Abstract
The present study analyzes the effect of highly active antiretroviral therapy (HAART) on restoration of cellular immunity in human immunodeficiency virus (HIV)-infected children over a 24-week period following initiation of HAART with ritonavir, nevirapine, and stavudine. The immunological parameters evaluated at four time points (at enrollment and at 4, 12, and 24 weeks of therapy) included cytokine production by monocytes as well as T-cell proliferation in response to mitogen, alloantigen, and recall antigens including HIV type 1 envelope peptides. Circulating levels of interleukin-16 (IL-16) were measured, in addition to CD4+ T-cell counts, plasma HIV RNA levels, and the delayed-type hypersensitivity (DTH) response. At enrollment the children exhibited defects in several immune parameters measured. Therapy increased CD4+ T-cell counts and decreased viral loads significantly. By contrast, the only immunological parameter that was significantly increased was IL-12 p70 production by monocytes; the DTH response to Candida albicans also showed a strong increase in patients becoming positive. In conclusion, these results demonstrate that HAART in HIV-infected children affects the dynamics of HIV replication and the CD4+ T-cell count over 24 weeks, similar to the pattern seen in HIV-infected adults. Furthermore, these data indicate improvement in antigen-presenting cell immunological function in HIV-infected children induced by HAART.
It is well established that treatment of human immunodeficiency virus (HIV)-infected individuals with highly active antiretroviral therapy (HAART) decreases the viral load (20). While an increase in total CD4+ T lymphocytes is readily detectable, the ability to regenerate immunocompetent T-cell populations remains unresolved (15, 34). Some clinical trials have demonstrated that the regenerated T cells consist predominantly of the memory (CD45RO+) type (14), while others have shown repopulation of naïve (thymus-dependent, CD45RA+) T cells as well (2, 13). When there is substantial thymic function, as can be the case in children, thymus-dependent T-cell regeneration can repopulate the host with normal numbers of naïve CD4+ T cells (19). Therefore, HIV-infected children may provide a unique population with which to study the potential for the immune reconstitution that may occur in the setting of nearly complete viral suppression with HAART. Recently, Cohen Stuart and coworkers (10) have reported high rates of recovery of naïve, memory, and total CD4+ T cells in children younger than 3 years of age. However, little is known about the regeneration potential of the immune systems of older children (25).
Previous studies have demonstrated that the loss of T-helper (Th)-cell function (proliferation and cytokine production in response to recall or HIV antigen) in HIV-infected patients precedes the loss of peripheral CD4+ T cells (9, 23, 35). These results suggest that characterization of immunological parameters other than the measurement of CD4+ T-cell counts and viral loads should be evaluated in HIV-infected patients receiving HAART (29). Relatively few reports on the use of HAART have described the analysis of parameters of immune function, and even fewer of these have described the analysis of the same parameters in pediatric AIDS patients (6). In several studies, some restoration of T-cell proliferation in response to recall antigens has been observed in adult patients treated with HAART (2, 28, 33). Most of the studies that examined HIV type 1 (HIV-1) antigen-specific T-cell proliferation found no improvement in the responses (2, 28). However, one study showed that HAART administered to patients with acute HIV-1 infection but not to patients with chronic infection leads to a strong HIV-1-specific Th-cell response (30).
Therefore, we asked to what extent effective suppression of HIV replication would lead to regeneration of T-cell populations with immune reconstitution, normalization of cytokine profiles, and T-cell function in children infected with HIV-1. This longitudinal study examined T-cell and antigen-presenting cell (APC) function in 11 pediatric HIV-infected patients receiving HAART for 24 weeks. Cytokine production by monocytes and T-cell proliferation in response to mitogen, alloantigen, and recall antigen (including HIV antigens) were evaluated. Plasma interleukin-16 (IL-16) levels were also measured, in addition to CD4+ T-cell counts, viral load, and delayed-type hypersensitivity (DTH) response, since the levels of IL-16 in the serum of HIV-1-infected adults are reported to increase with HAART (3). We observed significant decreases in viral loads and increases in total CD4+ T-cell counts as a result of HAART, in addition to a significant increase in the level of in vitro production of IL-12 p70 by these children. This is the first report to demonstrate improvement in APC function during 24 weeks of HAART in HIV-infected children.
MATERIALS AND METHODS
Study group.
Eleven HIV-infected children between the ages of 7.4 and 18.4 years were entered into a protocol conducted by the HIV and AIDS Malignancy Branch of the National Cancer Institute to study the effects of a HAART regimen. Written informed consent was obtained from the parent or legal guardian of each child. All children were protease inhibitor, nevirapine, and stavudine naïve, although all were heavily pretreated with nucleoside reverse transcriptase inhibitors. Stavudine and ritonavir were begun in escalating doses over 5 days on day 1, and nevirapine was added on day 8. Heparinized peripheral blood samples were obtained at four different time points: at the baseline (0 weeks) and at 4, 12, and 24 weeks. Blood samples collected from 30 healthy HIV-seronegative donors at the Transfusion Medicine Department of the National Institutes of Health were used as controls.
