Abstract
Objective
Recent studies have shown that early in the COVID-19 pandemic, rates of buprenorphine prescription dispensing for opioid use disorder (OUD) were relatively stable. However, whether that pattern continued later in the pandemic is unclear. This study examines the monthly rate of dispensed buprenorphine prescriptions during the early period and the later period of the pandemic.
Methods
The study uses interrupted time series analysis to examine buprenorphine prescription dispensed, average day's supply, payment source, and the number of patients with a dispensed buprenorphine prescription. The study utilized January 2019–April 2021 data from IQVIA National Prescription Audit, PayerTrack and Total Patient Tracker databases.
Results
After an initial increase in the number of patients prescribed buprenorphine in the early period of the pandemic, the monthly rate of patients prescribed buprenorphine increased at a lower rate compared to the pre-pandemic period (6100 vs 4600/month). The study observed a decline in the number of buprenorphine prescriptions dispensed both in levels and growth rate during the pandemic, but an increase occurred in the average day's supply of buprenorphine prescriptions (17 days pre-pandemic vs 18.6 day during the pandemic). Medicaid became the primary payer of buprenorphine prescriptions as the pandemic continued, while buprenorphine prescriptions paid for by private insurance declined.
Discussion
Expanding and maintaining access to treatment for OUD were key priorities in federal and state responses to the COVID-19 pandemic. The results of our study underscore the importance of policy efforts to help increase buprenorphine prescribing for OUD.
Keywords: Buprenorphine, Opioid use disorder, COVID-19
1. Introduction
Opioid misuse and opioid use disorder (OUD) remain major public health threats in the United States. In response to the crisis, interventions are being implemented at the state and federal levels to increase access to treatment among individuals with OUD (Haffajee et al., 2021). Expanded utilization of medications for opioid use disorder (MOUD) is an important component of this intervention, as research has demonstrated MOUD to be cost effective and associated with reduced opioid misuse and opioid related mortality (Wen, Borders, & Cummings, 2019). Despite current policy efforts, MOUD remains significantly underutilized, as some research has found that less than one-fifth of individuals with OUD received any treatment (Novak, Feder, Ali, & Chen, 2019; Saloner, Stoller, & Alexander, 2018).
Office-based treatment with buprenorphine has the potential to significantly increase access to treatment for OUD (Saloner et al., 2018). The Drug Addiction Treatment Act of 2000 (DATA 2000) created a program of waivers for qualified physicians to prescribe buprenorphine for OUD in an office-based setting, outside of federally registered opioid treatment programs. Until 2016, DATA-waivered physicians could treat up to 30 patients at a time during the first year of the waiver and could increase the limit to 100 patients after one year. In 2016, a regulatory change expanded these limits by allowing physicians to increase their patient limit to 275 (after a year at the 100-patient limit), and the Comprehensive Addiction and Recovery Act (CARA) included a provision that permitted nurse practitioners and physician assistants to obtain a DATA waiver after completing certain training requirements. The SUPPORT Act of 2018 also expanded waiver access to other mid-level providers, such as certified midwives and nurse anesthetists, and allowed mid-level providers to immediately begin treating up to 100 patients. The COVID-19 public health emergency further provided authorized practitioners flexibility in initiating and prescribing buprenorphine to patients through telehealth, which previously required an in-person visit. The pandemic saw a 30 % increase in drug-related overdose deaths compared to what it was prior to the pandemic (CDC, 2020), however, raising concerns about patients' continued access to treatment for OUD (Auty & Griffith, 2022). Specifically, in 2020, US drug-related deaths rose to >93,000, primarily driven by opioids; and in 2021, the United States surpassed 100,000 drug-related deaths for the first time (CDC, 2021). This figure further highlights the importance of access to treatment as opioid-related mortality has been exacerbated during the COVID-19 pandemic.
