Abstract
Enrollment rates for cancer clinical trials remain low, affecting the generalizability of new treatments. Research shows that many patients face significant challenges in understanding basic clinical trial vocabulary and making informed decisions about participation. Informational aids (IA) are developed to address these challenges and support decision making of cancer clinical trial participation. The present study proposed and tested a structural path model to explain the efficacy of three (i.e., interactive, non-interactive, non-cancer control) IAs. The results revealed that clinical trial participation intention was associated with attitudes and social constructs (i.e., social norm, social sharing, and cues to action). Ease of use, rather than knowledge, was the primary communication feature of IA that influenced the outcome variables. The path relations linking messages features, mediators, and outcome variables were different across all three IAs. The results therefore provide theoretical and practical implications for the use and development of IAs to support clinical trial accrual.
Keywords: informational aid, cancer clinical trials, knowledge, social sharing, interactivity
Cancer clinical trials are essential for assessing the efficacy of new treatments, but the enrollment is still low (Byrne, Tannenbaum, Glück, Hurley, & Antoni, 2014; Michaels et al., 2012). Insufficient participant accrual reduces the generalizability of study findings, affects the delivery of effective treatments (Murthy, Krumholz, & Gross, 2004; Oh et al., 2015), and further exacerbates existing health disparities (Ford et al., 2008; Occa, Morgan, & Potter, 2018).
Several key barriers could be associated with low accrual rates. Notably, patients experience difficulties understanding basic concepts related to clinical trials, such as randomization, placebo, and side effects (Krieger et al., 2015; Occa et al., 2018; Tam et al., 2015). Understanding of these topics is also impeded by the lack of effective communication strategies between providers and patients (Morgan, Mouton, Occa, & Potter, 2016; Morgan, Occa, Mouton, & Potter, 2017). When cancer treatment decisions need to be made quickly, patients often feel more pressured and may decide to receive standard treatment for less possible risks (Ellis & Butow, 1998; Mills et al., 2006). Despite numerous interventions implemented to address these barriers, systematic reviews have shown limited efficacy (Flory & Emanuel, 2004; Nishimura et al., 2013).
The barriers preventing effective communication in cancer clinical trials often cause uncertainty in the decision-making process (Miller et al., 2013). Shared decision making, in which patients’ personal needs and concerns are integrated and discussed, may be effective in improving informed consent (Flory & Emanuel, 2004). Rather than providing general education, clinical researchers should seek to clarify information concerning individual patients’ values, and facilitate an in-depth discussion with patients (Gillies, Cotton, Brehaut, Politi, & Skea, 2015). To this end, informational aid tools can effectively help patients understand clinical trials, support their decision making, and reduce conflict or anxiety (Gillies et al., 2015; Stacey et al., 2017).
The purpose of the present study was to examine the efficacy of an informational aid (IA) that was developed to help patients and their families understand basic concepts about clinical trial participation. While previous studies have supported the effectiveness of decision or informational aid tools (e.g., Elwyn et al., 2009; Stacey et al., 2017), some have noted potentially unintended effects, such as cognitive overload, confusion, and distress (Caldon et al., 2011; Lipstein, Brinkman, Sage, Lannon, & Morgan Dewitt, 2013; Melton, 2010). In light of these questions in the literature, the present study proposed and tested a model intended to explain how IAs might improve decision-making about cancer clinical trial participation.
Informational Decision Aids in Clinical Trials
In the context of clinical trial participation, decision making is related to perceived benefits and risks (Politi et al., 2016). Decision aid tools are developed to provide evidence-based information about treatment or research study options and outcomes based on personal preferences and values (O’Connor et al., 1999; Politi et al., 2016). A complete decision aid should be assessed on several key dimensions, including balanced presentations of options, the probabilities of associated benefits and harms, scientific uncertainties, and recognition of patient values (Elwyn et al., 2009; Stacey et al., 2017). In the context of cancer care, various formats of decision aids have been used to facilitate decision making, including pamphlets, audio or videotapes, prompts, audio‐guided workbooks, computer or web‐based programs, interactive videodiscs, decision boards, and group presentations (see review by Stacey et al., 2017). These decision aids vary only in format and share the same goal of informing patients and improving decision quality (Elwyn et al., 2009; O’Connor et al., 2007).
However, the issues preventing research accrual often stem from the lack of knowledge and general information about clinical trials, rather than the uncertainty about specific options (Gillies et al., 2015; Robertson et al., 2018; Stacey et al., 2017). Therefore, an informational aid tool, which considers patients’ needs (Gillies et al., 2015), uses lay language (Shneerson, Windle, & Cox, 2013), and rich visual cues (Kraft et al., 2017) to help patients understand clinical trials, should be more effective in supporting decision making and reaching mutual agreement between clinicians and patients in the context of clinical trial communication (J. S. Carpenter, Studts, & Byrne, 2011; Gillies et al., 2015). Informational aids (IAs) are one type of decision-support intervention that shares certain goals of traditional decision aids, including providing information that fits the patient’s processing preference and values, patient control over the decision-making process (Charles, Gafni, & Whelan, 1999) but that do not meet other criteria for true decision aids, such as the presentation of optimal treatment based on probabilities of outcomes on different patients (Elwyn et al., 2009).
Efficacy of Informational Aids
Drawing on the Theory of Planned Behavior (TPB; Ajzen, 1991), we propose several key processes that contribute to the efficacy of IAs in clinical trial communication. In this study, the intention to join a clinical trial study is jointly influenced by three key antecedent variables: attitudes, perceived behavioral control, and social norms. Attitude refers to the overall evaluation of participation in clinical trials and is further influenced by beliefs and judgment of the consequences. Perceived behavioral control refers to a person’s belief that he or she has the ability and control of clinical trial participation. Social norm indicates the degree to which one is influenced by others to join in clinical trials.
First, knowledge gained from the use of IAs may help patients develop more positive attitudes toward clinical trials. Research shows that attitudes are often influenced by low knowledge of clinical trials (Ellis & Butow, 1998; Miller et al., 2013). About 40% of patients do not fully understand clinical trials (Comis, Miller, Aldigé, Krebs, & Stoval, 2003). Negative misconceptions are common among patients (Krieger et al., 2015). IAs provide information that can effectively help patients learn more about clinical trials, as demonstrated in two Cochrane reviews (O’Connor et al., 1999; Stacey et al., 2017).
In addition to the provision of information, functional IAs should engage patients in active learning for decision making (Politi et al., 2016). Interactive IAs are advantageous in this perspective. In general, interactive communication environments can foster positive attitudes toward information than non-interactive ones (Sundar, Kalyanaraman, & Brown, 2003). Also, interactive tools personalize information about particular studies of interest and present tailored estimates of risks. As a result, patients are involved in information relevant to their concerns and more likely to develop positive attitudes toward study participation (Frew et al., 2010).
Knowledge learned from IAs may further help patients perceive more control of clinical trials. Understanding risks and benefits helps patients become more confident in decision making as they perceive stronger abilities to manage expectations from participation (J. S. Carpenter et al., 2011; O’Connor et al., 2007). This effect could be strengthened by the sense of control and participation resulting from the use of interactive IAs (Shneerson et al., 2013; Windle, McCormick, Dandrea, & Wharrad, 2011). Given this, interactive IAs that provide easier access to clinical trial knowledge can also facilitate patients’ control of decision making. Based on the rationales, we propose the following hypotheses.
H1: An interactive IA will lead to more knowledge about clinical trials than a non-interactive IA;
H2a: Higher levels of knowledge about clinical trials will be positively associated with attitudes toward clinical trial participation.
H2b: Knowledge will be positively associated with perceived behavioral control.
In addition, resources, skills, and opportunities are necessary to increase the confidence to perform specific behaviors (Bailey et al., 2016; Conner & Sparks, 2005). The increased sense of efficacy associated with necessary factors can foster stronger intentions to participate in research with fewer decisional conflicts (Miller et al., 2013). Also, efficacy is associated with the ability to communicate treatment preferences with providers (Meropol et al., 2003; Wright, Crooks, Ellis, Mings, & Whelan, 2002), which is another crucial potential outcome from using effective IAs. Patients are likely to feel supported in the decision making and develop more favorable attitudes after communicating with providers about their questions and concerns (Meropol et al., 2003). Thus, consistent with TPB (Ajzen, 1991), we expect IAs help patients to gain behavioral control over their participation in clinical trials, which should lead to stronger attitudes and participation intentions.
