Abstract
Background
Medication adherence is known to challenge treatment of human immunodeficiency virus (HIV)/AIDS and multidrug-resistant tuberculosis (MDR-TB). We hypothesized that adherence using electronic dose monitoring (EDM) would identify an antiretroviral therapy (ART) adherence threshold for emergent ART resistance and predict treatment outcomes in patients with MDR-TB and HIV on ART and bedaquiline-containing TB regimens.
Methods
A prospective cohort of adults with MDR-TB and HIV on ART and initiating MDR-TB treatment with bedaquiline were enrolled at a public hospital in KwaZulu-Natal, South Africa (PRAXIS Study). Participants received separate EDM devices that measure adherence to bedaquiline and ART (nevirapine or lopinavir/ritonavir). Adherence was calculated cumulatively over 6 months. Participants were followed through completion of MDR-TB treatment. HIV genome sequencing was performed at baseline and 2 and 6 months on samples with HIV RNA ≥1000 copies/mL.
Results
From November 2016 through February 2018, 198 persons with MDR-TB and HIV were enrolled and followed (median, 17.2 months; interquartile range, 12.2–19.6). Eleven percent had baseline ART resistance mutations, and 7.5% developed emergent ART resistance at 6 months. ART adherence was independently associated with ART resistance and mortality. Modeling identified a significant (P < .001), linear association between ART adherence and emergent resistance, suggesting a strong association without a specific threshold.
Conclusions
Our findings highlight the need for ART resistance testing, especially in patients with MDR-TB and HIV, which is currently not the standard of care in resource-limited settings. Despite short follow-up duration, reduced ART adherence was significantly associated with emergent resistance and increased mortality.
Clinical Trials Registration
Keywords: antiretroviral adherence, electronic dose monitoring, South Africa, patients with HIV/AIDS and multidrug-resistant tuberculosis, emergent resistance
In this prospective study, reduced adherence to antiretroviral therapy was associated with emergent drug resistance and increased mortality in patients with human immunodeficiency virus and multidrug-resistant tuberculosis. Targeted interventions are needed to improve adherence and reduce resistance emergence and mortality.
Multidrug-resistant tuberculosis (MDR-TB) represents a growing global public health threat. In 2019, close to half a million people developed rifampicin-resistant TB, of whom 78% had MDR-TB [1]. The introduction of new antimycobacterial treatment regimens has revolutionized MDR-TB treatment by reducing the number of required antimycobacterial drugs, eliminating the need for injectable agents, shortening treatment duration, improving mortality, and substantially improving treatment outcomes [2]. The diarylquinoline antibiotic bedaquiline, the first new antimycobacterial agent approved for TB treatment in 40 years, is a key component of all new World Health Organization (WHO)–recommended regimens for MDR-TB [3].
Living with MDR-TB and human immunodeficiency virus (HIV) is common in geographic areas where both are prevalent, including South Africa where an estimated 58% of incident cases of TB were coinfected with HIV in 2019 [1]. Treatment integration of antiretroviral therapy (ART) and anti-TB medications may effectively reduce mortality in patients with HIV and TB, but this opportunity to improve outcomes is often missed or poorly implemented [4–8]. It has been shown that ART adherence is challenged by MDR-TB/HIV cotreatment with increased pill burden, drug interactions, need for medication switches, and increased incidence of adverse reactions [9].
Patient adherence to ART is a critical determinant of HIV treatment outcomes, and low ART adherence is an important risk factor for HIV virologic failure [10]. Multiple studies have shown the connection between ART medication interruption (measured by patient self-report, electronic dose monitoring, and other methods) and the development of ART resistance in patient populations around the world, including in sub-Saharan Africa [11–13].
However, there is an important knowledge gap regarding the impact of MDR-TB–HIV cotreatment on ART adherence and emergent ART resistance. We hypothesized that cellular-enabled electronic dose monitoring in an operational setting in South Africa would effectively predict treatment outcomes and identify an ART adherence threshold for emergent ART resistance in patients with MDR-TB and HIV being treated with ART and bedaquiline-containing TB regimens.
METHODS
Study Setting and Participants
This prospective, observational, cohort study enrolled patients in a public hospital and associated MDR-TB clinics in KwaZulu-Natal, South Africa, from November 2016 through February 2018. Participants were adults (aged ≥18 years) with MDR-TB and HIV confirmed by conventional or molecular testing initiating bedaquiline-containing regimens. All enrolled participants were either already taking ART or started ART within 2 weeks of enrollment. Participants were enrolled with written informed consent in either isiZulu or English within 2 weeks of initiating bedaquiline. Each participant received 2 electronic dose-monitoring (EDM) devices (Wisepill RT2000) to measure medication adherence to bedaquiline and ART. Only ART adherence to nevirapine or lopinavir/ritonavir was measured with the EDM device due to logistical reasons (ie, limited EDM pill capacity). ART and TB treatment regimens were constructed per national guidelines and at the discretion of treating clinicians. ART regimens included either nevirapine or lopinavir/ritonavir, each accompanied by lamivudine and tenofovir, based on WHO recommendations due to concern for drug–drug interactions with bedaquiline. Integrase strand transfer inhibitors (INSTIs) were not available in the South African public health system during the study period.
