Abstract
Background
Oxygen therapy after extubation in the intensive care unit (ICU) is essential in order to maintain adequate oxygenation, especially in patients who have undertaken cardiovascular surgery. A Venturi mask (VM) has been routinely used as an oxygen therapy in the ICU. Recently, however, the high flow nasal cannula (HFNC) has become available, and this device can deliver up to 60 L/min of humidified oxygen. The aim of this study is to evaluate the short-term efficacy between HFNC and VM in cardiovascular surgical patients.
Methods
Forty patients who underwent cardiovascular surgery were randomized to either protocol A (HFNC followed by VM) or protocol B (VM followed by HFNC). After 60-minutes of use with either device, arterial blood gas analysis was performed, and the PaO2/FiO2 ratio (PFR) was calculated. Simultaneously, physiological data (respiratory rate, heart rate, mean arterial pressure, continuous cardiac index, and mixed venous oxygen saturation) were recorded. During this procedure, FiO2 and gas flow were maintained at a fixed rate. These variables were compared by using the paired t-test, and a p value < 0.05 was considered significant. All data were expressed as mean (standard deviation).
Results
Thirty-five patients (17 from protocol A and 18 from protocol B) were enrolled, and 5 patients were excluded from analysis in accordance with the exit criteria. PaO2 was significantly higher in the HFNC group than in the VM group [101.7 (25.9) vs. 91.8 (23.0), mean difference 9.87 (18.5), 95% confidence interval 3.5 to 16.2, p = 0.003]. Moreover, PFR was significantly higher in the HFNC group than in the VM group [265.9 (81.4) vs. 238.7 (68.5), p = 0.002]. Moreover, PaCO2 was significantly lower in the HFNC group than in the VM group [33.8 (3.5) vs. 34.7 (2.9), p = 0.033]. The respiratory rate was significantly lower in the HFNC group than in the VM group [18 (4) vs. 21 (4), p = 0.006], and no significant differences were seen in any of the other parameters.
Conclusions
Compared to VM, HFNC ameliorated oxygenation function and decreased patients’ effort in breathing. The hemodynamic state did not differ between HFNC and VM. Therefore, HFNC can be used safely in cardiovascular surgical patients.
Trial registration
This trial was registered with the UMIN Clinical Trials Registry (ID UMIN000016572).
Keywords: High flow nasal oxygen therapy, Venturi mask, Cardiovascular surgery, Oxygen therapy after extubation
Background
Oxygenation and gas exchange occasionally deteriorate after cardiovascular surgery due to the usage of cardiopulmonary bypass and perioperative blood transfusion. Postoperative optimal oxygen delivery in the intensive care unit (ICU) is essential for adequate oxygenation in order to prevent reintubation and postoperative adverse respiratory events. It is crucial to adjust the fraction of inspired oxygen (FiO2) and the oxygen flow rate when performing postoperative oxygen therapy. Minimalizing FiO2 is important in order to avoid absorption atelectasis – one of the possible respiratory complications after cardiovascular surgery [1]. In general, an oxygen flow rate of 30 L/min is necessary to accurately provide pre-specified FiO2 and to prevent the lungs from drawing ambient air. In recent years, the high flow nasal cannula (HFNC), which can deliver up to 60 L/min of humidified oxygen, has become available and widely used in the perioperative field. On the other hand, the Venturi mask (VM) has been routinely used as a high flow oxygen device for quite a while. VM needs a low flow rate of oxygen in order to create a large total flow rate, at predictable FiO2, entraining room air via the Venturi effect. Both devices can precisely regulate both FiO2 and flow rate and, therefore, both are thought to be comparable as a postoperative high-flow oxygen device. The aim of this study is to evaluate the short-term efficacy between HFNC and VM in cardiovascular surgical patients.
