Abstract
Objective
The study aimed to conduct a systematic review of the literature to verify the association between exposure to pesticides and allergic diseases (asthma, allergic rhinitis, and atopic dermatitis) in children and adolescents.
Method
A systematic review and meta-analysis were performed using the PRISMA method with the question “What is the association between exposure to pesticides and allergic diseases in children (asthma, allergic rhinitis, and atopic dermatitis)?” MEDLINE, EMBASE, SciELO, and Cochrane electronic databases were searched throughout the period in the literature up to September 2020. A total of 1296 studies were found, and 24 were selected.
Results
Exposure to pesticides showed a two-fold greater risk of developing or exacerbating asthma in children and adolescents (odds ratio [OR] = 2.14 95% confidence interval [CI] 1.26-3.64, p < 0.01). There was no association between exposure to pesticides and the development of allergic rhinitis (OR = 2.73, 95% CI 0.13-57.8, p = 0.52) and atopic dermatitis (OR = 2.19, 95% CI 0.51-9.36, p = 0.29).
Conclusions
Exposure to pesticides increases the risk of developing or exacerbating asthma in children and adolescents. There was no evidence of an association between exposure to pesticides and the development of allergic rhinitis and atopic dermatitis in children and adolescents, possibly due to the low number of studies found in this review.
Keywords: Agrochemicals; Asthma; Rhinitis; Dermatitis, atopic; Meta-analysis
Introduction
Pesticides were introduced in agriculture during the Green Revolution, which occurred in the late 1950s due to food shortages in several developing countries.1 From 1995, the Board of Directors of the United Nations Environment Program, concerned about the damage caused by persistent organic pollutants (POPs) to health and the environment, started a series of international actions culminating in the Stockholm Convention in 2001, which advocates for the restriction and banning of these substances. These initiatives demonstrate the global concern about the use of pesticides and their potential negative impact on health and the environment.2
Data from the Ministry of Agriculture, Livestock, and Supply show a significant increase in registrations of new pesticides from 2016 onwards in Brazil, reaching 493 new registrations in 2020.3 In addition, the use of pesticides per planted area increased from 3.2 kg per hectare to 6.7 kg/ha from 2005 to 2014.4
A pesticide can be considered highly toxic if it has acute toxicity, owing to its chronic toxic effects (even at low doses), or if it has the potential to contaminate the environment. Acute intoxications mostly occur in occupational settings. These are better known and easy to identify. However, chronic intoxications can affect the entire population since they are due to exposure to multiple pesticides present in food and the environment.2 Some of the possible consequences of chronic exposure to pesticides are cancer, neurotoxicity, infertility, miscarriage, malformation, hormonal imbalance, and effects on the immune system.5
Exposure to pesticides can also cause allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis. These diseases are characterized by type 2 inflammation, with a predominance of CD4+ T lymphocytes of the Th2 subtype, which recruit eosinophils to the site of inflammation by secreting IL-4, IL-5, and IL-13, besides stimulating B cells to produce IgE type antibodies.6,7
Data from a study8 conducted in Brazil with twenty thousand adolescents aged 13-14 years, nine years after phase III of the International Study of Asthma and Allergies in Childhood (ISAAC), showed a rapid increase in the prevalence of allergic diseases. Between 2003 and 2012, there was a 23% increase in the prevalence of physician-diagnosed asthma, a 12% increase in the prevalence of atopic eczema, and a 16% increase in the prevalence of nasal symptoms.
The objective of this study was to verify the association between exposure to pesticides and allergic diseases (asthma, allergic rhinitis, and atopic dermatitis) in children and adolescents, given their increasing prevalence.
Methods
A systematic review and meta-analysis were conducted using the PRISMA method (Preferred Reporting Items for Systematic Reviews and Meta-Analyses)9 to guide the preparation of the study. It was registered in PROSPERO (the International Database of Prospectively Registered Systematic Reviews) under the code CRD42021219890. The PECO strategy was used - P (Population of interest): children and adolescents; E (Exposure): exposure to pesticides; C (Comparator): children and adolescents with lower exposure to pesticides; O (Outcome): allergic diseases (asthma, rhinitis, and dermatitis) - to formulate the following research question: “What is the association between exposure to pesticides and allergic diseases in children (asthma, allergic rhinitis, and atopic dermatitis)?” MEDLINE, EMBASE, SciELO, and Cochrane electronic databases were searched. The descriptors “asthma,” “allergic rhinitis,” “atopic dermatitis,” and “pesticide” were used separately as follows: (asthma) AND (pesticide); (allergic rhinitis) AND (pesticide); and (atopic dermatitis) AND (pesticide).
The inclusion criteria were as follows: I) Studies that evaluated the association between exposure to pesticides and allergic diseases and II) Articles published up to September 2020 (the month in which this search was conducted). The exclusion criteria were as follows: I) Studies involving only adults; II) Articles that analyzed only domestic pesticides/insecticides; III) Studies with children working in agriculture; IV) Studies on acute intoxication; V) Review articles, case reports, editorials, letters, abstracts, and congress annals; VI) Articles in languages other than English, Portuguese, and Spanish.
After the search, the title and abstract of every article were read. The methodology was then evaluated. Finally, a list of relevant articles was compiled for a full reading. All these steps were performed by two independent researchers. After the articles were selected, a quality analysis was performed using the Methodological Items for Non-Randomized Studies (MINORS) tool,10 appropriate for non-randomized studies. This tool is composed of 8 items for non-comparative studies and 12 items for comparative studies, and each item carries a score of 0, 1, or 2 points. The total score can vary from 0 to 16 for non-comparative studies, and from 0 to 24 for comparative studies. This tool assesses the quality of the study design and execution as well as the results and data analysis. The higher the score, the better the quality of the analyzed article.
Meta-analysis was performed using the Review Manager 5.4 software. The association measures were analyzed by calculating the odds ratio (OR) with a confidence interval (CI) of 95%. The association measures were combined, resulting in a forest plot. The I-square (I²) was calculated for heterogeneity research, in which values equal to or greater than 50% were considered heterogeneous.