Hospital- and clinic-based physicians, nurses, and social workers, with additional support and training from study staff, provided adherence counseling to participants. Adherence support for MDR-TB medications at home and in the community was provided by directly observed treatment supporters, per South African provincial health guidelines, and through study participation with monthly adherence-support counseling at clinic visits. Additional adherence support for ART was provided through monthly clinic-based counseling delivered routinely. Treatment support was assessed monthly using a questionnaire for the first 6 months and at the end of bedaquiline treatment.
Patients were followed monthly with study visits through 6 months during which clinical evaluations were performed. Sputum for TB culture was collected at baseline and monthly through 6 months, and blood samples to quantify HIV RNA (HIV viral load) were collected at baseline and 2 and 6 months after study enrollment. Subsequently, participants were followed with monthly clinic visits in the public health system and telephonically by study staff until the end of MDR-TB treatment (full protocol available in the Supplementary Material). Adherence data were captured daily using EDM, through monthly questionnaires about pill-taking practices, and pharmacy records. If patients did not have a 6-month blood sample (due to death, loss to follow-up, or other reason), HIV viral load was measured using the blood sample drawn at their month-2 study visit. If HIV viral loads were ≥1000 copies/mL, genotypic ART resistance testing was subsequently performed. ART resistance testing was not done in real time, so genotyping results were not available to guide clinical ART management.
Laboratory Methods
Plasma samples with viral loads ≥1000 copies/mL were retrieved from the −80°C biorepository and thawed to room temperature prior to viral RNA extraction. Each plasma sample was centrifuged to pellet the virus, as previously described [14]. Viral RNA was extracted from pelleted plasma using a Chemagic 360 (PerkinElmer, Waltham, MA) automated extraction system, and protease and reverse transcriptase genes were amplified using custom primers [15]. Successfully amplified polymerase chain reaction (PCR) products were purified using a QIAquick PCR purification kit (Qiagen, Hilden, Germany).
In preparation for capillary electrophoresis, sequencing reactions were performed using a BigDye Terminator v3.1 kit (Applied Biosystems, Foster City, CA), and sequencing reaction purifications was performed using a BigDye XTerminator v3.1 purification kit (Applied Biosystems). Capillary electrophoresis was performed on an ABI 3730 genetic analyzer, and the quality of sequences was assessed using Geneious software v8.1.9 (Biomatters Ltd, New Zealand) [16].
The Stanford University HIV Drug Resistance Database (version 8.6) algorithm was used to characterize phenotypic resistance profiles from genotypic results [17]. The algorithm scans each sequence for the presence of drug-resistant mutations characterized in the International Antiviral Society-United States of America (IAS-USA) listing [18] and assigns a final Stanford penalty score. The final degree of resistance was defined as “high” if the computed score was ≥60, “intermediate” if 30–59, or “low” if 15–29.
Clinical Methods
Adherence to ART and bedaquiline was measured using the EDM device with Wisepill RT2000, with TB and ART medications each loaded in a separate device. Opening of the device activated a cellular signal that was monitored centrally with a cellular phone or laptop. Daily dose windows for bedaquiline and ART were constructed to allow for flexibility around dose timing. Using a prespecified algorithm and scripted call, study staff contacted the participants by telephone if excess missed doses, defined by prespecified criteria, were detected by EDM (see the protocol in the Supplementary Material for details). The study calls confirmed that the device had not failed, that it was not being stored in such a way that it could not transmit cellular signal, and that there was no limitation due to the battery charge. Cumulative adherence was calculated as observed doses within the dose windows divided by expected doses through 6 months, censored for death or loss to follow-up at the time of the event. At 6 months post-bedaquiline initiation, both EDM devices were retrieved from all participants since the standard duration of bedaquiline treatment was 6 months. Adherence was also assessed by monthly questionnaires based on patient recall; pharmacy records were obtained as available.
Outcomes
The primary study outcome was emergent HIV antiretroviral resistance. This was defined as the presence of new antiretroviral resistance (Stanford penalty score ≥15) detected at 6 months following study initiation that was not present at baseline. Resistance-conferring mutations to any antiretroviral were included. However, patients with existing resistance at baseline were classified as having emergent resistance only when it occurred in a different antiretroviral class (nonnucleoside reverse transcriptase inhibitor [NNRTI] vs protease inhibitor [PI]). Secondary outcomes included all-cause mortality, ART adherence measures, and HIV virologic response [19].