Methods
This randomized crossover trial was performed in the ICU (8 beds) of Osaka Medical College. The protocol of this study was approved by the institutional ethics committee of Osaka Medical College (file number: RIN89–1635) and registered with the UMIN Clinical Trials Registry (ID UMIN000016572, February 18th, 2015). Written informed consent was obtained from each patient. From February to August 2015, the authors recruited 40 patients who underwent scheduled cardiovascular surgery using cardiopulmonary bypass with median sternotomy and mild hypothermia. After the operation, all participants were admitted to the ICU and received mechanical ventilation under a continuous infusion of sedatives (propofol and dexmedetomidine). The day after surgery, patients who fulfilled the following criteria before extubation were eligible for the randomization of this study: arterial blood pH 7.35 to 7.45, PaO2/FiO2 ratio (PFR) ≧ 250 (mmHg), FiO2 ≦ 0.4, positive end-expiratory pressure (PEEP) ≦ 5 cmH2O, and pressure support (PS) ≦ 5 cmH2O. Patients were excluded if they had bronchial asthma, chronic obstructive pulmonary disease, hemodynamic instability, end-stage renal failure requiring hemodialysis, or a duration of postoperative mechanical ventilation in the ICU > 24 hours. In this study, we used an Aerosol mask® (Smith Medical inc. Minnesota, US) and an EZ-Water® nebulizer (Japan Medicalnext Co., Ltd., Osaka, Japan) as a humidification and Venturi system. The HFNC system includes OA2060® (Sanyu technology Co., Ltd., Saitama, Japan) as an air/oxygen blender, and an F&P 850® system (Fisher & Paykel Healthcare, Co., Ltd., Auckland, New Zealand) as a circuit.
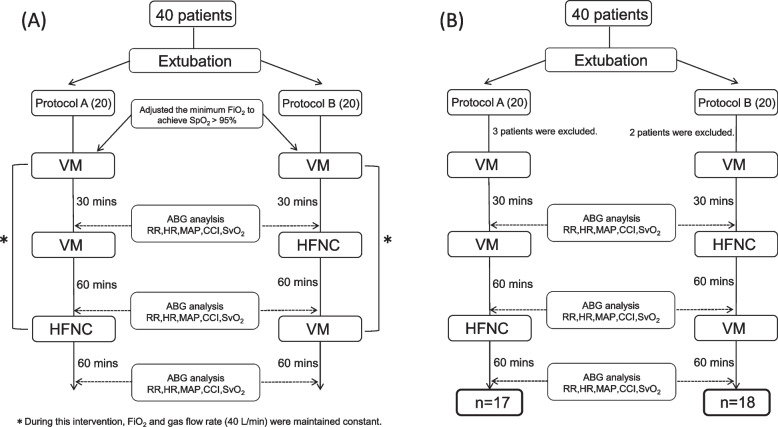
Before the study, we measured the oxygen flow rate of VM (Table 1), which is necessary to provide a total gas flow rate of 40 L/min using HALOSCALE® flowmeter (nSpire Health Ltd., Hertford, UK). After extubation, patients were provided with oxygen by VM at a rate of 40 L/min for 30 minutes. Targeted minimum FiO2 was adjusted to maintain SpO2 ≧ 95%, selecting from 0.33, 0.35, 0.4 and 0.5 (Table 1). After stabilization of this 30-minute oxygen administration, the arterial blood gas (ABG) analysis (pH, PaO2, PaCO2, and HCO3−) was performed, and PFR was calculated simultaneously. Respiratory rate (RR), heart rate (HR), mean arterial pressure (MAP), continuous cardiac index (CCI), and mixed venous oxygen saturation (SvO2) were also recorded. RR was measured using thoracic impedance pneumography (Life Scope®, Nihon Kohden, Tokyo, Japan). CCI and SvO2 were measured by a pulmonary artery catheter inserted after the induction of general anesthesia in the operating room. If the patients remained respiratorily and hemodynamically stable, they were then randomized into either protocol A (VM for 60 minutes, followed by HFNC for 60 minutes) or protocol B (HFNC for 60 minutes, followed by VM for 60 minutes) (Fig. 1A). During this intervention, FiO2 and a total gas flow rate of 40 L/min were maintained fixed and in similar fashion with the stabilization interval described above (Fig. 1A). At the end of the period of each oxygen device, PFR was calculated from the ABG analysis, and RR, HR, MAP, CCI, and SvO2 were recorded. The primary outcome of this study was PaO2, and the secondary outcomes were PFR, PaCO2, RR and hemodynamic parameters.
Table 1.
Necessary oxygen flow rate for VM to provide total gas flow of 40 L/min
| FiO2 | 33% | 35% | 40% | 50% |
|---|---|---|---|---|
| Flow rate (L/min) | 6 | 7 | 10 | 15 |
Fig. 1.
