Synopsis
Many teleost fish, such as gobies, have fused their paired pelvic fins into an adhesive disc. Gobies can use their pelvic suckers to generate passive adhesive forces (as in engineered suction cups), and different species exhibit a range of adhesive performance, with some even able to climb waterfalls. Previous studies have documented that, in the Hawaiian Islands, species capable of climbing higher waterfalls produce the highest passive pull-off forces, and species found at higher elevation sites are likely to have more rounded suction discs than those found in the lowest stream segments. Morphology of the pelvic girdle also varies between species, with more robust skeletons in taxa with superior passive adhesion. To investigate what factors impact the passive adhesive performance of waterfall climbing gobies, we tested biomimetic suction cups designed with a range of shapes and embedded bioinspired “skeletons” based on micro-CT scans of goby pelvic girdles. We found that while the presence of an internal skeleton may provide some support against failure, the performance of suction cups may be more strongly affected by their external shape. Nonetheless, factors besides external shape and skeletal morphology may still have a stronger influence on sucker tenacity. Our results suggest that the relationship between suction disc morphology and adhesive performance may be influenced by a variety of physical factors, and live animal performance likely is further complicated by muscle activation and climbing behavior. These results have implications for the evolution of suction disc shape in adhesive fish and for improving the design of biomimetic suction cups.
Introduction
The pelvic fins and girdles of fishes show tremendous diversity in form and function (Yamanoue et al. 2010). In many species, these fins can play important roles in stabilization and/or facilitating maneuvers during swimming (Standen 2008, 2010). However, the full diversity of pelvic fin structure and function spans from taxa that have greatly reduced or completely lost their pelvic fins (Bell et al. 1993), to lineages in which the pelvic fins have evolved additional, or alternative, specialized functions. For example, a variety of batoids and other elasmobranchs (Lucifora and Vassallo 2002; Macesic and Kajiura 2010; Macesic et al. 2013) use their pelvic fins to propel themselves across a benthic floor (Pridmore 1994; Goto et al. 1999; Macesic and Kajiura 2010; Macesic et al. 2013). Propulsive roles for the pelvic fins can be found in a variety of other lineages, ranging from lungfish (King et al. 2011; Aiello et al. 2014; King and Hale 2014) to loaches (Flammang et al. 2016). Among teleosts, however, a common specialization of pelvic fin function is their evolution as adhesive structures (Budney and Hall 2010). The adhesive behaviors used by such fishes vary greatly, as do the morphology and attachment strength of their pectoral fins. Some species exhibit combined adhesive structures formed from the pelvic and pectoral girdles, which may either work independently as in loaches (Wang et al. 2019), or are fused together as in clingfishes (Ditsche et al. 2014, 2017). Other groups, such as gobies, snailfishes, and lumpfishes (Budney and Hall 2010), have modified only the pelvic girdle and fused pelvic fins for adhesion. With adhesive capacity evolving independently in multiple teleost lineages, studies of the structure and function of adhesive pelvic fins have the potential to provide insight into a common, and possibly convergent, evolutionary transition in function.
The gobies provide an outstanding group for examining transitions in the role of the pelvic fins as adhesive structures. In all gobies, the pelvic fins are fused to form an adhesive disc (Thacker and Roje 2011) that attaches to surfaces via suction (Maie et al. 2012, 2013; Maie and Blob 2021). However, species vary in the ways that they use their discs. While marine and intertidal species may use the disc to adhere to substrates in reefs, tidepools, or estuaries, some amphidromous species use these discs to breach the surface of the water and climb structures such as waterfalls (Schoenfuss and Blob 2003; Greenfield and Randall 2004). In streams of the Hawaiian Islands in particular, four species of amphidromous gobies exhibit a range of climbing and adhesive abilities that result in elevation-dependent species stratification, where more proficient climbing species are found at higher elevations (Maie et al. 2013). These species include the non-climbing Stenogobius hawaiiensis mostly found in estuarine habitats, followed by the climbing species Awaous stamineus found in low-elevation streams, and Sicyopterus stimpsoni and Lentipes concolor achieving respectively higher elevations (Nishimoto and Fitzsimons 1999). Passive pull-off adhesive forces in these species follow the trend of their differences in elevation, with better climbers requiring higher forces to become dislodged (Palecek et al. 2021a). Sicyopterus stimpsoni also uses a distinctive mode of climbing. Whereas most climbing gobies use a mode called “powerburst climbing,” in which the pelvic sucker is sequentially attached and detached from the substrate while upward movement is achieved via axial undulations, S. stimpsoni use a mode called “inching,” in which there is little axial movement and the body is advanced through sequential attachment and detachment of the pelvic sucker and a second, oral sucker (Schoenfuss and Blob 2003).
Variation in adhesive performance between species can relate to a multitude of factors (Schoenfuss and Blob 2003; Blob et al. 2006,2008; Maie et al. 2011, 2012; Schoenfuss et al. 2013). For example, performance of a sucker depends on the contraction of pelvic elevator muscles once the sucker has contacted the substrate. Thus, adhesive performance can depend on factors such as sucker attachment kinematics (Griner et al. 2021), leverage, and fiber types of pelvic muscles (Maie et al. 2013; Schoenfuss et al. 2013). However, factors such as differences in the material properties and relative size and shape of the hard and soft tissue comprising the pelvic fins may also affect adhesive performance (Taft et al. 2017). One way to test the effects of tissue shape while controlling for differences in material properties is through physical modelling (Wainwright et al. 2013; Ditsche and Summers 2019; Sandoval et al. 2019). By fabricating biomimetic suction cups that vary in structural design, it may be possible to gain insight into the functional morphology of goby suction discs and, potentially, provide a foundation for designing bioinspired suction devices with the capability for enhanced performance on rough substrates or substrates under water.