Findings from recent studies show that during the early period of COVID-19 pandemic, buprenorphine prescriptions were relatively stable (Cantor, Dick, Haffajee, et al., 2021; Nguyen et al., 2021; Currie, Schnell, Schwandt, & Zhang, 2021). However, whether that pattern has persisted as the pandemic continued is unclear. Cremer et al. (2022) examined buprenorphine prescriptions for all of 2020 and found no statistically significant changes compared to 2019 levels. But this study did not examine any prescription characteristics, such as average day's supply or payer-type and did not explicitly model for the COVID-19 public health emergency (PHE). Explicitly modelling for the COVID-19 PHE is important in research, as it will allows us to decipher whether the changes observed in buprenorphine prescribing follows secular trends, reflects trends already observed in the pre-pandemic period, or whether the changes may relate to shifts in the health care landscape prompted by the pandemic. In addition, examining prescription characteristics, such as day's supply and payer type, provide us with a complete picture of how buprenorphine prescribing has changed during the pandemic.
This study expands on the previous literature by adopting a quasi-experimental research design using a national multi-payer pharmacy claims database to estimate trends in buprenorphine prescriptions from January 2019 to April 2021 after accounting for the March 2020 COVID-19 PHE. Specifically, the current study reports trends in the number of buprenorphine prescriptions dispensed, the average day's supply of buprenorphine prescriptions, and how this varied by payer-type (private insurance, Medicaid, Medicare and cash). The study also reports the number of patients prescribed buprenorphine.
2. Data & methods
The study drew the data for the analysis from IQVIA's National Prescription Audit (NPA), PayerTrak, and Total Patient Tracker (TPT) and data covered January 2019 to April 2021. NPA includes >3 billion prescriptions/year, representing >92 % of prescriptions dispensed at retail pharmacies (chain, independent, and food store pharmacies and mail-orders) and covers all 50 states and the District of Columbia. NPA is utilized to analyze the total number of buprenorphine prescriptions dispensed (excluding buprenorphine formulations commonly used to treat pain rather than OUD) and the average days' supply for dispensed prescriptions. Similar to NPA, PayerTrak also includes >3 billion prescriptions/year and covers >92 % of prescriptions dispensed in retail settings. Additionally, PayerTrak includes information on the source of payer for the prescriptions, enabling us to analyze how the number of buprenorphine prescriptions dispensed varied by payer-type, including private insurance, Medicaid, Medicare, and cash pay. TPT captures the total number of unique patients at the national level across all drugs and therapeutic classes in the retail outpatient setting. TPT eliminates duplicate patients and multiple prescription fills, allowing us to produce unique patient counts. TPT is utilized to estimate the total number of patients who were prescribed buprenorphine.
The research team conducted the analysis using an interrupted time series analysis (ITS), a quasi-experimental research design, that allowed us to account for the declaration of 2020 COVID-19 PHE (March 2020). We used the following equation for the regression:
where Yt is the outcome variable measured at each time point t, Tt is the time since the start of observation (in months), Xt is a dummy (indicator) variable representing the COVID-19 PHE declaration (pre–March 2020 periods = 0, post-March 2020 periods = 1), XtTt is an interaction term and εt is the error term. The study estimated the models to test for both a one-time change immediately after the COVID-19 PHE declaration (intercept/level change; β2) and the difference in the pre- and post-COVID-19 PHE trends (slope; β3). The study estimated generalized linear regression models with robust standard errors to account for autocorrelated error terms.
