Abstract
Objective:
Inflammatory arthritides exhibit hallmark patterns of affected and spared joints, but in each individual, arthritis affects only a subset of all possible sites. We studied patient-specific joint flare patterns to distinguish local from systemic drivers of disease chronicity.
Methods:
Patients with juvenile idiopathic arthritis followed without interruption from onset into adulthood were identified across two large academic centers. Joints inflamed at each visit were established by medical record review. Flare was defined as physician-confirmed joint inflammation following documented inactive disease.
Results:
Among 222 adult candidates, 95 had complete serial joint examinations since disease onset as children. Mean follow-up was 12.5 years (IQ range 7.9–16.7 years). 90 of 95 patients (95%) achieved inactive disease, after which 81% (73 patients) flared at least once. Among 940 joints affected in 253 flares, 74% had been involved previously. In flares affecting easily observed large joint pairs where only one side had been involved before (n=53), the original joint was affected in 83% and the contralateral joint in 17% (p<0.0001 vs. random laterality). However, disease extended to at least one new joint in approximately 40% of flares, a risk that remained stable even decades after onset and was greatest in flares that occurred off medications (54% vs. 36% on therapy, odds ratio 2.09, p=0.015).
Conclusions:
Arthritis flares preferentially affect previously inflamed joints but carry an ongoing risk of disease extension. These findings confirm joint-specific memory and suggest that prevention of new joint accumulation should be an important target for arthritis therapy.
Keywords: JIA, joint pattern, remission, arthritis flares, resident memory T cells
Introduction
Inflammatory arthritis encompasses a heterogeneous set of disorders affecting children and adults. While joint inflammation is the hallmark of these conditions, they can also involve other tissues, such as lung disease in rheumatoid arthritis (RA) and chronic anterior uveitis in early-onset juvenile idiopathic arthritis (JIA). Extensive study has therefore been dedicated to understanding the systemic immune drivers of autoimmune arthritis (1).
However, systemic autoimmunity is insufficient to explain the presentation of arthritis in the clinic. Beyond the broad disease patterns characterizing distinct forms of arthritis, each patient exhibits an individual pattern of joints that are involved or spared, both with respect to type of joint (e.g. wrist, knee) and laterality. This phenomenon was first described more than 30 years ago by Anderson and colleagues, who found that most joints ever involved in an individual with RA, including all joints needing subsequent joint replacement, were affected already in the first year of disease (2). Consistent with this result, analysis of RA patients in the Behandel Strategieen (BeSt) study showed that joints swollen at baseline were at higher risk of swelling subsequently, with wrists, metacarpophalangeal (MCP) and metatarsophalangeal (MTP) joints reaching recurrence rates of 60% (3). These observations suggest that, in addition to systemic autoimmunity, factors local to individual joints render them susceptible to subsequent inflammation.
The pathogenic implications of such “joint-specific memory” are profound. If individual joints retain a predilection to flare, even through a period of remission, then there must exist long-lived site-specific mechanisms that drive disease chronicity in addition to the “original sin” of systemic autoimmunity. Identification and treatment of these local mechanisms could offer new avenues to durable joint-specific therapy.
To understand the relative contributions of joint-specific vs. systemic factors to the localization of arthritis flares, we studied patients with JIA followed without interruption from onset to adulthood at Boston Children’s Hospital and at a specialized clinic in the adjoining Brigham and Women’s Hospital, the Center for Adults with Pediatric Rheumatic Illness (CAPRI). Historical and genetic evidence increasingly render a categorical division between childhood-onset and adult-onset arthritis biologically untenable (4, 5). However, the patterns of arthritis observed in children differ from those observed in adults, including a greater prevalence of oligoarticular large-joint arthritis for which it is simpler to identify laterality than in RA, a highly polyarticular disease with a predilection for small joints (1, 6). Further, the use of non-biologic and biologic disease-modifying antirheumatic drugs (DMARDs) has enabled achievement of inactive disease in most patients with JIA, although flares remain common, affording an ideal opportunity to evaluate whether individual joints retain a tendency to flare through periods of remission (7).
We demonstrate that JIA flares display unambiguous joint-specific memory as reflected in preserved joint laterality. However, many patients continue to develop inflammation in new joints, even a decade or more after disease onset, confirming both local and systemic drivers of arthritis chronicity. Paired together, these findings suggest a new paradigm, termed here the joint accumulation hypothesis, that provides a rationale for arthritis control that is both rapid and sustained.
Patients and Methods
We conducted a review of medical records from patients seen in the Brigham and Women’s Hospital’s CAPRI clinic between 2005–2017. Patients with JIA were evaluated to determine whether they also had records through the rheumatology clinic at Boston Children’s Hospital detailing their course since disease onset. Patients were excluded if care was interrupted, for example by care at another facility, or if insufficient documentation were available in the electronic medical record to determine clinical status at each clinic visit.