H3: Attitudes will be positively related to the intentions to join clinical trials.
H4: Perceived behavioral control will be positively related to (a) attitudes and (b) intentions to participate in a clinical trial.
Further, interactive IAs incorporating unique message features can make them particularly useful as a tool for patient education and empowerment. According to Shneerson and colleagues (2013), about 92% of prospective trial participants rated ease of use as an essential attribute of an informational tool. Ease of use incorporates high ratings on key design and message features that are intended to support human interactions with IAs, including simplicity of design, use of visual elements, and straightforward information (Shneerson et al., 2013; Windle et al., 2011). These design attributes remove the barriers to learning and promote a sense of ownership and control over patients’ own learning experiences (Windle et al., 2011). Research shows ease of use encourages the use of a tool to acquire knowledge as well as foster positive attitudes toward research participation (Agoritsas, Deom, & Perneger, 2011; Albrecht et al., 2008; Shneerson et al., 2013). Thus, we consider ease of use as an overall score of the messaging and communication features of IAs and propose the following hypotheses.
H5: Interactive IAs will be associated with greater ease of use than non-interactive IAs.
H6: Ease of use will be positively related to (a) knowledge, (b) perceived behavioral control and (c) attitudes toward research participation.
In the context of cancer treatment and care, participation decisions may extend beyond a reasoned appraisal of benefits versus risks. Prevailing social and cultural norms, for example, could influence enrollment in clinical trials (Frew et al., 2010; Simon et al., 2003; Sutherland, da Cunha, Lockwood, & Till, 1998). Acceptance of clinical trial participation by family and friends significantly influences clinical trial enrollment (Ford et al., 2008; Krieger et al., 2015). Supportive social norms also suggest a favorable assessment of risks and benefits in social and cultural rules (Kim et al., 2000; Paterniti et al., 2005). Positive normative beliefs could further help minority patients place more trust in medical researchers (Haynes-Maslow et al., 2014). Thus, after receiving support from their social networks, patients may be more likely to perceive a stronger control and to report more positive attitudes and increased intentions.
In this process, social norms can stimulate information seeking and sharing behaviors to understand more about clinical trials (Yang et al., 2012, 2010). Shared decision making cannot be achieved without interactive functions of IAs that harness the influence of social norms (Gillies et al., 2015; Shneerson et al., 2013). Interactive IAs customize information about clinical trials that integrates social values and cultural norms of patients (Obeidat, Finnell, & Lally, 2011), which could encourage sharing behaviors. Individuals patients are encouraged to consult significant others in their social networks before making a decision. Thus, social sharing should be an important mediating process that is jointly influenced by both the interactive functionality of IAs and social norms. That is, we expect both ease of use of interactive IAs and social norm could influence perceived control, attitudes, and participation intentions through social sharing behaviors.
H6d: Ease of use of IAs will be positively related to social sharing of information about clinical trial participation.
H7: Social norms that support research participation will be positively related to (a) social sharing of information, (b) attitudes toward research participation, and (c) intentions to participate in a clinical trial.
H8: Social sharing of information about clinical trial participation will be positively related to (a) perceived behavioral control, (b) attitudes, and (c) intentions to participate in a clinical trial.
H9: Social sharing of information will mediate the relationships between ease of use and outcome variables, including (a) perceived behavioral control, (b) attitudes, and (c) intentions to participate in a clinical trial.
H10: Social sharing of information will mediate the relationships between social norm and outcome variables, including (a) perceived behavioral control, (b) attitudes, and (c) intentions to participate in a clinical trial.
Lastly, we included cues to action in the model to assess how behavioral change could be triggered. According to the Health Belief Model (Rosenstock, 1974), a cue to action refers to a stimulus in the environment that spurs an individual to adopt a health-related behavior. External cues include stimuli such as mass media campaigns. Internal cues include negative physical symptoms or emotional feelings that prompt attention (C. J. Carpenter, 2010; Janz & Becker, 1984). In the present study, cues to action could be associated with stimuli from social norms that support research participation. Those who initiate social sharing and seek opinions from significant others are more likely to be influenced by their interpersonal interactions with members of their networks. Additionally, patients who perceive that clinical research participation is associated with benefits to their health or well-being will be more likely to develop positive attitudes.
H11: Cues to action will be positively related to (a) social norms, (b) social sharing, (c) attitudes, and (d) intentions to participate in a clinical trial.
Overview of the Proposed Model
Based on the Theory of Planned Behavior (TPB; Ajzen, 1991) and the literature of decision aids, we propose a model that the efficacy of IAs is related to knowledge, social norms, social sharing, and cues to action (see Figure 1). These factors are expected to positively influence outcome constructs, including attitudes, perceived behavioral control, and intention. Interactive IAs should also improve ease of use, which will subsequently increase social sharing behaviors, attitudes, and perceived behavioral control. The current study tested the model by comparing the efficacy of an interactive IA on cancer clinical trial enrollment with two other web-based IAs.
Figure 1.
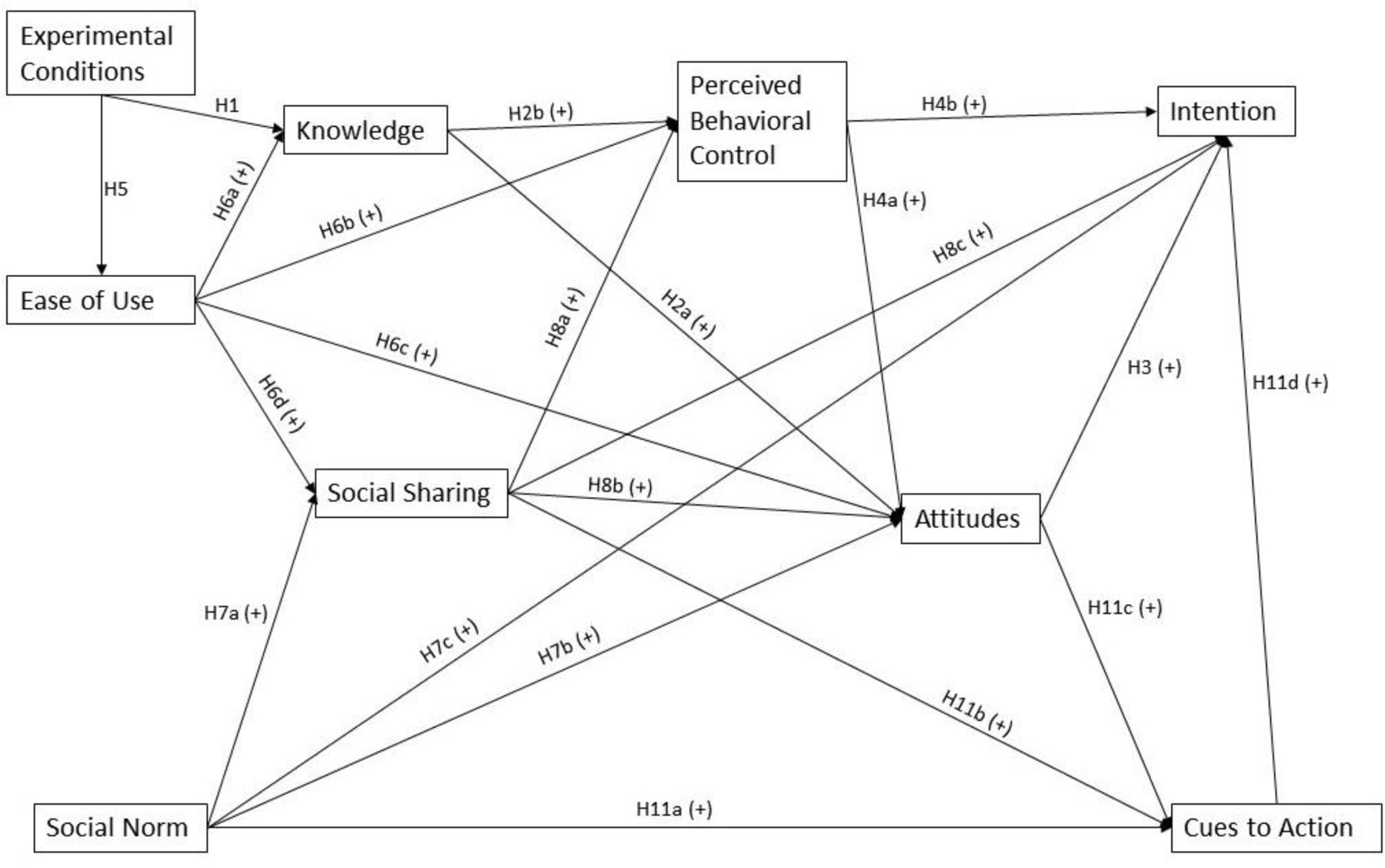
The Path Model with the Proposed Relationships Between Variables
Note: A “+” sign denotes a positive relationship proposed between two variables.
