Abstract
Background
Non-pharmacological strategies that have been proposed by complementary medical systems, can be effective in management of COVID-19.
Methods
This study was designed as a three-arm, assessor-blinded, randomized controlled trial. A total of 139 hospitalized COVID-19 patients were randomly assigned into three groups: (1) acupuncture (ACUG), (2) cupping (CUPG), and (3) control (CTRG). All participants received conventional treatment. The primary study endpoint included changes in respiratory signs including oxygen saturation (SpO2) and respiratory rate (RR). The secondary endpoints were COVID-19-related hospitalization duration and serious adverse events such as intensive care unit (ICU) admission, intubation or death, all up to day 30. Also, improvements in cough, dyspnea, chest tightness, oxygen demand, anorexia, headache, weakness, sore throat, and myalgia were evaluated.
Results
Forty-two patients in ACUG, 44 patients in CUPG, and 42 patients in CTRG completed the trial. After 3 days, SpO2 and RR improved significantly in CUPG and ACUG compared with CTRG (effect size: 8.49 (6.4 to 10.57) and 8.51 (6.67 to 10.34), respectively: p<0.001). Compared with CTRG, patients in CUPG and ACUG recovered faster (mean difference: 6.58 (4.8 to 8.35) and 9.16 (7.16 to 11.15), respectively) and except for two patients in ACUG who were admitted to ICU, none of patients in ACUG or CUPG needed ICU or intubation (p<0.001 in comparison to CTRG). Amelioration of clinical COVID-19 related symptoms reached a high level of statistical significance in CUPG and ACUG in comparison with CTRG (p<0.01).
Conclusion
Cupping and acupuncture are promising safe and effective therapies in management of COVID-19. Trial registration: This study was registered at Iranian Registry of Clinical Trials: IRCT20201127049504N1 (https://en.irct.ir/trial/52621).
Keywords: COVID-19, Persian medicine, Traditional Chinese Medicine, Cupping therapy, Acupuncture
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), which causes Coronavirus disease 2019 (COVID-19), has spread rapidly throughout the world since December 2019. The associated burden on health-care systems, especially intensive care units (ICU), has been overwhelming in several affected countries1. At present, no treatment options with strong evidence of clinical benefit exist. Thus, national and international guidelines recommend using experimental drugs as part of investigational trials2. There is a high demand and urgent global need for effective, safe and inexpensive treatment options for patients with moderate to severe COVID-19, especially in low- and middle-income countries (LMICs) with high disease burden and scarce resources3.
Patients with moderate-to-severe COVID-19 often suffer from breathlessness, dry cough and chest tightness that may progress to acute respiratory distress syndrome (ARDS) and septic shock. This is the result of a virus-induced cytokine storm, an overly aggressive immune response in the body4. Thus, any drug or medical manipulation that improves the body's ability to cope with the cytokine storm and modulate the immune system, can be beneficial. Many published reports have shown that Complementary and Alternative Medicine (CAM) can efficaciously help in the management of acute and critical illnesses, including infectious disease outbreaks5.
Once conventional medicine failed to deliver highly effective and approved treatment in past epidemic diseases including the Middle East Respiratory Syndrome (MERS), Severe Acute Respiratory Syndrome (SARS) and H1N1 influenza, CAM was used in combination with routine treatment to attain synergistic effects6, 7, 8. As a CAM modality against COVID-19, “China's model” has been fully confirmed as successful according to the World Health Organization (WHO) reports. The report of WHO-China Joint Mission published on February 2020, specifically points out the highly effective role of non-pharmaceutic measures such as acupuncture, moxibustion and cupping in management of COVID-199.
According to research reports, cupping contributes to activation of the complement system, as well as modulation of the cellular component of immunity. It reduces peripheral and local P-substance and inflammation significantly, and regulates the immune system. As an immunomodulator, cupping can significantly increase levels of immune products such as interferon and tumor necrotizing factor10. According to Persian Medicine (PM) resources such as “Exir-e Azam” and “Al-Havi”, warm cupping of the posterior thorax, can play an important role in treatment of acute respiratory diseases11, 12, 13.
Likewise, acupuncture is an effective adjunctive non-pharmacological treatment, with a wealth of experience in the prevention and control of epidemic diseases since ancient times. Having been used for various acute infectious diseases in the modern times, the efficacy of this CAM modality has been clearly and reliably reported14. Acupuncture decreases expression of the pro-inflammatory proteins such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6 and increases cytotoxicity of leukocytes and serum IL-10 level. It can also reduce pulmonary inflammation and alleviate airway inflammation15,16. It seems that cupping and acupuncture improve the body's ability to cope with a cytokine storm, a process that has been found to lead to lung inflammation, pneumonia and death in COVID-19.
Global randomized controlled trials of CAM manual therapies in hospitalized COVID-19 patients have shown conflicting results but reported potential 1) decrease in the number of severe cases and the intensive care burden; 2) increase in cure rate, and improvement in hospital discharge rate9. The COVID Iran Acupuncture and Cupping (COIRACCU) trial is, to our knowledge, the first randomized controlled trial of non-pharmacological treatment in COVID-19 performed entirely in an LMIC. For the first time, we evaluated the effectiveness of acupuncture -as a pure Traditional Chinese Medicine (TCM) method- versus warm cupping -as a PM method- to prevent progression of COVID-19, decrease symptom duration and improve respiratory signs including oxygen saturation (SpO2) and respiratory rate (RR) among hospitalized patients with moderate-to-severe SARS-CoV-2 infection.
2. Methods
The protocol of this study was registered at the Iranian Registry of Clinical Trials (IRCT) (identifier: IRCT20201127049504N1, https://en.irct.ir/trial/52621). This study is reported according to the CONSORT checklist of information when reporting a randomized trial assessing non-pharmacological treatments.
2.1. Trial design
COIRACCU was a randomized, three-arm trial, with blinded outcome assessment and 1:1:1 randomization to acupuncture, warm cupping and control groups. This study investigated the clinical outcomes of acupuncture or warm cupping plus standard care versus standard care alone in hospitalized COVID-19 patients with moderate-to-severe symptoms.
2.2. Participants
After approval by the Tehran University of Medical Science ethics committee (IR.TUMS.MEDICINE.REC.1399.1124) and receiving a trial registration code from IRCT, the study was conducted in the Department of infection diseases, Shohadaye Pakdasht Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran and Shahid Ziaeian Hospital, Tehran University of Medical Sciences, Tehran, Iran. Eligible patients included 18-75 year old men and non-pregnant women admitted to hospital with moderate-to-severe SARS-CoV-2 infection - defined according to the Iranian clinical management protocol for COVID-19 ninth version, December 202017. This guideline defines moderate disease as RR 15–24 per minute and SpO2 90-93%; and severe disease as RR ≥24 per minute or SpO2 <90% in ambient air. Other inclusion criteria comprised chest CT-scan with COVID-19 pattern. Patients with respiratory failure who required mechanical ventilation, pregnant or lactating women, and also patients with a positive history of allergies or any skin lesions in the area of manipulation were excluded. Patients with a diagnosis of pulmonary embolism and serious underlying diseases, such as coronary heart disease, chronic obstructive pulmonary disease, obstructive pulmonary disease, meningitis, encephalitis, or cirrhosis were not included. Laboratory exclusion criteria comprised platelet counts of less than 50000 cells per µL, and alanine aminotransferase or aspartate aminotransferase concentrations more than ten times upper normal limits within 24 h of screening or baseline.
All patients or their legally acceptable representatives provided written informed consent to participate in the study. The study was conducted in accordance with Iran regulatory requirements. The protocol and any amendments were approved by the Tehran University of Medical Sciences research and ethics committee at each study site in accordance with Iran regulations.
2.3. Randomization
A researcher explained the details of the trial and provided participants with informed consent forms to sign and complete. After applying the inclusion and exclusion criteria, eligible participants were randomly assigned in a 1:1:1 ratio to three groups using block randomization. The randomization sequence was generated using Random Allocation Software (RAS), version 9.4 by a researcher who was not in direct contact with patients and not involved in statistical analysis. Patients were divided into three intervention groups as A: acupuncture group (ACUG); B: cupping group (CUPG); and C: control group (CTRG). To perform random allocation, 23 blocks of 6 (AABBCC, AABCBC, AACBCB, etc.) were prepared and placed in envelopes. In order of entry and hospitalization (the time recorded in patient files), one envelope was randomly selected and based on the obtained block, six patients were assigned to three groups. If the number of patients to be randomized was more than 6, it was necessary to open more than one envelope.
