Abstract
Multi-modal imaging strategies integrating multiple imaging modalities have been recognized with improved feasibility in diagnosis, guiding therapy, and predicting outcomes. Magnetic resonance imaging (MRI) permits multi-parameter demonstration of anatomical structures, such as the T1 bright and T2 dark MRI programs. Due to the inherent black-and-white production of MR images, however, MRI detection is partially limited by the occurrence of false-positive diagnosis. Here, we introduce an interesting dual-modal program based on T1-T2 dual-modal MRI and the enhancement by contrast agents. We will focus on the interplay of T1 and T2 relaxation mechanism, which features the origin of T1-T2 dual-modal MRI from molecular basis to contrast agents. The discussion made in this Perspective paper may help to understand the T1-T2 dual-modal MRI and provoke the rational design of the contrast agents for sophisticated MRI applications.
Graphical Abstract
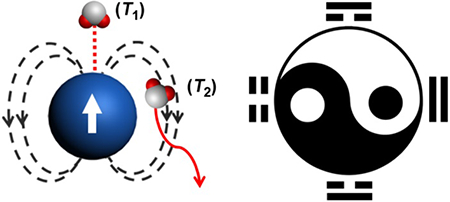
Since the first introduction of X-ray imaging, medicine has long been relying on molecular imaging techniques throughout the decision-making processes of diagnosis and prognosis.1–3 Along with the development of singular medical imaging methods, one of the most fascinating research fields is the development of multimodal imaging strategy to upgrade diagnostic accuracy.4 The increasing needs for imaging subtle details in clinical diagnosis further stimulate the essential requirement of multimodal imaging techniques in cutting-edge.5–7 The Imaging Council of the American College of Cardiology was in agreement that “multimodality imaging is the efficient integration of various methods of cardiovascular imaging to improve the ability to diagnose, guide therapy, or predict outcomes”.4 In general, multimodal imaging program benefits from the cross-validation of multiple parameters which are considered capable of making their individual advantages complementary to each other. The mutual confirmative diagnostic information by multiple imaging modalities holds great potential to exclude false-positive diagnosis as of singular imaging method. For example, the combination of positron emission tomography (PET) and computerized tomography (CT) or magnetic resonance imaging (MRI) for precise pinpointing region of interest with both molecular sensitivity and anatomic resolution.8
Advances in nanomedicine have spurred a number of nanomaterials as imaging agents to further augment the diagnostic sensitivity and accuracy.9, 10 However, contrast agents designed for multimodal imaging purposes have faced a lot of complicated situations.11, 12 For example, probes for PET can be 5~8 orders of magnitude more sensitive than those for MRI, which may lead PET-MRI dual-modal contrast agents to inadequate MRI detection at a low concentration and/or burdened radioactive PET tracers in an unnecessarily high level. Therefore, tying multiple imaging techniques together should avoid combining functions simply for convenience.12 Moreover, the past decades have witnessed multifarious design considerations of combining different imaging techniques in pre-clinical research, from dual-modal to hexa-modal combinations.13–16 Among the variety of multimodal imaging strategies, however, very few of them have earned admittance to clinical applications because of the mostly unparalleled spatial-temporal resolution and sensitivity from difficult imaging techniques. Image registration and comparsion across various imaging modalities are still challenging and time consuming now. In this Perspective paper, we introduce an interesting dual-modal imaging paradigm, T1-T2 dual-modal MRI, achieved by a MRI machine alone.