A Patient flowchart of this trial. VM, Venturi mask; HFNC, high flow nasal cannula; RR: respiratory rate; HR, heart rate; MAP, mean arterial pressure; CCI, continuous cardiac index; SvO2 mixed venous oxygen saturation. B Patient flow after randomization. VM, Venturi mask; HFNC, high flow nasal cannula; RR: respiratory rate; HR, heart rate; MAP, mean arterial pressure; CCI, continuous cardiac index; SvO2, mixed venous oxygen saturation
Randomization was performed using sequentially numbered sealed envelopes to preserve allocation concealment. The number of patients in this trial was calculated as follows: the overall average trial PaO2 in this setting was 95 ± 20 mmHg and obtained from our preliminary data. Thirty-three subjects were needed to show a PaO2 difference of 10 mmHg at a significance level of 0.05 and a power of 80%. The sample size was inflated to 40 patients to account for withdrawals and loss. Data are described as mean (standard deviation) and numbers with proportions (%), where appropriate. Baseline data of each protocol was assessed by the Welch’s t and Chi-square tests. Outcome variables were compared using the paired t test. Statistical analyses were performed separately for protocol A and protocol B, considering that a carry-over effect affected the results of this study.
All tests were two-tailed, and a p value < 0.05 was considered to be statistically significant.
Results
A total of 40 patients were recruited, and 20 each were randomized into either protocol A or B. Three patients from protocol A and two from protocol B were excluded from analyses due to early discharge from the ICU (Fig. 1B). Table 2 shows the patient background of each protocol at randomization, including age, gender, height, body weight, body mass index, ventilation time before extubation and type of operation. Table 3 shows the result of the ABG analysis and baseline physiologic data of each protocol before intervention.
Table 2.
Patient background of each protocol at randomization
| Protocol A | Protocol B | p value | |||
|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | ||
| Age | 66.0 | (11.0) | 71.9 | (8.9) | 0.100 |
| Gender (male/female) | 11/6 | 9/9 | 0.380 | ||
| Height (cm) | 162.2 | (8.8) | 157.0 | (11.8) | 0.156 |
| Body weight (kg) | 60.3 | (12.1) | 56.7 | (14.6) | 0.444 |
| BMI (kg/m2) | 21.3 | (5.8) | 22.8 | (3.7) | 0.401 |
| Ventilation time (min)* | 1057.8 | (333.7) | 983.2 | (204.7) | 0.436 |
| Operation time (min) | 368.4 | (149.4) | 384.4 | (89.8) | 0.708 |
| Operation | 0.187 | ||||
| CABG | 4 | 8 | |||
| Valve | 11 | 10 | |||
| Others | 2 | 0 | |||
SD Standard deviation, BMI Body mass index, CABG Coronary artery bypass grafting
Table 3.