Results
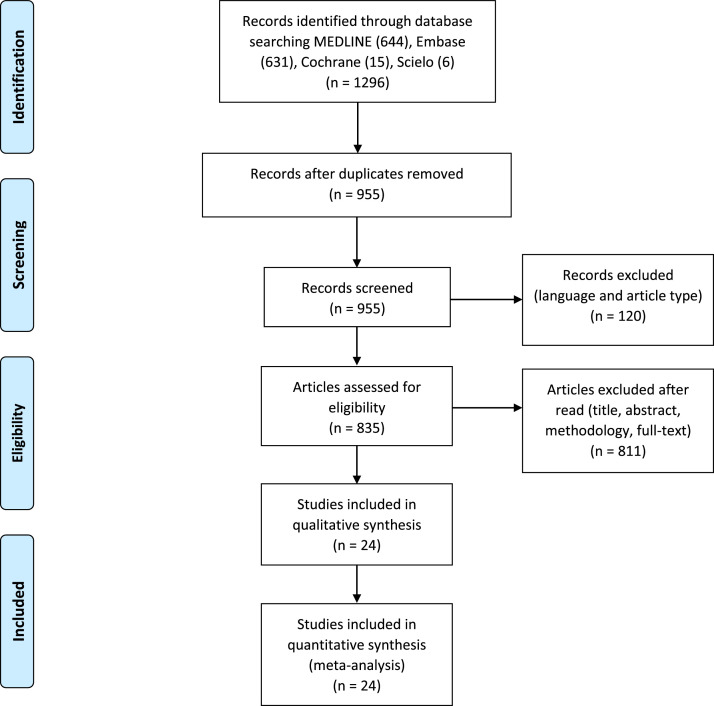
A total of 1,296 articles were found, 341 of which were duplicates and were excluded. A total of 120 articles in languages other than English, Portuguese, and Spanish, as well as review articles, editorials, letters, abstracts, congress annals, and case reports were excluded. After reading the title and abstract, 758 articles that did not address the topic of interest were excluded. Following the methodology evaluation, 40 were excluded for not having analyzed the association between exposure to pesticides and allergic diseases. Finally, 36 articles remained for a full reading. Of these, 24 were selected for this review (Figure 1).
Figure 1.
PRISMA flow diagram of the article selection process.
The selected articles were published between 2000 and 2019. All the studies were conducted in countries from the northern hemisphere: 12 from North America, 8 from Europe, 1 from Asia, 1 from the Middle East, and 1 mixed study from Europe and North America. The predominant study design was cohort (15 studies), corresponding to 62.5% of the total. The others were cross-sectional (6 studies) and case-control (3 studies) (Table 1).
Table 1.
Description of selected articles in the systematic review.
| Author, Year and Country | Study design | Objective | Sample | Results |
|---|---|---|---|---|
| Balte et al., 2017 Germany26 |
Cohort | Assess the association between DDE exposure and lung function | 344 children | DDE vs FVC Estimate 0.006; SE 0.02; p = 0.71 DDE vs FEV1 Estimate 0.004 SE 0.02; p = 0.81 |
| Benka-Coker et al., 2019 United States of America11 |
Cohort | Assess exposure to pesticides and asthma exacerbation using Leukotriene E4 in urine | 16 children (139 urine samples) | DAPs vs urinary LTE4 β: 4.1[0.6–7.6] pg/mg |
| Benka-Coker et al., 2020 United States of America12 |
Cohort | Assess exposure to pesticides and asthma exacerbation using Leukotriene E4 in urine | 16 children (139 urine samples) | βEDE: 8.7 (2.8, 14.6); CI=95% βEDM: 1.1 (0.5, 1.7) βEDAP: 4.1 (0.7, 7.5) |
| Bukalasa et al., 2018 Netherlands31 |
Cross-sectional | Assess exposure to pesticides and prevalence of asthma and related symptoms at 14 years old | 1470 adolescents | Distance from pesticide treated fields vs asthma (CI=95%) 100m OR = 0.31 (0.07, 1.32) 500m OR = 0.95 (0.57, 1.57) 1000m OR = 0.86 (0.52, 1.40) |
| Duramad et al., 2006 United States of America13 |
Cohort | Assess the association between organophosphate exposure and Th1 and Th2 cells | 239 2-year-old children | Th2 level in children living with farmers 0.8% (0.7–0.9%) CI 95% Th2 level in children who do not live with farmers 0.6% (0.5–0.7%) CI 95% p 0.02 Th2 vs eczema CI 95% 0.7% (0.6–1.0%) Th2 vs asthma CI 95%; p < 0.05 1.0% (0.7–1.2%) |
| Gascon et al., 2014 Spain28 |
Cohort | Assess prenatal effect of DDE, HCB, and PCB on the respiratory health of children from birth to 14 years old and assess the role of immune biomarkers | 405 (275 with blood samples at 4 years old) | DDE vs asthma CI 95% 10 years old RR=1.03 (0.71, 1.50) 14 years old RR=0.89 (0.61, 1.31) HCB vs asthma CI 95% 10 years old RR=1.21 (0.67, 2.18) 14 years old RR=1.08 (0.61, 1.90) ΣPCBs vs asthma CI 95% 10 years old RR=0.94 (0.82, 1.08) 14 years old RR=0.93 (0.82, 1.06) |
| Gharibi et al., 2020a United States of America15 |
Cross-sectional | Assess the impact of exposure to MBr (methyl bromide) in emergency asthma care | 4262 emergency visits of children and adolescents between 2 and 18 years old | 2-5 years OR = 1.024 (0.997, 1.052), CI 95% 6-18 years OR = 1.071 (1.016, 1.125), CI 95% |
| Gharibi et al., 2020b United States of America16 |
Cohort | Assess the association between 1,3-dichloropropene exposure and asthma emergency care in Southern California | 1331 2 to 18 years-old children and adolescents | Association between 1,3D and asthma emergency visits CI 95% 2-5 years OR = 1.065 (1.020, 1.113) 6-18 years OR = 1.142 (1.086, 1.196) |
| Gunier et al., 2018 United States of America17 |
Cohort | Assess pre- and postnatal exposure to pesticides and lung function and respiratory symptoms in 7-year-old children | 294 children | Prenatal exposure vs respiratory symptoms at 7 years old CI 95% Methyl Bromide OR = 1.1 (0.8, 1.6); p = 0.55 Chloropicrin OR = 1.1 (0.8, 1.4); p = 0.66 Metam sodium OR = 1.0 (0.8, 1.3); p = 0.88 1,3-DCP OR = 1.1 (0.8, 1.4); p = 0.58 Postnatal exposure vs respiratory symptoms at 7 years old CI 95% Methyl bromide OR = 1.0 (0.6, 1.6); p = 0.93 Chloropicrin OR = 1.0 (0.6, 1.5); p = 0.87 Metam sodium OR = 0.9 (0.6, 1.4); p = 0.72 1,3-DCP OR = 1.2 (0.6, 2.4); p = 0.64 Prenatal exposure vs asthma medication at 7 years old CI 95% Methyl bromide OR = 1.0 (0.6, 1.8); p = 0.87 Chloropicrin OR = 1.0 (0.6, 1.5); p = 0.94 Metam sodium OR = 1.2 (0.8, 1.8); p = 0.35 1,3-DCP OR = 1.3 (0.9, 2.0); p = 0.17 Postnatal exposure vs asthma medication at 7 years old CI 95% Methyl bromide OR = 0.8 (0.4, 1.7); p = 0.63 Chloropicrin OR = 0.9 (0.5, 1.6); p = 0.61 Metam sodium OR = 1.3 (0.7, 2.7); p = 0.41 1,3-DCP OR = 1.3 (0.4, 3.6); p = 0.66 |
| Hallit et al., 2017 Lebanon25 |
