evaluates change over time, focusing on the hippocampus and its role in tracking context. We leverage the anatomy and physiology of the hippocampal longitudinal axis, re-entrant loops, and amorphous networks to account for stimulus equivalence and the updating of an organism’s sense of its context. Place cells have a central role in tracking contextual continuities and discontinuities across multiple scales, a capacity beyond current models of pattern separation and completion. This perspective highlights the critical role of the hippocampus in both spatial cognition and episodic memory: tracking change and detecting boundaries separating one context, or episode, from another.
The Hippocampal Longitudinal Axis and Context Continuity
The problem of how the brain can produce perceptual equivalences across an indefinite number of input combinations has perplexed scholars for decades [1-5]. The stimulus equivalence problem led Lashley [2] and, subsequently, Hebb [3] to argue against linear stimulus–response formulations of brain and behavior. An example of equivalence at the perceptual level is the ‘reafference’ from action systems to the visual system that negates the shift in retinal location by ‘predicting’ where an object should fall on the retina given current movements of the organism (including eye, head, and full-body movements) [6-9]. Retinal location invariance allows us to maintain accurate knowledge of the location of immobile objects in the external world as we move through space and time. The corollary discharge that enables retinal invariance highlights the notion that input patterns are only meaningful in relation to other inputs, both past and present.
The perceived stability of stationary objects as we move through the world is itself nested within a broader framework; that is, a context or situation. Context refers to the state of the environment of the organism at any given moment, as reflected in the total set of brain activities, which is a direct function of the brain state just before that moment [10]. Local stimuli may change as an animal moves in the world, but the context remains the same unless some threshold of change has been exceeded or some boundary has been crossed [11]. In other words, the brain registers these varying inputs, but until that threshold has been reached or that boundary crossed, the default assumption appears to be the continuity of context. Clearly, tracking such continuity requires maintaining a sense of sameness at one scale (the context) in the face of change at other scales (local features within that context). While the neural mechanisms underlying the representation of thresholds and boundaries are poorly understood, there is increasing evidence that the hippocampus is influenced by spatial and temporal boundaries [12-15]. Critically, pattern separation and pattern completion models [16] are not sufficient to solve the stimulus equivalence problem. These models assume that patterns of brain activity represent unique states and that the hippocampus generates sharp transitions in activity between two well-learned states as input gradually changes [17-19]. This approach sees the hippocampus as making binary decisions, incapable of reconciling situations in which small changes must be tracked and incorporated within a continuous context (e.g., gradual changes in perceptual input while an environment is traversed). Even in a novel situation, the brain must relate similarities between different contexts to quickly adapt and generate appropriate behavior. Neither of these scenarios can be adequately solved with pattern completion or separation. Here, we propose a novel framework for how the problem of equivalence is solved at this crucial level of contexts.
The Physiology of Context Continuity
Considerable evidence links the hippocampus to the representation of context by the brain, and we argue here that the hippocampus is central to the ability of an organism to track the ‘sameness’ of context [11,20]. Hippocampal recordings from awake-behaving rats reveal a striking firing rate correlate of principal neurons to the location of the animal in space [21], leading to the idea that the hippocampus is the core of a cognitive mapping system [22]. Approximately 5% of neurons are active at any single location in space [23]. As the rat moves, the population of active cells ‘rolls over’, with some cells becoming silent and others active [24,25]. With each theta cycle, the population of cells changes in relation to displacement in the environment, either real [26-28] or imagined [29]. The change in the active population can be strongly driven by movement cues. Thus, little movement yields little change in cues and neural activity, while a lot of movement yields big change. Although we focus here on functions of the hippocampus related to movement, we do not mean to imply that this structure has no role in situations where movement is absent.
Moving through an environment, an animal ‘maps’ the fixed relations among the stable entities in that space. While moving, the position of various objects in the environment relative to the animal changes, even though their absolute position in space is unchanged. The sensitivity of the hippocampal network to motion allows it to predict the extent to which stable objects in the environment should appear to have been translocated as a result of the movement of the organism. This information helps solve the problem of context equivalence by off-setting the sensory consequences of movement through the world, allowing the hippocampus to generate and maintain patterns of activity that answer the question: ‘how equivalent is the current context to the last?’ That is, hippocampal neurons instantiate context equivalence. In our view, they do this by tightly linking displacement to shifts in the patterns of neuronal activity in direct proportion to the magnitude of sensory change driven via self-motion cues (Figure 1).
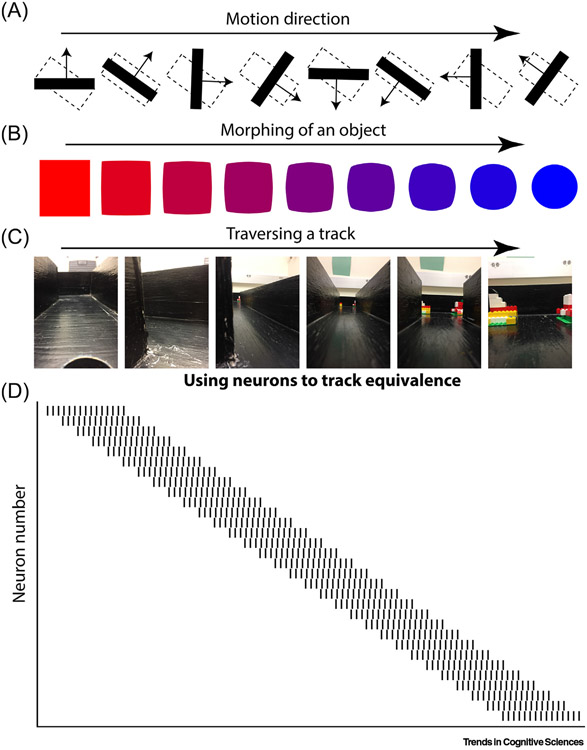
Figure 1. Population Vectors Evolve Based on Changing Input.