Patient records were reviewed for age at diagnosis of JIA, age at transfer to CAPRI, JIA classification by International League of Associations for Rheumatology 2001 classification criteria (8), treatments employed, and joints inflamed at each visit. Inactive disease was defined as a normal physical exam without joint swelling or other evidence of active arthritis, in the judgment of the attending physician. Disease activity scales were not routinely employed by physicians and therefore were not considered. “Arthritis flare” was defined as an episode of physician-confirmed joint inflammation following physician-documented inactive disease. MCP and MTP joints within each hand or foot were counted as one joint, as were proximal interphalangeal (PIP) joints and distal interphalangeal (DIP) joints, since in the individual joints affected these groups were often not documented precisely by the treating clinician.
Statistical comparisons were performed using two-tailed exact binomial test, Mantel-Cox test or Spearman correlation as indicated. Fisher’s exact test was used to evaluate independence of two variables. A p-value less than 0.05 was considered statistically significant.
This study was approved by the Institutional Review Boards of the Brigham and Women’s Hospital and Boston Children’s Hospital.
Results
Patient cohort.
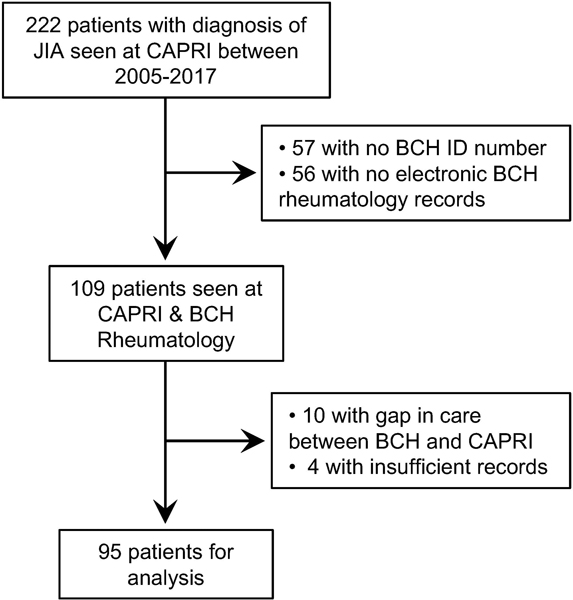
Of the 222 patients treated for JIA in CAPRI between 2005–2017, 109 had been cared for in the pediatric rheumatology program at Boston Children’s Hospital and had available medical records. Ten patients underwent care elsewhere before transferring to CAPRI and 4 patients had insufficient records to assess which joints were involved through the disease course, leaving 95 patients for detailed review (Figure 1).
Figure 1. Patient cohort.
Flowchart indicating patients with JIA seen in the Center for Adults with Pediatric Rheumatic Illness (CAPRI) who were followed longitudinally from Boston Children’s Hospital (BCH) Rheumatology.
Patient Demographics.
Most patients were female (77%). The mean age of JIA diagnosis was 11.5 years and the mean age of transition to CAPRI was 21.5 years. The mean duration of follow-up was 12.5 years, with the longest patient continuity at 21.4 years. 13% of patients had oligoarticular JIA, 43% polyarticular JIA (35% of whom were seropositive), 12% psoriatic JIA, 14% enthesitis related arthritis, 7% systemic JIA, and 11% undifferentiated JIA. 79% of patients had received prescription NSAIDs at any point during their disease course, 29% systemic steroids, and 24% intraarticular steroid injections. 89% of patients had received conventional synthetic DMARDs (csDMARDs: methotrexate, leflunomide, hydroxychloroquine, or sulfasalazine) at any point during their course, while 70% had received a biologic DMARD (bDMARDs: etanercept, adalimumab, infliximab, rituximab, abatacept, tocilizumab, anakinra, or canakinumab). Only one patient treated with bDMARDs never received csDMARDs at any point in their disease course (Table 1).
Table 1.
Patient Demographics
| n=95 | |
|---|---|
| Sex, female | 73 (77%) |
| Age at diagnosis, years, mean ±SD | 11.5 ±5.2 |
| Age at transition, years, mean ±SD | 21.5 ±2.5 |
| Age at last follow-up, years, mean ±SD | 23.7 ±3.4 |
| Duration of follow-up, years, mean (IQ range) | 12.5 (7.9–16.7) |
| JIA diagnosis, final | |
| Oligo, persistent | 5 (5%) |
| Oligo, extended | 8 (8%) |
| Poly, seronegative | 27 (28%) |
| Poly, seropositive | 14 (15%) |
| Psoriatic | 11 (12%) |
| ERA | 13 (14%) |
| Systemic | 7 (7%) |
| Undifferentiated | 10 (11%) |
| Medications | |
| NSAIDS | 75 (79%) |
| Steroids, intraarticular | 23 (24%) |
| Steroids, systemic | 28 (29%) |
| DMARDS, non-biologic | 85 (89%) |
| Biologics | 67 (70%) |
Availability of bDMARDs is associated with reduced time to inactive disease.