This study was assessor-blinded. Participants and researchers performing the procedures were not blind, but the researcher who completed the questionnaire and evaluated outcomes and the analyst were unaware of the grouping. Intervention groups were available to the outcome assessor as A, B, and C and the analyst had access to data as groups A, B and C.
2.4. Interventions
All patients in both intervention and control groups received conventional treatment based on the Iranian clinical management protocol for COVID-19, ninth version17. Participants in intervention group were allocated to either cupping therapy or acupuncture.
In the cupping group, we combined TCM and PM techniques of cupping in respiratory and infective diseases. The primary method included retained warm cupping on Urinary Bladder-13 (BL13) acupoint (on the back, 1.5 cun lateral to the lower border of the spinous process of the third thoracic vertebra) for one minute. This was supplemented with sliding (moving) cupping of paraspinal (1.5 cun lateral to the spinous process of thoracic vertebrae) and lung regions at least 1.5 cun lateral to the spinous process of thoracic vertebrae for five minutes. Patients were in sitting or lateral decubitus position. The procedure was performed three times a day for 3-7 consecutive days (based on patient hospitalization, from the first day of hospitalization to the maximum of the seventh day of hospitalization), with a medium glass (5 cm mouth diameter and 8 cm height), and a suction amount of 10 to 15 mm.
Cups had the texture of a transparent glass. Moving cupping involves standardized manipulations in PM10. First of all, vaseline is applied to the posterior thorax as a lubricant with no therapeutic effect. Using the flashing method, a 95% ethanol-soaked cotton ball is held with tweezers and the cup is held upside-down. After the cotton ball is ignited, it is immediately moved into the cup and removed; the cup is then quickly placed on the skin of apex of lungs, and the created vacuum causes the skin to be drawn into the cup. Then, the cup is held in one hand and pulled and pushed from the apex toward the base of the lungs and back while applying light force, such that the skin of the treatment area turns purple. Even force is applied when moving the cup to prevent falling off due to air leakage. This is repeated on the posterior thorax for five minutes.
In the acupuncture group, standard needling method (without electrical stimulation and by using disposable tube needles) was performed with 0.25 mm × 40 mm, single-use, sterile, stainless-steel needles. Selected acupoints included: Governor Vessel-20 (GV20; Baihui), Lung-5 (LU5; Chize), Lung-7 (LU7; Lieque), Large Intestine-4 (LI4; Hegu), Liver-3 (LR3; Taichong), Liver-14 (LR14; Qimen), Conception Vessel-12 (CV12; Zhongwan), Conception Vessel-17 (CV17; Tanzhong) and Stomach-36 (ST36; Zusanli). Patients were in recumbent position. Hand sanitizer was used for hand hygiene. Each acupoint was sterilized strictly according to the requirements of disinfection and protection in the negative-pressure ward. After disinfection, the cotton balls were disposed of into the infectious contaminant bucket in the inpatient ward buffer area.
The needles were inserted perpendicularly, at a 90 degree angle with the skin, 20-25 mm in depth at LR3, LI4, ST36 and LU5. At GV20, LU7, CV12, CV17 and LR14 the needle was inserted obliquely, at a 15-30 degree angle, with the needle tip toward the elbow at LU7 and toward the distal point at other points, 5 to 10 mm in depth. The acupoints, except GV20, were stimulated and manipulated after 10 minutes with even manipulating technique for 10 seconds. Needle manipulation was accomplished by means of a twirling, and up and down movement. Needles were retained for 20 minutes each session, and were then disposed of in the sharp instrument box in the inpatient ward buffer area. The acupuncture therapy was given once daily, for 3-7 days consecutively (based on patient hospitalization, from the first day of hospitalization to a maximum of the seventh day of hospitalization).
Interventions of this study were performed by one acupuncturist and two physicians performing cupping. The acupuncturist had more than two years of treatment experience, and the cupping physicians had more than eight years of cupping experience.
2.5. Outcomes
The primary endpoint was changes in respiratory signs (SpO2 percentage and RR). Secondary endpoints were COVID-19-related hospitalization duration and serious adverse events (SAEs) such as ICU admission, intubation or death, all up to day 30. Further secondary clinical outcomes included severity scores of respiratory symptoms, defined by cough, shortness of breath or dyspnea, unusual chest pain or chest tightness, and type of oxygen therapy, based on the National Institute of Health (NIH) endorsed Protocol to research Patient Experience of COVID-19, modified Medical Research Council (mMRC) Dyspnea Scale and Respiratory Symptoms Scale18,19. Moreover, severity of anorexia, headache, weakness, sore throat, and myalgia were recorded. Study evaluations were through intervention days (3-7 days) based on hospitalization duration. Symptom scores were self-reported by participants every day after cupping therapy or acupuncture on an ordinal scale: 0 = not affected, 1 = minimally affected, 2 = affected and 3 = severely affected, and the type of oxygen therapy was determined: 0 = not needed, 1 = nasal cannula, 2 = simple face mask, 3 = mask with reservoir and 4 = continuous positive airway pressure and non-invasive ventilation (CPAP/NIV) mask. We also checked SpO2 just before and five minutes after interventions. Additionally, SpO2 was recorded four hours after acupuncture and also the last cupping session each day. We collected longitudinal outcomes in the control group at the same time as intervention groups. Outcomes were evaluated at three time points: beginning of the study, day 3 and day 7 of intervention.
2.6. Statistical analysis
At the time of design and registration of the proposal, no study had been conducted to investigate the effect of cupping and acupuncture on COVID-19. Therefore, using Power Analysis and Sample Size 11 (PASS-11) software, a sample size of 30 patients in each arm and a total of 90 participants was calculated in order to achieve 90% power (α = 0.05). As decided by the epidemiologist consultant, an Interim Analysis was performed during the study, to adjust sample size based on calculated power.
After data collection, all patients who completed the interventions were included in statistical analysis, which was performed using IBM SPSS version 22 and MedCalc software. Data is presented as mean with standard deviation (SD) and confidence interval (CI) for quantitative variables and frequency with percentage for qualitative ones. In case of normal distribution, one-way ANOVA with Bonferroni as post-hoc. For non-normally distributed data, Friedman test and Kruskal-Wallis test were utilized. To compare the frequency distribution between groups, χ2 or Fisher's exact test were used.
3. Results
3.1. Patients
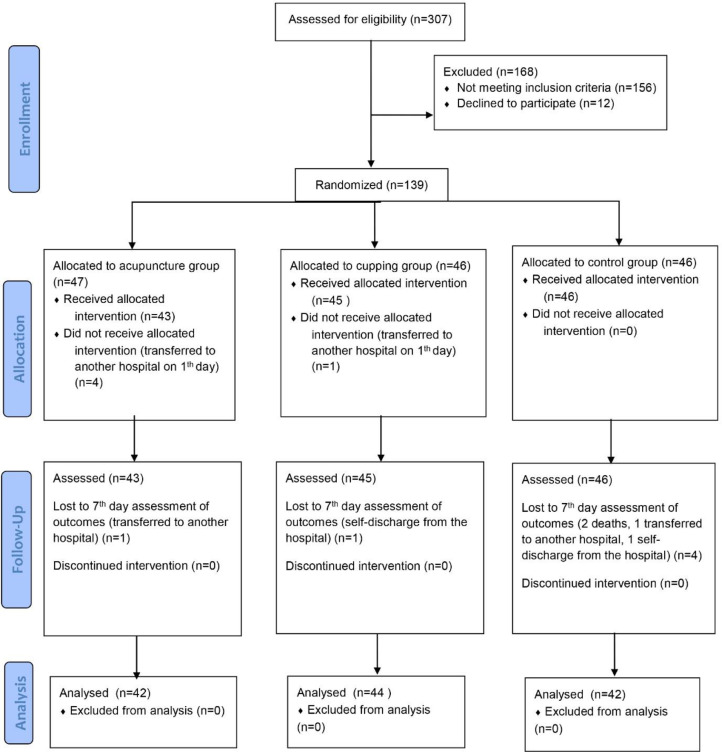
Initially, 90 COVID-19 patients were planned to be included. However, the power analysis performed during the study revealed low power, and thus, sample size was increased according to the consult of the epidemiologist. Finally, out of the 307 patients assessed for eligibility, 139 underwent randomization and were assigned to the groups between February 15 and May 15, 2021. Forty-three patients received acupuncture plus standard care, 45 patients received cupping plus standard care, and 46 patients received only standard care (as CTRG). Patients who were transferred to another hospital and lost to follow-up were excluded. Overall, 42 patients (89.36%) in the ACUG, 44 patients (95.65%) in the CUPG, and 42 patients (91.30%) in the CTRG completed the trial. Fig. 1 represents the patient selection process according to Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Fig. 1.
