Abstract
Background
The efficacy of exercise interventions in the treatment of mental health disorders is well known, but research is lacking on the most efficient exercise type for specific mental health disorders.
Objective
The present study aimed to compare and rank the effectiveness of various exercise types in the treatment of mental health disorders.
Methods
The PubMed, Web of Science, PsycINFO, SPORTDiscus, CINAHL databases, and the Cochrane Central Register of Controlled Trials as well as Google Scholar were searched up to December 2021. We performed pairwise and network meta-analyses as well as meta-regression analyses for mental health disorders in general and each type of mental health disorder, with alterations in symptom severity as the primary outcome.
Results
A total of 6456 participants from 117 randomized controlled trials were surveyed. The multimodal exercise (71%) had the highest probability of being the most efficient exercise for relieving depressive symptoms. While resistance exercise (60%) was more likely to be the most effective treatment for anxiety disorder, patients with post-traumatic stress disorder (PTSD) benefited more from mind–body exercise (52%). Furthermore, resistance exercise (31%) and multimodal exercise (37%) had more beneficial effects in the treatment of the positive and negative symptoms of schizophrenia, respectively. The length of intervention and exercise frequency independently moderated the effects of mind–body exercise on depressive (coefficient = 0.14, p = .03) and negative schizophrenia (coefficient = 0.96, p = .04) symptoms.
Conclusion
Multimodal exercise ranked best for treating depressive and negative schizophrenic symptoms, while resistance exercise seemed to be more beneficial for those with anxiety-related and positive schizophrenic symptoms. Mind–body exercise was recommended as the most promising exercise type in the treatment of PTSD. However, the findings should be treated with caution due to potential risk of bias in at least one dimension of assessment and low-to-moderate certainty of evidence.
Trial Registration This systematic review was registered in the PROSPERO international prospective register of systematic reviews (CRD42022310237).
Supplementary Information
The online version contains supplementary material available at 10.1186/s40798-022-00529-5.
Keywords: Depression, Anxiety disorder, Post-traumatic stress disorder, Schizophrenia, Exercise, Training
Key Points
The multimodal exercise had the highest probability of being the most effective exercise for relieving depression and negative symptoms of schizophrenia.
The resistance exercise had more benefits in the treatment of anxiety disorder and positive symptoms of schizophrenia.
The mind–body exercise was most likely to be the most efficient exercise for relieving post-traumatic stress syndrome.
Introduction
Mental health disorder (also termed mental disorder, mental illness, or psychiatric disorder) refers to a syndrome generated by clinical disturbances in cognition, emotion regulation, thinking, and behavior [1]. Prevalent mental health disorders include depression, anxiety disorder, post-traumatic stress disorder (PTSD), and schizophrenia [2, 3]. Approximately 29% of the population may suffer from at least one type of mental health disorder during their lifetime, and nearly half of the people with severe mental health disorders experience complications (e.g., cardiorespiratory diseases, stomatognathic diseases, and obesity-related cancers) [4], shortened life expectancy of 10–20 years, and excess mortality rates [5]. The substantial social and economic costs of mental health disorders pose significant burdens on individuals, families, and society [6]. During the coronavirus disease 2019 pandemic, lockdowns and socioeconomic stress not only led to less provision of medical care for psychiatric patients but also increased the morbidity of mental health disorders [7–9], which dealt an unprecedentedly severe blow to the global health care system [10–12]. Economic, practical, and effective treatment of mental health disorders is urgently needed.
Due to the uneven distribution of resources, side effects, and low patient compliance, currently pharmacological therapies lack stability and significant efficacy [13]. By contrast, evidence that non-pharmacological approaches (including exercise intervention) can improve mental health is extensive and growing [14]. According to the literature, exercise interventions benefit patients with mental health disorders through physiological, immune, neurobiological, and psycho-behavioral mechanisms [15, 16]. Exercise improves mental health via various pathways, for example, by increasing endocannabinoid levels [17], up-regulating mitochondrial numbers and related oxygenation capabilities [18], enhancing the mammalian target of rapamycin signaling [19], and improving the hypothalamic pituitary-adrenal axis function [20]. From an immune system perspective, exercise programs improve psychiatric symptoms principally by lowering inflammation, for example, by systemically increasing cytokines [21], decreasing visceral fat mass [22], down-regulating toll-like receptors [23], and increasing vagal tone [24]. Neurobiologically, exercise interventions promote neurogenesis, angiogenesis, synaptic plasticity, and cerebrovascular function, each of which contributes to enhanced connectivity within large-scale brain networks [16]. Psycho-behaviorally, exercise distracts patients with mental health disorders from negative moods and boosts their self-esteem through self-efficacy and mastery [25]. Notably, different types of exercise interventions generate mental health benefits through the above mechanisms disproportionately and may have different effects on the same mental health disorder [15, 16]. Moreover, mental health disorders with distinct pathologies and pathogenesis respond to the same type of exercise program in different ways [15, 16]. However, studies comparing the simultaneous mental health benefits of various exercises are lacking, and traditional pairwise meta-analyses cannot pool evidence from multiple interventions. Thus, the most efficient type of exercise for a particular mental health disorder has not been established, and this hinders clinical decision-making.
Network meta-analysis, a promising synthesis methodology in the field of sports science and health promotion, outperforms conventional pairwise meta-analysis by integrating and quantifying both direct and indirect evidence, estimating the relative effectiveness of multiple interventions simultaneously, and ranking treatments as part of the process of clinical decision-making [26]. Thus, we evaluated and compared the effectiveness of various exercise types in the improvement of primary mental health symptoms among populations diagnosed with mental health disorders (i.e., depression, anxiety disorder, PTSD, and schizophrenia) via network meta-analysis of relevant randomized controlled trials (RCTs). We also examined the moderating role of other exercise characteristics (frequency, intensity, session duration, and the length of intervention) in the association between exercise intervention and symptom severity.
Methods
We registered our systematic review and network meta-analysis with PROSPERO (CRD42022310237) and used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Network Meta-Analyses (PRISMA-NMA) checklist (Additional file 2: Appendix S1) [27].
Searching Strategy
The PubMed, Web of Science, PsycINFO (via EBSCOhost), SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases; the Cochrane Central Register of Controlled Trials (CENTRAL); and Google Scholar were searched online for the period up until December 2021. The search strategy was reviewed by experts from the fields of mental health and sports science (Additional file 3: Appendix S2). The reference lists of relevant systematic reviews published in the last three years were also cross-checked.
Study Selection and Eligibility Criteria
Duplicated references were removed using the deduplication function of EndNote 20 software (Clarivate Analytics, Philadelphia, PA, USA) and manually. Two investigators (QY and KW) independently screened titles and abstracts to identify all relevant studies and then assessed the full texts according to the pre-set criteria. Disagreements were resolved through discussion or consultation with a third expert (ZK).
To evaluate and compare the effectiveness of various exercise types in the improvement of primary mental health symptoms among patients with mental health disorders (i.e., depression, anxiety disorder, PTSD, and schizophrenia), the inclusion criteria were as follows: (1) participants: full diagnosis of depression, anxiety disorder, PTSD, or schizophrenia by professionals according to accepted criteria (i.e., Diagnostic and Statistical Manual of Mental Disorders [DSM; 4th and 5th eds.] and International Statistical Classification of Diseases and Relating Health Problems [ICD; 9th and 10th eds.]); (2) interventions: any type of exercise; (3) comparator: usual care, health education, or other type of exercise not applied in the experimental group; (4) outcomes: mental health symptom severity assessed by clinical assessment scales (i.e., depressive, anxiety, post-traumatic stress, and overall, positive, and negative schizophrenia symptoms); and (5) study design: RCTs comparing an exercise intervention with either a non-exercise intervention or other forms of exercise intervention, with a total sample size of more than 20 participants to reduce the risk of publication bias (Inheriting most methodological practices of pairwise meta-analysis, the network meta-analysis has greater complexity due to multiple comparisons [28].) The effective sample size refers to the number of participants that could provide the same degree and evidence strength in both direct and indirect comparisons [28, 29]. Considering the direct comparison, the indirect comparison, the interactions between direct and indirect comparisons, the study type and the characteristics of research topic, we followed the practice of Owen et al. [28–30]. All studies had to have been published in English in peer-reviewed journals. If multiple studies were determined to have used data from the same cohort, the study with the longest follow-up was included. When the studies had the same follow-up period, the one with the largest sample size was included.
To ensure that the included studies were homogeneous for statistical comparisons, the exclusion criteria were as follows: (1) participants diagnosed with more than one of the mental health disorders referred to in the inclusion criteria; (2) participants diagnosed with other type of physical or mental diseases (i.e., cancer, diabetes, hypertension, infection, osteoporosis, obsessive–compulsive disorder, autism, and mild cognitive impairment); (3) participants who had adopted an intervention (e.g., cognitive-behavior therapy, music therapy) other than exercise and daily medication; and (4) the study examined the effects of acute exercise.
Data Extraction
Two investigators (OL and JN) independently extracted the data from a self-designed statistical form. They enlisted the aid of a third investigator (ZK) when needed. The statistical form included study characteristics (author’s name and publication year); participant characteristics (disorder type, sample size and age); intervention (exercise type, frequency, intensity, session duration, length of intervention, and comparator information); and outcomes (the relevant pre-intervention and post-intervention statistics or post–pre-changed values for estimating effect size, and assessment tools). When the relevant statistics were not reported in the original article, the mean and standard deviation were estimated based on the sample size, median, range (the minimum and maximum values), and interquartile range [31, 32]. The ImageJ processing program [33] (V.1.50i, https://imagej.nih.gov/ij/) was used to calculate pixel value statistics of defined selections and to extract numerical data from figures in six of the studies [34–39]. We contacted the authors of five of the studies [40–44] via email and the ResearchGate network to request data twice a month; two responded positively [43, 44].
The DSMs and the ICDs were referred to for diagnosis, classification, and evaluation as part of the process of collecting data. If multiple assessment tools were applied in one study, the outcome from the most frequently used scale among all the studies was reported. The Physical Activity Guidelines for Americans [45] and previous systematic reviews [46, 47] for the classification of exercise interventions were referred to for information about exercise classification. We divided exercise interventions into the following broad categories [30, 46, 47] to compare their effects: (1) aerobic exercise (AE), which is used to improve cardiorespiratory fitness through walking, jogging, running, and cycling; (2) resistance exercise (RE), which is used to increase muscle strength and endurance through weight machines and resistance bands; (3) mind–body exercise (MBE), which is used to focus inwardly through, for instance, Tai Chi, yoga, Yijinjing, and dance [48]; (4) stretching (a static or dynamic exercise that increases muscle control, flexibility, and range of motion); (5) multimodal exercise (ME), wherein at least two types of exercise, such as AE and RE, are combined; and (6) other types of exercise (i.e., an exercise intervention that could not be placed into the above categories), for example, balance training or high-intensity interval exercise [HIIE].
Risk of Bias Assessment and GRADE
The risk of bias (RoB) for all studies was independently evaluated by two investigators (QS and LZ) using the Cochrane Collaboration’s risk-of-bias tool (RoB version 2.0) [49]. Bias arising from randomization; deviations from intended interventions; missing outcome data; outcome measurement; and selected reports were assessed. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of the evidence [50]. The GRADE certainty was rated down one (serious concern) or two grades (very serious concern) for reasons including risk of bias, inconsistency, indirectness, imprecision, and publication bias [50]. The effect estimate with sufficient, moderate, limited, or very little confidence was rated as high, moderate, low, and very low certainty, respectively [50]. Similarities between assessments of RoB and certainty of evidence between the two investigators were calculated, and the third investigator (ZK) was consulted in the event of disagreement.