T1-T2 DUAL-MODAL MRI
MRI is a noninvasive, non-ionized, and radiation-free technique that enables to reconstruct atomic nuclear magnetization signal into 2D/3D images, which is the most widely used anatomic tools in clinical diagnosis.17 The regular imaging protocols in MRI can be subdivided into weighted imaging based on longitudinal (T1) or transverse (T2) relaxation times, molecular diffusion, proton density, etc. The advantage of MRI is its high sensisitivity to soft tissue and its flexibility in designing contrast mechnishm of MRI images for various purpose. For example, T1 characterizes the time needed in the recovery of the longnitudinal magnetization from its excitation state to its equilibrium state after excitation with RF pulse, which is quite different among tissue types naturally and is commonly used in brain anatomic images. On the other hand, T2 characterizes the time needed for the transverse magnetization decaying to zero, which is also strongly dependent on tissue types. T1-weighted MRI is normally achieved by inversition-recovery MRI pulse sequences ref for MPRAGE sequence , which shows darker for longer T1, whereas T2-weighted MRI is normally achieved by echo time (TE) weighted MRI pulse sequences, which shows brigher for longer T2 ref for FLA1R sequence.
Another commonly used imaging procedure is proton density weighted MRI, which was designed to minimize the T1 and T2 effects using optimal parameters, generating an image dependent primarily on the density of protons of the imaging volume. Besides, there are lots of other imaging sequences developed as parameter packages for different imaging purposes.
Recently, novel MRI sequences that enables quantitatively parametrical imaging with clinically feasible time are also avalible, i.e., the paramters, like T1 and T2, can be quantitatively mapped rather than using the parameter-weighted imges. What’s more, the T1 and T2 can be quantitatively mapped simutanously in a single MRI sequence.(1, 2)
Lesion detection in practice requires multiscale integration of diagnostic information. The goal of all imaging strategies is to display contrast in images, which should emphasize certain contrast characteristics of anatomical structures and allow doctors to differentiate which structures are abnormal.18 Under an MR imaging session, both T1 and T2 weighted images can be obtained using one MRI machine by simply adjusting the acquisition sequences, even simultaneously with same spatial sampling (1). The combined T1 and T2 dual-modal MRI is considered with self-confirmed merits in practical diagnosis. Distinctly different from other dual-modal imaging strategies involving two different machines, T1-T2 dual-modal MRI performed on one machine promises to offer an accurate match of spatial and temporal imaging parameters between each other.19, 20
The imaging protocol made by MRI practitioners is highly dependent on their own empirical judgment. In general, T2 imaging protocol is used to assess water content which appears bright signal, while T1-weighted imaging is useful for assessing tissues with high fat contents, such as brain white matters. In many cases, both T1 and T2 MR images will be obtained in order to cross-validate the possible fault-positive information. Taking brain MRI diagnosis for an example, T1 image is acquired to differentiate gray matters between potential lesions and T2 image is used to show the cerebrospinal fluid (CSF) in the brain tissue. Owing to the precise tomographic algorithm of MRI, both T1 and T2 images of every slices can find exact match between each other, which accumulate the advantages of self-confirmed fault-free diagnosis. Although the procedure of T1-T2 dual-modal MRI has not gained acceptance as a standard, the combination of multiple contrast MR images in a way have potentiated the advantages of MRI diagnosis to its maximum extend.
MOLECULAR BASIS
MRI is usually default as referring to 1H MRI because 1H is the most widely studied (largest gyromagnetic ratio) and abundantly existed nucleus in human body. Other magnetic nucleus (e.g., 19F) obey the same rules as for 1H protons. In a typical MRI program, nucleus process around an axis along the direction of an external magnetic field, which features a Boltzmann equilibrium state with nucleus separated by the Zeeman splitting effect into high and low energy states. The net magnetization of this state is parallel to the external magnetic field. Subsequently, a radiofrequency pulse of 90 degrees perpendicularly to the external magnetic field is applied, which flips over the magnetization from longitudinal (z) direction to transverse (xy) plane. Afterwards, the tendency of nucleus to return to its equilibrium state makes up the definition of nuclear magnetic relaxation, including the magnetizations at xy plane and z direction (Figure 1a,b). To simplify, R1 and R2 were artificially nominated to represent for relaxation rate constants at z direction and xy plane, respectively. Correspondingly, the time it takes for longnitudinal magnetization recover to 63% or transverse magnetization decay to 37% of its original state were denoted as T1 and T2 relaxation time, respectively. Typically, T1 and T2 relaxation time differs from each other and varies in different subjects, which is dependent on the spin-lattice and spin-spin interactions between components and surroundings, respectively. A majority of T1 or T2 relaxation times recorded in routine MRI programs are between several microseconds to seconds.