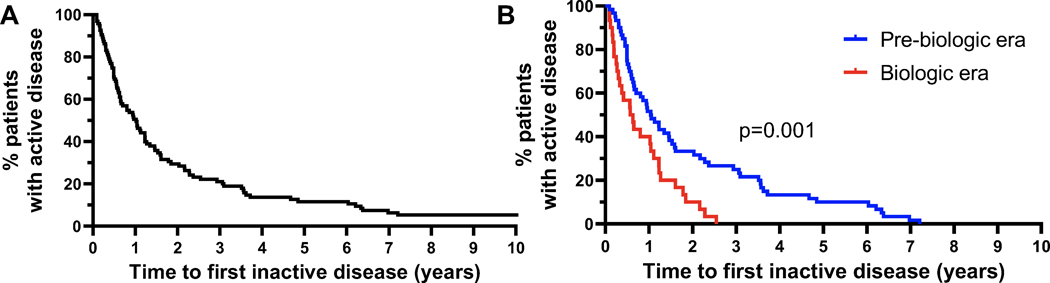
90 patients (95%) achieved inactive disease at some point in their disease course. We assessed duration between presentation to pediatric rheumatology clinic and first documented normal physical exam, finding that 50% of patients achieved inactive disease by 0.96 years after presentation (Figure 2A). Given the timeframe of this study, this cohort included patients diagnosed both before and after bDMARDs were available, since 45 (47%) patients were diagnosed with JIA before etanercept was approved by the U.S. Food and Drug Administration for JIA in May 1999 (9). Given the gradual uptake of these treatments in pediatric rheumatology, the threshold where 25% of patients in this cohort had received bDMARDs was achieved only in 2005 (Supplemental Figure 1). Considering those diagnosed with JIA before 2005 to fall within the “pre-biologic” era and those diagnosed after 2005 to fall within the “biologic” era, we observed a substantially shorter time to first capture in the biologic era (50% capture by 0.60 years in the biologic era vs. 1.05 years in the pre-biologic era, p=0.001, Figure 2B). Correspondingly, 56 of the 61 patients in the pre-biologic era (92%) received prescribed NSAIDs while 21 (34%) received systemic corticosteroids, compared with 19 (56%) and 7 (21%) of the 34 patients from the biologic era (contingency of NSAID use (p=0.0001) and systemic corticosteroid use (p=0.24) with pre-biologic era from two-tailed Fisher’s exact test) (Table 1). Since the CAPRI cohort is limited to patients who sought care as adults, these figures do not reflect JIA as a whole but are consistent with the known efficacy of biologic agents and thus support the face validity of the data.
Figure 2. Time to first episode of inactive disease.
(A) Time from first visit in rheumatology clinic to the first documented normal physical exam. n=95 patients, including 5 who never attained remission. (B) Time to first inactive disease for patients diagnosed with JIA before 2005 (pre-biologic era, n=60) and after 2005 (biologic era, n=30), among the 90 patients who attained inactive disease. 2005 is the year that 25% of the patient cohort had received biologic therapy. p=0.001 Mantel-Cox test.
Arthritis flares preferentially in previously inflamed joints.
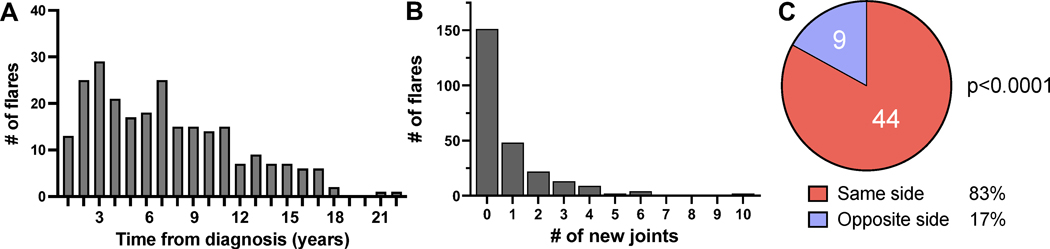
Among the 90 patients who achieved inactive disease, 73 (81%) experienced at least one arthritis flare, totaling 253 distinct flares involving 940 joints. Flares were most common soon after diagnosis but could also be observed late in disease (Figure 3A). While the triggers for 51% of flares were unknown or not documented, 4% of flares followed missed doses of medication, 16% occurred during the weaning of medications, and 18% occurred after discontinuation of medication either by the patient or the physician. 9% of flares were coincident with or followed an infection, 2% were associated with trauma, and 4% were attributed to emotional stress.