Data Synthesis and Analysis
Pairwise meta-analyses were conducted to compare the effect of one exercise type with another or the control condition if there was a minimum of two studies [51]. Effect size was estimated using standardized mean difference (SMD) and 95% confidence interval (95% CI) using post–pre-mean change and standard deviation (SD) difference by the random effects model. Effect size was considered small (SMD < 0.40); moderate (SMD = 0.40–0.70); or large (SMD > 0.70), in accordance with the Cochrane handbook [49]. Heterogeneity, as assessed by the I2 statistic, was classified as very low (I2 < 25%); low (I2 = 25–50%); moderate (I2 = 50–75%); or high (I2 ≥ 75%).
Bayesian network analysis was conducted using Aggregate Data Drug Information System software (ADDIS version 1.16.8, Drugis, Groningen, NL); the Markov chain Monte Carlo (MCMC) methods were used to estimate the models [52]. When the consistency assessed by node-splitting analysis was shown between direct and indirect comparisons (p > 0.05), the random effects and consistency model were implemented based on the following parameters: (1) 4 chains; (2) 20,000 tuning iterations; (3) 50,000 simulation iterations; (4) thinning interval of 10; (5) 10,000 inference samples; and (6) a variance scaling factor of 2.5 [53]. The rank probabilities were then calculated for the treatment effectiveness of each intervention type to provide the basis of alternative selection [52]. The Brooks–Gelman–Rubin diagnosis was used to estimate convergence, with the potential scale reduction factor close to 1 indicating approximate convergence [54]. When there was inconsistency between direct and indirect comparisons, the inconsistency model was used [53]. For the open-loop comparisons, the node-splitting analysis was not applicable as there was no direct evidence [52]. A 95% credible interval (CrI) was allowed for probability distribution. A network diagram was generated according to the network meta-analysis, where each node referred to one intervention type (i.e., AE, RE, MBE, stretching, ME, others, and the control) and the line referred to studies in which interventions were compared directly [55]. Node size was weighted by the number of participants receiving the specific intervention, while the line’s thickness was weighted by the number of RCTs [55]. In addition, the outcomes of network meta-analyses were retested by WinBUGS [56] (version 1.4, Medical Research Council, Imperial College of Science, Technology and Medicine, University of Cambridge, UK) and the relevant BUGS codes for network meta-analyses were provided (Additional file 4: Appendix S3).
We performed the analyses above both for the mental health disorders in general and each type of mental health disorder (i.e., subgroup analyses for depression, anxiety disorder, PTSD, and schizophrenia [overall, positive, and negative symptoms]), because the type of disorder may have influenced the intervention effects. To explore the causes of heterogeneity further, meta-regression analyses were conducted when there were more than 10 relevant studies, with participants’ age, exercise frequency, intensity, session duration, and length of interventions as covariates. Due to incomplete information and a limited number of studies, meta-regression analyses were conducted for aerobic and mind–body exercise for overall mental health disorders, depression, and negative schizophrenic symptoms, without consideration of exercise intensity.
Results
Study Selection and Characteristics
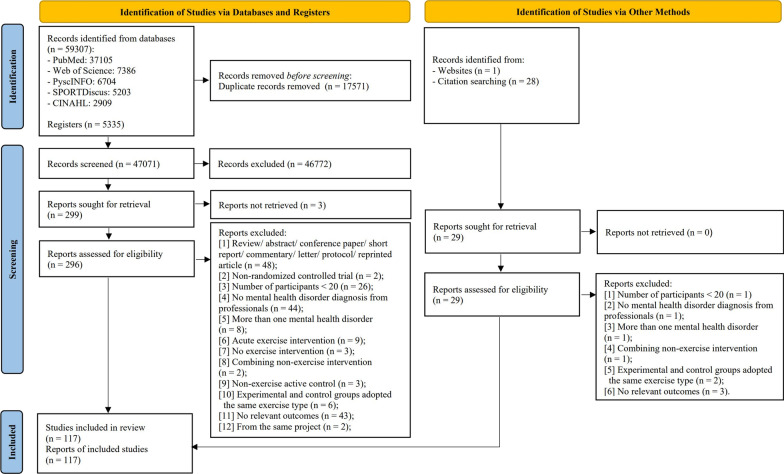
Possible studies for inclusion (n = 64,671) were identified in the databases (n = 59,307), the registers (n = 5335), and other sources (websites: n = 1; citation searching: n = 28) based on the requirements of the PRISMA 2020 flow diagram for new systematic review and meta-analysis. The search and selection process is shown in Fig. 1. A total of 6456 participants were finally included from 117 RCTs (depression: n = 70 [34–39, 57–120]; anxiety disorder: n = 5 [121–125]; PTSD: n = 11 [43, 126–135]; and schizophrenia: n = 31 [136–166] [overall symptoms: n = 16; positive symptoms: n = 26; negative symptoms: n = 28]) published between 1987 and 2021 (Additional files 5 and 6: Appendixes S4 and S5). The sample size of RCTs ranged from 20 to 244 participants. The age of participants ranged between 19 and 76 years old. Fifty-six studies (1510 participants) examined the effects of AE [34, 36–38, 57–59, 61, 64, 66, 67, 69–72, 74–78, 85, 86, 88–96, 98, 101, 102, 106, 107, 109, 111, 112, 114, 118, 121, 122, 124, 136–138, 141, 147, 150, 155–158, 160, 164], while 9 studies (161 participants) evaluated RE [57, 60, 63, 70, 122, 125, 134, 149, 166], 49 studies (1530 participants) evaluated MBE [35, 43, 62, 73, 79–84, 87, 93, 97, 99, 100, 103–105, 110, 115–117, 119, 120, 123, 126–131, 136, 138, 139, 141–144, 146, 148, 151–154, 159, 161–163, 165], 9 studies (212 participants) for stretching [37, 66, 68, 78, 92, 109, 111, 155, 157], 10 studies (276 participants) evaluated ME [39, 65, 108, 113, 132, 133, 135, 140, 145, 151], 3 studies (71 participants) evaluated other exercise types [38, 102, 164], and 98 studies (2696 participants) evaluated control conditions [34–36, 39, 43, 58–65, 67–69, 71–77, 79–91, 94–101, 103–108, 110, 112–121, 123–135, 137, 139, 140, 142–150, 152–154, 156, 158–163, 165, 166].
Fig. 1.
Flow diagram for systematic reviews which included searches of databases, registers, and other sources [27]
Risk of Bias and GRADE
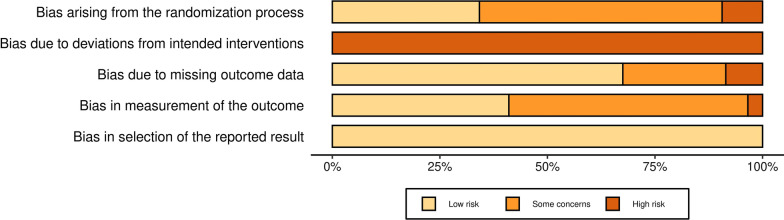
The outcome of the RoB assessment (Additional file 7: Appendix S6 and Fig. 2) was as follows: (1) bias from randomization (high risk: 9.40%, some concerns: 56.41%, low risk: 34.19%); (2) bias due to deviations from intended interventions (high risk: 100%); (3) bias due to missing outcome data (high risk: 8.55%, some concerns: 23.93%, low risk: 67.52%); (4) bias in measurement of outcome (high risk: 3.42%, some concerns: 55.56%, low risk: 41.03%); and (5) bias in selection of reported result (low risk: 100%). The certainty of evidence for outcomes ranged from very low-to-moderate levels (Additional file 8: Appendix S7). The similarities in RoB and GRADE assessments between the two investigators were 80.68% and 82.91%, respectively.
Fig. 2.
Risk of Bias Assessment. Notes: Domain 1: Bias from randomization process; Domain 2: Bias due to deviations from intended interventions; Domain 3: Bias due to missing outcome data; Domain 4: Bias in measurement of outcome; and Domain 5: Bias in selection of reported result
Treatment for Mental Health Disorders in General
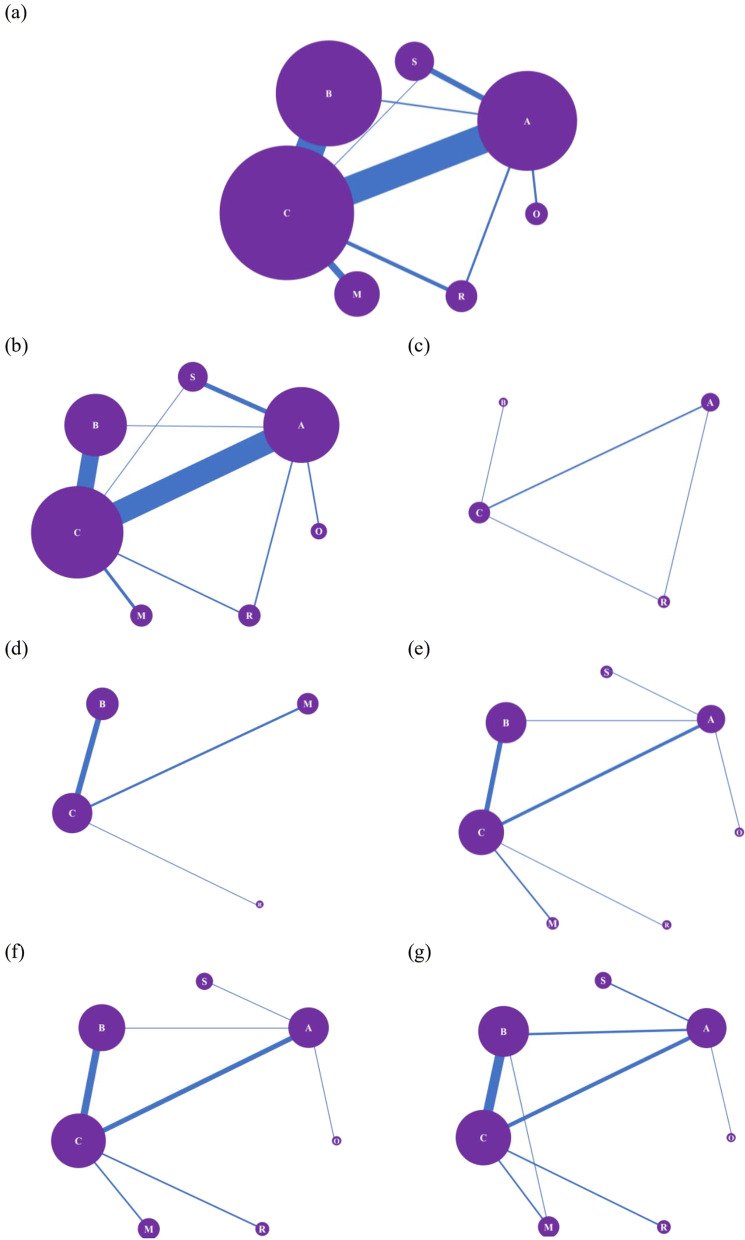
A total of 36 studies directly investigated the treatment effects on mental health symptoms of AE vs. control condition [34, 36, 58, 59, 61, 64, 67, 69, 71, 72, 74–77, 85, 86, 88–91, 94–96, 98, 101, 106, 107, 112, 114, 118, 121, 124, 137, 156, 158, 160], while 36 studies evaluated MBE vs. the control [35, 43, 62, 73, 79–84, 87, 97, 99, 100, 103–105, 110, 115–117, 119, 120, 123, 126–131, 142, 144, 152, 153, 161, 162], 5 studies evaluated RE vs. the control [60, 63, 125, 134, 149], 9 studies evaluated ME vs. the control [39, 65, 108, 113, 132, 133, 135, 140, 145], 1 study evaluated stretching vs. the control [68], 3 studies evaluated AE vs. RE [57, 70, 122], 2 studies evaluated AE vs. MBE [93, 141], 7 studies evaluated AE vs. stretching [37, 66, 78, 92, 109, 111, 157], and 3 studies evaluated AE vs. others [38, 102, 164] (Fig. 3a).
Fig. 3.