Consort flow diagram of the patient selection process.
The mean age of ACUG, CUPG, and CTRG were 52.74±12.72, 54.68±13.79, and 57.90±12.15 years, respectively (p=0.187). Seventeen patients in ACUG (40.47%), 16 patients in CUPG (36.36%), and 14 patients in CTRG (33.33%) were male (p=0.793). There was no significant difference between ACUG, CUPG, and CTRG in terms of underlying disease, radiologic lung involvement, or laboratory data (p>0.05) (Table 1 and Table S1).
Table 1.
Baseline characteristics of randomized participants.
| Characteristic | Group |
P value | |||
|---|---|---|---|---|---|
| Acupuncture | Control | Cupping | |||
| Age (years) (Mean ± SD) |
52.74±12.72 | 57.90±12.15 | 54.68±13.79 | 0.187† | |
| Sex N (%) |
Male | 17 (40.47) | 14 (33.33) | 16 (36.36) | 0.793* |
| Female | 25 (59.53) | 28 (66.67) | 28 (63.64) | ||
| Patients with coexisting disorders N (%) |
Diabetes mellitus | 13 (30.95) | 15 (35.71) | 10 (22.72) | 0.410* |
| Hypertension | 15 (35.71) | 17 (40.47) | 12 (27.27) | 0.425* | |
| Hypothyroid | 5 (11.90) | 1 (2.38) | 2 (4.54) | 0.167* | |
| Anemia | 2 (4.76) | 0 (0.0) | 3 (6.81) | 0.249* | |
| Asthma | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Cancer | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Chronic kidney disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Chronic obstructive pulmonary disease | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | |
| Duration of symptoms before admission1 (days) (Mean ± SD) |
5.29±3.71 | 7.02±3.50 | 7.26±3.64 | 0.026† | |
| Computed tomography (CT) scan findings N (%) |
Mild | 18 (31.0) | 22 (37.9) | 18 (31.0) | 0.524* |
| Moderate | 16 (32.7) | 17 (34.7) | 16 (32.7) | 0.926* | |
| Severe | 4 (28.6) | 4 (28.6) | 6 (42.9) | 0.778* | |
| Non typical COVID-19 | 3 (34.9) | 1 (14.3) | 3 (42.9) | 0.561* | |
*Chi-Square Tests; †ANOVA test; 1: data is represented by mean ± standard deviation or frequency (percentage).
3.2. Primary outcome (ACUG and CUPG vs. CTRG)
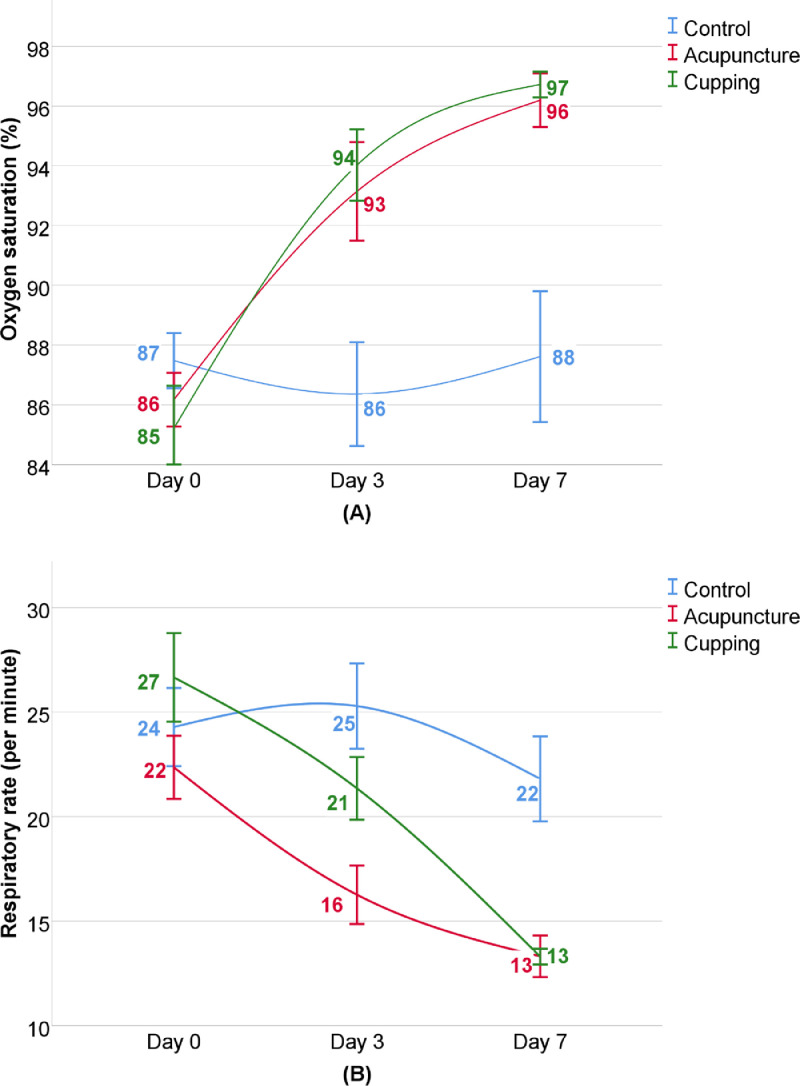
The improvement in primary outcome is shown in Table 2 and Fig. 2. Between-group analysis of RR showed no significant difference at the beginning of the study between CTRG and ACUG or CUPG, as well as SpO2 between CTRG and ACUG; however, the level of SpO2 in CTRG was significantly higher in comparison with CUPG (p=0.013), The SpO2 progression in ACUG and CUPG substantially increased after 3 days of intervention and lasted up to day 7 (mean change, 10% and 11%, respectively) (p<0.001); however, there was no significant difference even after day 7 in CTRG. No significant difference was reported between ACUG and CUPG (p>0.05). While RR did not change remarkably in CTRG within 3 days, it decreased significantly in CUPG and ACUG after 3 days (p<0.001). Although within group analysis showed significant reduction in RR in all groups after 7 days, (mean change: CTRG: 11.4%, ACUG: 40.64%, and CUPG: 50.41%), mean difference of RR in ACUG and CUPG were significantly higher than CTRG (p<0.001).
Table 2.
Statistical analysis of primary outcomes1.
| Symptom | Time | Group |
Mean difference |
||||
|---|---|---|---|---|---|---|---|
| Acupuncture | Control | Cupping | Δ1 | Δ2 | Δ3 | ||
| Oxygen saturation (%) |
Before intervention | 86.2±2.90a,b | 87.83±2.72a | 85.19±4.75b | 1.63 (0.40 to 2.85) |
2.64 (0.96 to 4.31)** |
-1.01 (-2.7 to 0.68) |
| Day 3 | 93.12±5.36a | 87.5±4.42b | 94.02±3.88a | -5.62 (-7.75 to -3.48)*** |
-6.52 (-8.30 to -4.73)*** |
0.9 (-1.09 to 2.89) |
|
| Day 7 | 96.2±2.84a | 87.61±6.47b | 96.72±1.40a | -8.59 (-10.75 to -6.42)*** |
-9.11 (-11.09 to -7.12)*** |
0.52 (-0.43 to 1.47) |
|
| P value (within groups) | <0.001 | 0.194 | <0.001 | ||||
| Respiratory rate (per minute) |
Before intervention | 22.44±4.88a | 24.64±6.06a,b | 26.79±6.99b | 2.2 (-0.18 to 4.58) |
-2.15 (-4.96 to 0.66) |
4.35 (1.75 to 6.94)** |
| Day 3 | 16.32±4.53a | 24.81±5.83b | 21.35±4.87c | 8.49 (6.22 to 10.75)*** |
3.46 (1.16 to 5.75)** |
5.03 (3.01 to 7.04)*** |
|
| Day 7 | 13.32±3.15a | 21.81±6.01b | 13.3±1.22a | 8.49 (6.4 to 10.57) |
8.51 (6.67 to 10.34)*** |
-0.02 (-1.03 to 0.99) |
|
| P value (within groups) | <0.001 | 0.007 | <0.001 | ||||
Data is represented by mean ± standard deviation for groups and mean (95% confidence interval) for mean difference; different letters (a-c) show significant statistical difference between groups and significance levels are represented as Δ1: acupuncture compared to control; Δ2: cupping compared to control; Δ3: acupuncture compared to cupping: * P<0.05, ** P<0.01, or *** P<0.001.
