Abstract
Objective
To identify the factors influencing the completion rate of the course of rabies vaccinations without considering the economic factors or the drug supplies.
Methods
Rabies vaccination data from the Fourth Medical Center of the PLA General Hospital from January 1 to December 31, 2020, were collected. This includes demographics, information on injury-causing animals, vaccination schemes, and injury assessments. Data on completed the course of rabies vaccinations were compared with data on uncompleted. Internal analysis of Zagreb regimen and Essen regimen was performed. The key factor affecting the completion of the course of rabies vaccinations was analyzed.
Results
A total of 1633 patients completed the course of rabies vaccinations, while 462 patients did not. There were differences between the two groups in terms of the vaccination scheme, age, and previous history of rabies vaccinations. The results of a multivariate analysis of variance showed that only the vaccination scheme was significantly correlated with the completion rate of the course of rabies vaccinations. The internal analysis of the vaccination schemes showed that the duration of the vaccination scheme directly affected the completion rate of the course of rabies vaccinations, and the vaccination duration was negatively correlated with the completion rate of rabies vaccinations.
Conclusion
The completion rate of the course of rabies vaccinations with Zagreb regimen was higher than Essen regimen. The completion rate was closely correlated with the vaccination scheme, but the duration of the vaccination scheme was the key factor. There was a linear relationship between the vaccination duration and the completion rate of the course of vaccinations, which can be represented by the following linear equation: the completion rate of the course of rabies vaccinations (%) = 98.342–0.999 × vaccination duration (d). Duration is the critical factor affecting the completion rate. To improve the completion rate of vaccination, it is recommended to use a shorter vaccination duration regimen.
Keywords: rabies, the completion rate, vaccination duration
Introduction
Rabies is an animal-borne infectious disease caused by the lyssavirus, with a mortality rate close to 100%. The most effective strategy for preventing rabies is timely, standardized post-exposure prophylaxis (PEP) after exposure to suspicious animals.1 In animal health live attenuated viral vaccines are preferred as they can be administered with a single vaccination. For humans, the most commonly used PEP vaccination schemes in clinical practice in China are the 2-1-1 “4 dose-3 visit” intramuscular injection (IM) procedure (ie, Zagreb regimen) and the 1-1-1-1-1 “5 dose-5 visit” IM procedure (ie, Essen regimen).2 It is known in both the field of animal as well as human health that vaccines with fewer administrations are more acceptable. Is Zagreb regimen more acceptable than Essen regimen? Is Zagreb regimen superior to Essen regimen because of dose and number of visits?
As a cornerstone, the World Health Organization (WHO) recommends that all humans who are exposed to known or suspected rabies animals should receive PEP.3 A full standardized course of vaccinations can play a preventive and immune-boosting role in people who have been exposed to rabies. It has been reported in the literature that the completion rate of standardized rabies vaccinations after an injury caused by an animal in China is 64.1% to 81.8%.4,5 For exposed people who do not complete the course, the intensity and duration of their immunity are uncertain, and they have a risk of infection. To further improve the vaccination completion rate, it is necessary to identify the critical factors influencing people’s decisions to complete their course of rabies vaccinations. Therefore, this study compares and analyzes the available information of people who were vaccinated against rabies at our center in 2020 to identify correlations.
Subjects and Methods
Subjects
Rabies vaccination data from the Fourth Medical Center of the PLA General Hospital from January 1 to December 31, 2020, were collected. The subjects were divided into two groups based on whether they completed a course of rabies vaccinations or not. The factors influencing the completion of the course of rabies vaccinations were analyzed in terms of the vaccination scheme, population informatics, and injury-related factors.
The inclusion criteria were (1) presented for a rabies vaccination at the Fourth Medical Center of the PLA General Hospital in 2020, (2) vaccinated with the Vero- cell-adapted virus, and (3) aged ≥18 y at the time of vaccination. The exclusion criteria were (1) severe immune deficiency, (2) received the course of rabies vaccinations within the preceding 3 years, (3) pre-exposure prophylaxis (PrEP), and (4) no full capacity for civil conduct (mainly refers to people with mental illness).