All engineered and organismal suction discs work similarly, wherein the disc is pressed against a substrate to form a seal, after which it is pulled away from the substrate while remaining attached (Kier and Smith 1990). This reduces pressure within the disc to below the atmospheric pressure outside of it, producing a pressure differential that keeps the disc stuck to the substrate. Failure of suction in a disc may happen in multiple ways. Often, air or water leaking into the disc (from a gap at the disc-substrate interface) will prevent a pressure differential from forming. A soft disc wall may prevent a gap between the disc and substrate, as conformability of the disc margins could fill any gaps in a rough or irregular contact surface. However, buckling can occur when the walls of the disc are too soft, causing the disc to detach. Previous studies have found that a disc with a strong, rigid wall and a soft, compliant edge can outperform traditionally engineered homogenous soft suction cups, as well as the suction discs of fish that inspired the novel cup design (Ditsche and Summers 2019). Moreover, the suction discs of fishes often perform better than human manufactured suction cups on rough or irregular substrates, and maintain strong attachments under water (Wainwright et al. 2013; Ditsche and Summers 2014; Ditsche et al. 2014; Ditsche and Summers 2019). Thus, bioinspired suction cups based on designs from fishes could have wide applications. Mimicking the mechanical properties, textures, and shapes of teleost suckers may improve the performance of manufactured suction cups, especially on challenging substrates. Biological suction discs are not only capable of generating suction, but often have the remarkable ability to form a strong seal against surfaces that are rough, wet, or fouled (Wainwright et al. 2013; Ditsche and Summers 2019; Palecek et al. 2021a).
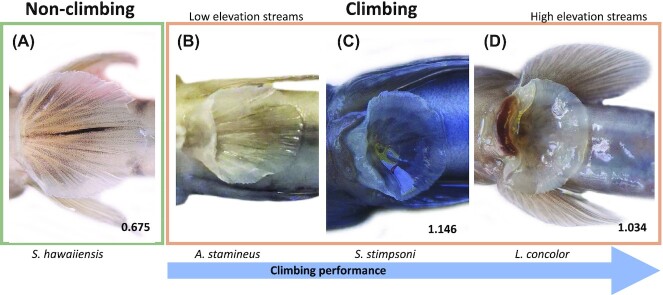
Human manufactured suction cups are typically circular, but biological suction cups can exhibit a range of shapes. Circular adhesive discs can be found in taxa like octopuses (Tramacere et al. 2013,2014), sea urchins (Santos and Flammang 2006), and sea stars (McCurley and Kier 1995), whereas oval discs are used by lineages such as bats (Riskin and Fenton 2001; Schliemann and Goodman 2011), and remoras (Beckert et al. 2015; Gamel et al. 2019; Su et al. 2020). Suction disc shapes also vary across the species of Hawaiian stream gobies (Taft et al. 2017). Nonclimbing Stenogobius possess an oval shaped disc, whereas discs are more circular among climbing species that reach higher elevations, with the most circular discs among the highest climbers, L. concolor (Fig. 1). Although both circular and oval shapes may be successful in nature, the prevalence of circular shapes among waterfall-climbing species may indicate that circular discs provide an advantage for generating ample suction and superior adhesive performance. In addition, variation in the internal pressure generated among discs with different morphologies could impact their ability to produce the pressure differentials responsible for adhesion. For example, if the internal volume of a suction cup is larger, it is possible that it may produce greater pull-off forces; however, if the greater volume also leads to a greater surface area contacting the substrate, then greater pull-off forces might be accompanied by lower tenacity (i.e., force normalized by contact area) due to the increased surface area. Previous studies on adhesive fish typically measure the length, width, and area of the suction disc, but volume is often more difficult to measure, so it is of interest to discover how this variable might affect adhesion.
Fig. 1.
Hawaiian waterfall climbing goby pelvic sucker shape diversity. (A) Stenogobious hawaiiensis. (B) Awaous stamineus. (C) Sicyopterus stimpsoni. (D) Lentipes concolor. Individuals from each species were CT scanned to obtain pelvic girdle morphology for 3D printing. Climbing performance is greatest in species found towards the right-hand side of the figure. Values in the lower right portion of each species panel indicate average width divided by average length values (n = 5 per species), with values closer to 1 indicating a more circular sucker. Some images modified from (Palecek et al. 2021a).
In addition to overall disc shape, in teleost fishes the internal skeletal elements of the pelvic fins might also enhance adhesive performance by providing a rigid frame that may prevent buckling of the cup walls, yet allow flexibility while being compressed against a substrate (Ditsche and Summers 2019). For example, shorter lepidotrichia (bony rays comprising the fin skeleton) with greater degrees of branching, like those found in better climbing species, may increase adhesive performance (Lundberg and Marsh 1976; Taft and Taft 2012; Taft et al. 2017). Thus, sucker models with an internal skeleton modeled after proficient climbers would be expected to outperform the pelvic girdles based on nonclimbing species, or species that are found at lower elevations.
In this study, we compare adhesive performance across physical models of goby suckers that vary in internal and external structural design to address two major questions. First, we test how overall disc shape affects adhesive performance. These data can improve understanding of how diversity in disc shape can be used to predict how adhesive discs are used in nature. Second, we test how the internal skeletal configurations of species that differ in climbing ability contribute to the adhesive performance of suction discs. These data can provide insight into how internal structure, independent of external shape, might contribute to successful adhesive performance in gobies and in biomimetic designs.
Materials and methods
We explored how internal skeletal pelvic girdle morphology, external soft tissue cup shape, and internal cup volume affected adhesive performance using 3D printed pelvic girdle skeletons embedded in silicone suction cups. To study the effect of these aspects of disc morphology, we used three different suction cup shapes—an oval cup, a low-volume circular cup, and a high-volume circular cup—to test effects of both shape and internal volume on adhesive performance. Examples of each shape were embedded with internal skeletons representing the morphology of each of the four Hawaiian goby taxa. We produced replicates of each configuration, as well as of each cup shape without embedded internal skeletons to serve as controls, allowing us to assess variation potentially related to our manufacturing or testing processes. Our sample ultimately included 42 cups from which performance data were successfully collected.