Statistical Methods
Univariate and multivariable analyses were performed using logistic regression and Cox proportional hazards modeling. Factors known to be associated with treatment failure were selected for univariate analysis. For inclusion in the multivariable model, we included univariate factors with a significant association with the outcome (P value < .05) or an effect size modification >10% (ie, odds ratio >1.1 or hazards ratio <0.9). We also included conventionally important factors in the multivariable analysis that did not meet these criteria (eg, age and gender). Linearity of association of ART adherence to emergence of resistance was tested with a generalized additive model as well as cubic splines. We considered ART adherence in multiple ways including as a continuous variable, with decile and quartile cutoffs, and using >95% adherence based on historical HIV adherence trials [20–22]. Statistical analyses were performed using IBM SPSS Statistics (version 27.1.00) and SAS (version 9.4). Heat maps to illustrate emergent ART resistance patterns were created using R (version 4.0.5).
RESULTS
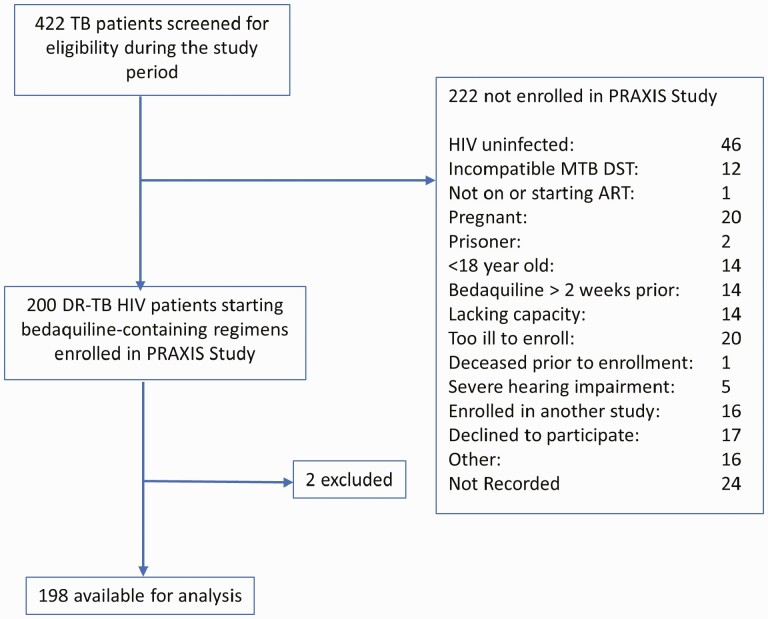
From November 2016 through February 2018, 422 patients were evaluated for inclusion (Figure 1). A total of 198 MDR-TB–HIV participants were enrolled and followed to the end of treatment, defined by standard MDR-TB treatment guidelines (median follow-up time, 17.2 months; interquartile range [IQR], 12.2–19.6). Consistent with other studies of drug-resistant TB and HIV in South Africa, participants were predominantly young and the majority were female (Table 1). Ninety-three percent (n = 183) of enrolled participants were on ART at the time of enrollment, with 63% (n = 124) on ART for more than 6 months at time of enrollment. Seven percent (n = 15) of participants were started on ART at the time of enrollment.
Figure 1.
Enrollment flowchart showing number of patients screened, enrolled, and included in the analysis. *One patient died 11 days after enrollment and had not initiated treatment; 1 patient was enrolled but subsequently was found to be ineligible. Abbreviations: ART, antiretroviral therapy; DR, drug resistant; DST, Drug sensitive; HIV, human immunodeficiency virus; MTB, Mycobacterium Tuberculosis; TB, tuberculosis.
Table 1.
Baseline Characteristics of Patients With Drug-Resistant Tuberculosis and Human Immunodeficiency Virus on Bedaquiline and Antiretroviral Therapy (N = 198)
| Characteristic | N (%) |
|---|---|
| Gender | |
| Male | 85 (42.9) |
| Female | 113 (57.1) |
| Age, years | |
| Median (IQR) | 35 (29–43) |
| Education | |
| No schooling | 6 (3.0) |
| Attended primary | 29 (14.7) |
| Attended secondary | 148 (74.8) |
| Attended college or university | 15 (7.6) |
| TB drug-susceptibility pattern | |
| MDR-TB | 98 (49.5) |
| Pre-XDR TB | 58 (29.3) |
| XDR-TB | 42 (21.2) |
| Baseline HIV | |
| Undetectable HIV viral load (%)a | 123 (62.1) |
| CD4 T-cell count, median (IQR)b | 238 (105–451) |
| ART | |
| Nevirapine | 147 (74.2) |
| Lopinavir/ritonavir | 36 (18.8) |
| No baseline ART | 15 (7.6) |
| ART for >6 months at enrollment | 124 (62.6) |
| Baseline body mass index, kg/m2 | |
| Median (IQR) | 20.5 (18.3–23.8) |
| Previous TB history | |
| None reported | 33 (16.6) |
| Drug-susceptible TB | 125 (62.8) |
| MDR/XDR TB | 41 (20.6) |
| Number of TB medicines in regimen | |
| Median medicines (IQR) | 7 (7–8) |
| Directly observed treatment supporterc,d | 137 (70.3) |
| History of imprisonment | |
| Yes | 22 (11.1) |
| History of substance use | |
| Alcohol | 90 (45.7) |
| Drugs | 17 (8.6) |
| Cigarettes | 53 (26.8) |
| None | 83 (41.9) |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug resistant; pre-XDR, pre-extensively drug resistant; TB, tuberculosis; XDR, extensively drug resistant.