Method
Participants and Procedures
The university Institutional Review Board approved the study before data collection. A total of 460 cancer patients and survivors were recruited from a Qualtrics Panel. All participants were required to be at least 18 years old (Mage = 60, SDage = 64), have had a cancer diagnosis in their lives, and to live in the United States at the time of study participation. Median household income was reported as 40,000 USD/year. Additional demographic information about participants appears in Table 1.
Table 1.
Demographics of Recruited Participants (N = 460)
| Demographic Variables | N (%) |
|---|---|
| Race/ethnicity | |
| Hispanic | 18 (3.9%) |
| Non-Hispanic | 435 (94.6%) |
| Prefer not to say | 7 (1.5%) |
| African American | 34 (7.4%) |
| Asian or Pacific Islander | 4 (.9%) |
| Hispanic/Latinx | 8 (1.7%) |
| White | 397 (86.3%) |
| Multiracial | 3 (.7%) |
| Other/prefer not to say | 9 (2.0%) |
| Sex | |
| Female | 336 (73%) |
| Male | 124 (27%) |
| Education level | |
| Some high school | 11 (2.4%) |
| High school | 79 (17.2% |
| Some college | 168 (36.5%) |
| College | 127 (27.6%) |
| Masters degree | 63 (13.7%) |
| Doctoral degree | 3 (.7%) |
| Professional degree | 4 (.9%) |
| Other | 5 (1.1%) |
| Cancer stage at diagnosis | |
| 0 | 36 (7.8%) |
| I | 127 (27.6%) |
| II | 97 (21.1%) |
| III | 62 (13.5%) |
| IV | 40 (8.7%) |
| Not sure/Not Applicable | 98 (21.3%) |
After participants completed a pretest survey, they were then randomly assigned to one of three online informational aids: an interactive and a non-interactive IA for cancer clinical trials, and an IA for flu vaccination. After using one IA, participants completed a posttest survey after using one of three IAs.
Stimuli
The Authors’ Informational Aid (AIA; real name hidden for anonymous review; see a screenshot in Appendix A) was developed based on the literature on the significant barriers to clinical trial participation as well as data from formative focus group research studies. The AIA provided tailored messages in response to a set of seven questions plus demographics. The seven questions included, for example, whether a patient had health insurance, the level of trust in physicians and cancer-related researchers, and whether a patient has a strong preference for either established treatments or the newest treatments. Multiple iterations of the developed scripts, branching logic, and interactive interface had been reviewed by oncologists and researchers who recruit patients for clinical trials. The branching logic for each participant’s response provides specific messages that acknowledge patients’ values and attitudes and provides available resources and an assessment of whether or not these circumstances are compatible with study participation. Additional information that is relevant to patient concerns is also provided.
The second IA was developed by the National Cancer Institute (NCI) (2015). This IA was provided information to assist cancer patients on how to enroll in a clinical trial. Compared to AIA, NCI was non-interactive and provided non-tailored messages based on individual patient’s circumstances and preferences. The third IA was a Flu Vaccine Interactive Informational Aid (FLU) was developed by healthwise.org (2017) and served as the control condition for this study. FLU was of similar length as the AIA, focused on a health topic of general interest, and prepared participants to make a decision that impacted their health.
Measures
Knowledge.
Knowledge about clinical trial participation was evaluated using a 9-item assessment developed by Cameron et al. (2013). Example items include “In a clinical trial, a patient will always get the experimental drug” and “Doctors personally receive money if I join a clinical trial.” Participants indicated whether they thought each statement was true, false, or that they did not know. Correct responses were coded as “1.”
Attitudes.
A 4-item scale adapted from Jenkins and Fallowfield (2000) was used to assess attitudes toward clinical trial participation. Items included “I think clinical trials offer the best treatment available for cancer” and “I feel that others with my illness will benefit from the results of a clinical trial” (ɑ = .70).
Perceived behavioral control.
Two items adapted from Umphrey (2004) were used to assess perceived behavioral control: “I am confident in my ability to enroll in a clinical trial” and “I feel well-informed about how to enroll in a clinical trial” (ɑ = .85).
Ease of use.
Ease of use was assessed by a scale adapted from Yi and Hwang (2003). Participants rated two items, “The decision aid was easy for me to use,” “I found it easy to understand the decision aid” (ɑ = .90).
Social sharing.
Social sharing was measured by a scale adapted from Hopp and Gallicano (2016). Participants rated how likely they were to discuss IA offline or online on social media (ɑ = .90).
Social norm.
Eight items were used to measure social norm. Four items were adapted from previous research (Jenkins & Fallowfield, 2000; Manne et al., 2010) and four additional ones were developed for this study. Items included “I am worried that my family would not want me to go on a clinical trial,” “Others have wanted me to join a clinical trial,” etc. (ɑ = .7).
Cues to action.
Cues to action were measured by the scale adapted from Jones et al. (2000). Participants rated the extent to which several sources of information that shaped their thinking about clinical trial participation. The sources included a doctor or nurse, friend, website, media, etc. (ɑ = .89).
Intention to join a study.
The intention was assessed using one item from Cameron et al. (2013) “If I had the option, I would definitely consider joining a clinical trial,” as well as an additional item developed for this study: “If a cancer study were offered to me, I would agree to take part in it” (ɑ = .96).
Analysis
A multi-group path analysis of Structural Equation Modeling (SEM) was conducted in Mplus using the Maximum Likelihood, which is robust to non-normality of the data (Kline, 2015). SEM is a multivariate technique designed to test hypothesized relations between latent or observed variables simultaneously as a system (Duncan, 1975). This analytic approach can understand the communication effects of informational aids as an omnibus model, rather than separate effects (Stephenson, Holbert, & Zimmerman, 2006).
Results
Model fit
Descriptive statistics and correlation results of measured variables were summarized in Table 2. After excluding one missing case, data from 459 participants were entered in path analysis. We analyzed two multiple-group models, each of which compared three experimental conditions. Model 1 allowed all structural paths to vary freely among three conditions. This model had possibly different path coefficients for different conditions. Model 2 constrained all parameters to be equal, suggesting no difference across the three conditions.
Table 2.
Descriptive Statistics and Correlations of Observed Variables in Three Experimental Conditions (AIA, NCI, and FLU) (N = 460)
| Condition | 1. Social Sharing |
2. Cues to Action |
3. Knowledge |
4. Attitudes |
5. Perceived Behavioral Control |
6. Intention |
7. Ease of Use |
8. Social Norm |
|
|---|---|---|---|---|---|---|---|---|---|
| AIA | 1 | - | |||||||
| 2 | .36** | - | |||||||
| 3 | −.28** | −.25** | - | ||||||
| 4 | .44** | .46** | −.20* | - | |||||
| 5 | .42** | .28** | −.47** | .55** | - | ||||
| 6 | .53** | .47** | −.29** | .55** | .38** | - | |||
| 7 | .31** | .29** | −.30** | .57** | .76** | .33** | - | ||
| 8 | .18* | .00 | .01 | −.09 | −.11 | −.03 | −.13 | - | |
| M | 3.73 | 2.19 | 1.67 | 5.03 | 5.59 | 4.44 | 5.96 | 3.14 | |
| SD | 1.57 | .39 | .37 | .94 | 1.09 | 1.44 | 1.06 | 1.35 | |
|
| |||||||||
| NCI | 1 | - | |||||||
| 2 | .38** | - | |||||||
| 3 | −.10 | −.06 | - | ||||||
| 4 | .45** | .50** | −.24** | - | |||||
| 5 | .38** | .22** | −.39** | .46** | - | ||||
| 6 | .60** | .59** | −.16* | .67** | .48** | - | |||
| 7 | .33** | .13 | −.26** | .37** | .75** | .39** | - | ||
| 8 | .05 | −.10 | .09 | −.26** | −.18* | −.11 | −.13 | - | |
| M | 3.39 | 2.11 | 1.63 | 4.73 | 5.40 | 4.43 | 5.70 | 3.33 | |
| SD | 1.62 | .39 | .30 | .99 | 1.16 | 1.43 | 1.15 | 1.32 | |
|
| |||||||||
| FLU | 1 | - | |||||||
| 2 | .46** | - | |||||||
| 3 | −.21** | −.19* | - | ||||||
| 4 | .43** | .31** | −.27** | - | |||||
| 5 | .44** | .24** | −.33** | .46** | - | ||||
| 6 | .64** | .40** | −.28** | .54** | .42** | - | |||
| 7 | .27** | .14 | −.19* | .28** | .47** | .18* | - | ||
| 8 | .19* | −.01 | −.08 | −.08* | −.08 | .02 | −.18* | - | |
| M | 3.30 | 2.13 | 1.71 | 4.68 | 5.17 | 4.09 | 6.06 | 3.28 | |
| SD | 1.70 | .44 | .36 | 1.08 | 1.12 | 1.59 | 1.07 | 1.32 | |
Note.
denotes correlation is significant at the .01 level (2-tailed).
denotes correlation is significant at the .05 level (2-tailed). Results exclude a case of missing data.