Specimen collection
Adult specimens from each of the four species (S. hawaiiensis, A. stamineus, S. stimpsoni, and L. concolor) were collected from their native streams in March 2020 on the Island of Hawai'i, using o'pae (prawn) nets (Fig. 1A–D). Stenogobius hawaiiensis and A. stamineus were collected from Waiakea Pond using nets attached to long-handled poles. Sicyopterus stimpsoni and L. concolor were collected from Hakalau and Nanue Streams while snorkeling. Specimens from the latter two species, therefore, were individuals that had successfully scaled a waterfall to reach adult breeding habitats. Consistent with prior studies, fish were maintained in aerated stream water with feeding rocks and housed at the Fisheries Research Station of the Hawai'i Division of Aquatic Resources in Hilo, Hawai'i, until euthanized and preserved. Collections were conducted under Hawai'i Special Activity Permit 2021–07, and all animal collection and care procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Clemson University (AUP 2017–085).
Micro-CT data processing and adhesive force data collection
A representative adult specimen from each species was selected (out of five total specimens scanned of each species) to generate skeletal reconstructions. Scans were performed on a Bruker SkyScan 1173 micro-CT scanner (Bruker Corp., Billerica, MA, USA) at a resolution of 24 μm (Fig. 2). Skeletal reconstructions of the pelvic girdles were segmented in 3D Slicer (Buser et al. 2020) and processed as files for 3D printing in Autodesk CAD software MeshMixer. Skeletal pelvic girdles included the basipterygium and all skeletal elements distal to it. Due to the light skeletal density of the finest rays toward the distal end of the fins, we were unable to distinguish the most distal portions of the fins. Skeletal pelvic girdle models were printed in a high-resolution, clear resin (Formlabs Inc., Somerville, MA, USA) at 50 μm resolution and embedded in a silicone suction cup (Ecoflex 00–30 silicone, Smooth-On, Inc., Macungie, PA, USA) in one of three shapes (Fig. 2). Skeletal girdles were printed to be embedded in larger suction cups (approximately 100x area) than equivalent live animal suction discs for ease of handling and to properly collect appropriately scaled forces on our available Instron mechanical testing system (Instron Corp., Norwood, MA, USA).
Fig. 2.
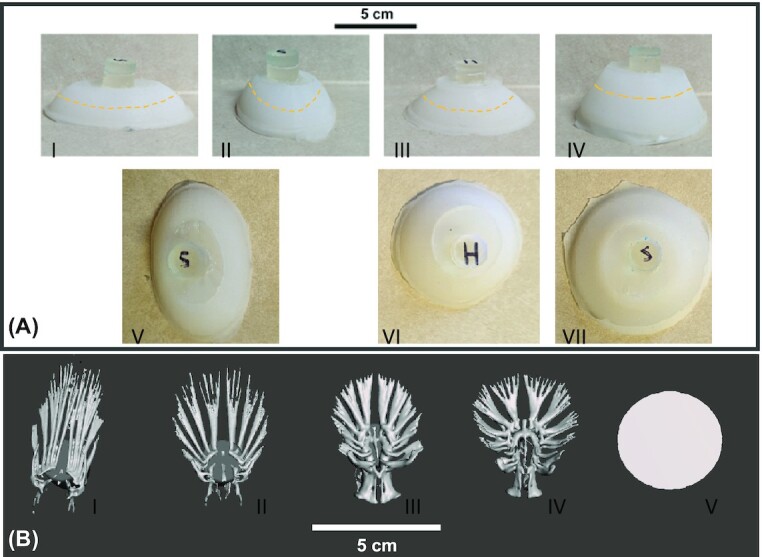
Biomimetic suction cup shapes and 3D pelvic girdles used in tenacity tests. (A) Silicone suction cups embedded with 3D pelvic skeletal girdles. Yellow dashed lines indicate approximate area that skeletal girdles end from within the cups. I: Long edge view of the oval suction cup. II: Short edge view of the oval suction cup. III: Low volume circular suction cup. IV: High volume circular suction cup. V: Top view of oval suction cup. VI: Top view of low volume circular suction cup. VII: Top view of high volume circular suction cup. (B) CT scans of pelvic skeletal girdles. I: Stenogobious hawaiianensis. II: Awaous stamineus. III: Sicyopterus stimpsoni. IV: Lentipes concolor. V: Control.
Models of pelvic girdle skeletons were embedded into the suction cups according to previously published methods (Palecek et al. 2021b; Huie and Summers 2022). 3D-printed pelvic girdle models were suspended within 3D-printed overmolds based on three suction cup shapes. A flexible silicone was poured into the overmolds and cured for four hours before being removed. The cup shapes included an oval cup (0.0054 m2 area while engaged, 0.0767 m3 volume) and circular cup (0.0050 m2 area while engaged, 0.0737 m3 volume) with similar inner volume values, and a high-volume circular cup (0.0060 m2 area while engaged, 0.1029 m3 volume). Surface area was similar between the circular suction cups while not engaged with a substrate, but we used the engaged surface area in our calculations of sucker performance (see below). Full details of suction cup sizing are provided in Table 1.
Table 1.
Size and shape measurements of biomimetic suction cup designs used in pull-off tests
| Shape | Area (m2) while adhered | Volume (m3) | Diameter (m) | Taper angle |
|---|---|---|---|---|
| Oval | 0.0054 | 0.0767 | 0.1027 (long edge) 0.0636 (short edge) | 49° (long edge) 63° (short edge) |
| Low volume circular | 0.0050 | 0.0737 | 0.7940 | 51° |
| High volume circular | 0.0060 | 0.1029 | 0.0885 | 66° |
For each of the four species-specific skeletal models, three examples of each of our three test shapes were produced (n = 12 per shape). However, one oval (modeled after Stenogobius) and one high-volume circular cup (modeled after Awaous) were ultimately excluded from analyses because their resin grips that connected to our testing apparatus fractured during testing. In addition to cups with embedded skeletons, suction cups without embedded skeletons were also produced to serve as controls that could give insight into how cup shape, independent of the supporting skeleton, could influence adhesion (n = 2 per shape, for a total of 8 control cups). Without rays extending into the cup walls, the configuration of the resin grip for control cups needed to be more robust than those for cups with embedded skeletons. This precluded formal comparisons of performance between control and skeleton-embedded cups, but still allowed comparisons of the impact of sucker shape on adhesion among the control specimens, independent of potential interactions with embedded skeletons.