Figure 1:
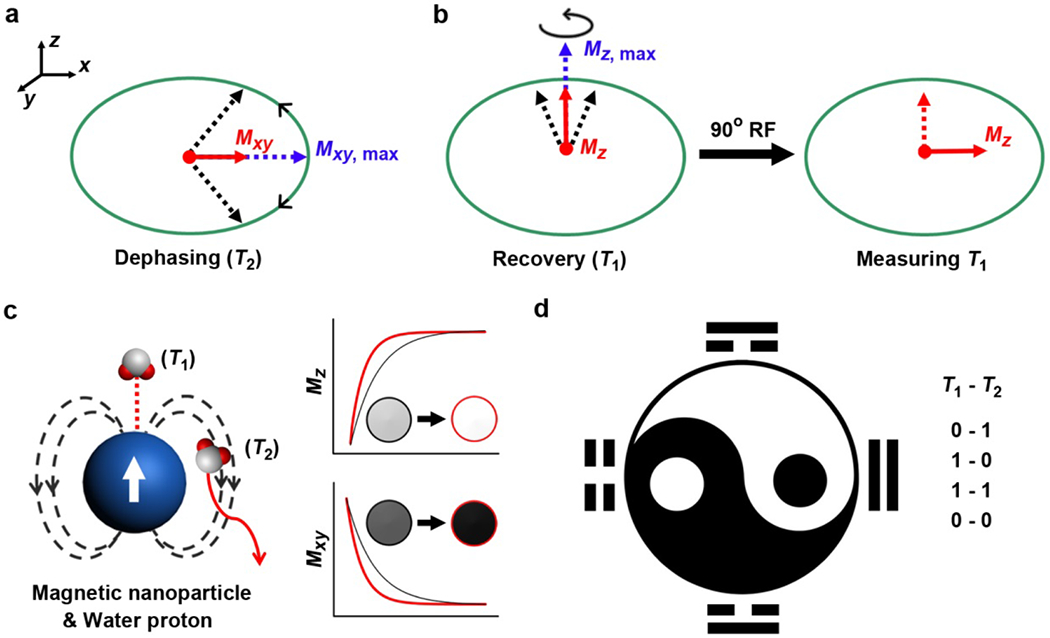
(a) The phenomenon of T2 relaxation correlates to the dephasing of the magnetization at xy plane (Mxy). The Mxy, max is the Mxy immediately after the nuclear magnetic resonance upon a 90° radiofrequency (RF) pulse. (b) The phenomenon of T1 relaxation describes the recovery of magnetization at z direction (Mz) from zero to the Mz, max along with nuclear spin. A second 90° RF is required to flip over the magnetization from z direction to xy plane in order to measure the Mz, so that strong T2 dephasing effect would attenuate T1 due to this inherent process. (c) The direct coordination with and the diffusion around a magnetic nanoparticle are related to the T1 and T2 relaxation enhancement of water protons, respectively. The effective T1 and T2 relaxation enhancement result in brighter contrast in T1 and darker contrast in T2 imaging. (d) The logic of T1-T2 dual-modal imaging can be described as OFF-ON (0-1), ON-OFF (1-0), ON-ON (1-1), OFF-OFF (0-0) states, featuring the multiple-parameter demonstration of MRI.
