Abstract
In humans, expansion of circulating Vγ9Vδ2 T cells seems to be a pathophysiological denominator shared by protozoan and intracellular bacterial diseases. The assumption was tested here on legionellosis, a condition conforming to the category but not yet described with respect to γδ T cells. Levels of Vγ9Vδ2 T cells in peripheral blood were measured at various intervals in 14 subjects undergoing a Pontiac fever-like disease, shown by serological investigation to be caused by Legionella micdadei. In samples obtained 4 to 6 days after the onset of the disease, the mean percentage (± the standard deviation) of Vγ9Vδ2+ T cells among CD3+ cells was 1.0% ± 0.5%, compared to 5.0% ± 3.9% in healthy control subjects (P < 0.001). Thereafter, a pronounced increase occurred and at 2 to 7 weeks after onset, mean peak levels were as high as ≈15%. During the next 6 months, values slowly declined, although without reaching the normal range. Percentages of γδ+ T cells expressing tumor necrosis factor alpha or gamma interferon in response to phorbol myristate acetate were assayed in vitro. At 14 to 16 days after the onset of disease, the expression of both cytokines was increased (P < 0.01), whereas at 5 to 7 weeks, the expression of tumor necrosis factor alpha was decreased (P < 0.05), possibly reflecting modulation of an inflammatory response. In conclusion, Pontiac fever was found to be associated with a pronounced and long-lasting expansion of Vγ9Vδ2 T cells, implying that the subset may also be pathophysiologically important in a mild and transient form of intracellular bacterial diseases. Surprisingly, the expansion was preceded by a depletion of circulatory Vγ9Vδ2 T cells. Possibly, Vγ9Vδ2 T cells are initially recruited to a site of infection before they expand in response to antigen and occur in high numbers in blood.
Legionella is a genus of gram-negative bacteria and the cause of two different clinical entities. One of them is Legionnaires' disease, a severe pneumonic disease associated with a relatively low attack rate, a long incubation period, and a significant mortality rate (8, 9, 39). Pontiac fever, the other entity, is an acute, influenza-like disease with a brief incubation period, a high attack rate, and a self-limiting course (12, 16). Although both cause a high fever, the difference in clinical expression between the two forms of legionellosis is striking. Due to the transient nature of Pontiac fever, it has even been questioned whether this entity is indeed associated with invasive infection and not a host response to dead bacteria, bacterial toxins, or other bacterial products present in an inhaled aerosol (23, 29). In Legionnaires′ disease, as well as Pontiac fever, Legionella pneumophila has been the species most frequently identified. In two reported outbreaks of whirlpool-associated Pontiac fever, however, L. micdadei was implicated as the causative agent (17, 27).
Members of the genus Legionella are facultatively intracellular pathogens, and consequently, the pathogenesis of legionellosis bears similarity to that of tuberculosis, listeriosis, brucellosis, tularemia, and Q fever. The host control of all of these infections depends, to a large extent, on T cells. Like other facultatively intracellular pathogens, Legionella bacteria replicate in mononuclear phagocytes, thereby inducing an αβ T-cell-dependent, major histocompatibility complex-restricted immune response to bacterial peptides (20).
Besides αβ T cells, 1 to 5% of circulating T cells express the γδ T-cell receptor (TCR). Increased levels of γδ T cells are found in the circulation of patients with protozoan (19, 33, 35) and intracellular bacterial infections, including mycobacterial disease (21), listeriosis (22), brucellosis (2), tularemia (32, 37), and Q fever (36).
Unlike that of αβ T cells, the role of γδ T cells in host-parasite interactions is poorly understood. In humans, a sentinel role is ascribed to γδ T cells, i.e., a major histocompatibility complex-independent recognition of broadly cross-reactive antigens (6). Cells of one single subset of γδ T cells, Vγ9Vδ2 T cells, account for the increased levels in protozoan and intracellular bacterial diseases. These cells recognize phosphorylated metabolic intermediates, so-called phosphoantigens, which are produced by the causative agents (5, 30, 32, 38).
Like αβ T cells, γδ T cells are endowed with the ability to produce cytokines. In response to microbial antigens, Vγ9Vδ2 T cells produce large amounts of tumor necrosis factor alpha (TNF-α) (25) and gamma interferon (IFN-γ) (11, 14). Although this would indicate a role primarily in the acute phase of disease, they are also believed to be involved in a later down regulation of the inflammatory response of macrophages in bacterial and viral infections (4). A majority of Vγ9Vδ2 T cells express CD94, a member of the type C lectin family of killer inhibitory receptor molecules. Signaling through CD94 interferes with the activation of Vγ9Vδ2 T cells, suggesting a role of the surface receptor in the control of Vγ9Vδ2 T-cell reactivity (31).
