Abstract
Background
Propionibacterium freudenreichii is used in biotechnological applications to produce vitamin B12. Although cultured mainly in anaerobic conditions, microaerobic conditions can greatly enhance biomass formation in P. freudenreichii. Since B12 yields may be coupled to biomass formation, microaerobic conditions show great potential for increasing B12 yields in P. freudenreichii.
Results
Here we show biomass formation increases 2.7 times for P. freudenreichii grown in microaerobic conditions on lactate versus anaerobic conditions (1.87 g/L vs 0.70 g/L). Consumption of lactate in microaerobic conditions resulted first in production of pyruvate, propionate and acetate. When lactate was depleted, pyruvate and propionate were oxidised with a concomitant sixfold increase in the B12 titer compared to anaerobic conditions, showing potential for propionate and pyruvate as carbon sources for B12 production. Consequently, a fed-batch reactor with anaerobically precultured lactate-grown cells was fed propionate in microaerobic conditions resulting in biomass increase and production of B12. Vitamin yields increased from 0.3 B12 per mmol lactate in anaerobic conditions to 2.4 B12 per mmol lactate and 8.4 B12 per mmol propionate in microaerobic conditions. Yield per cell dry weight (CDW) increased from 41 per g CDW in anaerobic conditions on lactate to 92 per g CDW on lactate and 184 per g CDW on propionate in microaerobic conditions.
Conclusions
Here we have shown both B12 yield per substrate and per CDW were highest on cells oxidising propionate in microaerobic conditions, showing the potential of propionate for biotechnological production of vitamin B12 by P. freudenreichii.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12934-022-01945-8.
Keywords: Propionibacterium freudenreichii, Vitamin B12, Respiration, Propionate, Wood-Werkman cycle
Background
Vitamin B12 (B12), or cobalamin, is an essential vitamin for humans which is exclusively produced by some Bacteria and Archaea. It acts as a co-factor in enzymatic processes, which can be divided into carbon rearrangement reactions, intramolecular methyl transfer reactions and reduction of ribonucleotide triphosphate to 2-deoxyribonucleotide triphosphate [1]. In propionic acid bacteria B12 acts as a co-factor in the characteristic Wood-Werkman cycle used to ferment substrates such as lactate. B12 is essential in the isomerisation of succinyl-CoA to methylmalonyl-CoA [2], as it acts as a co-factor of methylmalonyl-CoA mutase. B12 thus plays an essential role in the main metabolism of propionic acid bacteria under anaerobic fermentation conditions.
B12 can be synthesised in bacteria through an aerobic and an anaerobic pathway, of which the anaerobic pathway is used by Propionibacterium freudenreichii [3, 4]. Although the B12 production pathway in P. freudenreichii is anaerobic, yield increments have been reported for P. freudenreichii grown under aerobic conditions [5]. On the other hand, Quesada-Chanto et al. [6] and Menon et al. [7] found decreased B12 production when oxygen was present. The presented studies on B12 production have in common that relatively high amounts of oxygen are used, resulting in decreased cytochrome synthesis [5, 8] potentially caused by diminished -aminolevulinic acid dehydratase activity [7], resulting in lower growth rates and at higher oxygen levels even in diminished growth. Since heme and B12 share the same precursors produced by -aminolevulinic acid dehydratase, a decreased B12 yield could be expected when oxygen diminishes the respective dehydratase activity.
Recently Dank et al. [9] have shown lactate can be completely oxidised using a continuous flow of low amounts of oxygen in a three phase cultivation. Under these conditions, large proportions of lactate are fermented to propionate and acetate, after which, when lactate is depleted, propionate starts being oxidised and lastly acetate is being oxidised. The production and subsequent consumption of propionate shown by Dank et al. [9] can be explained by operation of the Wood-Werkman cycle in reverse direction [10] and a functional electron transport chain. The terminal oxidase of P. freudenreichii in the electron transport chain is cytochrome bd [11], which contains heme [12]. Since a functional electron transport chain is required for oxidising propionate with oxygen as terminal electron acceptor, it is conceivable that the conditions used by Dank et al. [9] allow heme, and thus also B12 synthesis. As B12 is required as co-factor for methylmalonyl-CoA mutase, reversing the Wood-Werkman cycle conceivably still results in a metabolic demand for B12 and consequently B12 production. This led us to test the hypothesis that B12 is actively produced by P. freudenreichii utilising propionate. In this study we confirm the ‘propionate switch’ observed by Dank et al. [9] in microaerobic conditions on lactate and consequently show microaerobic conditions enhance B12 yield on lactate. Furthermore we show propionate can be used as sole carbon source for the production of B12 and we show B12 yields are drastically improved using propionate as sole carbon source under microaerobic conditions compared to lactate in microaerobic and anaerobic conditions.
Results
Biomass formation and B12 yield on lactate drastically increase in microaerobic conditions
Biomass formation and B12 yield were monitored in P. freudenreichii cultures grown on lactate in anaerobic and microaerobic conditions.
