Abstract
A positive follow-up blood culture for methicillin-resistant Staphylococcus aureus (MRSA) while on seemingly appropriate therapy is a common and ominous development. However, the definition and management of persistent MRSA bacteremia is unstandardized. In this Opinion Paper, we identify the presence of bacteremia for > 1 calendar day as a “worry point” that should trigger an intensive diagnostic evaluation to identify metastatic infection sites. Next, we define the duration of MRSA bacteremia that likely constitutes antibiotic failure and outline a potential management algorithm for such patients. Finally, we propose pragmatic clinical trial designs to test treatment strategies for persistent MRSA bacteremia.
Keywords: Staphylococcus aureus bacteremia, methicillin-resistance
In this Opinion Paper, we define the “worry point” and appropriate diagnostic evaluation for persistent methicillin-resistant Staphylococcus aureus bacteremia and outline a proposed management algorithm for these patients, including when to declare antibiotic failure.
Methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections are common, and they carry high associated morbidity, mortality, and economic burdens [1]. These infections have become more complicated over time, in part because of increasing rates of prosthetic devices and metastatic sites of infection [2]. Even with appropriate therapy based on in vitro susceptibility testing, blood cultures in patients with MRSA bacteremia often remain positive for multiple days on seemingly appropriate therapy [3]. Faced with a patient with such ongoing MRSA bacteremia, clinicians must decide if, when, and how to change management.
Despite its frequency and severity, surprisingly little is known about persistent MRSA bacteremia. Key details such as the optimal timing and frequency of obtaining follow-up blood cultures, best clinical management practices, and even a standardized definition of the syndrome are unclear. In this paper, we review the current data informing these issues and highlight how a stepwise approach to persistent MRSA bacteremia could inform antibiotic selection, patient stratification, and execution of pivotal clinical trials in this field. This presentation will not focus on the separate and unique scenario of “recurrent or relapsing” MRSA bacteremia that has recently been examined elsewhere [4]. Although our focus here is on MRSA, the proposed principles discussed are likely applicable to methicillin-susceptible Staphylococcus aureus bacteremia (SAB) as well.
Defining Persistent MRSA Bacteremia – A Changing Standard
Historical Context
In trials published in the early 1990s, patients with MRSA endocarditis had a median duration of bacteremia of ∼7 days [3, 5]. On this basis, Fowler et al proposed that persistent MRSA bacteremia be defined as ≥ 7 days of positive blood cultures while the patient was receiving antibiotics to which the isolate was susceptible in vitro [6] (Table 1). The recommendation to wait a week before changing the diagnostic and therapeutic approach for persistent MRSA bacteremia was reasonable at a time when few alternatives to vancomycin existed. Trimethoprim-sulfamethoxazole (with or without rifampin) was inferior to vancomycin for S aureus infections [5], whereas the use of quinupristin/dalfopristin [7] or adjunctive antibiotics (rifampin [3], gentamicin [8]) was associated with substantial toxicities without clear benefit. As a result of these therapeutic limitations, a majority of infectious disease consultants surveyed in 2005 elected to continue vancomycin in the face of > 7 days of MRSA bacteremia, often with the addition of rifampin or gentamicin [9]. In this period, the primary management strategy for persistent MRSA bacteremia emphasized (1) adequate source control, (2) increased vancomycin dosing strategies with trough-level targeting, and (3) extending treatment duration.
Table 1.
Clinical Definitions and Ongoing Questions Related to MRSA Bacteremia
| Term | Definition | Unresolved Issues |
|---|---|---|
| MRSA bacteremia | Growth of MRSA from a blood culture specimen | Should detection of cell-free microbial DNA in blood be considered evidence of ongoing bacteremia in patients with negative blood cultures? |
| Uncomplicated MRSA bacteremia | Per consensus IDSA guidelines:
|
Is transesophageal echocardiography or other advanced cardiac imaging required to exclude endocarditis? |
| Persistent MRSA bacteremia | Positive follow-up blood culture > 1 day after initiation of seemingly appropriate antibiotic therapy based on in vitro susceptibility testing | Can an early intensified diagnostic strategy improve outcomes? |
| Skip phenomenon | Intermittently positive blood cultures over several days | What are the clinical implications of the skip phenomenon? |
Abbreviations: IDSA, Infectious Diseases Society of America; MRSA, methicillin-resistant Staphylococcus aureus.