Suction cups were tied to an Instron Model 5944 (Instron Corp., Norwood, MA, USA) for tensile testing on a 500 N load cell using zero stretch braided fishing line (Calamus Bastion store on Amazon, Seattle, WA, USA). Data were collected using Bluehill 2 Material Testing Software (Instron Corp., Norwood, MA, USA). Adhesive force of each suction cup was measured from the pull-off force at a constant speed (1 m/min) while stuck to smooth Plexiglas (Fig. 3). Each cup was tested once, and the maximum pull-off forces of the suction cups were used for analysis. To maintain consistency in preloading suction cups, cups were pressed so that the center of the suction cup touched the substrate.
Fig. 3.

Suction cup performance testing. Bioinspired suction cups were placed on a Plexiglass substrate and maximum pull-off forces were measured using an Instron Model 5944.
Statistical analysis
To account for differences in size, adhesive performance of each cup was normalized by calculating the tenacity of each pull. Tenacity values were generated by dividing the raw pull-off forces (measured in N) by the area of the suction cup while adhering. We used R version 4.0.5 (http://www.R-project.org/) to evaluate the impact of suction cup configuration (oval, low-volume circular, high-volume circular) and embedded pelvic girdle shape on adhesive performance. Model selection using linear models was conducted with Akaike's information criterion corrected for small sample sizes (AICc) through the R package “MuMIn” (v.1.43.17, https://CRAN.R-project.org/package = MuMIn). Modelling to evaluate the factors that best explained suction cup tenacity considered embedded skeletal structure, suction cup shape, and the interactive effects between these variables. Models were selected based on which model had the greatest weight (Table 3). We also calculated r2 values for our linear models to provide additional context for understanding factors that influenced tenacity. In our control suction cups, without an embedded skeleton, we measured the effects of suction cup shape on tenacity using a one way ANOVA. All R statistical analysis files are available in Supplementary Information.
Table 3.
Model selection results to determine the effects of suction cup shape and embedded species-inspired pelvic skeletons on suction cup tenacity.
| Model | df | AICc | Delta | Weight | P and adj R2 |
|---|---|---|---|---|---|
| Shape | 4 | 666.6 | 0.00 | 0.726 | 0.4171 |
| < 0.0001 | |||||
| Pelvic skeleton | 5 | 668.8 | 2.19 | 0.243 | 0.5253 |
| < 0.0001 | |||||
| Shape + Pelvic skeleton | 7 | 673.0 | 6.32 | 0.031 | 0.5603 |
| < 0.0001 | |||||
| Shape*Pelvic skeleton | 13 | 697.3 | 30.70 | 0.000 | 0.9464 |
| < 0.0001 |
Results
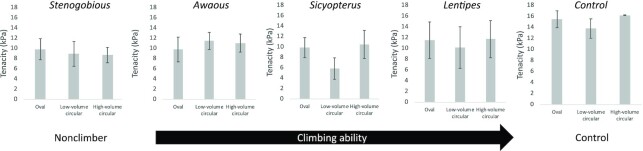
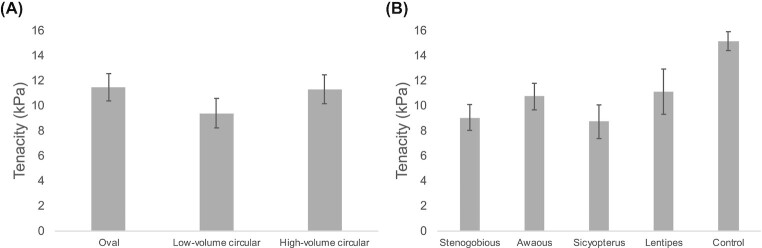
For almost all combinations of sucker shape and embedded skeleton, the average tenacity of each group of samples showed a modest range of variation, between 8 and 12 kPa (Figs. 4 and 5, Table 2). The only exception was the low-volume circular suckers embedded with Sicyopterus girdles (the closest replication of natural design in this species), for which average tenacity was just under 6 kPa. For cups with embedded biomimetic skeletal pelvic girdles, the model that provided the best explanation of variation in tenacity (i.e., lowest AICc) included only cup shape as an explanatory factor. Models that included pelvic skeleton design as the only factor provided poorer explanations of the variance in the data, and models that included both cup shape and embedded species-inspired pelvic skeletons, or the interaction between these variables, provided even worse explanations of variance in tenacity (Table 3). In this context, there was not consistent evidence that sucker designs closest to those found in a species (e.g., circular suckers in Sicyopterus) conveyed the best adhesive performance. Moreover, although shape by itself may have provided the best explanation of variance in tenacity, the AICc value for this model was still quite high (Table 3), indicating that the explanation it provided was weak. This point is reinforced by comparisons of tenacity in control suction cup models without embedded skeletons. Our ANOVA did not indicate a significant effect of suction cup shape on tenacity among control samples (P = 0.5083, F = 0.8552, and R2 < 0.0001), for which average values across the three shapes showed a range of only 2.4 kPa.
Fig. 4.
Average tenacity plotted across suction cup shapes by species. From left to right: average tenacity in oval, low volume circular, and high volume circular suction cups embedded with 3D printed S. hawaiiensis pelvic girdles, average tenacity in oval, low volume circular, and high volume circular suction cups embedded with 3D printed A. stamineus pelvic girdles, average tenacity in oval, low volume circular, and high volume circular suction cups embedded with 3D printed S. stimpsoni pelvic girdles, average tenacity in oval, low volume circular, and high volume circular suction cups embedded with 3D printed L. concolor pelvic girdles, and average tenacity in oval, low volume circular, and high volume circular suction cups embedded with 3D printed controls. Error bars are ± 1 S.E.M.
Fig. 5.
Variation in average tenacity for biomimetic suction cups depicted in Fig. 4, replotted to summarize variation across (A) the three suction cup shapes, and (B) cup designs embedded with four species-inspired skeletal pelvic girdles or the embedded control. Error bars are ± 1 S.E.M.
Table 2.