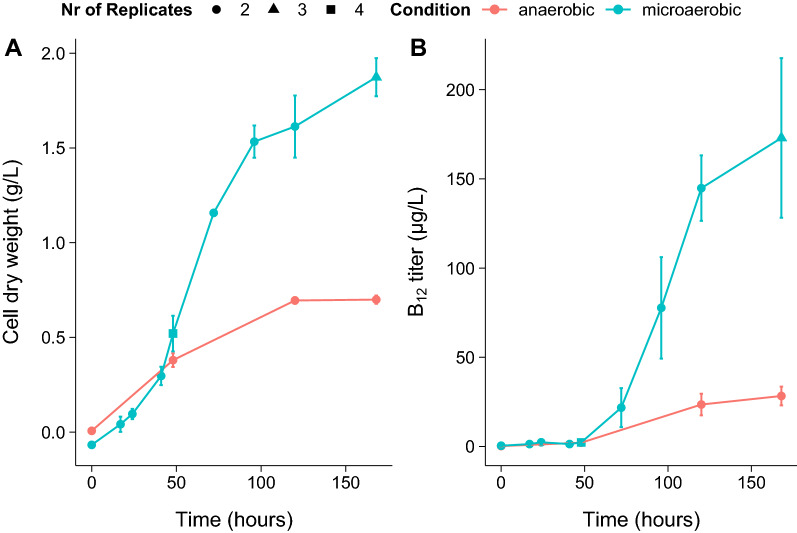
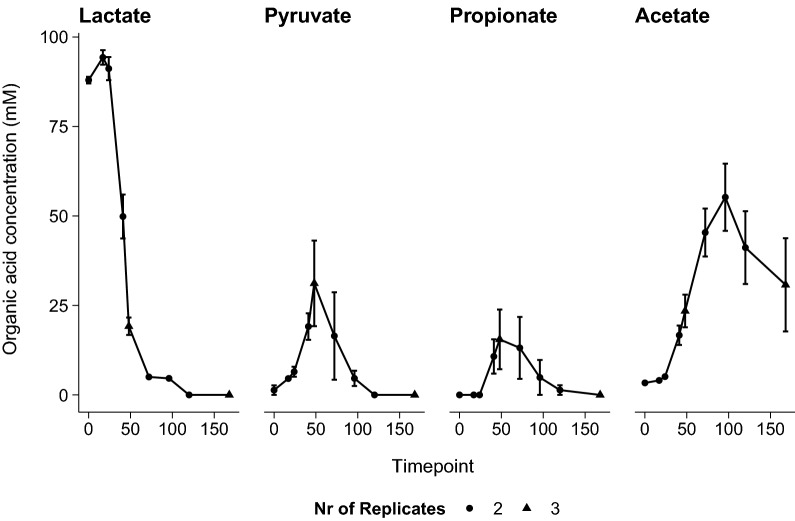
Biomass formation was found to increase 2.7-fold in microaerobic conditions in MM-lac compared to anaerobic conditions (Fig. 1A). In anaerobic conditions lactate was metabolised to propionate and acetate in a molar ratio of 1.98:1, close to the theoretical value of 2:1 (data not shown). In microaerobic conditions lactate was metabolised to propionate, acetate and pyruvate (Fig. 2). Contrary to the results of Dank et al. [9] obtained in rich medium, in our chemically defined medium the production of pyruvate was observed and propionate production declined. Biomass formation for anaerobic and microaerobic conditions did not differ significantly (0.38 vs 0.52 g CDW/L, independent student’s t-test (p = 0.38)) for cells growing on lactate at 48 h. When lactate was depleted no further biomass formation was observed in anaerobic conditions. In microaerobic conditions depletion of lactate was followed by oxidation of pyruvate and propionate to acetate and and a significant (independent student’s t-test (p < 0.01)) further increase in biomass (0.70 g/L anaerobic vs 1.87 g/L microaerobic). Total biomass formation after oxidation of propionate and pyruvate thus increased 2.7 times compared to anaerobic conditions, in line with result of Dank et al. [9], who observed an increase of 2.4.
Fig. 1.
Biomass formation (A) and B12 titer (B) in anaerobic and microaerobic conditions for growth of P. freudenreichii on lactate. Error bars represent standard error from biological replicates. Number of replicates per timepoint are displayed by circles (n = 2), triangles (n = 3) or squares (n = 4)
Fig. 2.
Substrate consumption and primary metabolite production in microaerobic conditions for P. freudenreichii growing on lactate. Error bars represent standard error from biological replicates. Number of replicates per timepoint are displayed by circles (n = 2) and triangles (n = 3)
Oxidation of propionate and pyruvate obviously resulted in an energetic benefit as shown by the increase in biomass formation. The increase in biomass formation was accompanied by a large increase of the B12 titer (/L), see Fig. 1B. The B12 titer during lactate metabolism in microaerobic conditions (t = 48 h) was found to be similar to that in anaerobic conditions (independent student’s t-test (p = 0.89)). However, a further increase of B12 was observed in microaerobic conditions, whilst in anaerobic conditions the B12 yield was minimally increased. The B12 titer increased sixfold in microaerobic conditions compared to anaerobic conditions (p = 0.088, independent student’s t-test), which means cells in microaerobic conditions produced on average two times more B12. As shown in Fig. 1B and Fig. 2 this may be attributed mainly to the oxidation of propionate and pyruvate. Pyruvate serves as major intermediate metabolite in carbon metabolism and thus is expected to contribute to biomass production and potentially production of B12. Propionate however is considered the metabolic end-product of anaerobic fermentation of lactate in propionic acid bacteria and is not linked directly as major carbon source for the production of biomass and B12. Our results thus raised the question whether propionate would serve as a suitable carbon source for P. freudenreichii for biomass formation and production of B12 in microaerobic conditions.