Stimulus equivalence refers to the situation where different sensory inputs yield the same output. Here, we propose that the nervous system solves this problem by (i) expressing a pattern of activity influenced by the stimulus; and (ii) progressively transforming the pattern of activity in proportion to ongoing change in the stimulus. (A) For instance, if the nervous system were to track the equivalence of motion direction, it would contain a population of neurons that progressively shifted as the angle changes. While this activity has been observed by Hubel and Wiesel [31], our approach provides the novel extension that V1 is not providing a ‘reduced representation’ of a complex environment that is built back up as activity moves to downstream cortical areas. Rather, it is part of a larger dynamic, iterative process that allows changes to be tracked in real time ensuring that an object is considered equivalent despite changes in motion direction. (B) Equivalence illustrated with the morphing of an object. As done on computer monitor screen savers a decade ago, a single stimulus changes shape and color as it traverses the screen, although simultaneously retalning the capability to be described as the same object. (C) The same sort of description can be applied to a rat traversing an L-shaped track. Each step yields changing environmental stimuli and simultaneously provides a measure of the amount of change that should have occurred. (D) A proposed stimulus/context equivalence solution: a population of neurons maintains and updates a pattern of activity as a function of the amount of change in external stimuli. As the pattern of activity for the red square slowly shifts in the first part of the morph, the remaining similarity supports continuity (it is the same object) while accounting for change. Of course, the blue circle yields a completely different pattern of activity than the red square. In the same way, the end of the track of the rat has a different pattern than the start. How does the nervous system maintain equivalence across such large changes? By allowing the neural pattern to change in concert with the stimuli, it provides a scaffold that enables the maintenance by the nervous system of equivalence across large changes by, essentially, packaging small changes into larger containers.
Both external sensory cues and internal states, including its own prior state, influence the hippocampus as it monitors and maintains equivalence (or continuity) over contiguous inputs. Unless there is a significant change, generated either by external or internal sources, the system signals ‘sameness’; that is, contextual equivalence. Thus, the animal continues to act as though it is still in the same context. Several problems need to be solved to accomplish context equivalence. First, some amount of change must be tolerated in the service of maintaining context stability. Second, rapid shifting from one context representation to another must be accomplished when change exceeds a threshold. Here, we suggest a solution to these problems that relies upon known anatomic and dynamic features of the hippocampus.
Maintaining Stability in the Face of Change
Consider a rat trained to alternate to obtain rewards on a figure-of-eight maze, and the sensory-motor experiences of which differ when it runs to the far sides of the maze. In both cases, the rat moves in pursuit of a reward associated with the global context. Even though sensory experience is different, the context and task situation remain the same. The rat makes the contextually appropriate turn at the central stem regardless of the sequence of local sensory and motor cues it experiences, indicating that its behavior continues to be determined by the stable global context. We propose that the hippocampus, along with its nearest neighbor, the entorhinal cortex, has architectural features that provide a basis for solving the context equivalence problem. The hippocampus can be ‘sliced’ in two ways (Figure 2A). Along its transverse axis lies the well-known tri-synaptic loop, while its longitudinal axis goes from the septal (dorsal or posterior) end to the temporal (ventral or anterior) end. An important feature of the cells along this longitudinal axis is that the size of the place fields systematically increases as one moves from dorsal to ventral in the rat hippocampus [30]. Similarly, the grain of the grid cells that provide inputs to the hippocampus also increases as one moves from dorsal to ventral within the medial entorhinal cortex [32,33].
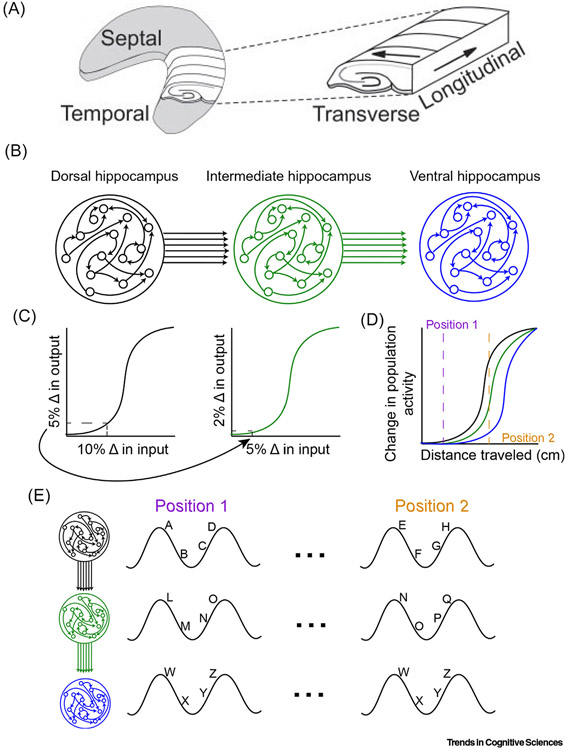
Figure 2. Variable Threshold Detection along the Hippocampal Long Axis.
(A) The 3D organization of the hippocampus. Theta activity propagates as a traveling wave along the longitudinal axis. (B) Consider a series of networks capable of both pattern completion and pattern separation (more appropriately, ‘pattern formation’; see main text) indicated by the sigmoidal response to changing input (C). The consequence of linking these networks in series is that each region becomes progressively less sensitive to the input (or distance traveled in terms of the hippocampus) indicated by the 5% change in the dorsal output serving as the input to the intermediate region (arrow), which only registers as a 2% change. This notion, which overlaps with the recently described convergence activity within recurrent neural networks [39], supposes that the activity in the ventral hippocampus is as much a consequence of intrahippocampal input, conveyed as a traveling wave of afferent activity, as it is a consequence of extrahippocampal input. (D) Operationalizing this model as a function of spatial movement, the change in the pattern of active neurons is less dramatic in more ventral regions [compare colors to (A)]. That is, based on (B), place cell activity will change more slowly in the ventral region. (E) Considering hippocampal look ahead, or the sequence of cellular activity within each theta cycle (with the activity of an individual cell assigned a letter), we can actualize how this model might work. Between positions ‘1’ and ‘2’ in (A), the input changes enough to alter the manner in which the network responds in the dorsal hippocampus. The intermediate hippocampus performs a sort of ‘low pass filter’ on this change, converging on a mildly altered population. That is, some cells are still active, some cells have gone silent, and new ones are active. However, this partially changed population in the intermediate hippocampus is seen as being the ‘same’ in the more ventral regions. Therefore, despite dorsal hippocampal population activity changing while moving between positions 1 and 2, ventral hippocampal population activity shows little change. Thus, the hippocampus is capable of tracking continuity across a continuum of scales. Reproduced from [40] (A).