Fig. 2.
Progression of primary outcomes: (A) Oxygen saturation (%), (B) Respiratory rate (per minute).
3.3. Secondary outcomes (ACUG and CUPG vs. CTRG)
Patients who received acupuncture plus standard care recovered a mean of 6 days faster than CTRG (mean difference: 6.16, CI: 4.16 to 8.15) (p<0.001). The fastest recovery time belonged to patients who received cupping plus standard care (mean: 3.82±1.45 days). Throughout the intervention period, COVID-19-related SAEs were significantly lower in ACUG and CUPG in comparison with CTRG: intubations and deaths were reported in 19% of CTRG (8 patients), but neither in ACUG (relative risk: 0.05), nor in CUPG (relative risk: 0.05). None of the patients in the CUPG (relative risk:0.03), two patients in ACUG (4.8%; relative risk: 0.15), and 13 patients in CTRG (31%) received ICU care (p<0.001).
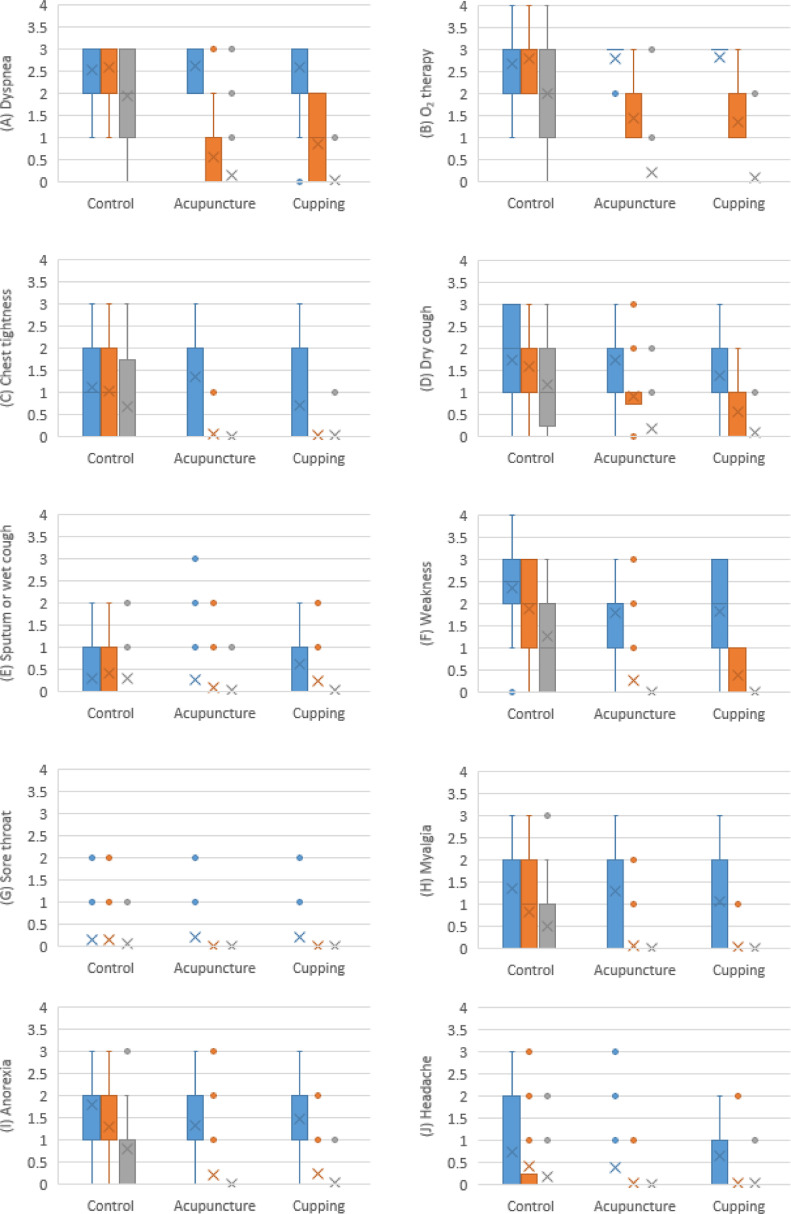
Among key secondary outcomes, dyspnea, type of oxygen therapy, and cough did not differ between groups at the beginning of the study, but mean score of chest tightness was higher in ACUG. Within-group analysis showed that severity score of dyspnea, type of oxygen therapy, chest tightness, dry cough, and wet cough declined significantly among all three intervention groups (p<0.01), except wet cough in CTRG patients (p=0.472) after 7 days; however, between-group analysis demonstrated that after 3 days of intervention, reduction of the severity score of all symptoms was statistically greater in both ACUG and CUPG in comparison with CTRG (dyspnea: p<0.001, type of oxygen therapy: p<0.001, chest tightness: p<0.001, dry cough: p<0.001, and sputum: p=0.002), and this significant difference remained up to day 7. The mean score of other secondary outcomes - including sore throat, myalgia and headache - did not vary between groups at the beginning of the study, except weakness (compared to ACUG, p=0.01, and CUPG, p=0.014) and anorexia (compared to ACUG, p=0.009) that were higher in CTRG. After 3 days of intervention, within-group analysis showed significant reduction in severity score of weakness, sore throat, myalgia, anorexia, and headache in all three intervention groups (p<0.01) - except sore throat in CTRG (p=0.135) – and this significant difference remained up to day 7 of intervention. Between-group analysis showed greater symptom severity change in ACUG and CUPG compared with CTRG in all symptoms (weakness: p<0.001, sore throat: p<0.001, myalgia: p<0.001, anorexia: p<0.001, and headache: p=0.004) (Table 3, Fig. 3).
Table 3.
Statistical analysis of secondary outcomes1.