Case-control | Assess exposure to pesticides and respiratory symptoms (asthma) | 1503 children and adolescents | OR = 3.307 (1.848, 5.918), p < 0.001 (live in area treated with pesticides) OR = 0.5 (0.337, 0.744), p = 0.001 (live with someone who works with pesticides) |
| Karmaus et al., 2001 Germany27 |
Cross-sectional | Assess the association between exposure to organochlorine pesticides and infections and allergic diseases | 343 children (340 IgE) | Asthma (CI 95%) DDE ≥ 0.3 pg/l vs. < 0.3 pg/l OR = 3.71 (1.10, 12.56) DDE < 0.3 p g / h HCB > 0.2 pg/l vs. ≤ 0.2 pg/l OR = 1.21 (0.11, 13.82) DDE ≥ 0.3 pg/k HCB > 0.2 pg/l vs. ≤ 0.2 pg/l OR = 0.53 (0.13, 2.1 6) DDE < 0.3 pg/l CPCB > 0.48 pg/l vs. ≤ 0.48 pg/1§ no valid estimation DDE ≥ 0.3 pg/k ZPCB > 0.48 pg/l vs. ≤ 0.48 pg/l§ OR = 0.56 (0.13, 2.52) IgE ≥ 200kU/l (CI=95%) DDE ≥ 0.3 pg/l vs. < 0.3 pg/l OR = 2.28 (1.20, 4.31) DDE < 0.3 p g / h HCB > 0.2 pg/l vs. ≤ 0.2 pg/l OR = 0.91 (0.30, 2.73) DDE ≥ 0.3 pg/k HCB > 0.2 pg/l vs. ≤ 0.2 pg/l OR = 0.49 (0.19, 1.23) DDE < 0.3 pg/l CPCB > 0.48 pg/l vs. ≤ 0.48 pg/1§ OR = 1.08 (0.35, 3.31) DDE ≥ 0.3 pg/k ZPCB > 0.48 pg/l vs. ≤ 0.48 pg/l§ OR = 0.82 (0.32, 2.08) |
| Lu et al., 2018 Romania33 |
Cross-sectional | Assess domestic exposure to environmental pollutants and the impact on respiratory health of school-age children | 280 children | Proximity of pesticide sprayed crops vs asthma symptoms CI 95%, p < 0.05 Single-pollutant model OR = 1.11 (0.51, 2.29) Multi-pollutant model OR = 3.53 (1.27, 10.29) Multi-pollutant model controlled to CO2 OR = 4.17 (1.43, 13.26) |
| Masley et al., 2000 Canada22 |
Cross-sectional | Describe the health and local conditions of a rural area in Canada | 393 children and adolescents | Asthma in children from non-agricultural families vs agricultural families OR = 2.2 (0.8, 5.7); p = 0.12; CI 95% Bronchitis in children from non-farm vs agricultural families OR = 2.8 (1.6, 4.8); p < 0.001; CI 95% |
| Meng et al., 2016 China23 |
Case-control | Compare the serum concentration of certain pesticides in asthmatic and non-asthmatic children | Children 3-6 years 124 cases 109 controls |
Asthma CI 95% p,p'-DDE OR = 1.02 (1.01, 1.03); p = 0.0004 p,p'-DDD OR = 1.01 (0.99, 1.03); p = 0.62 o,p'-DDT OR = 0.99 (0.97, 1.01); p = 0.17 p,p'-DDT OR = 1.01 (0.99, 1.03); p = 0.23 HCB OR = 1.02 (1.01, 1.04); p = 0.01 α-HCH OR = 1.06 (1.02, 1.10); p = 0.001 β-HCH OR = 1.01 (1.00, 1.01); p = 0.02 γ-HCH OR = 1.02 (1.00, 1.05); p = 0.04 Heptachlor OR = 1.06 (1.02, 1.11); p = 0.003 Total OCPs OR = 1.00 (1.00, 1.00); p = 0.002 Severe asthma CI 95% HCB OR = 1.01 (0.99, 1.03); p = 0.06 α-HCH OR = 1.01 (0.99, 1.02); p = 0.23 β-HCH OR = 1.00 (0.99, 1.00); p = 0.34 γ-HCH OR = 1.01 (0.97, 1.04); p = 0.78 Heptachlor OR = 1.04(0.99, 1.09); p = 0.11 p,p'-DDE OR = 1.00 (1.00, 1.00); p = 0.25 p,p'-DDD OR = 0.97 (0.95, 0.99); p = 0.01 o,p'-DDT OR = 0.97 (0.96, 0.99); p = 0.01 p,p'-DDT OR = 0.98 (0.95, 1.02); p = 0.36 Total OCPs OR = 1.00 (0.99, 1.002); p = 0.89 |
| Perla et al., 2015 United States of America14 |
Cohort | Association between urine pesticide biomarkers, blood DDE and asthma | 1484 children 6-11 years-old 1239 children 12-15 years-old |
DDE blood vs asthma (940 adolescents 12-15 years-old) CI 95% Ever Asthma <40th percentile RR=1.00 Referent 40th–80th percentile RR+0.63 (0.35, 1.14) >80th percentile RR=0.81 (0.33, 2.00) Current Asthma <40th percentile RR=1.00 Referent 40th–80th percentile RR=0.47 (0.21, 1.05) ** >80th percentile RR=0.78 (0.24, 2.55) **025 < p ≤ .10. Marginally statistically significant DAP urine vs asthma (1484 children 6 -11 years-old) CI 95% Ever Asthma <25th percentile RR=1.00 Referent 25th–50th percentile RR=1.19 (0.65, 2.18)) 50th–75th percentile RR=1.71 (0.97, 2.99) >75th percentile RR=1.16 (0.62, 2.17) Current Asthma <25th percentile RR=1.00 25th–50th percentile RR=1.49 (0.72, 3.06) 50th–75th percentile RR=1.89 (1.01, 3.53) >75th percentile RR=1.42 (0.64, 3.16) DAP urine vs asthma (1293 adolescents 12-13 years-old) CI 95% Ever Asthma <25th percentile 1.00 Referent 25th–50th percentile 1.14 (0.69, 1.90) 50th–75th percentile 1.44 (1.01, 2.06)** >75th percentile 1.20 (0.66, 2.20) **0.025 < p ≤ 0.10. Marginally statistically significant Current Asthma <25th percentile 1.00 Referent 25th–50th percentile 1.04 (0.54, 1.99) 50th–75th percentile 1.33 (0.74, 2.40) >75th percentile 1.12 (0.59, 2.12) |
| Raanan et al., 2015 United States of America18 |
Cohort | Assess the association between early exposure to organophosphate pesticides and respiratory symptoms | Between 270 to 331 depending on the analysis | Total DAP pregnant urine vs symptoms at 5 or 7 years old OR = 1.28 (0.77, 2.13); CI 95%; p = 0.34 Total DAP in children vs symptoms at 5 and 7 years old OR = 2.53 (1.32, 4.86); CI 95%; p = 0.005 |
| Raanan et al., 2017 United States of America19 |
Cohort | Association between sulfur exposure, respiratory symptoms and lung function in children living in an agricultural community | 237 7-year-old children | Sulfur exposure before 1 year vs respiratory symptoms at 7 years by different distances CI 95% 0.5Km OR = 1.71 (1.14, 2.57); p = 0.009 1Km OR = 2.09 (1.27, 3.46); p = 0.004 3Km OR = 0.96 (0.40, 2.26); p = 0.92 Sulfur exposure before 1 year vs use of asthma medication at 7 years by different distances CI 95% 0.5km OR = 2.23 (1.19, 4.21); p = 0.01 1Km OR = 3.51 (1.50, 8.23); p = 0.004 3Km OR = 2.10 (0.39, 11.30); p = 0.39 |