In the past 2 decades, however, at least 8 antibiotics with confirmed efficacy against MRSA have become clinically available. As a consequence, more than half of infectious disease practitioners surveyed in 2017 would discontinue vancomycin altogether for a patient with MRSA endocarditis and persistent bacteremia on day 6 of treatment [10]. During the same period, several studies proposed reduced durations (3–5 days) to define when MRSA bacteremia was “persistent” [11–14]. Thus, the length of time that clinicians consider to best represent persistent MRSA bacteremia has shortened as the number of therapeutic alternatives to vancomycin has increased.
MRSA Bacteremia: Duration and Outcome
A key question with persistent MRSA bacteremia is when to change treatment. The impact of ongoing bacteremia on patient outcome is clear. In a prospective observational cohort of 884 adults hospitalized with SAB (33% with MRSA bacteremia), each additional day of bacteremia increased mortality risk by 16% [15]. In another large prospective cohort, 30-day mortality for patients with more than 1 calendar day of SAB was more than double that of patients whose SAB resolved after a single calendar day [16]. Taken together, these 2 studies suggest that the identification of bacteremia even a single day beyond the initial day of diagnosis represents a critical “worry point” for metastatic infections and poor outcomes in patients with MRSA bacteremia, and should trigger a prompt diagnostic workup for metastatic sites of infection (see the following section).
Skip Phenomenon
Occasionally, patients with SAB have blood cultures that are intermittently positive over the course of several days. The significance of this occurrence, termed the skip phenomenon, is unknown. One theoretical concern is that the skip phenomenon could lead to treatment errors based on the erroneous assumption that a patient’s bacteremia had cleared. Alternatively, the skip phenomenon may simply represent a decreasing bacterial load that is clinically irrelevant. Thus, intermittently positive cultures may be a sign of progressively resolving bacteremia and extending the duration of treatment could be unnecessary.
The skip phenomenon was originally described in 4%–13% of patients in 2 retrospective SAB cohorts [17, 18]. In the first, among 1071 patients in an Australian health system, the skip phenomenon was more common with MRSA than with methicillin-sensitive S aureus and was associated with subsequent relapse > 14 days after the initial episode. A single negative blood culture 1 day after a positive blood culture had a negative predictive value of 87% for excluding intermittent bacteremia; 5% of all SAB cases would have been misclassified as “uncomplicated” infections if there were no additional blood cultures collected after the first negative one. The second study used a case-control design to compare 29 patients (12 with MRSA) who experienced the skip phenomenon with 87 controls matched for sex, age, and duration of bacteremia. Mortality was nominally higher among patients with the skip phenomenon. The study found that the skip phenomenon occurred predominately among older men, with a predilection for those receiving immunosuppressive therapy and with a longer initial duration of bacteremia.
Considered together, it appears that the skip phenomenon occurs in a relatively small proportion of MRSA bacteremia patients. Its presence should prompt the clinician to consider treatment-emergent resistance (especially when using daptomycin), an occult focus of infection, or even a new infection altogether. When previous blood cultures are positive for MRSA, clinicians should obtain follow-up blood cultures at least 24–48 hours apart to document clearance of bacteremia. Until the significance and optimal management of the skip phenomenon are better understood, such patients should not be considered for short course or oral step-down therapy.
Take-home Points for Duration of MRSA Bacteremia
A key clinical message of this narrative is the critical importance of follow-up blood cultures in patients with MRSA bacteremia. The finding of a positive follow-up blood culture for S aureus, even a single day after initiating therapy, identifies probable complicated bacteremia. Patients with a positive follow-up blood culture should undergo a thorough evaluation for occult foci of infection and receive a longer treatment duration than would be used for uncomplicated bacteremia.
Whether changing or intensifying antibiotic therapy can improve outcomes for patients with persistent MRSA bacteremia is unknown. Adding new antibiotics confers both potential harm and benefit [19–22]. Although multiple trials and cohort studies have demonstrated that adding an adjunctive antibiotic to standard monotherapy can reduce the duration of SAB, none has shown improved clinical outcomes [23–26]. As recently reviewed by Rose et al, a one-size-fits-all approach with combination antibiotic therapy risks overtreating patients at low risk of poor outcomes, as well as diluting our ability to demonstrate benefit (if any) among higher risk patients [27]. Definitive clinical trials are thus needed to evaluate the optimal strategies for persistent MRSA bacteremia.