The hippocampus most likely operates as a continuum, with activity propagating from the dorsal to ventral hippocampus in a peristalsis-like process [34-37]. Along this longitudinal axis, hippocampal organization can be considered as a discrete set of amorphous pools connected in series [33]. Modules project to other modules with larger place fields. Each module engages in a pattern formation process, being driven by current inputs and previously trained states. Pattern formation succeeds and is maintained when there are only modest changes in the input, allowing patterns to evolve without losing connection to the particular features of the outside world. This is how ‘sameness’ is achieved in the face of continuously changing input. Similarly, when environmental contexts are slowly morphed from one to another, hippocampal ensemble activity smoothly changes between these contexts [17]. The change from the pattern observed at the start to the one seen at the end is not a competitive process (pattern separation or completion) but the pattern formation that emerges from recurrent hippocampal neural networks.
In our view, the recurrent circuits of the hippocampus are capable of self-organization, such that they do not require a priori learning to form a spatiotemporal pattern. Input to such networks triggers nonlinear interplay between the neurons of the network, resulting in emergent order and structured activity [38]. This emergent pattern is not only robust, that is, recoverable in the face of mild perturbations, but also adaptable. If the pattern is changed slightly, some number of neurons cease to fire while others begin. Thus, organisms can detect both similarity and difference, generating a physiological response that maintains and adapts a pattern of activity in accordance with changes in input. This is best thought of as pattern formation, rather than pattern completion or separation.
How does the hippocampal system determine the switch from ‘modest’ to ‘substantial’ change; that is, when one has transitioned to a new context? In our view, this capacity emerges from the varying thresholds for change detection available in a system with varying scales of representation. The most dorsal module, with the smallest place fields, recreates its trained pattern up to, say, a threshold of 5% change, beyond which it transitions to a different pattern to be transmitted to the next module. This next module, with larger fields, will 'smooth over' 50% (let's say) of any change it receives, so in effect receives an input signal that is only 2.5% changed. As a result, this level transmits a ‘same’ context message to the next module; that is, it sustains its ongoing, trained, pattern. However, a 10% change in the first module would yield a 5% change in the second, sufficient to shift its output, and so on. This convergence activity has been modeled in recurrent neural networks as ‘contraction’ or the stabilization of the trajectory as activity moves through the brain [38]. A system wired this way would dampen relatively minor changes detected at a fine scale, in the service of preserving sameness at the situational or contextual scale (Figure 2).
The extent of the change detected by the organism determines how far a ‘change’ signal is propagated along the module stream characterizing the hippocampal long axis. Only substantial change (e.g., an actual context shift) will make it to, and out of, the modules at the ventral (anterior) end of the hippocampus. This proposal is consistent with evidence linking ventral/anterior hippocampus to context representation [41] and recent resting-state functional connectivity studies [42] suggesting privileged communication channels between the anterior (ventral) hippocampus and those areas in cortex concerned with context, prominently including the ventromedial prefrontal cortex (vmPFC). In our view, this mechanism, based on known anatomy and physiology of the hippocampus, can account for how the brain represents contexts, maintains their continuity and signals their discontinuity. This formulation is similar to the notion of hierarchical prediction error (PE) signaling, at the core of current ‘free energy’ approaches to brain function [43]. The hippocampus receives dopamine-mediated PE signals from locus coeruleus (LC) [44,45], and is affected as much by novelty as by reward PE. Hippocampal prediction errors are propagated to the vmPFC. Elaborating on these aspects of the model is beyond the scope of this paper.
To summarize, in the mechanism we sketched earlier, sharp transitions can occur anywhere along the longitudinal axis of the hippocampus (Figure 2B-E), but only transitions at the most anterior end are registered psychologically as a change in context. The circuit involving the anterior hippocampus and the vmPFC concerns contexts because the anterior end of the hippocampus has large ‘fields’, and the ventromedial part of the PFC to which it projects, is itself concerned with contexts and situational frameworks. The recently described syndrome of developmental topographical disorientation [46], which involves an inability to sense where one is in the world, reflects diminished connectivity between the PFC and the hippocampus, although the region within hippocampus was not sufficiently specified to tie this effect to the anterior end [47]. More specific data come from a recent study [48] investigating the acquisition and retrieval of trace-fear conditioning in the rat, which explored the role of inputs from ventral hippocampus to prelimbic areas. The authors concluded that ‘the VH [ventral hippocampus] continuously updates the pre-limbic area with the current contextual state of the animal, which, when disrupted during memory acquisition, is detrimental to the subsequent rapid retrieval of aversive contextual associations’. Although more research is clearly needed, these data provide support for our proposal. (See [49] for a related discussion of how the brain registers and deals with contexts and context shifts in the domain of memory.) Importantly, change signals from more dorsal aspects of the hippocampus project to target structures that are not concerned with contexts and, hence, should not affect the understanding of the organism of its situational and spatial context.
Changing State Space
Our focus to this point has been on what it takes to stay in the same state space when confronted with variation. Equally important, however, is the ability to rapidly recognize when these variations exceed some threshold. A system structured to track variation and adjust to minor perturbation, appears well placed to determine the presence of major perturbations. The mechanism specified earlier reacts to such a determination by generating a different pattern of activity in the network, in what is commonly referred to as ‘remapping’. In our view, understanding how one part of the brain, the hippocampus, both forms and evolves patterned signals relevant to its role in cognition demands a dynamic approach to the nature of neural connectivity. Such a perspective focuses on the fact that activity in the nervous system involves highly recurrent patterns projecting iteratively back onto themselves in a manner that is swayed by ongoing experience.
Critically, amorphous, highly recurrent networks can implement pathways that optimize for robust, yet adaptable dynamics [50-52]. Robust coding implies that modest change in the pattern of input has little to no effect on the output of a network. Adaptable coding suggests that a network has a high degree of pliability in terms of structure and function. For instance, a purely adaptable network could be trained to develop a specific pattern of activity by modulating specific synaptic weights. While this network can be precisely tuned, it is not necessarily fault tolerant (not robust).
How then can the brain achieve both adaptation and robust dynamics when, at first glance, favoring one may come at the detriment of the other (as pattern completion/separation would suggest)? The answer may reside in having convergence and divergence within the same network. Kozachkov and Miller [39] suggested that, when the internal weights of a recurrent neural network are either held fixed or slowly varied (ensuring contraction within the network) while the feed-forward weights between networks are free to adapt with the ‘ever-changing world’, the system will indeed have both robust and adaptable dynamics. Allowing multiple neurons to project into a network with amorphous connectivity favors robust operations that remain capable of adaptation [53,54]. Densely connected amorphous networks provide degenerate connections by which the brain forms and transmits analog patterns through large recurrent loops. Degenerate connectivity allows different components to perform either similar or distinct functions depending on conditions.