Undetectable HIV viral load is defined as HIV RNA <40 copies/mL.
N = 181.
Cumulative number of participants reporting use of directly observed treatment supporter over first 6 months of treatment.
N = 195.
At the time of enrollment (baseline), 197 of 198 (99.5 %) had HIV viral load testing, and of these, 42 of 197 (21.3%) had HIV viral loads ≥1000 copies/mL, including the 15 participants not on ART at baseline. There were175 of 198 initial participants who had HIV viral load testing at 6 months, and 23 of 198 did not (22 died prior to 6 months, 1 did not leave an available sample). Two-month samples were retrieved for 17 of 23 participants without a 6-month follow-up sample (5 died prior to month 2, 1 did not leave an available sample). A total of 192 participants had follow-up HIV viral load testing, and 34 of 192 (17.7%) had HIV viral loads ≥1000 copies/mL.
Overall, cumulative median ART adherence measured by EDM through 6 months was 89.0% (IQR, 66.3–96.2). There was a significant difference between the median ART adherence of participants on first-line NNRTI-based regimens (89.8%; IQR, 69.6–96.2) compared with second-line PI-based regimens (68.6%; IQR, 47.7–95.5; P = .023). ART adherence was highly associated with undetectable HIV viral load at 6 months, even when adjusted for age, gender, TB type, and education (P < .001; Supplementary Table 1). Participants’ ART adherence was significantly lower than bedaquiline adherence (median 89.0% vs 97.0%, P < .001) [23].
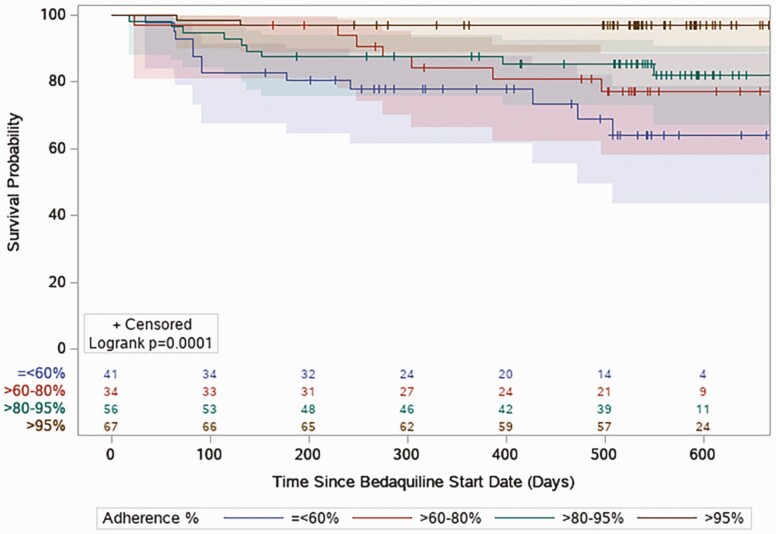
By Cox proportional hazards modeling, ART adherence was significantly protective for mortality with ART adherence considered a continuous variable (for each percent change in adherence, adjusted hazard ratio [aHR], 0.98; 95% confidence interval [CI], .970–.997; P = .017). Baseline ART resistance was significantly associated with mortality after adjusting for potential confounders (aHR, 3.15; 95% CI: 1.32–7.57; P = .01; Supplementary Table 2). Furthermore, there was statistically longer survival time in those with higher levels of ART adherence observed by Kaplan-Meier analysis (P = .0001; Figure 2).
Figure 2.
Kaplan-Meier analysis comparing survival probabilities across 4 levels of antiretroviral adherence (≤60%, 60%–80%, 80%–95%, >95%) by log-rank test.
There were 22 of 198 (11%) participants who had at least 1 antiretroviral resistance mutation at baseline. Nineteen of the 22 (86%) participants with baseline resistance had been on ART, 3 of 19 (14%) were ART naive. All (22 of 22) had high-level resistance to nevirapine (Supplementary Figure 1). On multivariable logistic regression, older age and CD4 T-cell count >200 were independently associated with decreased odds of the presence of a baseline ART resistance conferring mutation (Table 2).
Table 2.