| Secondary outcomes | Time | Group |
Mean difference |
||||
|---|---|---|---|---|---|---|---|
| Acupuncture | Control | Cupping | Δ1 | Δ2 | Δ3 | ||
| Hospitalization duration | - | 4.24±3.02a | 10.4±5.75b | 3.82±1.45a | 6.16 (4.16 to 8.15)*** |
6.58 (4.8 to 8.35)*** |
-0.42 (-1.42 to 0.58) |
| ICU admission | up to day 30 | 2 (4.8)a | 13 (31)b | 0 (0)a | RR: 0.15 (0.03 to 0.64) ** ARR: 3.81 (2.4 B to 9.26 B) |
RR:0.03 (0 to 0.57) * ARR: 3.3 (2.26 B to 6.11 B) |
RR: 5.23 (0.25 to 105.89) ARR: 21.26 (8.18 H to 35.48 B) |
| Intubation | up to day 30 | 0 (0)a | 8 (19)b | 0 (0)a | RR: 0.05 (0 to .098)* ARR: 5.37 (3.23 B to 15.93 B) |
RR: 0.05 (0 to 0.94) * ARR: 5.36 (3.25 B to 15.13 B) |
RR: 1.04 (0.02 to 51.57) ARR: 1935 (22.31 H to 22.83 B) |
| Death | up to day 30 | 0 (0)a | 8 (19)b | 0 (0)a | RR: 0.05 (0 to 0.98)* ARR: 5.37 (3.23 B to 15.93 B) |
RR: 0.05 (0 to 0.94) * ARR: 5.36 (3.25 B to 15.13 B) |
RR: 1.04 (0.02 to 51.57) ARR: 1935 (22.31 H to 22.83 B) |
| Dyspnea or2 breathlessness |
Before intervention | 2.62±0.49a | 2.52±0.71a | 2.59±0.66a | -0.1 (-0.36 to 0.16) |
-0.07 (-0.36 to 0.22) |
-0.03 (-0.28 to 0.22) |
| Day 3 | 0.57±0.86a | 2.6±0.66b | 0.86±0.86a | 2.03 (1.69 to 2.36)*** |
1.74 (1.41 to 2.06)*** |
0.29 (-0.07 to 0.65) |
|
| Day 7 | 0.15±0.57a | 1.94±1.09b | 0.05±0.21a | 1.79 (1.41 to 2.16)*** |
-0.1 (-0.28 to 0.08)*** |
-0.1 (0.28 to 0.08) |
|
| P value (within groups) | <0.001 | 0.001 | <0.001 | ||||
| Oxygen therapy3 | Before intervention | 2.79±0.42a | 2.69±0.6a | 2.82±0.39a | -0.1 (-0.32 to 0.12) |
-0.13 (-0.34 to 0.08) |
0.03 (-0.14 to 0.2) |
| Day 3 | 1.45±0.71a | 2.8±0.6b | 1.35±0.53a | 1.35 (1.06 to 1.63)*** |
1.45 (1.2 to 1.69)*** |
-0.1 (-0.36 to 0.16) |
|
| Day 7 | 0.22±0.69a | 2±1.22b | 0.09±0.43a | 1.78 (1.34 to 2.21)*** |
1.91 (1.52 to 2.29)*** |
-0.13 (-0.37 to 0.11) |
|
| P value (within groups) | <0.001 | <0.001 | <0.001 | ||||
| Chest tightness | Before intervention | 1.36±1.08a | 1.12±1.13a,b | 0.73±1.02b | -0.24 (-0.71 to 0.23) |
0.39 (-0.07 to 0.85) |
-0.63 (-1.08 to -0.17)** |
| Day 3 | 0.07±0.26a | 1.02±1.12b | 0.05±0.21a | 0.95 (0.59 to 1.3)*** |
0.97 (0.62 to 1.31)*** |
-0.02 (-0.12 to 0.08) |
|
| Day 7 | 0a | 0.69±1.04b | 0.02±0.15a | 0.69 (0.37 to 1)*** |
0.67 (0.35 to 0.98)*** |
0.02 (-0.02 to 0.06) |
|
| P value (within groups) | <0.001 | 0.002 | <0.001 | ||||
| Dry cough | Before intervention | 1.74±0.89a | 1.74±1.06a | 1.39±0.95a | 0 (-0.42 to 0.42) |
0.35 (-0.08 to 0.78) |
-0.35 (-0.74 to 0.04) |
| Day 3 | 0.93±0.68a | 1.6±1.06b | 0.56±0.59c | 0.67 (0.28 to 1.05)*** |
0.17 (-0.1 to 0.44)*** |
1.04 (0.67 to 1.4)* |
|
| Day 7 | 0.17±0.44a | 1.19±0.89b | 0.09±0.29a | 1.02 (to 1.32)*** |
1.1 (0.81 to 1.38)*** |
-0.08 (-0.23 to 0.07) |
|
| P value (within groups) | <0.001 | <0.001 | <0.001 | ||||
| Sputum or Wet cough | Before intervention | 0.29±0.74a | 0.31±0.56a | 0.61±1.02a | 0.02 (-0.26 to 0.3) |
-0.3 (0.65 to 0.05) |
0.32 (-0.06 to 0.7) |
| Day 3 | 0.1±0.37a | 0.4±0.7b | 0.26±0.54a,b | 0.3 (0.05 to 0.54)* |
0.14 (-0.12 to 0.4) |
0.16 (-0.03 to 0.35) |
|
| Day 7 | 0.02±0.16a | 0.31±0.62b | 0.05±0.21a | 0.29 (0.09 to 0.48)** |
0.26 (0.06 to 0.45)** |
0.03 (-0.05 to 0.11) |
|
| P value (within groups) | 0.004 | 0.472 | <0.001 | ||||
| Weakness | Before intervention | 1.81±0.83a | 2.36±0.82b | 1.84±1.16a | 0.55 (0.19 to 0.9)* |
0.52 (0.08 to 0.95)* |
0.03 (-0.4 to 0.46) |
| Day 3 | 0.26±0.66a | 1.88±0.94b | 0.4±0.49a | 1.62 (1.26 to 1.97)*** |
0.88 (0.53 to 1.22)*** |
0.14 (-0.1 to 0.38) |
|
| Day 7 | 0a | 1.28±1.03b | 0a | 1.28 (0.96 to 1.59)*** |
1.28 (0.97 to 1.58)*** |
0 (0) | |
| P value (within groups) | <0.001 | <0.001 | <0.001 | ||||
| Sore throat | Before intervention | 0.21±0.52a | 0.17±0.49a | 0.23±0.57a | -0.04 (-0.25 to 0.17) |
-0.06 (-0.28 to 0.16) |
0.02 (-0.21 to 0.25) |
| Day 3 | 0a | 0.14±0.42b | 0a | 0.14 (0.01 to 0.26)** |
0.14 (0.01 to 0.26)** |
0 (0) | |
| Day 7 | 0a | 0.06±0.23a | 0a | 0.06 (-0.01 to 0.13) |
0.06 (0 to 0.12) |
0 (0) | |
| P value (within groups) | 0.001 | 0.135 | 0.001 | ||||
| Myalgia | Before intervention | 1.29±1.13a | 1.36±1.03a | 1.07±1.15a | 0.07 (-0.39 to 0.53) |
0.29 (-0.17 to 0.75) |
-0.22 (-0.7 to 0.26) |
| Day 3 | 0.07±0.34a | 0.83±0.93b | 0.05±0.21a | 0.76 (0.45 to 1.06)*** |
0.78 (0.49 to 1.06)*** |
-0.02 (-0.14 to 0.1) |
|
| Day 7 | 0a | 0.5±0.85b | 0a | 0.5 (0.23 to 0.76)*** |
0.5 (0.24 to 0.75)*** |
0 (0) | |
| P value (within groups) | <0.001 | <0.001 | <0.001 | ||||
| Anorexia | Before intervention | 1.33±0.79a | 1.81±0.8b | 1.48±0.88a,b | 0.48 (0.13 to 0.82)** |
0.33 (-0.03 to 0.69) |
0.15 (-0.2 to 0.5) |
| Day 3 | 0.21±0.61a | 1.31±0.9b | 0.23±0.53a | 1.1 (0.76 to 1.43)*** |
1.08 (0.76 to 1.39)*** |
0.02 (-0.22 to 0.26) |
|
| Day 7 | 0a | 0.81±0.86b | 0.05±0.21a | 0.81 (0.54 to 1.07)*** |
0.76 (0.49 to 1.02)*** |
0.05 (-0.01 to 0.11) |
|
| P value (within groups) | <0.001 | <0.001 | <0.001 | ||||
| Headache | Before intervention | 0.38±0.85a | 0.74±1.04a | 0.66±0.99a | 0.36 (-0.05 to 0.77) |
0.08 (-0.35 to 0.51) |
0.28 (-0.11 to 0.67) |
| Day 3 | 0.05±0.22a | 0.43±0.83b | 0.05±0.3a | 0.38 (0.11 to 0.64)** |
0.38 (0.11 to 0.64)** |
0 (-0.11 to 0.11) |
|
| Day 7 | 0a | 0.19±0.47b | 0.02±0.15a | 0.19 (0.04 to 0.33)** |
0.17 (0.02 to 0.31)** |
0.02 (-0.02 to 0.06) |
|
| P value (within groups) | 0.001 | 0.002 | <0.001 | ||||
Data is represented by mean± standard deviation or frequency (percentage) for groups and mean (95% confidence interval) for mean difference.
Severity score of symptoms is described as: 0=not affected; 1= minimally-affected; 2=affected; and 3=severely-affected. 3. Severity score of oxygen therapy is described as: 0 = not needed; 1= nasal cannula; 2= simple face mask; 3= mask with reservoir; and 4= continuous positive airway pressure and non-invasive ventilation (CPAP/NIV) Mask; different letters (a-c) show significant statistical difference between groups and significance levels are represented as Δ1: acupuncture compared to control; Δ2: cupping compared to control; Δ3: acupuncture compared to cupping: * P<0.05, ** P<0.01, or *** P<0.001; RR: Relative Risk; ARR: Absolute Relative Reduction; H: Harm; B: Benefit.
Fig. 3.
Progression of secondary outcomes: (A) Dyspnea, (B) Type of oxygen therapy, (C) Chest tightness, (D) Dry cough, (E) Sputum or wet cough, (F) Weakness, (G) Sore throat, (H) Myalgia, (I) Anorexia, and (J) Headache. For each group, the first column (blue) is day 0, the second column (orange) is day 3, and the third column (gray) is day 7. The cross sign and circle sign are representative for mean score and outlier points, respectively. Numbers are mean score in each time: severity score of symptoms is described as: 0=not affected; 1= minimally-affected; 2=affected; and 3=severely-affected. Severity score of oxygen therapy is described as: 0 = not needed; 1= nasal cannula; 2= simple face mask; 3= mask with reservoir; and 4= continuous positive airway pressure and non-invasive ventilation (CPAP/NIV) Mask.