Implications for Definitive Randomized Clinical Trials for Persistent MRSA Bacteremia
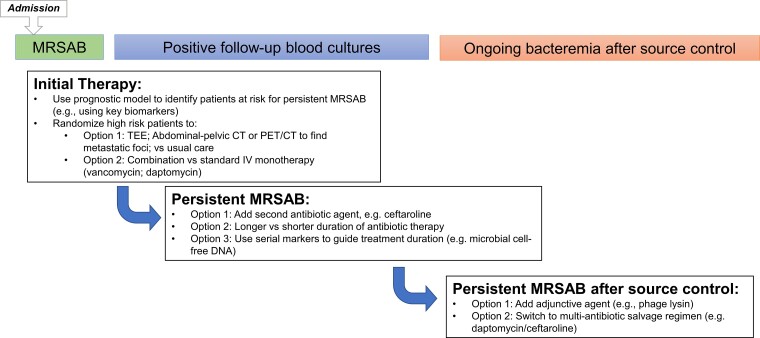
Questions around the most effective treatment strategies for persistent MRSA bacteremia should ideally be addressed by pragmatic randomized clinical trials. Potential points of intervention include (1) selection of initial diagnostic or therapeutic strategies, (2) time-dependent clearance of follow-up blood cultures, and (3) optimal interventions for persistent MRSA bacteremia (Figure 1).
Figure 1.
Potential pragmatic MRSA bacteremia trial designs. Abbreviations: CT, computed tomography; MRSAB, methicillin-resistant Staphylococcus aureus bacteremia; PET, positron emission tomography; TEE, transesophageal echocardiography.
Prognostic Biomarkers to Guide Clinical Trials in Persistent MRSA Bacteremia
A growing number of biological analytes have been identified as promising biomarker candidates to identify ultimate clinical outcomes in patients with MRSA bacteremia. In 1 recent prospective cohort, 8 serum proteins, including levels of the circulating cytokines, interleukin-17A and interleukin-10, as well as sE-selectin levels, discriminated between patients who went on to develop persistent SAB at 5 days from those who cleared their blood cultures by that timepoint [28]. In a subsequent cohort of > 200 patients with SAB, metabolomic and proteomic analyses of samples collected on the day of initial presentation were used to identify biomarkers predictive of mortality [29]. If ultimately validated in randomized trials, these biomarkers might one day identify patients (1) at highest risk of persistent MRSA bacteremia, (2) who are most likely to benefit from combination therapy, and 3) who require an initial intensive diagnostic evaluation for metastatic foci (eg, using positron emission tomography/computed tomography [PET/CT]).
Other assays might one day help to guide the duration of antibiotic therapy in patients with MRSA bacteremia. For example, next-generation sequencing can now identify and quantify the presence of DNA of infecting pathogens from the plasma of patients with a variety of infectious syndromes [30]. This microbial cell-free DNA (mcfDNA) can identify the causative pathogen for significantly longer durations than conventional blood cultures. In a recent series of patients with bacterial bloodstream infection, mcfDNA identified S aureus almost 2 weeks longer than conventional blood cultures (median 15 days vs. 2 days; P < .0001). Importantly, each additional day of detectable mcfDNA was associated with increased odds of metastatic infection (odds ratio, 2.89; 95% confidence interval, 1.53–5.46; P = .0011) [31]. Ultimately, such mcfDNA assays might be used to individualize duration of antibiotic therapy in patients with MRSA bacteremia by harmonizing the clearance of bacterial DNA from patients’ bloodstream to the discontinuation or deescalation of antibiotic therapy.
Stratification on Persistent MRSA Bacteremia in Clinical Trials
The occurrence of persistent MRSA bacteremia in patients enrolled in randomized trials represents an appropriate timepoint to assess the value of receipt of an adjunctive therapy as compared with standard monotherapy. For example, the potential role of adjunctive treatment was evaluated in a recent phase 2, double-blind randomized, control trial (RCT) featuring the addition of Exebacase, a bacteriophage-derived lysin versus placebo to standard anti-staphylococcal treatment in patients with complicated SAB or endocarditis [32]. Patients in the subgroup of MRSA bacteremia who received Exebacase were more likely to be clinically improved at day 14 than those receiving standard of care alone (74.1% vs. 31.3%; difference 42.8; 90% confidence interval, 14.3–71.4; P = .01). If this finding is confirmed in an ongoing phase 3 trial, Exebacase might represent an important future adjunctive therapy for patients with persistent MRSA bacteremia. Other adjunctive antibiotics, such as ceftaroline, would also ideally be studied in similar pragmatic RCTs. Bacteriophage treatments have demonstrated promise in preliminary studies [33, 34], and their adjunctive use is another potential strategy to improve outcomes in persistent MRSA bacteremia.