The γδ T-cell response seems not to have been studied in legionellosis. When faced with an outbreak of Pontiac fever-like disease, we analyzed levels of Vγ9Vδ2 T cells in blood. We investigated whether such a transient and mild condition caused by a facultatively intracellular bacterium would indeed induce a Vγ9Vδ2 T-cell response similar to what had been previously described in more invasive and severe intracellular infections. In the outbreak, comprising 14 cases of Pontiac fever-like disease with a short and well-defined incubation period, we observed an initial decline in Vγ9Vδ2 T cells in peripheral blood, followed by a dramatic increase and a slow decline over several months, suggesting a dynamic redistribution and expansion of the subset.
MATERIALS AND METHODS
Subjects.
Fourteen patients (six men, eight women; 22 to 57 [median, 46] years old) were included in the study. After spending a weekend at a hotel and visiting a whirlpool facility at the establishment on 16 to 17 April 1999, all patients developed symptoms of disease on 17 to 19 April and were admitted to our hospital on 19 to 22 April. Six patients were hospitalized for a median period of 3 (range, 2 to 4) days, and the others were managed as outpatients. After 14 to 16 days, the patients received a questionnaire to retrospectively determine the duration of the fever, confinement to bed, and symptoms experienced during the illness. Informed consent was obtained from all patients by using a protocol approved by the Committee on Research Ethics at the Medical Faculty, Umeå University, Umeå, Sweden.
A retrospective cohort study suggested that the whirlpool was the source of the outbreak (18).
Two groups of healthy control individuals were included. For measurement of γδ and Vγ9Vδ2 T-cell counts, values from 12 male and 14 female adults (mean age, 39.7 years) were available at the laboratory. These values were quite similar to those previously reported from a previous investigation of a Swedish normal adult population (10). For assay of cytokine production ex vivo, samples from six male and four female adults (mean age, 34.3 years) were obtained concomitantly with patient samples.
Blood chemistry.
Blood neutrophil and platelet counts were determined by standard Coulter counter technique on a Sysmex SE-9000 instrument (Toa Medical Electronics Co., Kobe, Japan), and serum C-reactive protein levels were measured with a dry-chemistry enzymatic sandwich immunoassay technique on an Ectachem Instrument (Johnson & Johnson Clinical Diagnostics Inc., Rochester, N.Y.).
Microbiology.
By using heat-killed L. pneumophila serogroups 1 to 8, L. bozemanii, L. micdadei, and L. longbeachae serogroups 1 and 2 as antigens, an immunofluorescent immunoglobulin G (IgG) antibody test was performed at the Swedish Institute for Infectious Disease Control, Stockholm, Sweden, as previously described (7). From each patient, serum samples were obtained 2 to 4 days after onset of disease, at days 14 to 16, and at 5 to 6 weeks after onset. In the first sample, all 14 patients showed a titer of serum antibodies to all legionella antigens of <32. In the second or third sample, all 14 patients showed a ≥4-fold increase in the titer of serum antibodies specific to L. micdadei, in three cases to a titer of 64, in six cases to 128, and in the remaining five cases to 256. The titers of all samples toward the remaining 11 legionella antigens were <32 up to 6 weeks after the onset of disease.
In 7 of 14 cases, nasopharyngeal aspirates or swabs that were obtained upon admission to the hospital for culture on selective legionella BCYE and BMP agar (6a) were consistent with no growth. Bacterial culture of blood samples obtained from five patients with febrile disease showed no growth. From each of the 14 patients, two to five urine samples were obtained for assay of L. pneumophila soluble antigen (Biotest AG, Dreieich, Germany), and all were negative. Complement fixation tests of paired serum samples from each of 14 patients showed no antibody response to influenza virus A or B, parainfluenza virus 3, adenovirus, Mycoplasma sp., or Chlamydia psittaci. Enzyme-linked immunosorbent assays of paired samples from 11 patients showed no evidence of hantavirus infection.
MAbs.
Monoclonal antibodies (MAbs) to the αβ TCR (clone WT31, fluorescein isothiocyanate [FITC] conjugated) and the γδ TCR (11F2, FITC and phycoerythrin [PE] conjugated) were purchased from Becton Dickinson, Sunnyvale, Calif. Antibodies to the αβ TCR (BMA 031, FITC conjugated) and the γδ TCR (5.A6.E9, PE conjugated) were also obtained from Serotec, Oxford, United Kingdom. From this source, CD3 (UCH-T1, PE conjugated), TCR-Vγ2 (7A5, FITC conjugated), and TCR-Vδ2 (15D, PE conjugated) antibodies were purchased as well. Antibodies to human IFN-γ (4S.B3, PE conjugated), TNF-α (MAb11, PE conjugated), and CD94 (cloneHP-3D9, FITC conjugated) and a mouse IgG1 PE-conjugated isotype control were obtained from Pharmingen, San Diego, Calif. Antibodies to interleukin-4 (clone 8604B312) and a mouse IgG2 isotype control came from Biosource, Fleurus, Belgium. TCR Vγ9 (B3, FITC conjugated) and TCR Vδ2 (B6, PE conjugated) antibodies were purchased from Pharmingen. Antibodies to CD94 (cloneHP-3D9, FITC conjugated), reacting with the 70-kDa dimer Kp43, were also from Pharmingen.