Figure 3. Arthritis flares preferentially in previously inflamed joints.
(A) Timing of flare from diagnosis. n=253 flares in JIA patients who attained inactive disease (n=90). (B) Number of new joints per flare. (C) Distribution of joint inflammation in 53 flares involving paired large joints of which only one had been affected prior to a period of inactive disease. p<0.0001 from two-tailed exact binomial test against a stochastic 50/50 distribution.
Among the 940 flaring joints, 698 (74%) had been inflamed at some point before the patient entered inactive disease, while 242 (26%) were new. 151 of 253 flares (60%) involved no new joints, while flares that extended disease typically recruited only a few new joints (Figures 3B).
To evaluate for joint-specific memory, we analyzed flares involving paired joints wherein only one side had been inflamed previously (n=116 flares). In n=30 (26%) of these events, inflammation developed on both sides; these flares were uninformative with respect to preserved laterality. However, in n=86 (74%), only one side became inflamed, allowing us to test whether flare distribution was stochastic (equal chance for either side) or skewed toward the side affected previously. Among these unilateral flares, 86% affected the side previously involved (p<0.0001 vs. stochastic). Since DIP, PIP, MCP, and MTP joints were grouped in our analysis, prohibiting us from assessing individual joints, we repeated the analysis in flares involving paired large joints (shoulder, elbow, wrist, hips, knee, ankle; n=53), finding that 44 (83%) of flares affected only the original joint, a strikingly non-random joint distribution (p<0.0001) (Figure 3C).
Intraarticular steroid injection did not evidently alter joint-specific memory. In the 13 patients with oligoarticular JIA, 11 of 12 distinct knee joints that were injected with intraarticular steroids flared again at least once. Several of the joints were injected multiple times, but this did not prevent future flares from recurring in the same joint.
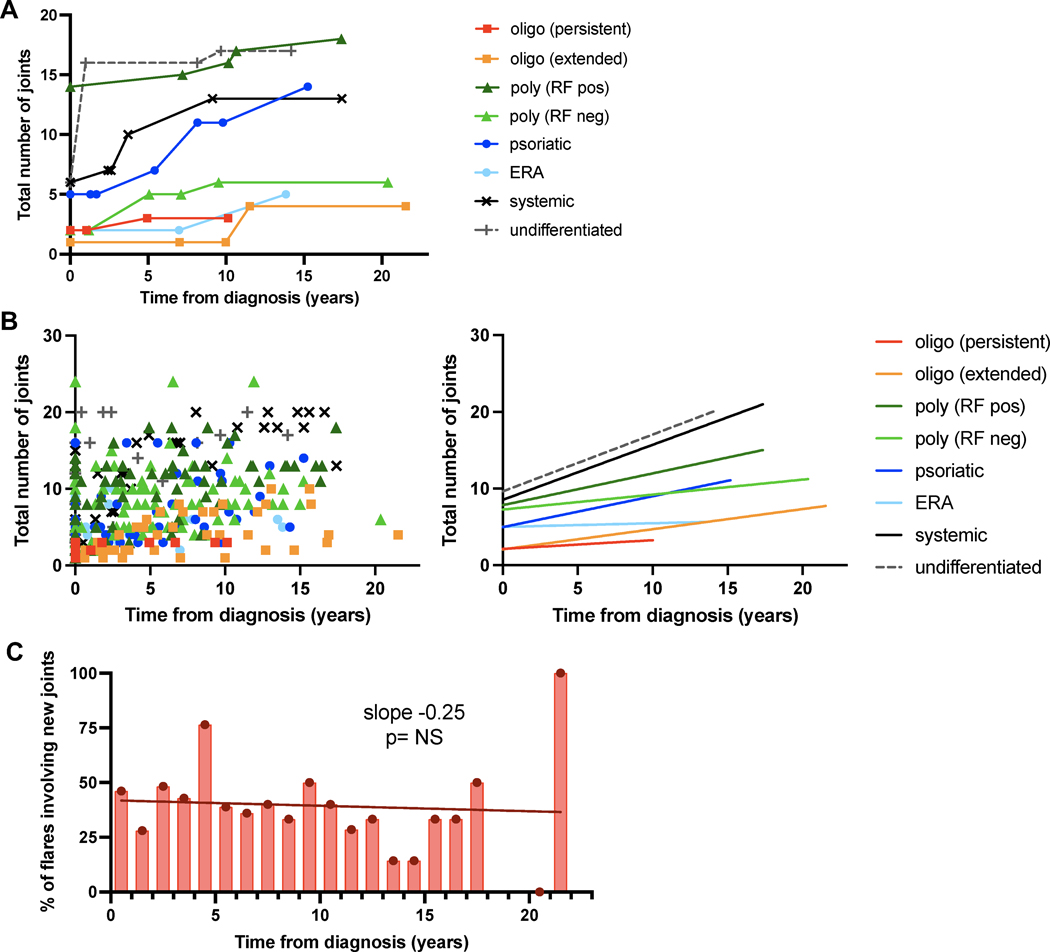
New joint accumulation continues years after onset.