Network meta-analyses of eligible comparisons for a mental health disorders in general, b depression, c anxiety disorder, d post-traumatic stress disorder, e overall, f positive and g negative schizophrenic symptoms. The network diagram was generated according to network meta-analyses, where each node referred to intervention type and the line referred to studies in which interventions were compared directly. Node size was weighted by the number of participants receiving the specific intervention, while the line’s thickness was weighted by the number of RCTs. Abbreviations: A, aerobic exercise; R, resistance exercise; B, mind–body exercise; S, stretching; M, multimodal exercise; O, other types of exercise; and C, control
Pairwise meta-analyses revealed significant differences in the six comparisons of AE vs. the control (SMD, 0.91; 95% CI 0.48–1.34), MBE versus the control (SMD, 0.81; 95% CI 0.56, 1.05), RE versus the control (SMD, 1.72; 95% CI 0.49, 2.95), ME versus the control (SMD, 1.62; 95% CI 0.89, 2.36), AE versus RE (SMD, − 0.49; 95% CI − 0.82, − 0.16), and AE versus stretching (SMD, 0.55; 95% CI 0.11, 0.99) (Additional file 9: Appendix S8.1.1). The remaining two direct comparisons—AE versus MBE (SMD, − 0.14; 95% CI − 1.23, 0.94) and AE versus others (HIIE) (SMD, 0.55; 95% CI − 0.53, 1.63)—were not significantly different (Additional file 9: Appendix S8.1.1).
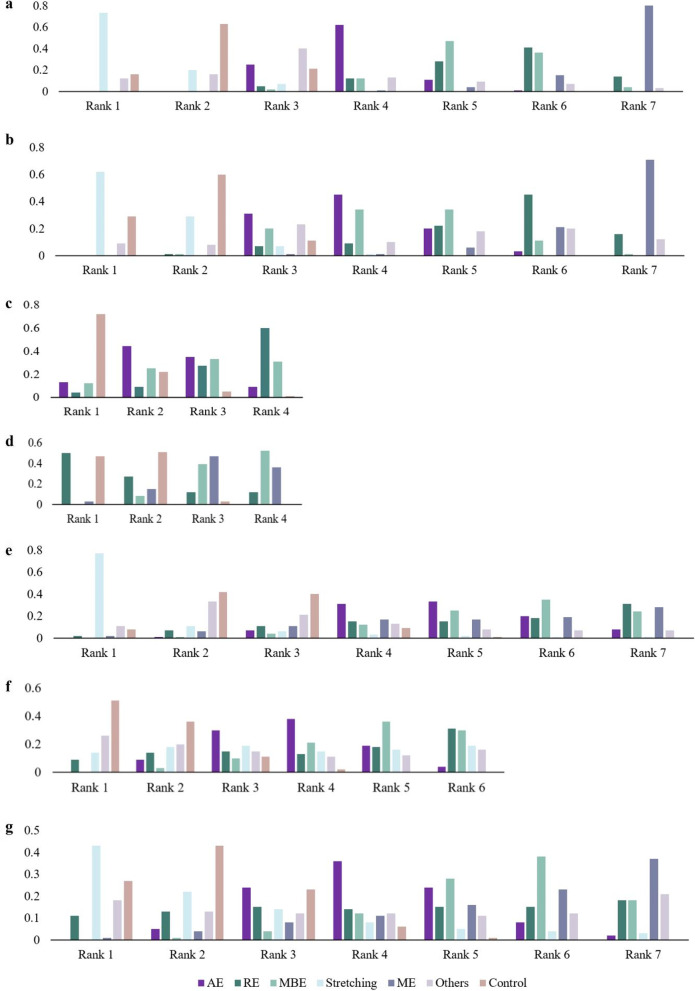
As node-splitting analysis revealed no significant inconsistency (all the p values were > 0.05), a network meta-analysis based on the consistency model was conducted (Additional file 9: Appendix S8.1.2). The pooled results from the network meta-analysis suggested that ME was superior to AE (SMD, − 4.14; 95% CrI − 7.59, − 0.77) and stretching was inferior to all the other exercise interventions (Additional file 9: Appendix S8.1.2). According to the rank probabilities (Fig. 4a and Additional file 9: Appendix S8.1.2), ME was most likely to rank best, followed by RE, MBE, AE, and stretching. Meta-regressions were conducted separately for AE and MBE, with participants’ age, exercise frequency, session duration, and length of the intervention as covariates (Additional file 9: Appendix S8.1.3). A meta-regression analysis of AE indicated that the length of intervention could be one of the moderators affecting treatment effects on mental health symptoms in general, whereas no covariate significantly influenced the efficacy of MBE (coefficient, 0.07; p = 0.04) (Additional file 9: Appendix S8.1.3).
Fig. 4.
Rank probabilities for treatment of a mental health disorders in general, b depression, c anxiety disorder, d post-traumatic stress disorder, e overall schizophrenic symptom, f positive schizophrenic symptom, and g negative schizophrenic symptom. Notes: Rank 1 is the worst, and rank last is the best. AE, aerobic exercise; RE, resistant exercise; MBE, mind–body exercise; and ME, multimodal exercise
Treatment for Depression
A total of 30 studies directly examined the efficacy on depressive symptoms of AE vs. control condition [34, 36, 58, 59, 61, 64, 67, 69, 71, 72, 74–77, 85, 86, 88–91, 94–96, 98, 101, 106, 107, 112, 114, 118], while 2 studies evaluated RE versus the control [60, 63], 22 studies evaluated MBE versus the control [35, 62, 73, 79–84, 87, 97, 99, 100, 103–105, 110, 115–117, 119, 120], 4 studies evaluated ME versus the control [39, 65, 108, 113], 1 study evaluated stretching versus the control [68], 2 studies evaluated AE versus RE [57, 70], 1 study evaluated AE versus MBE [93], 6 studies evaluated AE versus stretching [37, 66, 78, 92, 109, 111], and 2 studies evaluated AE versus others [38, 102] (Fig. 3b). Pairwise meta-analyses revealed significant differences in the following five comparisons: AE versus the control (SMD, 0.98; 95% CI 0.46–1.50), MBE vs. the control (SMD, 0.78; 95% CI 0.46, 1.11), ME vs. the control (SMD, 2.66; 95% CI 0.96, 4.37), AE versus. RE (SMD, − 0.56; 95% CI − 0.92, − 0.20), and AE versus stretching (SMD, 0.49; 95% CI 0.00, 0.99). There was no significant difference between AE versus other types of exercise (SMD, − 0.06; 95% CI − 0.43, 0.31) (Additional file 9: Appendix S8.2.1). A network meta-analysis based on the consistency model indicated that ME led to more improvement in depressive symptoms than AE (Additional file 9: Appendix S8.2.2). The rank probabilities of all exercise interventions in terms of depressive symptom improvement were generated as follows (from the best to the worst): ME, RE, MBE, AE, and stretching (Fig. 4b and Additional file 9: Appendix S8.2.2). The meta-regression for MBE suggested that the length of intervention positively moderated the effectiveness of MBE on depressive symptoms (coefficient, 0.14; p = 0.03) (Additional file 9: Appendix S8.2.3).
Treatment for Anxiety Disorder
Comparisons between the effects of different protocols on anxiety disorder [121–125] are shown in Fig. 3c. Both the pairwise analysis and network analysis based on the consistency model revealed no significant differences among AE, RE, MBE, and the control (Additional file 9: Appendix S8.3.1 and 8.3.2). The ranking of exercise interventions suggested that RE had the highest probability (60%) of being the most effective form of exercise in treating anxiety-related symptoms (Fig. 4c).
Treatment for Post‐traumatic Stress Disorder
For PTSD, a total of 11 studies with 576 participants were included [43, 126–135] (Fig. 3d). In the pairwise meta-analyses, MBE (SMD, 0.80; 95% CI 0.47, 1.13) and ME (SMD, 1.25; 95% CI 0.78, 1.72) were both directly compared to the control and had significant positive effects on post-traumatic stress symptoms (Additional file 9: Appendix S8.4.1). As the closed loop was not constructed in the network meta-analysis for PTSD, the node-splitting analysis was not applicable. Network meta-analysis based on the consistency model did not reveal any significant differences between RE, MBE, and ME (Additional file 9: Appendix S8.4.2). The ranking probability plot indicated that the MBE was most likely to rank best, followed by ME and RE (Fig. 4d).
Treatment for Schizophrenia
Among the 31 studies that targeted schizophrenia [136–166], 16 reported results associated with overall schizophrenic symptoms [137, 140–142, 144, 145, 149, 152, 153, 156–158, 160–162, 164], 26 associated with positive symptoms [136–138, 140–152, 155–158, 160, 162–166], and 28 associated with negative symptoms [136, 138–152, 154–160, 162–166]. The evidence structure of comparisons in overall, positive, and negative schizophrenic symptoms is provided in Fig. 3e–g. The pairwise meta-analyses for overall, positive, and negative schizophrenic symptoms are shown in Additional file 9: Appendix S8.5.1, S8.6.1, and S8.7.1. The results of the node-splitting analyses indicate inconsistencies between direct and indirect comparisons in the model for positive symptoms, but not in the models for overall and negative symptoms. After adjusting the model for positive symptoms by removing arms connected with ME, all network meta-analyses were conducted using the consistency model (Additional file 9: Appendix S8.5.2, S8.6.2, and S8.7.2). The ranking probability plots suggest that RE was most likely to rank best in the treatment of overall and positive symptoms, whereas ME had the highest probability to rank best in the case of negative symptoms (Fig. 4e–g). MBE ranked second in the treatment of overall, positive, and negative schizophrenia symptoms. For negative symptoms, meta-regression revealed that the effectiveness of MBE was moderated by exercise frequency (coefficient, 0.96; p = 0.04) (Additional file 9: Appendix S8.7.3).
Discussion
Network meta-analysis was conducted to evaluate and compare the effectiveness of various exercise interventions on mental health disorders (depression, anxiety disorder, PTSD, and schizophrenia). Pairwise and network analyses were conducted separately for overall, positive, and negative schizophrenia symptoms. The present review included 6456 participants from 117 RCTs published in the last 35 years. It is the first to examine the efficacy of distinct exercise types (AE, RE, MBE, ME, stretching, and others) in the treatment of four common mental health disorders. The findings demonstrate that the response to exercise interventions differed according to the condition: ME ranked best in the treatment of overall mental health, depressive, and negative schizophrenia symptoms; RE, anxiety-related and positive schizophrenia symptoms; and MBE, PTSD symptoms. The length of intervention positively moderated the effects of AE on overall mental health disorders and the effects of MBE on depression, while exercise frequency moderated the effects of MBE on negative schizophrenia symptoms.
Aerobic exercise, MBE, and ME all showed significant effects on depressive symptoms when compared with control conditions, with moderate-to-large effect sizes—all SMDs > 0.78. This suggested that an array of distinct exercise modalities may have clinically meaningful benefits in the treatment of depression and that clinicians working with depressive patients should consider exercise as complement to daily medication. According to the present network meta-analysis, ME had the highest probability of being the optimal exercise for improving depressive symptoms of all the interventions (i.e., AE, RE, MBE, ME, stretching, and other types of exercise [such as HIIE]), which went further than a previous network meta-analysis that examined the effects of AE, RE, and MBE on depression [167]. Hence, it is reasonable to infer that a combination of various exercise types was more likely to contribute to greater improvements in depression. Additionally, the outcome of the meta-regression indicated that the length of intervention (4–12 weeks) rather than exercise frequency and session duration positively moderated the effectiveness of MBE. This was partly consistent with previous meta-analyses in which the length of exercise was the only characteristic related to the effect size for depression treatment [168, 169], and where 9–12 weeks were associated with the largest reduction. Future researchers might retest these findings when studies with longer intervention periods are available; those involving extended MBE programs for depression (i.e., > 12 weeks) are currently lacking.