Interactions between inhibitory and excitatory neurons in these amorphously connected pools of neurons are critical for complex pattern formation, and supportive of robust and adaptable dynamics. A network solely comprised of recurrently connected excitatory cells is liable to kindle excitation to epileptic levels (Figure 3A). By contrast, a predominantly inhibitory network will rapidly quench activity. The ratio between these two populations of neurons is what gives rise to more complex dynamics. These networks go beyond ‘digital-like’ pattern separation versus pattern completion mechanisms [19], to instead express an analog quality in which the firing properties of any individual neuron has minimal consequences for the pattern of the whole. As long as the output is ‘close enough’, local mismatches are tolerated. However, as described later, the network is capable of converging onto other patterns. The nonlinear nature of excitatory–inhibitory interactions [38] (Figure 3B) makes it difficult to predict how much the input must vary to initiate substantial change in the network’s pattern.
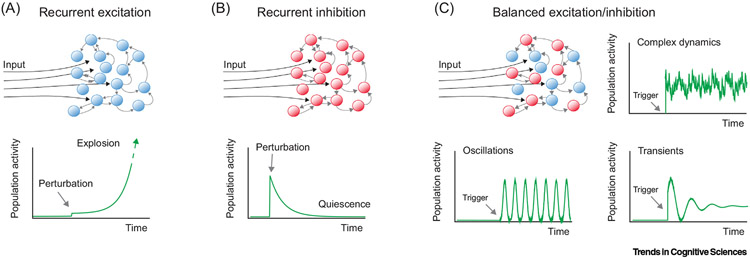
Figure 3. Network Dynamics.
As described in Rhythms of the Brain [38], (A) a network of ‘excitation-only’ neurons will spiral out of control, whereas (B) driving a network of inhibitory neurons will generate a transient pattern. However, the combination of these two populations of cells offers the ability to achieve complex spatiotemporal patterns, transient events, and/or oscillations (C). This figure was used by Berg and colleagues [55] to describe spinal motor circuits, but the amorphous connectivity of the nervous system lends it application to other systems, such as the hippocampus. In the absence of a hard-wired pattern from neuron to neuron, cells can connect to each other in an ‘approximate’ and amorphous manner. The dynamics of the amorphous connectivity network provides the first aspect of the solution to stimulus equivalence: to develop a spatiotemporal pattern of activity that can be roughly recreated (i.e., the pattern is recoverable, although with some noise) given a stable pattern of input. Should the input change, so does the amorphous network. The dynamics of how the amorphous network changes in relation to input is most likely nonlinear. Adapted from [55].
The dynamics described earlier link the ideas of Hebb and Lashley [56-58] (Box 1) with contemporary research into ‘reservoir computing’ [59] models, such as liquid-state machines [60] and echo-state networks [61], in describing how hippocampal networks function. Consider the ‘chaotic neural networks’ of Sussillo and Abbott [62] (Figure 4) in which a recurrent network generates spatiotemporal patterns of neural activity that could support either walking or running motions depending on the input. This example shows that these networks can spontaneously generate complex dynamic activity patterns that are also recoverable. Furthermore, tolerance is built into these network dynamics, such that inputs that are ‘close enough’ count, because the ability to approximately recreate a spatiotemporal pattern of activity is an emergent property of the excitatory and inhibitory connections within the network.
Box 1. Linear Cognition: Does Sensation → Perception → Memory?
Cognitive models emerging from the 1940s and 1950s adopted an information-processing approach in which information is relayed bottom up, from the environment, through the periphery of the nervous system, and then to the deeper structures of the brain, including the hippocampus and neocortex. This feed-forward view was captured, and reinforced (perhaps unintentionally), in the iconic wiring diagram of the visual system published by Felleman and Van Essen [79]. Behavioral evidence emerging during the 1990s showed that feedback influences even the earliest stages of perception, including figure–ground segregation [80]. Such data made it increasingly difficult to sustain a feedforward, linear, view of cognition.
The limits of a linear cognitive framework have been widely discussed [81-83]. Edelman rejected it for requiring too much ‘agency’. He argued that the point-to-point connectivity of the nervous system is never precise enough for any specific anatomical connection to have inherent meaning. Freeman also dismissed the approach for being too rigid. Patterns, he argued, are amorphous, and the classic perception → encoding → consolidation → memoryscheme cannot work with neural representations that are effectively moving targets [84]. Buzsaki noted that interneurons add nonlinearity to how activity is distributed within a small circuit, and also described the nested, circular loop architecture prominent in the brain.
In point of fact, connectivity between the primary visual cortex and hippocampus is as circular and re-entrant as it is hierarchical, and the contemporary neuroanatomical literature documents feed-forward and feedback connections between pairs of neurons, small clusters, and larger re-entrant networks, including the hippocampus and scaling up to include cortical-cerebellar loops [85-91]. The largest loop of all incorporates both the organism and the environment.
Re-entrant circuitry gibes well with Hebb’s perspective that activity circulates through loops in the brain all the time. Incoming information, from any source, is necessarily superimposed on, and interacts with, ongoing activity in a way that can change the brain’s trajectory: ‘…excitation might theoretically continue for an indefinite period, ‘chasing its tail,’ and not leave the circuit until some other excitation came along with which it might combine to produce a motor effect that neither could produce alone’ ([58] p. 56).
The presence of neural loops allows the system to largely drive itself, thereby maintaining robust circulation of information. This provides a means by which the models of the brain of the world (i.e., its ‘priors’ in Bayesian terms) are refined and ‘filled in’ by activity at one time-step informing the next. The loop structure of the brain shows that any simple linear understanding of its function must be incorrect. Our approach envisions brain activities that are continuously ‘chasing their own tails’, which renders any simple separation between such things as perception and memory implausible. Although such distinctions continue to populate the textbooks, it has been obvious for some time that there is at least as much feedback in the brain as there is feed-forward. The loops of various sizes that we assume in our model build in both, and allow for modeling space and time at multiple scales. Exactly how this way of thinking about the brain will map most appropriately onto psychological terms, such as perception and memory, remains to be seen. However, one thing is certain: traditional models that see these as wholly distinct activities no longer make sense.