While recurrence in established joints was a dominant pattern during flares, many patients continued to develop arthritis in previously uninvolved joints. This observation is illustrated by individual patients within each JIA category with the longest follow-up (Figure 4A) and was confirmed in the cohort as a whole (Figure 4B). Of the 73 patients who experienced at least one arthritis flare, 60 (82%) developed new joint involvement. Extension to new joints was not restricted to early disease, with one patient developing arthritis in a previously spared joint 21.5 years after diagnosis. We evaluated the proportion of flares that affect new joints as a function of disease duration. Approximately 40% of flares involved at least one new joint, irrespective of time from diagnosis, consistent with a sustained propensity to recruit previously unaffected joints during periods of resurgent disease activity (Figure 4C, Supplemental Table 1).
Figure 4. Arthritic joints accumulate over time during disease flares.
(A) Illustrative patients from each JIA subtype followed for at least 10 years, exhibiting a gradual increase in the number of affected joints with time. Each line represents an individual patient; each data point reflects an encounter for arthritis diagnosis or disease flare. (B) Total number of arthritic joints involved over time. (Left) Each patient contributes multiple data points and each dot represents an encounter for arthritis diagnosis or flare (n=348 encounters). (Right) Linear regression curve for each JIA subtype. Spearman correlation p<0.05 for all subtypes except enthesitis related arthritis (ERA). (C) Proportion of flares that recruit new joints over time (n=102 new joints, 253 flares). RF, rheumatoid factor.
23% of arthritis flares occurred off medications while 77% occurred in patients receiving standing NSAIDs, oral steroids, and/or conventional or biologic DMARDs. Intriguingly, 54% of flares that occurred off medications (32 of 59) involved previously unaffected joints, compared with only 36% of flares that occurred while on medications (70 of 194) (odds ratio 2.09 of new joint involvement if off medication at time of flare, 95% CI 1.16–3.80, p=0.015 from two-tailed Fisher’s exact test), suggesting that recurrent arthritis in the absence of a protective agent poses a particularly high risk of disease extension to new sites.
Discussion
Here we provide a detailed analysis of the pattern of arthritis flares in patients with JIA who had uninterrupted longitudinal follow-up into adulthood. Our data demonstrate unambiguous joint-specific memory, reflected most clearly in the preserved laterality of flares in paired large joints through periods of remission. However, patients also exhibited ongoing risk for accumulation of newly inflamed joints, revealing that pathways mediating disease extension remain active in many patients even years into the course of arthritis.
Mechanisms of joint-specific memory are not yet elucidated fully. Distler and colleagues showed that arthritis induces a persistent “primed” state in synovial fibroblasts, changing their function to intensify disease in previously inflamed joints (10). This work builds on the earlier demonstration that fibroblasts from RA joints exhibit epigenetic reprogramming (11, 12). The role of local fibroblast priming in determining the location of arthritis flares is unknown.
In psoriasis and fixed drug eruptions, recurrent localized inflammation is mediated by infiltrating T cells that become anchored in tissues as resident memory T cells (TRM) (13–16). We recently identified TRM in RA synovium (17). Using several distinct murine arthritis models, we found that synovial TRM accumulate in inflamed joints and persist indefinitely during remission, nucleating site-specific recurrence when triggered by antigen through elaboration of the chemokine CCL5; correspondingly, TRM deletion abrogated local disease flares (17). These findings show that TRM form at least a part of the mechanism by which joint-specific memory arises, representing an interesting new target for site-specific disease ablation (18).
Beyond local memory, however, many patients accrue new joints when disease flares. Perhaps surprisingly, the propensity to recruit previously unaffected joints appears not to wane, remaining at ∼40% with each flare even decades after disease onset in our cohort, with an even greater risk in patients off therapy at the time of flare. These observations indicate that systemic factors underlying the development of arthritis persist even through periods of clinical inactive disease, and raise the possibility that suppressive therapy plays an important role in limiting disease extension even if control remains imperfect.
