Abstract
Background
Interleukin (IL)-1β is a proinflammatory cytokine that has a role in disease-related inflammation, including malaria. However, reports on the effect of IL-1β on malaria severity are inconsistent. Therefore, meta-analyses to compare differences in IL-1β levels between patients with severe malaria, patients with uncomplicated malaria and healthy controls were performed.
Methods
The PRISMA standards were used to perform a systematic review and meta-analysis. A search of PubMed, Scopus, EMBASE and reference lists was conducted for articles providing data on IL-1β levels between patients with severe malaria, patients with uncomplicated malaria and healthy controls between January 1988 and March 2022, using a combination of search terms. The quality of all studies included in this review was determined using the Strengthening the Reporting of Observational Studies in Epidemiology statement: guidelines for reporting observational studies. The evidence was synthesized quantitatively and qualitatively. The differences in IL-1 levels across participant groups were recounted narratively for qualitative synthesis. For quantitative synthesis, the mean difference in IL-1β levels across groups of participants was calculated using a random effects meta-analysis. The publication bias was assessed using funnel plots, Egger’s test and a contour-enhanced funnel plot.
Results
A total of 1281 articles were discovered, and the 17 that satisfied the inclusion criteria were included for syntheses. The meta-analysis results using data from 555 cases of severe malaria and 1059 cases of uncomplicated malaria showed that severe malaria had a higher mean of IL-1β levels than uncomplicated malaria (P < 0.01, pooled mean difference: 1.92 pg/mL, 95% confidence interval: 0.60–3.25 pg/mL, I2: 90.41%, 6 studies). The meta-analysis results using data from 542 cases of uncomplicated malaria and 455 healthy controls showed no difference in mean IL-1β levels between the two groups (P = 0.07, pooled mean difference: 1.42 pg/mL, 95% confidence interval: − 0.1–2.94 pg/mL, I2: 98.93%, 6 studies).
Conclusion
The results from the meta-analysis revealed that IL-1β levels were higher in patients with severe malaria than in patients with uncomplicated malaria; however, IL-1β levels were similar in patients with uncomplicated malaria and healthy controls. Based on the limitations of the number of studies included in the meta-analysis and high levels of heterogeneity, further studies are needed to conclude that differences in IL-1β levels can be useful for monitoring the malaria severity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12936-022-04325-0.
Keywords: IL-1β, Malaria severity, Severe malaria, Meta-analysis
Background
Severe malaria is defined as Plasmodium falciparum asexual parasitaemia in combination with one or more of the following complications: impaired consciousness, prostration, multiple convulsions, acidosis, hypoglycaemia, severe malarial anaemia, renal impairment, jaundice, pulmonary oedema, significant bleeding, shock or hyperparasitaemia [1]. Moreover, severe Plasmodium vivax and Plasmodium knowlesi malaria are classified similarly to falciparum malaria, except that parasite density criteria are not used [1].
Interleukin-1 (IL-1) is a critical regulator of inflammation because it is involved in a variety of innate immune responses [2]. According to the human sequencing algorithm technology, the IL-1 family includes 11 members: IL-1α, IL-1β, IL-1Ra, IL-18, IL-33, IL-36α, IL-36β, IL-36γ, IL-36Ra, IL-37 and IL-38, all of which have comparable or unique biological effects [3, 4]. There are two distinct forms of IL-1, IL-1α and IL-1β, which demonstrate similar biological functions [5]. Although IL-1α and IL-1β share only 27% amino acid sequence homology, they are physically similar and accomplish the same functions through the IL-1 type 1 receptor [6, 7]. IL-1β is primarily synthesized by macrophages, epithelial cells, fibroblasts and endothelial cells in response to pathogen-associated molecular patterns or damage-associated molecular patterns that signal through pattern recognition receptors (PRRs) [8]. IL-1β is expressed in a variety of tissues and cells, but is particularly abundant in macrophages and lymphoid organs, such as the bone marrow, thymus, spleen and lymph nodes. Additionally, it is secreted by non-lymphoid organs such as the digestive system, lung and liver [9, 10]. IL-1β is synthesized as a 269 amino acid precursor protein that is proteolytically cleaved by caspase-1 or other serine proteases activated during inflammation into the active form, which has 153 amino acids at the C-terminus [5, 11–15]. Significant effects of IL-1β include the following: (1) induction of endothelial cells; (2) activation of neutrophil diapedesis and (3) stimulation of cytokine production in the lymphocytes (T and B) [8].
IL-1β is a proinflammatory cytokine that has a role in disease-related inflammation, fever and discomfort [16, 17]. It participates in cellular processes such as proliferation, differentiation and death [18]. Additionally, IL-1β plays a key role in homeostasis, regulating appetite, sleep and body temperature [19]. High levels of IL-1β have been observed in patients with bacterial, viral, fungal and parasitic infections; several forms of malignancies; autoimmune disorders; trauma (surgery); ischaemic illnesses (myocardial infarction) and UV radiation [19]. Studies on IL-1β in the context of malaria are limited and the results are inconsistent; therefore, conclusions on IL-1β in various types of malaria are unclear. Therefore, meta-analyses to assess differences in IL-1β levels between various types of malaria, including between patients with severe malaria, patients with uncomplicated malaria and healthy controls were performed. The findings of this study will inform future research on IL-1β and its function in malaria infection and severity.
Methods
Protocol and search strategy
PRISMA standards were used to perform a systematic review and meta-analysis (Additional files 10, 11) [20]. The systematic review was registered at PROSPERO (CRD42022318871). A search of PubMed, Scopus, EMBASE and reference lists was conducted for articles providing data on IL-1β levels between patients with severe malaria, patients with uncomplicated malaria and healthy controls between January 1988 and March 2022. Broad search terms ‘(‘Interleukin 1 beta’ OR ‘Interleukin 1beta’ OR ‘IL-1 beta’ OR ‘Interleukin-1 beta’ OR Catabolin) AND (malaria OR plasmodium) were combined as a search strategy for different databases (Additional file 7: Table S1). Relevant article citations were manually searched to ensure that relevant articles were not missed. Additionally, authors of published articles were contacted to get data that could not be extracted directly from the source. The search began on 7 March 2022, and concluded on 20 March 2022.