ABG analysis and baseline physiological data of each protocol before intervention
| Protocol A | Protocol B | p value | |||
|---|---|---|---|---|---|
| mean | (SD) | mean | (SD) | ||
| PaO2 (mmHg) | 95.9 | (23.4) | 97.6 | (25.7) | 0.380 |
| FiO2 (0.35/0.4/0.5) | 9/7/1 | 9/4/5 | 0.177 | ||
| PFR (mmHg) | 252.8 | (62.4) | 230.0 | (51.6) | 0.248 |
| PaCO2 (mmHg) | 33.5 | (3.4) | 34.9 | (2.9) | 0.923 |
| pH | 7.428 | (0.02) | 7.434 | (0.04) | 0.641 |
| HCO3− (mEq/L) | 22.4 | (2.4) | 23.1 | (1.7) | 0.606 |
| RR (rates/min) | 19.2 | (4.2) | 20.4 | (4.7) | 0.428 |
| HR (beats/min) | 92.4 | (11.6) | 94.6 | (9.0) | 0.073 |
| MAP (mmHg) | 65 | (10) | 66 | (8) | 0.940 |
| CCI (L/min/m2) | 3.8 | (0.8) | 3.5 | (0.6) | 0.199 |
| SvO2 (%) | 71.9 | (10.1) | 73.2 | (4.5) | 0.674 |
ABG Arterial blood gas, SD Standard deviation, PFR PaO2/FiO2 ratio, RR Respiratory rate, HR heart rate, MAP Mean arterial pressure, CCI Continuous cardiac index, SvO2 Mixed venous oxygen saturation
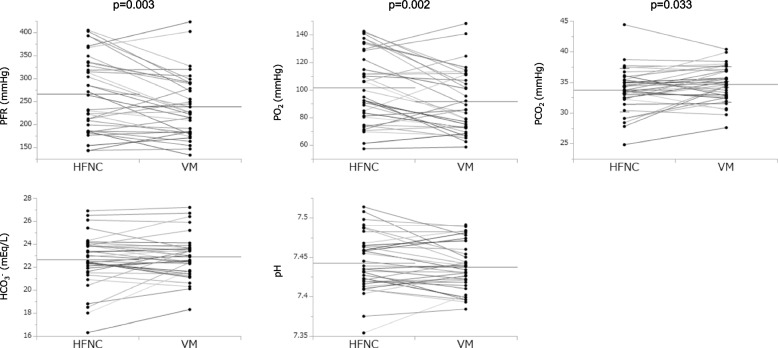
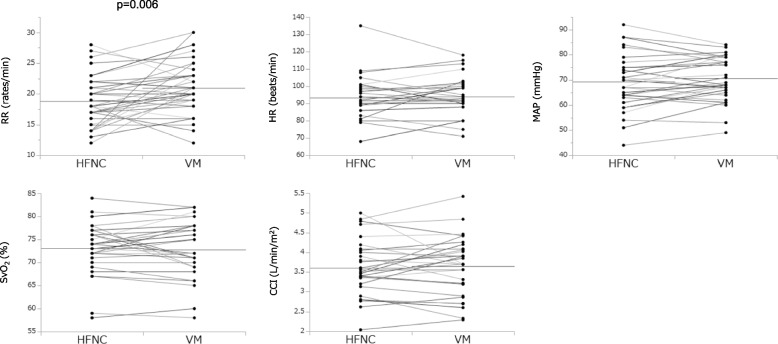
PaO2 was significantly higher in the HFNC group than in the VM group [101.7 (25.9) vs. 91.8 (23.0), mean difference 9.87 (18.5), 95% confidence interval 3.5 to 16.2, p = 0.003]. As well, PFR was significantly higher in the HFNC group than in the VM group [265.9 (81.4) vs. 238.7 (68.5), mean difference 27.2 (49.1), 95% confidence interval (10.3 to 44.1), p = 0.002]. Moreover, PaCO2 was slightly, but significantly, lower in the HFNC group than in the VM group [33.8 (3.5) vs. 34.7 (2.9), mean difference − 0.95 (2.5), 95% confidence interval (− 1.84 to − 0.06), p = 0.033] (Fig. 2). As for the physiological data, the respiratory rate was significantly lower in the HFNC group than in the VM group [18 (4) vs. 21 (4), mean difference − 2.2 (4.47), 95% confidence interval (− 3.74 to − 0.66), p = 0.006], and no significant differences were seen in any of the other parameters (Fig. 3). Tables 4, 5, 6 and 7 show a comparison of outcome variables separately performed for protocol A and protocol B. Similarly to the crossover analysis, PFR was significantly higher in HFNC for both protocols. PCO2 was significantly lower in HFNC for protocol A but not for protocol B. RR was significantly lower in HFNC for protocol B, but not for protocol A.
Fig. 2.
HFNC vs. VM (ABG analysis). HFNC, high flow nasal cannula; VM, Venturi mask; PFR, PaO2/FiO2 ratio. Horizontal lines indicate the mean value of each device
Fig. 3.
HFNC vs. VM (physiological data). HFNC, high flow nasal cannula; VM, venture mask; RR, respiratory rate; HR, heart rate; MAP, mean arterial pressure; CCI, continuous cardiac index; SvO2, mixed venous oxygen saturation. Horizontal lines indicate the mean value of each device
Table 4.