3.4. Primary and secondary outcomes (ACUG vs. CUPG)
The mean difference of SpO2 (mean: 0.52, CI: -0.43 to 1.47, p=0.416) and RR (mean: -0.02, CI: -1.03 to 0.99, P=0.976) were not significant between ACUG and CUPG after 7 days. Moreover, statistical analysis showed no significant difference between ACUG and CUPG in means of secondary outcomes including dyspnea (mean: -0.1, CI: 0.28 to 0.08, p=0.835), type of oxygen therapy (mean: -0.13, CI: -0.37 to 0.11, p=0.481), chest tightness (mean: 0.02, CI: -0.02 to 0.06, p=0.853), dry cough (mean: -0.08, CI: -0.23 to 0.07, p=0.539), sputum (mean: 0.03, CI: -0.05 to 0.11, p= 0.788), weakness (mean: 0, CI: 0, p>0.05), sore throat (mean: 0, CI: 0, p>0.05), myalgia (mean: 0, CI: 0, p>0.05), anorexia (mean: 0.05, CI: -0.01 to 0.11, p=0.661), and headache (mean: 0.02, CI: -0.02 to 0.06, p=0.695). Although no patient in ACUG or CTRG underwent intubation or died, two patients in ACUG but no patient in CUPG were admitted to ICU (relative risk: 5.23, CI: 0.25 to 105.89, p= 0.241)
In CUPG, 91% of the participants experienced the side effect of ecchymosis. Although some patients expressed concern, this ecchymosis was completely resolved after 3-5 days. In ACUG, 47% of the patients reported mild focal numbness or tingling. No important harms or unintended effects were reported.
4. Discussion
This clinical research confirmed that the two non-pharmaceutical therapies including warm cupping and acupuncture provide opportunities to improve respiratory signs (SpO2 and RR), reduce hospitalization duration and SAEs, and also alleviate symptoms in COVID-19 patients.
As a major component of TCM, acupuncture has a wealth of experience in prevention and treatment of acute respiratory infectious diseases. Its efficacy in control of epidemic disease and relieving respiratory symptoms has been clearly reported14,20. Since the outbreak of COVID-19 in China, acupuncture has played a significant role in management of COVID-19-related respiratory symptoms as an adjuvant treatment and many researchers have published guidelines on its potential benefits20.
From the TCM point of view, pathogenesis of COVID-19 involves blood stasis, heat, dampness, toxicity, and Qi stagnation – Qi is commonly explained as life energy, one of the functions of which is very close to the function of immune system21. COVID-19 is divided into different stages consisting of cold-damp with accumulation of heat, damp-toxin, and Qi stagnation22. Acupoints used in this study were proposed according to a specific differential diagnosis based on our previous study: GV20 decreases liver-Yang (heat symptoms); LU5 reduces lung heat and phlegm (inflammation in lungs); LU7 expels wind and raises Qi up to the head; LI4 expels wind, removes coldness, helps in first stages of upper respiratory infections, and regulates Qi movements; LR3 controls Liver-Yang, manages Qi flow, resolves dampness, and reduces anxiety; LR14 promotes Qi flow; CV12 expels dampness and phlegm, and reduces anxiety; CV17 tonifies Qi, and opens the chest (reducing breathlessness); ST36 tonifies Qi, increases the vigor against exterior pathogens, and expels cold and dampness23. A systematic review by Chen et al. (2020) showed that LI4 and ST36 acupoints are among the most frequent acupoints used in trials performed on COVID-19 patients20.
On the other hand, modern medicine literature describes the mechanism of action of acupuncture. In a systematic review and meta-analysis, Wu et al. (2020) showed that a combination of acupuncture and standard care in respiratory diseases reduces cytotoxic T lymphocyte CD8+ associated with airway inflammation and obstruction, improves the ratio of T-cell CD4+/CD8+, and enhances respiratory functions24. Results of another study indicated that parameters of spirometry (FEV1, FVC, and PEF), anti-inflammatory biomarkers (IL-4, IL-8, and interferon gamma), and T-cells (CD3+ and CD4+/CD8+ ratio) improve significantly with acupuncture25. Also, an animal study by Wei et al. (2015) demonstrated that via regulating serum cytokine levels, acupuncture reduces hypersensitive and inflammatory conditions and excessive mucus excretion in airways26.
A study by Jin et al. (2020) revealed that high levels of TNF-α and IL-6 are associated with higher mortality rate in COVID-19 patients. Hence, via inhibiting macrophage activation and regulating the level of proinflammatory cytokines such as TNF-α, IL- 6, IL-1b, etc., acupuncture significantly reduces duration of hospitalization, ICU admission and mortality rate in COVID-19 patients27. Furthermore, Wang et al. (2020) conducted an acupuncture-based clinical trial on 93 hospitalized COVID-19 patients. This study revealed that acupuncture significantly shortens duration of hospitalization compared with standard treatment28. Our study found similar results in comparing duration of hospitalization, ICU admission and mortality rate between ACUG and CTRG.
Additionally, we found that adding acupuncture to standard care, resulted in improvement of SpO2 and RR. Previous studies have reported regulation of IL-12 and TNF-α, and subsequent improvement in oxygen levels of pneumonia patients undergoing acupuncture29. Similar results have been demonstrated in the studies evaluating acupuncture as a therapeutic intervention to treat dyspnea and hypoxia in COVID-19 patients30,31. For example, Gong et al. (2020) reported the case of an 81-year-old COVID-19 patient suffering from respiratory symptoms and hypoxia (SpO2 69%). After one week of acupuncture, chest tightness relieved and SpO2 raised to 99% with 5 liter/minute oxygen therapy, and after two weeks of treatment, SpO2 remained 98% without oxygen therapy32.
As reported in the results section, recovery rate of dyspnea, cough, chest tightness, weakness, sore throat, myalgia, anorexia and headache were higher in ACUG in comparison with CTRG on days 3 and 7. Research by Guan et al. (2020), Gong et al. (2021), Zha et al. (2020) and Tao et al. (2020) have also reported promising efficacy for adding acupuncture to standard care in COVID-19 patients. Their results were indicative of respiratory and gastrointestinal symptom improvement after 7-10 days33. Moreover, no adverse events34 or recurrence were observed in at least a 4-week follow-up30.
As a non-pharmacological treatment in TCM, acupuncture has been clinically used to improve respiratory symptoms in COVID-19. However, this intervention is not common and easily-available in Iran. So, there was a high demand to develop a specific and suitable non-pharmacological PM-based treatment. We retrieved previously published studies and found that cupping therapy has been used for many purposes including common symptoms (cough and dyspnea) of a number of respiratory diseases such as asthma, bronchitis, and pneumonia in PM13,31,35.
Recent studies have reported that cupping significantly improves the severity scores of respiratory symptoms (cough, sputum disposal, rhinitis, and sore throat) and spirometry parameters (FEV1, FVC, and PEF) in comparison with control groups34,36. Similar results were shown in our case series that evaluated the effectiveness cupping in COVID-19 patients. The case series suggests that warm cupping of the posterior thorax is effective in improving respiratory symptoms and reducing mortality and intubation in COVID-19 patients with ARDS37. Furthermore, a case report by Cheng demonstrated amelioration of dyspnea and chest tightness in a COVID-19 patient with cupping38. These promising outcomes led to this randomized clinical trial, the results of which suggest that adding cupping to standard care shortens hospitalization duration and has significant preventive effects on severe COVID-19 outcomes (ICU admission and death)- according to the relative risk presented in Table 3, acupuncture and cupping decrease risk of ICU admission by 85% and 97%, respectively, and adding acupuncture or cupping to standard care declines the risk of intubation and death by 95%.
The cupping protocol used in this study was proposed according to our previous study. In this study, respiratory signs including RR and SpO2 significantly improved in CUPG in comparison with the control group. These effects might be attributed to the anti-inflammatory effects of warm cupping. Cupping increases anti-inflammatory lipids (such as prostaglandin E1) and decreases production of pro-inflammatory lipids (such as 12-HETE and Thromboxane B2), inflammatory cytokines (e.g., IL-4, IL-5, IL-6 and TNF-α), immunoglobulin (Ig)-E, and the number, activity and cytotoxicity of natural killer lymphocytes31,39,40.