Eligibility criteria
To be considered for inclusion in the review, articles had to report IL-1β levels among patients with severe malaria, patients with uncomplicated malaria and healthy controls. The following articles were excluded: (i) studies providing data on IL-1β levels in patients with uncomplicated malaria only, (ii) studies providing data on IL-1β levels in pregnancy/cord blood, because these participants had a diverse immune response to malaria infection, (iii) in vitro studies measuring IL-1β production, (iv) studies from which IL-1β data could not be extracted, (v) conference abstracts on IL-1β, (vi) studies providing data on IL-1β levels in patients with asymptomatic malaria only, (vii) studies providing data on IL-1β levels in patients with severe malaria only and (viii) studies providing data on IL-1β levels after malaria treatment.
Study selection and data extraction
The selection procedure began with the examination of titles and abstracts from three databases. The complete text of all qualified articles was then read and compared with the eligibility criteria. Additionally, the reference lists of papers included were evaluated to confirm that no study was omitted. Two authors (AM and MK) independently reviewed articles for inclusion and extracted data based on the following: first author name, publication year, research location, country, age range, number of patients, Plasmodium spp., IL-1β levels, technique used for diagnosing malaria and method used for quantifying IL-1β. Disagreements between the two authors were settled by consensus-building conversations.
Critical appraisal
The quality of all studies included in this review was determined using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [21]. Each study was evaluated on 22 items; a score of 1 (yes) or 0 (no) was awarded to each item and the aggregate of all values generated an overall quality score ranging from 0 to 22. The summed scores were classed as having high (> 75 percentile), moderate (50–75 percentile) or low (< 50 percentile) quality based on the total score.
Data syntheses
The evidence was synthesized quantitatively and qualitatively. The differences in IL-1 levels across participant groups were recounted narratively for qualitative synthesis. For quantitative synthesis, the mean difference (MD) in IL-1β levels across groups of participants was calculated using a random effects meta-analysis, which is a type of meta-analysis where each study is weighted according to variations between and within studies. The mean and standard deviation (SD) of IL-1β levels were used to calculate MD in IL-1β levels between studies. When the median or interquartile range (IQR) was reported in the studies, the mean and SD were computed using the previously established approach [22]. If just SD was missing from the study, SD was derived from one or more studies with comparable mean values [23]. The degree of heterogeneity was determined using Cochran’s Q statistic and the I2 statistic. The forest plot was used to depict the MDs and confidence intervals (CIs). Outliers were detected using the leave-one-out strategy, which involved iteratively rerunning meta-analysis and deleting studies. The publication bias was assessed by visualizing funnel plot symmetry. The funnel plot would be asymmetric in case of publication bias [24, 25]. Egger’s test was used to assess funnel plot symmetry [25]. Egger’s test with statistical significance (p < 0.05) might indicate that funnel plot asymmetry was due to a small-study effect [26]. A contour-enhanced funnel plot was used to explore the cause (s) of funnel plot symmetry [27]. To investigate potential sources of variation, research designs, study sites, Plasmodium spp., age groups and methodologies for IL-1β measurement were used as covariates. Stata, version 17, was used to analyse the data (Stata Corporation, College Station, TX).
Results
Search results
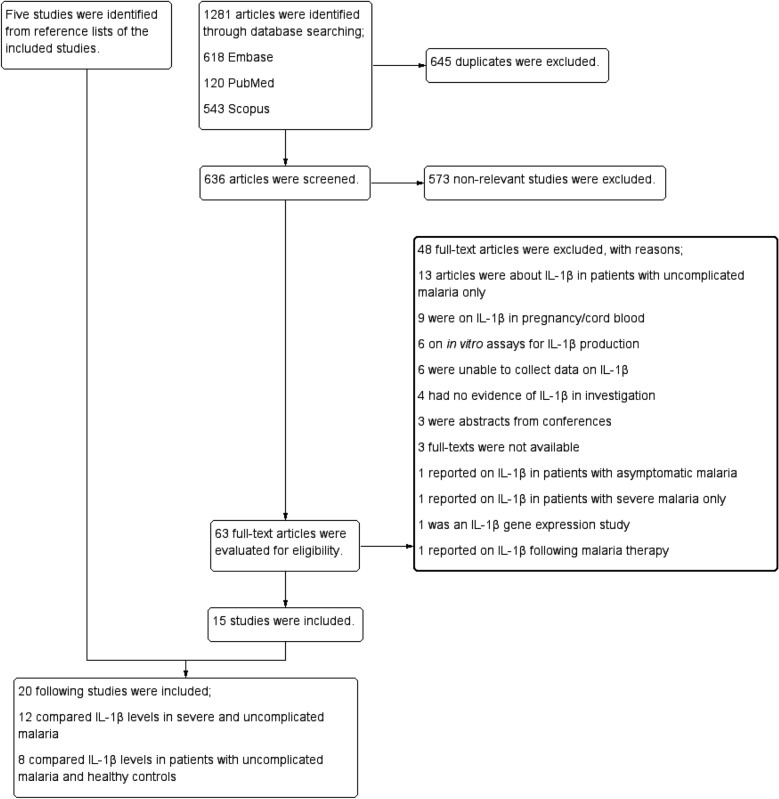
Through database searches, a total of 1281 articles were discovered, including 618 from Embase, 120 from PubMed and 543 from Scopus. After eliminating 645 duplicates, 636 articles were examined for titles and abstracts, followed by the exclusion of 573 irrelevant articles. The remaining 63 full-text articles were evaluated for eligibility, and 48 articles were eliminated for a variety of reasons; 13 articles were about IL-1β in patients with uncomplicated malaria only, 9 were on IL-1β in pregnancy/cord blood, 6 on in vitro assays for IL-1β production, 6 were unable to collect data on IL-1β, 4 had no evidence of IL-1β in the investigation, 3 were abstracts from conferences, 3 full-texts were not available, 1 reported on IL-1β in patients with asymptomatic malaria, 1 reported on IL-1β in patients with severe malaria only, 1 was an IL-1β gene expression study and 1 reported on IL-1β following malaria therapy. Fifteen studies [28–42] satisfied the inclusion criteria. Five articles [43–47] were found using the reference lists of the studies that were included. Finally, 20 studies [28–47] were included for qualitative and quantitative syntheses. These studies included 12 [28–36, 44, 46, 47] that compared IL-1β levels in severe and uncomplicated malaria and 8 [37–43, 45] that compared IL-1β levels in patients with uncomplicated malaria and healthy controls (Fig. 1).