The paired findings of joint-specific memory and ongoing susceptibility to extension have important implications for arthritis therapy. A leading model to conceptualize the benefit of early aggressive arthritis therapy in RA is the window of opportunity hypothesis, which postulates that effective early therapy induces a durable change in natural history by interrupting an autoimmune response that is not yet well established (19). Clinical trials have suggested benefit of early intervention in terms of structural injury and, to a much more modest extent, for drug-free remission, although a specific timeframe for intervention has proven difficult to define (20–22). Our data do not directly address whether such a window of opportunity exists, but do identify an important complementary principle that we term the joint accumulation hypothesis (Figure 5). Irrespective of any effect on the underlying autoimmune drivers of disease, effective and sustained treatment prevents inflammation from spreading to new joints that would then develop elevated risk for arthritis recurrence, for example through acquisition of synovial TRM. Consistent with this possibility, delay in securing inactive disease correlated in our cohort with a greater final number of joints accumulated, though this observation is difficult to interpret because some of this effect will reflect the greater difficulty of controlling highly polyarticular disease (data not shown). Nevertheless, since inflamed joints likely remain indefinitely at higher risk for recurrent disease than joints that had never been affected, the prevention of disease extension is plausibly a key priority in arthritis care. This priority is independent of any theoretical window for systemic immune re-programming and supports the importance not only of rapid achievement of inactive disease but also of sustained maintenance of remission.
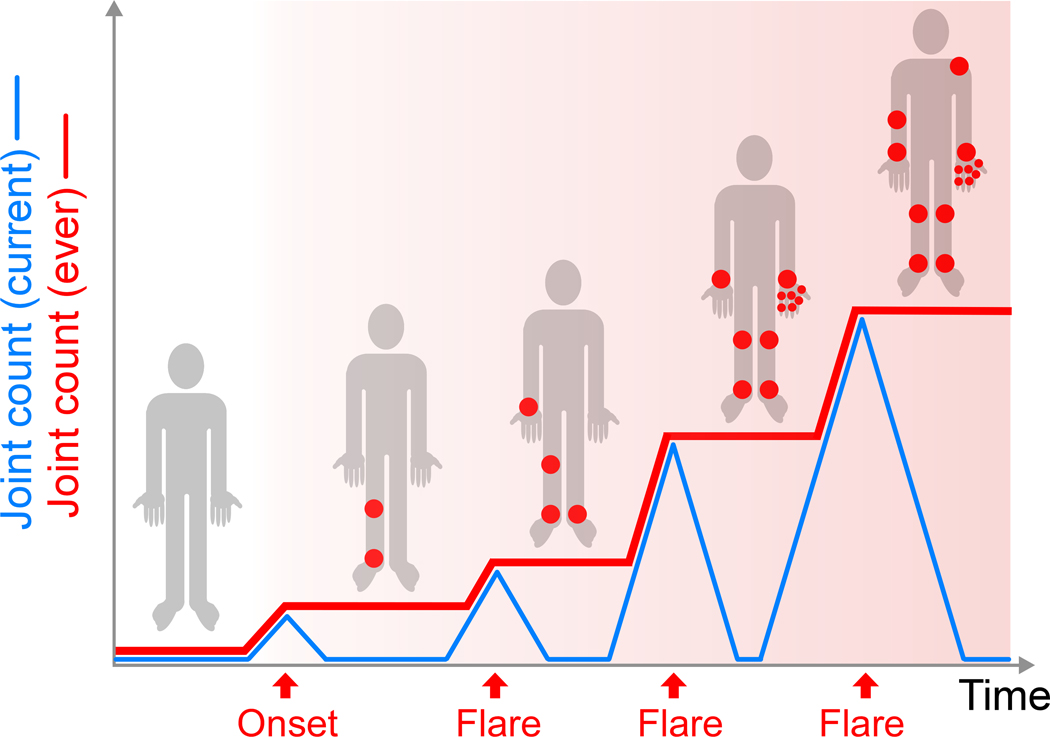
Figure 5. Joint accumulation hypothesis.
Over the course of arthritis, the number of joints currently inflamed varies with disease activity (blue line) but the number of joints ever involved increases stepwise, leaving each patient with a progressively greater number of joints at elevated risk for subsequent flare (joints marked red in homunculus during periods of inactive disease). Rapid, sustained disease control that prevents accumulation of at-risk joints may render arthritis easier to control in the long term, irrespective of any “window of opportunity” in the underlying autoimmune process.
Our data provide observational evidence supporting the key role of bDMARDs in the achievement of remission in JIA. We found that patients diagnosed in an era when biologics were in routine use, after 2005 in our center, achieved inactive disease 5.4 months earlier than those who did not have access to biologics and instead received more NSAIDs and, at least by trend, more systemic steroids. Other factors could have contributed to this finding, including evolving standards of care with respect to use of csDMARDs. However, a central role for biologics is consistent with their contribution to early achievement of good control in studies including ACUTE-JIA, BeSt for Kids, and STOP-JIA (23–27).