For anxiety disorder, direct and indirect comparisons between AE, RE, MBE, and controls did not show significant differences in treatment effects, but a reduction in anxiety-related symptoms could be observed in every case. Previous meta-analyses also demonstrated little to no benefits in exercise interventions for anxiety disorder [170, 171]. Based on the ranking probability plot, RE had the highest probability to be the most effective form of exercise, partly because it increases IGF-1 levels [172] and reduces inflammation [173] during training. Meanwhile, psychosocial factors such as improved self-efficacy [174], goal achievement [175], and mastery [176] also contribute to a relief in symptoms. It should be noted, however, that the above trials involved limited sample sizes, so the findings should be treated with caution.
Both pairwise and network meta-analyses suggested the MBE might ameliorate the symptoms of PTSD. Correspondingly, MBE was recommended as the most promising form of exercise based on the current ranking probability plot. In line with our findings, existing pairwise meta-analyses have demonstrated MBE to be an efficient adjuvant therapy in the improvement of PTSD-related symptoms, with effect sizes ranging from − 0.41 to − 0.39 [177, 178]. Subgroup analyses in a previous meta-analysis indicated that 60–150 min of MBE 1–3 times per week over 8–16 weeks resulted in benefits to those experiencing PTSD symptoms [178]. The possible psychophysiological mechanisms underlying the efficacy of MBE may include the down-regulation of inflammatory markers [179]; corticotropin-releasing hormone concentration [180]; the up-regulation of cortisol level [181]; and desensitization to inner arousal [182]. In addition, a closed loop was not generated in the present network meta-analysis for PTSD because distinct exercise types were not compared directly. Future researchers might evaluate and compare directly the efficacy of various exercise programs in the treatment of PTSD.
Schizophrenia is a psychiatric disorder characterized by positive symptoms of delusion, hallucination, and disorganized speech and by negative symptoms of blunted affect, alogia, anhedonia, asociality, avolition, among others [183]. For positive schizophrenic symptoms, RE was considered the preferred exercise intervention based on the present ranking probabilities. One explanation for this effect was that RE had more competitive advantages in attention control facilitation than other exercise interventions [184] because schizophrenia patients with positive symptoms have significant disturbances in sensory-motor gating, shifting, spatial focus, and cue detection [185]. According to the findings, ME outperformed the other exercise types for negative symptoms. Given the fact that schizophrenia patients tended to be more sedentary than healthy controls [186], this conclusion may encourage this population to engage in exercise by offering them multiple daily exercise program choices. It was also observed that MBE ranked the second most efficient exercise intervention for treating positive and negative symptoms, with exercise frequency moderating the effects on negative symptoms. Previous meta-analyses [187, 188] have shown that MBE, which is based on the exercise trinity (i.e., body, mind, and breathing), is a well-established complementary therapy for people with schizophrenia. In addition, the meditation nature of MBE alleviates psychotic symptoms by improving autonomic nerve function and reconstructing large-scale brain networks [189].
Future Recommendations for Reporting of Outcomes
Our Bayesian network meta-analysis has implications for further clinical practice and research in the field of mental health disorders. First, and in general, exercise interventions—regardless of type, frequency, or duration—help relieve psychiatric symptoms and should therefore be recommended as a routine complementary therapy for patients suffering from mental health disorders. Second, responses to intervention vary according to exercise type. While ME seems to be more suitable for patients with depression than other exercise interventions, those with anxiety disorder and PTSD might benefit more from RE and MBE, respectively. For schizophrenia, the selection of exercise program should be determined according to the specific symptoms: RE appears to be more efficacious in relieving positive symptoms, while ME is preferred for negative symptoms. Third, given the general effectiveness of MBE in curing mental health disorders, the combination of psychotherapeutic techniques (i.e., meditation and breathing) and exercise intervention should be further investigated. Fourth, the length of intervention positively moderates the efficacy of certain exercise interventions (i.e., AE and MBE). Thus, clinicians who prescribe exercise should take the interests of different populations and the practicability of regimens into consideration to encourage consistency. Fifth, multidimensional factors relating to exercise adherence should be explored further. Finally, the subsyndromal mental health symptoms (e.g., subsyndromal depression, subsyndromal generalized anxiety disorder, subsyndromal PTSD), failing to meet the full diagnostic criteria of mental health disorders, are broadly regarded as risk factors for developing clinically significant mental health disorders [190–192]. However, the population with subsyndromal mental health symptoms is underdiagnosed and undertreated globally, which results in public health hazards [190–192]. Further studies the mental health field are awaited to establish the recognized and unified diagnosis and evaluation criteria of subsyndromal mental health symptoms and further explore the potential therapeutic effects of exercise interventions.
Limitations
The present study has several limitations. First, some previous researchers have measured and described exercise intensity using multiple approaches, while others did not report exercise intensity numerically. Thus, exercise intensity has not been used as a moderator in the conduct of meta-regression analyses. As a result, the present study could not fully apply the frequency, intensity, type, and time (FITT) principle [193] and consider every exercise characteristic, which may have influenced the transitivity in the indirect comparisons. The standard for assessing the intensity of various exercise types awaits to be proposed. Second, for ethical and scientific reasons, the studies that were included in the present analysis allowed patients with psychiatric symptoms to take daily medications. Perhaps unsurprisingly, their regimens varied to a considerable degree. Hence, interactions between symptom, exercise, and medication were not explored in the present study. Third, a large proportion of the studies were evaluated with a high RoB in at least one dimension and low-to-moderate certainty of evidence. In light of these reservations, the outcomes of the present network meta-analysis should be interpreted with caution. Fourth, considering the publication bias and the effective sample size in multiple direct and indirect comparisons of network meta-analysis, the studies with a total sample size of less than 20 participants were not included in the current research [30]. The issue of effective sample size and power of network meta-analysis may be a hindrance to resolving uncertainty by synthetizing all available evidence and exploring causes of between-study heterogeneity [194]. Fifth, the protocol of this current systematic review and network meta-analysis was not published a prior, which may have resulted in publication bias and low reproducibility.
Conclusion
The results indicate the significance of exercise interventions as a complementary therapy in patients with mental health disorders. The selection of exercise program should be determined by disorder type. Multimodal exercise has the highest probability of being the most efficacious exercise for relieving depressive symptoms; RE for anxiety disorder; MBE for PTSD; and RE and ME for the positive and negative symptoms of schizophrenia, respectively. Finally, it was discovered that the length of intervention and exercise frequency positively moderated the effects of MBE on depressive and negative schizophrenia symptoms, respectively. The current findings should be treated with caution due to potential risk of bias in at least one dimension of assessment and low-to-moderate certainty of evidence.
Supplementary Information
Additional file 2: Appendix 1. PRISMA Network Meta-Analysis Checklist.
Additional file 3: Appendix 2. Searching Strategy.
Additional file 4: Appendix 3. WinBUGS Code for Network Meta-Analyses.
Additional file 5: Appendix 4. Included Studies List.
Additional file 6: Appendix 5. Basic Information of Included Studies.
Additional file 7: Appendix 6. Risk of Bias Assessment.
Additional file 8: Appendix 7. Grading of Recommendations, Assessment, Development and Evaluations (GRADE).
Additional file 9: Appendix 8. Outcomes of Pairwise Meta-Analysis, Network Meta-Analysis and Meta Regression.
Acknowledgements
Not applicable.
Abbreviations
- ADDIS
Aggregate Data Drug Information System Software
- AE
Aerobic exercise
- CI
Confidence interval
- CrI
Credible interval
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- HIIE
High-intensity interval exercise
- FITT
Frequency, intensity, type, and time
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- ICD
International Statistical Classification of Diseases and Relating Health Problems
- MBE
Mind–body exercise
- MCMC
Markov chain Monte Carlo
- ME
Multimodal exercise
- PRISMA-NMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses-Network Meta-Analyses
- PTSD
Post-traumatic stress disorder
- ROB
Risk of bias
- RCT
Randomized controlled trials
- RE
Resistance exercise
- SD
Standard deviation
- SMD
Standardized mean difference
Author contributions
QY and ZK conceptualized the review and criteria. QY, KW, and ZK completed the screening, whereas OL, JN, and ZK extracted the data. QS, LZ, and ZK evaluated the risk of bias for all studies. QY created the figures. ZK acquired the funding. QY, KW, OL, JN, QS, and LZ completed the draft. All authors contributed to the manuscript revision as well as read and approved the final version of the manuscript.
Funding
This work was supported with funding from the University of Macau (MYRG2017-00199-FED).
Availability of Data and Materials
All data and material reported in this systematic review are from peer-reviewed publications. The WinBUGS code used are available in Additional file 4: Appendix S3.
Declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing interests
Qian Yu, Ka-Kit Wong, On-Kei Lei, Jinlei Nie, Qingde Shi, Liye Zou and Zhaowei Kong declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Qian Yu, Email: yc17102@um.edu.mo.
Ka-Kit Wong, Email: yc17107@um.edu.mo.
On-Kei Lei, Email: yb97106@um.edu.mo.
Jinlei Nie, Email: jnie@ipm.edu.mo.
Qingde Shi, Email: qdshi@ipm.edu.mo.
Liye Zou, Email: liyezou123@gmail.com.
Zhaowei Kong, Email: zwkong@um.edu.mo.
References
- 1.Association AP. Diagnostic and statistical manual of mental disorders, 5th edn. 2013.
- 2.Organization WH. Mental Disorders. 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/mental-disorders.