Flow cytometry analysis of lymphocyte subsets.
Surface phenotyping of cells of EDTA-treated blood was performed with conjugated MAbs. Aliquots (50 μl) of blood were incubated with 10 μl of normal mouse serum at a dilution of 1:500 (DAKO, Glostrup, Denmark) for 15 min at room temperature, and 5 μl of appropriate MAbs was added, followed by incubation for 25 min at room temperature. Two milliliters of FACS lysing solution (Becton Dickinson) containing 1% paraformaldehyde was then added to each tube. After 10 min of incubation, cells were washed twice with Cell-Wash solution (Becton Dickinson), resuspended in 500 μl of FACS-flow solution (Becton Dickinson), and stored at 4°C before analysis.
With a FACSort instrument (Becton Dickinson), 10,000 events per sample were recorded. The data were collected and analyzed by use of CellQuest software (Becton Dickinson). Lymphocytes were gated according to their morphologic parameters by means of forward scatter and side scatter analysis or by expression of CD3. Results were expressed as the percentage of CD3 cells stained by a given label.
Analysis of cytokine expression by T cells.
For enumeration of cytokine-producing cells, a 100-μl blood sample was diluted with 400 μl of RPMI 1640 medium. Cells were stimulated with 1 ng of phorbol myristate acetate (Sigma, Madison, Wis.) per ml and 1 μM ionomycin (Sigma). Extracellular cytokine transport was blocked by addition of 3 μM monensin (Sigma), and cells were incubated for 4 h at 37°C. This time period was found to be optimal when the technique was standardized for demonstration of TNF-α and IFN-γ expression (28). After stimulation, cells were washed once and stained with conjugated anti-CD3 antibody and anti-TCR αβ or anti-TCR γδ antibody for 15 min on ice. Erythrocytes were then lysed by addition of 1 ml of FACS lysing solution (Becton Dickinson) for 10 min, washed once, and fixed for 10 min with ice-cold phosphate-buffered saline containing 4% paraformaldehyde and 0.1% saponin (Sigma). Fixed cells were washed once in Cell-Wash solution (Becton Dickinson) containing 0.1% saponin and stained for 15 min on ice with anti-cytokine or isotype control PE-conjugated antibody. Staining was followed by two washes in Cell-Wash solution–saponin and one wash in Cell-Wash solution containing 1% bovine serum albumin. Finally, cells were resuspended in 500 μl of FACS flow solution (Becton Dickinson). Cytokine analysis was performed on CD3-gated cells. Thresholds for cytokine signals were assessed after staining of samples with irrelevant isotype-specific antibodies. Results were expressed as the percentage of cytokine-positive cells in the respective subpopulation. Due to saponin permeabilization, both cytoplasmic and membrane-bound cytokines were detected.
Statistical analysis.
For statistical evaluation, the Wilcoxon paired test was used.
RESULTS
Clinical data.
All patients presented with fever (maximum, 39.4 to 40.5°C; mean, 39.9°C; n = 14) and influenza-like symptoms. Vital functions were never affected. In three cases, auscultation disclosed discrete pulmonary rales. By radiography, discrete pulmonary infiltrates were found in two of these patients and in a third patient. In one further patient, a small amount of pleural fluid occurred. At follow-up, all chest radiographs were normalized. Macrolides were prescribed for 5 to 10 days in 12 patients, whereas two patients received no antibiotics. The two latter patients did not deviate from the others regarding blood chemistry or γδ and Vγ9Vδ2 T-cell values. According to the patient-completed questionnaire, the most frequent symptoms were myalgia (14), headache (13), arthralgia (12), dizziness (12), fatigue (11), breathing discomfort (7), nausea, vomiting or diarrhea (5), cough (4), and disturbance of short-term memory (3). Defervescence occurred after a median period of 4 days (range, 2 to 7 days; n = 14), and the patients were bedridden for a medium of 5 days (range, 2 to 8 days).
Inflammatory parameters.