| Raherison et al., 2019 France32 |
Cohort | Assess the association between exposure to airborne pesticides and asthma and rhinitis | 281 children (96 children with analyzed urine samples in both phases and complete questionnaire) | Exposure to pesticides in air vs symptom score CI 95% Asthma OR = 3.93 (0.40, 38.44); p = 0.2398 Rhinitis OR = 0.27 (0.03, 2.35); p = 0.2362 ETU urinary concentration vs symptoms score CI 95% Asthma OR = 2.01 (0.54, 7.52); p = 0.2936 Rhinitis OR = 2.83 (0.75, 10.75); p = 0.1262 |
| Salam et al., 2004 United States of America20 |
Case-control | Assess risk factors for early life asthma | 338 cases 570 controls |
Pesticide vs asthma OR = 1.61 (0.93, 2.79); CI 95% Pesticide vs persistent asthma in the first year of life OR 3.58 (1.59–8.06); CI 95% Pesticide vs asthma in the first year of life OR = 2.39 (1.17, 4.89); CI 95% |
| Salameh et al., 2003 Lebanon24 |
Cross-sectional | Assess the association between exposure to pesticides and chronic respiratory diseases in children and adolescents | 407 children with diagnosed chronic respiratory disease 2016 children without respiratory symptoms |
Asthma OR 1.73 (1.02, 2.97) CI 95%; p < 0.01 |
| Smit et al., 201534 Greenland and Ukraine |
Cohort | Explore the association between prenatal exposure to environmental contaminants and asthma and eczema | Children 5-9 years old (1024 pairs mother-child, 492 from Ukraine and 532 from Greenland) | Prenatal exposure to organochlorines vs association between respiratory symptoms and eczema (CI 95%) Ever asthma OR = 0.96 (0.77, 1.20) Ever eczema OR = 0.89 (0.72, 1.10) Current eczema OR = 1.01 (0.80, 1.27) |
| Sunyer et al., 2005 Spain29 |
Cohort | Assess the association between levels of DDE and other organochlorines in cord blood and atopy and asthma in early childhood | 468 children | Cord blood DDE vs wheezing at 4 years old CI 95% (DDE at each concentration fold) All RR=1.32 (1.13, 1.54) Non-atopic RR=1.30 (1.05, 1.62) DDE by quartiles All < 0.57 RR=1 0.57-1.03 RR=1.00 (0.41, 2.43) 1.03-1.90 RR=1.62 (0.70, 3.74) <1.90 RR=2.36 (1.19, 4.69) Non-atopic < 0.57 RR=1 0.57-1.03 RR=1.32 (0.37, 4.70) 1.03-1.90 RR=2.63 (0.96, 7.20) <1.90 RR=2.49 (1.00, 6.19) |
| Sunyer et al., 2006 Spain30 |
Cohort | Assess the association between exposure to DDE and asthma at age 6 years | 462 children | DDE at birth vs asthma at 6 years OR = 1.18 (1.01, 1.39); CI 95% |
| Weselak et al., 2007 Canada21 |
Cohort | To assess the association between parental exposure to pesticides during pregnancy and asthma, bronchitis, and allergic rhinitis in children | 3405 children | CI 95% Any pesticide vs asthma OR = 1.00 (0.71, 1.40) Fungicide x asthma OR = 1.25 (0.74, 2.12) Insecticide x asthma OR = 1.06 (0.73, 1.54) Herbicide x asthma OR = 0.84 (0.55, 1.30) Other pesticides vs asthma OR = 0.55 (0.23, 1.31) Any pesticide vs allergic rhinitis OR = 1.58 (1.19, 2.08) Fungicide vs allergic rhinitis OR = 1.69 (1.15, 2.47) Insecticide vs allergic rhinitis OR = 1.48 (1.07, 2.03) Herbicide vs allergic rhinitis OR = 1.56 (1.15, 2.11) Other pesticides vs allergic rhinitis OR = 1.25 (0.68, 2.31) |
Among the studies that comprised the final sample, 41.6% were conducted in the United States of America (USA), two of which analyzed the relationship between exposure to pesticides and asthma exacerbations using the measurement of urinary leukotriene E4, both belonging to the same cohort (aggravating factors of asthma in a rural environment), showing an association between pesticide metabolites in urine and leukotriene E4.11,12 A cohort study from the USA evaluated organophosphorus pesticide exposure and Th1 and Th2 levels in children living with farmers and children not living with farmers. It reported higher Th2 levels in children living with farmers.13 Another cohort study conducted in the USA evaluated the association between pesticide metabolites in urine, dichlorodiphenyldichloroethylene (DDE) in blood, and asthma. It found no association between the metabolites and the outcomes in asthma.14 Two other American studies from the same group evaluated the impact of 1,3-dichloropropene (1,3D)15 and methylbromide (Mbr)16 exposure and asthma exacerbations and emergency department visits and found a positive association in both. Two studies, both with data from the same cohort (Center for the Health Assessment of Mothers and Children of Salinas - CHAMACOS), evaluated prenatal exposure to pesticides and respiratory symptoms in childhood. The first showed no association between the variables, and the second showed an association between early exposure to pesticides and asthma in childhood.17,18 Based on data from the same cohort, the impact of sulfur pesticide (considered as relatively safe and used in both conventional and organic agriculture) on pulmonary function and respiratory symptoms was evaluated, showing adverse effects of this substance on the respiratory health of children.19 A case-control study conducted in the USA showed an association between pesticide exposure in the first year of life and diagnosis of asthma before 5 years of age.20