Logistic Regression Multivariable Model Identifying Risk Factors for Baseline Antiretroviral Therapy Resistance
| Variable | Univariate OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|
| Agea | 0.92 (0.86–0.97) | .005 | 0.99 (0.93–1.07) | .97 |
| Male gender | 1.12 (0.46–2.74) | .80 | 2.15 (0.63–7.28) | .22 |
| Tuberculosis type | ||||
| Multidrug resistant | Ref | - | ||
| Pre-extensively drug resistant | 1.02 (0.35–2.96) | .98 | 1.37 (0.39–4.84) | .62 |
| Extensively drug resistant | 1.47 (0.50–4.34) | .49 | 0.71 (0.13–3.81) | .69 |
| CD4 T-cell count ≤200 cells/mL |
3.99 (1.41–11.28) | .00 | 3.82 (0.98–14.87) | .053 |
| On antiretroviral therapy <6 months | 1.18 (0.48–2.92) | .72 | 2.63 (0.80–8.67) | .11 |
| Substance use+ | 3.16 (1.02–9.76) | .046 | 0.81 (0.09–7.40) | .85 |
| History of opportunistic infections | 1.63 (0.59–4.47) | .35 | 0.48 (0.10–2.41) | .37 |
| Body mass index | 0.86 (0.75–0.98) | .024 | 0.94 (0.80–1.09) | .39 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Per year of increased age.
Substance use is defined as self-reported current use of psychoactive drugs, including alcohol, at baseline.
Fifteen of 198 (7.5%) participants developed emergent antiretroviral resistance at 2 or 6 months. Median cumulative adherence in participants with emergent antiretroviral resistance was 53.4% (IQR, 28.6–79.6), while the median adherence in those without emergent resistance was 90.0% (IQR, 68.6–96.2; Supplementary Table 3). On multivariable logistic regression, decreased ART adherence and CD4 T-cell count <200 were independently associated with emergent ART resistance conferring mutation. When considered as a continuous variable, ART adherence was an independent risk factor for ART resistance emergence, with a 5% reduced odds of emergent ART resistance per unit of increased adherence (OR, 0.95; 95% CI: .93–.98; Table 3). The most frequently occurring resistance-conferring mutations were M184V/I and K103N/S, which confer high-level resistance to lamivudine/emtricitabine and nevirapine/efavirenz, respectively (Supplementary Table 4). Two maps were created to illustrate phenotypic resistance that emerged to both NNRTIs and nucleoside reverse-transcriptase inhibitors (NRTIs) during the study period (Supplementary Figure 2A, 2B).
Table 3.
Logistic Regression Multivariable Model Identifying Risk Factors for Emergent Antiretroviral Therapy Resistance at 6 Months
| Variable | Univariate OR (95% CI) | P Value | Multivariable OR (95% CI) | P Value |
|---|---|---|---|---|
| Agea | 0.99 (0.93–1.05) | .66 | 0.97 (0.90–1.05) | .45 |
| Male gender | 2.11 (0.72–6.18) | .17 | 3.89 (0.84–18.07) | .083 |
| CD4 T-cell count ≤200 cells/mL |
4.51 (1.23–16.52) | .022 | 5.21 (1.13–24.01) | .034 |
| On ART <6 months | 2.72 (0.93–7.99) | .068 | 3.08 (0.85 –11.21) | .088 |
| ART adherenceb (%) | 0.96 (0.94-0.98) | .0002 | 0.95 (0.93–0.98) | .0002 |
| Substance usec | 0.62 (0.08–4.95) | .65 | 0.72 (0.07–7.37) | .78 |
| History of opportunistic infections | 0.61 (0.13–2.81) | .52 | 0.38 (0.07–2.22) | .28 |
| Body mass index | 0.90 (0.79–1.04) | .15 | 0.97 (0.82–1.15) | .76 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Per year of increased age.
Per percent of increased adherence, cumulative adherence measured by electronic dose measuring through 6 months.
Substance use is defined as self-reported current use of psychoactive drugs, including alcohol, at baseline.