Algorithm for the Definition and Management of Persistent MRSA Bacteremia
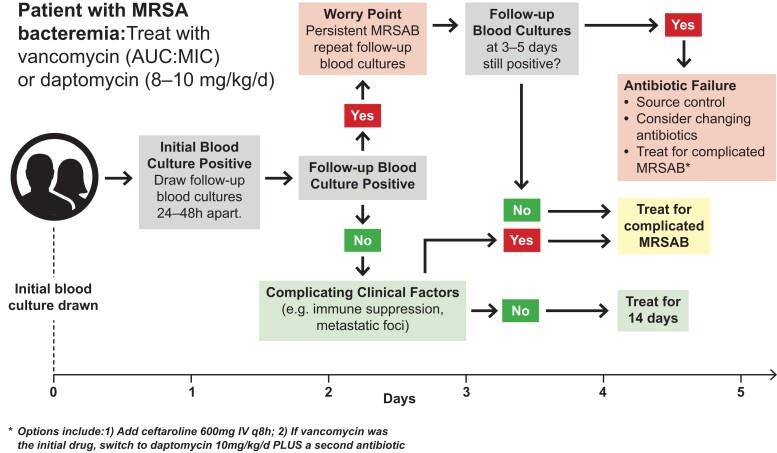
The intuitive appeal of initial combination therapy for MRSA bacteremia is offset by both the absence of high-quality evidence supporting its efficacy [19, 20, 35] and the documented risk of additional toxicity [8, 19, 20]. For this reason, we believe that current initial standard of care should remain monotherapy with either vancomycin (dosed by area under the curve:minimum inhibitory concentration ratios) [36, 37] or daptomycin (8–10 mg/kg/d), plus source control as appropriate (Figure 2). After selection of initial therapy, follow-up blood cultures should then inform the next diagnostic and therapeutic decisions.
Figure 2.
Suggested management algorithm for MRSA bacteremia. Abbreviations: AUC, area under the curve; MIC, minimum inhibitory concentration; MRSAB, methicillin-resistant Staphylococcus aureus bacteremia.
The First 24–48 Hours of Therapy: Documenting Persistent MRSA Bacteremia
Blood cultures should be drawn 24–48 hours after the initiation of appropriate antibiotic therapy. Obtaining follow-up blood cultures on separate days will enable detection of the skip phenomenon. If any are positive, these follow-up blood cultures define a worry point and designate the patient a priori as having (1) persistent MRSA bacteremia, (2) complicated MRSA bacteremia, and (3) a higher risk for poor outcomes. This event should trigger a thorough diagnostic workup (Table 2) to find and address metastatic sites of infection. The evidence base of appropriate diagnostic evaluations is strongest for transesophageal echocardiography, which can diagnose underlying endocarditis even in cases in which it is not clinically suspected. In addition, PET/CT is promising for evaluating periannular complications of endocarditis (especially in patients with prosthetic cardiac valves in place), as well as metastatic foci of endocarditis [49]. In many parts of the world, however, the use of PET/CT is limited by availability and cost. Persistent MRSA bacteremia, which is associated with high rates of metastatic foci of infection, is likely the most cost-effective group in which to obtain PET/CT. We propose that patients with persistent MRSA bacteremia should be evaluated with PET/CT if (1) there is diagnostic uncertainty about possible metastatic sites of infection, especially with prosthetic material in-place (eg, vascular graft), (2) there is a contraindication to transesophageal echocardiography, (3) there is ongoing bacteremia despite source control procedures for known sites of infection. Finally, an abdominal-pelvic CT scan may identify other occult foci, such as visceral abscesses, aortic or mycotic aneurysms, pseudoaneurysm (especially in sites of femoral vascular cannulation), or pelvic septic thrombophlebitis. After completion of the above diagnostic evaluation, clinicians should then select a treatment duration for complicated MRSA bacteremia.
Table 2.