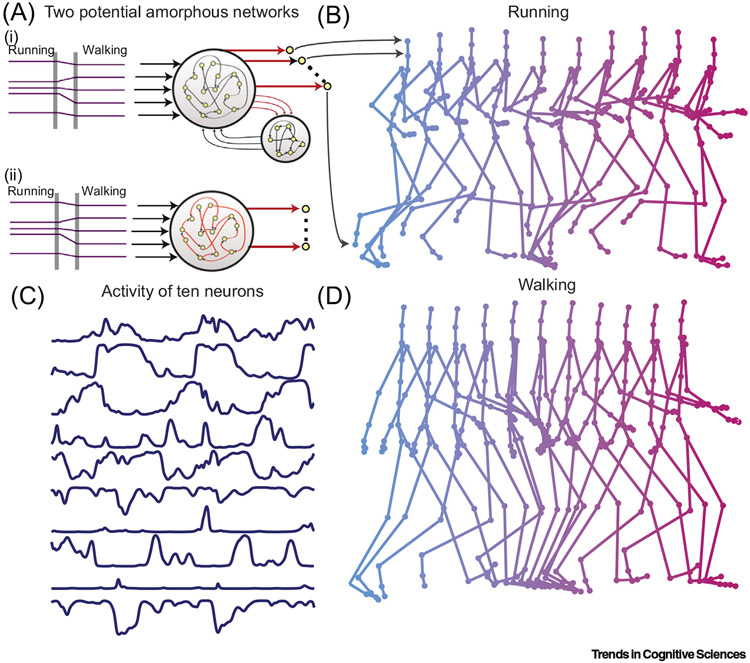
Figure 4. Amorphous Networks Have the Ability to Recover Dynamic Patterns Based on the Input.
(A) Constant neural input (e.g., either ‘running’ or ‘walking’) provides the activity into either linked amorphous networks [with modifiable connections between them (i)] or a single amorphous network with modifiable connections between neurons [red connections denote modifiability (ii)]. After training, the dynamic activity of recurrent neural networks can be relayed to motor units, with motor patterns that match human motion capture data (arrows from A to B), to supporting complex actions, such as running (B) or walking (D). Importantly, the network can respond with a time-evolving pattern as shown. (C) Activity of ten neurons during walking motion shows the transition or activity within the network in relation to walking. Akin to the sequences of cell firing within a cycle of theta, the input into this network kicks off a pattern of activity. While some neurons appear to correlate to behavior, others do not. The network is simply evolving a pattern of activity in response to an input. Reproduced from [65].
As already indicated, the temptation to consider this merely a ‘pattern completion’ network should be resisted: when the active inputs into such a network are redistributed, an entirely different yet equally stable pattern can emerge. To reiterate, the term ‘pattern formation’ more accurately describes what these networks do. Sussillo and Abbott [62] had the insight that these randomly connected networks will not exhibit any obvious relationship between the function they are currently performing (e.g., running versus walking) and their fixed anatomical connectivity alone is insufficient to precisely specify function [63].
Amorphous neural ensembles of this type in the hippocampus could accomplish the goals of tracking context continuity and discontinuity in the following way: (i) given a set of inputs, an amorphous network would converge on a stable activity pattern. This accounts for the rapid formation of place fields in a novel environment; (ii) such an ensemble would gradually change its pattern in a conserved manner, generating predictable sequences linked to the interaction of the organism with the environment. Sequences are inherent to amorphous, recurrent networks, which have asymmetric connectivity that render the appearance of repeatable sequences, such as phase precession, replay and preplay (e.g., [64]) inevitable; (iii) afferent input initiates spatiotemporal patterns of activity in the hippocampus, redistributing activity from one moment to the next (Figures 2 and 3). The strength of afferent input determines how many neurons are active within a particular sequence, while the initial starting spatiotemporal pattern is determined by the pattern of afferent input interacting with the present state of the network (which in itself is a function of the prior activity history). Firing rates and look-ahead would increase with velocity. If input comes into the network at theta frequency, a sequence or ‘look-ahead’ is the consequence. This model has implications for theta phase precession [64] that will not be covered here; and (iv) should afferent input change enough, a different stable pattern, also capable of generating reliable sequences, would emerge. Remapping is a change in the pattern of the active population that occurs as a function of pattern formation, or how a different pattern results in a different spatio-temporal pattern of input.
That is, the network ensemble has the property of multistability, allowing for multiple states with substantially different outcomes. Work by Jezek et al. [65], subjecting rats to instantaneous context shifts, showed that the network flickered back and forth between states representing the two contexts, finally settling into the one the animal was in at the moment. When the system determines that the organism is no longer in context X, given the extent of unexpected stimulation, it shifts into a brain state appropriate for the context it is now in, or its best guess at what that might be. This need for a specified context puts a premium on a multistable network. While correlates between individual neuron activity and behavior may exist, they can be spurious by-products of the larger network dynamics functioning to maintain context equivalence. While it is tempting to link hippocampal place cell patterns to specific behavioral correlates, this approach loses sight of the operation of the network as a whole.
Predictions
Our proposal specifies the roles of different segments of the hippocampus, because they enable the internal modeling of experienced events and environments, both novel and largely familiar. Limiting the function of either the dorsal or ventral hippocampus in rodents should yield rather different effects, something known for 50 years [66]. Early speculation focused on the role of the dorsal hippocampus in spatial tasks, and a putative role of the ventral hippocampus in regulating anxiety [67]. More recent views emphasize the multiscale nature of hippocampal organization (e.g., [41]), as does the present proposal. We and many others would expect damage to the dorsal hippocampus to impair precise behavior in space, while damage to the ventral hippocampus should primarily affect the ability of the animal to utilize contexts adaptively. Recent studies support these predictions [48,68,69].
Similarly, in humans, the posterior hippocampus should be especially important in the retention of detailed information about past events and environments, while the anterior hippocampus should reflect the engagement of more coarse-grained representations. A novel prediction about boundary detection tests the assertion of the model that a contextual boundary is signaled from the anterior (ventral) hippocampus only when mismatches along the length of the axis are extensive enough to reach the anterior end. Novelty, that might or might not be signaling a new context, will yield large increases in blood oxygen level-dependent (BOLD) activity in posterior hippocampus, possibly larger than those seen in anterior hippocampus, but only in anterior hippocampus will the extent of activation be related to the perception of a boundary. In addition, damage to the anterior hippocampus would have a greater impact than damage to the posterior hippocampus on the perception of a boundary. There is evidence consistent with these predictions [70-73] but critical studies remain to be done.