According to Hu & Bentler (1999), model 1 provided overall good fit, χ2 (24, N = 459) = 34.05, p = .08, CFI = .99, TLI = .97, RMSEA = .05 (CI 90% = .0 - .09), SRMR = .04. Model 2 provided a suboptimal fit to the data, χ2 (68, N = 459) = 106.78, p < .01, CFI = .97, TLI = .96, RMSEA = .06 (CI 90% = .04 - .08), SRMR = .09. The chi-square difference test indicated that model 1 fit significantly better than model 2, ∆χ2 (44) = 72.73, p < .01, suggesting the structural coefficients should vary between two models. Therefore, the proposed model was tested with paths varying freely among three conditions.
Path Analysis Results
Test statistics, coefficients of individual structural paths, and explained variance of endogenous variables were summarized in Table 3. The effects of ease of use as an exogenous variable were found to be significantly different between AIA and NCI, ∆M = .26, t = 2.10, p < .05. Knowledge did not differ between the AIA and any of the other IAs (∆MAIA-NCI = .29, t = 1.45, p = .15; ∆MAIA-FLU = .22, t = .92, p = .36). Thus, H1 was not supported but H5 was supported.
Table 3.
Summary of Direct Structural Path Analysis Coefficients of Three Experimental Conditions (AIA, NCI, and FLU; N = 460)
| Conditions | AIA | NCI | FLU | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome / | |||||||||
| Predictors | b (beta) | t | R 2 | b (beta) | t | R 2 | b (beta) | t | R 2 |
| Attitudes / | .42 | .34 | .29 | ||||||
| Knowledge | .19 (.08) | 1.08 | −.27 (−.08) | .23 | −.35 (−.12) | .22 | |||
| Social Sharing | .17 (.29)*** | 4.17 | .22 (.36)*** | .04 | .20 (.31)*** | .05 | |||
| Social Norm | −.05 (−.07) | −1.12 | −.17 (−.23)** | .05 | −.10 (−.12) | .06 | |||
| Perceived Behavioral Control | .16 (.18) | 1.74 | .21 (.24)* | .09 | .25 (.26)** | .08 | |||
| Ease of Use | .32 (.35)*** | 3.75 | .01 (.02) | .08 | .03 (.03) | .08 | |||
| Perceived Behavioral Control / | .66 | .62 | .35 | ||||||
| Social Sharing | .11 (.16)** | 3.09 | .10 (.14)** | .04 | .20 (.30)*** | .05 | |||
| Knowledge | −.69 (−.24)*** | −4.75 | −.81 (−.21)*** | .20 | −.62 (−.20)** | .21 | |||
| Ease of Use | .66 (.65)*** | 12.77 | .66 (.65)*** | .05 | .37 (.35)*** | .07 | |||
| Cues to Action / | .24 | .28 | .24 | ||||||
| Attitudes | .15 (.37)*** | 4.68 | .16 (.41)*** | .03 | .05 (.12) | .03 | |||
| Social Sharing | .05 (.20)* | 2.47 | .05 (.20)* | .02 | .11 (.43)*** | .02 | |||
| Social Norm | −.001 (−.01) | −.07 | < .001(< .001) | .02 | −.03 (−.08) | .03 | |||
| Social Sharing / | .14 | .12 | .13 | ||||||
| Social Norm | .25 (.22)** | 2.92 | .11 (.09) | .09 | .32 (.25)** | .10 | |||
| Ease of Use | .50 (.34)*** | 4.52 | .49 (.35)*** | .11 | .50 (.31)*** | .12 | |||
| Intention / | .44 | .64 | .49 | ||||||
| Social Norm | −.06 (−.06) | −.97 | .02 (.02) | .06 | −.04 (−.04) | .07 | |||
| Social Sharing | .31 (.33)*** | 4.60 | .25 (.29)*** | .05 | .42 (.46)*** | .07 | |||
| Attitudes | .45 (.31)*** | 3.73 | .48 (.33)*** | .09 | .42 (.28)*** | .10 | |||
| Cues to Action | .76 (.21)** | 2.97 | 1.05 (.28)*** | .21 | .31 (.09) | .24 | |||
| Perceived Behavioral Control | .02 (.01) | .18 | .20 (.16)** | .07 | .09 (.06) | .10 | |||
| Knowledge / | .09 | .07 | .04 | ||||||
| Ease of Use | −.11 (−.30)*** | −3.98 | −.07 (−.26)** | .02 | −.06 (−.19)* | .03 | |||
Note:
p < .05
p < .01
p < .001.
Outcome variables are italicized. Standardized coefficients (beta) are provided in parentheses. T-statistics (t) are calculated based on unstandardized coefficients (B). R-squared (R2) value denotes the proportion of the variance for an outcome variable that is explained by all of its predictors in the model. Results exclude a case of missing data.
In the condition of AIA (see Figure 2a), ease of use was found to be positively associated with participation intention through two paths. First, ease of use was positively related to attitudes (H6c); attitudes were related to intention (H3). Second, ease of use was related to social sharing (H6d); the latter was positively associated with attitude (H8b), cues to action (H11b), and intention (H8c). Contrary to our predictions in H6a and H2b, ease of use was negatively related to knowledge, which was also negatively related to perceived behavioral control. Social norms contributed to social sharing (H7a) but not directly to attitudes (H7b) or intention (H7c). Cues to action were positively related to attitudes (H11c) and intention (H11d).
Figure 2.