From a molecular viewpoint, spin-lattice (T1) relaxation implies energy exchange of spins with environment, while spin-spin (T2) relaxation is the loss of phase coherence as spins interacting with each other and surrounding environments. Therefore, it is conceivable that factors causing T1 relaxation will also influence T2 relaxation, whereas some factors that cause T2 relaxation do not involve T1 relaxation, such as static dipolar fields created by neighboring dipoles. Therefore, in most cases but not in principle, the T2 for a given object is always shorter than its T1 relaxation time. It is noteworthy that T1 and T2 relaxations take place independently and immediately after the removal of the 90 degrees radiofrequency pulse. However, only magnetization at transverse plane (T2) can be directly recorded by MRI machine because it exactly perturbs the external magnetic field when it is decaying. To measure magnetization at longitudinal direction (T1) which is parallel to the external magnetic field, another radiofrequency pulse is needed to flip over the restored magnetization at longitudinal direction to transverse plane (Figure 1b). Assuming that an object has larger R2 (shorter T2), its longitudinal magnetization can be partially attenuated by the strong T2 decaying effect when it is measured at the transverse plane after magnetization flipping. Therefore, MRI physicists and radiologists usually have to tune the parameters on each MRI sequence or design new MRI sequence to acquire MRI with satisfied imaging contrast.
A general procedure of T1-T2 dual-modal MRI strategy is to acquire T1 and T2 MRI successively in one MRI study. However, reliable contrasts for T1 bright and T2 dark images are highly dependent on the interplay between the intrinsic T1 and T2 relaxation times of region-of-interest, which are determined by the structural features of the region-of-interest as well as the magnet strength of MRI scanner. In an extreme circumstance, T1-T2 dual-modal MRI should avoid to obtain dual-dark or dual-bright T1-T2 images, which otherwise would cause ambiguous illustration and therefore the loss of mutual confirmative merits in practice. This phenomenon could happen when a tissue has extremely long T1 and short T2 relaxation times (resulting in dual-dark T1-T2 images), or has extremely short T1 and long T2 relaxation times (resulting in dual-bright T1-T2 images). As we noted above, short T2 relaxation time would largely attenuate T1 signal under MRI test. An ideal T1-T2 dual-modal MRI program is thus desirable for tissues with moderate T1 and T2 relaxation times, for examples, liver, kidney, muscle, and brain matters with T1 of 500-900 and T2 of 40-80 ms at a magnet field of 1.5 T. Pathological abnormalities or inflammations usually accompany with water content alternation. Because free water has both long T1 and T2 relaxation times, which are both of about 3 s, which may appear dark in T1 and bright in T2 images. By T1-T2 dual-modal MRI, it is assumable that the diagnostic accuracy can be largely augmented by orthogonal complement of T1 and T2 signal contrasts between normal and abnormal tissues.ref?
CONTRAST AGENTS
Contrast agents are a series of materials enabling to generate imaging contrasts between the region-of-interest and the surroundings. The mechanism of MRI contrast agents is to alter the magnetization relaxation at T1 and T2 planes, wherein the effectiveness is determined by its relaxivity r1 and r2 values, respectively.21, 22 Magnetic materials are usually assorted as T1 or T2 contrast agents based on their dominated function in T1 or T2 MRI. Empirically speaking, T1 contrast agents are usually paramagnetic metal-chelating molecules (e.g., Gd3+, Mn2+ chelates) or organic radicals, which shorten T1 and cause bright contrast in conventional T1-weighted image;23, 24 T2 contrast agents are usually superparamagnetic nanoparticles (e.g., Fe3O4, FeCx), which give dark contrast in T2-weighted image.22, 25 We briefly summarized here that T1 contrast enhancement is mainly related to the direct chemical exchange of protons with the paramagnetic centers at the innersphere regime, and T2 is mainly attributed to the proton’s effective diffusion and interaction with the magnetic dipolar moment at the outersphere regime (Figure 1c).26 Although both T1 and T2 contrast agents have individual r1 and r2 values, few of them is able to exhibit feasible dual-modal contrasts. The reason is complicated in physics and can be easily understood by the followings: (i) T1 contrast agents usually have low r2 values, less than 10 mM−1s−1 for most of Gd chelates, which make it have very limited effect (i.e., percentage change) on R2 , while the effect on R1 is large enough to visibal contrast on T1-weighted images, due to the fact that the natural R1 (without contrast agent) is much smaller than R2; (ii) T2 contrast agents have apparently high r2 value, hundreds to thousands mM−1s−1, which significantly constrained the T1 contrast even though their r1 values are not necessarily low.