Average (± S.E.M.) surface area measurements and tenacity values from fish specimens and biomimetic suction cups.
| Fish sucker surface area (cm2) (Palecek et al. 2021a) | Oval cup surface area (cm2) | Low-volume circular cup surface area (cm2) | High-volume circular cup surface area (cm2) | Fish tenacity specimen on glass (Pa) (Palecek et al. 2021a) | Oval cup tenacity on Plexiglas (kPa) | Low-volume circular cup tenacity on Plexiglas (kPa) | High-volume circular cup tenacity on Plexiglas (kPa) | |
|---|---|---|---|---|---|---|---|---|
| Stenogobius hawaiiensisTL 55–70 mm 3D printdimensions: 55 x 35 mm | 0.6582 ± 0.0660 | 53.6 | 50.3 | 60.1 | 0 ± 0 | 9.8498 ± 2.9139 | 8.9343 ± 2.4428 | 8.6914 ± 1.5121 |
| Awaous stamineusTL 60–75 mm 3D printdimensions: 60 x 40 mm | 0.7762 ± 0.1044 | 53.6 | 50.3 | 60.1 | 0.0859 ± 0.04345 | 9.7918 ± 2.4295 | 11.4722 ± 1.6741 | 11.0551 ± 1.7763 |
| Sicyopterus stimpsoniTL 50–65 mm 3D printdimensions: 55 x 35 mm | 0.5304 ± 0.0348 | 53.6 | 50.3 | 60.1 | 0.4440 ± 0.1197 | 9.8673 ± 1.9239 | 5.8530 ± 2.0672 | 10.4692 ± 2.7112 |
| Lentipes concolorTL 60–70 mm 3D printdimensions: 50 x 40 mm | 0.5298 ± 0.0439 | 53.6 | 50.3 | 60.1 | 0.4264 ± 0.1685 | 11.5107 ± 3.4027 | 10.1635 ± 3.8561 | 11.7115 ± 3.4449 |
N = 3 for all suction cup groups except oval Stenogobius and high-volume Awaous (N = 2).
Discussion
Gobies show considerable variation in the structure of their pelvic skeletons (Maie et al. 2013; Taft et al. 2017). However, the impact of these structural differences on the adhesive performance of live fish remains uncertain after our trials with biomimetic suckers. The embedded skeletal designs that we employed, which were based on species-specific CT scans, were not clearly correlated with differences in sucker tenacity. It is possible that the impacts of different skeletal designs across goby taxa may be related primarily to changes in muscle attachment areas or leverage and, thus, are only likely to be observed through their effects on active suction mediated by muscle contraction (Maie et al. 2012, 2013; Schoenfuss et al. 2013; Maie and Blob 2021). A possible exception to this conclusion, potentially contributing to the explanatory weight that our statistical models attributed to skeletal design, is the generally lower tenacity found among suction cups embedded with skeletal girdles based on Sicyopterus compared to cups with girdles based on other taxa (Fig. 5B). It is noteworthy that this taxon is the only one of our models that was based on a species that climbs by “inching,” meaning that they alternate attachment to substrates between the pelvic sucker and an oral sucker (Schoenfuss and Blob 2003; Cullen et al. 2013). This might reduce the demands placed on pelvic sucker morphology in Sicyopterus, though pull-off tests showed the suckers of this species were generating high adhesive tenacity (Palecek et al. 2021a). Viewed in a different context, our results also indicate that the nonclimbing species, Stenogobius, possesses a pelvic girdle morphology that does not negatively affect the ability to adhere, with a design that produced tenacity values comparable to those of Sicyopterus in particular (Fig. 5B). Instead, some other factor may be contributing to the poor performance of Stenogobius suckers in live animals, such as the mechanical properties of the skeletal supports (Taft et al. 2017), the muscular structure within the pelvic sucker (Maie et al. 2013), or even differences in their epidermal morphology or mucus production. Even greater differences in skeletal structure than those across our samples might be necessary to affect passive adhesion performance; if so, then testing bioinspired suction cups that mimic more distantly related groups (i.e., clingfishes vs gobies) might provide better insight into the role of pelvic girdle shape on passive adhesion (Palecek et al. 2021b). Alternatively, it is also possible that the specific morphology of embedded support structures is of little importance to the adhesive performance of suction discs in fish. Previous studies have emphasized the importance of having some kind of rigid support to enhance suction cup tenacity (Ditsche and Summers 2019), but additional research appears necessary to clarify which sizes and shapes of rigid embedded support structure provide the best improvements in tenacity for suction discs.
With respect to external cup shape, high-volume cups did not trend towards greater tenacity values despite having greater average pull-off forces (Fig. 5A). This pattern likely results because tenacity depends on the area of a surface contacted by a cup; when pressed against a surface, the steeper walls that generated higher volumes for the circular cups that we tested also produced increases in the area contacting the substrate. Because increased forces were paralleled by increasing contact areas, tenacity changed little between low- and high-volume cups.
Contrary to our original hypothesis, for many species configurations oval shaped suction cups had slightly greater average tenacity values than circular suction cups of similar area and volume (Fig. 5A). Part of this trend may be due to differences in tapering angles (i.e., the angle between the cup wall and the substrate that the cup is pressed against) across the cups. In soft gripping structures, tapered grippers (based on octopus suckers) performed better than cylindrical grippers (Xie et al. 2020). While circular suction cups will have the same angles along the entirety of the cup, oval suction cups will likely have a gradation of angles when comparing from the center to the edge across the major and minor axes, with angles toward the edges of the major axis smaller than those toward the minor axis. Greater taper angles have been shown to produce greater forces, at the cost of the ability to bend to curved surfaces (Xie et al. 2020). Thus, differences in taper angle for oval shapes may promote increased tenacity.