Propionate oxidation supports biomass formation and production
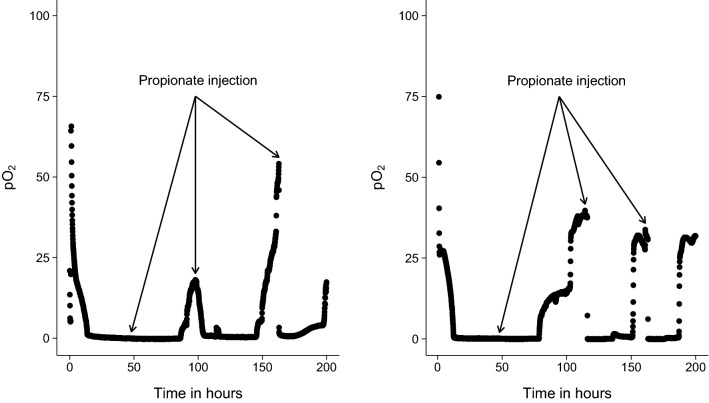
To study whether P. freudenreichii can grow on propionate as carbon source a bioreactor setup using minimal medium containing 100 mM propionate (MM-prop) was attempted. Surprisingly, no growth was observed under these conditions which may be attributed to a combination of inhibition by propionate [13], toxicity by oxygen [8] and low inoculum (see discussion). To minimise the product inhibition of propionate a fed-batch reactor was set up. Cells were pre-cultured in anaerobic conditions on lactate and transferred to a bioreactor with MM-propionate in microaerobic conditions. The inoculum size to the bioreactor was increased from 2% (v/v) to 10% (v/v). Propionate was fed to these cells to a final concentration of 10 mM at specific time points (t = 0 h, t = 48 h, t = 120 h and t = 168 h) whilst keeping the flux of oxygen constant. Injection of cells led to consumption of oxygen (Fig. 3) and complete consumption of propionate with a concomitant increase of biomass and B12 as shown in Fig. 4. The oxidation of propionate resulted first in the formation of acetate (data not shown). Oxygen was readily consumed after the primary injection and remained at the lower detection limit until depletion of both propionate and the formed acetate, after which oxygen levels steadily rose again. Injection of fresh propionate resulted in instantaneous consumption of oxygen, which confirmed respiratory pathways were used for metabolism of propionate and acetate with oxygen as terminal electron acceptor (Fig. 3). These results also indicate no loss of electron transport chain functionality at the oxygen fluxes used in our studies.
Fig. 3.
Dissolved oxygen measurement in bioreactors throughout cultivation on propionate. Dissolved oxygen as expressed as percentage of content measured at 100% air at 0.1 L/min at 30 ºC using 300 RPM and 0% air. Arrows indicate at which time new propionate was injected to an end concentration of 10 mM. Samples for biomass, HPLC and B12 quantification were taken directly prior to each new propionate injection
Fig. 4.
Biomass formation (A) and B12 titer (B) in microaerobic conditions for P. freudenreichii growing on propionate in a fed-batch reactor. Error bars represent standard error from biological duplicates
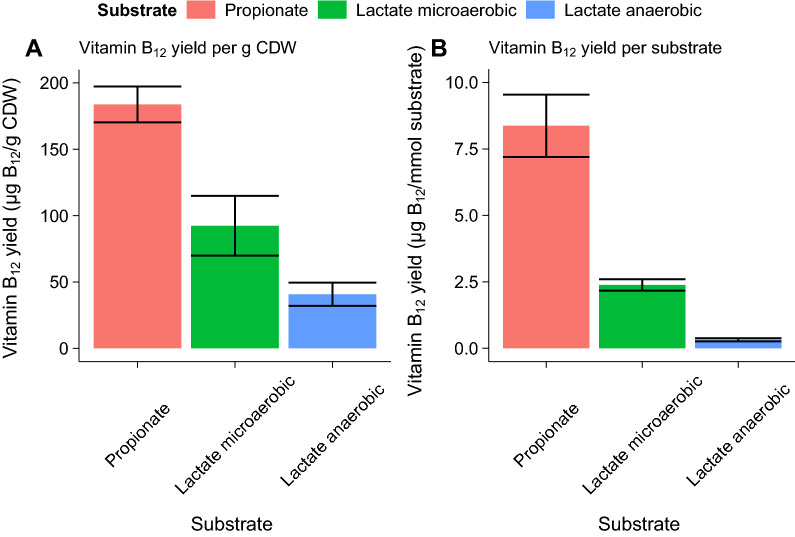
Propionate is the substrate with the highest B12 yield per substrate and per biomass
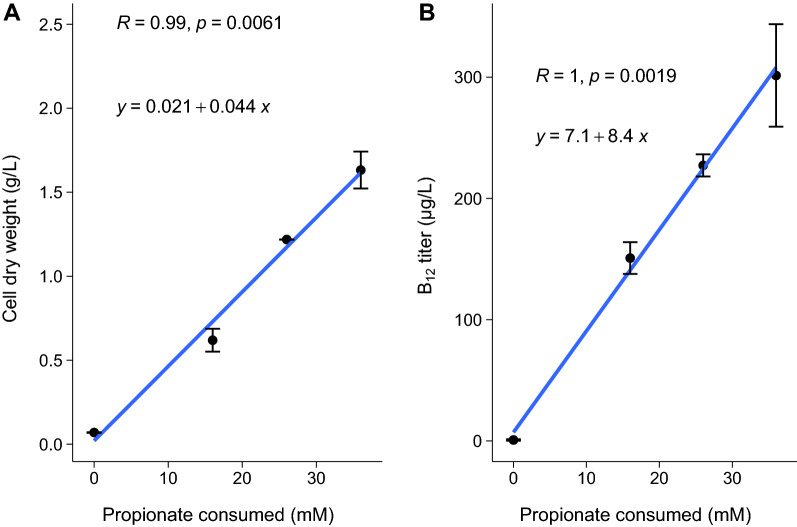
The propionate fed-batch cultivation clearly shows the potential of propionate as a carbon source for B12 production. To compare the B12 yield on different substrates correctly the B12 yield per substrate was calculated at 168 h (Fig. 5). A 7.5-fold increased yield per substrate was found for microaerobic lactate-grown cells versus anaerobic grown cells. An increased yield of 26.3 times was found for microaerobic propionate-grown cells versus anaerobic lactate-grown cells. The yield per CDW increased two-fold for propionate-grown microaerobic cells versus lactate-grown microaerobic cells and 4.5 times for lactate-grown anaerobic cells. Both the productivity per substrate (p < 0.01 for propionate as substrate, multiple linear regression) and productivity per cell biomass (p < 0.05 for propionate as substrate, multiple linear regression) thus increases drastically when metabolising propionate in microaerobic conditions compared to lactate in anaerobic and microaerobic conditions.