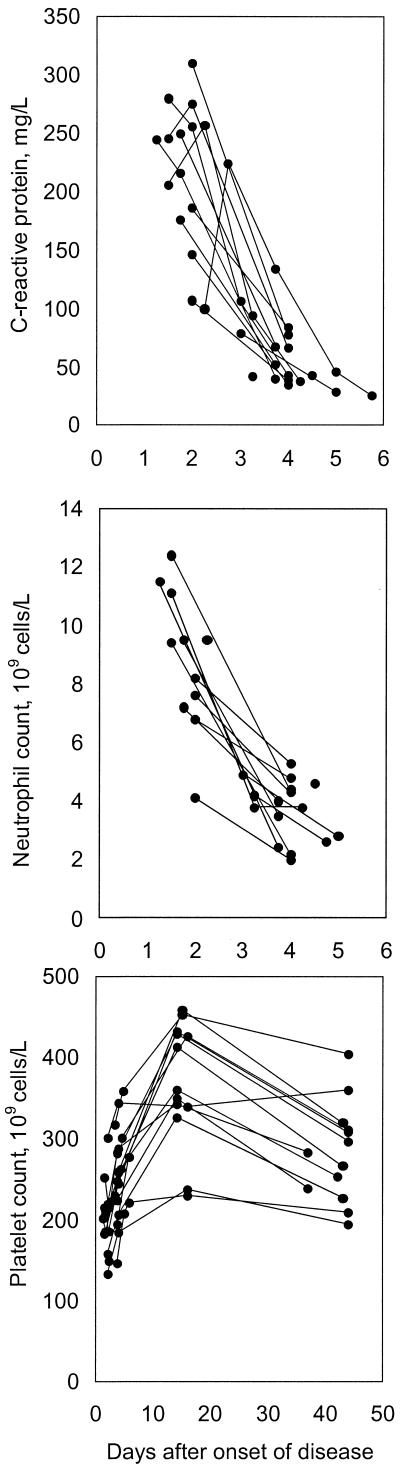
On admission to the hospital, i.e., 1 to 2 days after the onset of disease, neutrophil counts and levels of C-reactive protein were markedly elevated, compared to normal laboratory values of healthy adults (Fig. 1). Within a few days, both reactants normalized and they remained normal throughout the study period. Relative thrombocytosis was demonstrated later in the course (Fig. 1).
FIG. 1.
Neutrophil counts (normal values, 1.8 × 109 to 6.3 × 109 cells/liter, C-reactive protein levels (normal values, <10 mg/liter) and platelet counts (normal values, 150 × 109 to 350 × 109 cells/liter) at various intervals after onset of Pontiac fever-like disease.
Proportions of γδ TCR-expressing T cells at various intervals after onset of disease.
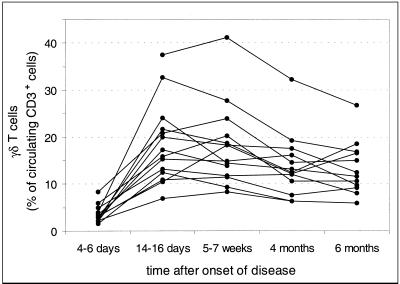
From days 4 to 6 to days 14 to 16 after onset of disease, a rise in the proportion of γδ T cells was generally observed (Fig. 2). Thereafter, the proportion declined gradually over a 6-month-period. The magnitude of the γδ T-cell expansion varied considerably among individuals. This variation was not the result of a day-to-day variation inherent in the assay, because differences among individuals were retained from one sample to another (Fig. 2). Results were similar when expressed as the absolute numbers of γδ T cells per microliter of blood (data not shown), indicating that the percentage of γδ T cells increased because of expansion and not as a result of a concomitant decrease in the number of αβ T cells.
FIG. 2.
Relative numbers of γδ+ T cells in peripheral blood of patients with legionellosis. Blood samples from 14 patients were obtained at various intervals after onset of legionellosis and analyzed by two-color flow cytometry using anti-γδ TCR MAb and anti-CD3 MAb.
When Vγ9Vδ2 T cells were analyzed separately, these cells were found to account for the changes in γδ T-cell percentages (Table 1). Irrespective of the interval, γδ T cells other than Vγ9Vδ2 T cells remained stationary at ≈2% of the total number of CD3+ cells. An unexpected finding was a marked decrease in Vγ9Vδ2 T cells early after onset. In the first sample, obtained 4 to 6 days after onset, the mean percentage was as low as 1.0% ± 0.5% (Table 1) and significantly (P < 0.001) lower than in control subjects (5.0% ± 3.9%). Thus, an initial reduction and a subsequent dramatic, long-lasting increase in Vγ9Vδ2 T cells were observed in the peripheral blood of legionellosis patients. At 6 months, values were still significantly (P = 0.001) higher than those of the control group (Table 1).
TABLE 1.
Percentages of peripheral blood T cells that were Vγ9Vδ2 T cells at various intervals after the onset of legionellosis
| Time after onset of disease | Mean % (± SD) of CD3+ cells that were:
|
No. of subjects | |
|---|---|---|---|
| γδ+ T cellsa | Vγ9Vδ2+ T cells | ||
| 4–6 days | 3.6 ± 1.8 | 1.0 ± 0.5b | 13 |
| 5–7 wk | 18.0 ± 8.3 | 16.1 ± 9.0b | 14 |
| 4 mo | 13.9 ± 6.6 | 12.0 ± 7.6b | 13 |
| 6 mo | 13.1 ± 5.3 | 11.2 ± 5.5b | 13 |
| Control group | 5.4 ± 4.1 | 5.0 ± 3.9 | 26 |
Cells were gated according to morphology.
P < 0.001 compared to control group.