Previous studies show performance differences in passive pull-off forces among Hawaiian waterfall climbing gobies (Palecek et al. 2021a), where better climbing species require higher pull-off forces to become dislodged. But if circular suckers do not have clearly higher pull-off forces than other shapes, then why do Hawaiian goby species with the best climbing ability (e.g., L. concolor) have the most circular suckers? It is possible that other factors not considered in our models play roles in the adhesive performance of live fish that complicate seemingly straightforward predictions. For example, mucus or surfactants on the sucker surface, tissue material properties specific to live fish (e.g., Young's modulus), and microscopic structures on the surface of the suction disc could contribute to friction or seal retention. Also, as noted earlier, muscle activation of the pelvic suction disc and behavioral modifications are also likely to affect adhesive performance, such that passive adhesion might not completely reflect the demonstrated abilities of live animals. It is also worth noting that our comparisons were only made in the context of pull-off forces applied in tension. Gobies in nature might be exposed to forces from a variety of directions (Palecek et al. 2021a), for which circular discs might provide advantageous versatility. Such versatility might be of greater importance among species that climb higher and are exposed to more intense flow. Adhesion must also be quickly reversed in live fish while climbing or moving from a fixed position, so a disc that is capable of these quick changes may be advantageous over one that is able to adhere strongly, but unable to quickly detach. Oval shaped suction cups, even if they had higher peak capacity than circular cups, could carry a cost if they are slower to unstick. Goby suckers already appear to have a high safety against failure, being able to produce suction forces >2.5x body mass (Maie and Blob 2021). The overbuilt nature of suction discs in all species may allow for sufficient adhesive performance while climbing or adhering to challenging surfaces. Moreover, live fish may rarely (if ever) use their maximum abilities in nature as this may risk costly damage to their tissues (Husak 2006). Thus, aspects of performance such as detachment speed may actually be more critical for living fish than maximum tenacity.
Some further qualifications of our biomimetic approach are important to acknowledge. Models derived from CT scans of specimens present some limitations, as the scans are unable to capture morphological configurations during live adhesion, and artifacts from drying in the CT scanner may cause the fins to move away from a neutral position. Additional limitations include the centering of the base on the skeletal girdle prints—centering had to be modified as the proportions of each species were different. Nonetheless, the variety of sucker configurations exhibited by fish provide a diverse range of models for bioinspired designs. Recognizing that goby suckers do not provide optimized performance highlights several important issues. As expressed through the principle of many-to-one mapping of structure to function, many structural designs can achieve comparable performance (Wainwright et al. 2005). In this context, although attention in comparisons of structural design often focuses on maximal performance, a wider range of alternative designs might be capable of achieving adequate performance, particularly when large margins of safety against failure are present (Maie et al. 2012; Blob et al. 2014). It is also possible that alternative aspects of performance besides those typically considered (e.g., detachment speed versus tenacity) may contribute to potential discrepancies between designs exhibited by natural systems and the designs implemented in engineered structures. For example, trade-offs have been noted in aspects of goby performance (e.g., escape from predation versus climbing) that could relate to such aspects of sucker design (Blob et al. 2010). Nonetheless, approaches such as bioinspired physical modeling have outstanding potential for generating insights for both biology and engineering, highlighting unresolved questions that require further examination. Through these efforts, we aim for a deepened understanding of the diverse underpinnings of organismal performance and its evolutionary transitions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the staff of the Hilo Division of Aquatic Resources Research Station (N. Ahu, L. Nishiura, T. Sakihara, T. Shimoda and T. Shindo) for facility access, assistance and hospitality, and T. Maie for assistance in catching fish in the field. CT scans were conducted at the Karel F. Liem Bioimaging Facility at Friday Harbor Laboratories, and 3D printing for model construction was supported by the Clemson Makerspace. We thank K. Diamond for access to CT specimen files and assistance with CT methods, K. Barrett and M. Novak for advice on statistical analyses, A. Summers and J. Huie for advice in suction cup construction and design (including the overmolding technique), G. Korneva for assistance with Instron data collection, and two anonymous reviewers for their suggestions to improve the manuscript.
Notes
From the symposium “Lesser known transitions: organismal form and function across abiotic gradients’’ presented at the annual meeting of the Society for Integrative and Comparative Biology virtual annual meeting, January 3–February 28, 2022.
Contributor Information
A M Palecek, Department of Biological Sciences, Clemson University, Clemson, SC 29634, USA.
H L Schoenfuss, Aquatic Toxicology Laboratory, Saint Cloud State University, Saint Cloud, MN 56301, USA.
R W Blob, Department of Biological Sciences, Clemson University, Clemson, SC 29634, USA.
Funding
This work was supported by, Clemson University Creative Inquiry [grant number 479] and Creative Inquiry support from the South Carolina Translational Research Improving Musculoskeletal Health Center. Physical model data presented in this manuscript were collected using SC BioCRAFT facilities supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health under award number P30 GM131959.
Conflicts of Interest
The authors declare no conflicts of interest.
Data availability statement
The data underlying this article are available in the online supplementary material.