Fig. 1.
Study selection process
Characteristics and quality of the included studies
The characteristics of the included studies are shown in Table 1. Briefly, the included studies were published between 1994 and 2020. The included studies were case–control studies (8/20, 40%) [29, 33, 36, 37, 40, 43, 44, 46], cross-sectional studies (6/20, 30%) [30, 31, 34, 39, 41, 45], prospective observational studies (5/20, 25%) [28, 35, 38, 42, 47] and a cohort study [32]. The included studies were conducted in Africa (10/20, 50%) [28, 30, 31, 33, 34, 36, 38, 43, 44, 47], South America (4/20, 20%) [37, 40, 41, 45], Asia and Oceania [29, 35, 46], Europe [39, 42] and North America [32]. Most of the included studies enrolled patients with P. falciparum (13/20, 65%) [28, 30–36, 38, 39, 43, 44, 47] and children aged between less than 1 and 17 years (11/20, 55%) [28, 30–34, 36, 38, 44, 46, 47]. Finally, most of the included studies (16/20, 80%) used microscopy alone for to identify the malaria parasites [28–35, 37–40, 42–44, 47] and bead-based assay (13/20, 65%) for IL-1β quantification [28, 31–34, 38–41, 44–47]. Details of the included studies are shown in Additional file 8: Table S2. Among 20 studies included for the review, 19 were of high quality [28–42, 44–47] and 1 was of low quality [43] based on the STROBE checklist (Additional file 9: Table S3).
Table 1.
Characteristics of the included studies
| Characteristics | N | % |
|---|---|---|
| Study designs | ||
| Case–control studies | 8 | 40 |
| Cross-sectional studies | 6 | 30 |
| Prospective observational studies | 5 | 25 |
| Cohort study | 1 | 5 |
| Study areas | ||
| Africa | 10 | 50 |
| South America | 4 | 20 |
| Asia and Oceania | 3 | 15 |
| Europe | 2 | 10 |
| North America | 1 | 5 |
| Plasmodium spp. | ||
| P. falciparum | 13 | 65 |
| P. falciparum/P. vivax | 4 | 20 |
| P. vivax | 2 | 10 |
| P. knowlesi/P. falciparum | 1 | 5 |
| Participants | ||
| Children | 11 | 55 |
| Adults | 7 | 35 |
| All age groups | 2 | 10 |
| Methods for malaria detection | ||
| Microscopy | 16 | 80 |
| Microscopy/PCR | 4 | 20 |
| Methods for TGF-β quantification | ||
| Bead-based assay | 13 | 65 |
| ELISA | 7 | 35 |
ELISA enzyme-linked immunosorbent assay, PCR polymerase chain reaction
IL-1β levels in severe and uncomplicated malaria
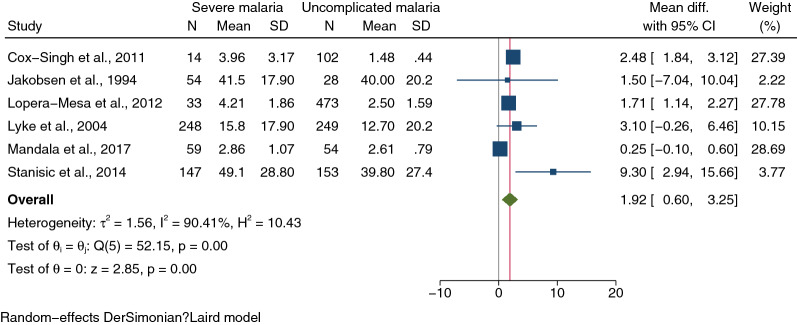
Of the 20 studies included, 12 [28–36, 44, 46, 47] that compared IL-1β levels between severe and uncomplicated malaria were included for qualitative and quantitative syntheses. A qualitative synthesis of the 12 studies revealed that severe malaria had higher IL-1β levels than uncomplicated malaria [28, 29, 31, 32, 35, 46]. No difference between groups was demonstrated by other studies [30, 33, 34, 36, 44, 47]. Moreover, 6 studies [29, 30, 32, 33, 44, 46] provided quantitative data on IL-1β levels in severe (555 cases) and uncomplicated malaria (1059 cases) and were included in the meta-analysis. The meta-analysis results showed that severe malaria had a higher mean of IL-1β levels than uncomplicated malaria (P < 0.01, pooled MD: 1.92 pg/mL, 95% CI: 0.60–3.25 pg/mL, I2: 90.41%, 6 studies, Fig. 2).
Fig. 2.
Forest plot demonstrating pooled MD of IL-1β levels (pg/mL) between patients with severe and uncomplicated malaria. Horizontal line (whiskers) encircling the square on both sides, CI; the squares’ centres and the values of the study’s effect sizes; green diamond, the overall effect size; the values of the overall effect size are indicated by the red vertical dashed line; vertical gray line with no effect on the overall composition (0); I2, H2, τb2, heterogeneity measures; under the green diamond: the test of I also known as the homogeneity test, is based on the Q statistic; the test of θ, the overall magnitude of the effect. CI confidence interval, Mean Diff. mean difference, SD standard deviation
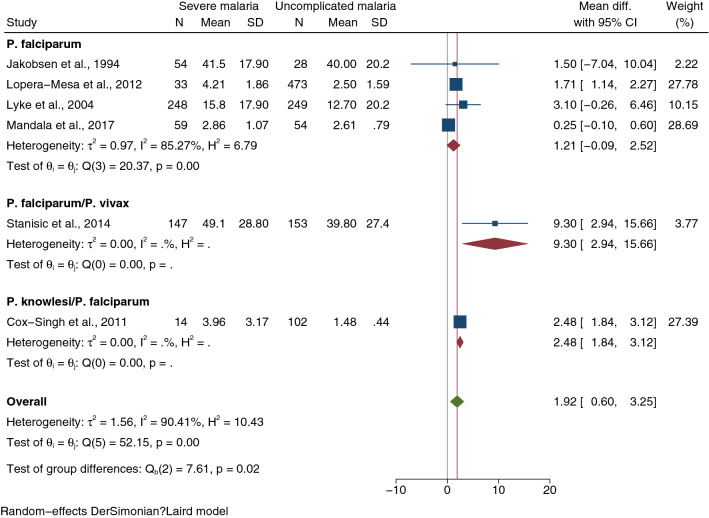
Meta-regression analysis using study design, continents, Plasmodium spp., age group and method for IL-1β quantification as covariates showed that only Plasmodium spp. confounded pooled MD (P = 0.047). Subgroup analysis of Plasmodium spp. showed no difference in IL-1β levels between the two groups in patients infected with P. falciparum (pooled MD: 1.21 pg/mL, 95% CI − 0.09–2.52 pg/mL, I2: 85.27%, four studies, Fig. 3). Data from other subgroups could not be interpreted because the number of studies was less than two.