Comparison of arterial blood gas analysis in protocol A
| VM | HFNC | mean diffference | 95%CI | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| PFR (mmHg) | 243.7 | (62.5) | 272.0 | (88.6) | −28.3 | − 53.8 | to | − 2.9 | 0.031 |
| PO2 (mmHg) | 90.7 | (18.8) | 101.0 | (27.4) | −10.3 | −19.7 | to | −0.9 | 0.034 |
| PCO2 (mmHg) | 34.6 | (3.0) | 32.4 | (3.6) | 2.1 | 0.9 | to | 3.3 | 0.002 |
| HCO3− (mEq/L) | 22.7 | (2.3) | 22.1 | (2.6) | 0.5 | −0.2 | to | 1.2 | 0.129 |
| pH | 7.44 | (0.03) | 7.45 | (0.03) | −0.01 | −0.03 | to | −0.00 | 0.003 |
HFNC High flow nasal cannula, VM Venture mask, CI Confidence interval, PFR PaO2/FiO2 ratio
Data are presented as mean (standard deviation)
Table 5.
Comparison of physiological data in protocol A
| VM | HFNC | mean diffference | 95%CI | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| RR (rates/min) | 20.5 | (5.3) | 18.7 | (3.6) | 1.8 | −0.5 | to | 4.2 | 0.118 |
| HR (beats/min) | 91.2 | (10.6) | 93.6 | (13.0) | −2.4 | −6.6 | to | 1.9 | 0.262 |
| MAP (mmHg) | 69.1 | (9.7) | 69.4 | (12.1) | −0.3 | −2.9 | to | 2.3 | 0.811 |
| SvO2 (%) | 70.3 | (12.4) | 70.7 | (12.4) | −0.4 | −2.6 | to | 1.7 | 0.673 |
| CCI (L/min/m2) | 3.7 | (0.9) | 3.7 | (0.7) | 0.0 | −0.2 | to | 0.2 | 0.970 |
HFNC High flow nasal cannula, VM Venture mask, CI Confidence interval, RR Respiratory rate, HR Heart rate, MAP Mean arterial pressure, SvO2 Mixed venous oxygen saturation, CCI Continuous cardiac index
Data are presented as mean (standard deviation)
Table 6.
Comparison of arterial blood gas analysis in protocol B
| HFNC | VM | mean difference | 95%CI | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| PFR (mmHg) | 260.2 | (76.2) | 234.0 | (75.3) | 26.2 | 1.3 | to | 51.1 | 0.041 |
| PO2 (mmHg) | 102.3 | (25.1) | 92.8 | (26.9) | 9.5 | −0.1 | to | 19.1 | 0.052 |
| PCO2 (mmHg) | 35.0 | (3.0) | 34.9 | (2.9) | 0.1 | −1.0 | to | 1.3 | 0.791 |
| HCO3− (mEq/L) | 23.1 | (2.0) | 23.1 | (1.5) | 0.0 | −0.7 | to | 0.7 | 0.935 |
| pH | 7.43 | (0.04) | 7.44 | (0.03) | −0.01 | −0.02 | to | 0.00 | 0.254 |
HFNC High flow nasal cannula, VM Venture mask, CI Confidence interval, PFR PaO2/FiO2 ratio
Data are presented as mean (standard deviation)
Table 7.
Comparison of physiological data in protocol B
| HFNC | VM | mean difference | 95%CI | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| RR (rates/min) | 18.9 | (4.5) | 21.4 | (3.0) | −2.6 | −4.8 | to | −0.3 | 0.028 |
| HR (beats/min) | 92.9 | (9.0) | 96.3 | (9.1) | −3.4 | −5.4 | to | −1.4 | 0.002 |
| MAP (mmHg) | 72.2 | (6.8) | 69.2 | (9.3) | 3.0 | 0.3 | to | 5.7 | 0.033 |
| SvO2 (%) | 73.2 | (3.5) | 72.9 | (4.8) | 0.3 | −1.5 | to | 2.1 | 0.728 |
| CCI (L/min/m2) | 3.5 | (0.7) | 3.6 | (0.6) | −0.1 | −0.3 | to | 0.2 | 0.626 |
HFNC High flow nasal cannula, VM Venture mask, CI Confidence interval, RR Respiratory rate, HR Heart rate, MAP Mean arterial pressure, SvO2 Mixed venous oxygen saturation, CCI Continuous cardiac index
Data are presented as mean (standard deviation)
Discussion
This is the first randomized crossover trial to compare the short-term efficacy of HFNC and VM for cardiovascular surgical patients. Our study revealed that, compared with VM, HFNC ameliorates gas exchange, and that the hemodynamic state did not differ between these devices in cardiovascular surgical patients after extubation. In addition, using HFNC reduced the respiratory rate when the patient was switched from VM. These findings do not contradict a previous report that HFNC generates a flow-dependent effect of continuous positive airway pressure [2] and an upper airways deadspace washout effect [3, 4]. In addition, delivering humidified and heated oxygen reduces patient effort and oxygen consumption. The most distinctive characteristic of this study is that we directly measured the flow rate of VM by using the HALOSCALE® flowmeter when comparing the rate with HFNC. As for those studies [5, 6] using HFNC compared with VM, the method application of VM was not mentioned in detail. VM cannot provide pre-set oxygen concentration with inappropriate total flow rate of < 30 L/min.