T-cell proliferation.
Peripheral blood mononuclear cells (PBMCs) were separated on lymphocyte separation medium (Organon Teknika, Rockville, Md.) and resuspended at 3 × 106 cells/ml in RPMI 1640 culture medium containing 100 U of penicillin per ml, 100 μg of streptomycin per ml, 5 mM HEPES, and 2 mM glutamine (Gibco, Grand Island, N.Y.)
Cells were cultured in 96-well flat-bottom tissue culture plates (Costar, Cambridge, Mass.) in the medium described above supplemented with 5% human type AB serum (complete medium) at a final concentration of 1.5 × 106 cells/ml and were stimulated with phytohemagglutinin (PHA-M; dilution 1:80; Life Technologies, Grand Island, N.Y.), alloantigen (a pool of irradiated [5,000 Gy] PBMCs derived from three unrelated healthy blood bank donors; final concentration, 1 × 106 cells/ml), Candida albicans antigen (10 μg/ml; Greer Laboratories, Lenoir, N.C.), tetanus toxoid (dilution, 1:800; Connaught Laboratories, Pasadena, Calif.), and a pool of four HIV-1 envelope peptides (T1, Th4.1, P18IIIB, and P18MN; concentration, 5 μM each). The sequences and synthesis of these peptides are described in detail elsewhere (8). After 5 days, the cultures were pulsed overnight with [3H]thymidine (1 μCi/well), and the cells were harvested the next day. Results are expressed as stimulation indices (SIs; calculated as counts per minute of stimulated culture/counts per minute of unstimulated culture). An SI of ≥3.0 is considered a positive response.
IL-10, IL-12 p70, and TNF-α production.
PBMCs (1.5 × 106 cells/ml) were cultured in complete medium and either were unstimulated to determine spontaneous cytokine production or were stimulated with Staphylococcus aureus Cowan strain 1 (final dilution, 0.01%; Pansorbin; Calbiochem-Behring Corporation, La Jolla, Calif.). Supernatants were harvested after 24 h and were stored at −70°C. Cytokine production was measured by enzyme-linked immunosorbent assay (ELISA) (ELISAs for IL-10 and tumor necrosis factor alpha [TNF-α], Endogen, Woburn, Mass.; ELISA for IL-12 p70, Biosource International, Camarillo, Calif.). The detection limits were 20 pg/ml for IL-10 and TNF-α and 4 pg/ml for IL-12. All values less than the detection limit were assigned an arbitrary value of one-half the detection limit.
Circulating IL-16.
Plasma IL-16 was quantified by ELISA (Biosource International). The detection limit was 12 pg/ml.
Tests for DTH responses.
Tests for DTH responses to C. albicans antigen (1:100) and tetanus toxoid (1:5) were performed at week 0 and week 24. Skin tests were considered positive if the induration was >5 mm in diameter at 72 h following placement, regardless of erythema.
Viral load.
Plasma HIV RNA levels were determined in real time by a reverse transcriptase PCR assay (lower limit of detection, 200 copies/ml; Roche Amplicor; Roche Molecular Systems, Branchburg, N.J.).
Statistical considerations.
The data for the present study are derived from patients enrolled in a trial of HAART, which was designed to evaluate the safety, tolerability, and efficacy of the regimen in HIV-1-infected, protease inhibitor-naïve children. Although the trial was originally designed to enroll 25 patients in order to evaluate changes in naïve CD4+ T cells, 12 were enrolled, of whom 11 were fully evaluable for up to 24 weeks with respect to immune parameters. Statistical comparisons of the paired differences between immune parameters at the baseline and at later time points were done by the Wilcoxon signed rank test because of the lack of normality in the distributions of several of the parameters. The difference between the baseline value for the study patients and the control subjects with respect to both SIs and cytokine production was evaluated by the Wilcoxon rank sum test. Because five SIs and five different cytokine production parameters were evaluated, these comparisons between patients and controls were adjusted for the number of statistical tests performed by using the Bonferroni method in order to provide a more conservative interpretation of the statistical significance of these comparisons. The correlation between various parameters was assessed by the Spearman rank correlation method. Correlation coefficients are interpreted as follows: |r| > 0.7, strong correlation; 0.5 < |r| < 0.7, moderate correlation; 0.3 < |r| < 0.5, weak to moderate correlation; and |r| < 0.3, weak correlation. The P value for a correlation coefficient indicates the result of a test of whether the correlation is equal to 0. Comparisons of DTH evaluations at the baseline versus those at 24 weeks were performed by McNemar's test for paired categorical data. All P values are two tailed.
RESULTS
Baseline clinical parameters.