3. Results
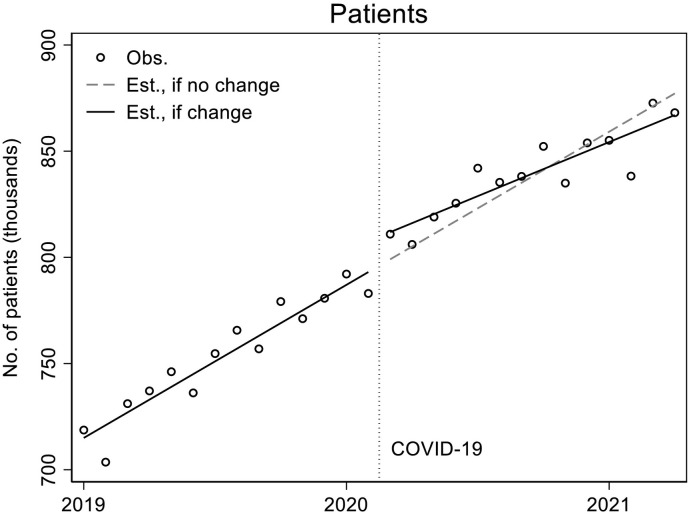
Fig. 1 (observed data points and unadjusted model predictions) and Table 1 (adjusted model estimates) show that the number of patients with filled buprenorphine prescriptions was increasing in the pre-pandemic period at a statistically significant rate of about 6100 additional patients/month (p < 0.001), from approximately 719,000 in January 2019 to 792,000 in January 2020. At the start of the pandemic, in March 2020, a statistically significant immediate upward shift occurred in the level of patients with buprenorphine prescription fills/month of about 8720 (p = 0.026). During the pandemic, however, the rate of increase in the number of patients dispensed buprenorphine for OUD slowed compared to the pre-pandemic rate by about 1490 patients per month (p = 0.002).
Fig. 1.
Patients.
Table 1.
ITS Models for Total Patients, Total Prescriptions, and Average Rx Days.
| Patients |
Prescriptions |
Rx days |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | P | Coef. | SE | P | Coef. | SE | P | |
| Intercept | 719.95 | 1.87 | <0.001 | 1326.46 | 13.17 | <0.001 | 17.07 | 0.04 | <0.001 |
| Slopes | |||||||||
| Pre-COVID | 6.10 | 0.28 | <0.001 | 6.39 | 1.36 | <0.001 | 0.05 | 0.01 | <0.001 |
| Post-COVID | 4.61 | 0.35 | 0.000 | 4.56 | 2.25 | 0.055 | 0.02 | 0.01 | 0.193 |
| Difference | −1.49 | 0.43 | 0.002 | −1.83 | 2.69 | 0.505 | −0.03 | 0.02 | 0.088 |
| Level | |||||||||
| Difference | 8.72 | 3.64 | 0.026 | −39.21 | 20.28 | 0.066 | 0.90 | 0.15 | <0.001 |
| Days per month | |||||||||
| 29 | −21.78 | 2.96 | <0.001 | −118.42 | 10.03 | <0.001 | |||
| 30 | −8.64 | 2.29 | 0.001 | −54.04 | 16.85 | 0.004 | |||
Notes: Interrupted time series regression models with Newey-West standard errors to account for autocorrelation (lag: 1 month). N = 28 for all models. The reference value for the days/month variable was 31.
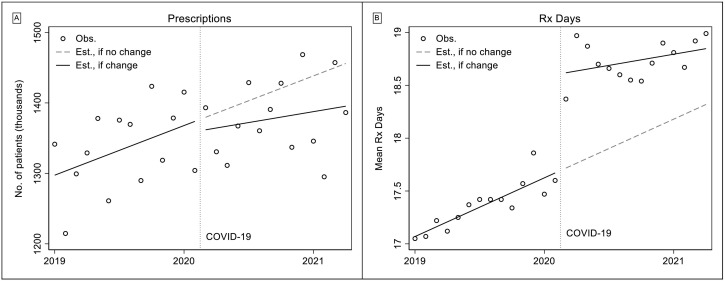
We present the observed and predicted values of monthly buprenorphine prescriptions dispensed in Fig. 2 (predicted values based on unadjusted ITS models). From the figure we see an increase in the number of buprenorphine prescriptions dispensed in the pre-COVID-19 period, from 1.341 million in January 2019 to 1.415 million in January 2020. After controlling for length of month (Table 1), this amounted to a significant average increase of about 6390 prescriptions/month (p < 0.001). We did not observe statistically significant declines in level or monthly rates of buprenorphine dispensed after the start of the pandemic. During the pandemic, the rate of increase was not significantly different than zero.