Diagnostic Workup for Patients with Persistent MRSA Bacteremia
| Diagnostic Test | Recommended? | Evidence Base | Comments |
|---|---|---|---|
| TTE | For all patients with MRSA bacteremia | Endocarditis is common among patients with persistent MRSA bacteremia and affects prognosis and treatment [0–0] | Noninvasive and readily available |
| TEE | For patients with negative TTE, or in all patients with prosthetic valve in place. | Better sensitivity than TTE for detection of vegetations, particularly involving prosthetic valves [3–3] | Clinical prediction rules such as VIRSTA and PREDICT can help quantify endocarditis risk and need for TEE [43] |
| PET/CT | For:
|
PET/CT at 7–14 days after SAB diagnosis associated with lower mortality in observational cohorts [36, 44, 45] | May be limited by availability; costs; false positive results in the first several months after prosthetic device placement |
| CT or MRI spine | For patients with back pain or sciatica syndromes | S aureus is the most common cause of vertebral osteomyelitis and merits prolonged antibiotic therapy [46, 47] | |
| Ultrasound of vascular catheter sites | For patients suspected of septic thrombophlebitis (eg, indwelling vascular catheters) | Persistent bacteremia is a cardinal clue [48] | Readily available |
| Abdominal-pelvic CT | May identify occult intra-abdominal abscess (eg, renal, splenic) | Especially for patients with symptoms/signs referable to these anatomic sites | Readily available |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; MRSA, methicillin-resistant Staphylococcus aureus; PET/CT, Positron emission tomography/computed tomography; SAB, Staphylococcus aureus bacteremia; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography.
The Next 3–5 Days of Therapy: Defining Monotherapy Failure
We propose that ongoing bacteremia 3–5 days after initiation of appropriate therapy (with adequate source control) represents a reasonable timepoint to define an inadequate response to monotherapy, and to consider alternative therapeutic steps. These alternative approaches will usually involve adding a second antibiotic or switching to a different anti-MRSA drug. In patients with persistent MRSA bacteremia, switching from vancomycin to daptomycin monotherapy carries risk for treatment-emergent resistance to daptomycin [50, 51]. This is particularly true among S aureus isolates with intermediate susceptibility to vancomycin [52]. For this reason, we do not generally favor a monotherapy switch between vancomycin and daptomycin. Instead, a reasonable alternative in this scenario is the addition of ceftaroline (600 mg intravenously every 8 hours) to either vancomycin or high-dose daptomycin (8–10 mg/kg), with renal dose-adjustments as appropriate [53, 54]. Potential future options, currently under evaluation in clinical trials, include the following: ceftobiprole (a novel cephalosporin with anti-MRSA activity) [55]; anti-staphylococcal phage lysins as described previously [32, 56]; step-down therapy with oral anti-MRSA antibiotics such as linezolid [57]; or treatment with long half-life parenteral lipoglycopeptide agents, such as dalbavancin (NCT04775953).
CONCLUSIONS
MRSA bacteremia is associated with high mortality rates that have not changed appreciably over several decades. Duration of bacteremia is an important prognostic factor; patients with even 1 additional day of MRSA bacteremia on therapy are at higher risk of poor outcomes, compared with cohorts that rapidly clear MRSA bacteremia. In the current era, therefore, the worry point for persistent MRSA bacteremia should be moved earlier, and “the clock” for defining this syndrome and modifying therapy regimens appropriately turned back. Those patients with follow-up blood cultures positive after even 1–2 days of appropriate antibiotic therapy should be carefully evaluated for metastatic sites of infection, screened for treatment-emergent antibiotic resistance; receive aggressive source control, and be considered for antibiotic modifications as indicated. Although the clinical significance of the skip phenomenon is unknown, repeating blood cultures on separate and successive calendar days can help to ensure unambiguous clearance of bacteremia.
Pragmatic, investigator-initiated clinical trials offer the clinical community the most rigorous path forward toward answering important questions about the management of persistent MRSA bacteremia. These trial questions include (1) the optimal diagnostic evaluation, (2) choice of initial antibiotic therapy, (3) response to positive follow-up blood cultures, and (4) salvage treatment options for persistent MRSA bacteremia. Encouragingly, clinical trial platforms such as SNAP (SNAPtrial.com.au) are beginning to address these critical questions. Rigorously conducted RCTs that yield definitive answers will ultimately improve outcomes for our patients with MRSA bacteremia.
Contributor Information
Thomas L Holland, Department of Medicine, Duke University, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Arnold S Bayer, The Lundquist Institute for Biomedical Innovation at Harbor-UCLA, Torrance, California, USA; The Geffen School of Medicine at UCLA, Los Angeles, California, USA.