Eleven children, all over 7 years of age, were enrolled in the present study. Baseline clinical data for these children are shown in Table 1. In general, most of the patients were not highly immunosuppressed. Six of 11 patients were classified into Centers for Disease Control and Prevention (CDC) clinical category A (mildly symptomatic), and only 2 patients had CD4 T-cell counts below 200/μl. Median CD4 T-cell counts were 598/μl (range, 63 to 835/μl). The viral load was also relatively low in most patients, with 6 of 11 patients having a viral load of less than 4 log10 copies/ml (median, 3.94 log10 copies/ml; range, 2.50 to 5.40 log10 copies/ml).
TABLE 1.
Clinical data for HIV-1-infected children at enrollment
| Individual | Age (yr) | Route of acquisition of HIV infection | CDC stage | CD4+ T cell count/μl | Plasma HIV load (log10 copies/ml) |
|---|---|---|---|---|---|
| 1 | 18.4 | Blood products | A2 | 338 | 4.47 |
| 2 | 8.1 | Vertical | A3 | 140 | 3.80 |
| 3 | 12.5 | Blood products | B2 | 768 | 3.42 |
| 4 | 8.6 | Vertical | A2 | 835 | 4.69 |
| 5 | 14.0 | Blood products | B2 | 626 | 3.94 |
| 6 | 7.9 | Vertical | C2 | 648 | 5.31 |
| 7 | 12.6 | Vertical | B2 | 579 | 3.94 |
| 8 | 10.5 | Vertical | A2 | 396 | 3.68 |
| 9 | 11.1 | Vertical | C2 | 598 | 4.22 |
| 10 | 17.5 | Blood products | A3 | 63 | 5.40 |
| 11 | 7.4 | Vertical | A2 | 665 | 2.50 |
| Median | 11.1 | 598 | 3.94 | ||
| Range | 7.4–18.4 | 63–835 | 2.50–5.40 |
T-cell proliferation at baseline.
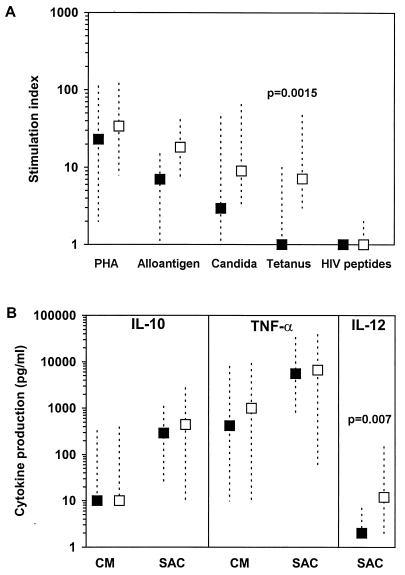
There was no statistically significant difference between the levels of PHA-stimulated proliferation of PBMCs from patients and those from HIV-1-seronegative control adults (P = 1.0 after adjustment for multiple comparisons) (Fig. 1A). It should be noted that in a recent study by our laboratory (6), comparable results were obtained for proliferative responses to mitogen and recall antigens between HIV-seronegative adults and HIV-seronegative children whose age span was similar to that of the children in the present study. Responses to alloantigen (median SI, 6.5; range, 1 to 15) and C. albicans (median SI, 3; range, 1 to 45) were somewhat reduced in HIV-infected children compared to those in controls (for HIV-infected children, median SI, 18; range, 7 to 41; for controls, median SI, 9; range, 3 to 65), but the reduction was not statistically significantly different (P = 0.11 and 0.13, respectively, after adjustment). Of nine patients tested for a response to tetanus toxoid, only one responded, with an SI of ≥3, to this recall antigen (median SI, 1; range, 1 to 10), while all of the control individuals had positive proliferative responses to tetanus toxoid (median SI, 7; range, 3 to 47) (P = 0.0015, after adjustment) (Fig. 1A). No response was detected after stimulation with HIV-1 envelope peptides in either HIV-infected or control individuals (all SIs, <3) (Fig. 1A). At the baseline, moderately strong positive correlations between T-cell proliferative responses to alloantigen and the CD4+ T-cell counts (r = 0.58; P = 0.23) and between T-cell proliferative responses to C. albicans and the CD4+ T-cell counts (r = 0.51; P = 0.11) were observed in these patients.
FIG. 1.
Baseline values of immune functions for HIV-infected children enrolled in the HAART protocol. (A) Proliferative responses (SIs) after stimulation of PBMCs with PHA, alloantigen, C. albicans, tetanus toxoid, and a pool of four HIV-1 synthetic envelope peptides (HIV peptides) (8). (B) Spontaneous (complete medium [CM]) or S. aureus Cowan (SAC)-stimulated cytokine production. The statistical significance of the difference between HIV-infected children (▪) and HIV-seronegative control donors (□) was determined by the Wilcoxon rank sum test, after adjustment for multiple comparisons. P values less than 0.05 are shown. The data shown are the medians and ranges.