A Canadian study evaluated the association of parental exposure during pregnancy and asthma and rhinitis in children, showing a positive association for rhinitis but no association for asthma.21 A significant predominance of asthma was identified in children from farming families in another Canadian study.22
A case-control study conducted in China compared blood concentrations of pesticides in asthmatic and non-asthmatic children, showing a positive association between exposure to pesticides and diagnosis of asthma.23
Two Lebanese studies evaluated the exposure of children and adolescents to pesticides through occupational exposure of their parents and by living in an area treated with pesticides. Both the studies showed pesticides as factors associated with asthma.24,25
Among the European studies, a study from Germany evaluated the association between DDE in blood and lung function. DDE was seen to have a height-reducing effect in children, with a consequent effect on forced vital capacity (FVC) and forced expiratory volume in one second (FEV1).26 Another German study studied the association between organochlorines in blood, IgE, and asthma, showing a positive association between pesticides and asthma and between pesticides and increased IgE.27 A study from Spain evaluated prenatal exposure using cord blood tests for DDE, hexachlorobenzene (HCB), and polychlorinated biphenyls (PCB) and the presence of asthma up to the age of 14 years and found a positive association between prenatal exposure and adverse effects on the respiratory system.28 Two other studies from Spain.29,30 evaluated the association between DDE in cord blood and asthma in children, indicating a positive association. A study from the Netherlands evaluated prenatal exposure in residential areas within 100 m, 500 m, and 1000 m of pesticide-treated agricultural fields and asthma at 14 years of age. It reported no association.31 A study from France showed an association between ethylene thiourea (ETU - a substance resulting from the degradation of certain fungicides) in urine and asthma and rhinitis in children.32 In Romania, a study showed that living near spraying areas may be a risk factor for the development of asthma symptoms.33 A study from Greenland and Ukraine assessed prenatal pesticide exposure and childhood asthma and eczema symptoms, finding a negative association between organochlorine exposure and eczema.34
Table 2 shows the scores of the quality assessment using the MINORS tool. The non-comparative studies scored between 11 and 14 (total possible score is 0-16) and the three comparative studies scored between 20 and 22 (total possible score is 0-24).
Table 2.
Scores of articles selected through the MINORS tool criteria (C).
| Studies | C 1 | C 2 | C 3 | C 4 | C 5 | C 6 | C 7 | C 8 | Total Score |
|---|---|---|---|---|---|---|---|---|---|
| Balte et al., 201726 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 0 | 12 |
| Benka-Coker et al., 201911 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Benka-Coker et al., 202012 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Bukalasa et al., 201831 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Duramad et al., 200613 | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 12 |
| Gascon et al., 201428 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Gharibi et al., 2020a15 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Gharibi et al., 2020b16 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Gunier et al., 201817 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Hallit et al., 201725 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 21a |
| Karmaus et al., 200127 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 2 | 12 |
| Lu et al., 201833 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 14 |
| Masley et al., 200022 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Meng et al., 201623 | 2 | 2 | 2 | 2 | 0 | 2 | 2 | 2 | 22b |
| Perla et al., 201514 | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 12 |
| Raanan et al., 201518 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Raanan et al., 201719 | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 12 |
| Raherison et al., 201932 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Salam et al., 200420 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 2 | 20c |
| Salameh et al., 200324 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
| Smit et al., 201534 | 2 | 1 | 2 | 2 | 0 | 2 | 1 | 2 | 12 |
| Sunyer et al., 200529 | 2 | 2 | 2 | 1 | 0 | 2 | 1 | 2 | 12 |
| Sunyer et al., 200630 | 2 | 1 | 2 | 1 | 2 | 0 | 1 | 2 | 11 |
| Weselak et al., 200721 | 2 | 2 | 2 | 2 | 0 | 2 | 1 | 2 | 13 |
Additional criteria for comparative studies (Hallit et al., 201725): C 9: 2; C 10: 2; C 11: 2; C 12: 2; Total Score: 21.
Additional criteria for comparative studies (Meng et al., 201623): C 9: 2; C 10: 2; C 11: 2; C 12: 2; Total Score: 22.
Additional criteria for comparative studies (Salam et al., 200420): C 9: 2; C 10: 2; C 11: 2; C 12: 2; Total Score: 20.