Fig. 3.
Forest plot demonstrating pooled MD of IL-1β levels (pg/mL) between patients with severe and uncomplicated malaria stratified by Plasmodium species. Horizontal line (whiskers) encircling the square on both sides, CI; the squares’ centres and the values of the study's effect sizes; green diamond, the overall effect size; the values of the overall effect size are indicated by the red vertical dashed line; vertical gray line with no effect on the overall composition (0); I2, H2, τb2, heterogeneity measures; under the crimson diamond: I2, H2, τb2, heterogeneity measures of subgroup; test of θi, the homogeneity test based on the Q statistic; under the green diamond: the test of θi also known as the homogeneity test, is based on the Q statistic; The test of θ, the overall magnitude of the effect. CI confidence interval, Mean Diff. mean difference, SD standard deviation
IL-1β levels in patients with uncomplicated malaria and healthy controls
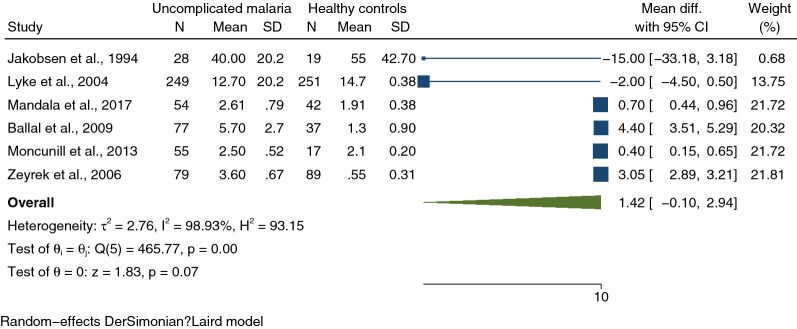
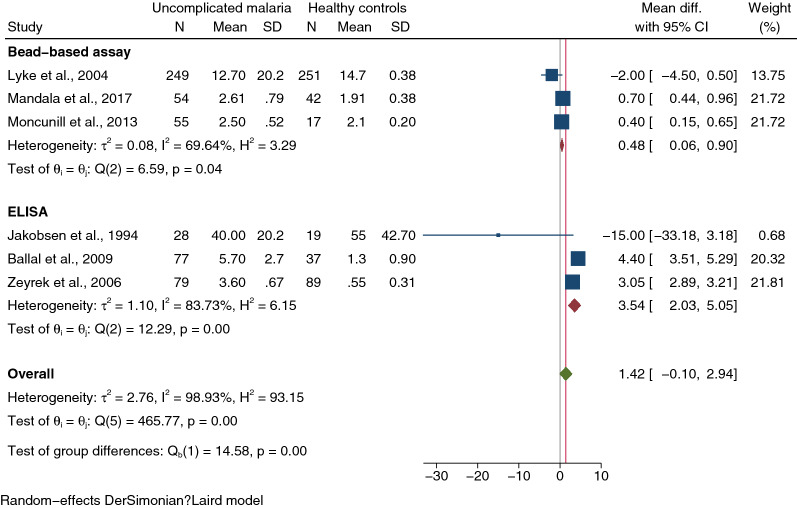
Of 20 studies included in this study, 10 [30, 33, 37, 38, 40–45] that compared IL-1β levels between patients with uncomplicated malaria and healthy controls were included for qualitative and quantitative syntheses. A qualitative synthesis of 10 studies providing data on IL-1β levels between patients with uncomplicated malaria and healthy controls revealed that 6 [37, 41–45] reported higher IL-1β levels in patients with uncomplicated malaria than healthy controls. No difference in IL-1β levels between the two groups was reported by 4 studies [30, 33, 38, 40]. Of the 10 studies that compared IL-1β levels between patients with uncomplicated malaria and healthy controls, 6 [30, 33, 39, 42–44] provided quantitative data on IL-1β levels in uncomplicated malaria (542 cases) and healthy controls (455 individuals). Meta-analysis results showed no difference in mean IL-1β levels between the two groups (P = 0.07, pooled MD: 1.42 pg/mL, 95% CI − 0.1–2.94 pg/mL, I2: 98.93%, 6 studies, Fig. 4).
Fig. 4.
Forest plot demonstrating pooled MD of IL-1β levels (pg/mL) between uncomplicated and healthy control participants. The x-axis of the forest plot is on a logged scale 0–10, black x-axis line; the squares’ centres and the values of the study’s effect sizes in logged scale; green diamond; I2, H2, τb2, heterogeneity measures; under the green diamond: the test of I also known as the homogeneity test, is based on the Q statistic; the test of θ, the overall magnitude of the effect. CI confidence interval, Mean Diff. mean difference, SD standard deviation
Meta-regression analysis using study design, continents, Plasmodium spp., age group and method for IL-1β quantification as covariates showed that only method for IL-1β quantification confounded pooled MD (P < 0.001). Subgroup analysis of the method for IL-1β quantification showed a subgroup difference (P < 0.01). A higher mean of IL-1β levels in uncomplicated malaria than healthy controls was observed in studies using the bead-based assay (pooled MD: 0.48 pg/mL, 95% CI 0.06–0.90 pg/mL, I2: 69.64%, three studies, Fig. 5) and studies using ELISA for IL-1β quantification (pooled MD: 3.54 pg/mL, 95% CI 2.03–5.05 pg/mL, I2: 83.73%, three studies, Fig. 5).
Fig. 5.
