Abstract
Background
Surfactant protein-D (SP-D) is a lung-resident protein that has emerged as a potential biomarker for COVID-19. Previous investigations on acute respiratory distress syndrome patients demonstrated a significant increment of SP-D serum levels in pathological conditions. Since SP-D is not physiologically permeable to alveoli-capillary membrane and poorly expressed by other tissues, this enhancement is likely due to an impairment of the pulmonary barrier caused by prolonged inflammation.
Methods
A retrospective study on a relatively large cohort of patients of Hospital Pio XI of Desio was conducted to assess differences of the hematic SP-D concentrations among COVID-19 patients and healthy donors and if SP-D levels resulted a risk factor for disease severity and mortality.
Results
The first analysis, using an ANOVA-model, showed a significant difference in the mean of log SP-D levels between COVID-19 patients and healthy donors. Significant variations were also found between dead vs survived patients. Results confirm that SP-D concentrations were significantly higher for both hospitalized COVID-19 and dead patients, with threshold values of 150 and 250 ng/mL, respectively. Further analysis conducted with Logistic Mixed models, highlighted that higher SP-D levels at admission and increasing differences among follow-up and admission values resulted the strongest significant risk factors of mortality (model predictive accuracy, AUC = 0.844).
Conclusions
The results indicate that SP-D can be a predictive marker of COVID-19 disease and its outcome. Considering its prognostic value in terms of mortality, the early detection of SP-D levels and its follow-up in hospitalized patients should be considered to direct the therapeutic intervention.
Keywords: COVID-19, Mortality, SARS-CoV-2 infection, Surfactant protein-D (SP-D)
1. Introduction
On July 2022 the number of confirmed coronavirus disease 2019 (COVID-19) cases, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), exceeds 565 million [1] and still increases. In COVID-19 patients, the identification of valid biomarkers able to predict patient prognosis is still a main challenge.
To identify specific markers for pulmonary diseases, the surfactant protein D (SP-D) was broadly investigated [2]. SP-D is a low abundant (0.6 %) hydrophilic protein strongly involved in lung homeostasis through two specific functions: 1) SP-D is able to bind highly conserved glycosidic residues exposed on pathogens surface triggering their clearance by agglutination and/or opsonization; 2) SP-D directly modulates the activity of lung resident immune cells: under physiological conditions, it has an anti-inflammatory effect, whereas under several specific stimuli (e.g., pathogens infection) it promotes inflammation [2], [3], [4]. SP-D has long been used as a diagnostic and/or prognostic biomarker for interstitial lung diseases (ILDs) [5], [6], [7] and acute respiratory distress syndrome (ARDS) [8], [9]. In particular, an enhancement of serum levels of SP-D was observed in these pathologic conditions [2]. Given the extremely low permeability of SP-D to alveoli-capillary membrane and its poor expression in extrapulmonary tissues, the previously mentioned effect is generally attributed to an impairment of the pulmonary barrier caused by prolonged inflammation [2], [10], [11]. COVID-19 pandemic of the last two years led to an intensification of the research activity aimed to correlate pulmonary surfactant proteins with the clinical manifestations of the virus and, in this context, several studies indicated SP-D as a prognostic biomarker of COVID-19 pneumonia severity. A retrospective study on 39 laboratory-confirmed COVID-19 cases from The Fourth Hospital of Yiyang, Hunan China (between February and March 2020) demonstrated that in the acute phase, the circulating SP-D levels were higher in patients with severe COVID-19 pneumonia compared to mild patients, suggesting that the serum SP-D was strictly correlated to the disease severity [12].
Another study aimed to investigate whether the serum level of SP-D could stratify the disease severity at an early stage, was performed on a small cohort of COVID-19 patients from Sapporo Medical University Hospital (46 between March and April 2020) demonstrated that SP-D levels increased with the aggravation of symptoms and disease severity, as indicated by radiological evidence [13].
A more recent study on 64 COVID-19 confirmed patients (between September 2020 and February 2021) versus patients diagnosed with community-acquired pneumonia and healthy controls, suggested that SP-D could be a predictive factor in differentiating COVID-19 patients and it also associates with the severity of disease compared to patients diagnosed with community-acquired pneumonia and healthy controls [14].
Here is reported a retrospective study on the patients admitted at Hospital Pio XI of Desio from November 2020 to January 2021 and correlates the levels of SP-D in hospitalized COVID-19 confirmed patients to the grade of severity, mortality and other clinical parameters relevant to the disease evolution and risk factors. We aimed to determine the role of SP-D in discriminating the disease severity and mortality in COVID-19 patients, and also to study how clinical covariates affect SP-D levels, suggesting a specific role of SP-D as a prognostic marker of severity and/or of mortality risk.