- 3.Charlson F, van Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S. New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet. 2019;394(10194):240–248. doi: 10.1016/S0140-6736(19)30934-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dehert M, Correll CU, Bobes J, Cetkovich-Bakmas M, Cohen D, Asai I, et al. Physical illness in patients with severe mental disorders: I—prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77. doi: 10.1002/j.2051-5545.2011.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulland A. Action is urged to improve physical health in severe mental illness. BMJ. 2016;355:i5729. doi: 10.1136/bmj.i5729. [DOI] [PubMed] [Google Scholar]
- 6.Killaspy H, Harvey C, Brasier C, Brophy L, Ennals P, Fletcher J, et al. Community-based social interventions for people with severe mental illness: a systematic review and narrative synthesis of recent evidence. World Psychiatry. 2022;21(1):96–123. doi: 10.1002/wps.20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santomauro DF. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398(10312):1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Connor RC, Wetherall K, Cleare S, McClelland H, Melson AJ, Niedzwiedz CL, et al. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry. 2021;218(6):326–333. doi: 10.1192/bjp.2020.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong ASF, Pearson RM, Adams MJ, Northstone K, Tilling K, Smith D, et al. Mental health before and during the COVID-19 pandemic in two longitudinal UK population cohorts. Br J Psychiatry. 2021;218(6):334–343. doi: 10.1192/bjp.2020.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Druss BG. Addressing the COVID-19 pandemic in populations with serious mental illness. JAMA Psychiat. 2020;77(9):891–892. doi: 10.1001/jamapsychiatry.2020.0894. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Xu R, Volkow ND. Increased risk of COVID-19 infection and mortality in people with mental disorders: analysis from electronic health records in the United States. World Psychiatry. 2021;20(1):124–130. doi: 10.1002/wps.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan AJ, Reavley NJ, Ross A, Too LS, Jorm AF. Interventions to reduce stigma towards people with severe mental illness: Systematic review and meta-analysis. J Psychiatr Res. 2018;103:120–133. doi: 10.1016/j.jpsychires.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Dixon LB, Holoshitz Y, Nossel I. Treatment engagement of individuals experiencing mental illness: review and update. World Psychiatry. 2016;15(1):13–20. doi: 10.1002/wps.20306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biddle S. Physical activity and mental health: evidence is growing. World Psychiatry. 2016;15(2):176–177. doi: 10.1002/wps.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mikkelsen K, Stojanovska L, Polenakovic M, Bosevski M, Apostolopoulos V. Exercise and mental health. Maturitas. 2017;106:48–56. doi: 10.1016/j.maturitas.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45–62. doi: 10.1146/annurev-med-060619-022943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuss J, Steinle J, Bindila L, Auer MK, Kirchherr H, Lutz B, et al. A runner's high depends on cannabinoid receptors in mice. Proc Natl Acad Sci USA. 2015;112(42):13105–13108. doi: 10.1073/pnas.1514996112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vina J, Gomez-Cabrera MC, Borras C, Froio T, Sanchis-Gomar F, Martinez-Bello VE, et al. Mitochondrial biogenesis in exercise and in ageing. Adv Drug Deliv Rev. 2009;61(14):1369–1374. doi: 10.1016/j.addr.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd BA, Hake HS, Ishiwata T, Farmer CE, Loetz EC, Fleshner M, et al. Exercise increases mTOR signaling in brain regions involved in cognition and emotional behavior. Behav Brain Res. 2017;323:56–67. doi: 10.1016/j.bbr.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32(12):741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- 21.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 22.Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 23.Gleeson M, McFarlin B, Flynn M. Exercise and Toll-like receptors. Exerc Immunol Rev. 2006;12:34–53. [PubMed] [Google Scholar]
- 24.Routledge FS, Campbell TS, McFetridge-Durdle JA, Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26(6):303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Middelkamp J, van Rooijen M, Wolfhagen P, Steenbergen B. The effects of a self-efficacy intervention on exercise behavior of fitness club members in 52 weeks and long-term relationships of transtheoretical model constructs. J Sports Sci Med. 2017;16(2):163–171. [PMC free article] [PubMed] [Google Scholar]
- 26.Su X, McDonough DJ, Chu H, Quan M, Gao Z. Application of network meta-analysis in the field of physical activity and health promotion. J Sport Health Sci. 2020;9(6):511–520. doi: 10.1016/j.jshs.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorlund K, Mills EJ. Sample size and power considerations in network meta-analysis. Syst Rev. 2012;1(1):1–13. doi: 10.1186/2046-4053-1-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tonin FS, Rotta I, Mendes AM, Pontarolo R. Network meta-analysis: a technique to gather evidence from direct and indirect comparisons. Pharmacy Practice (Granada) 2017;15(1):943. doi: 10.18549/PharmPract.2017.01.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen PJ, Miller CT, Mundell NL, Verswijveren S, Tagliaferri SD, Brisby H, et al. Which specific modes of exercise training are most effective for treating low back pain? Netw Meta Anal Br J Sports Med. 2020;54(21):1279–1287. doi: 10.1136/bjsports-2019-100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid- range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 33.Vucic K, Jelicic Kadic A, Puljak L. Survey of Cochrane protocols found methods for data extraction from figures not mentioned or unclear. J Clin Epidemiol. 2015;68(10):1161–1164. doi: 10.1016/j.jclinepi.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 34.Blumenthal JA, Babyak MA, Moore KA, Craighead E, Herman S, Khatri P, et al. Effects of exercise training on older patients with major depression. Arch Intern Med. 1999;159(19):2349–2356. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 35.Sarubin N, Nothdurfter C, Schule C, Lieb M, Uhr M, Born C, et al. The influence of Hatha yoga as an add-on treatment in major depression on hypothalamic-pituitary-adrenal-axis activity: A randomized trial. J Psychiatr Res. 2014;53:76–83. doi: 10.1016/j.jpsychires.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 36.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Zimmermann AB, Rocha NS, Fleck MP. Exercise and severe major depression: effect on symptom severity and quality of life at discharge in an inpatient cohort. J Psychiatr Res. 2015;61:25–32. doi: 10.1016/j.jpsychires.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Olson RL, Brush CJ, Ehmann PJ, Alderman BL. A randomized trial of aerobic exercise on cognitive control in major depression. Clin Neurophysiol. 2017;128(6):903–913. doi: 10.1016/j.clinph.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 38.Minghetti A, Faude O, Hanssen H, Zahner L, Gerber M, Donath L. Sprint interval training (SIT) substantially reduces depressive symptoms in major depressive disorder (MDD): A randomized controlled trial. Psychiatry Res. 2018;265:292–297. doi: 10.1016/j.psychres.2018.04.053. [DOI] [PubMed] [Google Scholar]
- 39.Bruchle W, Schwarzer C, Berns C, Scho S, Schneefeld J, Koester D, et al. Physical activity reduces clinical symptoms and restores neuroplasticity in major depression. Front Psych. 2021;12:935. doi: 10.3389/fpsyt.2021.660642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Herring MP, Kline CE, O'Connor PJ. Effects of exercise on sleep among young women with Generalized Anxiety Disorder. Ment Health Phys Act. 2015;9:59–66. doi: 10.1016/j.mhpa.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caponnetto P, Auditore R, Maglia M, Pipitone S, Inguscio L. Psychological wellness, yoga and quality of life in patients affected by schizophrenia spectrum disorders: a pilot study. Ment Illn. 2019;11(1):22–25. doi: 10.4081/mi.2019.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gokcen A, Ekici G, Abaoglu H, Sen DT. The healing effect of goal-oriented dance and movement therapy in schizophrenia: a rater-blinded randomized controlled trial. Arts Psychother. 2020;71:101702. [Google Scholar]
- 43.Nguyen-Feng VN, Hodgdon H, Emerson D, Silverberg R, Clark CJ. Moderators of treatment efficacy in a randomized controlled trial of trauma-sensitive yoga as an adjunctive treatment for posttraumatic stress disorder. Psychol Trauma. 2020;12(8):836–846. doi: 10.1037/tra0000963. [DOI] [PubMed] [Google Scholar]
- 44.Imboden C, Gerber M, Beck J, Eckert A, Lejri I, Puhse U, et al. Aerobic exercise and stretching as add-on to inpatient treatment for depression have no differential effects on stress-axis activity, serum-BDNF, TNF-alpha and objective sleep measures. Brain Sci. 2021;11(4):411. doi: 10.3390/brainsci11040411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Services. DoHaH. Physical activity guidelines for Americans. 2nd edn. Washington, DC: Department of Health and Human Services.; 2018.
- 46.Zou L, Loprinzi PD, Yeung AS, Zeng N, Huang T. The beneficial effects of mind-body exercises for people with mild cognitive impairment: a systematic review with meta-analysis. Arch Phys Med Rehabil. 2019;100(8):1556–1573. doi: 10.1016/j.apmr.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Yin H, Wang X, Jia Y, Wang C, Wang L, et al. Efficacy of different types of exercises on global cognition in adults with mild cognitive impairment: a network meta-analysis. Aging Clin Exp Res. 2019;31(10):1391–1400. doi: 10.1007/s40520-019-01142-5. [DOI] [PubMed] [Google Scholar]
- 48.Falck RS, Davis JC, Best JR, Crockett RA, Liu-Ambrose T. Impact of exercise training on physical and cognitive function among older adults: a systematic review and meta-analysis. Neurobiol Aging. 2019;79:119–130. doi: 10.1016/j.neurobiolaging.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.2: Cochrane; 2021.
- 50.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9(7):e99682. doi: 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 52.Van Valkenhoef G, Tervonen T, Zwinkels T, De Brock B, Hillege H. ADDIS: a decision support system for evidence-based medicine. Decis Support Syst. 2013;55(2):459–475. [Google Scholar]
- 53.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. 2010;29(7–8):932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 54.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Gr Stat. 1998;7(4):434–455. [Google Scholar]
- 55.Shim S, Yoon BH, Shin IS, Bae JM. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Winters M, Holden S, Lura CB, Welton NJ, Caldwell DM, Vicenzino BT, et al. Comparative effectiveness of treatments for patellofemoral pain: a living systematic review with network meta-analysis. Br J Sports Med. 2020;55:3697. doi: 10.1136/bjsports-2020-102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Doyne EJ, Ossip-Klein DJ, Bowman ED, Osborn KM, McDougall-Wilson IB, Neimeyer RA. Running versus weight lifting in the treatment of depression. J Consult Clin Psychol. 1987;55(5):748–754. doi: 10.1037//0022-006x.55.5.748. [DOI] [PubMed] [Google Scholar]
- 58.McNeil JK, LeBlanc EM, Joyner M. The effect of exercise on depressive symptoms in the moderately depressed elderly. Psychol Aging. 1991;6(3):487–488. doi: 10.1037//0882-7974.6.3.487. [DOI] [PubMed] [Google Scholar]
- 59.Veale D, Le Fevre K, Pantelis C, de Souza V, Mann A, Sargeant A. Aerobic exercise in the adjunctive treatment of depression: a randomized controlled trial. J R Soc Med. 1992;85(9):541–544. doi: 10.1177/014107689208500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh NA, Clements KM, Fiatarone MA. A randomized controlled trial of progressive resistance training in depressed elders. J Gerontol A Biol Sci Med Sci. 1997;52(1):M27–35. doi: 10.1093/gerona/52a.1.m27. [DOI] [PubMed] [Google Scholar]
- 61.Armstrong K, Edwards H. The effects of exercise and social support on mothers reporting depressive symptoms: a pilot randomized controlled trial. Int J Ment Health Nurs. 2003;12(2):130–138. doi: 10.1046/j.1440-0979.2003.00229.x. [DOI] [PubMed] [Google Scholar]