Forest plot demonstrating pooled MD of IL-1β levels (pg/mL) between patients with severe and uncomplicated malaria stratified by methods for IL-1β measurement. Horizontal line (whiskers) encircling the square on both sides, CI; the squares’ centres and the values of the study’s effect sizes; Green diamond, the overall effect size; the values of the overall effect size are indicated by the red vertical dashed line; vertical gray line with no effect on the overall composition (0); I2, H2, τb2, heterogeneity measures; under the crimson diamond: I2, H2, τb2, heterogeneity measures of subgroup; test of θi, the homogeneity test based on the Q statistic; under the green diamond: the test of θi also known as the homogeneity test, is based on the Q statistic; The test of θ, the overall magnitude of the effect. CI confidence interval, Mean Diff. mean difference, SD standard deviation
Sensitivity analysis and certainty of the evidence
The sensitivity analysis using the leave-one-out method showed robust meta-analysis results, demonstrating a higher mean of IL-1β levels in severe malaria than uncomplicated malaria (P < 0.05 when each study was removed and the meta-analysis was rerun, 6 studies, Additional file 1: Fig. S1). However, meta-analysis results demonstrating differences in mean IL-1β levels between patients with uncomplicated malaria and healthy controls were not robust. P < 0.05 when three studies [30, 33, 42] were removed and the meta-analysis was rerun, P > 0.05 when three studies [39, 43, 44] were removed and the meta-analysis was rerun (Additional file 2: Fig. S2).
Reporting bias
Reporting bias (publication bias) was assessed by visualizing funnel plot asymmetry, Egger’s test, Begg’s rank test and contour-enhanced funnel plot. For the meta-analysis of the difference in mean of IL-1β levels between severe malaria and uncomplicated malaria, the funnel plot was asymmetric (Additional file 3: Fig. S3); Egger’s test demonstrated no small-study effects (P = 0.07) and contour-enhanced funnel plot demonstrated the distribution of the MDs in both significant and non-significant areas (Additional file 4: Fig. S4), indicating that funnel plot asymmetry was due to other causes, such as heterogeneity of the MDs in the included studies, rather than publication bias.
For the meta-analysis of the difference in mean of IL-1β levels between patients with uncomplicated malaria and healthy controls, the funnel plot was asymmetric (Additional file 5: Fig. S5); Egger’s test demonstrated small-study effects (P = 0.03) and contour-enhanced funnel plot demonstrated the distribution of the MDs in both significant and non-significant areas (Additional file 6: Fig. S6), indicating that the cause of funnel plot asymmetry was due to publication bias and heterogeneity of the MDs in the included studies.
Discussion
The meta-analysis confirmed that patients with severe malaria had higher IL-1β levels than those with uncomplicated malaria. Studies have indicated that IL-1β plays a crucial role in parasite clearance when combined with other cytokines, including interferon-gamma (IFN-γ), IL-2, IL-12 and TNF-α [48–50]. In the recent meta-analysis, significantly increased TNF-α [51] and decreased IL-12 levels were found in patients with severe malaria compared with patients with uncomplicated malaria. These results indicate that increased TNF-α and IL-1β production contributes to the pathogenesis of severe malaria. However, the overall meta-analysis result confirmed higher IL-1β levels than those in uncomplicated malaria. There were high levels of heterogeneity in IL-1β levels in the studies included in the meta-analysis (90.41%). The meta-regression analysis was used to test whether study design, continents, Plasmodium spp., age group and method for IL-1β quantification were confounders in the meta-analysis. Unfortunately, only Plasmodium spp. was a candidate confounder in the meta-analysis, and the results showed no difference in IL-1β levels between patients with severe and uncomplicated malaria caused by P. falciparum based on 4 studies [30, 32, 33, 44]. Because a limited number of studies were included in the meta-analysis, it could not be conclude that the infection by different Plasmodium spp. causes differences in IL-1β levels. There was a possibility that infection with different Plasmodium spp. may cause differences in IL-1β levels. For example, there was a low detection rate of IL-1β levels in patients infected with P. knowlesi, P. vivax and P. falciparum; however, the rate was higher for patients infected with P. falciparum than P. vivax and P. knowlesi [29]. Further studies are needed to confirm the differences in Plasmodium spp. and different cytokine levels in malaria severity.
In the literature, inconsistent reports exist on the influence of IL-1β on malaria severity. Studies have reported an increase in IL-1β in patients with severe malaria, notably cerebral malaria [31, 35]. In cerebral malaria, increased IL-1β production was shown to associate with malaria pathogenesis [52, 53]. Increased IL-1β levels in cerebral malaria were either directly or indirectly connected with brain oedema [54]. Vogetseder et al. reported that anti-malarial therapy for 5 d decreased IL-1β levels in patients with severe malaria, indicating that increased IL-1β levels involve malaria severity [55]. However, some studies indicated that no statistically significant differences in IL-1β levels existed between patients with different severity of malaria and healthy controls [28, 30]. Lyke et al. suggested that no correlation between IL-1β levels and malaria severity might be because IL-1β was downregulated by IL-10 [33].
Meta-analysis results confirmed that patients with uncomplicated malaria had comparable IL-1β levels to healthy controls. Nevertheless, a high level of heterogeneity in IL-1β levels was observed in the studies included in the meta-analysis (98.93%). The meta-regression analysis was used to test whether study design, continents, Plasmodium spp., age group and method for IL-1β quantification were confounder in the meta-analysis. We found that the method for IL-1β quantification was a confounder in the meta-analysis, and the results showed higher IL-1β levels in uncomplicated malaria than healthy controls in studies using both the bead-based assay and ELISA for IL-1β quantification. There was a difference in pooled MD in studies using the bead-based assay (0.48 pg/mL) and those using ELISA (3.54 pg/mL) for IL-1β quantification. The reason for the difference in the performance of the bead-based assay and ELISA for the detection of IL-1β is unknown. The bead-based assay is suitable for detection of several cytokines in a single platform, and ELISA can detect only one cytokine in a single platform [56]. Therefore, bead-based assays are preferred for screening several cytokines and may become increasingly commonplace [57].