It is important to emphasize that our study population consisted of patients with JIA who sought rheumatology follow-up in CAPRI as adults (28). Among all JIA patients, approximately 15–20% have the polyarticular form, of whom only 15% are seropositive (29). By contrast, in our CAPRI cohort 42% had polyarticular JIA, of whom 36% were seropositive, an enrichment that almost certainly reflects the fact that some children with less aggressive JIA “outgrow” their disease (30). For this reason, our data are not representative of JIA as a whole. However, since 81% experienced at least one flare after achieving inactive disease, our cohort provided an optimal opportunity to characterize patterns of disease recurrence. The generalizability of the conclusions beyond JIA is supported by the broad clinical and biological similarities between pediatric and adult arthritis and the concordance between our results and those from RA (2–5).
These findings suggest several directions for future investigation. It will be important to understand in detail the conceptually orthogonal mechanisms underlying local joint memory vs. the persistent drive to accumulate new joints. Our cohort included fewer patients with the least aggressive form of childhood arthritis, persistent oligoarticular JIA, underscoring the intriguing question of how many of these children succeed in maintaining drug-free remission. For example, it may be that local arthritis memory fails to form, or that the triggering antigens disappear, or that memory mechanisms are somehow cleared or suppressed. Finally, since flares off medication appear to carry the highest risk for disease extension, it will be important to study whether patients in remission who are tolerating therapy should be continued on treatment rather than attempting to discontinue medications, especially if risk for relapse is predictably substantial, comparing outcomes including long-term remission, medication exposure, side effects, and cost.
Taken together, our data show two interwoven patterns in inflammatory arthritis: a predilection for joints that have been inflamed previously to flare again, which we term joint-specific memory, and an ongoing predilection in many patients to accumulate new joints with disease flares. These findings support therapeutic efforts targeting mechanisms of synovial memory while also defining a new paradigm of disease chronicity, the joint accumulation hypothesis, that may favor early and sustained treatment to forestall arthritis extension by preventing disease flares.
Supplementary Material
Acknowledgements:
MHC was supported by a Rheumatology Research Foundation Scientist Development Award, Boston Children’s Hospital Lovejoy Award, NIH/NIAID T32AI007512, NIH/NICHD K12HD052896, NIH/NIAMS K08AR080992 and a Translation Accelerator Grant from the Human Skin Disease Research NIH/NIAMS P30AR069625. AVB was by a Medical Student Preceptorship from the Rheumatology Research Foundation. PAN was funded by an Innovative Research Grant from the Rheumatology Research Foundation; NIH/NIAMS 2R01AR065538, R01AR075906, R01AR073201, and P30AR070253; the Fundación Bechara; and the Arbuckle Family Fund for Arthritis Research.
Footnotes
Conflicts of interest: The authors declare no conflicts.
References
- 1.Chang MH, and Nigrovic PA. Antibody-dependent and -independent mechanisms of inflammatory arthritis. JCI Insight. 2019;4(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts WN, Daltroy LH, and Anderson RJ. Stability of normal joint findings in persistent classic rheumatoid arthritis. Arthritis Rheum. 1988;31(2):267–71. [DOI] [PubMed] [Google Scholar]
- 3.Heckert SL, Bergstra SA, Matthijssen XME, Goekoop-Ruiterman YPM, Fodili F, Ten Wolde S, et al. Joint inflammation tends to recur in the same joints during the rheumatoid arthritis disease course. Ann Rheum Dis. 2021. [DOI] [PubMed] [Google Scholar]
- 4.Nigrovic PA, Raychaudhuri S, and Thompson SD. Review: Genetics and the Classification of Arthritis in Adults and Children. Arthritis Rheumatol. 2018;70(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nigrovic PA, Colbert RA, Holers VM, Ozen S, Ruperto N, Thompson SD, et al. Biological classification of childhood arthritis: roadmap to a molecular nomenclature. Nat Rev Rheumatol. 2021;17(5):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigrovic PA, and White PH. Care of the adult with juvenile rheumatoid arthritis. Arthritis Rheum. 2006;55(2):208–16. [DOI] [PubMed] [Google Scholar]
- 7.Guzman J, Oen K, Tucker LB, Huber AM, Shiff N, Boire G, et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis. 2015;74(10):1854–60. [DOI] [PubMed] [Google Scholar]
- 8.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 9. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. [Google Scholar]