Vance G Fowler, Jr, Department of Medicine, Duke University, Durham, North Carolina, USA; Duke Clinical Research Institute, Durham, North Carolina, USA.
Notes
Financial support. V. G. F. was supported in part by National Institutes of Health (NIH) Grant 1R01AI165671. A. S. B. was supported in part by NIH grant 1RO1 AI146078.
References
- 1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clinical Microbiology Reviews 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Souli M, Ruffin F, Choi SH, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019; 69:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med 1991; 115:674–80. [DOI] [PubMed] [Google Scholar]
- 4. Choi SH, Dagher M, Ruffin F, et al. Risk factors for recurrent Staphylococcus aureus bacteremia. Clin Infect Dis 2021; 72:1891–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Markowitz N, Quinn EL, Saravolatz LD. Trimethoprim-sulfamethoxazole compared with vancomycin for the treatment of Staphylococcus aureus infection. Ann Intern Med 1992; 117:390–8. [DOI] [PubMed] [Google Scholar]
- 6. Fowler VG J, Sakoulas G, McIntyre LM, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–9. [DOI] [PubMed] [Google Scholar]
- 7. Drew RH, Perfect JR, Srinath L, et al. Treatment of methicillin-resistant Staphylococcus aureus infections with quinupristin-dalfopristin in patients intolerant of or failing prior therapy. J Antimicrob Chemother 2000; 46:775–84. [DOI] [PubMed] [Google Scholar]
- 8. Cosgrove SE, Vigliani GA, Fowler VG Jr., et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis 2009; 48:713–21. [DOI] [PubMed] [Google Scholar]
- 9. Hageman JC, Liedtke LA, Sunenshine RH, et al. Management of persistent bacteremia caused by methicillin-resistant Staphylococcus aureus: a survey of infectious diseases consultants. Clin Infect Dis 2006; 43:e42-5. [DOI] [PubMed] [Google Scholar]
- 10. Liu C, Strnad L, Beekmann SE, Polgreen PM, Chambers HF. Clinical practice variation among adult infectious disease physicians in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2019; 69:530–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Siegman-Igra Y, Reich P, Orni-Wasserlauf R, Schwartz D, Giladi M. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand J Infect Dis 2005; 37:572–8. [DOI] [PubMed] [Google Scholar]
- 12. Khatib R, Johnson LB, Fakih MG, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis 2006; 38:7–14. [DOI] [PubMed] [Google Scholar]
- 13. Minejima E, Bensman J, She RC, et al. A dysregulated balance of proinflammatory and anti-inflammatory host cytokine response early during therapy predicts persistence and mortality in Staphylococcus aureus bacteremia. Crit Care Med 2016; 44:671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neuner EA, Casabar E, Reichley R, McKinnon PS. Clinical, microbiologic, and genetic determinants of persistent methicillin-resistant Staphylococcus aureus bacteremia. Diagn Microbiol Infect Dis 2010; 67:228–33. [DOI] [PubMed] [Google Scholar]
- 15. Minejima E, Mai N, Bui N, et al. Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin Infect Dis 2020; 70:566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuehl R, Morata L, Boeing C, et al. Defining persistent Staphylococcus aureus bacteraemia: secondary analysis of a prospective cohort study. Lancet Infect Dis 2020; 20:1409–17. [DOI] [PubMed] [Google Scholar]
- 17. Stewart JD, Graham M, Kotsanas D, Woolley I, Korman TM. Intermittent negative blood cultures in Staphylococcus aureus bacteremia; a retrospective study of 1071 episodes. Open Forum Infect Dis 2019; 6:ofz494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fiala J, Palraj BR, Sohail MR, Lahr B, Baddour LM. Is a single set of negative blood cultures sufficient to ensure clearance of bloodstream infection in patients with Staphylococcus aureus bacteremia? The skip phenomenon. Infection 2019; 47:1047–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tong SYC, Lye DC, Yahav D, et al. Effect of vancomycin or daptomycin with vs without an antistaphylococcal beta-lactam on mortality, bacteremia, relapse, or treatment failure in patients with MRSA bacteremia: a randomized clinical trial. JAMA 2020; 323:527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thwaites GE, Scarborough M, Szubert A, et al. Adjunctive rifampicin for Staphylococcus aureus bacteraemia (ARREST): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391:668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pujol M, Miro JM, Shaw E, et al. Daptomycin plus fosfomycin versus daptomycin alone for methicillin-resistant Staphylococcus aureus bacteremia and endocarditis: a randomized clinical trial. Clin Infect Dis 2021; 72:1517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Davis JS, Sud A, O'Sullivan MVN, et al. Combination of vancomycin and beta-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis 2016; 62:173–80. [DOI] [PubMed] [Google Scholar]