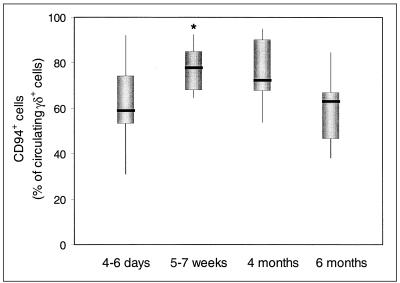
The majority of circulating Vγ9Vδ2 T cells express the inhibitory CD94/NKG2-A receptor for HLA class I molecules. The percentage of CD94+ cells of Vγ9Vδ2 T cells increased significantly (P = 0.01) from days 4 to 6 to the interval of 5 to 7 weeks after onset and normalized at 6 months (Fig. 3).
FIG. 3.
Frequency of CD94+ T cells of Vγ9Vδ2 T cells at various intervals after onset of legionellosis. The thick line through each box shows the median, with quartiles at either end. The vertical lines indicate maximum and minimum values. ∗, significantly different (P = 0.01) from the 4- to 6-day interval.
Cytokine expression of γδ T cells.
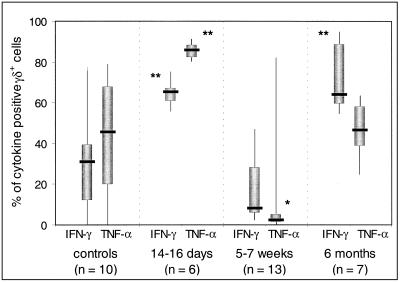
At intervals after the onset of disease, cytokine expression by γδ T cells in response to short-term stimulation with phorbol myristate acetate and ionomycin was measured in various numbers of patients. On days 14 to 16, the proportions of cells expressing TNF-α and/or IFN-γ were high (Fig. 4). At 5 to 7 weeks, a decrease in cytokine-expressing cells occurred and at this interval, the percentage of TNF-α-expressing cells was significantly (P = 0.02) lower than the values of control subjects. In samples obtained at 6 months, the percentages of cells expressing the cytokines were restored (Fig. 4). Irrespective of the time of sampling, only a few percent of the cells expressed interleukin-4 (data not shown).
FIG. 4.
Frequency of cytokine-producing γδ+ T cells at various intervals after onset of legionellosis. Cytokine analysis was performed on CD3-gated cells double stained with anti-γδ antibody and anti-cytokine antibody or an isotype control antibody. The thick line through each box shows the median, with quartiles at either end. The vertical lines indicate maximum and minimum values. ∗, significantly different from controls at P < 0.05. ∗∗, significantly different from controls at P < 0.01.
DISCUSSION
The present outbreak was characterized by a brief incubation period (24 to 48 h) and a transient, influenza-like disease. Neutrophil counts and levels of C-reactive protein increased rapidly and were also quickly normalized. Although pulmonary changes were occasionally found by radiography, they were discrete and symptoms conformed better to Pontiac fever than to Legionnaires′ disease. As in two previous outbreaks of whirlpool-associated Pontiac fever in Scotland and Denmark (17, 27), L. micdadei was implicated as the causative agent. In spite of our failure to isolate the causative agent, the consistency of the serological findings on the patient material, together with a uniform clinical expression well in accordance with Pontiac disease, makes the etiology highly likely. It should be added that the specificity of the immunofluorescence test is extremely high (39). Similar to the present experience, attempts to isolate L. micdadei from patients also failed in the two previous outbreaks (17, 27).
All of the changes in γδ T cells recorded here refer to the Vγ9Vδ2 T-cell subset and show a uniform pattern among the patients. An initial decrease was followed by a rapid and prolonged increase. In blood samples obtained 4 to 6 days after onset of disease, the mean percentage of Vγ9Vδ2 T cells was only 20% of the values of control subjects. Except for this occasion, samples from all patients, as well as control subjects, showed a predominance of Vγ9Vδ2 cells among γ9Vδ T cells. The simplest explanation for the depletion early after the onset of the disease would be an extravasal migration of the cells into the site of infection, preceding an antigen-induced expansion and reentry into the circulation. It remains to be shown whether the cells accumulate in bronchoalveolar fluid and also whether such an initial depletion of Vγ9Vδ2 T cells occurs in other intracellular bacterial infections. In experimental Plasmodium falciparum infection, two infected humans showed a transient decrease in Vγ9Vδ2 T cells in peripheral blood during the second week of infection (34).
The pronounced and protracted increase in Vγ9Vδ2 T cells, in both absolute numbers and proportions of cells, was similar to that previously recorded in patients with ulceroglandular tularemia, an invasive febrile disease caused by another facultatively intracellular bacterium (24). Such a dramatic and long-standing elevation of Vγ9Vδ2 T cells in two intracellular bacterial diseases, widely differing in their clinical pictures, supports the assumption that Vγ9Vδ2 T cells are pathophysiologically important in this kind of infection. No clinical data are available regarding the possible relevance of the long-standing increase in Vγ9Vδ2 T cells. Increased proportions of the subset have been reported in studies of healthy individuals living in regions with increased exposure to intracellular pathogens (10).