Cytokine production at baseline.
The levels of spontaneous or S. aureus Cowan-stimulated production of IL-10 and TNF-α by PBMCs collected at the baseline from HIV-infected children were similar to the levels of production of these cytokines in control individuals (Fig. 1B). We were unable to detect spontaneous production of IL-12 p70 (the biologically active heterodimer) in the supernatants of PBMCs from either control or HIV-infected individuals cultured in complete medium only. S. aureus Cowan is known to induce IL-12 p70 production, although the quantities detected are relatively small (21). After stimulation with S. aureus Cowan, the level of production of IL-12 by PBMCs obtained from 11 HIV-infected children (median, 2 pg/ml; range, 2 to 7 pg/ml) was reduced significantly compared to the level of IL-12 production by PBMCs from HIV-seronegative individuals (median, 11.5 pg/ml, range, 2 to 149 pg/ml [P = 0.007, after adjustment]) (Fig. 1B).
HAART-associated changes in CD4+ T-cell count and viral load.
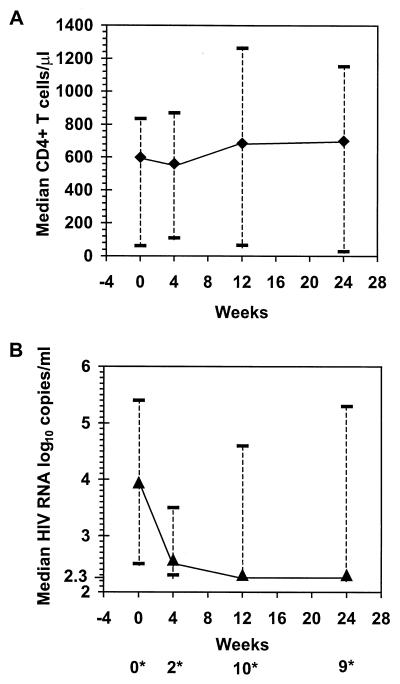
A significant increase in the peripheral CD4+ T-cell count at week 24 (median change, 231 cells/μl; range, −33 to 548 cells/μl [P = 0.0029, Wilcoxon signed rank test]) was accompanied by a substantial decrease in the HIV load (median change, −1.64 log10 copies/ml; range, −2.98 to −0.12 log10 copies/ml [P = 0.001]) (Fig. 2). Nine of the 11 children had undetectable viral loads (2.30 log10 copies/ml) at week 24.
FIG. 2.
HAART-associated changes in CD4+ T-cell counts (A) and plasma HIV RNA levels (B) for 11 HIV-infected children. ∗, number of patients with undetectable viral loads (2.30 log10 copies/ml) at 0, 4, 12, and 24 weeks. Data shown are the medians and ranges.
HAART-associated changes in immunological functions.
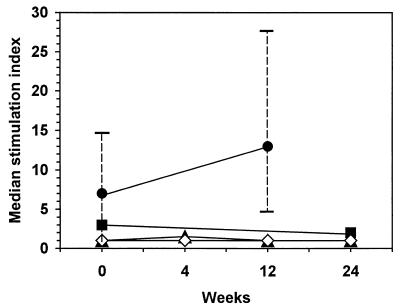
The HIV-infected children showed no significant change in the proliferative responses to PHA during therapy (data not shown). Proliferative responses to alloantigen were increased at week 12 in four of the six patients (median SI, 12.5; range, 5 to 28 at 12 weeks) compared to those at the baseline (median SI, 6.5; range, 1 to 15) (Fig. 3), but the change was not statistically significant (P = 0.84, after adjustment) due to the limited number of subjects to whom these data applied and the variation in the changes (median change, 8.5 pg/ml; range, −7 to 19 pg/ml). Data are not available for week 24 because of a lack of sufficient numbers of samples. T-cell responses to C. albicans, tetanus toxoid, or HIV-1 antigen also did not change over the 24 weeks of therapy (Fig. 3).
FIG. 3.
HAART-associated changes in T-cell proliferative response to alloantigen (●; n = 6), C. albicans (▪; n = 11), tetanus toxoid (▴; n = 9), and HIV peptides (⋄; n = 8) in HIV-infected children. Data shown are the medians and ranges.