- 10.Filer A, Ward LSC, Kemble S, Davies CS, Munir H, Rogers R, et al. Identification of a transitional fibroblast function in very early rheumatoid arthritis. Ann Rheum Dis. 2017;76(12):2105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakano K, Whitaker JW, Boyle DL, Wang W, and Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann Rheum Dis. 2013;72(1):110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ai R, Laragione T, Hammaker D, Boyle DL, Wildberg A, Maeshima K, et al. Comprehensive epigenetic landscape of rheumatoid arthritis fibroblast-like synoviocytes. Nat Commun. 2018;9(1):1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebhardt T, Palendira U, Tscharke DC, and Bedoui S. Tissue-resident memory T cells in tissue homeostasis, persistent infection, and cancer surveillance. Immunol Rev. 2018;283(1):54–76. [DOI] [PubMed] [Google Scholar]
- 14.Park CO, and Kupper TS. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nature medicine. 2015;21(7):688–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mizukawa Y, Yamazaki Y, Teraki Y, Hayakawa J, Hayakawa K, Nuriya H, et al. Direct evidence for interferon-gamma production by effector-memory-type intraepidermal T cells residing at an effector site of immunopathology in fixed drug eruption. Am J Pathol. 2002;161(4):1337–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, and Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. 2004;199(5):731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang MH, Levescot A, Nelson-Maney N, Blaustein RB, Winden KD, Morris A, et al. Arthritis flares mediated by tissue-resident memory T cells in the joint. Cell Rep. 2021;37(4):109902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onuora S. Erasing the memory of arthritis in joints. Nat Rev Rheumatol. 2022;18(1):5. [DOI] [PubMed] [Google Scholar]
- 19.Boers M. Understanding the window of opportunity concept in early rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1771–4. [DOI] [PubMed] [Google Scholar]
- 20.van Nies JA, Krabben A, Schoones JW, Huizinga TW, Kloppenburg M, and van der Helm-van Mil AH. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis. 2014;73(5):861–70. [DOI] [PubMed] [Google Scholar]
- 21.Bergstra SA, Van Der Pol JA, Riyazi N, Goekoop-Ruiterman YPM, Kerstens P, Lems W, et al. Earlier is better when treating rheumatoid arthritis: but can we detect a window of opportunity? RMD Open. 2020;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimian S, Salami A, Malek Mahdavi A, Esalatmanesh K, Khabbazi A, and Hajialilo M. Can treating rheumatoid arthritis with disease-modifying anti-rheumatic drugs at the window of opportunity with tight control strategy lead to long-term remission and medications free remission in real-world clinical practice? A cohort study. Clin Rheumatol. 2021;40(11):4485–91. [DOI] [PubMed] [Google Scholar]
- 23.Tynjala P, Vahasalo P, Tarkiainen M, Kroger L, Aalto K, Malin M, et al. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): a multicentre randomised open-label clinical trial. Ann Rheum Dis. 2011;70(9):1605–12. [DOI] [PubMed] [Google Scholar]
- 24.Hissink Muller P, Brinkman DMC, Schonenberg-Meinema D, van den Bosch WB, Koopman-Keemink Y, Brederije ICJ, et al. Treat to target (drug-free) inactive disease in DMARD-naive juvenile idiopathic arthritis: 24-month clinical outcomes of a three-armed randomised trial. Ann Rheum Dis. 2019;78(1):51–9. [DOI] [PubMed] [Google Scholar]
- 25.Kimura Y, Schanberg LE, Tomlinson GA, Riordan ME, Dennos AC, Del Gaizo V, et al. Optimizing the Start Time of Biologics in Polyarticular Juvenile Idiopathic Arthritis: A Comparative Effectiveness Study of Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans. Arthritis Rheumatol. 2021;73(10):1898–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hissink Muller PC, Brinkman DM, Schonenberg D, Koopman-Keemink Y, Brederije IC, Bekkering WP, et al. A comparison of three treatment strategies in recent onset non-systemic Juvenile Idiopathic Arthritis: initial 3-months results of the BeSt for Kids-study. Pediatr Rheumatol Online J. 2017;15(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong MS, Ringold S, Kimura Y, Schanberg LE, Tomlinson GA, Natter MD, et al. Improved Disease Course Associated With Early Initiation of Biologics in Polyarticular Juvenile Idiopathic Arthritis: Trajectory Analysis of a Childhood Arthritis and Rheumatology Research Alliance Consensus Treatment Plans Study. Arthritis Rheumatol. 2021;73(10):1910–20. [DOI] [PubMed] [Google Scholar]
- 28.Hazel E, Zhang X, Duffy CM, and Campillo S. High rates of unsuccessful transfer to adult care among young adults with juvenile idiopathic arthritis. Pediatr Rheumatol Online J. 2010;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petty RE, Laxer RM, Lindsley CB, Wedderburn LR, Mellins E, and Fuhlbrigge R. Textbook of pediatric rheumatology. Philadelphia: Elsevier, Inc; 2020. [Google Scholar]
- 30.Wallace CA, Huang B, Bandeira M, Ravelli A, and Giannini EH. Patterns of clinical remission in select categories of juvenile idiopathic arthritis. Arthritis Rheum. 2005;52(11):3554–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.