The present results imply that Pontiac fever is due to infection rather than exposure to dead bacteria or bacterial toxins. Several pieces of evidence support this line. In guinea pigs, which are highly susceptible to L. pneumophila, aerogenic exposure to dead organisms of the species caused no signs of disease (1). Moreover, strains of L. pneumophila causing Pontiac fever did not differ from those causing Legionnaires′ disease (12), and pneumonic and nonpneumonic forms of legionellosis have been described in patients with a common-source exposure (15). Finally, urine samples from patients with Pontiac fever have shown the presence of Legionella antigen (13).
The function of Vγ9Vδ2 T cells in host response to intracellular infections remains elusive. In the samples obtained here 14 to 16 days after the onset of legionellosis, a majority of the cells produced TNF-α and IFN-γ after being exposed for 4 h to phorbol myristate acetate. One month later, the cytokine expression capability of the Vγ9Vδ2 T cells was decreased, TNF-α expression in particular. These data may reflect an initial cytokine response important in the activation of bactericidal macrophages, followed by down regulation or modulation of the inflammatory response. A similar change has been observed in tularemia (24). In vitro studies suggest that γδ T cells are subjected to a fine-tuned combination of stimulation and inhibition by their interaction with different membrane receptors (26, 31). One of the receptors involved in down regulation is CD94, a type C lectin. In vitro data suggest that CD94 expression is increased on Vγ9Vδ2 T cells as a result of antigen stimulation (3). We found that its expression increased at 5 to 7 weeks after onset of disease, compared with 4 to 6 days after onset, i.e., concomitantly with the decrease in cytokine expression. In fact, experimental work on intracellular infections has suggested a dual role for γδ T cells. After contributing to an early inflammatory response by cytokine expression and activation of macrophages, γδ T cells are suggested to participate in termination of the immune response (4).
In conclusion, patients with Pontiac fever-like disease showed an early depletion of Vγ9Vδ2 T cells from the circulation, followed by a dramatic increase and a subsequent slow decline over the next 6 months. The capability of the cells to express IFN-γ and TNF-α seemed to be down-regulated after the acute phase of the disease. These results support the assumption that Vγ9Vδ2 T cells are pathophysiologically important in intracellular bacterial infections, including a mild and transient condition such as Pontiac fever.
ACKNOWLEDGMENTS
Financial support for this study was received from the Swedish Medical Research Council (no. 9485), Västerbottens läns landsting, and the Medical Faculty, Umeå University, Umeå, Sweden.
REFERENCES
- 1.Berendt R F, Young H W, Allen R G, Knutsen G L. Dose-response of guinea pigs experimentally infected with aerosols of Legionella pneumophila. J Infect Dis. 1980;141:186–192. doi: 10.1093/infdis/141.2.186. [DOI] [PubMed] [Google Scholar]
- 2.Bertotto A, Gerli R, Spinozzi F, Muscat C, Scalise F, Castellucci G, Sposito M, Candio F, Vaccaro R. Lymphocytes bearing the γδ T cell receptor in acute Brucella melitensis infection. Eur J Immunol. 1993;23:1177–1180. doi: 10.1002/eji.1830230531. [DOI] [PubMed] [Google Scholar]
- 3.Boullier S, Poquet Y, Halary F, Bonneville M, Fournie J-J, Gougeon M-L. Phosphoantigen activation induces surface translocation of intracellular CD94/NKG2A class I receptor on CD94 − peripheral Vγ9 Vδ2 T cells but not on CD94 − thymic or mature γδ T cell clones. Eur J Immunol. 1998;28:3399–3410. doi: 10.1002/(SICI)1521-4141(199811)28:11<3399::AID-IMMU3399>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.Carding S R, Egan P J. The importance of γδ T cells in the resolution of pathogen-induced inflammatory immune responses. Immunol Rev. 2000;173:98–108. doi: 10.1034/j.1600-065x.2000.917302.x. [DOI] [PubMed] [Google Scholar]
- 5.Constant P, Davodeau F, Peyrat M A, Poquet Y, Puzo G, Bonneville M, Fournie J-J. Stimulation of human gamma delta T cells by nonpeptidic mycobacterial ligands. Science. 1994;264:267–270. doi: 10.1126/science.8146660. [DOI] [PubMed] [Google Scholar]