Study Methods
Adopting a single-center retrospective continuous case study design, the following information was collected from people who met the inclusion criteria: demographics, injury sites, wound exposure grades, rabies immunoglobulin use, rabies vaccine administration, the animal that caused the injury, the ownership status of the injury-causing animal, and missing injections during the vaccination process. The factors influencing the completion of the course of vaccinations were analyzed. To calculate and compare the completion rate of the course of vaccinations, Zagreb regimen and Essen regimen were evaluated internally. The critical factors affecting the completion of the course of rabies vaccinations were analyzed.
Vaccination Regimen
Drugs Used
The human rabies vaccine (purified Vero cell cultured rabies vaccine) was used (Liaoning Chengda Co., Ltd., national drug approval no. S20043089, drug registration standard WS4-(S-030)-2010Z). In China, this rabies vaccine is legally applicable to Zagreb regimen and Essen regimen.
Injection Method
The vaccination process of the Zagreb regimen is as follows: On day 0 (the day of exposure), two doses are injected into the deltoid muscles of the left and right upper arms. On day 7 and day 21, one dose is injected into the deltoid muscles successively. The course of rabies vaccinations requires three visits and four doses, and the theoretical duration of this regimen is 21 days.
The vaccination process of the Essen regimen is as follows: On day 0 (the day of exposure), one dose is injected into the deltoid muscle of the upper arm. On days 3, 7, 14, and 28, one dose is injected into the deltoid muscle successively. The course of rabies vaccinations requires five visits and five injections, and the theoretical duration of the regimen is 28 days.
Executive Standard
The treatment of the rabies injury site, vaccination, and the use of passive immune agents complied with the Technical Guidelines for Rabies Exposure Prevention and Treatment in Beijing (Trial protocol).6
Statistical Analysis
The data were statistically analyzed using SPSS 20.0 statistical software. Normally distributed data were compared using a t-test, non-normally distributed data were compared using a Mann–Whitney U-test, and categorical count data were compared using a Chi-squared test. As Zagreb regimen has different completion rates of the course rabies vaccinations with Essen regimen, the internal evaluation of the two regimens is carried out. The partial correlation analysis of the completion rate with the number of visit and vaccination duration was carried out. A linear regression analysis was conducted for those with linear relationships. A value of P < 0.05 was considered statistically significant.
Results
Comparison of the Effects of Demographics, Vaccination Information, and the Cause of the Injury on the Completion of the Course of Rabies Vaccinations
From January 1 to December 31, 2020, a total of 3495 patients visited the rabies clinic at the Fourth Medical Center of the PLA General Hospital. A total of 637 patients who re-exposured and received booster vaccination after completing rabies vaccination in the past three years were excluded. Seven hundred and fifty-seven minors and 6 duplicates were excluded, too. A total of 2095 adults were vaccinated using Zagreb regimen or Essen regimen. Of these, 1633 patients completed the course of rabies vaccinations, and 462 patients did not complete the course.
We divided the data into two groups according to whether all vaccination procedures have been completed. There were differences between the two groups in terms of the vaccination scheme (P < 0.001), age (P < 0.001), and history of prior rabies vaccination (P = 0.035). A multivariate analysis of variance between these three factors and the completion rate revealed that only the vaccination scheme was significantly correlated with the completion rate (P = 0.015), while age (P = 0.156) and history of prior rabies vaccination (P = 0.103) were not (see Table 1).
Table 1.