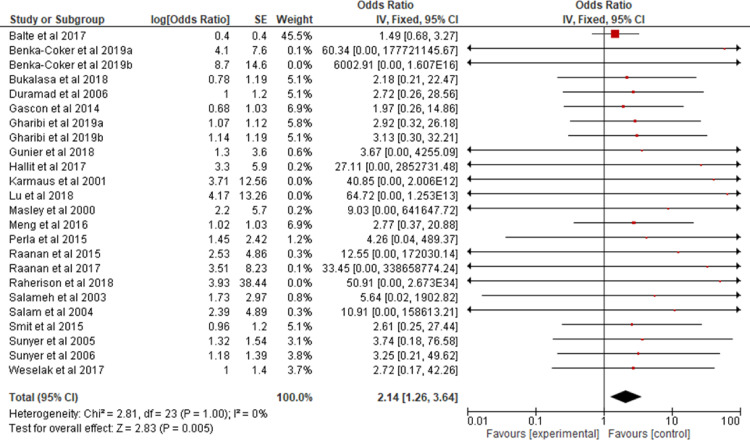
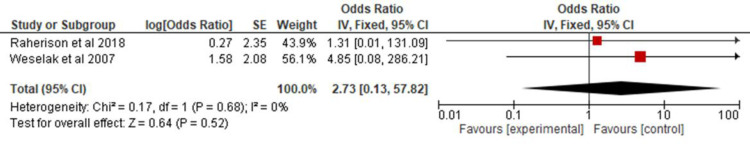
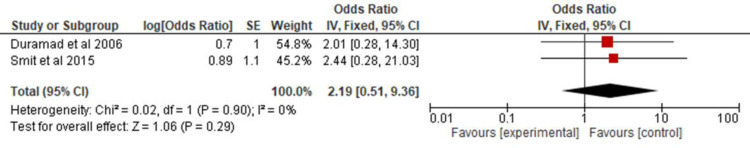
All the studies selected for this review were combined in a meta-analysis (Figure 2) to analyze the association of asthma in children and adolescents exposed to pesticides. A 2.14 OR (CI 95% 1.26, 3.64) and p = 0.005 were found. Two of the studies32,21 provided data on allergic rhinitis and were combined in a meta-analysis (Figure 3) to verify the association between exposure to pesticides and allergic rhinitis in children and adolescents (OR 2.73; CI 95% 0.13, 57.8; p = 0.52). Two other studies13,34 were combined in a meta-analysis (Figure 4) to evaluate the association of atopic dermatitis in children and adolescents exposed to pesticides (OR, 2.19; 95% CI 0.51, 9.36; p = 0.29).
Figure 2.
Odds ratio of asthma among children and adolescents exposed to pesticides.
Figure 3.
Odds ratio of allergic rhinitis among children and adolescents exposed to pesticides.
Figure 4.
Odds ratio of atopic dermatitis among children and adolescents exposed to pesticides.
Discussion
The association of exposure to pesticides and allergic diseases in children (asthma, allergic rhinitis, and atopic dermatitis) is not well established. However, the high prevalence of these allergic diseases in children and adolescents worldwide and the increased use of these chemicals in crops raises the possibility of a relationship between them. The 24 studies that were selected based on the criteria indicated in the methodology are concentrated entirely in the northern hemisphere, with half coming from North America and one-third from Europe. Three studies were conducted in developing countries: two in Lebanon25,24 and a mixed one in Ukraine-Greenland,34 the former being considered a developing country. In Brazil, although there is a widespread use of pesticides and a significant prevalence of asthma, rhinitis, and dermatitis, no studies were found on the subject, highlighting the need to conduct national studies.
The analyzed studies used different ways to measure exposure to pesticides, and they can be divided into various classes: concentration of pesticides in blood, the concentration of pesticide metabolites in urine, exposure of the child through occupational exposure of parents, estimates based on official monitoring data, and living near areas treated with pesticides. Some studies used more than one way to measure exposure. Such methodological differences resulted in heterogeneous results, some more robust than others, thereby making it difficult to evaluate the association between exposure to pesticides and allergic diseases, which is a limitation of the present study. Another limitation was the fact that many articles did not specify the pesticide studied, hindering the performance of more in-depth analyses of classes of pesticides and allergic diseases. The proposed methodology and language restrictions also reduced the number of articles selected for review. All the articles were concerned with analyzing and correcting possible confounding factors, such as exposure to tobacco smoke or pollution.
Seven studies evaluated prenatal exposure and the development of respiratory symptoms in children and adolescents. This evaluation is relevant since women, not only those working or living with someone who works in agriculture, may be exposed to varying degrees and bring consequences to their offspring. During the first half of gestation, the bronchi are developing and branching; in the second half, the alveoli begin to develop, and for several years after birth, they continue to mature, increasing in number, size, and complexity.35 Studies with animal models showed that organophosphate doses lower than those required to cause acetylcholinesterase inhibition was able to induce airway hyperreactivity.36,37 A study involving 331 children aged 7-10 years in Germany showed a relationship between increased IgE levels and serum levels of DDE. These findings indicate that DDE changes the response of the immune system towards the production of Th2 lymphocytes, causing allergic diseases such as asthma.38 Thus, further studies on pesticide use that can go beyond acute toxicity are necessary to understand its long-term effects from gestation to adulthood.
Another point addressed in one of the studies39 is the immunological impact of pesticides. An increase in Th2 cells, characteristic of an allergic inflammatory pattern, was identified in children living with people who worked in agriculture. This finding may indicate how pesticides trigger or exacerbate asthma, showing potential paths for future research in the area.
Figure 2 shows the result of the meta-analysis with a narrow CI, low heterogeneity, and significance in exposure to pesticides, with a 2.14-fold increased risk of children and adolescents presenting or exacerbating asthma. Asthma is a chronic disease with high prevalence in Brazil and the rest of the world in both adults and children/adolescents. It is responsible for reduced quality of life, absenteeism from school and work, hospitalization, and death. Thus, it is extremely important to conduct more studies on the impact of pesticides on the respiratory health of children and adolescents, especially in Brazil, a country where these compounds are widely used.
Regardless of the country of origin and how pesticide exposure and respiratory status were analyzed, all studies were to the right of the line of null effect of the forest plot. This study indicates that this is a global issue that requires international efforts to determine and mitigate the impact of pesticides on health and the need for expanding and continuing the actions of the Board of Directors of the United Nations Environment Program, which aims at reducing and banning these compounds as stated in the Stockholm and Rotterdam Conventions.
As for the analyses of allergic rhinitis and atopic dermatitis, there were few studies selected by the methodology applied, and the combined results of the two studies with data on rhinitis showed a three times higher risk, but with a wide CI and no statistical significance. Considering that about 80% of asthma patients have rhinitis, and its control impacts asthma, further studies are needed to determine the association of pesticides on rhinitis symptoms. The combined result of the data on atopic dermatitis identified a two times higher risk, with a smaller CI compared to that of rhinitis, but also with no statistical significance, indicating the need for further studies to evaluate this association.