In recent years, HFNC has been widely and rapidly propagated as a standard oxygen delivery system, especially for those patients with deteriorated oxygenation function. The results of recent randomized control trials show that HFNC, at a minimum, is not inferior to noninvasive ventilation (NIV) [7, 8]. Especially with regard to its comfortability, HFNC was thought to be superior to NIV. However, Elie Azoulay et al. demonstrated that HFNC therapy did not significantly decrease mortality among critically ill patients with acute respiratory failure, compared with standard oxygen therapy [9]. The greatest advantage of HFNC is its capability of adjusting both oxygen concentration (0.21 to 1.0) and total gas flow (0 to 60 L/min). Using a high flow rate of over 30–40 L/min, HFNC can provide a gas flow rate without decreasing oxygen concentration due to air entrainment. VM is also capable of adjusting both oxygen concentration and total gas flow rate; however, its adjustable range is restricted (Table.1). Although several randomized control trials for cardiovascular surgical patients were carried out comparing HFNC with conventional oxygen therapy such as VM or face mask with a reservoir bag, HFNC ameliorated oxygenation but did not decrease perioperative mortality [5, 10]. On the other hand, focusing on short-term therapeutic effects, various verifications have been made. After cardiothoracic surgery, a postoperative routine use of HFNC did not yield improvement in oxygenation nor reduce the rate of atelectasis; however, it did reduce the requirement for an escalation of respiratory support, such as a high flow face mask, HFNC, NIV, and reintubation [11]. Maggiore et al. demonstrated that HFNC could provide an improvement in oxygenation only after 24 h of treatment for hypoxemic patients in their weaning from mechanical ventilation after acute respiratory failure [5]. A recent study compared the preemptive use of HFNC and VM after thoracotomy for lung resection. In the study, HFNC did not reduce the incidence of postoperative hypoxemia but did reduce the incidence of postoperative hypercapnia, compared to VM [12]. Although these findings suggest that HFNC does not ameliorate the long-term prognosis, it was beneficial for the postoperative patient to avoid hypoxemia or hypercapnia after extubation.
On the other hand, taking advantage of its excellent oxygenation, the validity of HFNC as a preoxygenation device has been reported [13]. Recently, the indication of HFNC usage has been developing, not only for the treatment of respiratory failure after extubation in the ICU, but also for preoxygenation before intubation in the emergent or operating room.
This study has some limitations, however. In it, we provided a 60-minute wash-out period after each device usage in order to eliminate the effects of the prior oxygen delivery. A previous study demonstrated that, after either an increase or decrease in FiO2 in stable condition, 5 to 10 minutes is adequate to accurately measure arterial blood samples [14]. Hence, 60 minutes of wash-out period is considered to be sufficient. During both protocols, FiO2 and total gas flow rate were maintained fixed and similar; however, FiO2 was not similar, actually, between these devices because the entrainment of room air varied during breathing when VM was used. In addition, the entrainment of room air with HFNC at 40 L/min could be substantially lower, as the peak inspiratory flow in stable patients after extubation should not exceed 40 L/min and, in this study, was lower than the HFNC setting. Therefore, the higher actual FiO2 could explain the higher PaO2 (Fig. 2) when using HFNC.
Conclusion
Compared to VM, HFNC ameliorates oxygenation function and gas exchange and decreased patients’ effort in breathing. The hemodynamic state did not differ between HFNC and VM and, therefore, HFNC can be used safely in cardiovascular surgical patients after extubation.
Acknowledgements
Not applicable.
CONSORT guidelines
This study adheres to CONSORT guidelines (supplementary material).