The study has limitations. First, although changes in IL-1β levels in several groups of patients were examined, each cytokine performs a distinct function and contributes to a complex cytokine network. Therefore, predicting an association between cytokine levels and malaria severity is challenging. Second, the heterogeneity of outcome between the studies included in the meta-analysis may restrict the conclusion reached. The study was also limited due to publication bias, as indicated by the funnel plot. Third, publication bias was caused due to small-study effects in the meta-analysis of MDs between patients with uncomplicated malaria and healthy controls, indicating the need for more studies providing data on IL-1β levels in the meta-analysis. Fourth, the meta-analysis results revealing a higher mean of IL-1β levels in severe malaria compared with uncomplicated malaria had more certainty than those demonstrating a difference in mean IL-1β levels between patients with uncomplicated malaria and healthy controls. Fifth, the study excluded studies with pregnant women from the analysis. Future studies to determine differences in IL-1β levels in malaria in pregnancy are suggested, because pregnant women are a frequently neglected and highly vulnerable population.
Conclusion
The meta-analysis results suggested that IL-1β levels were higher in patients with severe malaria than patients with uncomplicated malaria. However, IL-1β levels were not different between patients with uncomplicated malaria and healthy controls. Based on the limitations of the number of studies included in the meta-analysis and high levels of heterogeneity, further studies are needed to conclude that differences in IL-1β levels can be useful for monitoring malaria severity.
Supplementary Information
Additional file 1: Figure S1. Sensitivity analysis using the leave-one-out method demonstrated the difference in mean IL-1β levels (pg/mL) between patients with severe malaria and those with uncomplicated malaria after excluding each study. Horizontal green line extending on either side of the green dot, CI; The green dots, the values of the overall effect size. CI: confidence interval; Mean Diff.: mean difference (MD); red vertical line: the overall effect size.
Additional file 2: Figure S2. Sensitivity analysis using the leave-one-out method demonstrated the difference in mean IL-1β levels (pg/mL) between patients with uncomplicated malaria and healthy control participants after excluding each study. Horizontal green line extending on either side of the green dot, CI; the green dots, the values of the overall effect size. CI: confidence interval; Mean Diff.: mean difference (MD); red vertical line, the overall effect size.
Additional file 3: Figure S3. Funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between severe and uncomplicated malaria. CI: confidence interval; Mean Diff.: mean difference (MD); estimated θIV: the overall effect size.
Additional file 4: Figure S4. Contour enhanced funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between severe and uncomplicated malaria. CI: confidence interval; Mean Diff.: mean difference (MD).
Additional file 5: Figure S5. Funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between uncomplicated malaria and healthy control participants. CI: confidence interval; Mean Diff.: mean difference (MD); estimated θIV: the overall effect size.
Additional file 6: Figure S6. Contour enhanced funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between uncomplicated malaria and healthy control participants. CI: confidence interval; Mean Diff.: mean difference (MD).
Additional file 7: Table S1. Search terms.
Additional file 8: Table S2. Quality of the included studies.
Additional file 9: Table S3. Details of the included studies.
Additional file 10. PRISMA 2020 abstract checklist.
Additional file 11. PRISMA 2020 checklist.
Acknowledgements
We thank the New Strategic Research (P2P) Project Fiscal year 2022, Walailak University, Thailand, for providing partial funds for this study.
Abbreviations
- CIs
Confidence intervals
- DAMPs
Damage-associated molecular patterns
- IFN-γ
Interferon gamma
- IL
Interleukin
- IQR
Interquartile range
- MD
Mean difference
- PAMPs
Pathogen-associated molecular patterns
- PRRs
Pattern recognition receptors
- SD
Standard deviation
- STROBE
Strengthening the Reporting of Observational Studies in Epidemiology
Author contributions
MK, PK and AM carried out the study design, selection, data extraction, and statistical analysis; and drafted the manuscript. FRM, KUK and PR participated in approving the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Mahidol University, Thailand.
Availability of data and materials
All data and related materials are presented in this manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Aongart Mahittikorn and Pattamaporn Kwankaew contributed equally to this work
Contributor Information
Aongart Mahittikorn, Email: aongart.mah@mahidol.ac.th.
Pattamaporn Kwankaew, Email: pattamaporn.kw@wu.ac.th.
Pongruj Rattaprasert, Email: pongruj.rat@mahidol.ac.th.
Kwuntida Uthaisar Kotepui, Email: kwuntida.ut@wu.ac.th.
Frederick Ramirez Masangkay, Email: frederick_masangkay2002@yahoo.com.
Manas Kotepui, Email: manas.ko@wu.ac.th.
References
- 1.WHO. Guidelines for malaria. Geneva: World Health Organization; 2022. https://www.who.int/publications/i/item/guidelines-for-malaria. Accessed 12 Mar 2022.
- 2.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello C, Arend W, Sims J, Smith D, Blumberg H, O'Neill L, et al. IL-1 family nomenclature. Nat Immunol. 2010;11:973. doi: 10.1038/ni1110-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lachman LB, Hacker MP, Handschumacher RE. Partial purification of human lymphocyte-activating factor (LAF) by ultrafiltration and electrophoretic techniques. J Immunol. 1977;119:2019–2023. [PubMed] [Google Scholar]
- 6.Priestle JP, Schär HP, Grütter MG. Crystallographic refinement of interleukin 1 beta at 2.0 A resolution. Proc Natl Acad Sci USA. 1989;86:9667–9671. doi: 10.1073/pnas.86.24.9667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graves BJ, Hatada MH, Hendrickson WA, Miller JK, Madison VS, Satow Y. Structure of interleukin 1 alpha at 2.7-A resolution. Biochemistry. 1990;29:2679–2684. doi: 10.1021/bi00463a009. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77(8):1627–1652. [PubMed] [Google Scholar]
- 9.Dinarello CA. The interleukin-1 family: 10 years of discovery. FASEB J. 1994;8:1314–1325. [PubMed] [Google Scholar]
- 10.Takács L, Kovacs EJ, Smith MR, Young HA, Durum SK. Detection of IL-1 alpha and IL-1 beta gene expression by in situ hybridization. Tissue localization of IL-1 mRNA in the normal C57BL/6 mouse. J Immunol. 1988;141:3081–3095. [PubMed] [Google Scholar]
- 11.March CJ, Mosley B, Larsen A, Cerretti DP, Braedt G, Price V, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 12.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 13.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasula SM, Poyet JL, Razmara M, Datta P, Zhang Z, Alnemri ES. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J Biol Chem. 2002;277:21119–21122. doi: 10.1074/jbc.C200179200. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11:633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenstreich DL, Vogel SN, Jacques AR, Wahl LM, Oppenheim JJ. Macrophage sensitivity to endotoxin: genetic control by a single codominant gene. J Immunol. 1978;121:1664. [PubMed] [Google Scholar]
- 18.Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:12. doi: 10.1186/s41232-019-0101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. Version 6.3 (updated February 2022). Cochrane; 2022. www.training.cochrane.org/handbook.