Comparison of Influencing Factors of the Whole Course of Rabies Vaccination
| Completed | Uncompleted | P | |
|---|---|---|---|
| Scheme (person-time, %) | <0.001 | ||
| Zagreb | 1326 (80.41) | 323 (19.59) | |
| Essen | 307 (68.83) | 139 (31.17) | |
| Gender (person-time, %) | 0.973 | ||
| Male | 772 (47.27) | 218 (47.19) | |
| Female | 861 (52.73) | 244 (52.81) | |
| Average age (years old) | 39.91 (19–89) | 36.84 (19–89) | <0.001 |
| Personnel category (person-time, %) | 0.337 | ||
| Student | 76 (4.65) | 28 (6.06) | |
| In service personnel | 1308 (80.10) | 377 (81.60) | |
| Retired | 214 (13.10) | 39 (8.44) | |
| Others | 35 (2.14) | 18 (3.90) | |
| Injured site (person-time, %) | 0.236 | ||
| Head, neck and face | 42 (2.57) | 11 (2.38) | |
| Hand | 900 (55.11) | 273 (59.09) | |
| Upper limb | 230 (14.08) | 59 (12.77) | |
| Trunk | 48 (2.94) | 10 (2.16) | |
| Lower limb | 399 (24.43) | 107 (23.16) | |
| Others | 14 (0.86) | 2 (0.43) | |
| Category exposure | 0.176 | ||
| Category II exposure | 593 (36.31) | 152 (32.90) | |
| Category III exposure | 1040 (63.69) | 310 (67.10) | |
| Type of injury-causing animals (times, %) | 0.070 | ||
| Dog | 696 (42.62) | 183 (39.61) | |
| Cat | 812 (49.72) | 230 (49.78) | |
| Others | 125 (7.65) | 49 (10.61) | |
| Ascription of injury-causing animals (times, %) | 0.206 | ||
| Animal with an owner | 1330 (81.45) | 369 (79.87) | |
| Ownerless animal | 262 (16.04) | 74 (16.02) | |
| Others | 41 (2.51) | 19 (4.11) | |
| Whether immunoglobulin is used (person-time, %) | 0.549 | ||
| Yes | 685 (41.95) | 201 (43.51) | |
| No | 948 (58.05) | 261 (56.49) | |
| History of prior rabies vaccination (person-time, %) | 0.035 | ||
| Yes | 440 (26.94) | 102 (22.08) | |
| No | 1193 (73.06) | 360 (77.92) |
The Impact of the Regimen on the Completion Rate
A total of 1649 people were vaccinated using Zagreb regimen, and 80.41% of them completed the course of vaccinations. A total of 446 people were vaccinated using the Essen regimen, and 68.83% of them completed the course of vaccinations. The differences between the two regimens were the reasons for the difference in the completion rate.
The impacts of the injection sequence and the vaccination duration of Zagreb regimen and Essen regimen on the completion rate of the course of rabies vaccinations were analyzed, and the results are shown in Table 2. It can be seen that the differences between the schemes had an impact on the completion rate, and the non-completion rate in the Essen regimen (31.17%) was 1.59 times that of the Zagreb regimen (19.59%).
Table 2.
Statistics of Completion of the Whole Course of Rabies Vaccination Caused by Different Regimens
| Regimen | Zagreb | Essen | ||||
|---|---|---|---|---|---|---|
| Project | Injection Sequence | Vaccination Time (d) | Completion (Person-Time, %) | Injection Sequence | Vaccination Time (d) | Completion (Person-Time, %) |
| Data | 1 | 0 | 1630 (98.85) | 1 | 0 | 441 (98.88) |
| 2 | 0 | 1623 (98.42) | 2 | 3 | 422 (94.62) | |
| 3 | 7 | 1511 (91.63) | 3 | 7 | 401 (89.91) | |
| 4 | 21 | 1326 (80.41) | 4 | 14 | 373 (83.63) | |
| 5 | 28 | 307 (68.83) | ||||
| Termination | 4 | 21 | 1326 (80.41) | 5 | 28 | 307 (68.83) |
The Impact of the Vaccination Duration on the Completion Rate
There are three differences between the two vaccination regimens: (1) a different number of injections on day 0, (2) differences in the injection sequence, and (3) a different total duration of the vaccination regimen.
When an exposed person presents at a rabies vaccination clinic, they are indicating their willingness to be vaccinated. Therefore, on day 0, the vaccination scheme is accepted after informed consent is obtained, and theoretically, the completion rate of vaccination on day 0 is 100%. The actual clinical statistics in this study showed that the completion rate of Zagreb and Essen regimens on day 0 was 98.42% (1623 out of 1649) and 98.88% (441 out of 446), respectively, which is close to 100% for both regimens. The difference in the dose and injection site between the two schemes had no impact on the completion rate of the course of rabies vaccinations.
Table 2 shows that in both vaccination regimens, the completion rate decreased over time as the vaccination sequence progressed. However, it is not clear whether the difference in the completion rate was related to the injection sequence or vaccination duration.
A bivariate correlation analysis of the injection sequence, the vaccination duration, and the completion rate showed that there was a significant correlation between injection sequence and vaccination duration (P < 0.01), between the vaccination duration and the completion rate (P < 0.01), between the injection sequence and the completion rate (P < 0.01).