Fig. 2.
Total Rx and Rx days.
Fig. 2 also shows that the average days' supply of buprenorphine prescriptions dispensed was about 17.4 days in the pre-pandemic period and about 18.7 days during the pandemic. Model-based estimates (Table 1) indicate that the average length of a prescription was significantly increasing by about 0.05 days/month (p < 0.001) in the pre-period, and a significant, immediate level shift of about 0.9 days occurred at the beginning of the pandemic.
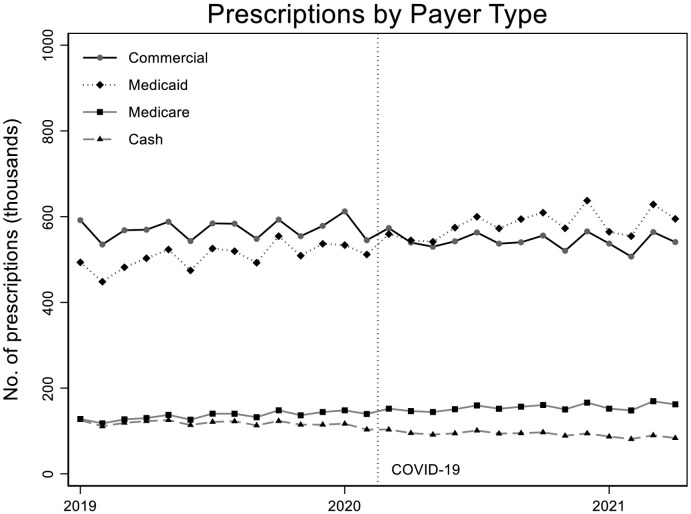
From Fig. 3 we see that commercial insurance was the primary payer of buprenorphine prescriptions during the pre-pandemic period, but after the pandemic Medicaid became the primary payer and it continued to increase in both level and monthly growth rate. Table 2 shows ITS model-based estimates of levels and rates of change before and during the pandemic. Specifically, buprenorphine prescriptions paid for by commercial insurance had a significant decrease in level of about −33,430 prescriptions/month (p < 0.001) but no significant change in slope (and slope was not significantly different from zero in both the pre- and postpandemic periods). The number of prescriptions paid for by Medicaid was increasing in the pre-pandemic period by an average of 4420 prescriptions/month (p < 0.001), and we did not find statistically significant evidence of a change in this trajectory after the pandemic began in either immediate level or slope. The average number of prescriptions paid for per month by Medicare increased significantly during both the pre- and postpandemic periods (pre: 1710, p < 0.001; post: 1220, p < 0.001), but the rate of increase during the pandemic was not significantly different than the pre-period trend. Prescriptions for buprenorphine paid in cash were already declining during the pre-pandemic period (an average of about 660 fewer/month, p = 0.001). At the beginning of the pandemic, cash declined further by a significant amount of about 14,800 (p < 0.001). TA significantly negative trend continued to occur during the pandemic as well (−910/month, p < 0.001), though this rate was not significantly different than the pre-pandemic trend.
Fig. 3.
Prescriptions by payer type.
Table 2.
ITS models for total prescriptions by payer.