Abbreviations
- ICU
Intensive care unit
- HFNC
High flow nasal canulla
- VM
Venturi mask
- PFR
PaO2/FiO2 ratio
- ABG
Arterial blood gas
- RR
Respiratory rate
- HR
Heart rate
- MAP
Mean arterial pressure
- CCI
Continuous cardiac index
- SvO2
Mixed venous oxygen saturation
- NIV
Noninvasive ventilation
Authors’ contributions
YK and OU designed this study. SD recruited patients. SD and SN performed measurements and recorded clinical data. SD and YK analyzed the data, prepared figures and tables and contributed to the manuscript. OU reviewed the manuscript. The author(s) read and approved the final manuscript.
Funding
This reserch did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets and analyses of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Osaka Medical and Pharmaceutical University (file number: RIN89–1635). Written informed consent was obtained.
Consent for publication
Consent for publication has been obtained from all patients.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Joshi P, Fraser JF, Mullany DV. The high risk cardiac surgical patient. Curr Anaesth Crit Care. 2005;16:369–383. doi: 10.1016/j.cacc.2006.05.001. [DOI] [Google Scholar]
- 2.Corley A, Caruana LR, Barnett AG, et al. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth. 2011;107:998–1004. doi: 10.1093/bja/aer265. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Rehder KJ, Williford L, et al. Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature. Intensive Care Med. 2013;39:247–257. doi: 10.1007/s00134-012-2743-5. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura M. High-flow nasal cannula oxygen therapy devices. Respir Care. 2019;64:735–742. doi: 10.4187/respcare.06718. [DOI] [PubMed] [Google Scholar]
- 5.Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. 2014;190:282–288. doi: 10.1164/rccm.201402-0364OC. [DOI] [PubMed] [Google Scholar]
- 6.Theologou S, Ischaki E, Zakynthinos SG, et al. High flow oxygen therapy at two initial flow settings versus conventional oxygen therapy in cardiac surgery patients with Postextubation hypoxemia: a single-center, Unblinded, randomized, controlled trial. J Clin Med. 2021;10:2079. doi: 10.3390/jcm10102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernández G, Vaquero C, Colinas L, et al. Effect of Postextubation high-flow nasal cannula vs. noninvasive ventilation on reintubation and Postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316:1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 8.Stéphan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs. noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313:2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 9.Azoulay E, Lemiale V, Mokart D, et al. Effect of high-flow nasal oxygen vs standard oxygen on 28-day mortality in immunocompromised patients with acute respiratory failure: the HIGH randomized clinical trial. JAMA. 2018;320:2099–2107. doi: 10.1001/jama.2018.14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vourc'h M, Nicolet J, Volteau C, et al. High-flow therapy by nasal Cannulae versus high-flow face mask in severe hypoxemia after cardiac surgery: a single-center randomized controlled study-the HEART FLOW study. J Cardiothorac Vasc Anesth. 2019;34:157–165. doi: 10.1053/j.jvca.2019.05.039. [DOI] [PubMed] [Google Scholar]
- 11.Parke R, McGuinness S, Dixon R, et al. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth. 2013;111:925–931. doi: 10.1093/bja/aet262. [DOI] [PubMed] [Google Scholar]
- 12.Pennisi MA, Bello G, Congedo MT, et al. Early nasal high-flow versus Venturi mask oxygen therapy after lung resection: a randomized trial. Crit Care. 2019;23:68. doi: 10.1186/s13054-019-2361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frat J-P, Ricard J-D, Quenot J-P, et al. Non-invasive ventilation versus high-flow nasal cannula oxygen therapy with apnoeic oxygenation for preoxygenation before intubation of patients with acute hypoxaemic respiratory failure: a randomized, multicenter, open-label trial. Lancet Respir Med. 2019;7:303–312. doi: 10.1016/S2213-2600(19)30048-7. [DOI] [PubMed] [Google Scholar]
- 14.Cakar N, Tuŏrul M, Demirarslan A, et al. Time required for partial pressure of arterial oxygen equilibration during mechanical ventilation after a step change in fractional inspired oxygen concentration. Intensive Care Med. 2001;27:655–659. doi: 10.1007/s001340100900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and analyses of this study are available from the corresponding author upon reasonable request.