A partial correlation analysis of the injection sequence, the vaccination duration, and the completion rate showed that there was no significant correlation between the injection sequence and the completion rate (partial correlation P = 0.306), but there was a significant correlation between the vaccination duration and the completion rate (partial correlation P < 0.001). The vaccination duration is clearly related to the completion rate. Increasing the injection sequence can decrease the completion rate by prolonging the vaccination duration.
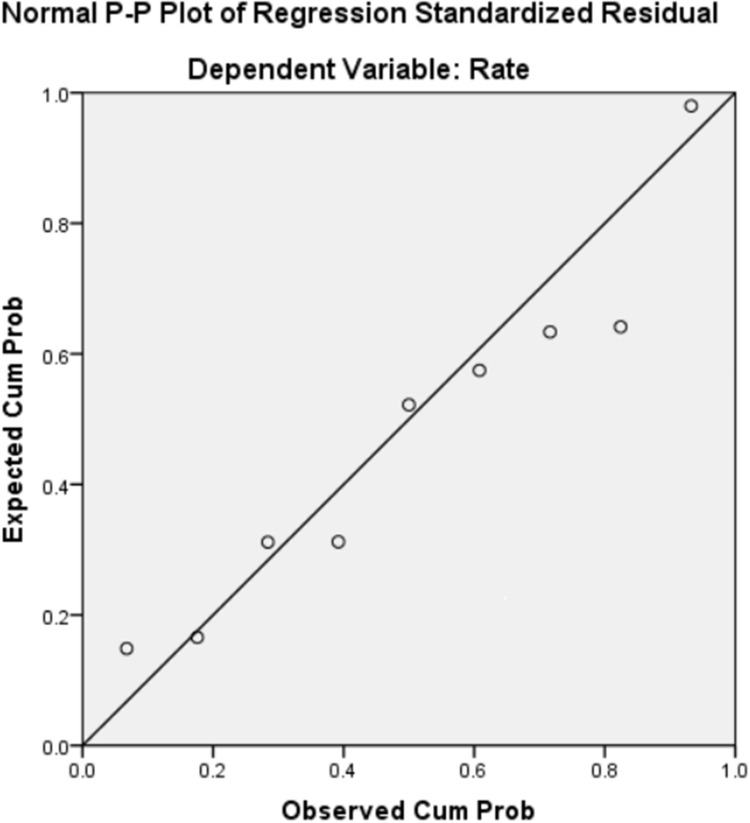
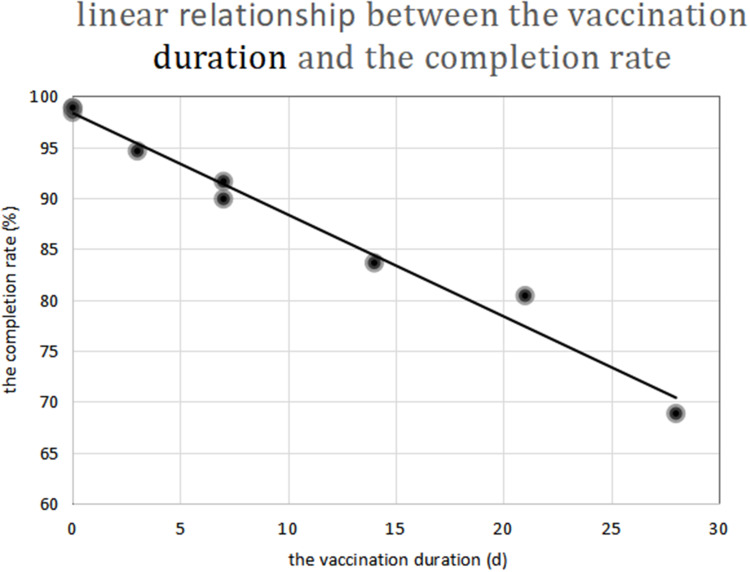
A linear correlation analysis of the vaccination duration (d) and the completion rate (%) showed that the value of Adjusted R-squared by the regression model was 0.979, the Durbin-Watson value was 0.996, the data had a normal distribution (see Figure 1), the degree of fit of the linear regression was very high, and the residuals exhibited positive autocorrelation. The constant function of the linear regression analysis was 98.342 (P = 0.001), and the coefficient was −0.999 (P = 0.003). The following conclusive formula was obtained (see Figure 2):
Figure 1.