The obtained results can be crucial to propose the detection of SP-D plasmatic concentration in the first stages of SARS-CoV-2 infection as an important hallmark of disease useful for the definition of personalized protocols in the preventive treatment of these patients.
2. Patients and methods
2.1. Patients
Peripheral blood samples were obtained from SARS-COV-2 patients admitted from November 2020 up to January 2021 at the Hospital Pio XI of Desio, in the proximity of Milan (Italy). Our study included 226 participants, 147 males and 79 females, divided in three groups, two of them are identified according to COVID-19 severity (Group 1: 79 mild COVID-19 patients; Group 2: 123 severe COVID-19 patients) and one composed by 24 healthy patients (Group 3).
COVID-19 patients have been diagnosed through RT-PCR of nasopharyngeal swab and classified upon admission into mild and severe after the evaluation of the respiratory symptoms. In particular, patients were classified as severe if they presented one or more of the following symptoms (according to Chinese guidelines): respiratory rate (RR) ≥ 30 breaths/min, finger oxygen saturation (SpO2) ≤ 93 % at rest and arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FIO2) ≤ 300 ratio [15].
Blood samples were collected upon admission for all the patients included in the study (T0), while the follow-up sampling was conducted after 5 ± 3 days (T1). A total of 299 samples from COVID-19 were considered (202 at T0 and 97 at T1). At T1 the mild COVID-19 samples were 37, while severe COVID-19 samples were 60.
Demographic and clinical characteristics of study participants are reported in Table 1 . In detail, 42 (53.1 %), 85 (69.1 %) and 20 (83.3 %) of the patients included, in Groups 1, 2 and 3, respectively, were men. Mean ages were 75.6 ± 14.0 years in Group 1, 75.4 ± 11.2 in Group 2, and 45.1 ± 14.1 in the control group (Group 3). According to the clinical characteristics, COVID-19 patients were not generally discernable into mild and severe (there was no significant difference between the two groups for all parameters except for obesity), while an increased percentage of treatments (e.g., intubation and tracheostomy) was associated to the worst clinical status of Group 2.
Table 1.
Demographic and clinical characteristics of study participantsa.
|
Group1 (n = 79) |
Group 2 (n = 123) |
P-value |
Group 3 (n = 24) |
||||
|---|---|---|---|---|---|---|---|
| % | % | Group 2 vs Group 1 |
% | ||||
| Age | 75.6 ± 14.0 | 75.4 ± 11.2 | 0.223 | 45.1 ± 14.1 | |||
| Male | 42 | 53.2 | 85 | 69.1 | 0.112 | 20 | 83.3 |
| Comorbidities | |||||||
| Hypertension | 30 | 38.0 | 60 | 48.8 | 0.554 | 0 | 0 |
| Cardiovascular diseases | 20 | 25.3 | 26 | 21.1 | 0.100 | 0 | 0 |
| Diabetes | 11 | 13.9 | 31 | 25.2 | 0.282 | 0 | 0 |
| Respiratory diseases | 8 | 10.1 | 6 | 4.9 | 0.255 | 0 | 0 |
| Cancer | 2 | 2.5 | 9 | 7.3 | 0.317 | 0 | 0 |
| Sepsis | 3 | 3.8 | 6 | 4.9 | 0.716 | 0 | 0 |
| Kidney diseases | 15 | 19.0 | 14 | 11.4 | 0.335 | 0 | 0 |
| Obesity | 5 | 6.3 | 14 | 11.4 | <0.001 | 0 | 0 |
| Treatments | |||||||
| CPAP | 17 | 21.5 | 60 | 48.8 | 0.307 | 0 | 0 |
| Intubation | 4 | 5.1 | 22 | 17.9 | <0.001 | 0 | 0 |
| Tracheostomy | 1 | 1.3 | 3 | 2.4 | <0.001 | 0 | 0 |
Group1: mild COVID-19; Group 2: severe COVID-19; Group 3: healthy subjects.
2.2. Analyses of plasma samples
Whole blood was collected in tubes with citrate as anticoagulant. Plasma was isolated by centrifugation (10 min, 1500 × g) and store at –80 °C until analysis. The quantification of SP-D concentration in plasma was performed with the Human Surfactant Protein D (SP-D) ELISA kit (BioVendor, Czech Republic). The samples, diluted or not as needed, were analyzed according to manufacturer’s instructions. Since the mean coefficient of variation calculated from the analysis of 76 samples in duplicate was 3.14 %, all the other measurements were performed in single run. Data are reported as ng/mL.