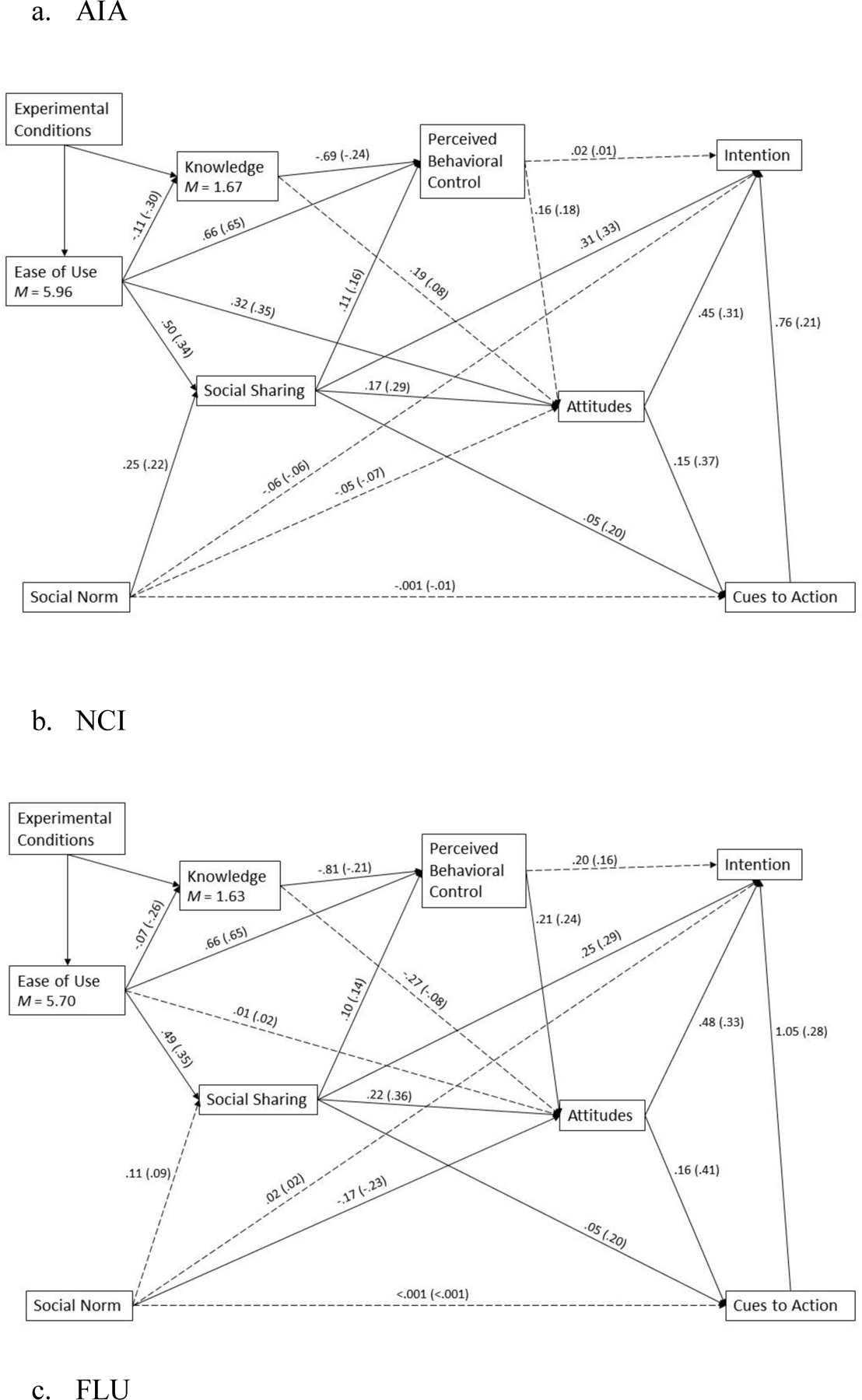
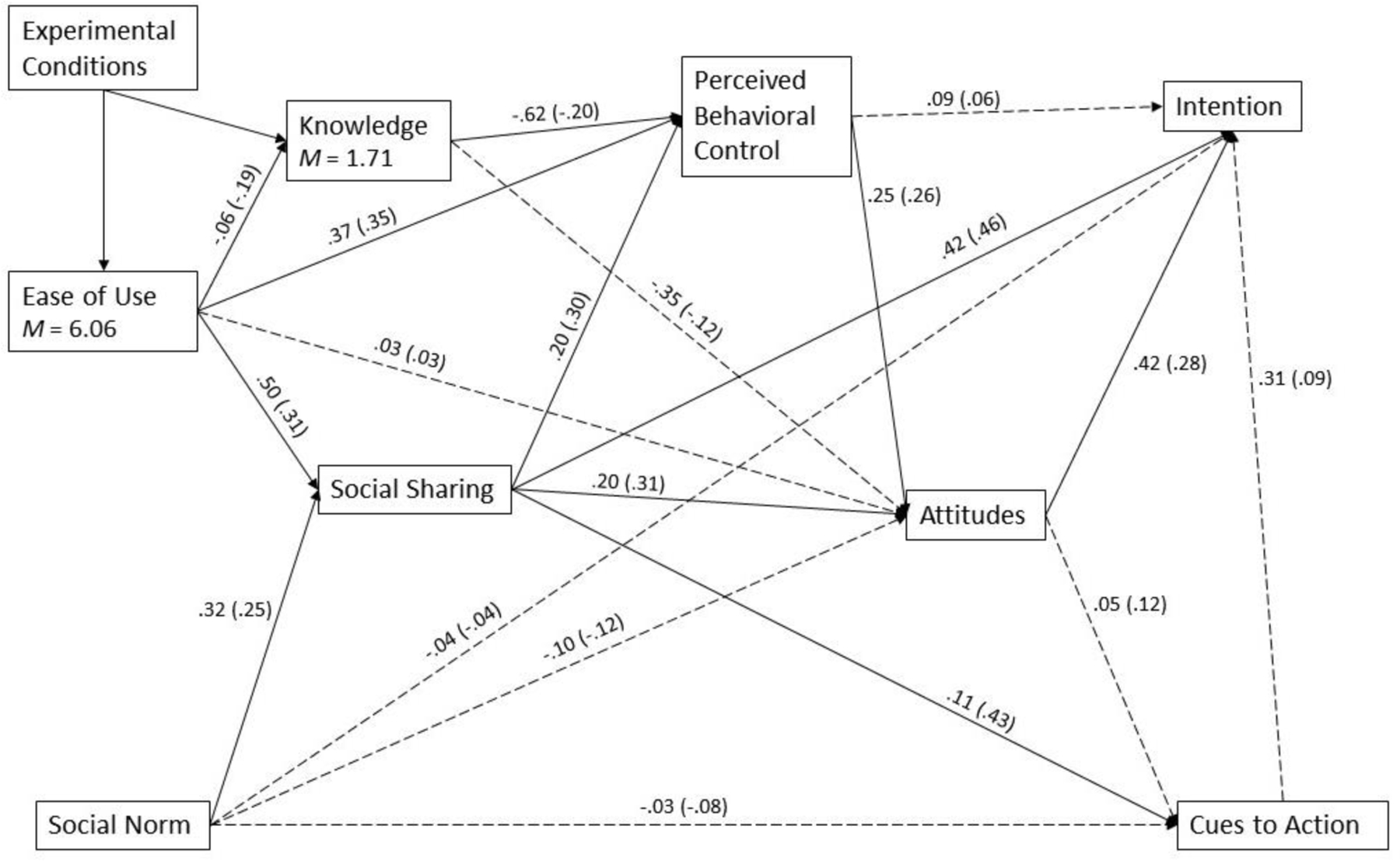
Results of the Path Model of Three Experimental Conditions (AIA, NCI, and FLU; N = 460)
Note: All solid paths are significant at .05 level. The coefficients outside parentheses are unstandardized. The coefficients inside parentheses are standardized. Results exclude a case of missing data.
In the condition of NCI (see Figure 2b), the intention was related to different antecedents through several paths. First, ease of use was positively related to perceived behavioral control (H6b) and social sharing (H6d). Both perceived behavioral control and social sharing were further positively related to attitudes (H4a and H8b, respectively); attitudes was positively associated with intention (H3). Second, knowledge was negatively related to ease of use (H6a) and perceived behavioral control (H2b). Third, social norms were directly related to attitudes (H7b) but not to social sharing (H7a). Fourth, social sharing was positively related to attitudes (H8b), cues to action (H11b), and intention (H8c).
In the control condition of flu vaccine IA (see Figure 2c), ease of use was related to perceived behavioral control (H6b) and social sharing (H6d). Perceived behavioral control was positively associated with attitudes (H4a), which was related to intention (H3). Social sharing was positively associated with perceived behavioral control (H8a), attitudes (H8b), intention (H8c), and also cues to action (H11b). Also, similar to other conditions, knowledge was negatively related to ease of use (H6a) and perceived behavioral control (H2b).
Lastly, we investigated the mediational relations of social sharing (see Table 4). Supporting H9s, across three conditions social sharing mediated the relationships between ease of use and outcome variables. Additionally, H10s were supported in AIA and FLU that social sharing mediated the associations between social norm and outcome variables. H10s were not supported in the NCI condition.
Table 4.
Summary of Indirect Structural Path Analysis Coefficients of Three Experimental Conditions (AIA, NCI, and FLU; N = 460)
| AIA | NCI | FLU | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Indirect path | b | t | b | t | b | t |
| Ease of use - Social Sharing - Perceived Behavioral Control | .05* | 2.55 | .05* | 2.33 | .10** | 2.94 |
| Ease of use - Social Sharing - Attitudes | .09** | 3.07 | .11** | 3.38 | .10** | 2.79 |
| Ease of use - Social Sharing - Intention | .15** | 3.23 | .12** | 3.34 | .21** | 3.33 |
| Social Norm - Social Sharing – Perceived Behavioral Control | .03* | 2.13 | .01 | 1.11 | .06* | 2.57 |
| Social Norm - Social Sharing - Attitudes | .04* | 2.39 | .02 | 1.19 | .06* | 2.47 |
| Social Norm - Social Sharing - Intention | .08* | 2.47 | .03 | 1.19 | .14** | 2.82 |
Note:
p < .05
p < .01
p < .001.
Results exclude a case of missing data.