Standard P-P chart of standardized residuals of linear regression between the vaccination duration and the completion rate of the course of rabies vaccination.
Figure 2.
Negative proportional function linear chart of the vaccination duration and the completion rate.
The completion rate for the course of rabies vaccinations (%) = 98.342–0.999 × vaccination duration (d)
There was a negative proportional function relationship between the vaccination duration and the completion rate.
Discussion
Rabies is a zoonotic infectious disease with a fatality rate of 100%. About 60,000 people worldwide die of rabies every year, resulting in a loss of more than 3.7 million disability-adjusted life years.7 PEP is the most important treatment to prevent rabies. The standardized use of vaccines in the process can cause the positive conversion rate of neutralizing antibodies in rabies serum to reach 100%.8 Zagreb and Essen regimens are widely recommended and used in China for post-exposure prophylactic immunization, and they are safe with good preventative immune effects.9 However, after exposure, the completion rate of the course of rabies vaccinations is not high. An investigation into rabies vaccination in 31 provinces (autonomous regions and municipalities) in China from 2016 to 2018 showed that the average proportion of people who completed the course of vaccinations was 81.11%,10 but this proportion varied between provinces and cities (71.83–99.53%).11
Beijing has a sound economic foundation. Therefore, the completion rates of courses of rabies vaccinations in Beijing are less affected by economic factors and insufficient drug supplies. Comparing and analyzing the rabies vaccination data from our center showed that the completion rate was correlated with the vaccination scheme adopted. The completion rate was 80.41% when using the Zagreb regimen and 68.83% when using the Essen regimen. Therefore, the completion rate of the Zagreb regimen was significantly higher than the Essen regimen, which is consistent with the completion rates for the two schemes reported in China. For example, by examining the data of 600 rabies vaccination cases in Hubei, Yan Xiong demonstrated that the completion rate was 94.31% for the Zagreb regimen and 84.62% for the Essen regimen.5 The study also confirmed that the compliance and health economic benefits of Zagreb regimen were better than those of Essen regimen.5 In the present study, analyzing the demographic information and clinical data (see Table 1) and comparing them between people in the completed group and the uncompleted group demonstrated that there were significant differences between the two groups in terms of vaccination scheme. The completion rate of Zagreb regimen was higher than Essen regimen. To ensure compliance and full rabies vaccination completion rate, consider using the Zagreb regimen instead of the Essen regimen for PEP.
An internal analysis of the two regimens was conducted, and the results revealed that the difference in the number of injections on day 0 between the two regimens was not correlated with the completion rate. The difference in the number of injections (4 vs 5) was not correlated with the completion rate.8 However, there was a linear relationship between the vaccination duration and the completion rate. The longer the vaccination lasts, the lower the proportion of people completing vaccination. For example, the booster vaccination of COVID-19, at last 2 months apart, reduce compliance.12 After conducting a regression analysis, the following correlation was obtained: the completion rate of the course of rabies vaccinations (%) = 98.342–0.999 × vaccination duration (d). This suggests that as the vaccination duration increases, the completion rate decreases gradually, ie, there is a negative proportional relationship between the vaccination duration and the completion rate. Based on this data, at the start of the course of rabies vaccinations, 1.66% of people are expected to choose not to complete the course of vaccinations. As the duration of the course of rabies vaccinations increases, the completion rate decreases by approximately 1% each day, which is consistent with China’s big data. The completion rate of the course of rabies vaccinations in China is about 77–78%,13 which is ≤77.36% for the 21-day vaccination scheme and >70.37% for the 28-day vaccination scheme. Lili Yang reported that the final completion rate for the Essen regimen was 64.16%.4 Therefore, the longer duration of the course of rabies vaccinations, the greater the impact on the completion rate.
Different vaccination durations will lead to different completion rates, and different schemes have different durations, leading to different completion rates by different duration. The data analysis clearly revealed that the vaccination duration is the key factor affecting the completion rate of the course of rabies vaccinations rather than the doses of injection (4 or 5) or the number of visits (3 or 5).