We believe that our proposal, based on known anatomy and physiology of the hippocampus, can account for how the brain represents contexts, and monitors their continuity or discontinuity. Within this perspective, there is no ‘solid index’ or engram stored in synapses or a definable population of neurons, but rather a ‘liquid’ congruence capable of maintaining continuity in an approximate, but updatable format. As long as the densely recurrent networks of the hippocampus respond in the way described by Sussillo and Abbott [62], the exact neurons within the hippocampus that are active are of little consequence. The iterative ‘dialog’ between hippocampus and neocortex, each step informing the next, either brings these two into ever-closer registration, to reduce prediction error; or drives them completely apart, forcing the system to utilize separate representations (see Figure 2 of [74]). Within this view, place cells track equivalence in the midst of change and, hence, are key elements in the continuity of contexts. Remapping when exposed to distinct environments reflects the response of a recurrent neural network to input from two ‘unique, non-continuous contexts’. As already noted, the brain is capable of driving itself during sleep, evolving a pattern based on internal states. Our place cell description can be extended to the process of imagining the future, in which the brain moves from a familiar ‘context’ to a ‘novel’ one based on internal dynamics. Thus, our model is consistent with the burgeoning evidence of a role for the hippocampus in imagining future states [75].
To summarize: the hippocampus accomplishes context continuity through mechanisms leveraging the changing scale of spatial representation along the longitudinal axis of the hippocampus. It accomplishes context discontinuity by utilizing amorphous networks that not only keep pattern formation within bounds while managing a certain level of fault tolerance, but also have the capacity to rapidly shift to a different pattern of activity when sufficient prediction error indicates a new context.
Concluding Remarks
What are the implications of our position for the role of the hippocampus in memory? First, our approach sees the physiological basis of episodic memories as fluctuating from moment to moment, even as the ‘content’ of any given memory may be held constant. Second, our view stresses ongoing linkages between hippocampus and cortex as a main contributor to the evolving hippocampal state. This implies that, as long as a memory for a given episode persists, both hippocampus and cortex will necessarily be engaged, and this in turn has implications for current debates about the role of the hippocampus in remote episodic memory [76-78].
Barry and Maguire [76] argued that the physiological flux observed in hippocampus rendered it unsuitable as a site of long-term memories. While they were correct in pointing out the challenge created by an unstable substrate, there appears to be little reason to think things are much better in the neocortical sites involved in long-term memory. Instead, we need to understand exactly how dynamic ‘representations’ can undergird a stable mental life. The approach adopted here is an attempt to provide such an account, and it depends upon continued engagement of both hippocampal and associated neocortical sites throughout the life of a ‘memory’.
Here, we have offered not a fine-tuned theory of how the hippocampus accomplishes its role in context or episodic memory, but rather a conceptual framework that leverages dynamics, re-entrant loops, and amorphous networks. However, this framework raises several issues (see Outstanding Questions). We close by noting that our perspective accepts Hebb’s admonition about assigning functions to isolated brain structures: ‘There is a trap……the student must be warned about. No psychological function can exist within a segment of cortex by itself.’ ([58] p. 83). The representation of context by the brain resides neither in the hippocampus nor the entorhinal cortex, nor in isolation in any of other structures in the loop.
Outstanding Questions.
If the brain is a series of nested anatomical loops, where in these loops does ‘meaning’ reside? Can we assign representational content to specific parts of these loops?
How do the multiscale amorphous pools in the hippocampus emerge during development?
Given that brain regions described as subserving ‘perception’, ‘sensation’, and ‘memory’ continually circulate information between one another, is there any virtue to maintaining these classical divisions moving forward?
What role does inhibitory–excitatory balance have in shaping the subtle dynamics of the amorphous pools, in the region between seizures and flatlining?
Highlights.
Local stimuli may change as an animal moves in the world, but the context remains the same unless some threshold of change has been exceeded or some boundary has been crossed.
How the brain can tolerate a certain amount of change in the service of context stability while also rapidly shifting from one context to another when the change exceeds a certain threshold is not well understood.
This ‘equivalence’ function was identified by both Lashley and Hebb as critical to understanding how the brain generates adaptive behavior.
Considerable evidence links the hippocampus to the representation of context by the brain, suggesting that the hippocampus has a central role in solving the equivalence problem with respect to context.
We propose a novel solution to the context equivalence problem, leveraging the anatomy and physiology of the hippocampus, with a critical role attributed to the shifting representational scales observed along the longitudinal axis of this structure.
Acknowledgments
We thank Sara Burke, Mary Peterson, Gyuri Buzsaki, Leo Kozachkov, and Earl Miller for their suggestions on earlier drafts.