Fig. 5.
B12 yield per g cell dry weight (CDW) (A) and per mmol substrate (B) for P. freudenreichii in anaerobic and microaerobic conditions. Error bars represent standard error from biological replicates
Discussion
P. freudenreichii has been extensively studied as a producer of B12 as it favours production of the human active form of B12 [14] and has the generally recognised as safe status [11].
Many different strategies for increasing B12 yield by P. freudenreichii have been attempted, such as genetic engineering [15], genome shuffling [16], media optimalisations [17] and changing environmental conditions such as presence or absence of oxygen [5] or activation of riboswitches using blue light [18]. In anaerobic processes the production of propionic acid (and conceivably acetic acid [19]) by P. freudenreichii causes product inhibition, resulting in decreased cell growth and reduced B12 synthesis [5, 20]. However, a decreased B12 yield per gram cells has also been reported for processes removing propionic acid efficiently [21]. Indeed, Wang et al. [20] have found maintaining propionic acid concentrations at specific levels can increase B12 production. The role and effect of propionic acid on final B12 yield thus remains complex, but points towards higher production of B12 with minimal presence of propionate in the environment. What most studies have in common is a goal to remove propionic acid (and acetic acid) or decrease its negative effect on cell growth in anaerobic conditions. Usually, this is done by mechanical means such as removing effluent whilst returning or immobilizing cells [22, 23]. Here we attempt to solve this problem in a bioenergetically favourable way; removal of propionic acid (and acetic acid) by oxidation, resulting in ATP generation and lower inhibition potential and possible activation of pathways requiring B12, such as the Wood-Werkman cycle.
Results obtained in the current study support the findings of Dank et al. [9] in chemically defined lactate medium in microaerobic conditions and show that during these conditions biomass production and B12 production drastically increases. However, contrary to Dank et al. [9] we also observed accumulation of pyruvate. Similar observations were found for Acidipropionibacterium acidipropionici by van Gent-Ruijters et al. [24], who attribute the accumulation of pyruvate to a lack of oxidative decarboxylation of pyruvate. Indeed, oxygen inhibits pyruvate-ferredoxin oxidoreductase (PFOR) [25, 26], which has been proposed to be a key enzyme during the utilisation of lactate by propionic acid bacteria [27]. Alternatively, in microaerobic conditions pyruvate may be directly oxidised using oxygen as acceptor by pyruvate oxidase (PO), producing CO2, H2O2 and acetyl-phosphate [27]. Consequently if the anaerobic route for pyruvate dissimilation is disabled due to inactive PFOR, accumulation of pyruvate can be expected when oxygen contents are limited and pyruvate dissimilatory pathways requiring oxygen directly (PO) or indirectly (through oxygen-dependent NADH:dehydrogenase activity (pyruvate dehydrogenase)) are limited in flux and compete with other processes requiring regeneration of NADH to NAD+. This hypothesis is supported by the observations of Ye et al. [28], who reported accumulation of pyruvate after injection of propionate in microaerobic conditions, implying the rate-limiting step during propionate oxidation to acetate occurs at the pyruvate node. Therefore, the most likely explanation is a stochiometric limitation of oxygen, limiting the amount of oxygen available for NADH dehydrogenase-coupled electron transport activity in combination with potential competition for oxygen by pyruvate oxidase and (partial) inactivation of other key metabolic enzymes such as PFOR, resulting in small NAD+ pools and pyruvate accumulation and production of propionate. The described stochiometric limitations in oxygen levels are in line with the reported sensitivity of P. freudenreichii to oxygen, while efficient substrate metabolism is supported in microaerobic conditions.