- 62.Sharma VK, Das S, Mondal S, Goswampi U, Gandhi A. Effect of Sahaj Yoga on depressive disorders. Indian J Physiol Pharmacol. 2005;49(4):462–468. [PubMed] [Google Scholar]
- 63.Singh NA, Stravinos TM, Scarbek Y, Galambos G, Liber C, Singh MAF, et al. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60(6):768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- 64.Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, et al. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69(7):587–596. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brenes GA, Williamson JD, Messier SP, Rejeski WJ, Pahor M, Ip E, et al. Treatment of minor depression in older adults: a pilot study comparing sertraline and exercise. Aging Ment Health. 2007;11(1):61–68. doi: 10.1080/13607860600736372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Knubben K, Reischies FM, Adli M, Schlattmann P, Bauer M, Dimeo F. A randomised, controlled study on the effects of a short-term endurance training programme in patients with major depression. Br J Sports Med. 2007;41(1):29–33. doi: 10.1136/bjsm.2006.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daley A, Winter H, Grimmett C, McGuinness M, McManus R, MacArthur C. Feasibility of an exercise intervention for women with postnatal depression: a pilot randomised controlled trial. Br J Gen Pract. 2008;58(548):178–183. doi: 10.3399/bjgp08X277195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heh S, Huang L, Ho S, Fu Y, Wang L. Effectiveness of an exercise support program in reducing the severity of postnatal depression in Taiwanese women. Birth Issues Perinatal Care. 2008;35(1):60–5. doi: 10.1111/j.1523-536X.2007.00192.x. [DOI] [PubMed] [Google Scholar]
- 69.Da Costa D, Lowensteyn I, Abrahamowicz M, Ionescu-Ittu R, Dritsa M, Rippen N, et al. A randomized clinical trial of exercise to alleviate postpartum depressed mood. J Psychosom Obstet Gynaecol. 2009;30(3):191–200. doi: 10.1080/01674820903212136. [DOI] [PubMed] [Google Scholar]
- 70.Krogh J, Saltin B, Gluud C, Nordentoft M. The DEMO trial: a randomized, parallel-group, observer-blinded clinical trial of strength versus aerobic versus relaxation training for patients with mild to moderate depression. J Clin Psychiatry. 2009;70(6):790–800. doi: 10.4088/jcp.08m04241. [DOI] [PubMed] [Google Scholar]
- 71.Callaghan P, Khalil E, Morres I, Carter T. Pragmatic randomised controlled trial of preferred intensity exercise in women living with depression. BMC Public Health. 2011;11:465. doi: 10.1186/1471-2458-11-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de la Cerda P, Cervelló E, Cocca A, Viciana J. Effect of an aerobic training program as complementary therapy in patients with moderate depression. Percept Mot Skills. 2011;112(3):761–769. doi: 10.2466/02.15.PMS.112.3.761-769. [DOI] [PubMed] [Google Scholar]
- 73.Lavretsky H, Alstein LL, Olmstead RE, Ercoli LM, Riparetti-Brown M, Cyr NS, et al. Complementary use of tai chi chih augments escitalopram treatment of geriatric depression: a randomized controlled trial. Am J Geriatr Psychiatry. 2011;19(10):839–850. doi: 10.1097/JGP.0b013e31820ee9ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mota-Pereira J, Silverio J, Carvalho S, Ribeiro JC, Fonte D, Ramos J. Moderate exercise improves depression parameters in treatment-resistant patients with major depressive disorder. J Psychiatr Res. 2011;45(8):1005–1011. doi: 10.1016/j.jpsychires.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 75.Roshan VD, Pourasghar M, Mohammadian Z. The efficacy of intermittent walking in water on the rate of MHPG sulfate and the severity of depression. Iran J Psychiatry Behav Sci. 2011;5(2):26–31. [PMC free article] [PubMed] [Google Scholar]
- 76.Schuch FB, Vasconcelos-Moreno MP, Borowsky C, Fleck MP. Exercise and severe depression: preliminary results of an add-on study. J Affect Disord. 2011;133(3):615–618. doi: 10.1016/j.jad.2011.04.030. [DOI] [PubMed] [Google Scholar]
- 77.Hemat-Far A, Shahsavari A, Mousavi SR. Effects of selected aerobic exercises on the depression and concentrations of plasma serotonin in the depressed female students aged 18 to 25. J Appl Res. 2012;12(1):47–52. [Google Scholar]
- 78.Krogh J, Videbech P, Thomsen C, Gluud C, Nordentoft M. DEMO-II trial: aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS ONE. 2012;7(10):e48316. doi: 10.1371/journal.pone.0048316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell J, Field T, Diego M, Bendell D, Newton R, Pelaez M. Yoga reduces prenatal depression symptoms. Psychology. 2012;3(9A):782–786. [Google Scholar]
- 80.Yeung A, Lepoutre V, Wayne P, Yeh G, Slipp LE, Fava M, et al. Tai chi treatment for depression in Chinese Americans: a pilot study. Am J Phys Med Rehabil. 2012;91(10):863–870. doi: 10.1097/PHM.0b013e31825f1a67. [DOI] [PubMed] [Google Scholar]
- 81.Field T, Diego M, Delgado J, Medina L. Tai chi/yoga reduces prenatal depression, anxiety and sleep disturbances. Complement Ther Clin Pract. 2013;19(1):6–10. doi: 10.1016/j.ctcp.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Field T, Diego M, Delgado J, Medina L. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther. 2013;17(4):397–403. doi: 10.1016/j.jbmt.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 83.Gangadhar BN, Naveen GH, Rao MG, Thirthalli J, Varambally S. Positive antidepressant effects of generic yoga in depressive out-patients: a comparative study. Indian J Psychiatry. 2013;55(7):S369–S373. doi: 10.4103/0019-5545.116312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Naveen GH, Thirthalli J, Rao MG, Varambally S, Christopher R, Gangadhar BN. Positive therapeutic and neurotropic effects of yoga in depression: a comparative study. Indian J Psychiatry. 2013;55(7):S400–S404. doi: 10.4103/0019-5545.116313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ho CWH, Chan SC, Wong JS, Cheung WT, Chung DWS, Lau TFO. Effect of aerobic exercise training on Chinese population with mild to moderate depression in Hong Kong. Rehabil Res Practice. 2014 doi: 10.1155/2014/627376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belvederi Murri M, Amore M, Menchetti M, Toni G, Neviani F, Cerri M, et al. Physical exercise for late-life major depression. Br J Psychiatry. 2015;207(3):235–242. doi: 10.1192/bjp.bp.114.150516. [DOI] [PubMed] [Google Scholar]
- 87.Buttner MM, Brock RL, O'Hara MW, Stuart S. Efficacy of yoga for depressed postpartum women: a randomized controlled trial. Complement Ther Clin Pract. 2015;21(2):94–100. doi: 10.1016/j.ctcp.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Carter T, Guo B, Turner D, Morres I, Khalil E, Brighton E, et al. Preferred intensity exercise for adolescents receiving treatment for depression: a pragmatic randomised controlled trial. BMC Psychiatry. 2015;15:247. doi: 10.1186/s12888-015-0638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doose M, Ziegenbein M, Hoos O, Reim D, Stengert W, Hoffer N, et al. Self-selected intensity exercise in the treatment of major depression: a pragmatic RCT. Int J Psychiatry Clin Pract. 2015;19(4):266–275. doi: 10.3109/13651501.2015.1082599. [DOI] [PubMed] [Google Scholar]
- 90.Kerling A, Tegtbur U, Gützlaff E, Kück M, Borchert L, Ates Z, et al. Effects of adjunctive exercise on physiological and psychological parameters in depression: a randomized pilot trial. J Affect Disord. 2015;177:1–6. doi: 10.1016/j.jad.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 91.Majumder P, Sharma I, Vostanis P, Bone C. The effect of aerobic exercise in the maintenance treatment of depression. Bjpsych Int. 2015;12(S1):S3–S6. doi: 10.1192/s2056474000000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Legrand FD, Neff EM. Efficacy of exercise as an adjunct treatment for clinically depressed inpatients during the initial stages of antidepressant pharmacotherapy: an open randomized controlled trial. J Affect Disord. 2016;191:139–144. doi: 10.1016/j.jad.2015.11.047. [DOI] [PubMed] [Google Scholar]
- 93.Schuver KJ, Lewis BA. Mindfulness-based yoga intervention for women with depression. Complement Ther Med. 2016;26:85–91. doi: 10.1016/j.ctim.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Siqueira CC, Valiengo LL, Carvalho AF, Santos-Silva PR, Missio G, de Sousa RT, et al. Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS ONE. 2016;11(5):e0154195. doi: 10.1371/journal.pone.0154195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toni G, Belvederi Murri M, Piepoli M, Zanetidou S, Cabassi A, Squatrito S, et al. Physical exercise for late-life depression: effects on heart rate variability. Am J Geriatric Psychiatry. 2016;24(11):989–997. doi: 10.1016/j.jagp.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 96.Forsyth J, Boath E, Henshaw C, Brown H. Exercise as an adjunct treatment for postpartum depression for women living in an inner city—a pilot study. Health Care Women Int. 2017;38(6):635–639. doi: 10.1080/07399332.2017.1295049. [DOI] [PubMed] [Google Scholar]
- 97.Prathikanti S, Rivera R, Cochran A, Tungol JG, Fayazmanesh N, Weinmann E. Treating major depression with yoga: a prospective, randomized, controlled pilot trial. PLoS ONE. 2017;12(3):e0173869. doi: 10.1371/journal.pone.0173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Turner D, Carter T, Sach T, Guo B, Callaghan P. Cost-effectiveness of a preferred intensity exercise programme for young people with depression compared with treatment as usual: an economic evaluation alongside a clinical trial in the UK. BMJ Open. 2017;7(11):e016211. doi: 10.1136/bmjopen-2017-016211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uebelacker LA, Tremont G, Gillette LT, Epstein-Lubow G, Strong DR, Abrantes AM, et al. Adjunctive yoga vs health education for persistent major depression: a randomized controlled trial. Psychol Med. 2017;47(12):2130–42. doi: 10.1017/S0033291717000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeung AS, Feng R, Kim DJH, Wayne PM, Yeh GY, Baer L, et al. A Pilot, randomized controlled study of Tai Chi With passive and active controls in the treatment of depressed Chinese americans. J Clin Psychiatry. 2017;78(5):e522.e8. doi: 10.4088/JCP.16m10772. [DOI] [PubMed] [Google Scholar]
- 101.Cheung LK, Lee S. A randomized controlled trial on an aerobic exercise programme for depression outpatients. Sport Sci Health. 2018;14(1):173–181. [Google Scholar]
- 102.Gerber M, Minghetti A, Beck J, Zahner L, Donath L. Sprint interval training and continuous aerobic exercise training have similar effects on exercise motivation and affective responses to exercise in patients with major depressive disorders: a randomized controlled trial. Front Psych. 2018;9:694. doi: 10.3389/fpsyt.2018.00694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tolahunase MR, Sagar R, Dada R. 5-HTTLPR and MTHFR 677C > T polymorphisms and response to yoga-based lifestyle intervention in major depressive disorder: arandomized active-controlled trial. Indian J Psychiatry. 2018;60(4):410–426. doi: 10.4103/psychiatry.IndianJPsychiatry_398_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bressington D, Mui J, Yu C, Leung SF, Cheung K, Wu CST, et al. Feasibility of a group-based laughter yoga intervention as an adjunctive treatment for residual symptoms of depression, anxiety and stress in people with depression. J Affect Disord. 2019;248:42–51. doi: 10.1016/j.jad.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 105.Kumar S, Subramaniam E, Bhavanani AB, Sarkar S, Balasundaram S. Effect of adjunct yoga therapy in depressive disorders: findings from a randomized controlled study. Indian J Psychiatry. 2019;61(6):592–597. doi: 10.4103/psychiatry.IndianJPsychiatry_173_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tasci G, Baykara S, Gurok MG, Atmaca M. Effect of exercise on therapeutic response in depression treatment. Psychiatry Clin Psychopharmacol. 2019;29(2):137–143. [Google Scholar]
- 107.Zhang JL, Chen TX. Effect of aerobic exercise on cognitive function and symptoms in patients with depression. Natl Acad Sci Lett India. 2019;42(5):419–421. [Google Scholar]