In the study by Hyland et al.,40 an organic diet was found to reduce urinary excretion of insecticide, herbicide, and fungicide metabolites in adults and children, demonstrating how ingestion of pesticide-contaminated water and food can cause or exacerbate asthma, allergic rhinitis, and atopic dermatitis.
A limitation of this study is that it is a review, though it was systematized and the best available guidelines were followed while developing it. Another limiting factor is that studies in languages other than English, Portuguese, and Spanish were excluded from the review. There were only two studies that evaluated allergic rhinitis and two studies that analyzed atopic dermatitis. This may have influenced the results found in the evaluation of these two diseases.
The results showed that there is an association between exposure to pesticides and the risk of development or exacerbation of asthma in children and adolescents. It is essential to study the exposure to pesticides when taking the medical history of children and adolescents with asthma. The association between exposure to pesticides and the development of allergic rhinitis and atopic dermatitis in children and adolescents has not been proven. Better control and supervision of pesticide use in Brazil and the rest of the world are necessary, aiming at health promotion and prevention of potentially serious allergic diseases such as asthma in children and adolescents.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at Universidade Federal do Paraná, Curitiba, PR, Brazil.
References
- 1.Pimentel D. Green revolution agriculture and chemical hazards. Sci Total Environ. 1996;188:S86–S98. doi: 10.1016/0048-9697(96)05280-1. [DOI] [PubMed] [Google Scholar]
- 2.Food and Agriculture Organization of the United Nations . Food and Agriculture Organization of the United States and World Health Organization; Rome (IT): 2016. Guidelines on highly hazardous pesticides [Internet]http://www.fao.org/documents/card/en/c/a5347a39-c961-41bf-86a4-975cdf2fd063/ [cited 2021 Jan 20]. Available from: [Google Scholar]
- 3.Brasil. Coordenação Geral de Agrotóxicos. Ministério da Agricultura, Pecuária e Abastecimento. Registros concedidos 2005-2021 [cited 2021 Jan 20]. Available from:https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-agricolas/agrotoxicos/informacoes-tecnicas
- 4.Valadares A, Alves F, Galiza M. Instituto de Pesquisa Econômica Aplicada; Diretoria de Estudos e Políticas Sociais (Disoc); Brasília: 2020. Nota técnica n.° 65. O Crescimento do uso de agrotóxicos: uma análise descritiva dos resultados do censo agropecuário 2017; p. 42.https://www.ipea.gov.br/portal/index.php?option=com_content&view=article&id=35512 [cited 2021 Dec 7]. Available from: [Google Scholar]
- 5.Carneiro F, Rigotto R, Giraldo L, Pignati W, Rizzolo A, Alexander V, et al. Associação Brasileira de Saúde Coletiva (ABRASCO); Rio de Janeiro: 2012. Parte 1 - Agrotóxicos, Segurança Alimentar e Nutricional e Saúde [Internet]https://www.abrasco.org.br/site/wp-content/uploads/2015/03/Dossie_Abrasco_01.pdf [cited 2021 Dec 7]. Available from: [Google Scholar]
- 6.Reis AP dos, Machado JA. Biomarkers and immunobiological agents in asthma. Arq Asma Alerg Imunol. 2018;2:405–415. [Google Scholar]
- 7.Saglani S, Lloyd CM, et al. In: Kendig's Disorders of the Respiratory Tract in Children. Wilmott RW, Bush A, Deterding RR, Ratjen F, Sly P, Zar H, et al., editors. Elsevier Inc.; Philadelphia: 2019. The Immunopathogenesis of Asthma; pp. 665–676.e3. [Google Scholar]
- 8.Solé D, Rosário Filho NA, Sarinho ES, Camelo-Nunes IC, Barreto BA, Medeiros ML, et al. Prevalence of asthma and allergic diseases in adolescents: nine-year follow-up study (2003-2012) J Pediatr (Rio J) 2015;91:30–35. doi: 10.1016/j.jped.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10:89. doi: 10.1186/s13643-021-01626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 11.Benka-Coker WO, Loftus C, Karr C, Magzamen S. Characterizing the joint effects of pesticide exposure and criteria ambient air pollutants on pediatric asthma morbidity in an agricultural community. Environ Epidemiol. 2019;3:e046. doi: 10.1097/EE9.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benka-Coker W, Loftus C, Karr C, Magzamen S. Association of organophosphate pesticide exposure and a marker of asthma morbidity in an agricultural community. J Agromedicine. 2020;25:106–114. doi: 10.1080/1059924X.2019.1619644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perla ME, Rue T, Cheadle A, Krieger J, Karr CJ. Biomarkers of insecticide exposure and asthma in children: a national health and nutrition examination survey (NHANES) 1999-2008 analysis. Arch Environ Occup Health. 2015;70:309–322. doi: 10.1080/19338244.2014.910490. [DOI] [PubMed] [Google Scholar]
- 15.Gharibi H, Entwistle MR, Schweizer D, Tavallali P, Cisneros R. The association between 1,3-dichloropropene and asthma emergency department visits in California, USA from 2005 to 2011: a bidirectional-symmetric case crossover study. J Asthma. 2020;57:601–609. doi: 10.1080/02770903.2019.1590596. [DOI] [PubMed] [Google Scholar]
- 16.Gharibi H, Entwistle MR, Schweizer D, Tavallali P, Thao C, Cisneros R. Methyl-bromide and asthma emergency department visits in California, USA from 2005 to 2011. J Asthma. 2020;57:1227–1236. doi: 10.1080/02770903.2019.1645167. [DOI] [PubMed] [Google Scholar]