References
- Aiello BR, King HM, Hale ME. 2014. Functional subdivision of fin protractor and retractor muscles underlies pelvic fin walking in the African lungfish Protopterus annectens. J Exp Biol. 217:3474–82. [DOI] [PubMed] [Google Scholar]
- Beckert M, Flammang BE, Nadler JH. 2015. Remora fish suction pad attachment is enhanced by spinule friction. J Exp Biol. 218:3551–8. [DOI] [PubMed] [Google Scholar]
- Bell MA, Orti G, Walker JA, Koenings JP. 1993. Evolution of pelvic reduction in threespine stickleback fish: a test of competing hypotheses. Evolution. 47:906–14. [DOI] [PubMed] [Google Scholar]
- Blob RW, Rai R, Julius ML, Schoenfuss HL. 2006. Functional diversity in extreme environments: effects of locomotor style and substrate texture on the waterfall-climbing performance of Hawaiian gobiid fishes. J Zool. 268:315–24. [Google Scholar]
- Blob RW, Bridges WC, Ptacek MB, Maie T, Cediel RA, Bertolas MM, Julius ML, Schoenfuss HL. 2008. Morphological selection in an extreme flow environment: body shape and waterfall-climbing success in the Hawaiian stream fish Sicyopterus stimpsoni. Integr Comp Biol. 48:734–49. [DOI] [PubMed] [Google Scholar]
- Blob RW, Kawano SM, Moody KN, Bridges WC, Maie T, Ptacek MB, Julius ML, Schoenfuss HL. 2010. Morphological selection and the evaluation of potential tradeoffs between escape from predators and the climbing of waterfalls in the Hawaiian stream goby Sicyopterus stimpsoni. Integr Comp Biol. 50:1185–99. [DOI] [PubMed] [Google Scholar]
- Blob RW, Espinoza NR, Butcher MT, Lee AH, D'Amico AR, Baig F, Sheffield KM. 2014. Diversity of limb-bone safety factors for locomotion in terrestrial vertebrates: evolution and mixed chains. Integr Comp Biol. 54:1058–71. [DOI] [PubMed] [Google Scholar]
- Budney LA, Hall BK. 2010. Comparative morphology and osteology of pelvic fin-derived midline suckers in lumpfishes, snailfishes and gobies. J Appl Ichthyol. 26:167–75. [Google Scholar]
- Buser TJ, Boyd OF, Donatelli CM, Kolmann MA, Luparell JL, Pfeiffenberger JA, Sidlauskas BL, Summers AP. 2020. The natural historian's guide to the CT galaxy: step-by-step instructions for preparing and analyzing computed tomographic (CT) data using cross-platform, open access software. Integr Org Biol. 2:doi: 10.1093/iob/obaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen JA, Maie T, Schoenfuss HL, Blob RW. 2013. Evolutionary novelty versus exaptation: oral kinematics in feeding versus climbing in the waterfall-climbing Hawaiian goby Sicyopterus stimpsoni. PLoS One. 8:e53274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsche P, Summers AP. 2014. Aquatic versus terrestrial attachment: water makes a difference. Beilstein J Nanotechnol. 5:2424–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsche P, Summers A. 2019. Learning from Northern clingfish (Gobiesox maeandricus): bioinspired suction cups attach to rough surfaces. Philos Trans R Soc B Biol Sci. 374:20190204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditsche P, Wainwright DK, Summers AP. 2014. Attachment to challenging substrates – fouling, roughness and limits of adhesion in the northern clingfish (Gobiesox maeandricus). J Exp Biol. 217:2548–54. [DOI] [PubMed] [Google Scholar]
- Ditsche P, Hicks M, Truong L, Linkem C, Summers A. 2017. From smooth to rough, from water to air: the intertidal habitat of Northern clingfish (Gobiesox maeandricus). Sci Nat. 104. doi: 10.1007/s00114-017-1454-8. [DOI] [PubMed] [Google Scholar]
- Flammang BE, Suvarnaraksha A, Markiewicz J, Soares D. 2016. Tetrapod-like pelvic girdle in a walking cavefish. Sci Rep. 6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamel K, Garner A, Flammang BE. 2019. Bioinspired remora adhesive disc offers insight into evolution. Bioinspir Biomim. 14:056014. [DOI] [PubMed] [Google Scholar]
- Goto T, Nishida K, Nakaya K. 1999. Internal morphology and function of paired fins in the epaulette shark, Hemiscyllium ocellatum. Ichthyol Res. 46:281–7. [Google Scholar]
- Greenfield DW, Randall JE. 2004. The marine gobies of the Hawaiian Islands. Proc Calif Acad Sci. 55:498–549. [Google Scholar]
- Griner J, Palecek A, Diamond K, Schoenfuss H, Blob R. 2021. Geometric morphometrics of climbing kinematics in waterfall climbing goby fishes. Proceedings of the Society for Integrative and Comparative Biology, Washington, DC. (https://sicb.org/abstracts/geometric-morphometrics-of-climbing-kinematics-in-waterfall-climbing-goby-fishes/). [Google Scholar]
- Huie JM, Summers AP. 2022. The effects of soft and rough substrates on suction-based adhesion. J Exp Biol. 225:doi: 10.1242/jeb.243773. [DOI] [PubMed] [Google Scholar]
- Husak JF. 2006. Does survival depend on how fast you can run or how fast you do run?. Funct Ecol. 20:1080–6. [Google Scholar]
- Kier WM, Smith AM. 1990. The morphology and mechanics of octopus suckers. Biol Bull. 178:126–36. [DOI] [PubMed] [Google Scholar]
- King HM, Hale ME. 2014. Musculoskeletal morphology of the pelvis and pelvic fins in the lungfish Protopterus annectens. J Morphol. 275:431–41. [DOI] [PubMed] [Google Scholar]
- King HM, Shubin NH, Coates MI, Hale ME. 2011. Behavioral evidence for the evolution of walking and bounding before terrestriality in sarcopterygian fishes. Proc Natl Acad Sci. 108:21146–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucifora LO, Vassallo AI. 2002. Walking in skates (Chondrichthyes, Rajidae): anatomy, behaviour and analogies to tetrapod locomotion. Biol J Linn Soc. 77:35–41. [Google Scholar]
- Lundberg JG, Marsh E. 1976. Evolution and functional anatomy of the pectoral fin rays in Cyprinoid fishes, with emphasis on the suckers (Family Catostomidae). Am Midl Nat. 96:332–49. [Google Scholar]
- Macesic LJ, Kajiura SM. 2010. Comparative punting kinematics and pelvic fin musculature of benthic batoids. J Morphol. 271:1219–28. [DOI] [PubMed] [Google Scholar]
- Macesic LJ, Mulvaney D, Blevins EL. 2013. Synchronized swimming: coordination of pelvic and pectoral fins during augmented punting by the freshwater stingray Potamotrygon orbignyi. Zoology. 116:144–50. [DOI] [PubMed] [Google Scholar]