- 23. Holland TL, Davis JS. Combination therapy for MRSA bacteremia: to β or not to β? Clin Infect Dis 2020; 71:11–3. [DOI] [PubMed] [Google Scholar]
- 24. Dilworth TJ, Sliwinski J, Ryan K, Dodd M, Mercier RC. Evaluation of vancomycin in combination with piperacillin-tazobactam or oxacillin against clinical methicillin-resistant Staphylococcus aureus Isolates and vancomycin-intermediate S. aureus isolates in vitro. Antimicrob Agents Chemother 2014; 58:1028–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casapao AM, Jacobs DM, Bowers DR, Beyda ND, Dilworth TJ, Group R-IS. Early administration of adjuvant beta-lactam therapy in combination with vancomycin among patients with methicillin-resistant Staphylococcus aureus bloodstream infection: a retrospective, multicenter analysis. Pharmacotherapy 2017; 37:1347–56. [DOI] [PubMed] [Google Scholar]
- 26. Zasowski EJ, Trinh TD, Atwan SM, et al. The impact of concomitant empiric cefepime on patient outcomes of methicillin-resistant Staphylococcus aureus bloodstream infections treated with vancomycin. Open Forum Infect Dis 2019; 6:ofz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rose W, Fantl M, Geriak M, Nizet V, Sakoulas G. Current paradigms of combination therapy in methicillin-resistant Staphylococcus aureus (MRSA) bacteremia: does it work, which combination, and for which patients? Clin Infect Dis 2021; 73:2353–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guimaraes AO, Cao Y, Hong K, et al. A prognostic model of persistent bacteremia and mortality in complicated Staphylococcus aureus bloodstream infection. Clin Infect Dis 2019; 68:1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wozniak JM, Mills RH, Olson J, et al. Mortality risk profiling of Staphylococcus aureus bacteremia by multi-omic serum analysis reveals early predictive and pathogenic signatures. Cell 2020; 182:1311–27 e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Blauwkamp TA, Thair S, Rosen MJ, et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat Microbiol 2019; 4:663–74. [DOI] [PubMed] [Google Scholar]
- 31. Eichenberger EM, de Vries CR, Ruffin F, et al. Microbial cell-free DNA identifies etiology of bloodstream infections, persists longer than conventional blood cultures, and its duration of detection is associated with metastatic infection in patients with Staphylococcus aureus and gram-negative bacteremia. Clin Infect Dis 2022; 74:2020–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fowler VG J, Das AF, Lipka-Diamond J, et al. Exebacase for patients with Staphylococcus aureus bloodstream infection and endocarditis. J Clin Invest 2020; 130:3750–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kebriaei R, Lev KL, Stamper KC, et al. Bacteriophage AB-SA01 cocktail in combination with antibiotics against MRSA-VISA strain in an in vitro pharmacokinetic/pharmactodynamic model. Antimicrob Agents Chemother 2020; 65:e01863-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fabijan AP, Lin RCY, Ho J, et al. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat Microbiol 2020; 5:465–72. [DOI] [PubMed] [Google Scholar]
- 35. Fowler VG J, Boucher HW, Corey GR, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med 2006; 355:653–65. [DOI] [PubMed] [Google Scholar]
- 36. Lodise TP, Rosenkranz SL, Finnemeyer M, et al. The emperor's new clothes: prospective observational evaluation of the association between initial vancomycin exposure and failure rates among adult hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infections (PROVIDE). Clin Infect Dis 2020; 70:1536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-system Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2020; 71:1361–4. [DOI] [PubMed] [Google Scholar]