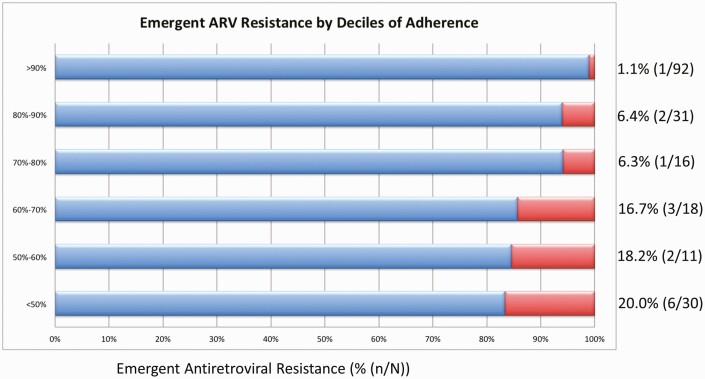
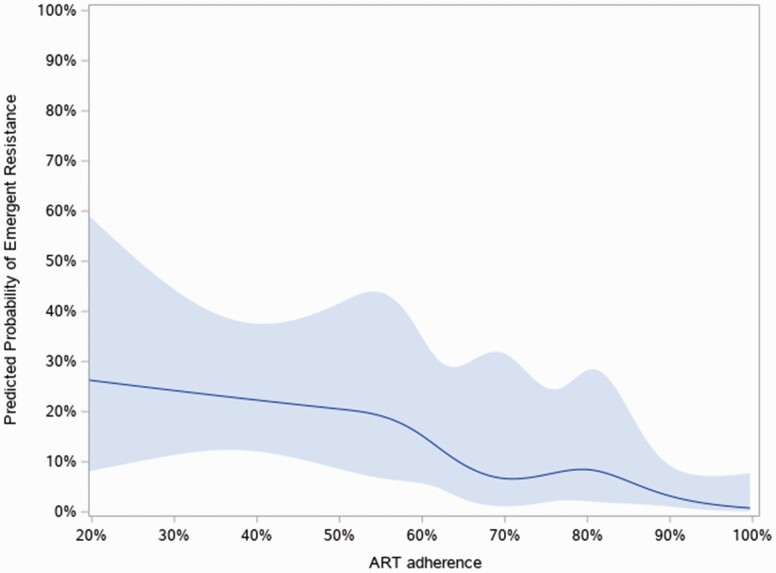
The proportion of participants with emergent resistance increased with each decreasing decile of adherence as follows: 1.1% of participants with greater than 90% adherence developed emergent ART resistance, 6.4% in those with between 80% and 90% adherence, 6.3% in those with between 70% and 80% adherence, 16.7% in those with between 60% and 70% adherence, 18.2% in those with between 50% and 60% adherence, and 20% in those with less than 50% adherence developed emergent ART resistance (Figure 3). Using a generalized additive model and a cubic spline model adjusted for multiple variables, we found a strong association between adherence and resistance emergence (P < .0001), even after adjustment for potential confounding factors. However, the relationship was linear (P = .20 for nonlinearity) and therefore there was no “natural” ART adherence threshold seen in our data (Figure 4).
Figure 3.
Bar graph showing the percentage of participants who developed resistance emergence at different deciles* of adherence. *Less than 50% adherence was used as a cutoff because of the small number of participants in deciles below 50%.
Figure 4.
Likelihood of emergent resistance at 6 months by percent adherence.* Effect plot of continuous association of percent treatment adherence with emergent resistance measured at 6 months using a logistic model with cubic splines indicating predicted probability (solid line) and the 95% confidence interval (shaded area). The P for linearity = .20** and P for association <.0001. **A P for linearity suggests the association is linear. Abbreviations: ART, antiretroviral therapy.
DISCUSSION
In this prospective, observational, cohort study, we found that reduced ART adherence measured using an EDM device was independently associated with emergent ART resistance, HIV treatment failure, and mortality. Eleven percent of study participants had baseline antiretroviral resistance (93% of participants were on ART at enrollment, 7% were not on ART), and an additional 7.5% developed emergent resistance during early treatment. We did not identify an ART adherence threshold cut-point, which suggests that increased medication adherence is associated with reduced emergence of ART-resistant HIV through the range of measured ART adherence.
Compared with surveys of antiretroviral resistance in pretreatment patients living with HIV in South Africa, our cohort had a higher prevalence of baseline ART resistance when adjusted to comparable denominators, reflecting our participants’ ART treatment experience [24, 25]. Similar to our findings, the most frequent mutations detected in these surveys conferred high-level resistance to the NNRTIs (eg, K103N). Similar to incidence data reported in high-burden settings [26], emergent ART resistance was seen in 7.5% of our participants during treatment. Of note, emergent resistance in our study was conservatively defined as new resistance to a new class of ART during treatment, so a patient with baseline NNRTI resistance would only be considered to have emergent ART resistance if they developed emergent mutations conferring resistance to NRTI or PI during treatment.
The increasing prevalence of NNRTI resistance in high-burden HIV settings [27], along with the advantageous safety, potency, and cost-effectiveness characteristics of INSTIs [28], has prompted the WHO to recommend INSTI-based ART as a preferred first-line regimen [29], including for patients with drug-resistant TB and HIV. However, a recent study suggested that NNRTI resistance prior to treatment may be associated with long-term failure of INSTI regimens, which could portend high rates of treatment failure in areas with high NNRTI resistance, creating an urgency to improve overall adherence to ART even with the availability of new regimens [30].