2.3. Statistical analysis
Two main analyses were conducted. Firstly, an ANOVA model was adopted to compare the (logged) SP-D means of three groups (mild and severe COVID-19, and healthy volunteers) at admission in an unbalanced framework, using as covariates only the dummy variable to identify the group. Significance of mean differences were assessed with p-values and a Bonferroni adjusted paired t-tests was adopted to compare means among groups. Confidence intervals of estimated means were used to identify possible thresholds that discriminates between COVID-19 and healthy patients. The assumption of normality and sphericity of residuals has been verified by using Shapiro-Wilk and Mauchly’s tests. The same ANOVA analysis was repeated to assess if log of SP-D mean levels change among mortality and severity conditions at admission.
Secondly, for a more complete picture, limiting the attention to COVID-19 patients (Group 1 and 2: mild and severe), we assessed the effect of clinical covariates (including SP-D, measured both upon admission and as difference from follow-up to admission) both on risk of mortality and severity of COVID-19 (binary outcomes). The aim of this part of our study is to analyze if SP-D (and its evolution over time) can be considered a significant predictor (possibly interacting with other risk factors) for mortality or severity.
Methodologically, mortality status was modelled by generalized mixed models (logistic regression for repeated measures), whereas a simple logistic regression has been adopted to study patients’ severity, since severity was assessed only once (i.e., upon admission).
Significance of covariates was assessed with p-values, whereas the area under the ROC curve (AUC) has been considered as a measure of predictive accuracy.
2.4. Ethics
The study was approved by the Ethical Committee of the Istituto Nazionale Malattie Infettive Lazzaro Spallanzani, Roma and stemmed as a sub-project of the Observational cohort study on the natural history of hospitalized SARS-CoV-2 patients: the STORM trial of the University of Milano Bicocca, Milan, Italy.
3. Results
3.1. Differences in SP-D plasma levels among patient groups, mortality and severity condition at admission
In such analyses, to confirm the assumptions of ANOVA, SP-D was transformed in base-10 logarithmic form to assure normality and sphericity. Diagnostic tests confirm the assumptions: p = 0.181, p = 0.414, p = 0.535 for normality of three groups, p = 0.079 for sphericity test.
The group variable was found strongly significant (p < 0.0001). In particular, significant difference between (log) means of COVID-19 cases (mild and severe) versus healthy individuals was found (p < 0.0001) at admission, but not among the two classes of COVID-19 patients (p = 0.177). This means that we can identify the difference between healthy donors and COVID-19 patients, but we cannot identify difference among mild and severe patients.
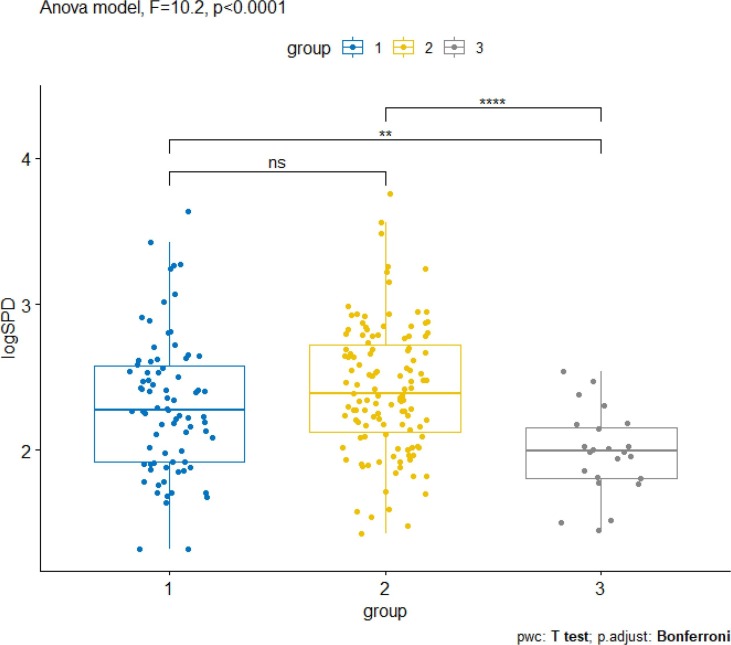
The 95 % confidence intervals for the mean level of SP-D were 244.5 ng/mL-327.7 ng/mL for severe, 187.8 ng/mL-271.3 ng/mL for mild and 64.4 ng/mL-144.6 ng/mL for healthy individuals, respectively (Fig. 1 ). Thus, a SP-D level ranging between 144.6 ng/mL and 187.8 ng/mL can be identified as threshold that best discriminates between COVID-19 hospitalized patients and healthy donors.
Fig. 1.
Distributions of SP-D levels in plasma (expressed as logSP-D) by COVID-19 incidence and (Bonferroni adjusted) paired comparisons of ANOVA (1: mild COVID-19 patients; 2: severe COVID-19 patients; 3: healthy donors). Significance: *** p < 0.01, **** p < 0.001.
Repeating the analysis and aggregating COVID-19 patents, 144.6–206.2 ng/mL was identified as threshold range to best discriminate this group from healthy donors.