The past decades have witnessed a number of design and applications of T1-T2 dual-modal MRI contrast agents, which are eligible to show both T1 bright and T2 dark contrasts. 19, 20, 26–31 Due to the relatively different performance of T1 and T2 contrast agents, one can simply realize that integrating both T1 and T2 contrast materials into one nanoentity could achieve both T1 and T2 contrast abilities. For example, Cheon group reported that MnFe2O4@SiO2 nanoparticles decorated with Gd agents showed distance-dependent T1-T2 dual-modal contrast logics by modulating the thickness of the SiO2 layer.20 Most recently, the same group reported the utilization of distance-dependent magnetic resonance tuning for sensing a wide range of biological targets.32 However, this strategy is established on the compromising of T2 contrast ability to minimize the quenching effect to T1 contrast.20, 29 Alternatively, Gao group reported a serious of Gd2O3 nanocrystals embedded iron oxide nanostructures which exhibited mutual-enhanced T1 and T2 contrast ability compared with that of their single components.19, 28, 33, 34 Besides the paradigm of combining T1 and T2 contrast agents, it was found that certain magnetic nanoparticles inherently display both T1 and T2 contrasts, propagating the family and theory of MRI contrast agents.35 Dated back to a decade ago, Dai group demonstrated that FeCo nanoparticles enabled to serve as both T1 and T2 contrast agents, in which the underlying mechanism is still ambiguous.36 In other attempts, the integration of paramagnetic T1 contrast materials with nonmagnetic matrix (e.g., polymers, porous silica, proteins) would result geometrically confined diffusion for surrounding water molecules, which in turn manifest T1-T2 dual-modal contrast ability of the complex.37, 38
Apparently, the mechanism of T1-T2 dual-modal MRI contrast agents differs from one to another, whereas a few general rules could be applied for assessing the contrast. First, due to the interference of T1 relaxation by T2 decaying effect, magnetic nanoparticles should be optimized with conservative r2 values to compromise the T2 decaying effect to T1 relaxation, which otherwise would vanish the T1 contrast effect. Second, magnetic nanoparticles with small size or magnetic metal chelates with clustering structure would benefit from the altered structural parameters, which may therefore exhibit T1-T2 dual-modal contrasts. Third, the T1-T2 dual-modal manner is highly dependent on the magnetic field strength of the used MRI scanner. Due to the fact that stronger magnetic field would have much more significant T2* effect, which would largely attenuate the T1 relaxivity especially for nano-sized particles and macromolecules. Last but not least, the optimization of sequences used for acquiring T1 and T2 images are required to highlight the differentiation between T1 and T2 contrast images. In practice, the optimal concentration of a given T1-T2 dual-modal contrast agents should have benefited from the phantom study, which is considered to be critical in this system. The use of dual-modal MRI contrast agents may promise to build up an artificial logic program in MRI program with T1-T2 OFF-ON (0-1), ON-OFF (1-0), ON-ON (1-1), OFF-OFF (0-0) states (Figure 1d), featuring the multiple-parameter demonstration of MRI.
CONLUSION AND OUTLOOK
Diagnosis plays a pivotal role in modern medicine, which to some extend will determine the therapeutic process and the outcomes. Different imaging technologies possessing various diagnostic resolution and sensitivity at different levels are often used as a combination to achieve complement accuracy and precision in diagnosis. However, most of the current imaging modalities are difficult to compare the obtained diagnostic information with each other in a parallel level, which underscores the synergistic advantages of combination. On the contrary, T1-T2 dual-modal MRI program is able to provide a pair of anatomical images at exactly the same levels but with different contrasts. Further assisted by the contrast agents, this imaging program can output mutual-confirmative information from both the T1 bright and T2 dark images of pre-contrast and post-contrast. In this respect, the diagnostic accuracy and precision may be significantly enhanced through the orthogonal algorithms. The development of T1-T2 dual-modal contrast agents have attracted numerous attention from chemists and materials scientists for a variety of biomedical applications. After visiting the molecular basis behind T1-T2 dual-modal MRI, the evaluation of T1-T2 dual-modal contrast efficiency of a given magnetic material should take into considerations the magnetic property, the individual T2 decaying effect, and the structural parameters. It is of noted that the study on T1-T2 dual-modal MRI program is still in its infant stage due to the lack of clinical verification.