Strengths of our study include its prospective design, long follow-up interval, use of cellular-enabled EDM devices to enable minimally biased adherence measurement, and study of HIV-specific variables in a cohort with MDR-TB and HIV. There are also limitations to our study. One potential limitation is that we did not perform genotyping in patients with HIV viral load <1000 copies/mL. In these patients, we assumed that there was no ART resistance. However, one South African study of 125 participants found that 79% of those with low-level viremia (defined as 5–999 copies/mL) had resistance mutations [31], indicating that ART resistance may have been underestimated in our study. Another limitation is that use of EDM pillbox openings as a surrogate for medication adherence could overcount adherence. However, this would presumably bias the effect of adherence on emergent resistance, HIV viral response, and mortality toward the null hypothesis. The intense burden of HIV disease on communities and health systems in KwaZulu-Natal, South Africa, may limit the generalizability of our findings. A second limit to generalizability is that INSTI-based regimens (which were not included in our study) are now recommended as first-line ART, including in the setting of living with both MDR-TB and HIV. However, many features of the TB-HIV epidemic in KwaZulu-Natal are common to other southern African regions, and our findings may be broadly applicable in these settings. Further, although INSTI-based regimens are considered first-line ART, NNRTI-based ART regimens are still frequently prescribed in clinical settings in sub-Saharan Africa.
In conclusion, we found that poor adherence to ART is significantly associated with emergent ART resistance, HIV treatment failure, and mortality in patients with MDR-TB and HIV. Management of these patients with complex HIV and TB drug-resistance patterns in a setting with limited alternate therapeutic options is challenging. These data highlight the need for close monitoring and early intervention to support adherence for ART as well as TB medications [32], especially in patients receiving complex treatment and dosing. As first-line ART moves to INSTI-based regimens throughout the world, these findings highlight the urgency of targeted interventions to improve adherence and prevent emergence of ART resistance.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. M. R. O. and N. P. are supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID; R01AI124413). M. R. O. is supported by the National Center for Advancing Translational Sciences (UL1TR001873). J. C. M. B. is supported by NIH/NIAID (R01AI145679) and Einstein-Rockefeller-City University of New York Center for AIDS Research (CUNY CFAR) (P30AI124414). A. W. reports support for the present work from the NIH. A.W. receives support from the same NIH/NIAID grant (R01AI24413) that M.R.O and N.P. do. She does not have her own separate grant.
Contributor Information
Mark Bateman, Department of Medicine, Columbia University Irving Medical Center, New York, New York, USA.
Allison Wolf, Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Irving Medical Center, New York, New York, USA.
Benjamin Chimukangara, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, Maryland, USA; Centre for the AIDS Programme of Research in South Africa Medical Reseach Council - Human Immunodeficiency Virus - Tuberculosis Pathogenesis and Treatment Research Unit, Durban, South Africa.
James C M Brust, Department of Medicine, Albert Einstein College of Medicine, New York, New York, USA.
Richard Lessells, KwaZulu-Natal Research Innovation and Sequencing Platform, School of Laboratory Medicine & Medical Sciences, University of KwaZulu-Natal, Durban, South Africa.
Rivet Amico, Department of Health Behavior & Health Education, School of Public Health, University of Michigan, Ann Arbor, Michigan, USA.
Resha Boodhram, Centre for the AIDS Programme of Research in South Africa Medical Reseach Council - Human Immunodeficiency Virus - Tuberculosis Pathogenesis and Treatment Research Unit, Durban, South Africa.
Nalini Singh, King Dinuzulu Hospital Complex, Durban, South Africa.
Catherine Orrell, Desmond Tutu Health Foundation, Cape Town, South Africa.
Gerald Friedland, Yale University School of Medicine, New Haven, Connecticut, USA.
Kogieleum Naidoo, Centre for the AIDS Programme of Research in South Africa Medical Reseach Council - Human Immunodeficiency Virus - Tuberculosis Pathogenesis and Treatment Research Unit, Durban, South Africa.
Nesri Padayatchi, Centre for the AIDS Programme of Research in South Africa Medical Reseach Council - Human Immunodeficiency Virus - Tuberculosis Pathogenesis and Treatment Research Unit, Durban, South Africa.
Max R O’Donnell, Division of Pulmonary, Allergy, and Critical Care Medicine, Columbia University Irving Medical Center, New York, New York, USA; Centre for the AIDS Programme of Research in South Africa Medical Reseach Council - Human Immunodeficiency Virus - Tuberculosis Pathogenesis and Treatment Research Unit, Durban, South Africa; Department of Epidemiology, Mailman School of Public Health, Columbia University Irving Medical Center, New York, New York, USA.
References
- 1. World Health Organization. Global tuberculosis report 2020. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 17 February 2022.