Receiver Operating Characteristic (ROC) curve analysis was applied to determine the diagnostic sensitivity and specificity of plasma SP-D levels (as unique predictor) in patients diagnosed with COVID-19 at admission.
SP-D exhibits a satisfactory predictive power in differentiating COVID-19 cases from healthy individuals (AUC = 0.763, 95 % confidence interval [CI] = 0.682–0.845).
In particular, at a cut-off value of 150 ng/mL plasma SP-D levels exhibited sensitivity (event: COVID-19 case) of 78.5 % and specificity of 69.2 %. Increasing threshold over 150, sensitivity decreases and specificity increases (e.g. at 200.0, sensitivity of 53.6 %, specificity of 83.1 %).
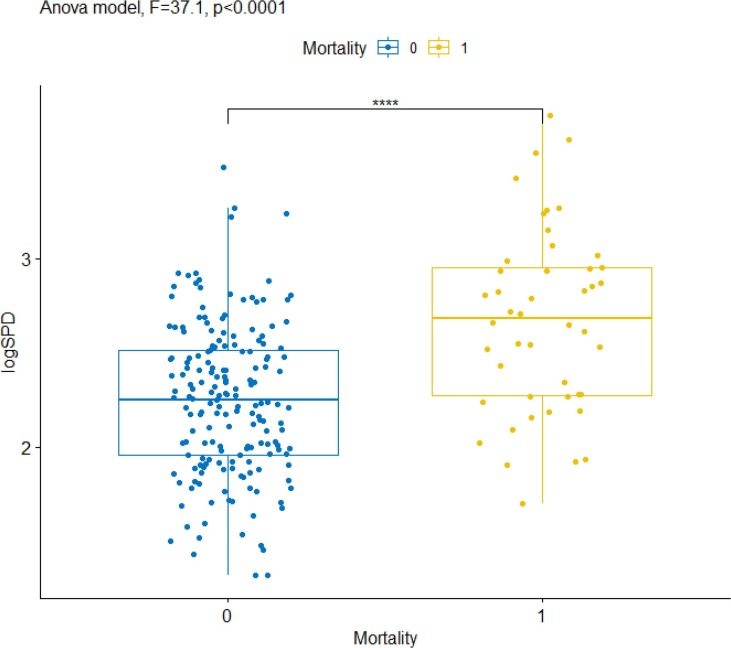
Moreover, strong significant differences among (log) SP-D mean levels were found when we compared dead and survived patients (F = 37.1, p < 0.0001, see Fig. 2 ): the 95 % confidence intervals for the mean level of SP-D were 417.9 ng/mL–661.5 ng/mL for death patients and 173.8 ng/mL–220.3 for survived.
Fig. 2.
Distributions of SP-D levels in plasma (expressed as logSP-D) by mortality condition and contrast among means of ANOVA. Significance: **** p < 0.001.
ROC curve analysis, applied to determine the diagnostic sensitivity and specificity of SP-D levels (as unique predictor) regarding mortality, shows a discrete predictive power (AUC = 0.738, 95 % CI = 0.621–0.850). In particular, at a cut-off value of 220, SP-D levels exhibited sensitivity (event: death) of 70.8 %, but poor specificity (50.6 %). At 250 of SP-D levels, sensitivity is of 70.5 % and specificity is of 58.2 %. The latter can be considered as threshold value to more accurately predict the disease outcome.
As expected, negligible difference among (log) SP-D mean levels was found by severity conditions within COVID-19 patients (F = 2.82, p-value = 0.095; AUC = 0.578), whereas stronger difference was observed among severe and non-severe (aggregating mild COVID-19 and healthy) patients (F = 9.69, p-value = 0.002), although with weak performance in term of predictive accuracy (AUC = 0.624). In this case, the suggested threshold varies over the range 208.5 ng/mL–218.1 ng/mL.
3.2. Predicting mortality and severity by SP-D and other clinical covariates
Clinical covariates available to assess the risk of mortality and COVID-19 severity include age, sex, prior diseases (Hypertension, Cardiovascular diseases, Diabetes, Respiratory diseases, Cancer, Sepsis, Kidney diseases, Obesity), procedures during the acute phase of COVID-19, as Intubation, Tracheotomy and CPAP (Continuous Positive Airway Pressure, a treatment for sleep apnea), platelets count, white blood cells and the levels of C-reactive protein C (CRP), an indicator of the inflammatory status.
Age, SP-D, platelets count, white blood cells and CRP are time-varying covariates, measured both upon admission and over time. In particular, these covariates were specified in the model both at T0 and as difference between T1 and T0 values (for SP-D, retained in its original measurement level, these variables were labeled as SP-D1 and Diff_SP-D, respectively).
The results of the Logistic Mixed model fitted on 299 observations (97 patients with SP-D at admission and follow-up) are described in Table 2 .