References
- 1.Adrian ED (1932) The Mechanism of Nervous Action, Electrical Studies of the Neurone, University of Pennsylvania Press [Google Scholar]
- 2.Lashley K (1942) The problem of cerebral organization in vision. In Visual Mechanisms (Kluver H, ed.), pp. 301–322, Jacques Cattell [Google Scholar]
- 3.Hebb DO (1949) The Organization of Behavior, Wiley [Google Scholar]
- 4.Sokolov EN (1960) Neuronal models and the orienting reflex. In The Central Nervous System and Behavior (Brazier MAB, ed.), pp. 187–276, Josiah Macy Jr. Foundation [Google Scholar]
- 5.Konorski J (1967) Integrative Activity of the Brain: An Interdisciplinary Approach, The University of Chicago Press [Google Scholar]
- 6.Sperry R (1950) Neural basis of the spontaneous optokinetic response produced by visual inversion. J. Comp. Physiol. Psychol 43, 482–489 [DOI] [PubMed] [Google Scholar]
- 7.von Holst E and Mittelstaedt H (1971) The principle of reafference: Interactions between the central nervous system and the peripheral organs. In Perceptual Processing: Stimulus Equivalence and Pattern Recognition (Dodwell PC, ed.), pp. 41–72, Appleton-Century-Crofts [Google Scholar]
- 8.Evarts EV (1971) Central control of movement: feedback and corollary discharge: a merging of the concepts. Neurosci. Res. Program Bull 9, 86–112 [PubMed] [Google Scholar]
- 9.Brooks JX and Cullen K (2019) Predictive sensing: the role of motor signals in sensory processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 4, 842–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nickolic D (2010) The brain is a context machine. Rev. Psychol 17, 33–38 [Google Scholar]
- 11.Nadel L (2008) Hippocampus and context revisited. In Hippocampal Place Fields: Relevance to Learning and Memory (Mizumori SJY, ed.), pp. 3–5, Oxford University Press [Google Scholar]
- 12.Zacks JM (2020) Event perception and memory. Annu. Rev. Psychol 71, 165–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clewett D et al. (2019) Transcending time in the brain: how event memories are constructed from experience. Hippocampus 29, 162–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldassano C et al. (2017) Discovering event structure in continuous narrative perception and memory. Neuron 95, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunec IK et al. (2018) Boundaries shape cognitive representations of spaces and events. Trends Cogn. Sci 22, 637–650 [DOI] [PubMed] [Google Scholar]
- 16.Rolls ET (2016) Pattern completion and pattern separation mechanisms in the hippocampus. In The Neurobiological Basis of Memory: A System, Attribute, and Process Analysis (Jackson PA et al. , eds), pp. 77–113, Springer [Google Scholar]
- 17.Leutgeb JK et al. (2005) Progressive transformation of hippocampal neuronal representations in ‘morphed’ environments. Neuron 48, 345–358 [DOI] [PubMed] [Google Scholar]
- 18.Amit DJ et al. (1987) Statistical mechanics of neural networks near saturation. Ann. Phys 173, 30–67 [Google Scholar]
- 19.McNaughton BL and Morris RG (1987) Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 10, 408–415 [Google Scholar]
- 20.Nadel L and Willner J (1980) Context and conditioning: a place for space. Physiol. Psychol 8, 218–228 [Google Scholar]
- 21.O'Keefe J and Dostrovsky J (1971) The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175 [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe J and Nadel L (1978) The Hippocampus as a Cognitive Map, The Clarendon Press [Google Scholar]
- 23.Wilson MA and McNaughton BL (1993) Dynamics of the hippocampal ensemble code for space. Science 261, 1055–1058 [DOI] [PubMed] [Google Scholar]
- 24.Gothard KM et al. (2001) Dentate gyrus and CA1 ensemble activity during spatial reference frame shifts in the presence and absence of visual input. J. Neurosci 21, 7284–7292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maurer AP et al. (2005) Self-motion and the origin of differential spatial scaling along the septo-temporal axis of the hippocampus. Hippocampus 15, 841–852 [DOI] [PubMed] [Google Scholar]
- 26.Maurer AP et al. (2012) Greater running speeds result in altered hippocampal phase sequence dynamics. Hippocampus 22, 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maurer AP et al. (2014) Back to the future: preserved hippocampal network activity during reverse ambulation. J. Neurosci 34, 15022–15031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skaggs WE et al. (1996) Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6, 149–172 [DOI] [PubMed] [Google Scholar]
- 29.Kay K et al. (2020) Constant sub-second cycling between representations of possible futures in the hippocampus. Cell 180, 552–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung MW et al. (1994) Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J. Neurosci 14, 7347–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubel DH and Wiesel TN (1962) Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J. Physiol 160, 106–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brun VH et al. (2008) Progressive increase in grid scale from dorsal to ventral medial entorhinal cortex. Hippocampus 18, 1200–1212 [DOI] [PubMed] [Google Scholar]
- 33.Stensola H et al. (2012) The entorhinal grid map is discretized. Nature 492, 72. [DOI] [PubMed] [Google Scholar]
- 34.Petsche H and Stumpf C (1960) Topographic and toposcopic study of origin and spread of the regular synchronized arousal pattern in the rabbit. Electroencephalogr. Clin. Neurophysiol 12, 589–600 [DOI] [PubMed] [Google Scholar]
- 35.Lubenov EV and Siapas AG (2009) Hippocampal theta oscillations are travelling waves. Nature 459, 534–539 [DOI] [PubMed] [Google Scholar]
- 36.Patel J et al. (2012) Traveling theta waves along the entire septotemporal axis of the hippocampus. Neuron 75, 410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patel J et al. (2013) Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci 33, 17029–17041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzsaki G (2006) Rhythms of the Brain, Oxford University Press [Google Scholar]
- 39.Kozachkov L et al. (2020) Achieving stable dynamics in neural circuits. PLoS Comput. Biol 16, e1007659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gloveli T et al. (2005) Orthogonal arrangement of rhythm-generating microcircuits in the hippocampus. Proc. Natl. Acad. Sci 102, 13295–13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poppenk J et al. (2013) Long-axis specialization of the human hippocampus. Trends Cogn. Sci 17, 230–240 [DOI] [PubMed] [Google Scholar]
- 42.Dalton MA et al. (2019) Functional connectivity along the anterior–posterior axis of hippocampal subfields in the ageing human brain. Hippocampus 29, 1049–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Friston KJ (2019) Waves of prediction. PLoS Biol. 17, e3000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dupret D and McNamara CG (2017) Two sources of dopamine for the hippocampus. Trends Neurosci. 40, 383–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kempadoo KA et al. (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc. Natl. Acad. Sci 113, 14835–14840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iaria G and Burles F (2016) Developmental topographical disorientation. Trends Cogn. Sci 20, 720–722 [DOI] [PubMed] [Google Scholar]