The WHO issued its “Rabies vaccines: WHO position paper – 2018” recommends three vaccination regimens: (1) 2-sites intradermal injection (ID) on days 0, 3 and 7 day (ie, 2-2-2-0-0 “6 dose-3 visit” ID procedure) or (2) 1-site IM on days 0, 3, 7 day and between day 14–28 day (ie, 1-1-1-1(0)-1(0) “4 dose-4 visit” IM procedure) or (3) 2-sites IM on days 0 and 1-site IM on days 7, 21 day (ie, Zagreb regimen).14 The duration of the “2-2-2-0-0” ID injection procedure is 7 days, and its seroconversion rate, safety, and health economic benefits have been verified.15,16 I am sure that completion rate in both intramuscular (IM) regimens studied, are compatible to one week regimen. The ID approach has the advantages of shorter program time and less vaccine usage.17 But, at present, the only approved vaccination method for rabies in China is IM, no matter PrEP or PEP.18 The duration of the 1-1-1-1(0)-1(0) IM procedure is 14–28 days; it is safe and effective,19 and it has been studied in China to a certain extent.20 The Zagreb regimen lasts for 21 days, is widely employed in China, and is also the most widely used vaccination regimen in clinical practice. These regimens have reduced the vaccination duration and have great significance in improving the completion rate of the course of rabies vaccinations. Vaccination regimen with shorter duration have higher compliance and are more likely to be accepted by people, which reduces time costs and increases economic benefits. In order to improve the complete rate of rabies vaccination, it is recommended to use the vaccination regimen like 2-2-2-0-0 ID procedure which is the shortest time regimen, at present.
This report was a single-center retrospective continuous case study; therefore, it lacks the statistical results that could be generated by a multi-center large-sample study. The study subjects were mainly Beijing residents, who have a good economic foundation and awareness of the need for rabies vaccination after exposure, and it thus lacked statistical data in rural and remote areas. The vaccination schemes included in the study were Zagreb regimen and Essen regimen, and there was a lack of data on the simple four-dose regimen, the two-point intradermal injection, and other vaccination schemes. The relationship between the vaccination duration and the completion rate of the course of rabies vaccinations obtained in this study could provide a preferred direction for the vaccination of high-risk groups after exposure and present suggestions for standardizing and improving the course of rabies vaccinations for adults in clinical practice.
Conclusion
The vaccination scheme is significantly correlated with the completion rate of the course of rabies vaccinations. Thus, the completion rate of Zagreb regimen was higher than Essen regimen. Through an internal analysis of the schemes, a linear relationship was found between the completion rate and the vaccination duration, which can be represented by the following linear equation: the completion rate of the course of rabies vaccinations (%) = 98.34–0.999 × vaccination duration (d). An increase vaccination duration will result in a decrease in the completion rate of the course of rabies vaccinations. The vaccination duration is the critical factor affecting the completion rate. In order to improve the completion rate of vaccination, it is recommended to use a shorter vaccination duration regimen.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yin W, Wang C, Chen Q, et al. Expert consensus on prevention and treatment of rabies exposure. Chin J Prev Vet Med. 2019;53(7):668–679. doi: 10.3760/cma.j.issn.0253-9624.2019.07.00 [DOI] [PubMed] [Google Scholar]
- 2.Zhou H, Li Y, Chen RF, et al. Technical Guide for prevention and control of rabies (2016 edition). Chin J Epidemiol. 2016;37(2):139–163. doi: 10.3760/cma.j.issn.0254-6450.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO Expert Consultation on Rabies. 3rd ed. Geneva: WHO Press; 2018. [Google Scholar]