Table 2.
Generalized mixed model results on Mortality status (parameters of significant covariates).
| Effect | Estimate | Std. Error | Wald Chi-square | p-value | Odds Ratio* | 95 % OR | |
|---|---|---|---|---|---|---|---|
| Intercept | −8.0944 | 2.8150 | 8.2681 | 0.0040 | |||
| SP-D1 | 0.0025 | 0.0010 | 6.1171 | 0.0134 | 1.132 | 1.026 | 1.249 |
| Diff_SP-D | 0.0026 | 0.0009 | 8.7308 | 0.0031 | 1.141 | 1.045 | 1.245 |
| Age | 0.0713 | 0.0356 | 4.0142 | 0.0451 | 1.074 | 1.002 | 1.151 |
aOdds ratios are calculated at means of Age (73.4), of level of SP-D at T0 (150 ng/mL) and of difference among follow-up and admission values (Diff_SP-D). Odds ratios indicate how risk of mortality varies by unitary increase of Age, increases of 50 points of Diff_SP-D and increases of 50 points of SP-D levels from their base values.
Among all, Diff_SP-D is the most significant covariate in terms of risk of mortality: specifically, the risk of death increases by 14 % (4.5 %-24.5 %, 95 % confidence limits) for every increase of 50 SP-D points over time for a patient of mean age. The effect of SP-D at T0 (SP-D1) was also found strongly significant: the risk of death increases by 13.2 % (2.6 %-24.9 %, 95 % confidence limits) for every increase of 50 SP-D points from a base value of 150 ng/mL for a patient of mean age. No interactions were found significant among SP-D1, Diff_SP-D and Age.
The risk slightly increases with age (7.4 % more each year), although this effect is at the limit of classical significance levels (p = 0.045). Other covariates (measured at baseline or their variations from baseline) were not significant. Particularly, this also holds for CPAP or interactions with SP-D1, Diff_SP-D and Age.
The predictive accuracy of the estimated model is excellent (AUC = 0.844, 95 % confidence interval: 0.732–0.955), confirming that SP-D levels and variations play a significant role in identifying the main risk factors of mortality.
Regarding the outcome severity (the analysis was performed only at T0), the estimated AUC (0.651; 95 % CI: 0.584–0.718) demonstrates a modest predictive accuracy. Table 3 reports the significant covariates. Firstly, notice the strong significance of CPAP (in a similar model without interaction the CPAP main effect had OR = 3.44; 95 % CI: 1.79–6.62) on the risk of severe status.
Table 3.
Logistic regression results on Severity status (parameters of significant covariates).
| Effect | Coefficient | Std. Error |
Wald Chi-square |
p-value |
|---|---|---|---|---|
| Intercept | 0.2396 | 0.2861 | 0.7012 | 0.4024 |
| Kidney diseases | −0.4476 | 0.2339 | 3.6621 | 0.0557 |
| CPAP | 0.7977 | 0.2068 | 14.879 | 0.0001 |
| SP-D1 | 0.0004 | 0.0003 | 1.4366 | 0.2307 |
| SP-D1*CPAP | −0.0005 | 0.0003 | 2.7646 | 0.0964 |
Moreover, SP-D1 was found not significant, also exhibiting a weak significant interaction (p = 0.096) among SP-D and CPAP. More specifically, when SP-D increases (by 50 points) the risk of severity increase for patients without treatment for sleep apnea (OR = 1.04; 95 % CI: 0.98–1.09) and decreases for patients with the treatment (OR = 0.97; 95 % CI: 0.93–1.02). Both effects were significant only at the 10 % significant level. Finally, past Kidney diseases seem associated with (although modest in term of significance) protective factor for severity.
4. Discussion
The strong impact of COVID-19 on public health worldwide has attracted tremendous attention of the scientific community in the attempt to establish a reliable predictive strategy for this life-threatening disease. In the effort to identify a valid hallmark of COVID-19, our attention was focused on SP-D for several reasons: 1) SP-D is mainly produced by lung-resident cells and secreted in alveolar space [10], [16]; 2) loss of air–blood barrier integrity is responsible for the outward intravascular leakage of lung-secreted proteins [11]; 3) our previous observations from clinical samples analyses pointed out that COVID-19 patients under mechanical ventilation exhibited decreased pulmonary levels of SP-D [17]. Thus, SP-D may be a good indicator for alveolar damage, recognized as one of the causes for extrapulmonary manifestations and complications of COVID-19 [18].