In addition to RR and SpO2, we found that respiratory symptoms improved in CUPG in comparison with CTRG after 3 days of intervention. Some patients expressed that after receiving cupping they felt more comfortable breathing. A 53-year-old female questioned whether she could continue cupping at home after discharge, as she had a really good feeling with cupping due to ease of breathing. Many patients stated that: "Cupping opens my lungs, I can breathe easier, and I cough less than before". Cupping helps alleviate respiratory symptoms by decreasing deoxy-hemoglobin and enhancing blood microcirculation, endothelial repair, angiogenesis, and oxygenation10,41, 42, 43. Additionally, cupping regulates parasympathetic activity, vasodilation, interstitial fluid exertion, and lung ventilation44.
In the present clinical trial, cupping relieved chest tightness, headache, weakness and myalgia. Recent studies have demonstrated that cupping ameliorates muscle weakness, fatigue45,46, reduces frequency and intensity of headache, and improves quality of life47. According to an article that has described effects and mechanisms of action, cupping controls pain by increasing levels of brain opioid production, improving blood circulation, and eliminating toxins from the bloodstream10.
Limitations of this study include restricting outcomes to clinical symptoms. Inflammatory markers such as C-reactive protein (CRP), lactate dehydrogenase (LDH) or procalcitonin, and follow-up chest CT scan were not considered herein because of the regulations imposed by isolation policies and lack of sufficient funding. All symptom reports were based on subjective assessment.
To our knowledge, this is the first randomized controlled trial that compared the effects of acupuncture and cupping in COVID-19 patients and represented the equal effectiveness of these two methods on COVID-19 symptoms. This study suggests the feasibility and reliability of cupping and acupuncture for management of COVID-19 respiratory symptoms. In addition to being safe and effective, cupping and acupuncture are low-cost and noninvasive modalities. Although COVID-19 is a worldwide outbreak with increasing number of infected patients, there are still controversies regarding treatments such as antiviral and antibiotic medications, corticosteroids, and healing plasma48. Hence, CAM methods with strong literature support seem promising adjunctive treatments.
In conclusion, the COIRACCU trial provides evidence that cupping or acupuncture in combination with standard care improve respiratory signs, reduce progression to mechanical ventilation or death, hospital stay, hypoxemia, tachypnea, dyspnea, cough, sore throat, weakness, myalgia, anorexia, headache, and need for oxygen therapy in hospitalized COVID-19 patients.
Acknowledgments
This study was Dr. Reihane Alipour's PhD thesis. We would like to thank Tehran University of Medical Sciences for its financial support. We are grateful to Dr. Anoshirvan Sadeghi, Shohadaye Pakdasht Hospital manager, and Dr. Rezvan Hashemi, Research Deputy of Shahid Ziaeian Hospital. We appreciate assistance from Dr. Mehran Arab Ahmadi for reporting patient chest CT scans, from Ms. Mohebat Vali for productive cooperation in data analysis, and from Mrs. Fateme Hemmat for assessment of outcomes. We especially thank the patients and their families who participated in this study for their altruism and dedication in such a challenging situation as the COVID-19 pandemic.
CRediT authorship contribution statement
Reihane Alipour: Data curation, Writing – original draft, Writing – review & editing. Saeidreza Jamalimoghadamsiahkali: Supervision. Mehrdad Karimi: Conceptualization, Investigation, Supervision, Writing – review & editing. Asma Asadi: Supervision. Haleh Ghaem: Formal analysis, Methodology, Validation, Writing – review & editing. Mohammad Sadegh Adel-Mehraban: Formal analysis, Writing – review & editing. Amir Hooman Kazemi: Conceptualization, Investigation, Supervision, Writing – review & editing.
Acknowledgments
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This study was supported by Tehran University of Medical Sciences (Grant ID: 99-3-147-50353).
Ethical statement
This study was approved by the Tehran University of Medical Science ethics committee (IR.TUMS.MEDICINE.REC.1399.1124).
Data availability
The data associated with this study can be made available by authors upon reasonable request.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.imr.2022.100898.
Supplementary Table S1. Baseline characteristics.
Appendix. Supplementary materials
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kang JE, Rhie SJ. “Practice considerations on the use of investigational anti-COVID-19 medications: dosage, administration and monitoring.” J Clin Pharm Ther vol. 45,5: 1199–1205. doi: 10.1111/jcpt.13199.2020. [DOI] [PMC free article] [PubMed]
- 3.Soin AS, Kumar K, Choudhary NS, Sharma P, Mehta Y, Kataria S, et al. Tocilizumab plus standard care versus standard care in patients in India with moderate to severe COVID-19-associated cytokine release syndrome (COVINTOC): an open-label, multicenter, randomized, controlled, phase 3 trial. Lancet Respir Med. 2021;9(5):511–521. doi: 10.1016/S2213-2600(21)00081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y, Wang C-Z, Hesse-Fong J, Lin J-G, C-SJTAjoCm Yuan. Application of Chinese medicine in acute and critical medical conditions. Am J Chin Med. 2019;47(06):1223–1235. doi: 10.1142/S0192415X19500629. [DOI] [PubMed] [Google Scholar]
- 6.Arora R, Chawla R, Marwah R, Arora P, Sharma R, Kaushik V, et al. Potential of complementary and alternative medicine in preventive management of novel H1N1 flu (Swine flu) pandemic: thwarting potential disasters in the bud. Evid Based Complement Alternat Med. 2011 doi: 10.1155/2011/586506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. SARS: clinical trials on treatment using a combination of traditional Chinese medicine and Western medicine: report of the WHO International Expert Meeting to review and analyse clinical reports on combination treatment for SARS, 8-10 October 2003, Beijing, People's Republic of China. 2004.
- 8.Hwang JH, Cho HJ, Im HB, Jung YS, Choi SJ, DJBcm Han, et al. Complementary and alternative medicine use among outpatients during the 2015 MERS outbreak in South Korea: a cross-sectional study. BMC Complement Med. 2020;20:1–10. doi: 10.1186/s12906-020-02945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W-h, Guo S-n, F Wang, Hao YjWJoA-M. Understanding of Guidance for acupuncture and moxibustion interventions on COVID-19 issued by China Association of Acupuncture-Moxibustion. World J Acupunct. 2020;30(1):1–4. doi: 10.1016/j.wjam.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Bedah AM, Elsubai IS, Qureshi NA, Aboushanab TS, Ali GI, El-Olemy AT, et al. The medical perspective of cupping therapy: effects and mechanisms of action. J Tradit Complement Med. 2019;9(2):90–97. doi: 10.1016/j.jtcme.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammadi kenari H, Kordafshar G. Aamal Yadavi. Qom: Yaghot, 2017. Persian.
- 12.Razi M.Al-Havi. Persian; 2001. [The Large Comprehensive]. Tehran: Shahid Besheshti University of Medical Sciences & Health Services Research Institute for Traditional Medicine. [Google Scholar]
- 13.Tehran University of Medical Sciences; Tehran: 2013. Nazim Jahan MA. Exireh Azam. Persian. [Google Scholar]
- 14.Liu B, Wang H, Zhou ZY, Chang XR, Zhang W, Liu BY. [Analysis on the theory and clinical ideas of acupuncture and moxibustion for the prevention and treatment of coronavirus disease 2019] Zhongguo Zhen Jiu. 2020;40(6):571–575. doi: 10.13703/j.0255-2930.20200305-k0004. [DOI] [PubMed] [Google Scholar]
- 15.Karst M, Schneidewind D, Scheinichen D, Juettner B, Bernateck M, Molsberger A, et al. Acupuncture induces a pro-inflammatory immune response intensified by a conditioning-expectation effect. Complement Med Res. 2010;17(1):21–27. doi: 10.1159/000264657. [DOI] [PubMed] [Google Scholar]
- 16.Feng D, Zhou H, Jin X, Wei J, Zhang Q, Gu Y, et al. Electroacupuncture pretreatment alleviates LPS-induced acute respiratory distress syndrome via regulating the PPAR Gamma/NF-Kappa B signaling pathway. Evid Based Complement Alternat Med. 2020;2020 doi: 10.1155/2020/4594631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iranian National Guideline for Diagnosis and Treatment of COVID-19 in Outpatient and Inpatient Services, 9th ed.. Available at: https://irimc.org/news/id/45952/[Date accessed: December 15, 2020]. [Persian].