- 38. Rasmussen RV, Host U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12:414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Showler A, Burry L, Bai AD, et al. Use of transthoracic echocardiography in the management of low-risk Staphylococcus aureus bacteremia: results from a retrospective multicenter cohort study. JACC Cardiovasc Imaging 2015; 8:924–31. [DOI] [PubMed] [Google Scholar]
- 40. Fowler VG Jr, Li J, Corey GR, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol 1997; 30:1072–8. [DOI] [PubMed] [Google Scholar]
- 41. Kaasch AJ, Fowler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Incani A, Hair C, Purnell P, et al. Staphylococcus aureus bacteraemia: evaluation of the role of transoesophageal echocardiography in identifying clinically unsuspected endocarditis. Eur J Clin Microbiol Infect Dis 2013; 32:1003–8. [DOI] [PubMed] [Google Scholar]
- 43. Peinado-Acevedo JS, Hurtado-Guerra JJ, Hincapie C, et al. Validation of VIRSTA and predicting risk of endocarditis using a clinical tool (PREDICT) scores to determine the priority of echocardiography in patients with Staphylococcus aureus bacteremia. Clin Infect Dis 2021; 73:e1151-e7. [DOI] [PubMed] [Google Scholar]
- 44. Ghanem-Zoubi N, Kagna O, Abu-Elhija J, et al. Integration of FDG-PET/CT in the diagnostic workup for Staphylococcus aureus bacteremia: a prospective interventional matched-cohort study. Clin Infect Dis 2021; 73:e3859–e66. [DOI] [PubMed] [Google Scholar]
- 45. Berrevoets MAH, Kouijzer IJE, Aarntzen E, et al. (18)F-FDG PET/CT optimizes treatment in Staphylococcus Aureus bacteremia and is associated with reduced mortality. J Nucl Med 2017; 58:1504–10. [DOI] [PubMed] [Google Scholar]
- 46. Bernard L, Dinh A, Ghout I, et al. Antibiotic treatment for 6 weeks versus 12 weeks in patients with pyogenic vertebral osteomyelitis: an open-label, non-inferiority, randomised, controlled trial. Lancet 2015; 385:875–82. [DOI] [PubMed] [Google Scholar]
- 47. Park KH, Cho OH, Lee JH, et al. Optimal duration of antibiotic therapy in patients with hematogenous vertebral osteomyelitis at low risk and high risk of recurrence. Clin Infect Dis 2016; 62:1262–9. [DOI] [PubMed] [Google Scholar]
- 48. Crowley AL, Peterson GE, Benjamin DK Jr, et al. Venous thrombosis in patients with short- and long-term central venous catheter-associated Staphylococcus aureus bacteremia. Crit Care Med 2008; 36:385–90. [DOI] [PubMed] [Google Scholar]
- 49. Duval X, Le Moing V, Tubiana S, et al. Impact of systematic whole-body 18F-fluorodeoxyglucose PET/CT on the management of patients suspected of infective endocarditis: the prospective multicenter TEPvENDO study. Clin Infect Dis 2021; 73:393–403. [DOI] [PubMed] [Google Scholar]
- 50. Sharma M, Riederer K, Chase P, Khatib R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2008; 27:433–7. [DOI] [PubMed] [Google Scholar]
- 51. Gasch O, Camoez M, Dominguez MA, et al. Emergence of resistance to daptomycin in a cohort of patients with methicillin-resistant Staphylococcus aureus persistent bacteraemia treated with daptomycin. J Antimicrob Chemother 2014; 69:568–71. [DOI] [PubMed] [Google Scholar]
- 52. Patel JB, Jevitt LA, Hageman J, McDonald LC, Tenover FC. An association between reduced susceptibility to daptomycin and reduced susceptibility to vancomycin in Staphylococcus aureus. Clin Infect Dis 2006; 42:1652–3. [DOI] [PubMed] [Google Scholar]
- 53. Zasowski EJ, Trinh TD, Claeys KC, et al. Multicenter observational study of ceftaroline fosamil for methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2017; 61:e02015–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Geriak M, Haddad F, Rizvi K, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2019; 63:e02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hamed K, Engelhardt M, Jones ME, et al. Ceftobiprole versus daptomycin in Staphylococcus aureus bacteremia: a novel protocol for a double-blind, phase III trial. Future Microbiol 2020; 15:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wire MB, Jun SY, Jang IJ, Lee SH, Hwang JG, Huang DB. A phase 1 study to evaluate the safety and pharmacokinetics following administration of single and multiple doses of the anti-staphylococcal lysin, LSVT-1701, in healthy adult subjects. Antimicrob Agents Chemother 2022:AAC0184221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dagher M, Fowler VG Jr, Wright PW, Staub MB. A narrative review of early oral stepdown therapy for the treatment of uncomplicated Staphylococcus aureus bacteremia: yay or nay? Open Forum Infect Dis 2020; 7:ofaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]