- 108.Chau RMW, Tsui AYY, Wong EYW, Cheung EYY, Chan DYC, Lau PMY, et al. Effectiveness of a structured physical rehabilitation program on the physical fitness, mental health and pain for Chinese patients with major depressive disorders in Hong Kong: a randomized controlled trial with 9-month follow-up outcomes. Disabil Rehabil. 2020;44:1294. doi: 10.1080/09638288.2020.1800833. [DOI] [PubMed] [Google Scholar]
- 109.Gerber M, Imboden C, Beck J, Brand S, Colledge F, Eckert A, et al. Effects of aerobic exercise on cortisol stress reactivity in response to the trier social stress test in inpatients with major depressive disorders: a randomized controlled trial. J Clin Med. 2020;9(5):1419. doi: 10.3390/jcm9051419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hyvonen K, Pylvanainen P, Muotka J, Lappalainen R. The Effects of dance movement therapy in the treatment of depression: a multicenter, randomized controlled trial in Finland. Front Psychol. 2020;11:1687. doi: 10.3389/fpsyg.2020.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Imboden C, Gerber M, Beck J, Holsboer-Trachsler E, Pühse U, Hatzinger M. Aerobic exercise or stretching as add-on to inpatient treatment of depression: Similar antidepressant effects on depressive symptoms and larger effects on working memory for aerobic exercise alone. J Affect Disord. 2020;276:866–876. doi: 10.1016/j.jad.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 112.Ozkan SA, Kucukkelepce DS, Korkmaz B, Yilmaz G, Bozkurt MA. The effectiveness of an exercise intervention in reducing the severity of postpartum depression: a randomized controlled trial. Perspect Psychiatr Care. 2020;56(4):844–850. doi: 10.1111/ppc.12500. [DOI] [PubMed] [Google Scholar]
- 113.Rao UT, Noronha JA, Adiga K. Effect of aerobic exercises on depressive symptoms, anxiety, self-esteem, and quality of life among adults with depression. Clin Epidemiol Global Health. 2020;8(4):1147–1151. [Google Scholar]
- 114.Adagide S, Karatas N. The effects of physical exercise on the depressive symptoms and quality of life of individuals diagnosed with depression. J Psychiatr Nurs. 2021;12(2):122–131. [Google Scholar]
- 115.Bieber M, Gorgulu E, Schmidt D, Zabel K, Etyemez S, Friedrichs B, et al. Effects of body-oriented yoga: a RCT study for patients with major depressive disorder. Eur Arch Psychiatry Clin Neurosci. 2021;271(7):1217–1229. doi: 10.1007/s00406-021-01277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kang H, Jang S. Effect of mindfulness yoga on depression severity, self-esteem, and quality of life in middle-aged men. Iran J Public Health. 2021;50(7):1334–1342. doi: 10.18502/ijph.v50i7.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavretsky H, Milillo MM, Kilpatrick L, Grzenda A, Wu P, Nguyen SA, et al. A randomized controlled trial of tai chi chih or health education for geriatric depression. Am J Geriatric Psychiatry. 2021;30:392. doi: 10.1016/j.jagp.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis BA, Schuver K, Dunsiger S, Samson L, Frayeh AL, Terrell CA, et al. Randomized trial examining the effect of exercise and wellness interventions on preventing postpartum depression and perceived stress. BMC Pregnancy Childbirth. 2021;21(1):785. doi: 10.1186/s12884-021-04257-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ravindran AV, McKay MS, da Silva T, Tindall C, Garfinkel T, Paric A, et al. Breathing-focused yoga as augmentation for unipolar and bipolar depression: a randomized controlled trial: Le yoga axe sur la respiration comme traitement d'appoint pour la depression unipolaire et bipolaire: Un essai randomise controle. Can J Psychiatry Revue Canadienne De Psychiatrie. 2021;66(2):159–169. doi: 10.1177/0706743720940535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Srivastava A, Kuppili PP, Gupta T, Nebhinani N, Chandani A. Kriya yoga in patients with depressive disorders: a pilot study. J Neurosc Rural Practice. 2021;12(02):362–367. doi: 10.1055/s-0041-1726618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Broocks A, Bandelow B, Pekrun G, George A, Meyer T, Bartmann U, et al. Comparison of aerobic exercise, clomipramine, and placebo in the treatment of panic disorder. Am J Psychiatry. 1998;155(5):603–609. doi: 10.1176/ajp.155.5.603. [DOI] [PubMed] [Google Scholar]
- 122.Herring MP, Jacob ML, Suveg C, O'Connor PJ. Effects of short-term exercise training on signs and symptoms of generalized anxiety disorder. Ment Health Phys Act. 2011;4(2):71–77. [Google Scholar]
- 123.Song QH, Shen GQ, Xu RM, Zhang QH, Ma M, Guo YH, et al. Effect of Tai Chi exercise on the physical and mental health of the elder patients suffered from anxiety disorder. Int J Physiol Pathophysiol Pharmacol. 2014;6(1):55–60. [PMC free article] [PubMed] [Google Scholar]
- 124.Ma W-F, Wu P-L, Su C-H, Yang T-C. The effects of an exercise program on anxiety levels and metabolic functions in patients with anxiety disorders. Biol Res Nurs. 2017;19(3):258–268. doi: 10.1177/1099800416672581. [DOI] [PubMed] [Google Scholar]
- 125.Gordon BR, McDowell CP, Lyons M, Herring MP. Resistance exercise training among young adults with analogue generalized anxiety disorder. J Affect Disord. 2021;281:153–159. doi: 10.1016/j.jad.2020.12.020. [DOI] [PubMed] [Google Scholar]
- 126.Carter J, Gerbarg P, Brown R, Ware R, Ambrosio C, Anand L, et al. Multi-component yoga breath program for vietnam veteran post traumatic stress disorder: randomized controlled trial. J Traumatic Stress Disorders Treatment. 2013;2:1–10. [Google Scholar]
- 127.Mitchell KS, Dick AM, DiMartino DM, Smith BN, Niles B, Koenen KC, et al. A pilot study of a randomized controlled trial of yoga as an intervention for PTSD symptoms in women. J Trauma Stress. 2014;27(2):121–128. doi: 10.1002/jts.21903. [DOI] [PubMed] [Google Scholar]
- 128.Thordardottir K, Gudmundsdottir R, Zoega H, Valdimarsdottir UA, Gudmundsdottir B. Effects of yoga practice on stress-related symptoms in the aftermath of an earthquake: a community-based controlled trial. Complement Ther Med. 2014;22(2):226–234. doi: 10.1016/j.ctim.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 129.van der Kolk BA, Stone L, West J, Rhodes A, Emerson D, Suvak M, et al. Yoga as an adjunctive treatment for posttraumatic stress disorder: a randomized controlled trial. J Clin Psychiatry. 2014;75(6):e559–e565. doi: 10.4088/JCP.13m08561. [DOI] [PubMed] [Google Scholar]
- 130.Jindani F, Turner N, Khalsa SB. A yoga intervention for posttraumatic stress: a preliminary randomized control trial. Evid Based Complement Alternat Med. 2015;2015:351746. doi: 10.1155/2015/351746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Quinones N, Maquet YG, Velez DM, Lopez MA. Efficacy of a satyananda yoga intervention for reintegrating adults diagnosed with posttraumatic stress disorder. Int J Yoga Therap. 2015;25(1):89–99. doi: 10.17761/1531-2054-25.1.89. [DOI] [PubMed] [Google Scholar]
- 132.Rosenbaum S, Sherrington C, Tiedemann A. Exercise augmentation compared with usual care for post-traumatic stress disorder: a randomized controlled trial. Acta Psychiatr Scand. 2015;131(5):350–359. doi: 10.1111/acps.12371. [DOI] [PubMed] [Google Scholar]
- 133.Goldstein LA, Mehling WE, Metzler TJ, Cohen BE, Barnes DE, Choucroun GJ, et al. Veterans group exercise: a randomized pilot trial of an Integrative exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345–352. doi: 10.1016/j.jad.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 134.Whitworth JW, Nosrat S, SantaBarbara NJ, Ciccolo JT. High intensity resistance training improves sleep quality and anxiety in individuals who screen positive for posttraumatic stress disorder: a randomized controlled feasibility trial. Mental Health Physical Activity. 2019;16:43–49. [Google Scholar]
- 135.Hall KS, Morey MC, Bosworth HB, Beckham JC, Pebole MM, Sloane R, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648–659. doi: 10.1007/s10865-019-00073-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Duraiswamy G, Thirthalli J, Nagendra HR, Gangadhar BN. Yoga therapy as an add-on treatment in the management of patients with schizophrenia—a randomized controlled trial. Acta Psychiatr Scand. 2007;116(3):226–232. doi: 10.1111/j.1600-0447.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 137.Acil AA, Dogan S, Dogan O. The effects of physical exercises to mental state and quality of life in patients with schizophrenia. J Psychiatr Ment Health Nurs. 2008;15(10):808–815. doi: 10.1111/j.1365-2850.2008.01317.x. [DOI] [PubMed] [Google Scholar]
- 138.Behere RV, Arasappa R, Jagannathan A, Varambally S, Venkatasubramanian G, Thirthalli J, et al. Effect of yoga therapy on facial emotion recognition deficits, symptoms and functioning in patients with schizophrenia. Acta Psychiatr Scand. 2011;123(2):147–153. doi: 10.1111/j.1600-0447.2010.01605.x. [DOI] [PubMed] [Google Scholar]
- 139.Ho RTH, Au Yeung FSW, Lo PHY, Law KY, Wong KOK, Cheung IKM, et al. Tai-Chi for residential patients with schizophrenia on movement coordination, negative symptoms, and functioning: a pilot randomized controlled trial. Evid Based Complem Altern Med. 2012;2012:1. doi: 10.1155/2012/923925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Takahashi H, Sassa T, Shibuya T, Kato M, Koeda M, Murai T, et al. Effects of sports participation on psychiatric symptoms and brain activations during sports observation in schizophrenia. Transl Psychiatry. 2012;2(3):e96. doi: 10.1038/tp.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Varambally S, Gangadhar BN, Thirthalli J, Jagannathan A, Kumar S, Venkatasubramanian G, et al. Therapeutic efficacy of add-on yogasana intervention in stabilized outpatient schizophrenia: randomized controlled comparison with exercise and waitlist. Indian J Psychiatry. 2012;54(3):227–232. doi: 10.4103/0019-5545.102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ikai S, Uchida H, Suzuki T, Tsunoda K, Mimura M, Fujii Y. Effects of yoga therapy on postural stability in patients with schizophrenia-spectrum disorders: a single-blind randomized controlled trial. J Psychiatr Res. 2013;47(11):1744–1750. doi: 10.1016/j.jpsychires.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 143.Jayaram N, Varambally S, Behere RV, Venkatasubramanian G, Arasappa R, Christopher R, et al. Effect of yoga therapy on plasma oxytocin and facial emotion recognition deficits in patients of schizophrenia. Indian J Psychiatry. 2013;55(7):S409–S413. doi: 10.4103/0019-5545.116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ikai S, Suzuki T, Uchida H, Saruta J, Tsukinoki K, Fujii Y, et al. Effects of weekly one-hour Hatha yoga therapy on resilience and stress levels in patients with schizophrenia-spectrum disorders: an eight-week randomized controlled trial. J Altern Complement Med. 2014;20(11):823–830. doi: 10.1089/acm.2014.0205. [DOI] [PubMed] [Google Scholar]
- 145.Kaltsatou A, Kouidi E, Fountoulakis K, Sipka C, Theochari V, Kandylis D, et al. Effects of exercise training with traditional dancing on functional capacity and quality of life in patients with schizophrenia: a randomized controlled study. Clin Rehabil. 2015;29(9):882–891. doi: 10.1177/0269215514564085. [DOI] [PubMed] [Google Scholar]
- 146.Lee HJ, Jang SH, Lee SY, Hwang KS. Effectiveness of dance/movement therapy on affect and psychotic symptoms in patients with schizophrenia. Arts Psychother. 2015;45:64–68. [Google Scholar]
- 147.Loh SY, Abdullah A, Abu Bakar AK, Thambu M, Nik Jaafar NR. Structured walking and chronic institutionalized schizophrenia inmates: a pilot RCT study on quality of life. Glob J Health Sci. 2015;8(1):238–248. doi: 10.5539/gjhs.v8n1p238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Paikkatt B, Singh AR, Singh PK, Jahan M, Ranjan JK. Efficacy of Yoga therapy for the management of psychopathology of patients having chronic schizophrenia. Indian J Psychiatry. 2015;57(4):355–360. doi: 10.4103/0019-5545.171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Silva BA, Cassilhas RC, Attux C, Cordeiro Q, Gadelha AL, Telles BA, et al. A 20-week program of resistance or concurrent exercise improves symptoms of schizophrenia: results of a blind, randomized controlled trial. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil: 1999) 2015;37(4):271–9. doi: 10.1590/1516-4446-2014-1595. [DOI] [PubMed] [Google Scholar]