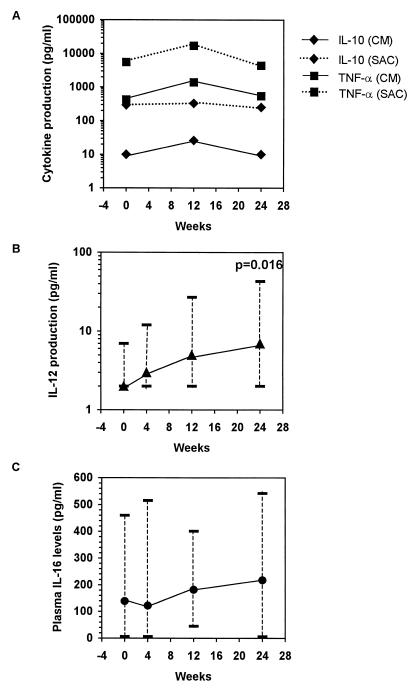
The levels of spontaneous and S. aureus Cowan-induced production of IL-10 and TNF-α (Fig. 4A) throughout therapy showed no significant changes compared to the baseline levels (for all comparisons, P > 0.05). However, at week 24 the level of S. aureus Cowan-stimulated IL-12 production increased significantly from that at the baseline (at the baseline, median, 2 pg/ml; range, 2 to 7 pg/ml; at week 24, median, 7 pg/ml; range, 2 to 43 pg/ml) (P = 0.016) (Fig. 4B), and at 24 weeks the level of S. aureus Cowan-stimulated IL-12 production was similar to that in HIV-seronegative control individuals (median, 11.5 pg/ml; range, 2 to 149 pg/ml) (P = 0.21, by Wilcoxon rank sum test). In fact, while only 2 of 11 (18%) HIV-infected children produced detectable IL-12 at enrollment (4 and 7 pg/ml), 7 of 11 (64%) produced detectable IL-12 (range, 6 to 43 pg/ml) at week 24 of therapy.
FIG. 4.
HAART-associated changes in spontaneous (complete medium [CM]) and S. aureus Cowan (SAC)-induced IL-10 and TNF-α production (A), S. aureus Cowan-induced IL-12 production (B), and plasma IL-16 levels (C) in 11 HIV-infected children. Results are expressed as the median concentration and range for panels B and C. The statistical significance of the paired difference versus the baseline (week 0) was determined by the Wilcoxon signed rank test.
Plasma IL-16 levels.
IL-16 is secreted by activated CD8+ T cells and has been shown to inhibit HIV replication in infected cells in vitro (36). It has also been demonstrated that serum IL-16 levels increase with HAART (3). Thus, the IL-16 levels in the plasma of infected children were measured at all four time points (Fig. 4C). Although initially decreasing by a median of 25 pg/ml, we found a sustained trend toward increases in the IL-16 levels in plasma after the first 4 weeks of therapy. However, none of the three measured changes were statistically significant (P > 0.20 for each comparison).
DTH responses.
None of the patients received tetanus toxoid immunization within the 1 month prior to or during the study. At the baseline most HIV-infected children failed to have DTH responses to C. albicans (response rate, 36.4%) or tetanus toxoid (response rate, 9.1%). This level of reactivity is much lower than that reported for HIV-seronegative children of similar ages and is in agreement with the reactivity reported for HIV-infected children (6). The unresponsiveness to tetanus toxoid in the children persisted through the entire treatment period (18.2% response rate at week 24). By contrast, the proportion of patients with a positive response to C. albicans increased to 81.8% at week 24. Five patients who had previously been negative experienced positive responses to C. albicans at week 24, while six retained their initial reactivity (P = 0.0625 by McNemar's test). There was also a trend toward a greater decrease in viral load at 24 weeks in patients with a positive test for C. albicans (median, −1.65 log10 copies/ml; range, −0.21 to −2.98 log10 copies/ml [n = 9]) compared to the load in those with a negative response (two values, −1.38 and −0.11 log10 copies/ml, respectively [P = 0.13]).
DISCUSSION
The objective of the present study was to assess whether treatment of HIV-infected children with HAART for a 6-month period would improve their immune function. Prior to treatment with HAART, the 11 children enrolled in the study exhibited polyclonal T-lymphocyte proliferative responses to mitogen comparable to the responses of HIV-seronegative controls. However, the proliferative responses to alloantigen and C. albicans were somewhat reduced, while tetanus toxoid- and HIV-1-specific responses were absent. In addition, at enrollment most children had negative DTH responses to C. albicans and tetanus toxoid.
The effect of 24 weeks of HAART on each of the immune parameters studied was examined. While therapy had a beneficial effect on CD4 T-cell counts and viral loads by significantly increasing the total CD4+ T-cell counts and decreasing the viral loads, in agreement with other reports (15, 20), T-cell proliferative responses to tetanus toxoid were not induced in vitro. We may not exclude the possibility that the ability to detect changes in proliferative responses were affected by the small number of patients studied. Most of the children enrolled in our study continued to have negative DTH responses to tetanus toxoid antigen, although there was increased DTH reactivity to C. albicans antigen at week 24. The improvement in the DTH response to C. albicans was moderately well associated with a decreased viral load, as demonstrated earlier (33).