- 24.Leucht S, Kissling W, Davis JM. How to read and understand and use systematic reviews and meta-analyses. Acta Psychiatr Scand. 2009;119:443–450. doi: 10.1111/j.1600-0447.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- 25.Wilairatana P, Kwankaew P, Kotepui KU, Kotepui M. Low interleukin-12 levels concerning severe malaria: a systematic review and meta-analysis. Int J Environ Res Public Health. 2022;19:9345. doi: 10.3390/ijerph19159345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilairatana P, Masangkay FR, Kotepui KU, De Jesus MG, Kotepui M. Prevalence and risk of Plasmodium vivax infection among Duffy-negative individuals: a systematic review and meta-analysis. Sci Rep. 2022;12:3998. doi: 10.1038/s41598-022-07711-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilairatana P, Mala W, Milanez GJ, Masangkay FR, Kotepui KU, Kotepui M. Increased interleukin-6 levels associated with malaria infection and disease severity: a systematic review and meta-analysis. Sci Rep. 2022;12:5982. doi: 10.1038/s41598-022-09848-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armah HB, Wilson NO, Sarfo BY, Powell MD, Bond VC, Anderson W, et al. Cerebrospinal fluid and serum biomarkers of cerebral malaria mortality in Ghanaian children. Malar J. 2007;6:147. doi: 10.1186/1475-2875-6-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox-Singh J, Singh B, Daneshvar C, Planche T, Parker-Williams J, Krishna S. Anti-inflammatory cytokines predominate in acute human Plasmodium knowlesi infections. PLoS ONE. 2011;6:e20541. doi: 10.1371/journal.pone.0020541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jakobsen PH, McKay V, Morris-Jones SD, McGuire W, Van Hensbroek MB, Meisner S, et al. Increased concentrations of interleukin-6 and interleukin-1 receptor antagonist and decreased concentrations of beta-2-glycoprotein I in Gambian children with cerebral malaria. Infect Immun. 1994;62:4374–4379. doi: 10.1128/iai.62.10.4374-4379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John CC, Opika-Opoka R, Byarugaba J, Idro R, Boivin MJ. Low levels of RANTES are associated with mortality in children with cerebral malaria. J Infect Dis. 2006;194:837–845. doi: 10.1086/506623. [DOI] [PubMed] [Google Scholar]
- 32.Lopera-Mesa TM, Mita-Mendoza NK, van de Hoef DL, Doumbia S, Konaté D, Doumbouya M, et al. Plasma uric acid levels correlate with inflammation and disease severity in Malian children with Plasmodium falciparum malaria. PLoS ONE. 2012;7:e46424. doi: 10.1371/journal.pone.0046424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyke KE, Burges R, Cissoko Y, Sangare L, Dao M, Diarra I, et al. Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1β), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun. 2004;72:5630–5637. doi: 10.1128/IAI.72.10.5630-5637.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ong'echa JM, Davenport GC, Vulule JM, Hittner JB, Perkins DJ. Identification of inflammatory biomarkers for pediatric malarial: anemia severity using novel statistical methods. Infect Immun. 2011;79:4674–4680. doi: 10.1128/IAI.05161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prakash D, Fesel C, Jain R, Cazenave PA, Mishra GC, Pied S. Clusters of cytokines determine malaria severity in Plasmodium falciparum-infected patients from endemic areas of central India. J Infect Dis. 2006;194:198–207. doi: 10.1086/504720. [DOI] [PubMed] [Google Scholar]
- 36.Rovira-Vallbona E, Moncunill G, Bassat Q, Aguilar R, MacHevo S, Puyol L, et al. Low antibodies against Plasmodium falciparum and imbalanced pro-inflammatory cytokines are associated with severe malaria in Mozambican children: a case–control study. Malar J. 2012;11:181. doi: 10.1186/1475-2875-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa AG, Chaves YO, Teixeira-Carvalho A, Ramasawmy R, Antonelli LRV, Barbosa L, et al. Increased platelet distribution width and reduced IL-2 and IL-12 are associated with thrombocytopenia in Plasmodium vivax malaria. Mem Inst Oswaldo Cruz. 2020;115:e200080. doi: 10.1590/0074-02760200080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davenport GC, Hittner JB, Otieno V, Karim Z, Mukundan H, Fenimore PW, et al. Reduced parasite burden in children with falciparum malaria and bacteremia coinfections: role of mediators of inflammation. Mediat Inflamm. 2016;2016:4286576. doi: 10.1155/2016/4286576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moncunill G, Mayor A, Bardají A, Puyol L, Nhabomba A, Barrios D, et al. Cytokine profiling in immigrants with clinical malaria after extended periods of interrupted exposure to Plasmodium falciparum. PLoS ONE. 2013;8:e73360. doi: 10.1371/journal.pone.0073360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodrigues-da-Silva RN, Lima-Junior JC, Fonseca BP, Antas PR, Baldez A, Storer FL, et al. Alterations in cytokines and haematological parameters during the acute and convalescent phases of Plasmodium falciparum and Plasmodium vivax infections. Mem Inst Oswaldo Cruz. 2014;109:154–162. doi: 10.1590/0074-0276140275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sánchez-Arcila JC, De Souza P-D-S, Vasconcelos MPA, Rodrigues-Da-Silva RN, Pereira VA, Aprígio CL, et al. Intestinal parasites coinfection does not alter plasma cytokines profile elicited in acute malaria in subjects from endemic area of Brazil. Mediat Inflamm. 2014;2014:857245. doi: 10.1155/2014/857245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.YildizZeyrek F, Kurcer MA, Zeyrek D, Simsek Z. Parasite density and serum cytokine levels in Plasmodium vivax malaria in Turkey. Parasite Immunol. 2006;28:201–207. doi: 10.1111/j.1365-3024.2006.00822.x. [DOI] [PubMed] [Google Scholar]
- 43.Ballal A, Saeed A, Rouina P, Jelkmann W. Effects of chloroquine treatment on circulating erythropoietin and inflammatory cytokines in acute Plasmodium falciparum malaria. Ann Hematol. 2009;88:411–415. doi: 10.1007/s00277-008-0636-z. [DOI] [PubMed] [Google Scholar]