- 150.Areshtanab HN, Ebrahimi H, Farnam AR, Mohammadpoorasl A, Jamali B, Piri S. The effect of regular aerobic exercise on both positive and negative symptoms of male patients with chronic Schizophrenia: a double blinded study. Int J Med Res Health Sci. 2016;5(11):529–535. [Google Scholar]
- 151.Ho Rainbow TH, Fong Ted CT, Wan Adrian HY, Au-Yeung Friendly SW, Wong Cathy PK, Ng Winnie YH. A randomized controlled trial on the psychophysiological effects of physical exercise and tai-chi in patients with chronic schizophrenia. Schizophren Res. 2016:. [DOI] [PubMed]
- 152.Kang R, Wu Y, Li Z, Jiang J, Gao Q, Yu Y, et al. Effect of community-based social skills training and tai-chi exercise on outcomes in patients with chronic schizophrenia: a randomized. One Year Study Psychopathol. 2016;49(5):345–355. doi: 10.1159/000448195. [DOI] [PubMed] [Google Scholar]
- 153.Kavak F, Ekinci M. The effect of yoga on functional recovery level in schizophrenic patients. Arch Psychiatr Nurs. 2016;30(6):761–767. doi: 10.1016/j.apnu.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 154.Martin LA, Koch SC, Hirjak D, Fuchs T. Overcoming disembodiment: the effect of movement therapy on negative symptoms in schizophrenia-a multicenter randomized controlled trial. Front Psychol. 2016;7:483. doi: 10.3389/fpsyg.2016.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Su C-Y, Wang P-W, Lin Y-J, Tang T-C, Liu M-F, Chen M-D. The effects of aerobic exercise on cognition in schizophrenia: a 3-month follow-up study. Psychiatry Res. 2016;244:394–402. doi: 10.1016/j.psychres.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 156.Curcic D, Stojmenovic T, Djukic-Dejanovic S, Dikic N, Vesic-Vukasinovic M, Radivojevic N, et al. Positive impact of prescribed physical activity on symptoms of schizophrenia: randomized clinical trial. Psychiatr Danub. 2017;29(4):459–465. doi: 10.24869/psyd.2017.459. [DOI] [PubMed] [Google Scholar]
- 157.Wang P-W, Lin H-C, Su C-Y, Chen M-D, Lin KC, Ko C-H, et al. Effect of aerobic exercise on improving symptoms of individuals with schizophrenia: a single blinded randomized control study. Front Psychiatry. 2018;9:167. doi: 10.3389/fpsyt.2018.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shimada T, Ito S, Makabe A, Yamanushi A, Takenaka A, Kobayashi M. Aerobic exercise and cognitive functioning in schizophrenia: a pilot randomized controlled trial. Psychiatry Res. 2019;282:112638. doi: 10.1016/j.psychres.2019.112638. [DOI] [PubMed] [Google Scholar]
- 159.Bryl K, Bradt J, Cechnicki A, Fisher K, Sossin KM, Goodill S. The role of dance/movement therapy in the treatment of negative symptoms in schizophrenia: a mixed methods pilot study. J Mental Health (Abingdon, England). 2020;31:613. doi: 10.1080/09638237.2020.1757051. [DOI] [PubMed] [Google Scholar]
- 160.Shimada T, Ito S, Makabe A, Yamanushi A, Takenaka A, Kawano K, et al. Aerobic exercise and cognitive functioning in schizophrenia: results of a 1-year follow-up from a randomized controlled trial. Psychiatry Res. 2020;286:1. doi: 10.1016/j.psychres.2020.112854. [DOI] [PubMed] [Google Scholar]
- 161.Akbas E, Erdem EU, Gunes E, Ozkan TD, Kinikli GI. Effects of pilates-based exercises on functional capacity and mental health in individuals with schizophrenia: a pilot study. Physiother Theory Practice. 2021;2021:1–9. doi: 10.1080/09593985.2021.1929613. [DOI] [PubMed] [Google Scholar]
- 162.Gao H, Luo C, Tu SJ, Lu RP, Jiang LN, Qiao HJ, et al. The effect of Yijinjing on the cognitive function of patients with chronic schizophrenia. Front Psych. 2021;12:1. doi: 10.3389/fpsyt.2021.739364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Govindaraj R, Naik SS, Mehta UM, Sharma M, Varambally S, Gangadhar BN. Yoga therapy for social cognition in schizophrenia: an experimental medicine-based randomized controlled trial. Asian J Psychiatr. 2021;62:102731. doi: 10.1016/j.ajp.2021.102731. [DOI] [PubMed] [Google Scholar]
- 164.Lo LLH, Lee EHM, Hui CLM, Chong CSY, Chang WC, Chan SKW, et al. Effect of high-endurance exercise intervention on sleep-dependent procedural memory consolidation in individuals with schizophrenia: a randomized controlled trial. Psychol Med. 2021;2021:1. doi: 10.1017/S0033291721003196. [DOI] [PubMed] [Google Scholar]
- 165.Rao NP, Ramachandran P, Jacob A, Joseph A, Thonse U, Nagendra B, et al. Add on yoga treatment for negative symptoms of schizophrenia: a multi-centric, randomized controlled trial. Schizophr Res. 2021;231:90–97. doi: 10.1016/j.schres.2021.03.021. [DOI] [PubMed] [Google Scholar]
- 166.Senormanci G, Korkmaz N, Senormanci O, Ugur S, Topsac M, Gultekin O. Effects of exercise on resilience, insight and functionality in patients with chronic schizophrenia in a psychiatric nursing home setting: a randomized controlled trial. Issues Ment Health Nurs. 2021;42(7):690–698. doi: 10.1080/01612840.2020.1847221. [DOI] [PubMed] [Google Scholar]
- 167.Miller KJ, Goncalves-Bradley DC, Areerob P, Hennessy D, Mesagno C, Grace F. Comparative effectiveness of three exercise types to treat clinical depression in older adults: a systematic review and network meta-analysis of randomised controlled trials. Ageing Res Rev. 2020;58:100999. doi: 10.1016/j.arr.2019.100999. [DOI] [PubMed] [Google Scholar]
- 168.Craft LL, Landers DM. The effect of exercise on clinical depression and depression resulting from mental illness: a meta-analysis. J Sport Exerc Psychol. 1998;20:339–357. [Google Scholar]
- 169.North TC, McCullagh P, Tran ZV. Effect of exercise on depression. Exerc Sport Sci Rev. 1990;18:379–415. [PubMed] [Google Scholar]
- 170.Bartley CA, Hay M, Bloch MH. Meta-analysis: aerobic exercise for the treatment of anxiety disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:34–39. doi: 10.1016/j.pnpbp.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 171.Zika MA, Becker L. Physical activity as a treatment for social anxiety in clinical and non- clinical populations: a systematic review and three meta-analyses for different study designs. Front Hum Neurosci. 2021;15:653108. doi: 10.3389/fnhum.2021.653108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Cassilhas RC, Lee KS, Fernandes J, Oliveira MG, Tufik S, Meeusen R, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. doi: 10.1016/j.neuroscience.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 173.Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes (Lond) 2007;31(6):996–1003. doi: 10.1038/sj.ijo.0803534. [DOI] [PubMed] [Google Scholar]
- 174.Focht BC, Garver MJ, Cotter JA, Devor ST, Lucas AR, Fairman CM. Affective responses to acute resistance exercise performed at self- selected and imposed loads in trained women. J Strength Cond Res. 2015;29(11):3067–3074. doi: 10.1519/JSC.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 175.Lindheimer JB, Szabo A, Raglin JS, Beedie C. Advancing the understanding of placebo effects in psychological outcomes of exercise: lessons learned and future directions. Eur J Sport Sci. 2020;20(3):326–337. doi: 10.1080/17461391.2019.1632937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Petruzzello SJ, Landers DM, Hatfield BD, Kubitz KA, Salazar W. A meta-analysis on the anxiety-reducing effects of acute and chronic exercise. Outcomes Mech Sports Med. 1991;11(3):143–182. doi: 10.2165/00007256-199111030-00002. [DOI] [PubMed] [Google Scholar]
- 177.Gallegos AM, Crean HF, Pigeon WR, Heffner KL. Meditation and yoga for posttraumatic stress disorder: a meta-analytic review of randomized controlled trials. Clin Psychol Rev. 2017;58:115–124. doi: 10.1016/j.cpr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhu L, Li L, Li XZ, Wang L. Mind-body exercises for PTSD symptoms, depression, and anxiety in patients with PTSD: a systematic review and meta-analysis. Front Psychol. 2021;12:738211. doi: 10.3389/fpsyg.2021.738211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Streeter CC, Gerbarg PL, Saper RB, Ciraulo DA, Brown RP. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78(5):571–579. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 180.McEwen BS. The neurobiology and neuroendocrinology of stress: implications for post- traumatic stress disorder from a basic science perspective. Psychiatr Clin N Am. 2002;25(2):469–94. doi: 10.1016/s0193-953x(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 181.Yehuda R, Golier J. Is there a rationale for cortisol-based treatments for PTSD? Expert Rev Neurother. 2009;9(8):1113–1115. doi: 10.1586/ern.09.79. [DOI] [PubMed] [Google Scholar]
- 182.Hegberg NJ, Hayes JP, Hayes SM. Exercise intervention in PTSD: a narrative review and rationale for implementation. Front Psychiatry. 2019;10:133. doi: 10.3389/fpsyt.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381(18):1753–1761. doi: 10.1056/NEJMra1808803. [DOI] [PubMed] [Google Scholar]
- 184.Hsieh SS, Chang YK, Fang CL, Hung TM. Acute resistance exercise facilitates attention control in adult males without an age-moderating effect. J Sport Exerc Psychol. 2016;38(3):247–254. doi: 10.1123/jsep.2015-0282. [DOI] [PubMed] [Google Scholar]
- 185.Morris R, Griffiths O, Le Pelley ME, Weickert TW. Attention to irrelevant cues is related to positive symptoms in schizophrenia. Schizophr Bull. 2013;39(3):575–582. doi: 10.1093/schbul/sbr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Stubbs B, Firth J, Berry A, Schuch FB, Rosenbaum S, Gaughran F, et al. How much physical activity do people with schizophrenia engage in? A systematic review, comparative meta-analysis and meta-regression. Schizophr Res. 2016;176(2–3):431–440. doi: 10.1016/j.schres.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 187.Wei GX, Yang L, Imm K, Loprinzi PD, Smith L, Zhang X, et al. Effects of mind-body exercises on schizophrenia: a systematic review with meta-analysis. Front Psychiatry. 2020;11:819. doi: 10.3389/fpsyt.2020.00819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Li J, Shen J, Wu G, Tan Y, Sun Y, Keller E, et al. Mindful exercise versus non-mindful exercise for schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2018;32:17–24. doi: 10.1016/j.ctcp.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 189.Shen H, Chen M, Cui D. Biological mechanism study of meditation and its application in mental disorders. Gen Psychiatr. 2020;33(4):e100214. doi: 10.1136/gpsych-2020-100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.VanItallie TB. Subsyndromal depression in the elderly: underdiagnosed and undertreated. Metabolism. 2005;54(5 Suppl 1):39–44. doi: 10.1016/j.metabol.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 191.Kasckow JW, Karp JF, Whyte E, Butters M, Brown C, Begley A, et al. Subsyndromal depression and anxiety in older adults: health related, functional, cognitive and diagnostic implications. J Psychiatr Res. 2013;47(5):599–603. doi: 10.1016/j.jpsychires.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Greene DR, Petruzzello SJ. Working it out: acute exercise to combat anxiety and depressive symptoms in individuals living with subsyndromal post-traumatic stress disorder. Int J Sport Exercise Psychol. 2021;20:1–16. [Google Scholar]
- 193.Burnet K, Kelsch E, Zieff G, Moore JB, Stoner L. How fitting is FITT? A perspective on a transition from the sole use of frequency, intensity, time, and type in exercise prescription. Physiol Behav. 2019;199:33–4. doi: 10.1016/j.physbeh.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 194.Turner RM, Bird SM, Higgins JP. The impact of study size on meta-analyses: examination of underpowered studies in Cochrane reviews. PLoS ONE. 2013;8(3):e59202. doi: 10.1371/journal.pone.0059202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Appendix 1. PRISMA Network Meta-Analysis Checklist.
Additional file 3: Appendix 2. Searching Strategy.
Additional file 4: Appendix 3. WinBUGS Code for Network Meta-Analyses.
Additional file 5: Appendix 4. Included Studies List.
Additional file 6: Appendix 5. Basic Information of Included Studies.
Additional file 7: Appendix 6. Risk of Bias Assessment.
Additional file 8: Appendix 7. Grading of Recommendations, Assessment, Development and Evaluations (GRADE).
Additional file 9: Appendix 8. Outcomes of Pairwise Meta-Analysis, Network Meta-Analysis and Meta Regression.
Data Availability Statement
All data and material reported in this systematic review are from peer-reviewed publications. The WinBUGS code used are available in Additional file 4: Appendix S3.