- 6.De Libero G. Sentinel function of broadly reactive human γδ T cells. Immunol Today. 1997;18:22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 6a.Edelstein P H. Legionella. In: Lenette E H, Balows A, Hausler W J Jr, Shadomy H J, editors. Manual of clinical microbiology. 4th ed. Washington, D.C.: American Society for Microbiology; 1985. pp. 373–381. [Google Scholar]
- 7.Edelstein P H. Detection of antibodies to Legionella. In: Rose N R, de Macario E C, Fahey J L, Friedman H, Penn G M, editors. Manual of clinical laboratory immunology. 4th ed. Washington, D.C.: American Society for Microbiology; 1992. pp. 459–466. [Google Scholar]
- 8.Edelstein P H. Legionnaires' disease. Clin Infect Dis. 1993;16:741–747. doi: 10.1093/clind/16.6.741. [DOI] [PubMed] [Google Scholar]
- 9.England A C, III, Fraser D W, Plikaytis B D, Tsai T F, Storch G, Broome C V. Sporadic legionellosis in the United States: the first thousand cases. Ann Intern Med. 1981;94:164–170. doi: 10.7326/0003-4819-94-2-164. [DOI] [PubMed] [Google Scholar]
- 10.Esin S, Shigematsu M, Nagai S, Eklund A, Wigzell H, Grunewald J. Different percentages of peripheral blood γδ+ T cells in healthy individuals from different areas of the world. Scand J Immunol. 1996;43:593–596. doi: 10.1046/j.1365-3083.1996.d01-79.x. [DOI] [PubMed] [Google Scholar]
- 11.Follows G A, Munk M E, Gatrill A J, Conradt P, Kaufmann S H E. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in γ/δ T-cell cultures after activation with bacteria. Infect Immun. 1992;60:1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser D W, Deubner D C, Hill D L, Gilliam D K. Nonpneumonic, short-incubation-period legionellosis (Pontiac fever) in men who cleaned a steam turbine condenser. Science. 1979;205:690–691. doi: 10.1126/science.462175. [DOI] [PubMed] [Google Scholar]
- 13.Friedman S, Spitalny K, Barbaree J, Faur Y, McKinney R. Pontiac fever outbreak associated with a cooling tower. Am J Public Health. 1987;77:568–572. [PMC free article] [PubMed] [Google Scholar]
- 14.García V E, Sieling P A, Gong J H, Barnes P F, Uyemura K, Tanaka Y, Bloom B R, Morita C T, Modlin R L. Single-cell analysis of γδ T cell responses to nonpeptide mycobacterial antigens. J Immunol. 1997;159:1328–1335. [PubMed] [Google Scholar]
- 15.Girod J C, Reichman R C, Winn W C, Jr, Klaucke D N, Vogt R L, Dolin R. Pneumonic and nonpneumonic forms of legionellosis. The result of a common-source exposure to Legionella pneumophila. Arch Intern Med. 1982;142:545–547. [PubMed] [Google Scholar]
- 16.Glick T H, Gregg M B, Berman B, Mallison G, Rhodes W W, Jr, Kassanoff I. Pontiac fever: an epidemic of unknown etiology in a health department. I. Clinical and epidemiologic aspects. Am J Epidemiol. 1978;107:149–160. doi: 10.1093/oxfordjournals.aje.a112517. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg D J, Wrench J G, Collier P W, Emslie J A, Fallon R J, Forbes G I, McKay T M, Macpherson A C, Markwick T A, Reid D. Lochgoilhead fever: outbreak of non-pneumonic legionellosis due to Legionella micdadei. Lancet. 1989;i:316–318. doi: 10.1016/s0140-6736(89)91319-6. [DOI] [PubMed] [Google Scholar]
- 18.Götz H M, Tegnell A, De Jong B, Broholm K A, Kuusi M, Kallings I, Ekdahl K. A whirlpool associated outbreak of Pontiac fever at a hotel in Northern Sweden. Epidemiol Infect. 2001;126:241–247. doi: 10.1017/s0950268801005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho M, Webster H K, Tongtawe P, Pattanapanyasat K, Weidanz W P. Increased γδ T cells in acute Plasmodium falciparum malaria. Immunol Lett. 1990;25:139–141. doi: 10.1016/0165-2478(90)90105-y. [DOI] [PubMed] [Google Scholar]
- 20.Horwitz M A. Cell-mediated immunity in Legionnaires' disease. J Clin Investig. 1983;71:1686–1697. doi: 10.1172/JCI110923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito M, Kojiro N, Ikeda T, Ito T, Funada J, Kokubu T. Increased proportions of peripheral blood γδ T cells in patients with pulmonary tuberculosis. Chest. 1992;102:195–197. doi: 10.1378/chest.102.1.195. [DOI] [PubMed] [Google Scholar]
- 22.Jouen-Beades F, Paris E, Dieulois C, Lemeland J-F, Barre-Dezelus V, Marret S, Humbert G, Leroy J, Tron F. In vivo and in vitro activation and expansion of γδ T cells during Listeria monocytogenes infection in humans. Infect Immun. 1997;65:4267–4272. doi: 10.1128/iai.65.10.4267-4272.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaufmann A F, McDade J E, Patton C M, Bennett J V, Skaliy P, Feeley J C, Anderson D C, Potter M E, Newhouse V F, Gregg M B, Brachman P S. Pontiac fever: isolation of the etiologic agent (Legionella pneumophila) and demonstration of its mode of transmission. Am J Epidemiol. 1981;114:337–347. doi: 10.1093/oxfordjournals.aje.a113200. [DOI] [PubMed] [Google Scholar]