In our study propionate oxidation in microaerobic conditions resulted in a boost of B12 production, while in previous studies Ye et al. [5] observed ceased B12 production conceivably due to loss of -aminolevulinic acid dehydratase activity [7] and consequently loss of cytochrome synthesis [8] in the high oxygenation conditions used in their experiments. Our results suggest it is key to keep oxygen fluxes low in order to maintain the ability to oxidise substrates using oxygen as a terminal electron acceptor. These results are supported by results of Tangyu et al. [29], who report highest B12 production at specific oxygen regimes in their food product. Since oxygen is required as terminal electron acceptor for oxidation of propionate, constraining oxygen to low levels results in oxygen being the growth rate determining factor. Since oxygen is supplied at a constant rate, the observed growth of P. freudenreichii on propionate in the microaerobic conditions used is linear [9]. Hence, to increase biomass formation and B12 production in time, higher oxygen fluxes should be applied, which requires increasing aerotolerance and/or respiration rates in P. freudenreichii. It is therefore interesting to attempt to obtain mutants with increased respiration rates by genetic engineering or adaptive evolution approaches. We hypothesised that the utilisation of propionate as sole carbon source for P. freudenreichii in microaerobic conditions will result in forcing flux through the reversed Wood-Werkman cycle. This in turn will result in a demand for B12 in growing cells as co-factor in the methylmalonyl-CoA transferase reaction and consequently activation of B12 production. However, in our first setup using 100 mM propionate in combination with the same microaerobic conditions applied on lactate no growth was observed. Both propionate [13] and oxygen [8] are known to be toxic for P. freudenreichii. Since the same microaerobic conditions were used as in the experiments on lactate as a carbon source, oxygen itself is not deleterious enough to inhibit growth at the used oxygen regime. Furthermore, Dank et al. [9] have shown that propionate is oxidised at higher concentrations (~ 70 mM) when larger amounts of biomass are present and pO2 inside the system is 0. Initial cell numbers are also reported to influence the potential of P. freudenreichii to either grow or not grow in milk [30]. Environmental stresses limit microbial growth in either synergistic or even multiplicative manner [31, 32]. Hence, it is conceivable the imposed stress of both propionate and oxygen in our initial setup was too big of a ‘hurdle’ for the low inoculum used in our study. It is therefore key to minimise the imposed stresses on P. freudenreichii by using a combination of low oxygen fluxes, low propionic acid concentrations and high initial biomass numbers.
Using a fed-batch system and thus low concentrations of propionate (10 mM), thereby preventing excessive product inhibition, we provide evidence that oxidation of propionate leads to production of acetate, which was further oxidised to CO2 during prolonged incubation. The oxidation of propionate to acetate and consequently to leads to a significant increase of B12 production per cell biomass, i.e., an increased yield per g CDW of 184 /g CDW on propionate versus 92 /g CDW on lactate microaerobically and 41 /g CDW on lactate anaerobically. The presence of propionate as sole carbon source thus increased the yield considerably. To conclude, we have shown that propionate can serve as excellent carbon source for P. freudenreichii in microaerobic conditions. This opens up great potential for the application of microaerobic conditions in combination with controlled propionate feeding for efficient production of B12. Applications of (genetically) engineered B12-overproducing strains [15, 16] in combination with other optimisation strategies such as media optimisations [17] by addition of precursors or altering other environmental conditions such as light [18] as previously suggested are recommended to investigate further yield increments using propionate as substrate in microaerobic conditions. Further studies about the regulatory role of propionate in activation of B12 biosynthesis pathways in P. freudenreichii can provide clues for further optimisation.
Conclusions
Here we show minimal fluxes of oxygen can greatly enhance biomass and B12 production in P. freudenreichii with lactate or propionate as a substrate. Stochiometric constraints of oxygen cause triauxic growth on lactate as observed earlier by Dank et al. [9]. The formation and subsequent oxidation of propionate appeared to be linked to increased B12 titer and yield. Fed-batch experiments showed that propionate can serve as excellent carbon source for biomass production and B12 production. Further studies on the potential regulatory role of propionate in activation of B12 synthesis in P. freudenreichii need to be performed. Since the oxidation of propionate is limited by the stochiometric constraint of oxygen, optimizing oxygen fluxes, increasing aerotolerance and/or respiration rates in P. freudenreichii may aid in improving oxidation rates and thus biomass and B12 production.
Methods
Strain and preculture conditions
P. freudenreichii DSM 20271 was obtained from Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) and routinely grown on yeast extract lactate (YEL) consisting per liter of: 10 g tryptone, 5 g yeast extract, 5 g and 16 g 80% l-Lactate syrup (Sigma Aldrich) and 15 g bacteriological agar for plates. Cell cultures were grown for 3 days in liquid media and maintained in 30% (v/v) glycerol stocks at −80 °C. Cells were precultured for each experiment by streaking P. freudenreichii on YEL agar and incubating at 30 °C in anaerobic conditions for 7 days. Single colonies were inoculated in minimal medium with composition described below.
Minimal media
Minimal media (MM) used in this study consisted per liter of: 100 mM carbon l-lactate (MM-lac), 10 mL metal stock(100x), 10 mL nucleic acid stock(100x), 10 mL vitamin stock(100x) and 400 mL amino acids stock(2.5x) with the following compositions for each stock described below. Metal stock per kg: 20 g, 5 g, 0.5 g, 0.25 g, 1.6 g, 0.25 g, 0.25 g, 0.3 g, 0.3 g ( was first dissolved in 10 ml 17% HCl, before it was mixed with the other compounds). Nucleic acid stock per kg: 1 g of each dissolved in 0.1 M NaOH; adenanine, uracil, xanthine, guanine. Vitamin stock per kg: Ca-d-pantothenate 0.1 g, d-biotin 0.25 g, thiamin-HCl 0.1 g, na-p-aminobenzoate 1 g. Amino acids stock: 1 mM of l-Alanine, l-Arginine Hydrochloride, l-Asparagine monohydrate, l-Aspartic Acid, l-Cysteine hydrochloride, l-Cystine, l-Glutamic Acid, l-Glutamine, Glycine, l-Histidine hydrochloride, l-Isoleucine, l-Leucine, l-Lysine hydrochloride, l-Methionine, l-Proline, l-Serine, l-Threonine, l-Tryptophan, l-Tyrosine, l-Valine. For fed-batch experiments l-lactate was replaced by 10 mM propionate (MM-prop).