Of note was the lack of induction of proliferative responses to the HIV-1 antigens in our patients, even after 24 weeks of HAART. While repopulation by naïve CD4+ T lymphocytes from the thymus can demonstrably lead to immunoreconstitution (10, 19), generation of memory and effector Th cells, which are essential in providing help to B cells and cytotoxic T lymphocytes, will not occur unless there is sufficient antigenic exposure (27). This was demonstrated in studies of the in vitro T-cell proliferative responses of HAART-treated adults, in whom the responses to antigens to which the immune system was constantly exposed were restored more frequently (C. albicans) than the responses to an antigen to which the level of exposure was low (tetanus toxoid) ( 22, 27, 33). Thus, HIV-1-specific Th cells and cytotoxic T lymphocytes are poorly stimulated when the viral load is effectively suppressed by HAART (11, 16, 18, 27, 28). However, development of HIV-specific T-cell responses has been reported to occur after a brief interruption of antiviral treatment (24). Therefore, the lack of response to the pooled HIV-1 peptides in our patients under highly effective HAART may be due in part to the lack of antigenic stimulation by HIV. However, there is a possibility that responses to other HIV antigens like p24 would be induced, as the p24 responses are more prevalent in HIV-infected patients (30). These results suggest that strategies that use therapeutic immunization against HIV in patients treated with HAART are worth exploring, and our group is testing the use of such approaches in children (31) and adults (26).
To examine the potential pathogenic role of IL-10 and TNF-α in HIV infection, we measured spontaneous and S. aureus Cowan-stimulated levels of production of these cytokines by the cells of study patients before and after up to 24 weeks of therapy. Several groups have previously reported increased levels of production of IL-10 and TNF-α in HIV-infected adults and children (4, 6, 32). However, we did not find any differences between patients and control donors in the present study. Other studies have similarly shown unchanged or even decreased levels of production of IL-10 in HIV-infected persons (12), which is in agreement with our findings. A possible explanation may be the decreased level of immunosuppression in our HIV-infected children relative to those in patients studied previously.
In order to determine the effect of HAART on APC function and, in particular, the function necessary for the generation of cell-mediated immunity, IL-12 production by monocytes in response to S. aureus Cowan stimulation was evaluated. S. aureus Cowan is known to induce IL-12 p70, the biologically active heterodimer, although the quantities detected are quite low (21). We found that the level of S. aureus Cowan-induced IL-12 p70 production by monocytes was significantly reduced in the HIV-infected children enrolled in our study compared to that in the controls. Of interest is that the level of IL-12 production at week 24 of HAART was significantly increased compared to the baseline IL-12 levels. In vitro infection with HIV inhibits IL-12 production (5, 7), and PBMCs from HIV-infected persons produce less IL-12 than PBMCs from HIV-uninfected persons (21). The identified weak initial association of the increased level of IL-12 p70 production by monocytes stimulated with S. aureus Cowan and decreased viral load suggests that the IL-12 downregulation seen in HIV-infected patients might possibly be directly related to viral infection. Increased levels of IL-12 p40 production by stimulated monocytes have been reported in HIV-1-infected adults after treatment with HAART (1). The increase in the level of S. aureus Cowan-stimulated IL-12 p70 observed in our HIV-infected children after 24 weeks of HAART might represent an improvement in monocyte/macrophage function. It is also possible that increased levels of IL-12 may block HIV-induced apoptosis of CD4+ T cells (8) and therefore contribute to an increase in CD4+ T-cell numbers.
Our data suggest that the HAART-related suppression of HIV replication and the increase in total CD4+ T-cell numbers in children result in an increase in the level of ex vivo IL-12 production and an increased DTH response to C. albicans but otherwise do not restore cell-mediated immunity for up to 24 weeks of therapy. It is possible that treatment for periods longer than 24 weeks or exposure to certain antigens is required for the restoration of other immune responses. Also, the combination of HAART with specific immune-based therapy (17, 24, 27) may induce greater HIV-specific T-cell immunity in infected children.
ACKNOWLEDGMENTS
We first thank the patients who volunteered for this study and their families. We also thank Lauren Wood, Leslie Serchuck, Steven Zeichner, Perdita Taylor, and Corina Gonzales; Anne Marie Boler; Paul Jarosinski; the data managers Kim Mitchell and Sheila Keels; nurses, physicians, social workers, and other staff of the NCI pediatric service and Warren G. Magnuson Clinical Center, Bethesda, Md.; and David Waters and Mike Baseler of SAIC, Frederick, Md. The technical assistance of N. Torres and M. Trubey is gratefully acknowledged.