- 4.Yang L. Analysis of prevention and treatment of rabies exposed population. Med Inform. 2020;033(009):134–135. [Google Scholar]
- 5.Xiong Y. Comparison of compliance and economic cost of two different immunization procedures of rabies vaccine. Chin J Contemp Med. 2020;26(16):44–46. [Google Scholar]
- 6.Li X, Zhou T, Lu L. Guidelines for prevention and treatment of rabies exposure in Beijing (Trial). Capital Public Health. 2018;12(03):5–11. [Google Scholar]
- 7.Hampson K, Coudeville L, Lembo T, et al. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis. 2015;9(4):e0003709. doi: 10.1371/journal.pntd.0003709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi N, Zhang Y, Zheng H, et al. Immunogenicity, safety and antibody persistence of a purified vero cell cultured rabies vaccine (Speeda) administered by the Zagreb regimen or Essen regimen in post-exposure subjects. Hum Vaccin Immunother. 2017;13(6):1338–1345. doi: 10.1080/21645515.2017.1279770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Huang S, Cai L, et al. Safety, immunogenicity of lyophilized purified vero cell cultured rabies vaccine administered in Zagreb and Essen regimen in post-exposure subjects: a post-marketing, parallel control clinical trial. Hum Vaccin Immunother. 2021;25:1–7. doi: 10.1080/21645515.2021.1880200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu JJ, Du L, Tao XY, et al. Epidemiological characteristics of rabies in China from 2016 to 2018. Chin J Epidemiol. 2018;42(1):131–136. doi: 10.3760/cMA.j.cn11238-20200116-00037 [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Zhu L, Zhu W, et al. Analysis of the epidemiological characteristics of rabies in China in 2016. Chin J Epidemiol. 2018;39(1):40–43. doi: 10.3760/cma.j.issn.0254-6450.2018.01.008 [DOI] [Google Scholar]
- 12.Yi HY, Zhao JM, Liang XF, Ying TY. 新加坡新型冠状病毒疫苗免疫接种策略研究[J].中华流行病学杂志 [Research on COVID-19 vaccination strategies in Singapore]. Zhonghua Liu Xing Bing Xue Za Zhi. 2022;43(3):310–314. Chinese. doi: 10.3760/cma.j.cn112338-20211125-00919 [DOI] [PubMed] [Google Scholar]
- 13.Li RC, Li YP, Wen SQ, et al. Immunogenicity and safety of purified chick-embryo cell rabies vaccine under Zagreb 2-1-1 or 5-dose Essen regimen in Chinese children 6 to 17 years old and adults over 50 years: a randomized open-label study. Hum Vaccin. 2015;11(2):435–442. doi: 10.4161/21645515.2014.994460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. Rabies vaccines: WHO position paper, April 2018 – recommendations. Vaccine. 2018;36(37):5500–5503. doi: 10.1016/j.vaccine.2018.06.061 [DOI] [PubMed] [Google Scholar]
- 15.Quiambao BP, Ambas C, Diego S, et al. Intradermal post-exposure rabies vaccination with purified Vero cell rabies vaccine: comparison of a one-week, 4-site regimen versus updated Thai Red Cross regimen in a randomized non-inferiority trial in the Philippines. Vaccine. 2019;37(16):2268–2277. doi: 10.1016/j.vaccine.2019.02.083 [DOI] [PubMed] [Google Scholar]
- 16.Tarantola A, Tejiokem MC, Briggs DJ. Evaluating new rabies post-exposure prophylaxis (PEP) regimens or vaccines. Vaccine. 2019;37(3):A88–A93. doi: 10.1016/j.vaccine.2018.10.103 [DOI] [PubMed] [Google Scholar]
- 17.Ravish HS, Sudarshan MK, Madhusudana SN, et al. Assessing safety and immunogenicity of post‑exposure prophylaxis following interchangeability of rabies vaccines in humans. Hum Vaccin Immunother. 2014;10(5):1354–1358. doi: 10.4161/hv.28064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wenwu Y, Chuanlin W, Qiulan C, et al. Expert consensus on rabies exposure prophylaxis. Chin J Prev Med. 2019;53(7):668–679. doi: 10.3760/cma.j.issn.0253-9624.2019.07.004 [DOI] [PubMed] [Google Scholar]
- 19.Rupprecht CE, Briggs D, Brown CM, et al. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine. 2009;27(51):7141–7148. doi: 10.1016/j.vaccine.2009.09.029 [DOI] [PubMed] [Google Scholar]
- 20.Lu X, Chen Q, Zhu W, et al. Domestic rabies vaccine using four stitch ”simple” immune effect research. Proceedings 2020 China Make a Diagnosis and Give Treatment Way to Peak BBS; 2020: 25. doi: 10.26914/Arthurc.kihy.2020.016422. [DOI] [Google Scholar]