Bioreactor cultivations on lactate
Bioreactor cultivations were performed according to the methods described by [9]. A single colony of P. freudenreichii was inoculated from YEL agar plates in 10 mL MM-lac and incubated at 30 °C anaerobically for 5 days, after which 2% (v/v) was inoculated into bioreactors with a working volume of 500 mL (Multifors, Infors HT, Switzerland). The stirring speed was set at 300 RPM, the temperature was kept constant at 30 °C and the pH was controlled at 7.0 by automatic addition of 5 M NaOH and 1 M HCl. A gas mix containing 85% gas and 15% air was used for microaerobic conditions. Gas was supplied through a sparger at the bottom of the fermenter using a mass flow controller premixing gas at set values at a rate of 0.1 L/min. Dissolved oxygen was measured using a probe which was calibrated at 100% by flushing the system with pure air at 0.1 L/min for 2 h and at 0% by flushing the system with for 2 h. Samples were taken at various time points aseptically through a sampling port. P. freudenreichii was grown in anaerobic conditions in 50 mL greiner tubes in MM-lac as described above and sampled at the several timepoints as reference condition. All samples were stored at −20 °C.
Fed-batch cultivations on propionate
P. freudenreichii was precultured on MM-lac in anaerobic conditions as described before. P. freudenreichii was inoculated into bioreactors containing 10 mM propionate minimal medium (MM-prop). Bioreactor settings were equal to settings used for cultivation on lactate described above. 10 mL samples were taken at 0 h, 48 h, 120 h and 168 h. After each sample point a new injection with 10 mL of 500 mM propionate stock was made to establish an end concentration of 10 mM propionate in the reactor after the injection. All samples were stored at −20 °C.
Biomass quantification
Biomass was quantified by measuring the cell dry weight (CDW) concentration as described by van Mastrigt et al. [33]. Membrane filters with a pore size of 0.2 µm (Pall Corporation, Ann Arbor, MI, USA) were pre-dried in an oven at 80 °C and then weighed. Samples were passed through the pre-weighted membrane filters using a vacuum filtration unit. Residual cell material in the funnel was washed to the filter using approximately 30 mL of demi water. The filters containing the biomass were dried at 80 °C again, after which filters were weighed again. CDW was calculated using the following formula:
Analysis of organic acids
Lactate, acetate, propionate and pyruvate were quantified by High Performance Liquid Chromatography (HPLC) as described by van Mastrigt et al. [34]. Briefly, 500 µL of sample was deproteinized by addition of 250 µL Carrez A (0.1 M potassium ferrocyanide trihydrate), mixing, addition of 250 µL Carrez B (0.2 M zinc sulfate heptahydrate), mixing and centrifugation for 2 min at 17,000 × g. 200 µL supernatant was injected on a UltiMate 3000 HPLC (Dionex Germany) equipped with an Aminex HPX-87H column (300 × 7.8 mm) with guard column (Biorad). 5 mM H2SO4 was used as mobile phase with a flow rate of 0.6 mL/min at a column temperature of 40 °C. Compounds were detected using a refractive index detector (RefractoMax 520).
B12 quantification
B12 was detected using a Vitafist B12 biological assays (R-Biopharm). Samples were prepared for analysis by diluting them 10 × with demi water. Samples were beat-beaded (lysing matrix B, mp-bio) in FastPrep-24 instrument (MP Biomedicals) 3 times using 1-min intervals followed by centrifugation at 17,000 × g for 2 min. Supernatants were collected and diluted 4 × with demi water, after which they were heated for 30 min at 95 °C in a water bath. Samples were chilled on ice and diluted further to fall within the microbiological assay detection range. B12 assays were performed as described by the manufacturers protocol in technical replicates. Absorbance was measured in microtiter plates using Microwell Plate Reader SpectraMax M2 at 610 nm with SoftMax Pro software.
Statistical analysis
Statistical analysis was performed using R in combination with Rstudio. Data normality was tested using Shapiro-Wilk test. Equal or unequal variance was tested using F-tests. Both normality and equal variances were assumed when p > 0.05. Independent-students t-tests were used based on equal variances using R t.test function. The effects of microaerobic conditions and propionate as substrate were estimated using multiple linear regression in R using the lm function. Both yield per substrate and per cell were fitted using substrate and condition as dependent variables.
Supplementary Information
Additional file 1. Datasheet containing all data gathered and used for this study.
Acknowledgements
The authors would like to acknowledge Javier Huerta Lobregad for his excellent work in the lactate-grown batch cultures.
Abbreviations
- PFOR
Pyruvate: ferredoxin oxidoreductase
- PO
Pyruvate oxidase
- P
Propionibacterium
- CDW
Cell dry weight
- Vitamin B12
B12
- MM
Minimal medium
- DSMZ
Deutsche Sammlung von Mikroorganismen und Zellkulturen
- YEL
Yeast extract lactate
Author contributions
AD, GB, TA and ES designed experiments. AD and GB carried our experiments and analysed the data. AD wrote the draft manuscript. All authors read and commented on the draft manuscript. AD wrote the final version of the manuscript and all authors read and approved submission of the draft manuscript. AD, ES and TA revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was financially supported by Arla Foods Amba (Aarhus, Denmark).