- 18.Harris P. All of Us Research Program; Maryland: 2020. All of Us Research Program COVID-19 Participant Experience (COPE) Survey (PPI) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Zhu Q, Zhan C, Cheng W, Fang X, Fang M, et al. Acupressure therapy and Liu-Zi-Jue Qigong for pulmonary function and quality of life in patients with severe novel coronavirus pneumonia (COVID-19): study protocol for a randomized controlled trial. Trials. 2020;21(1):751. doi: 10.1186/s13063-020-04693-5. Aug 27PMID: 32854761; PMCID: PMC7450683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen C, Zhan J, Wen H, Wei X, Ding L, Tao C, et al. Current state of research about acupuncture for the treatment of COVID-19: a scoping review. Integr Med Res. 2021;10(Suppl) doi: 10.1016/j.imr.2021.100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.von Trott P, Oei SL, Ramsenthaler C. Acupuncture for breathlessness in advanced diseases: a systematic review and meta-analysis. J Pain Symptom Manag. 2020;59(2):327–338. doi: 10.1016/j.jpainsymman.2019.09.007. .e3. [DOI] [PubMed] [Google Scholar]
- 22.Leung EL-H, Pan H-D, Huang Y-F, Fan X-X, Wang W-Y, He F, et al. The scientific foundation of Chinese herbal medicine against COVID-19. Engineering. 2020;6(10):1099–1107. doi: 10.1016/j.eng.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maciocia G. Elsevier Health Sciences; 2015. The Foundations of Chinese Medicine: a Comprehensive Text. [Google Scholar]
- 24.Wu J-J, Zhang Y-X, Xu H-R, Li Y-X, Jiang L-D, Wang C-X, et al. Effect of acupoint application on T lymphocyte subsets in patients with chronic obstructive pulmonary disease: a meta-analysis. Medicine (Baltimore) 2020;99(16):e19537. doi: 10.1097/MD.0000000000019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin XJ, Qi HY. Effect of acupuncture combined with salmeterol on pulmonary function, serum inflammatory response and T lymphocyte subsets in patients with bronchial asthma. Shaanxi J Tradit Chin Med. 2018;39:1630–1633. [Google Scholar]
- 26.Wei Y, Dong M, Zhang H, Lv Y, Liu J, Wei K, et al. Acupuncture attenuated inflammation and inhibited Th17 and Treg activity in experimental asthma. Evid Based Complement Altern Med eCAM. 2015;2015 doi: 10.1155/2015/340126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin G-Y, Jin LL, Zheng J, He BJ. Advantages of anti-inflammatory acupuncture in treating sepsis of coronavirus disease 2019. World J Tradit Chin Med. 2020;6(2):188. [Google Scholar]
- 28.Wang R, Li X, Wang Z, Shi X, Jiang K, Deng YJSJTCM. Analysis o f clinical features, course of disease and hospitalization days of COVID-19 patients. J Shanghai J Tradit Chin Med. 2020;54:25–30. [Google Scholar]
- 29.Li L MR, Yu JB, Shao W, Lu S, Zhang GC. Effect of electro-acupuncture at Zusanli ST36 and Chize LU5 acupoints on sepsis-induced acute lung injury. Chin J Anesthesiol. 2013;33:626–629. [Google Scholar]
- 30.Zha L, Xu X, Wang D, Qiao G, Zhuang W, Huang S. Modified rehabilitation exercises for mild cases of COVID-19. Ann Palliat Med. 2020;9(5):3100–3106. doi: 10.21037/apm-20-753. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Wang X, Yan G, Lei J, Zhou Y, Wu L, et al. Anti- versus pro-inflammatory metabololipidome upon cupping treatment. Cell Physiol Biochem. 2018;45(4):1377–1389. doi: 10.1159/000487563. [DOI] [PubMed] [Google Scholar]
- 32.Gong Y-B, Yang Z-L, Liu Y, Zhang Y, Jiang K, Shi X-J, et al. Two cases of corona virus disease 2019 (COVID-19) treated with the combination of acupuncture and medication in bedridden patients2. World J Acupunct. 2020;30(3):171–174. doi: 10.1016/j.wjam.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan L, Liu N, Man N. The effect of ear acupressure combined with acupressure on the clinical symptoms of patients with severe COVID-19. Beijing Tradit Chin Med. 2020;39(11):1166–1168. [Google Scholar]
- 34.Xu Y, Cui ST, Bai LY, Yang JJ, Li J, Xie TL, et al. [Cupping treatment combined with antibiotics for bacterial pneumonia in children: a randomized controlled trial] Zhongguo Zhen Jiu. 2021;41(3):283–287. doi: 10.13703/j.0255-2930.20200804-k0004. [DOI] [PubMed] [Google Scholar]
- 35.Cao H, Li X, Liu J. An updated review of the efficacy of cupping therapy. PLoS One. 2012;7(2):e31793. doi: 10.1371/journal.pone.0031793. -e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong J, Fu M, Wang X, Gao Z. Effects of cupping therapy on the pulmonary functions in asthmatic children. J Tradit Chin Med. 2006;26(1):7. [PubMed] [Google Scholar]
- 37.Karimi M, Kazemi AM, Asadi A, Zarei A, Zargaran A, et al. Warm cupping of the posterior thorax in combination with standard conventional therapy for ARDS in COVID-19 patients in ICU: a case series. J Acupunct Meridian Stud. 2022 doi: 10.51507/j.jams.2022.15.3.194. [in press] [DOI] [PubMed] [Google Scholar]
- 38.Cheng SI. Medical acupuncture as a treatment for novel COVID-19-related respiratory distress: personal experience from a frontline anesthesiologist. Med Acupunct. 2021;33(1):83–85. doi: 10.1089/acu.2020.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dons'koi BV, Chernyshov VP, Osypchuk DV, Baksheev SM. Repeated cupping manipulation temporary decreases natural killer lymphocyte frequency, activity and cytotoxicity. J Integr Med. 2016;14(3):197–202. doi: 10.1016/S2095-4964(16)60250-9. [DOI] [PubMed] [Google Scholar]
- 40.Ke ZH, Long SH. [Medicinal vesiculation combined with quick cupping at Shenque (CV 8) for allergic rhinitis with syndrome of yang deficiency: a randomized controlled trial] Zhongguo Zhen Jiu. 2014;34(9):853–856. [PubMed] [Google Scholar]
- 41.Saguil A, Fargo MV. Acute respiratory distress syndrome: diagnosis and management. Am Fam Physician. 2020;101(12):730–738. [PubMed] [Google Scholar]
- 42.Wang S-z, Lu Y-h, Wu M, Chen K-j, Liu Y, Liu L-tJCjoim. Cupping therapy for diseases: an overview of scientific evidence from 2009 to 2019. Chin J Integr Med. 2021;27(5):394–400. doi: 10.1007/s11655-020-3060-y. [DOI] [PubMed] [Google Scholar]
- 43.Li T, Li Y, Lin Y, Li K. Significant and sustaining elevation of blood oxygen induced by Chinese cupping therapy as assessed by near-infrared spectroscopy. Biomed Opt Express. 2016;8(1):223–229. doi: 10.1364/BOE.8.000223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ismail AMA, Abdelghany AI, AMAJJoC Elfahl, Medicine I. Immediate effect of interscapular cupping on blood pressure, oxygen saturation, pulse rate and chest expansion in sedentary smoker students. J Complement Integr Med. 2021;18(2):391–396. doi: 10.1515/jcim-2020-0050. [DOI] [PubMed] [Google Scholar]
- 45.Hou X, Wang X, Griffin L, Liao F, Peters J, Jan Y-K. Immediate and delayed effects of cupping therapy on reducing neuromuscular fatigue. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.678153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilal M, Khan RA, Danial K. Hijama improves overall quality of life in chronic renal failure patients: a pilot study. Pak J Pharm Sci. 2015;28(5):1731–1735. [PubMed] [Google Scholar]
- 47.Seo J, Chu H, Kim C-H, Sung K-K, Lee S. Cupping therapy for migraine: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Evid Based Complement Altern Med eCAM. 2021;2021 doi: 10.1155/2021/7582581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jahan I, Onay A. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk J Biol. 2020;44(3):228–241. doi: 10.3906/biy-2005-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data associated with this study can be made available by authors upon reasonable request.