Moderation Analysis Results
We examined post hoc whether structural paths differed across three experimental conditions for potential moderation effects. Most of the moderation effects were not significant, suggesting the proposed path relations were not different across three IAs. Nevertheless, the relationship between ease of use and perceived behavioral control was significantly stronger in AIA than the one of FLU (b = .29, t = 3.30, p < .01). Also, the association of ease of use and attitude was stronger of AIA than the other two IA conditions (AIA-NCI: b = .29, t = 2.48, p < .05; AIA-FLU b = .28, t = 2.4, p < .02). These results were consistent with the main effect supporting H5, suggesting that for users of AIA, ease of use was a more important factor to explain the change in attitudes and behavioral control.
Discussion
The present study proposed and tested a model to explain the efficacy of informational aids. The findings provided empirical support to our proposed model. Variances in attitudes, perceived behavioral control, social sharing, and knowledge were explained more by the predictors in interactive AIA than in other IAs. Social sharing was an important mediator between interactive features and outcome variables across different IAs. Also, the path relations linking interactive features, mediators, and outcome variables were different among different IAs. Theoretical and practical implications are discussed as follows.
First, ease of use was related to important outcome variables in the model. The ties between ease of use and perceived behavioral control and attitudes were stronger in AIA than two other IAs. This finding suggests that interactive features of AIA eased the barriers to effective communication about clinical trial topics. Through presenting personalized information relevant to one’s interest and concern, AIA reduced information load and improved ease of use. Consequently, users developed more positive attitudes and a greater sense of control in decision making (Windle et al., 2011). Compared to flu vaccination, information about clinical trials is probably unfamiliar and more difficult to understand. Thus, cancer patients are more likely to need interactive communication technologies for understanding more complex concepts (i.e., clinical trials) than a common health topic (i.e., flu vaccination).
Also, social sharing emerged as a strong mediator linking ease of use or social norms with several outcome variables. Compared to NCI, social norms changed attitudes only indirectly through social sharing in AIA. This finding demonstrates the importance of social sharing in patient-centered shared health care. As Whelan et al. (2004) pointed out, a cancer patient’s decisional conflict may stem not only from their lack of information but also from feeling unsupported. Emotional or information support from peers or family was indispensable for improving cancer patients’ decision quality and reducing their distress (Stacey, O’Connor, & DeGrasse, 2003). Also, whether families or friends accept clinical trial participation significantly influences a patient’s likelihood of enrollment (Ford et al., 2008; Mills et al., 2006). In this process, interactive IAs consider the factors of social norms and integrate the values and preferences of individual patients to support their decision-making process. Thus, interactive IAs can empower patients to seek social support in the shared decision-making process and minimize decisional conflicts (Gillies et al., 2015; Shneerson et al., 2013).
Contrary to our prediction, attitudes were not associated with knowledge but with ease of use. This finding nevertheless showed a meaningful strength of interactive IAs. AIA provided tailored information that responded to users’ specific needs and circumstances, not to promoting general knowledge about clinical trials. The interactive function improved ease of use and perceived control through reducing confusion, distress, and cognitive overload from irrelevant information (Caldon et al., 2011; Lipstein et al., 2013; Melton, 2010). By contrast, traditional IAs may provide more comprehensive information, which may increase knowledge but not necessarily result in attitudinal changes. This explanation has been further supported by a more negative path coefficient between ease of use and knowledge in AIA than NCI as well as a significantly positive relationship between ease of use and attitudes in AIA only.
Moreover, perceived behavioral control was significantly related to attitudes and intention in NCI but not in AIA. Although this finding was inconsistent with the prediction based on TPB (Ajzen, 1991), the lack of association still suggests potential benefits provided by the interactive functionality of AIA. Interactive decision aid tools allow a more balanced presentation of benefits and risks related to each user than traditional formats (e.g., paper-based pamphlet) (Thomson et al., 2007). Thus, AIA could have helped users evaluate possible outcomes, leading to the decisions for or against participation. In other words, the efficacy of AIA could be offset by participation versus non-participation intentions. However, the non-interactive NCI might have presented overall effectiveness, hampering an effective evaluation of benefits or harms associated with a patient’s specific situations. This possible result illustrates the significance of interactive decisional tools in applied clinical settings.
Lastly, given the risk involved in clinical trials, the efficacy of IAs may not be translated to a particular outcome, such as participation intentions. Nevertheless, IAs should be considered effective when they can help individuals make an informed decision (Politi et al., 2016). For this reason, our analysis was able to uncover different processes involved in decision making after using an interactive versus non-interactive IA. In the present study, AIA was designed to present a balanced information about clinical trial participation based on a user’s preference. In addition, the interactive functions of AIA were perceived easy to use, which could facilitate stronger analytic reasoning for participants to reach more agreement between their values and decisions of clinical trial participation (Sepucha et al., 2013). Future studies should continue to focus on various constructs that are involved in a decision-making process to assess the overall strength of different IAs (Hersch et al., 2015; Politi et al., 2016).
Several limitations warrant attention. First, the intention to join a study was measured as a dependent variable. Future research should examine the efficacy of IAs in actual clinical enrollment settings. Second, although previous research found the Qualtrics Panel could provide a sample closest to the probability sampling on most demographic variables in the U.S. (Boas, Christenson, & Glick, 2018), our recruited sample consisted of 94% of Caucasian respondents. It could be related to lower interests in clinical trials and medical distrust due to significant barriers to research participation faced by minority patients (Murthy et al., 2004). Future research should test the model in diverse populations, especially among non-White populations and also individuals with less educational attainment and health literacy. Lastly, future research can replicate the findings among volunteers in clinical research registries (e.g., ResearchMatch.org).
Conclusion
In health care environments with limited resources and prominent challenges of clinical trial accrual, the development of informational aids to support shared decision making is of significant value for both patients and providers. Interactive informational aids for clinical trials, including the one developed and tested in the present study, can address specific barriers and concerns for individual participants. Based on the study findings, an easy-to-use informational aid has the potential to assist patients to evaluate benefits and risks, gain autonomy, seek social support, and facilitate meaningfully informed healthcare decision-making.
Appendix A
Screenshot of Authors’ Informational Aid (AIA)
Footnotes
Disclosures:
This study was partially funded by grants from Clinical and Translational Science Institute and Center for Communication, Culture, & Change at the University of Miami.