Availability of data and materials
The dataset supporting the conclusions of this article is included within Additional file 1.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martens J-H, Barg H, Ma W, Jahn D. Microbial production of vitamin B 12. Appl Microbiol Biotechnol. 2002;58:275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- 2.Marsh E, McKie N, Davis N, Leadlay P. Cloning and structural characterization of the genes coding for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Biochemical Journal. 1989;260:345–352. doi: 10.1042/bj2600345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore SJ, Warren MJ. The anaerobic biosynthesis of vitamin B12. Biochem Soc Trans. 2012;40:581–586. doi: 10.1042/BST20120066. [DOI] [PubMed] [Google Scholar]
- 4.Roessner CA, Huang K-x, Warren MJ, Raux E, Scott AI. Isolation and characterization of 14 additional genes specifying the anaerobic biosynthesis of cobalamin (vitamin B12) in Propionibacterium freudenreichii (P. shermanii) The GenBank accession numbers for the sequences reported in this paper are AY033235, AY033236, U13043 and U51164. Microbiology. 2002;148:1845–1853. doi: 10.1099/00221287-148-6-1845. [DOI] [PubMed] [Google Scholar]
- 5.Ye K, Shijo M, Jin S, Shimizu K. Efficient production of vitamin B12 from propionic acid bacteria under periodic variation of dissolved oxygen concentration. J Ferment Bioeng. 1996;82:484–491. doi: 10.1016/S0922-338X(97)86988-7. [DOI] [Google Scholar]
- 6.Quesada-Chanto A, Schmid-Meyer A, Schroeder A, Carvalho-Jonas M, Blanco I, Jonas R. Effect of oxygen supply on biomass, organic acids and vitamin B12 production by Propionibacterium shermanii. World J Microbiol Biotechnol. 1998;14:843–846. doi: 10.1023/A:1008868907251. [DOI] [Google Scholar]
- 7.Menon IA, Shemin D. Concurrent decrease of enzymic activities concerned with the synthesis of coenzyme B12 and of propionic acid in propionibacteria. Arch Biochem Biophys. 1967;121:304–310. doi: 10.1016/0003-9861(67)90080-X. [DOI] [PubMed] [Google Scholar]
- 8.De Vries W, Van Wijck-Kapteijn WM, Stouthamer A. Influence of oxygen on growth, cytochrome synthesis and fermentation pattern in propionic acid bacteria. Microbiology. 1972;71:515–524. doi: 10.1099/00221287-71-3-515. [DOI] [PubMed] [Google Scholar]
- 9.Dank A, van Mastrigt O, Boeren S, Lillevang SK, Abee T, Smid E. Propionibacterium freudenreichii thrives in microaerobic conditions by complete oxidation of lactate to CO2. Environ Microbiol. 2021 doi: 10.1111/1462-2920.15532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emde R, Schink B. Oxidation of glycerol, lactate, and propionate by Propionibacterium freudenreichii in a poised-potential amperometric culture system. Arch Microbiol. 1990;153:506–512. doi: 10.1007/BF00248435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falentin H, Deutsch S-M, Jan G, Loux V, Thierry A, Parayre S, Maillard M-B, Dherbecourt J, Cousin FJ, Jardin J. The complete genome of Propionibacterium freudenreichii CIRM-BIA1T, a hardy Actinobacterium with food and probiotic applications. PLoS ONE. 2010;5:e11748. doi: 10.1371/journal.pone.0011748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borisov VB, Gennis RB, Hemp J, Verkhovsky MI. The cytochrome bd respiratory oxygen reductases. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 2011;1807:1398–1413. doi: 10.1016/j.bbabio.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martínez-Campos R, de la Torre M. Production of propionate by fed-batch fermentation of Propionibacterium acidipropionici using mixed feed of lactate and glucose. Biotech Lett. 2002;24:427–431. doi: 10.1023/A:1014562504882. [DOI] [Google Scholar]
- 14.Deptula P, Kylli P, Chamlagain B, Holm L, Kostiainen R, Piironen V, Savijoki K, Varmanen P. BluB/CobT2 fusion enzyme activity reveals mechanisms responsible for production of active form of vitamin B12 by Propionibacterium freudenreichii. Microb Cell Fact. 2015;14:1–12. doi: 10.1186/s12934-015-0363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piao Y, Yamashita M, Kawaraichi N, Asegawa R, Ono H, Murooka Y. Production of vitamin B12 in genetically engineered Propionibacterium freudenreichii. J Biosci Bioeng. 2004;98:167–173. doi: 10.1016/S1389-1723(04)00261-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Liu J-Z, Huang J-S, Mao Z-W. Genome shuffling of Propionibacterium shermanii for improving vitamin B12 production and comparative proteome analysis. J Biotechnol. 2010;148:139–143. doi: 10.1016/j.jbiotec.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Kośmider A, Białas W, Kubiak P, Drożdżyńska A, Czaczyk K. Vitamin B12 production from crude glycerol by Propionibacterium freudenreichii ssp. shermanii: optimization of medium composition through statistical experimental designs. Bioresource Technol. 2012;105:128–133. doi: 10.1016/j.biortech.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 18.Yu Y, Zhu X, Shen Y, Yao H, Wang P, Ye K, Wang X, Gu Q. Enhancing the vitamin B12 production and growth of Propionibacterium freudenreichii in tofu wastewater via a light-induced vitamin B12 riboswitch. Appl Microbiol Biotechnol. 2015;99:10481–10488. doi: 10.1007/s00253-015-6958-6. [DOI] [PubMed] [Google Scholar]