- 47.Iaria G et al. (2014) Developmental topographical disorientation and decreased hippocampal functional connectivity. Hippocampus 24, 1364–1374 [DOI] [PubMed] [Google Scholar]
- 48.Twining RC et al. (2020) Ventral hippocampal input to the prelimbic cortex dissociates the context from the cue association in trace fear memory. J. Neurosci 40, 3217–3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nadel L and Sederberg PB Memory reconsolidation: making predictions better. In Handbook of Human Memory (Kahana M and Wagner A, eds), Oxford University Press; (in press) [Google Scholar]
- 50.Edelman GM and Gally JA (2001) Degeneracy and complexity in biological systems. Proc. Natl. Acad. Sci 98, 13763–13768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tononi G et al. (1999) Measures of degeneracy and redundancy in biological networks. Proc. Natl. Acad. Sci 96, 3257–3262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tononi G et al. (1998) Complexity and coherency: integrating information in the brain. Trends Cogn. Sci 2, 474–484 [DOI] [PubMed] [Google Scholar]
- 53.Whitacre J and Bender A (2010) Degeneracy: a design principle for achieving robustness and evolvability. J. Theor. Biol 263, 143–153 [DOI] [PubMed] [Google Scholar]
- 54.Whitacre JM et al. (2012) Evolutionary mechanics: new engineering principles for the emergence of flexibility in a dynamic and uncertain world. Nat. Comput 11, 431–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berg RW et al. (2019) When networks walk a fine line: balance of excitation and inhibition in spinal motor circuits. Curr. Opin. Physiol 8, 76–83 [Google Scholar]
- 56.Nadel L and Maurer AP (2018) Recalling Lashley and reconsolidating Hebb. Hippocampus 30, 776–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown RE (2020) Donald O. Hebb and the organization of behavior: 17 years in the writing. Mol. Brain 13, 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hebb DO (1958) A Textbook of Psychology, Saunders [Google Scholar]
- 59.Enel P et al. (2016) Reservoir computing properties of neural dynamics in prefrontal cortex. PLoS Comput. Biol 12, e1004967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maass W et al. (2002) Real-time computing without stable states: a new framework for neural computation based on perturbations. Neural Comput 14, 2531–2560 [DOI] [PubMed] [Google Scholar]
- 61.Jaeger H (2003) Adaptive nonlinear system identification with echo state networks. Networks 8, 17 [Google Scholar]
- 62.Sussillo D and Abbott LF (2009) Generating coherent patterns of activity from chaotic neural networks. Neuron 63, 544–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bargmann CI and Marder E (2013) From the connectome to brain function. Nat. Methods 10, 483–490 [DOI] [PubMed] [Google Scholar]
- 64.O'Keefe J and Recce ML (1993) Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus 3, 317–330 [DOI] [PubMed] [Google Scholar]
- 65.Jezek K et al. (2011) Theta-paced flickering between place-cell maps in the hippocampus. Nature 478, 246. [DOI] [PubMed] [Google Scholar]
- 66.Nadel L (1968) Dorsal and ventral hippocampal lesions and behavior. Physiol. Behav 3, 891–900 [Google Scholar]
- 67.Fanselow MS and Dong HW (2010) Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hauser J et al. (2020) Small lesions of the dorsal or ventral hippocampus subregions are associated with distinct impairments in working memory and reference memory retrieval, and combining them attenuates the acquisition rate of spatial reference memory. Hippocampus 30, 938–957 [DOI] [PubMed] [Google Scholar]
- 69.Meyer-Mueller C et al. (2020) Dorsal, but not ventral, hippocampal inactivation alters deliberation in rats. Behav. Brain Res 390, 112622. [DOI] [PubMed] [Google Scholar]
- 70.Collin SH et al. (2015) Memory hierarchies map onto the hippocampal long axis in humans. Nat. Neurosci 18, 1562–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reagh ZM et al. (2020) Aging alters neural activity at event boundaries in the hippocampus and posterior medial network. Nat. Commun 11, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grady CL (2020) Meta-analytic and functional connectivity evidence from functional magnetic resonance imaging for an anterior to posterior gradient of function along the hippocampal axis. Hippocampus 30, 456–471 [DOI] [PubMed] [Google Scholar]
- 73.Nadel L et al. (2013) Spatial cognition and the hippocampus: the anterior–posterior axis. J. Cogn. Neurosci 25, 22–28 [DOI] [PubMed] [Google Scholar]
- 74.Hwaun E and Colgin LL (2019) CA3 place cells that represent a novel waking experience are preferentially reactivated during sharp wave-ripples in subsequent sleep. Hippocampus 29, 921–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schacter DL et al. (2017) Episodic future thinking: mechanisms and functions. Curr. Opin. Behav. Sci 17, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Barry DN and Maguire EA (2018) Remote memory and the hippocampus: a constructive critique. Trends Cogn. Sci 23, 128–142 [DOI] [PubMed] [Google Scholar]
- 77.Barry DN and Maguire EA (2019) Consolidating the case for transient hippocampal memory traces. Trends Cogn. Sci 23, 635–636 [DOI] [PubMed] [Google Scholar]
- 78.Moscovitch M and Nadel L (2019) Sculpting remote memory: enduring hippocampal traces and vmPFC reconstructive processes. Trends Cogn. Sci 23, 634–635 [DOI] [PubMed] [Google Scholar]
- 79.Felleman DJ and Van Essen DE (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 [DOI] [PubMed] [Google Scholar]
- 80.Peterson MA and Gibson BS (1994) Must figure-ground organization precede object recognition? An assumption in peril. Psychol. Sci 5, 253–259 [Google Scholar]
- 81.Edelman GM (1987) Neural Darwinism: The Theory of Neuronal Group Selection, Basic Books; [DOI] [PubMed] [Google Scholar]
- 82.Freeman WJ (2000) How Brains Make Up Their Minds, Columbia University Press [Google Scholar]
- 83.Buzsaki G (2019) The Brain from Inside Out, Oxford University Press [Google Scholar]
- 84.Káli S and Dayan P (2004) Off-line replay maintains declarative memories in a model of hippocampal-neocortical interactions. Nat. Neurosci 7, 286–294 [DOI] [PubMed] [Google Scholar]
- 85.Amaral DG and Witter MP (1989) The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience 31, 571–591 [DOI] [PubMed] [Google Scholar]
- 86.Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society of London. Series B, containing papers of a biological character pp. 308–319 [Google Scholar]
- 87.Gutierrez GJ et al. (2013) Multiple mechanisms switch an electrically coupled, synaptically inhibited neuron between competing rhythmic oscillators. Neuron 77, 845–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marshall L et al. (2002) Hippocampal pyramidal cell-interneuron spike transmission is frequency dependent and responsible for place modulation of interneuron discharge. J. Neurosci 22, RC197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizuseki K et al. (2009) Theta oscillations provide temporal windows for local circuitcomputation in the entorhinal-hippocampal loop. Neuron 64, 267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat. Rev. Neurosci 7, 511–522 [DOI] [PubMed] [Google Scholar]
- 91.Witter M and Amaral D (2004) Hippocampal formation. In The Rat Nervous System (3rd edn) (Paxinos G, ed.), pp. 635–704, Gulf Professional Publishing [Google Scholar]