Despite for the low sensitivity of the typical MRI study, the sensitive responsiveness of T1 and T2 relaxation to environmental changes have stimulated numerous design of responsive MRI systems for analysis of a wide range of biological targets. However, the large variations during the responsive T1 or T2 MRI experiments make it suspicious to the broad applications. The opportunity to use T1-T2 dual-modal MRI scheme may shed light to the sophisticated design of responsive MRI systems using orthogonal T1 and T2 relaxation changes as two coherence parameters. Therefore, future directions on this topic may focus on the design of T1-T2 dual-modal responsive MRI applications. The mutual confirmative logic between T1 and T2 relaxation changes would render greatly improved sensitivity and accuracy, which may also open up new avenues to understanding the phenomenon of MRI contrast enhancement by magnetic materials.
ACKNOWLEDGMENT
This work was supported by the National Science Foundation of China (81571744 and 81601489), the National Basic Research Program of China (863 Program 2015AA020502), the Fundamental Research Funds for the Central Universities (20720170065), the Science Foundation of Fujian Province (No. 2014Y2004), and by the Intramural Research Program (IRP), National Institute of Biomedical Imaging and Bioengineering (NIBIB), National Institutes of Health (NIH).
Footnotes
The authors declare no competing financial interests.
REFERENCES
- 1.Weissleder R; Pittet MJ Imaging in the Era of Molecular Oncology. Nature 2008, 452, 580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weissleder R Molecular Imaging in Cancer. Science 2006, 312, 1168–1171. [DOI] [PubMed] [Google Scholar]
- 3.Willmann JK; van Bruggen N; Dinkelborg LM; Gambhir SS Molecular Imaging in Drug Development. Nat. Rev. Drug Discov 2008, 7, 591–607. [DOI] [PubMed] [Google Scholar]
- 4.Weissman NJ; Soman P; Shah DJ Multimodality Imaging: Opportunities and Challenges. JACC: Cardiovascular Imaging 2013, 6, 1022–1023. [DOI] [PubMed] [Google Scholar]
- 5.Sauter AW; Wehrl HF; Kolb A; Judenhofer MS; Pichler BJ Combined PET/MRI: One Step Further in Multimodality Imaging. Trends Mol. Med 2010, 16, 508–515. [DOI] [PubMed] [Google Scholar]
- 6.Fass L Imaging and Cancer: A Review. Mol. Oncol 2008, 2, 115–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weissleder R; Schwaiger MC; Gambhir SS; Hricak H Imaging Approaches to Optimize Molecular Therapies. Sci. Transl. Med 2016, 8, 355ps16–355ps16. [DOI] [PubMed] [Google Scholar]
- 8.Schulthess G. K. v.; Steinert HC; Hany TF Integrated PET/CT: Current Applications and Future Directions. Radiology 2006, 238, 405–422. [DOI] [PubMed] [Google Scholar]
- 9.Shin T-H; Choi Y; Kim S; Cheon J Recent Advances in Magnetic Nanoparticle-Based Multi-Modal Imaging. Chem. Soc. Rev 2015, 44, 4501–4516. [DOI] [PubMed] [Google Scholar]
- 10.Lee D-E; Koo H; Sun I-C; Ryu JH; Kim K; Kwon IC Multifunctional Nanoparticles for Multimodal Imaging and Theragnosis. Chem. Soc. Rev 2012, 41, 2656–2672. [DOI] [PubMed] [Google Scholar]