- 19.Pinhal S, Ropers D, Geiselmann J, de Jong H. Acetate metabolism and the inhibition of bacterial growth by acetate. J Bacteriol. 2019;201:e00147. doi: 10.1128/JB.00147-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Zhang Z, Jiao Y, Liu S, Wang Y. Improved propionic acid and 5, 6-dimethylbenzimidazole control strategy for vitamin B12 fermentation by Propionibacterium freudenreichii. J Biotechnol. 2015;193:123–129. doi: 10.1016/j.jbiotec.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Wang P, Wang Y, Liu Y, Shi H, Su Z. Novel in situ product removal technique for simultaneous production of propionic acid and vitamin B12 by expanded bed adsorption bioreactor. Biores Technol. 2012;104:652–659. doi: 10.1016/j.biortech.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 22.Yongsmith B, Sonomoto K, Tanaka A, Fukui S. Production of vitamin B 12 by immobilized cells of a propionic acid bacterium. Eur J Appl Microbiol Biotechnol. 1982;16:70–74. doi: 10.1007/BF00500729. [DOI] [Google Scholar]
- 23.Miyano K-i, Ye K, Shimizu K. Improvement of vitamin B12 fermentation by reducing the inhibitory metabolites by cell recycle system and a mixed culture. Biochem Eng J. 2000;6:207–214. doi: 10.1016/S1369-703X(00)00089-9. [DOI] [PubMed] [Google Scholar]
- 24.van Gent-Ruijters ML, de Meijere FA, de Vries W, Stouthamer A. Lactate metabolism in Propionibacterium pentosaceum growing with nitrate or oxygen as hydrogen acceptor. Antonie Van Leeuwenhoek. 1976;42:217–228. doi: 10.1007/BF00394118. [DOI] [PubMed] [Google Scholar]
- 25.Lu Z, Imlay JA. When anaerobes encounter oxygen: mechanisms of oxygen toxicity, tolerance and defence. Nat Rev Microbiol. 2021;19:774–785. doi: 10.1038/s41579-021-00583-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan N, Imlay JA. How does oxygen inhibit central metabolism in the obligate anaerobe Bacteroides thetaiotaomicron. Mol Microbiol. 2001;39:1562–1571. doi: 10.1046/j.1365-2958.2001.02343.x. [DOI] [PubMed] [Google Scholar]
- 27.McCubbin T, Gonzalez-Garcia RA, Palfreyman RW, Stowers C, Nielsen LK, Marcellin E. A pan-genome guided metabolic network reconstruction of five Propionibacterium species reveals extensive metabolic diversity. Genes. 2020;11:1115. doi: 10.3390/genes11101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye K, Shijo M, Miyano K, Shimizu K. Metabolic pathway of Propionibacterium growing with oxygen: enzymes, 1 3C NMR analysis, and its application for vitamin B12 production with periodic fermentation. Biotechnol Prog. 1999;15:201–207. doi: 10.1021/bp990012s. [DOI] [PubMed] [Google Scholar]
- 29.Tangyu M, Fritz M, Ye L, Aragão Börner R, Morin-Rivron D, Campos-Giménez E, Bolten CJ, Bogicevic B, Wittmann C. Co-cultures of Propionibacterium freudenreichii and Bacillus amyloliquefaciens cooperatively upgrade sunflower seed milk to high levels of vitamin B12 and multiple co-benefits. Microb Cell Fact. 2022;21:1–23. doi: 10.1186/s12934-022-01773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piveteau P, Condon S, Cogan TM. Inability of dairy propionibacteria to grow in milk from low inocula. J Dairy Res. 2000;67:65–71. doi: 10.1017/S0022029999004008. [DOI] [PubMed] [Google Scholar]
- 31.Biesta-Peters EG, Reij MW, Gorris LG, Zwietering MH. Comparing nonsynergistic gamma models with interaction models to predict growth of emetic Bacillus cereus when using combinations of pH and individual undissociated acids as growth-limiting factors. Appl Environ Microbiol. 2010;76:5791–5801. doi: 10.1128/AEM.00355-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leistner L, Gorris LG. Food preservation by hurdle technology. Trends Food Sci Technol. 1995;6:41–46. doi: 10.1016/S0924-2244(00)88941-4. [DOI] [Google Scholar]
- 33.van Mastrigt O, Abee T, Lillevang SK, Smid EJ. Quantitative physiology and aroma formation of a dairy Lactococcus lactis at near-zero growth rates. Food Microbiol. 2018;73:216–226. doi: 10.1016/j.fm.2018.01.027. [DOI] [PubMed] [Google Scholar]
- 34.van Mastrigt O, Mager EE, Jamin C, Abee T, Smid EJ. Citrate, low pH and amino acid limitation induce citrate utilization in Lactococcus lactis biovar diacetylactis. Microb Biotechnol. 2018;11:369–380. doi: 10.1111/1751-7915.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Datasheet containing all data gathered and used for this study.
Data Availability Statement
The dataset supporting the conclusions of this article is included within Additional file 1.