The authors declared no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Agoritsas T, Deom M, & Perneger TV (2011). Study design attributes influenced patients’ willingness to participate in clinical research: A randomized vignette-based study. Journal of Clinical Epidemiology, 64(1), 107–115. doi: 10.1016/j.jclinepi.2010.02.007 [DOI] [PubMed] [Google Scholar]
- Ajzen I (1991). The theory of planned behavior. Organizational Behavior and Human Decision Processes, 50(2), 179–211. doi: 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- Albrecht TL, Eggly SS, Gleason MEJ, Harper FWK, Foster TS, Peterson AM, … Ruckdeschel JC (2008). Influence of clinical communication on patients’ decision making on participation in clinical trials. Journal of Clinical Oncology, 26(16), 2666–2673. doi: 10.1200/JCO.2007.14.8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RA, Pfeifer M, Shillington AC, Harshaw Q, Funnell MM, VanWingen J, & Col N (2016). Effect of a patient decision aid (PDA) for type 2 diabetes on knowledge, decisional self-efficacy, and decisional conflict. BMC Health Services Research, 16, 10. doi: 10.1186/s12913-016-1262-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boas TC, Christenson DP, & Glick DM (2018). Recruiting large online samples in the United States and India: Facebook, Mechanical Turk, and Qualtrics. Political Science Research and Methods, 1–19. doi: 10.1017/psrm.2018.28 [DOI]
- Byrne MM, Tannenbaum SL, Glück S, Hurley J, & Antoni M (2014). Participation in cancer clinical trials: Why are patients not participating? Medical Decision Making: An International Journal of the Society for Medical Decision Making, 34(1), 116–126. doi: 10.1177/0272989X13497264 [DOI] [PubMed] [Google Scholar]
- Caldon LJM, Collins KA, Reed MW, Sivell S, Austoker J, Clements AM, … Group B (2011). Clinicians’ concerns about decision support interventions for patients facing breast cancer surgery options: Understanding the challenge of implementing shared decision-making. Health Expectations: An International Journal of Public Participation in Health Care and Health Policy, 14(2), 133–146. doi: 10.1111/j.1369-7625.2010.00633.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P, Pond GR, Xu RY, Ellis PM, & Goffin JR (2013). A comparison of patient knowledge of clinical trials and trialist priorities. Current Oncology, 20(3), e193–e205. doi: 10.3747/co.20.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter CJ (2010). A meta-analysis of the effectiveness of health belief model variables in predicting behavior. Health Communication, 25(8), 661–669. doi: 10.1080/10410236.2010.521906 [DOI] [PubMed] [Google Scholar]
- Carpenter JS, Studts JL, & Byrne MM (2011). A systematic review of menopausal symptom management decision aid trials. Maturitas, 69(1), 11–21. doi: 10.1016/j.maturitas.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Charles C, Gafni A, & Whelan T (1999). Decision-making in the physician-patient encounter: Revisiting the shared treatment decision-making model. Social Science & Medicine, 49(5), 651–661. doi: s0277-9536(99)00145-8 [DOI] [PubMed] [Google Scholar]
- Comis RL, Miller JD, Aldigé CR, Krebs L, & Stoval E (2003). [Review of Public attitudes toward participation in cancer clinical trials]. Journal of clinical oncology, 21(5), 830–835. doi: 10.1200/JCO.2003.02.105 [DOI] [PubMed] [Google Scholar]
- Conner M, & Sparks P (2005). Theory of planned behaviour and health behaviour. In Conner M & Norman P (Eds.), Predicting Health Behaviour: Research and Practice with Social Cognition Models (Second edition, pp. 170–222). New York, NY: Open University Press. [Google Scholar]
- Duncan OD (1975). Introduction to Structural Equation Models New York, NY: Academic Press. [Google Scholar]
- Ellis PM, & Butow PN (1998). Focus group interviews examining attitudes to randomised trials among breast cancer patients and the general community. Australian and New Zealand Journal of Public Health, 22(5), 528–531. doi: j.1467-842x.1998.tb01432.x [DOI] [PubMed] [Google Scholar]
- Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand M-A, … Edwards A (2009). Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PloS One, 4(3), e4705. doi: 10.1371/journal.pone.0004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory J, & Emanuel E (2004). Interventions to improve research participants’ understanding in informed consent for research: A systematic review. JAMA: The Journal of the American Medical Association, 292(13), 1593–1601. doi: 10.1001/jama.292.13.1593 [DOI] [PubMed] [Google Scholar]
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, … Bass EB (2008). Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer, 112(2), 228–242. doi: 10.1002/cncr.23157 [DOI] [PubMed] [Google Scholar]
- Frew PM, Hou S-I, Davis M, Chan K, Horton T, Shuster J, … del Rio C (2010). The likelihood of participation in clinical trials can be measured: The Clinical Research Involvement Scales. Journal of Clinical Epidemiology, 63(10), 1110–1117. doi: 10.1016/j.jclinepi.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies K, Cotton SC, Brehaut JC, Politi MC, & Skea Z (2015). Decision aids for people considering taking part in clinical trials. Cochrane Database of Systematic Reviews, (11), CD009736. doi: 10.1002/14651858.CD009736.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes-Maslow L, Godley P, Dimartino L, White B, Odom J, Richmond A, & Carpenter W (2014). African American women’s perceptions of cancer clinical trials. Cancer Medicine, 3(5), 1430–1439. doi: 10.1002/cam4.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch J, Barratt A, Jansen J, Irwig L, McGeechan K, Jacklyn G, … McCaffery K (2015). Use of a decision aid including information on overdetection to support informed choice about breast cancer screening: A randomised controlled trial. The Lancet, 385(9978), 1642–1652. doi: 10.1016/S0140-6736(15)60123-4 [DOI] [PubMed] [Google Scholar]
- Hopp T, & Gallicano TD (2016). Development and test of a multidimensional scale of blog engagement. Journal of Public Relations Research, 28(3–4), 127–145. doi: 10.1080/1062726X.2016.1204303 [DOI] [Google Scholar]
- Janz NK, & Becker MH (1984). The Health Belief Model: A decade later. Health Education Quarterly, 11(1), 1–47. doi: 10.1177/109019818401100101 [DOI] [PubMed] [Google Scholar]
- Jenkins V, & Fallowfield L (2000). Reasons for accepting or declining to participate in randomized clinical trials for cancer therapy. British Journal of Cancer, 82(11), 1783–1788. doi: 10.1054/bjoc.2000.1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T, Fowler MC, & Hubbard D (2000). Refining a tool to measure cues to action in encouraging health-promoting behavior—The CHAQ. American Journal of Health Promotion: AJHP, 14(3), 170–173. doi: 10.4278/0890-1171-14.3.170 [DOI] [PubMed] [Google Scholar]
- Kim M, Klingle RS, Sharkey WF, Park HS, Smith DH, & Cai D (2000). A test of a cultural model of patients’ motivation for verbal communication in patient‐doctor interactions. Communication Monographs, 67(3), 262–283. doi: 10.1080/03637750009376510 [DOI] [Google Scholar]
- Kline RB (2015). Principles and Practice of Structural Equation Modeling, Fourth Edition. New York, NY: Guilford Publications. [Google Scholar]
- Kraft SA, Constantine M, Magnus D, Porter KM, Lee SS-J, Green M, … Cho MK (2017). A randomized study of multimedia informational aids for research on medical practices: Implications for informed consent. Clinical Trials, 14(1), 94–102. doi: 10.1177/1740774516669352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JL, Palmer-Wackerly A, Dailey PM, Krok-Schoen JL, Schoenberg NE, & Paskett ED (2015). Comprehension of randomization and uncertainty in cancer clinical trials decision making among rural, Appalachian patients. Journal of Cancer Education, 30(4), 743–748. doi: 10.1007/s13187-015-0789-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipstein EA, Brinkman WB, Sage J, Lannon CM, & Morgan Dewitt E (2013). Understanding treatment decision making in juvenile idiopathic arthritis: A qualitative assessment. Pediatric Rheumatology Online Journal, 11(1), 34. doi: 10.1186/1546-0096-11-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne S, Jacobsen PB, Ming ME, Winkel G, Dessureault S, & Lessin SR (2010). Tailored versus generic interventions for skin cancer risk reduction for family members of melanoma patients. Health Psychology, 29(6), 583–593. doi: 10.1037/a0021387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton GB (2010). Biomedical and health informatics for surgery. Advances in Surgery, 44, 117–130. doi: 10.1016/j.yasu.2010.05.015 [DOI] [PubMed] [Google Scholar]
- Meropol NJ, Weinfurt KP, Burnett CB, Balshem A, Benson AB 3rd, Castel L, … Schulman KA (2003). Perceptions of patients and physicians regarding phase I cancer clinical trials: Implications for physician-patient communication. Journal of Clinical Oncology, 21(13), 2589–2596. doi: 10.1200/JCO.2003.10.072 [DOI] [PubMed] [Google Scholar]
- Michaels M, Weiss ES, Guidry JA, Blakeney N, Swords L, Gibbs B, … Patel S (2012). “The promise of community-based advocacy and education efforts for increasing cancer clinical trials accrual.” Journal of Cancer Education, 27(1), 67–74. doi: 10.1007/s13187-011-0271-6 [DOI] [PubMed] [Google Scholar]