| Commercial |
Medicaid |
Medicare |
Cash |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coef. | SE | P | Coef. | SE | P | Coef. | SE | P | Coef. | SE | P | |
| Intercept | 579.53 | 5.65 | <0.001 | 491.28 | 7.17 | <0.001 | 127.82 | 1.55 | <0.001 | 124.83 | 1.59 | <0.001 |
| Slopes | ||||||||||||
| Pre-COVID | 0.79 | 0.80 | 0.333 | 4.42 | 0.79 | <0.001 | 1.71 | 0.17 | <0.001 | −0.66 | 0.17 | 0.001 |
| Post-COVID | −0.18 | 1.00 | 0.859 | 4.41 | 1.11 | 0.001 | 1.22 | 0.31 | 0.001 | −0.91 | 0.21 | <0.001 |
| Difference | −0.97 | 1.28 | 0.455 | −0.01 | 1.54 | 0.994 | −0.50 | 0.38 | 0.199 | −0.25 | 0.28 | 0.382 |
| Level | ||||||||||||
| Difference | −33.43 | 10.88 | 0.006 | 10.50 | 11.62 | 0.376 | −1.75 | 2.91 | 0.554 | −14.82 | 2.44 | <0.001 |
| Days/month | ||||||||||||
| 29 | −46.15 | 3.28 | <0.001 | −47.42 | 8.10 | <0.001 | −12.58 | 1.94 | <0.001 | −11.92 | 1.38 | <0.001 |
| 30 | −24.53 | 6.02 | 0.001 | −19.46 | 7.97 | 0.023 | −5.80 | 1.82 | 0.004 | −4.09 | 1.34 | 0.006 |
Notes: Interrupted time series regression models with Newey-West standard errors to account for autocorrelation (lag: 1 month). N = 28 for all models. The reference value for the days/month variable was 31.
3.1. Robustness checks
We conducted several sensitivity analyses to assess the validity of our estimates and we report these results in the Supplemental Appendix. In addition to the ITS models with Newey-West standard errors reported in Table 1, Table 2, we estimated 3 other ARIMA models with autoregressive, moving average, and differenced components. We compared parameter estimates and information criteria between models. Models with other AR and MA components produced very similar results and small differences in information criteria (the models had no meaningful differences in fit). Based on these checks, we retained the Newey-West models. We also conducted a Wald test for structural break and rejected the null hypothesis of no structural break (χ2 = 8.65, p = 0.013). Finally, we selected a randomly chosen policy date (October 2019) and re-ran the ITS models and found no statistically significant relationship between that policy date and our outcome variables.
4. Discussion
Using a national multi-payer pharmacy claims database this study found that the number of patients prescribed buprenorphine for OUD remained stable during the COVID-19 pandemic. However, after an initial increase in the number of patients prescribed buprenorphine in the early period of the pandemic, the monthly rate of patients prescribed buprenorphine increased at a lower rate compared to the pre-pandemic period. The study found a decline in the number of buprenorphine prescriptions dispensed both in levels and growth rate during the pandemic, but an increase occurred in the average days' supply of buprenorphine prescriptions. This finding could account, at least in part, for the reduction in the number of prescriptions dispensed, as providers were writing prescription for longer periods. The increase in days' supply of buprenorphine prescriptions could also imply that the flexibility provided to authorized practitioners by the COVID-19 PHE resulted in them writing prescriptions for longer durations to help patients comply with the state's quarantine and stay-at-home policies without losing access to treatment.
The study also found Medicaid to be the primary payer of buprenorphine prescriptions during the COVID-19 pandemic, while buprenorphine prescriptions paid for by private insurance declined. This finding is consistent with the literature that has documented a decline in employer sponsored health insurance coverage, but higher enrollment in public health insurance programs after the initial shock to employment in March 2020 (Bundorf, Gupta, & Kim, 2021). Even before the pandemic Medicaid was a major payer for behavioral health services (CMS, 2021), but the pandemic may have accelerated this growth. To our knowledge most of the changes in Medicaid policies during our study period are related to the COVID-19 PHE. The unique nature of the pandemic, and the economic downturn it caused, prompted many states to adopt Medicaid emergency authorization to expand eligibility and/or modify eligibility rules, eliminate/waive premiums, and streamline the application and enrollment processes. All of this might help to explain the growing share of buprenorphine prescriptions being covered by Medicaid. Indeed, recent data show that 16.2 million more people were enrolled in Medicaid/CHIP since the beginning of the pandemic (CMS, 2022). By the end of 2020, 79.8 million individuals were enrolled under Medicaid/CHIP and by the end of 2021 this number increased to 86.4 million — whereas in 2019 it was 70.2 million (CMS, 2022). Another finding of the study was a steady and continued rise in Medicare's share of payment for patients prescribed buprenorphine, further documenting the important role played by the public insurance program during the pandemic. However, the field needs more research to understand the use of opioids and MOUD among older adults in the United States.