- 11.Pagel MD The Hope and Hype of Multimodality Imaging Contrast Agents. Nanomedicine 2011, 6, 945–948. [DOI] [PubMed] [Google Scholar]
- 12.Louie A Multimodality Imaging Probes: Design and Challenges. Chem. Rev 2010, 110, 3146–3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rieffel J; Chen F; Kim J; Chen G; Shao W; Shao S; Chitgupi U; Hernandez R; Graves SA; Nickles RJ; Prasad PN; Kim C; Cai W; Lovell JF Hexamodal Imaging with Porphyrin-Phospholipid-Coated Upconversion Nanoparticles. Adv. Mater 2015, 27, 1785–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Curvers WL; Herrero LA; Wallace MB; Wong Kee Song LM; Ragunath K; Wolfsen HC; Prasad GA; Wang KK; Subramanian V; Weusten BLAM; Ten Kate FJ; Bergman JJGHM Endoscopic Tri-Modal Imaging Is More Effective Than Standard Endoscopy in Identifying Early-Stage Neoplasia in Barrett’s Esophagus. Gastroenterology 2010, 139, 1106–1114. e1. [DOI] [PubMed] [Google Scholar]
- 15.Xie J; Chen K; Huang J; Lee S; Wang J; Gao J; Li X; Chen X PET/NIRF/MRI Triple Functional Iron Oxide Nanoparticles. Biomaterials 2010, 31, 3016–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ali Z; Abbasi AZ; Zhang F; Arosio P; Lascialfari A; Casula MF; Wenk A; Kreyling W; Plapper R; Seidel M; Niessner R; Knöll J; Seubert A; Parak WJ Multifunctional Nanoparticles for Dual Imaging. Anal. Chem 2011, 83, 2877–2882. [DOI] [PubMed] [Google Scholar]
- 17.Liang ZP; Lauterbur PC Principles of Magnetic Resonance Imaging: A Signal Processing Perspective. Wiley-IEEE Press: 1999. [Google Scholar]
- 18.Moseley M; Donnan G Multimodality Imaging Introduction. Stroke 2004, 35, 2632–2634. [Google Scholar]
- 19.Zhou Z; Huang D; Bao J; Chen Q; Liu G; Chen Z; Chen X; Gao J A Synergistically Enhanced T1-T2 Dual-Modal Contrast Agent. Adv. Mater 2012, 24, 6223–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JS; Lee JH; Shin TH; Song HT; Kim EY; Cheon J Self-Confirming “and” Logic Nanoparticles for Fault-Free MRI. J. Am. Chem. Soc 2010, 132, 11015–11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Na HB; Song IC; Hyeon T Inorganic Nanoparticles for MRI Contrast Agents. Adv. Mater 2009, 21, 2133–2148. [Google Scholar]
- 22.Lee N; Yoo D; Ling D; Cho MH; Hyeon T; Cheon J Iron Oxide Based Nanoparticles for Multimodal Imaging and Magnetoresponsive Therapy. Chem. Rev 2015, 115, 10637–10689. [DOI] [PubMed] [Google Scholar]
- 23.Villaraza AJ; Bumb A; Brechbiel MW Macromolecules, Dendrimers, and Nanomaterials in Magnetic Resonance Imaging: The Interplay between Size, Function, and Pharmacokinetics. Chem. Rev 2010, 110, 2921–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L; Liu R; Peng H; Li P; Xu Z; Whittaker AK The Evolution of Gadolinium Based Contrast Agents: From Single-Modality to Multi-Modality. Nanoscale 2016, 8, 10491–10510. [DOI] [PubMed] [Google Scholar]