- Miller SM, Hudson SV, Egleston BL, Manne S, Buzaglo JS, Devarajan K, … Others. (2013). The relationships among knowledge, self-efficacy, preparedness, decisional conflict, and decisions to participate in a cancer clinical trial. Psycho-Oncology, 22(3), 481–489. doi: 10.1002/pon.3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Seely D, Rachlis B, Griffith L, Wu P, Wilson K, … Wright JR (2006). Barriers to participation in clinical trials of cancer: A meta-analysis and systematic review of patient-reported factors. The Lancet Oncology, 7(2), 141–148. doi: 10.1016/S1470-2045(06)70576-9 [DOI] [PubMed] [Google Scholar]
- Morgan SE, Mouton A, Occa A, & Potter J (2016). Clinical trial and research study recruiters’ verbal communication behaviors. Journal of Health Communication, 21(7), 765–772. doi: 10.1080/10810730.2016.1157654 [DOI] [PubMed] [Google Scholar]
- Morgan SE, Occa A, Mouton A, & Potter J (2017). The role of nonverbal communication behaviors in clinical trial and research study recruitment. Health Communication, 32(4), 461–469. doi: 10.1080/10410236.2016.1140266 [DOI] [PubMed] [Google Scholar]
- Murthy VH, Krumholz HM, & Gross CP (2004). Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA: The Journal of the American Medical Association, 291(22), 2720–2726. doi: 10.1001/jama.291.22.2720 [DOI] [PubMed] [Google Scholar]
- NIH. (2015, May 14). The NIH Clinical Trials and You Retrieved April 11, 2019, from National Institutes of Health (NIH) website: https://www.nih.gov/health-information/nih-clinical-research-trials-you/basics
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, & McCormick JB (2013). Improving understanding in the research informed consent process: A systematic review of 54 interventions tested in randomized control trials. BMC Medical Ethics, 14, 28. doi: 10.1186/1472-6939-14-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeidat R, Finnell DS, & Lally RM (2011). Decision aids for surgical treatment of early stage breast cancer: A narrative review of the literature. Patient Education and Counseling, 85(3), e311–e321. doi: 10.1016/j.pec.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Occa A, Morgan SE, & Potter JE (2018). Underrepresentation of Hispanics and other minorities in clinical trials: Recruiters’ perspectives. Journal of Racial and Ethnic Health Disparities, 5(2), 322–332. doi: 10.1007/s40615-017-0373-x [DOI] [PubMed] [Google Scholar]
- O’Connor AM, Rostom A, Fiset V, Tetroe J, Entwistle V, Llewellyn-Thomas H, … Jones J (1999). Decision aids for patients facing health treatment or screening decisions: Systematic review. BMJ, 319(7212), 731–734. doi: 10.1136/bmj.319.7212.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AM, Wennberg JE, Legare F, Llewellyn-Thomas HA, Moulton BW, Sepucha KR, … King JS (2007). Toward the “tipping point”: Decision aids and informed patient choice. Health Affairs, 26(3), 716–725. doi: 10.1377/hlthaff.26.3.716 [DOI] [PubMed] [Google Scholar]
- Oh SS, Galanter J, Thakur N, Pino-Yanes M, Barcelo NE, White MJ, … Burchard EG (2015). Diversity in Clinical and Biomedical Research: A Promise Yet to Be Fulfilled. PLoS Medicine, 12(12), e1001918. doi: 10.1371/journal.pmed.1001918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottawa Hospital Research Institute. (2017). Patient Decision Aids Retrieved April 11, 2019, from Ottawa Hospital Research Institute website: https://decisionaid.ohri.ca/
- Paterniti DA, Chen MS, Jr, Chiechi, C., Beckett, L. A., Horan, N., Turrell, C., … Others. (2005). Asian Americans and cancer clinical trials: A mixed-methods approach to understanding awareness and experience. Cancer, 104(S12), 3015–3024. doi: 10.1002/cncr.21522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Politi MC, Kuzemchak MD, Kaphingst KA, Perkins H, Liu J, & Byrne MM (2016). Decision aids can support cancer clinical trials decisions: Results of a randomized trial. The Oncologist, 21(12), 1461–1470. doi: 10.1634/theoncologist.2016-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EG, Wakefield CE, Signorelli C, Cohn RJ, Patenaude A, Foster C, … Fardell JE (2018). Strategies to facilitate shared decision-making about pediatric oncology clinical trial enrollment: A systematic review. Patient Education and Counseling, 101(7), 1157–1174. doi: 10.1016/j.pec.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Rosenstock IM (1974). The Health Belief Model and Preventive Health Behavior. Health Education Monographs, 2(4), 354–386. doi: 10.1177/109019817400200405 [DOI] [PubMed] [Google Scholar]
- Sepucha KR, Borkhoff CM, Lally J, Levin CA, Matlock DD, Ng CJ, … Thomson R (2013). Establishing the effectiveness of patient decision aids: Key constructs and measurement instruments. BMC Medical Informatics and Decision Making, 13 Suppl 2, S12. doi: 10.1186/1472-6947-13-S2-S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shneerson C, Windle R, & Cox K (2013). Innovating information-delivery for potential clinical trials participants. What do patients want from multi-media resources? Patient Education and Counseling, 90(1), 111–117. doi: 10.1016/j.pec.2012.06.031 [DOI] [PubMed] [Google Scholar]
- Simon C, Zyzanski SJ, Eder M, Raiz P, Kodish ED, & Siminoff LA (2003). Groups potentially at risk for making poorly informed decisions about entry into clinical trials for childhood cancer. Journal of Clinical Oncology, 21(11), 2173–2178. doi: 10.1200/JCO.2003.03.003 [DOI] [PubMed] [Google Scholar]
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, … Trevena L (2017). Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews, 4, CD001431. doi: 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D, O’Connor AM, & DeGrasse C (2003). Development and evaluation of a breast cancer prevention decision aid for higher‐risk women. Health, 6(1), 3–18. doi: 10.1046/j.1369-6513.2003.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson MT, Holbert RL, & Zimmerman RS (2006). On the use of structural equation modeling in health communication research. Health Communication, 20(2), 159–167. doi: 10.1207/s15327027hc2002_7 [DOI] [PubMed] [Google Scholar]
- Sundar SS, Kalyanaraman S, & Brown J (2003). Explicating web site interactivity: Impression formation effects in political campaign sites. Communication Research, 30(1), 30–59. doi: 10.1177/0093650202239025 [DOI] [Google Scholar]
- Sutherland HJ, da Cunha R, Lockwood GA, & Till JE (1998). What attitudes and beliefs underlie patients’ decisions about participating in chemotherapy trials? Medical Decision Making, 18(1), 61–69. doi: 10.1177/0272989X9801800113 [DOI] [PubMed] [Google Scholar]
- Tam NT, Huy NT, Thoa LTB, Long NP, Trang NTH, Hirayama K, & Karbwang J (2015). Participants’ understanding of informed consent in clinical trials over three decades: Systematic review and meta-analysis. Bulletin of the World Health Organization, 93(3), 186–198H. doi: 10.2471/BLT.14.141390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RG, Eccles MP, Steen IN, Greenaway J, Stobbart L, Murtagh MJ, & May CR (2007). A patient decision aid to support shared decision-making on anti-thrombotic treatment of patients with atrial fibrillation: Randomised controlled trial. Quality & Safety in Health Care, 16(3), 216–223. doi: 10.1136/qshc.2006.018481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umphrey LR (2004). Message defensiveness, efficacy, and health‐related behavioral intentions. Communication Research Reports, 21(4), 329–337. doi: 10.1080/08824090409359997 [DOI] [Google Scholar]
- Whelan T, Levine M, Willan A, Gafni A, Sanders K, Mirsky D, … Dubois S (2004). Effect of a decision aid on knowledge and treatment decision making for breast cancer surgery: A randomized trial. JAMA: The Journal of the American Medical Association, 292(4), 435–441. doi: 10.1001/jama.292.4.435 [DOI] [PubMed] [Google Scholar]
- Windle RJ, McCormick D, Dandrea J, & Wharrad H (2011). The characteristics of reusable learning objects that enhance learning: A case-study in health-science education: Evaluation and effectiveness of RLOs. British Journal of Educational Technology, 42(5), 811–823. doi: 10.1111/j.1467-8535.2010.01108.x [DOI] [Google Scholar]
- Wright JR, Crooks D, Ellis PM, Mings D, & Whelan TJ (2002). Factors that influence the recruitment of patients to Phase III studies in oncology: The perspective of the clinical research associate. Cancer, 95(7), 1584–1591. doi: 10.1002/cncr.10864 [DOI] [PubMed] [Google Scholar]
- Yang ZJ, McComas KA, Gay GK, Leonard JP, Dannenberg AJ, & Dillon H (2012). Comparing decision making between cancer patients and the general population: Thoughts, emotions, or social influence? Journal of Health Communication, 17(4), 477–494. doi: 10.1080/10810730.2011.635774 [DOI] [PubMed] [Google Scholar]
- Yang ZJ, McComas K, Gay G, Leonard JP, Dannenberg AJ, & Dillon H (2010). Motivation for health information seeking and processing about clinical trial enrollment. Health Communication, 25(5), 423–436. doi: 10.1080/10410236.2010.483338 [DOI] [PubMed] [Google Scholar]
- Yi MY, & Hwang Y (2003). Predicting the use of web-based information systems: Self-efficacy, enjoyment, learning goal orientation, and the technology acceptance model. International Journal of Human-Computer Studies, 59(4), 431–449. doi: 10.1016/S1071-5819(03)00114-9 [DOI] [Google Scholar]
- Stacey D, O’Connor AM, & DeGrasse C (2003). Development and evaluation of a breast cancer prevention decision aid for higher‐risk women. Health Expectations, 6, 3–18. doi: 10.1046/j.1369-6513.2003.00195.x [DOI] [PMC free article] [PubMed] [Google Scholar]