- 17.Gunier RB, Raanan R, Castorina R, Holland NT, Harley KG, Balmes JR, et al. Residential proximity to agricultural fumigant use and respiratory health in 7-year old children. Environ Res. 2018;164:93–99. doi: 10.1016/j.envres.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raanan R, Harley KG, Balmes JR, Bradman A, Lipsett M, Eskenazi B. Early-life exposure to organophosphate pesticides and pediatric respiratory symptoms in the CHAMACOS cohort. Environ Health Perspect. 2015;123:179–185. doi: 10.1289/ehp.1408235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raanan R, Gunier RB, Balmes JR, Beltran AJ, Harley KG, Bradman A, Eskenazi B. Elemental sulfur use and associations with pediatric lung function and respiratory symptoms in an agricultural community (California, USA) Environ Health Perspect. 2017;125 doi: 10.1289/EHP528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salam MT, Li YF, Langholz B, Gilliland FD, Children's Health Study Early-life environmental risk factors for asthma: findings from the children's health study. Environ Health Perspect. 2004;112:760–765. doi: 10.1289/ehp.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weselak M, Arbuckle TE, Wigle DT, Krewski D. In utero pesticide exposure and childhood morbidity. Environ Res. 2007;103:79–86. doi: 10.1016/j.envres.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Masley ML, Semchuk KM, Senthilselvan A, McDuffie HH, Hanke P, Dosman JA, et al. Health and environment of rural families: results of a community canvass survey in the prairie ecosystem study (PECOS) J Agric Saf Health. 2000;6:103–115. doi: 10.13031/2013.3023. [DOI] [PubMed] [Google Scholar]
- 23.Meng G, Feng Y, Nie Z, Wu X, Wei H, Wu S, et al. Internal exposure levels of typical POPs and their associations with childhood asthma in Shanghai, China. Environ Res. 2016;146:125–135. doi: 10.1016/j.envres.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Salameh PR, Baldi I, Brochard P, Raherison C, Abi Saleh B, Salamon R. Respiratory symptoms in children and exposure to pesticides. Eur Respir J. 2003;22:507–512. doi: 10.1183/09031936.03.00107403a. [DOI] [PubMed] [Google Scholar]
- 25.Hallit S, Raherison C, Waked M, Salameh P. Association between caregiver exposure to toxics during pregnancy and childhood-onset asthma: a case-control study. Iran J Allergy Asthma Immunol. 2017;16:488–500. [PubMed] [Google Scholar]
- 26.Balte PP, Kühr J, Kruse H, Karmaus WJJ. Body burden of dichlorodiphenyl dichloroethene (DDE) and childhood pulmonary function. Int J Environ Res Public Health. 2017;14:1376. doi: 10.3390/ijerph14111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karmaus W, Kuehr J, Kruse H. Infections and atopic disorders in childhood and organochlorine exposure. Arch Environ Health. 2001;56:485–492. doi: 10.1080/00039890109602896. [DOI] [PubMed] [Google Scholar]
- 28.Gascon M, Sunyer J, Martínez D, Guerra S, Lavi I, Torrent M, Vrijheid M. Persistent organic pollutants and children's respiratory health: the role of cytokines and inflammatory biomarkers. Environ Int. 2014;69:133–140. doi: 10.1016/j.envint.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Sunyer J, Torrent M, Muñoz-Ortiz L, Ribas-Fitó N, Carrizo D, Grimalt J, et al. Prenatal dichlorodiphenyldichloroethylene (DDE) and asthma in children. Environ Health Perspect. 2005;113:1787–1790. doi: 10.1289/ehp.8127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunyer J, Torrent M, Garcia-Esteban R, Ribas-Fitó N, Carrizo D, Romieu I, et al. Early exposure to dichlorodiphenyldichloroethylene, breastfeeding and asthma at age six. Clin Exp Allergy. 2006;36:1236–1241. doi: 10.1111/j.1365-2222.2006.02560.x. [DOI] [PubMed] [Google Scholar]
- 31.Bukalasa JS, Brunekreef B, Brouwer M, Koppelman GH, Wijga AH, Huss A, et al. Associations of residential exposure to agricultural pesticides with asthma prevalence in adolescence: the PIAMA birth cohort. Environ Int. 2018;121:435–442. doi: 10.1016/j.envint.2018.09.029. [DOI] [PubMed] [Google Scholar]
- 32.Raherison C, Baldi I, Pouquet M, Berteaud E, Moesch C, Bouvier G, et al. Pesticides exposure by air in vineyard rural area and respiratory health in children: a pilot study. Environ Res. 2019;169:189–195. doi: 10.1016/j.envres.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Lin S, Lawrence WR, Lin Z, Gurzau E, Csobod E, et al. Evidence from SINPHONIE project: Impact of home environmental exposures on respiratory health among school-age children in Romania. Sci Total Environ. 2018;621:75–84. doi: 10.1016/j.scitotenv.2017.11.157. [DOI] [PubMed] [Google Scholar]
- 34.Smit LA, Lenters V, Høyer BB, Lindh CH, Pedersen HS, Liermontova I, et al. Prenatal exposure to environmental chemical contaminants and asthma and eczema in school-age children. Allergy. 2015;70:653–660. doi: 10.1111/all.12605. [DOI] [PubMed] [Google Scholar]
- 35.Schittny JC. Development of the lung. Cell Tissue Res. 2017;367:427–444. doi: 10.1007/s00441-016-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lein PJ, Fryer AD. Organophosphorus insecticides induce airway hyperreactivity by decreasing neuronal M2 muscarinic receptor function independent of acetylcholinesterase inhibition. Toxicol Sci. 2005;83:166–176. doi: 10.1093/toxsci/kfi001. [DOI] [PubMed] [Google Scholar]
- 37.Fryer AD, Lein PJ, Howard AS, Yost BL, Beckles RA, Jett DA. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. Am J Physiol Lung Cell Mol Physiol. 2004;286:L963–L969. doi: 10.1152/ajplung.00343.2003. [DOI] [PubMed] [Google Scholar]
- 38.Karmaus W, Brooks KR, Nebe T, Witten J, Obi-Osius N, Kruse H. Immune function biomarkers in children exposed to lead and organochlorine compounds: a cross-sectional study. Environ Health. 2005;4:5. doi: 10.1186/1476-069X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duramad P, Harley K, Lipsett M, Bradman A, Eskenazi B, Holland NT, et al. Early environmental exposures and intracellular Th1/Th2 cytokine profiles in 24-month-old children living in an agricultural area. Environ Health Perspect. 2006;114:1916–1922. doi: 10.1289/ehp.9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyland C, Bradman A, Gerona R, Patton S, Zakharevich I, Gunier RB, et al. Organic diet intervention significantly reduces urinary pesticide levels in U.S. children and adults. Environ Res. 2019;171:568–575. doi: 10.1016/j.envres.2019.01.024. [DOI] [PubMed] [Google Scholar]