- Maie T, Blob RW. 2021. Adhesive force and endurance of the pelvic sucker across different modes of waterfall-climbing in gobiid fishes: contrasting climbing mechanisms share aspects of ontogenetic change. Zoology. 149:125969. [DOI] [PubMed] [Google Scholar]
- Maie T, Meister AB, Leonard GL, Schrank GD, Blob RW, Schoenfuss HL. 2011. Jaw muscle fiber type distribution in Hawaiian gobioid stream fishes: histochemical correlations with feeding ecology and behavior. Zoology. 114:340–7. [DOI] [PubMed] [Google Scholar]
- Maie T, Schoenfuss HL, Blob RW. 2012. Performance and scaling of a novel locomotor structure: adhesive capacity of climbing gobiid fishes. J Exp Biol. 215:3925–36. [DOI] [PubMed] [Google Scholar]
- Maie T, Schoenfuss HL, Blob RW. 2013. Musculoskeletal determinants of pelvic sucker function in hawaiian stream gobiid fishes: interspecific comparisons and allometric scaling. J Morphol. 274:733–42. [DOI] [PubMed] [Google Scholar]
- McCurley RS, Kier WM. 1995. The functional morphology of starfish tube feet: the role of a crossed-fiber helical array in movement. Biol Bull. 188:197–209. [DOI] [PubMed] [Google Scholar]
- Nishimoto RT, Fitzsimons JM. 1999. Behavioral determinants of the instream distribution of native Hawaiian stream fishes. In: Seret B, Sire JY, editors. Proceedings of the 5th Indo-Pacific Fish Conference, Noume´a. Paris: Societe Francaise d’Ichtyologie. p. 813–8.. [Google Scholar]
- Palecek AM, Schoenfuss HL, Blob RW. 2021a. Sticking to it: testing passive pull-off forces in waterfall-climbing fishes across challenging substrates. J Exp Biol. 224:jeb.228718. [DOI] [PubMed] [Google Scholar]
- Palecek A, Huie J, Cohen K, Donatelli C, Summers A. 2021b. Stuck on you: How pelvic girdle morphology influences adhesion. Proceedings of the Society for Integrative and Comparative Biology, Washington, DC. (https://sicb.burkclients.com/meetings/2021/schedule/abstractdetails.php?id=259). [Google Scholar]
- Pridmore AP. 1994. Submerged walking in the epaulette shark Hemiscyllium ocellatum (Hemiscyllidae) and its implications for locomotion in rhipidistian fishes and early tetrapods. Zoology. 98:278–97. [Google Scholar]
- Riskin DK, Fenton MB. 2001. Sticking ability in Spix's disk-winged bat, Thyroptera tricolor (Microchiroptera: Thyropteridae). Can J Zool. 79:2261–7. [Google Scholar]
- Sandoval JA, Jadhav S, Quan H, Deheyn DD, Tolley MT. 2019. Reversible adhesion to rough surfaces both in and out of water, inspired by the clingfish suction disc. Bioinspir Biomim. 14: 066016. [DOI] [PubMed] [Google Scholar]
- Santos R, Flammang P. 2006. Morphology and tenacity of the tube foot disc of three common European sea urchin species: a comparative study. Biofouling. 22:173–86. [DOI] [PubMed] [Google Scholar]
- Schliemann H, Goodman SM. 2011. A new study on the structure and function of the adhesive organs of the old world sucker-footed bat (Myzopoda: Myzopodidae) of Madagascar. Verhandlungen des Naturwissenschaftlichen Vereins Hambg. 46:313–30. [Google Scholar]
- Schoenfuss HL, Blob RW. 2003. Kinematics of waterfall climbing in Hawaiian freshwater fishes (Gobiidae): Vertical propulsion at the aquatic-terrestrial interface. J Zool. 261:191–205. [Google Scholar]
- Schoenfuss HL, Maie T, Moody KN, Lesteberg KE, Blob RW, Schoenfuss TC. 2013. Stairway to heaven: evaluating levels of biological organization correlated with the successful ascent of natural waterfalls in the hawaiian stream goby Sicyopterus stimpsoni. PLoS One. 8:e84851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen EM. 2008. Pelvic fin locomotor function in fishes: three-dimensional kinematics in rainbow trout (Oncorhynchus mykiss). J Exp Biol. 211:2931–42. [DOI] [PubMed] [Google Scholar]
- Standen EM. 2010. Muscle activity and hydrodynamic function of pelvic fins in trout (Oncorhynchus mykiss). J Exp Biol. 213:831–41. [DOI] [PubMed] [Google Scholar]
- Su S, Wang S, Li L, Xie Z, Hao F, Xu J, Wang S, Guan J, Wen L. 2020. Vertical fibrous morphology and structure-function relationship in natural and biomimetic suction-based adhesion discs. Matter. 2:1207–21. [Google Scholar]
- Taft NK, Taft BN. 2012. Functional implications of morphological specializations among the pectoral fin rays of the benthic longhorn sculpin. J Exp Biol. 215:2703–10. [DOI] [PubMed] [Google Scholar]
- Taft NK, Taft BN, Henck H, Diamond KM, Schoenfuss HL, Blob RW. 2017. Comparative morphology and mechanical properties of the lepidotrichia of climbing and non-climbing Hawaiian gobioid fishes. Cybium. 41:107–15. [Google Scholar]
- Thacker CE, Roje DM. 2011. Phylogeny of Gobiidae and identification of gobiid lineages. Syst Biodivers. 9:329–47. [Google Scholar]
- Tramacere F, Beccai L, Kuba M, Gozzi A, Bifone A, Mazzolai B. 2013. The morphology and adhesion mechanism of Octopus vulgaris suckers. PLoS One. 8:e65074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramacere F, Kovalev A, Kleinteich T, Gorb SN, Mazzolai B. 2014. Structure and mechanical properties of Octopus vulgaris suckers. J R Soc, Interface. 11:20130816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainwright PC, Alfaro ME, Bolnick DI, Hulsey CD. 2005. Many-to-one mapping of form to function: a general principle in organismal design?. Integr Comp Biol. 45:256–62. [DOI] [PubMed] [Google Scholar]
- Wainwright DK, Kleinteich T, Kleinteich A, Gorb SN, Summers AP. 2013. Stick tight: suction adhesion on irregular surfaces in the Northern clingfish. Biol Lett. 9:20130234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ji C, Wang W, Zou J, Yang H, Pan M. 2019. An adhesive locomotion model for the rock-climbing fish, Beaufortia kweichowensis. Sci Rep. 9:16571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Domel AG, An N, Green C, Gong Z, Wang T, Knubben EM, Weaver JC, Bertoldi K, Wen L. 2020. Octopus arm-inspired tapered soft actuators with suckers for improved grasping. Soft Robotics. 7:639–48. [DOI] [PubMed] [Google Scholar]
- Yamanoue Y, Setiamarga DHE, Matsuura K. 2010. Pelvic fins in teleosts: structure, function and evolution. J Fish Biol. 77:1173–208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the online supplementary material.