In this study, we have demonstrated that plasmatic SP-D concentration correlates significantly with COVID-19 incidence. Notably, the analyses performed using an ANOVA model, showed a significant difference in the mean of log plasma SP-D upon admission between hospitalized patients and healthy donors (Fig. 1). Considering the 95 % confidence intervals, a value in the range of 144.6 and 206.2 ng/mL SP-D was identified as threshold to discriminate COVID-19 cases and healthy donors. In addition, significant discrepancies in SP-D levels at T0 were observed when comparing dead vs survived patients. In the mentioned conditions, SP-D hematic concentrations were significantly higher for COVID-19 patients and dead cases (Fig. 1, Fig. 2).
The augmented SP-D plasma levels here observed in COVID-19 hospitalized patients as compared with healthy individuals are in line with previous studies conducted on SARS-CoV and SARS-CoV-2 infected patients [14], [19], [20]. Moreover, our values result highly comparable to those obtained in other cases (e.g. patients of the Fourth Hospital of Yiyang of Hunan, China- February-March 2020) [12]. This enhanced amount of SP-D into the blood of COVID-19 patients is likely favored by the inflammatory framework associated with the pulmonary infection [2], [4]: it has been proved that activated inflammation state and cytokine release promote the secretion of SP-D into the blood circulation, which persists and increases over lung injury [21], [22]. It is worth noting that not all the studies reported in literature recorded high hematological levels of SP-D in COVID-19 patients and most of them assume this is caused by a downregulation in the surfactant production subsequent to SARS-CoV-2 infection of alveolar type II cells [23], [24], [25]. However, our data are supported by the fact that SP-D is secreted also by other lung resident cells (particularly Clara cells) [26], [27] and that in other pathological conditions, where the infective agent preferentially target type II pneumocytes (e.g., influenza A virus) [28], an enhancement of SP-D levels is observed in severe patients [25], [29].
Our study evidenced that SP-D plasma levels are not useful to distinguish the severity of COVID-19 patients, even using a log of their values (Fig. 1). On the other hand, a strong correlation of SP-D plasma levels with mortality has been observed (Fig. 2). It should be pointed out that an analogue significant correlation with mortality has been previously described only in one study, where higher plasma SP-D levels at the time of admission were detected in non-survivors versus survivor patients [20]. In the other investigations reported in literature, a significant increase of SP-D is generally associated to a stratification of disease severity [12], [13], [14]. Differences from case to case can be due to several reasons, including the methods used to determine the COVID-19 severity, the characteristics and the number of the patients recruited. With regard to the latter, it is important to highlight that our study includes a quite large number of patients (>200), compared to the ones previously quoted, which are based on a much smaller collection of samples (≤100).
Further analysis conducted with Logistic Mixed models, highlighted that SP-D at T0 (SP-D1) and the difference among follow-up and admission values (Diff_SP-D), in a model with Age, resulted the strongest significant risk factors of mortality (model predictive accuracy, AUC = 0.844). The latter result corroborates the hypothesis that high plasmatic level of SP-D and its increase over time correlate with an impaired clinical status and an unfavorable outcome. The higher significance recorded in Diff_SP-D reveals that it is of utmost importance to monitor the follow-up in COVID-19 patients besides measuring SP-D plasma levels at admission. As regards the severity, it has been observed that an increased risk does not correlate with SP-D values as well as with all the covariates considered in this study, except CPAP.
In conclusion, the overall data indicate SP-D as a valid marker of COVID-19 and its outcome. Notably, 150 ng/mL and 250 ng/mL SP-D in plasma can be proposed as cut-off values to predict the disease incidence and mortality. This is the first study in Europe in which a significant correlation between SP-D levels and mortality has been determined, by an accurate statistical analysis on a large number of patients. The latter evidence makes SP-D as an important prognostic factor in hospitalized patients. Therefore, the early detection of this protein should be considered for scheduling adequate preventive treatments for COVID-19 patients.
CRediT authorship contribution statement
Lucia Salvioni: Investigation, Methodology, Validation, Writing – original draft. Filippo Testa: Investigation, Methodology, Validation. Adela Sulejmani: Investigation, Methodology, Validation. Francesca Pepe: Investigation, Methodology. Pietro Giorgio Lovaglio: Formal analysis, Visualization, Writing – original draft. Paolo Berta: Validation, Writing – review & editing. Roberto Dominici: Conceptualization, Investigation. Valerio Leoni: Conceptualization, Resources, Validation, Writing – review & editing. Davide Prosperi: Funding acquisition, Writing – review & editing. Giorgio Vittadini: Conceptualization, Writing – review & editing. Miriam Colombo: Supervision, Writing – review & editing. Luisa Fiandra: Project administration, Conceptualization, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by Fondazione Cariplo (project ref. 2020-0934) and Fondazione Banco Farmaceutico, Italy.
Data availability
Data will be made available on request.
References
- 1.Ministero della Salute, Covid-19 - Situazione nel mondo, 18/05/2020. (2021) 1–7. http://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?lingua=italiano&id=5338&area=nuovoCoronavirus&menu=vuoto.