- 44.Mandala WL, Msefula CL, Gondwe EN, Drayson MT, Molyneux ME, MacLennan CA. Cytokine profiles in Malawian children presenting with uncomplicated malaria, severe malarial anemia, and cerebral malaria. Clin Vaccine Immunol. 2017;24:e00533–e616. doi: 10.1128/CVI.00533-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinna RA, dos Santos AC, Perce-da-Silva DS, da Silva LA, da Silva RNR, Alves MR, et al. Correlation of APRIL with production of inflammatory cytokines during acute malaria in the Brazilian Amazon. Immun Inflamm Dis. 2018;6:207–220. doi: 10.1002/iid3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanisic DI, Cutts J, Eriksson E, Fowkes FJI, Rosanas-Urgell A, Siba P, et al. γδ T cells and CD14+ monocytes are predominant cellular sources of cytokines and chemokines associated with severe malaria. J Infect Dis. 2014;210:295–305. doi: 10.1093/infdis/jiu083. [DOI] [PubMed] [Google Scholar]
- 47.Thuma PE, van Dijk J, Bucala R, Debebe Z, Nekhai S, Kuddo T, et al. Distinct clinical and immunologic profiles in severe malarial anemia and cerebral malaria in Zambia. J Infect Dis. 2011;203:211–219. doi: 10.1093/infdis/jiq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarangi A, Mohapatra PC, Dalai RK, Sarangi AK. Serum IL-4, IL-12 and TNF-alpha in malaria: a comparative study associating cytokine responses with severity of disease from the Coastal Districts of Odisha. J Parasit Dis. 2014;38:143–147. doi: 10.1007/s12639-013-0237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylvester B, Gasarasi DB, Aboud S, Tarimo D, Masawe S, Mpembeni R, et al. Interferon-gamma and interleukin-10 responses during clinical malaria episodes in infants aged 0–2 years prenatally exposed to Plasmodium falciparum: Tanzanian birth cohort. J Trop Med. 2018;2018:6847498. doi: 10.1155/2018/6847498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramharter M, Kremsner PG, Willheim M, Winkler H, Graninger W, Winkler S. Plasmodium falciparum-specific interleukin-2 and tumor necrosis factor-alpha expressing-T cells are associated with resistance to reinfection and severe malaria in healthy African children. Eur Cytokine Netw. 2004;15:189–196. [PubMed] [Google Scholar]
- 51.Mahittikorn A, Mala W, Srisuphanunt M, Masangkay FR, Kotepui KU, Wilairatana P, et al. Tumour necrosis factor-alpha as a prognostic biomarker of severe malaria: a systematic review and meta-analysis. J Travel Med. 2022;29:taac053. doi: 10.1093/jtm/taac053. [DOI] [PubMed] [Google Scholar]
- 52.Brown H, Turner G, Rogerson S, Tembo M, Mwenechanya J, Molyneux M, et al. Cytokine expression in the brain in human cerebral malaria. J Infect Dis. 1999;180:1742–1746. doi: 10.1086/315078. [DOI] [PubMed] [Google Scholar]
- 53.Maneerat Y, Pongponratn E, Viriyavejakul P, Punpoowong B, Looareesuwan S, Udomsangpetch R. Cytokines associated with pathology in the brain tissue of fatal malaria. Southeast Asian J Trop Med Public Health. 1999;30:643–649. [PubMed] [Google Scholar]
- 54.Harawa V, Njie M, Kessler A, Choko A, Kumwenda B, Kampondeni S, et al. Brain swelling is independent of peripheral plasma cytokine levels in Malawian children with cerebral malaria. Malar J. 2018;17:435. doi: 10.1186/s12936-018-2590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vogetseder A, Ospelt C, Reindl M, Schober M, Schmutzhard E. Time course of coagulation parameters, cytokines and adhesion molecules in Plasmodium falciparum malaria. Trop Med Int Health. 2004;9:767–773. doi: 10.1111/j.1365-3156.2004.01265.x. [DOI] [PubMed] [Google Scholar]
- 56.de Jager W, Rijkers GT. Solid-phase and bead-based cytokine immunoassay: a comparison. Methods. 2006;38:294–303. doi: 10.1016/j.ymeth.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Elshal MF, McCoy JP. Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sensitivity analysis using the leave-one-out method demonstrated the difference in mean IL-1β levels (pg/mL) between patients with severe malaria and those with uncomplicated malaria after excluding each study. Horizontal green line extending on either side of the green dot, CI; The green dots, the values of the overall effect size. CI: confidence interval; Mean Diff.: mean difference (MD); red vertical line: the overall effect size.
Additional file 2: Figure S2. Sensitivity analysis using the leave-one-out method demonstrated the difference in mean IL-1β levels (pg/mL) between patients with uncomplicated malaria and healthy control participants after excluding each study. Horizontal green line extending on either side of the green dot, CI; the green dots, the values of the overall effect size. CI: confidence interval; Mean Diff.: mean difference (MD); red vertical line, the overall effect size.
Additional file 3: Figure S3. Funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between severe and uncomplicated malaria. CI: confidence interval; Mean Diff.: mean difference (MD); estimated θIV: the overall effect size.
Additional file 4: Figure S4. Contour enhanced funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between severe and uncomplicated malaria. CI: confidence interval; Mean Diff.: mean difference (MD).
Additional file 5: Figure S5. Funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between uncomplicated malaria and healthy control participants. CI: confidence interval; Mean Diff.: mean difference (MD); estimated θIV: the overall effect size.
Additional file 6: Figure S6. Contour enhanced funnel plot of studies included in the meta-analysis of MD of IL-1β levels (pg/mL) between uncomplicated malaria and healthy control participants. CI: confidence interval; Mean Diff.: mean difference (MD).
Additional file 7: Table S1. Search terms.
Additional file 8: Table S2. Quality of the included studies.
Additional file 9: Table S3. Details of the included studies.
Additional file 10. PRISMA 2020 abstract checklist.
Additional file 11. PRISMA 2020 checklist.
Data Availability Statement
All data and related materials are presented in this manuscript.