REFERENCES
- 1.Angel J B, Kumar A, Parato K, Filion L G, Diaz-Mitoma F, Daftarian P, Pham B, Sun E, Leonard J M, Cameron D W. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J Infect Dis. 1998;177:898–904. doi: 10.1086/515244. [DOI] [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Bisset L R, Rothen M, Joller-Jemelka H I, Dubs R W, Grob P J, Opravil M. Change in circulating levels of the chemokine macrophage inflammatory proteins 1 alpha and 1 beta, RANTES, monocyte chemotactic protein-1 and interleukin-16 following treatment of severely immunodeficient HIV-infected individuals with indinavir. AIDS. 1997;11:485–491. doi: 10.1097/00002030-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Blazevic V, Heino M, Lagerstedt A, Ranki A, Krohn K J. Interleukin-10 gene expression induced by HIV-1 Tat and Rev in the cells of HIV-1 infected individuals. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:208–214. doi: 10.1097/00042560-199611010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Chehimi J, Starr S E, Frank I, D'Andrea A, Ma X, MacGregor R R, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chougnet C, Fowke K R, Mueller B U, Smith S, Zuckerman J, Jankelevitch S, Steinberg S M, Luban N, Pizzo P A, Shearer G M. Protease inhibitor and triple-drug therapy: cellular immune parameters are not restored in pediatric AIDS patients after 6 months of treatment. AIDS. 1998;12:2397–2406. doi: 10.1097/00002030-199818000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chougnet C, Wynn T A, Clerici M, Landay A L, Kessler H A, Rusnak J, Melcher G P, Sher A, Shearer G M. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 8.Clerici M, Sarin A, Berzofsky J A, Landay A L, Kessler H A, Hashemi F, Hendrix C W, Blatt S P, Rusnak J, Dolan M J, Coffman R L, Henkart P A, Sheraer G M. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS. 1996;10:603–611. doi: 10.1097/00002030-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Clerici M, Stocks N I, Zajac R A, Boswell R N, Bernstein D C, Mann D L, Shearer G M, Berzofsky J A. Interleukin-2 production used to detect antigenic peptide recognition by T-helper lymphocytes from asymptomatic HIV-seropositive individuals. Nature. 1989;339:383–385. doi: 10.1038/339383a0. [DOI] [PubMed] [Google Scholar]
- 10.Cohen Stuart J W, Slieker W A, Rijkers G T, Noest A, Boucher C A, Suur M H, de Boer R, Geelen S P, Scherpbier H J, Hartwig N G, Hooijkaas H, Roos M T, de Graeff-Meeder B, de Groot R. Early recovery of CD4+ T lymphocytes in children on highly active antiretroviral therapy. Dutch Study Group for Children with HIV Infections. AIDS. 1998;12:2155–2159. doi: 10.1097/00002030-199816000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Dalod M, Harzic M, Pellegrin I, Dumon B, Hoen B, Sereni D, Deschemin J C, Levy J P, Venet A, Gomard E. Evolution of cytotoxic T lymphocyte responses to human immunodeficiency virus type 1 in patients with symptomatic primary infection receiving antiretroviral triple therapy. J Infect Dis. 1998;178:61–69. doi: 10.1086/515587. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Mitoma F, Kumar A, Karimi S, Kryworuchko M, Daftarian M P, Greery W D, Filion L G, Cameron W. Expression of IL-10, IL-4 and interferon-gamma in unstimulated and mitogen-stimulated peripheral blood lymphocytes from HIV-seropositive patients. Clin Exp Immunol. 1995;102:31–39. doi: 10.1111/j.1365-2249.1995.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 14.Evans T G, Bonnez W, Soucier H R, Fitzgerald T, Gibbons D C, Reichman R C. Highly active antiretroviral therapy results in a decrease in CD8+ T cell activation and preferential reconstitution of the peripheral CD4+ T cell population with memory rather than naïve cells. Antivir Res. 1998;39:163–173. doi: 10.1016/s0166-3542(98)00035-7. [DOI] [PubMed] [Google Scholar]
- 15.Fleury S, Pantaleo G. T cell regeneration in HIV-infected subjects under highly active antiretroviral therapy. Int J Mol Med. 1999;4:91–97. doi: 10.3892/ijmm.4.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Paris M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 17.Isada C M, Calabrese L H. AIDS update 1999: viral reservoirs and immune-based therapies. Cleveland Clin J Med. 1999;66:267–269. doi: 10.3949/ccjm.66.5.267. [DOI] [PubMed] [Google Scholar]