- 2.Hartl D., Griese M. Surfactant protein D in human lung diseases. Eur. J. Clin. Invest. 2006;36:423–435. doi: 10.1111/j.1365-2362.2006.01648.x. [DOI] [PubMed] [Google Scholar]
- 3.Guo C.-J., Atochina-Vasserman E.N., Abramova E., Foley J.P., Zaman A., Crouch E., Beers M.F., Savani R.C., Gow A.J., Stamler J. S-Nitrosylation of surfactant Protein-D controls inflammatory function. PLoS Biol. 2008;6(11):e266. doi: 10.1371/journal.pbio.0060266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watson A., Madsen J., Clark H.W. SP-A and SP-D: dual functioning immune molecules with antiviral and immunomodulatory properties. Front. Immunol. 2021;11 doi: 10.3389/fimmu.2020.622598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okamoto T., Fujii M., Furusawa H., Tsuchiya K., Miyazaki Y., Inase N. The usefulness of KL-6 and SP-D for the diagnosis and management of chronic hypersensitivity pneumonitis. Respir. Med. 2015;109:1576–1581. doi: 10.1016/j.rmed.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H., Imai Y., Fujishima T., Shiratori M., Murakami S., Chiba H., Kon H., Kuroki Y., Abe S. Diagnostic significance of surfactant proteins A and D in sera from patients with radiation pneumonitis. Eur. Respir. J. 2001;17:481–487. doi: 10.1183/09031936.01.17304810. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi H., Kuroki Y., Tanaka H., Saito T., Kurokawa K., Chiba H., Sagawa A., Nagae H., Abe S. Serum levels of surfactant proteins A and D are useful biomarkers for interstitial lung disease in patients with progressive systemic sclerosis. Am. J. Respir. Crit. Care Med. 2000;162:258–263. doi: 10.1164/ajrccm.162.1.9903014. [DOI] [PubMed] [Google Scholar]
- 8.Greene K.E., Wright J.R., Steinberg K.P., Ruzinski J.T., Caldwell E., Wong W.B., Hull W., Whitsett J.A., Akino T., Kuroki Y., Nagae H., Hudson L.D., Martin T.R. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am. J. Respir. Crit. Care Med. 1999;160:1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 9.Eisner M.D. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003;58:983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The human protein atlas. https://www.proteinatlas.org/ENSG00000133661-SFTPD/tissue.
- 11.Sorensen G.L. Surfactant Protein D in respiratory and non-respiratory diseases. Front. Med. (Lausanne) 2018;5 doi: 10.3389/fmed.2018.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong M., Xiong Y., Zhu C., Xu H., Zheng Q., Jiang Y., Zou L., Xiao X., Chen F., Yan X., Hu C., Zhu Y. Serum surfactant protein D in COVID-19 is elevated and correlated with disease severity. BMC Infect. Dis. 2021;21:737. doi: 10.1186/s12879-021-06447-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito A., Kuronuma K., Moniwa K., Kodama K., Takahashi S., Takahashi H., Chiba H. Serum surfactant protein A and D may be novel biomarkers of COVID-19 pneumonia severity. Researchsquare Com. 2020:1–17. [Google Scholar]
- 14.Alay H., Laloglu E. The role of angiopoietin-2 and surfactant protein-D levels in SARS-CoV-2-related lung injury: a prospective, observational, cohort study. J. Med. Virol. 2021;93:6008–6015. doi: 10.1002/jmv.27184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sulejmani A., Galimberti E., Giacobone C., Milano A., Scopetta E., Intra J., Falbo R., Sarto C., Leoni V., Brambilla P. Baseline characteristics of COVID-19 Italian patients admitted to Desio Hospital, Lombardy: a retrospective study. Scand. J. Clin. Lab. Invest. 2021;81:18–23. doi: 10.1080/00365513.2020.1846211. [DOI] [PubMed] [Google Scholar]
- 16.Crouch E.C. Structure, biologic properties, and expression of surfactant protein D (SP-D) Biochimica et Biophysica Acta (BBA) – Mol. Basis Disease. 1998;1408(2-3):278–289. doi: 10.1016/s0925-4439(98)00073-8. [DOI] [PubMed] [Google Scholar]