The COVID-19 pandemic prompted numerous policy changes in the treatment delivery system for OUD at both the federal and state levels. These changes include flexibilities around buprenorphine prescriptions via telehealth, including increased reimbursement and coverage for telehealth services, increase in the number of days of take-home medications and home delivery options for patients, relaxed licensing laws for providers among others (Pessar, Boustead, Ge, Smart, & Pacula, 2021). Although how each of these specific policies have impacted initiation and retention of buprenorphine treatment for OUD is unclear, they might have played a role in keeping buprenorphine prescriptions steady during the pandemic, especially during the later periods. In particular, the increase in the number of patients prescribed buprenorphine, the increase in days' supply and Medicaid becoming the primary payer for buprenorphine indicates the importance of federal and state policies in helping patients to access treatment. An evaluation of the specific polices implemented during COVID-19 and how its continuation might impact treatment utilization for OUD will be an important avenue for future research to explore. In addition, how demand side policy initiatives can complement these supply side policies is also an area that is ripe for further exploration.
Despite the comprehensive nature and timeliness of the data, the findings of this study should be viewed in the context of some limitations. First, the study was only able to examine prescriptions dispensed but not consumed. However, recent research has shown that diversion of buprenorphine among individuals with OUD has declined in recent years (Han, Jones, Einstein, & Compton, 2021). As drug-related overdose deaths have soared during the pandemic (CDC, 2020), understanding how buprenorphine prescriptions might be correlated with opioid-related mortality is important. Second, the study examined buprenorphine; research should investigate the dispensing of other medications for OUD, such as naltrexone and methadone. Third, the race/ethnicity of the patients were not available in the IQVIA data sets used in the study. The literature has shown the impact of the COVID-19 pandemic to be more pronounced among people of color (Shim & Starks, 2021), and examining racial/ethnic differences in buprenorphine prescriptions during the pandemic will be an important direction for future studies (Nguyen, Ziedan, Simon, et al., 2022). Fourth, our study was not able to distinguish between buprenorphine treatment initiation vs continuation. Examining treatment initiation and continuation will have important policy implications as the pandemic continues to evolve. Finally, a methodological limitation of our ITS estimation strategy is the lack of a nontreatment or a control group. A quasi-experimental research design commonly includes a control group; however, finding a suitable control group in our case was difficult because COVID-19 impacted almost every aspect of the US health care system. Thus, our results demonstrate strong associations rather than causal relationships.
5. Conclusion
Expanding and maintaining access to treatment for OUD has been a key priority in the federal and state response to the COVID-19 pandemic. The results of our study show that the use of buprenorphine remained stable during the pandemic with public insurance programs playing an important role in helping patients to pay for their treatment.
Disclaimer
The views expressed here are those of the authors and do not necessarily reflect the views of the Office of the Assistant Secretary for Planning & Evaluation, or US Department of Health & Human Services.
Declaration of competing interest
The authors report no financial relationships with commercial interests and have no conflicts of interest relevant to this article to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsat.2022.108923.