- 2. WHO consolidated guidelines on tuberculosis: Module 4: Treatment—Drug-resistant tuberculosis treatment. WHO Guidelines Approved by the Guidelines Review Committee. Geneva, 2020.. Available at: https://www.who.int/publications/i/item/9789240007048. Accessed 9 February 2022. [PubMed] [Google Scholar]
- 3. World Health Organization. The use of bedaquiline in the treatment of multidrug-resistant tuberculosis: interim policy guidance. 2013. Available at: https://apps.who.int/iris/handle/10665/84879. Accessed 9 February 2022
- 4. Franke MF, Robins JM, Mugabo J, et al. . Effectiveness of early antiretroviral therapy initiation to improve survival among HIV-infected adults with tuberculosis: a retrospective cohort study. PLoS Med 2011; 8:e1001029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gadkowski LB, Hamilton CD, Allen M, et al. . HIV-specific health care utilization and mortality among tuberculosis/HIV coinfected persons. AIDS Patient Care STDS 2009; 23:845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Lettow M, Chan AK, Ginsburg AS, et al. . Timing and uptake of ART during treatment for active tuberculosis in HIV co-infected adults in Malawi. Public Health Action 2011; 1:6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varma JK, Nateniyom S, Akksilp S, et al. . HIV care and treatment factors associated with improved survival during TB treatment in Thailand: an observational study. BMC Infect Dis 2009; 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Velasco M, Castilla V, Sanz J, et al. . Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr 2009; 50:148–52. [DOI] [PubMed] [Google Scholar]
- 9. Naidoo K, Rampersad S, Karim SA.. Improving survival with tuberculosis & HIV treatment integration: a mini-review. Indian J Med Res 2019; 150:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nachega JB, Marconi VC, van Zyl GU, et al. . HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 2011; 11:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE.. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003; 37:1112–8. [DOI] [PubMed] [Google Scholar]
- 12. Harrigan PR, Hogg RS, Dong WW, et al. . Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis 2005; 191:339–47. [DOI] [PubMed] [Google Scholar]
- 13. Oyugi JH, Byakika-Tusiime J, Ragland K, et al. . Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS 2007; 21:965–71. [DOI] [PubMed] [Google Scholar]
- 14. Chimukangara B, Giandhari J, Lessells R, et al. . Impact of pretreatment low-abundance HIV-1 drug-resistant variants on virological failure among HIV-1/TB-co-infected individuals. J Antimicrob Chemother 2020; 75:3319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Manasa J, Danaviah S, Pillay S, et al. . An affordable HIV-1 drug resistance monitoring method for resource limited settings. J Vis Exp 2014:51242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearse M, Moir R, Wilson A, et al. . Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012; 28:1647–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stanford University HIV Drug Resistance Database: Genotypic Resistance Interpretation Algorithm. HIVdb Program. Available at: http://sierra2.stanford.edu/sierra/servlet/JSierra. Accessed on 21 March 2021.
- 18. Wensing AM, Calvez V, Ceccherini-Silberstein F, et al. . 2019 update of the drug resistance mutations in HIV-1. Top Antivir Med 2019; 27:111–21. [PMC free article] [PubMed] [Google Scholar]
- 19. O’Connor JL, Gardner EM, Mannheimer SB, et al. . Factors associated with adherence amongst 5295 people receiving antiretroviral therapy as part of an international trial. J Infect Dis 2013; 208:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arnsten JH, Demas PA, Grant RW, et al. . Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med 2002; 17:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bangsberg DR, Hecht FM, Charlebois ED, et al. . Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS 2000; 14:357–66. [DOI] [PubMed] [Google Scholar]
- 22. Paterson DL, Swindells S, Mohr J, et al. . Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med 2000; 133:21–30. [DOI] [PubMed] [Google Scholar]
- 23. Zelnick JR, Daftary A, Hwang C, et al. . Electronic dose monitoring identifies a high-risk subpopulation in the treatment of drug-resistant tuberculosis and HIV. Clin Infect Dis 2020; 73:e1901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chimukangara B, Kharsany ABM, Lessells RJ, et al. . Moderate-to-high levels of pretreatment HIV drug resistance in KwaZulu-Natal Province, South Africa. AIDS Res Hum Retroviruses 2019; 35:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steegen K, Carmona S, Bronze M, et al. . Moderate levels of pre-treatment HIV-1 antiretroviral drug resistance detected in the first South African National Survey. PLoS One 2016; 11:e0166305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stadeli KM, Richman DD.. Rates of emergence of HIV drug resistance in resource-limited settings: a systematic review. Antivir Ther 2013; 18:115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta RK, Gregson J, Parkin N, et al. . HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: a systematic review and meta-regression analysis. Lancet Infect Dis 2018; 18:346–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Phillips AN, Venter F, Havlir D, et al. . Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV 2019; 6:e116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. World Health Organization. Guidelines on the public health response to pretreatment HIV drug resistance. Available at: https://www.who.int/publications/i/item/9789241550055. Accessed 10 February 2022
- 30. Siedner MJ, Moorhouse MA, Simmons B, et al. . Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun 2020; 11:5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bangalee A, Hans L, Steegen K.. Feasibility and clinical relevance of HIV-1 drug resistance testing in patients with low-level viraemia in South Africa. J Antimicrob Chemother 2021; 76:2659–65. [DOI] [PubMed] [Google Scholar]
- 32. Meresse M, March L, Kouanfack C, et al. . Patterns of adherence to antiretroviral therapy and HIV drug resistance over time in the Stratall ANRS 12110/ESTHER trial in Cameroon. HIV Med 2014; 15:478–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.