- 17.Arroyo R., Grant S.N., Colombo M., Salvioni L., Corsi F., Truffi M., Ottolina D., Hurst B., Salzberg M., Prosperi D., Kingma P.S. Full-Length Recombinant hSP-D binds and inhibits SARS-CoV-2. Biomolecules. 2021;11:1114. doi: 10.3390/biom11081114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y., Freedberg D.E., Kirtane A.J., Parikh S.A., Maurer M.S., Nordvig A.S., Accili D., Bathon J.M., Mohan S., Bauer K.A., Leon M.B., Krumholz H.M., Uriel N., Mehra M.R., Elkind M.S.V., Stone G.W., Schwartz A., Ho D.D., Bilezikian J.P., Landry D.W. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y.P., Liu Z.H., Wei R., Pan S.D., Mao N.Y., Chen B., Han J.J., Zhang F.S., Holmskov U., Xia Z.L., de Groot P.G., Reid K.B.M., Xu W.B., Sorensen G.L. Elevated Plasma Surfactant Protein D (SP-D) levels and a direct correlation with anti-severe acute respiratory syndrome coronavirus-specific IgG antibody in SARS patients. Scand. J. Immunol. 2009;69:508–515. doi: 10.1111/j.1365-3083.2009.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerget B., Kerget F., Koçak A.O., Kızıltunç A., Araz Ö., Uçar E.Y., Akgün M. Are serum Interleukin 6 and surfactant Protein D levels associated with the clinical course of COVID-19? Lung. 2020;198:777–784. doi: 10.1007/s00408-020-00393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurt A., Turut H., Acipayam A., Kirbas A., Yuce S., Cumhur Cure M., Cure E. Investigation of surfactant protein-D and interleukin-6 levels in patients with blunt chest trauma with multiple rib fractures and pulmonary contusions: a cross-sectional study in Black Sea Region of Turkey. BMJ Open. 2016;6(10):e011797. doi: 10.1136/bmjopen-2016-011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaunsbaek M.Q., Rasmussen K.J., Beers M.F., Atochina-Vasserman E.N., Hansen S. Lung Surfactant Protein D (SP-D) response and regulation during acute and chronic lung injury. Lung. 2013;191:295–303. doi: 10.1007/s00408-013-9452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agustama A., Surgean Veterini A., Utariani A. Correlation of surfactant Protein-D (SP-D) serum levels with ARDS severity and mortality in Covid-19 patients in Indonesia. Acta Med. Acad. 2022;51:21–28. doi: 10.5644/ama2006-124.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togashi Y., Kono Y., Okuma T., Shioiri N., Mizushima R., Tanaka A., Ishiwari M., Toriyama K., Kikuchi R., Takoi H., Abe S. Surfactant protein D: A useful biomarker for distinguishing COVID-19 pneumonia from COVID-19 pneumonia-like diseases. Health Sci. Rep. 2022;5 doi: 10.1002/hsr2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choreño-Parra J.A., Jiménez-Álvarez L.A., Ramírez-Martínez G., Cruz-Lagunas A., Thapa M., Fernández-López L.A., Carnalla-Cortés M., Choreño-Parra E.M., Mena-Hernández L., Sandoval-Vega M., Hernández-Montiel E.M., Hernández-García D.L., Ramírez-Noyola J.A., Reyes-López C.E., Domínguez-Faure A., Zamudio-López G.Y., Márquez-García E., Moncada-Morales A., Mendoza-Milla C., Cervántes-Rosete D., Muñoz-Torrico M., Luna-Rivero C., García-Latorre E.A., Guadarrama-Ortíz P., Ávila-Moreno F., Domínguez-Cherit G., Rodríguez-Reyna T.S., Mudd P.A., Hernández-Cárdenas C.M., Khader S.A., Zúñiga J. Expression of surfactant Protein D distinguishes severe pandemic Influenza A(H1N1) from Coronavirus Disease 2019. J. Infect. Dis. 2021;224:21–30. doi: 10.1093/infdis/jiab113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown-Augsburger P., Chang D., Rust K., Crouch E.C. Biosynthesis of surfactant Protein D. J. Biol. Chem. 1996;271:18912–18919. doi: 10.1074/jbc.271.31.18912. [DOI] [PubMed] [Google Scholar]
- 27.Crouch E.C. Surfactant protein-D and pulmonary host defense. Respir. Res. 2000;1:6. doi: 10.1186/rr19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinheimer V.K., Becher A., Tonnies M., Holland G., Knepper J., Bauer T.T., Schneider P., Neudecker J., Ruckert J.C., Szymanski K., Temmesfeld-Wollbrueck B., Gruber A.D., Bannert N., Suttorp N., Hippenstiel S., Wolff T., Hocke A.C. Influenza A Viruses Target Type II Pneumocytes in the human lung. J. Infect. Dis. 2012;206:1685–1694. doi: 10.1093/infdis/jis455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Delgado C., Krötzsch E., Jiménez-Alvarez L.A., Ramírez-Martínez G., Márquez-García J.E., Cruz-Lagunas A., Morán J., Hernández C., Sierra-Vargas P., Avila-Moreno F., Becerril C., Montaño M., Bañales-Méndez J.L., Zúñiga J., Buendía-Roldán I. Serum Surfactant Protein D (SP-D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung. 2015;193:25–30. doi: 10.1007/s00408-014-9669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.