- 24.Kroca M, Tärnvik A, Sjöstedt A. The proportion of γδ T cells increases after the first week of onset of tularaemia and remains elevated for more than a year. Clin Exp Immunol. 2000;120:280–284. doi: 10.1046/j.1365-2249.2000.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lang F, Peyrat M A, Constant P, Davodeau F, David-Amelie J, Poquet Y, Vié H, Fournié J J, Bonneville M. Early activation of human Vγ9Vδ2 T cell broad cytotoxicity and TNF production by nonpeptidic mycobacterial ligands. J Immunol. 1995;154:5986–5994. [PubMed] [Google Scholar]
- 26.Lopez-Botet M, Perez-Villar J J, Carretero M, Rodriguez A, Melero I, Bellon T, Llano M, Navarro F. Structure and function of the CD94 C-type lectin receptor complex involved in recognition of HLA class I molecules. Immunol Rev. 1997;155:165–174. doi: 10.1111/j.1600-065x.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 27.Lüttichau H R, Vinther C, Uldum S A, Møller J, Faber M, Jensen J S. An outbreak of Pontiac fever among children following use of a whirlpool. Clin Infect Dis. 1998;26:1374–1378. doi: 10.1086/516354. [DOI] [PubMed] [Google Scholar]
- 28.Mascher B, Schlenke P, Seyfarth M. Expression and kinetics of cytokines determined by intracellular staining using flow cytometry. J Immunol Methods. 1999;223:115–121. doi: 10.1016/s0022-1759(98)00200-2. [DOI] [PubMed] [Google Scholar]
- 29.Miller L A, Beebe J L, Butler J C, Martin W, Benson R, Hoffman R E, Fields B S. Use of polymerase chain reaction in an epidemiologic investigation of Pontiac fever. J Infect Dis. 1993;168:769–772. doi: 10.1093/infdis/168.3.769. [DOI] [PubMed] [Google Scholar]
- 30.Pfeffer K, Schoel B, Gulle H, Kaufmann S H E, Wagner H. Primary responses of human T cells to mycobacteria: a frequent set of γ/δ T cells are stimulated by protease-resistant ligands. Eur J Immunol. 1990;20:1175–1179. doi: 10.1002/eji.1830200534. [DOI] [PubMed] [Google Scholar]
- 31.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, López-Botet M, Fournié J-J, Gougeon M-L. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and anti-tumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 32.Poquet Y, Kroca M, Halary F, Stenmark S, Peyrat M-A, Bonneville M, Fournie J-J, Sjöstedt A. Expansion of Vγ9Vδ2 T cells is triggered by Francisella tularensis-derived phosphoantigens in tularemia but not after tularemia vaccination. Infect Immun. 1998;66:2107–2114. doi: 10.1128/iai.66.5.2107-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raziuddin S, Telmasani A W, El-Hag El-Awad M, Al-Amari O, Al-Janadi M. γδ T cells and the immune response in visceral leishmaniasis. Eur J Immunol. 1992;22:1143–1148. doi: 10.1002/eji.1830220506. [DOI] [PubMed] [Google Scholar]
- 34.Rzepczyk C M, Stamatiou S, Anderson K, Stowers A, Cheng Q, Saul A, Allworth A, McCormack J, Whitby M, Olive C, Lawrence G. Experimental human Plasmodium falciparum infections: longitudinal analysis of lymphocyte responses with particular reference to γδ T cells. Scand J Immunol. 1996;43:219–227. doi: 10.1046/j.1365-3083.1996.d01-24.x. [DOI] [PubMed] [Google Scholar]
- 35.Scalise F, Gerli R, Castellucci G, Spinozzi F, Fabietti G M, Crupi S, Sensi L, Britta R, Vaccaro R, Bertotto A. Lymphocytes bearing the γδ T-cell receptor in acute toxoplasmosis. Immunology. 1992;76:668–670. [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider T, Jahn H-U, Liesenfeld O, Steinhoff D, Riecken E-O, Zeitz M, Ullrich R. The number and proportion of Vγ9Vδ2 T cells rise significantly in the peripheral blood of patients after the onset of acute Coxiella burnetii infection. Clin Infect Dis. 1997;24:261–264. doi: 10.1093/clinids/24.2.261. [DOI] [PubMed] [Google Scholar]
- 37.Sumida T, Maeda T, Takahashi H, Yoshida S, Yonaha F, Sakamoto A, Tomioka H, Koike T, Yoshida S. Predominant expansion of Vγ9/Vδ2 T cells in a tularemia patient. Infect Immun. 1992;60:2554–2558. doi: 10.1128/iai.60.6.2554-2558.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka Y, Sano S, Nieves E, De Libero G, Rosa D, Modlin R L, Brenner M B, Bloom B R, Morita C T. Nonpeptide ligands for human γδ T cells. Proc Natl Acad Sci USA. 1994;91:8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winn W C., Jr Legionnaires disease: historical perspective. Clin Microbiol Rev. 1988;1:60–81. doi: 10.1128/cmr.1.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]