- 25.Lee N; Hyeon T Designed Synthesis of Uniformly Sized Iron Oxide Nanoparticles for Efficient Magnetic Resonance Imaging Contrast Agents. Chem. Soc. Rev 2012, 41, 2575–2589. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z; Zhao Z; Zhang H; Wang Z; Chen X; Wang R; Chen Z; Gao J Interplay between Longitudinal and Transverse Contrasts in Fe3O4 Nanoplates with (111) Exposed Surfaces. ACS Nano 2014, 8, 7976–7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li F; Zhi D; Luo Y; Zhang J; Nan X; Zhang Y; Zhou W; Qiu B; Wen L; Liang G Core/Shell Fe3O4/Gd2O3 Nanocubes as T1-T2 Dual Modal MRI Contrast Agents. Nanoscale 2016, 8, 12826–12833. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Z; Wu C; Liu H; Zhu X; Zhao Z; Wang L; Xu Y; Ai H; Gao J Surface and Interfacial Engineering of Iron Oxide Nanoplates for Highly Efficient Magnetic Resonance Angiography. ACS Nano 2015, 9, 3012–3022. [DOI] [PubMed] [Google Scholar]
- 29.Shin T-H; Choi J.-s.; Yun S; Kim I-S; Song H-T; Kim Y; Park KI; Cheon J T1 and T2 Dual-Mode MRI Contrast Agent for Enhancing Accuracy by Engineered Nanomaterials. ACS Nano 2014, 8, 3393–3401. [DOI] [PubMed] [Google Scholar]
- 30.Im GH; Kim SM; Lee D-G; Lee WJ; Lee JH; Lee IS Fe3O4/MnO Hybrid Nanocrystals as a Dual Contrast Agent for Both T1- and T2-Weighted Liver MRI. Biomaterials 2013, 34, 2069–2076. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z; Liu H; Chi X; Chen J; Wang L; Sun C; Chen Z; Gao J A Protein-Corona-Free T1–T2 Dual-Modal Contrast Agent for Accurate Imaging of Lymphatic Tumor Metastasis. ACS Appl. Mater. Interfaces 2015, 7, 28286–28293. [DOI] [PubMed] [Google Scholar]
- 32.Choi J.-s.; Kim S; Yoo D; Shin T-H; Kim H; Gomes MD; Kim SH; Pines A; Cheon J Distance-Dependent Magnetic Resonance Tuning as a Versatile MRI Sensing Platform for Biological Targets. Nat. Mater 2017, DOI: 10.1038/nmat4846. [DOI] [PubMed] [Google Scholar]
- 33.Yang L; Zhou Z; Liu H; Wu C; Zhang H; Huang G; Ai H; Gao J Europium-Engineered Iron Oxide Nanocubes with High T1 and T2 Contrast Abilities for MRI in Living Subjects. Nanoscale 2015, 7, 6843–6850. [DOI] [PubMed] [Google Scholar]
- 34.Huang G; Li H; Chen J; Zhao Z; Yang L; Chi X; Chen Z; Wang X; Gao J Tunable T1 and T2 Contrast Abilities of Manganese-Engineered Iron Oxide Nanoparticles through Size Control. Nanoscale 2014, 6, 10404–10412. [DOI] [PubMed] [Google Scholar]
- 35.Li Z; Yi PW; Sun Q; Lei H; Li Zhao H; Zhu ZH; Smith SC; Lan MB; Lu GQ Ultrasmall Water-Soluble and Biocompatible Magnetic Iron Oxide Nanoparticles as Positive and Negative Dual Contrast Agents. Adv. Funct. Mater 2012, 22, 2387–2393. [Google Scholar]
- 36.Seo WS; Lee JH; Sun X; Suzuki Y; Mann D; Liu Z; Terashima M; Yang PC; McConnell MV; Nishimura DG; Dai H FeCo/Graphitic-Shell Nanocrystals as Advanced Magnetic-Resonance-Imaging and Near-Infrared Agents. Nat. Mater 2006, 5, 971–976. [DOI] [PubMed] [Google Scholar]
- 37.Niu D; Luo X; Li Y; Liu X; Wang X; Shi J Manganese-Loaded Dual-Mesoporous Silica Spheres for Efficient T1- and T2-Weighted Dual Mode Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2013, 5, 9942–9948. [DOI] [PubMed] [Google Scholar]
- 38.Wang L; Lin H; Ma L; Jin J; Shen T; Wei R; Wang X; Ai H; Chen Z; Gao J Albumin-Based Nanoparticles Loaded with Hydrophobic Gadolinium Chelates as T1-T2 Dual-Mode Contrast Agents for Accurate Liver Tumor Imaging. Nanoscale 2017, 9, 4516–4523. [DOI] [PubMed] [Google Scholar]