- 18.Kalams S A, Goulder P J, Shea A K, Jones N G, Trocka A K, Ogg K S, Walker B D. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackall C L, Fleisher T A, Brown M R, Andrich M P, Cleen C C, Fenerstein I M, Horowitz M E, Magrath I T, Shad A T, Steinberg S M. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz M, Saag M, Powderly W G, Hurley A M, Hsu A, Valdes J M, Henry D, Sattler F, La Marca A, Leonard J M. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 21.Marshall J D, Chehimi J, Gri G, Kostman J R, Montaner L J, Trinchieri G. The interleukin-12-mediated pathway of immune events is dysfunctional in human immunodeficiency virus-infected individuals. Blood. 1999;94:1003–1011. [PubMed] [Google Scholar]
- 22.Mezzaroma I, Carlesimo M, Pinter E, Alario C, Sacco G, Muratori D S, Bernardi M L, Paganelli R, Aiuti F. Long-term evaluation of T-cell subsets and T-cell function after HAART in advanced stage HIV-1 disease. AIDS. 1999;13:1187–1193. doi: 10.1097/00002030-199907090-00006. [DOI] [PubMed] [Google Scholar]
- 23.Miedema F, Petit A J, Terpstra F G, Schattenkerk J K, de Wolf F, Al B J, Ross M, Lange J M, Danner S A, Goudsmit J. Immunological abnormalities in human immunodeficiency virus (HIV)-infected asymptomatic homosexual men. HIV affects the immune system before CD4+ T helper cell depletion occurs. J Clin Investig. 1988;82:1908–1914. doi: 10.1172/JCI113809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz G M, Nixon D F, Trkola A, Binley J, Jin X, Bonhoeffer S, Kuebler P J, Donahoe S M, Demontie M A, Kakimoto W M, Ketas T, Clas B, Heymann J J, Zhang L, Car Y, Hurley A, Moore J P, Ho D D, Martowitz M. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Investig. 1999;104:R13–R18. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pelton S I, Johnson D, Chadwick E, Baldwin Z, Yogev R. A one year experience: T cell responses and viral replication in children with advanced human immunodeficiency virus type 1 disease treated with combination therapy including ritonavir. Pediatr Infect Dis. 1999;18:650–652. doi: 10.1097/00006454-199907000-00019. [DOI] [PubMed] [Google Scholar]
- 26.Pinto L A, Berzofsky J A, Fowke K R, Little R F, Merced-Galindez F, Humphrey R, Ahlers J, Dunlop N, Cohen R B, Steinberg S M, Nara P, Shearer G M, Yarchoan R. HIV-specific immunity following immunization with HIV synthetic envelope peptides in asymptomatic HIV-infected patients. AIDS. 1999;13:2003–2012. doi: 10.1097/00002030-199910220-00002. [DOI] [PubMed] [Google Scholar]
- 27.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 28.Potensilli O, Kerkhof-Garde S, Notermans D W, Foudraine N A, Roos M T, Klein M R, Danner S A, Lange J M, Miedema F. Functional T cell reconstitution and human immunodeficiency virus-1-specific cell-mediated immunity during highly active antiretroviral therapy. J Infect Dis. 1999;180:76–86. doi: 10.1086/314837. [DOI] [PubMed] [Google Scholar]
- 29.Richman D. The challenge of immune control of immunodeficiency virus. J Clin Investig. 1999;104:677–678. doi: 10.1172/JCI8242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 31.Sei S, Sandelli S L, Theofan G, Ratto-Kim S, Kumagai M, Loomis-Price L D, Cox J H, Jarosinski P, Walsek C M, Brouwers P, Venzon D J, Xu J, Pizzo P A, Moss R B, Robb M L, Wood L V. Preliminary evaluation of human immunodeficiency virus type 1 (HIV-1) immunogen in children with HIV-1 infection. J Infect Dis. 1999;180:626–640. doi: 10.1086/314944. [DOI] [PubMed] [Google Scholar]
- 32.Stylianou E, Aukrust P, Kvale D, Muller F, Froland S S. IL-10 in HIV infection: increasing serum IL-10 levels with disease progression-down-regulatory effect of potent anti-retroviral therapy. Clin Exp Immunol. 1999;116:115–120. doi: 10.1046/j.1365-2249.1999.00865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wendland T, Furrer H, Vernazza P L, Frutig K, Christen A, Matter L, Malinverni R, Pichler W J. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS. 1999;13:1857–1862. doi: 10.1097/00002030-199910010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Volberding P A, Deeks S G. Antiretroviral therapy for HIV infection: promises and problems. JAMA. 1998;279:1343–1344. doi: 10.1001/jama.279.17.1343. [DOI] [PubMed] [Google Scholar]
- 35.Yarchoan R, Klecker R W, Weinhold K J, Markham P D, Lyerly H K, Durack D T, Gelmann E, Lehrman S N, Blum R M, Barry D W. Administration of 3′-azido-3′-deoxythymidine, an inhibitor of HTLV-III/LAV replication, to patients with AIDS or AIDS-related complex. Lancet. 1986;i:575–580. doi: 10.1016/s0140-6736(86)92808-4. [DOI] [PubMed] [Google Scholar]
- 36.Zhou P, Goldstein S, Devadas K, Tewari D, Notkins A L. Human CD4+ cells transfected with IL-16 cDNA are resistant to HIV-1 infection: inhibition of mRNA expression. Nat Med. 1997;3:659–664. doi: 10.1038/nm0697-659. [DOI] [PubMed] [Google Scholar]