Appendix A. Supplementary data
Robustness Checks
References
- Auty S.G., Griffith K.N. Medicaid expansion and drug overdose mortality during the COVID-19 pandemic in the United States [published online ahead of print, 2022 feb 2] Drug and Alcohol Dependence. 2022;232 doi: 10.1016/j.drugalcdep.2022.109340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundorf M.K., Gupta S., Kim C. Trends in US health insurance coverage during the COVID-19 pandemic. JAMA Health Forum. 2021;2(9) doi: 10.1001/jamahealthforum.2021.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor J., Dick A.W., Haffajee R., et al. Use of buprenorphine for those with employer-sponsored insurance during the initial phase of the COVID-19 pandemic. Journal of Substance Abuse Treatment. 2021;129 doi: 10.1016/j.jsat.2021.108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2020. Overdose deaths accelerating during COVID-19.https://www.cdc.gov/media/releases/2020/p1218-overdose-deaths-covid-19.html [Google Scholar]
- Centers for Disease Control and Prevention Drug overdose deaths in the U.S. top 100,000 annually. 2021. https://www.cdc.gov/nchs/pressroom/nchs_press_releases/2021/20211117.htm
- Center for Medicare and Medicaid Services Behavioral health services. 2021. https://www.medicaid.gov/medicaid/benefits/behavioral-health-services/index.html Accessed April 12, 2022.
- Center for Medicare and Medicaid Services February 2022 Medicaid and CHIP enrollment trends snapshot. 2022. https://www.medicaid.gov/medicaid/national-medicaid-chip-program-information/downloads/february-2022-medicaid-chip-enrollment-trend-snapshot.pdf Accessed June 16, 2022.
- Cremer L.J., Board A., Guy G.P., Jr, Schieber L., Asher A., Parker E.M. Trends in pharmacy-based dispensing of buprenorphine, extended-release naltrexone, and naloxone during the COVID-19 pandemic by age and sex - United States, March 2019 - December 2020. Drug Alcohol Depend. 2022;232 doi: 10.1016/j.drugalcdep.2021.109192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie J.M., Schnell M.K., Schwandt H., Zhang J. Prescribing of opioid analgesics and buprenorphine for opioid use disorder during the COVID-19 pandemic. JAMA Network Open. 2021;4(4) doi: 10.1001/jamanetworkopen.2021.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffajee R.L., Sherry T.B., Dubenitz J.M., White J.O., Schwartz D., Stoller B., Swenson-O'Brien A.J., Manocchio T.M., Creedon T.B., Bagalman E. Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services; Washington, DC: 2021. U.S. Department of Health and Human Services Overdose Prevention Strategy (Issue Brief) [Google Scholar]
- Han B., Jones C.M., Einstein E.B., Compton W.M. Trends in and characteristics of buprenorphine misuse among adults in the US. JAMA Network Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.29409. Published 2021 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P., Feder K.A., Ali M.M., Chen J. Behavioral health treatment utilization among individuals with co-occurring opioid use disorder and mental illness: Evidence from a national survey. Journal of Substance Abuse Treatment. 2019;98:47–52. doi: 10.1016/j.jsat.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.D., Gupta S., Ziedan E., et al. Assessment of filled buprenorphine prescriptions for opioid use disorder during the coronavirus disease 2019 pandemic. JAMA Internal Medicine. 2021;181(4):562–565. doi: 10.1001/jamainternmed.2020.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T., Ziedan E., Simon K., et al. Racial and ethnic disparities in buprenorphine and extended-release naltrexone filled prescriptions during the COVID-19 pandemic. JAMA Network Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessar S.C., Boustead A., Ge Y., Smart R., Pacula R.L. Assessment of state and federal health policies for opioid use disorder treatment during the COVID-19 pandemic and beyond. JAMA Health Forum. 2021;2(11) doi: 10.1001/jamahealthforum.2021.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B., Stoller K.B., Alexander G.C. Moving addiction care to the mainstream - Improving the quality of buprenorphine treatment. The New England Journal of Medicine. 2018;379(1):4–6. doi: 10.1056/NEJMp1804059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim R.S., Starks S.M. COVID-19, structural racism, and mental health inequities: Policy implications for an emerging syndemic. Psychiatric Services (Washington, D.C.) 2021;72(10):1193–1198. doi: 10.1176/appi.ps.202000725. [DOI] [PubMed] [Google Scholar]
- Wen H., Borders T.F., Cummings J.R. Trends in buprenorphine prescribing by physician specialty. Health Affairs (Millwood) 2019;38(1):24–28. doi: 10.1377/hlthaff.2018.05145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robustness Checks