Abstract
Objective:
CXCR4(X4)-tropic HIV-1 was found previously to herald CD4+ cell depletion and disease progression in individuals who were antiretroviral-naive or took combination antiretroviral therapy (cART) for <5 years. We updated this finding by investigating whether the deleterious effect of X4-tropic strains is mitigated by long-term cART.
Design:
We examined morbidity and mortality in relation to HIV-1 tropism and cART in 529 participants followed up to 18 years in the Women’s Interagency HIV Study; 91% were women of color.
Methods:
Plasma-derived HIV-1 tropism was determined genotypically.
Results:
We categorized participants according to number of visits reported on cART after initiation: Group 1) ≤3 visits, 74% of these participants reporting no cART; Group 2) ≥4 visits and <70% of visits on cART; Group 3) ≥70% of visits on cART. AIDS mortality rates for participants in each group with X4-virus compared to those with R5-virus exclusively were, respectively: 1) 62% vs 40% (P=0.0088); 2) 23% vs 22% [Nonsignificant (NS)]; 3) 7% vs 14% (NS). Kaplan-Meier curves showed accelerated progression to AIDS death or AIDS-defining illness in participants with ≤3 cART visits and X4-viruses (P=0.0028), but no difference in progression rates stratified by tropism in other groups. Logistic Regression found that HIV-1 suppression for ≥10 semiannual visits (≥5 years total) mitigated X4-tropism’s deleterious effect on mortality, controlling for maximal viral load and CD4 nadir.
Conclusions:
Long-term cART markedly mitigated the deleterious effect of X4-viruses on AIDS morbidity and mortality. Mitigation was correlated with duration of viral suppression, supporting HIV-1 suppression as a crucial goal.
Keywords: HIV-1, HIV-1 tropism, HIV-1 coreceptor usage, CXCR4, CCR5, combination antiretroviral therapy, cART, HAART, immunologic nonresponders, HIV infection in women
INTRODUCTION
HIV-1 coreceptor usage plays a critical role in viral pathogenesis and disease progression [1-7]. HIV-1 strains that initiate infection use the CCR5-coreceptor (R5-viruses) [1-7]. Strains using the CXCR4-coreceptor (X4-viruses) emerge in ~50-80% of persons during chronic infection, with X4- and R5-viruses generally coexisting within the viral swarm [3-6,8-11]. Emergence of X4-viruses usually heralds CD4 cell depletion and disease progression [3-6,8-10,12-15]. Deleterious effects of X4-viruses were primarily demonstrated in combination antiretroviral therapy (cART)-naïve individuals or those who took cART for <5 years. [14,16,18-21]. Initial studies of coreceptor usage, also called tropism, and cART focused on pretreatment tropism, with few years of follow-up on therapy; most showed that detection of X4-viruses before cART was a strong predictor of disease progression on treatment [16-17,19-21].
Sustained cART has led to dramatic reductions in HIV-1 disease [22], yet the question of whether the deleterious effect of X4-tropism persists despite long-term cART has not been studied in detail. Furthermore, most studies of tropism in vivo focused predominantly on men [10,14,18-21]. We therefore examined the relationship of HIV-1 tropism and long-term cART to morbidity and mortality in 529 women followed up to 18 years in the Woman’s Interagency HIV Study (WIHS), an investigation of inner city women in the US.
METHODS
We studied participants in the Bronx and Brooklyn, NY sites of WIHS, a longitudinal study of women with HIV-1. Interviews, examinations, and laboratory tests were performed semiannually; follow-up extended from enrollment in 1994-1995 until 2012 [23]. Each Institutional Review Board approved the investigation and each woman signed informed consent. cART was defined as any three-drug antiretroviral combination [24].
Plasma-derived HIV-1 tropism was determined using a DNA heteroduplex tracking assay. This assay, performed by Wadsworth Center coauthors, was validated by comparison with phenotypic assay results; both assay and validation were published previously [19]. The assay was licensed to Quest Diagnostics, where it was approved by the Food and Drug Administration (FDA) and Clinical Laboratory Improvement Amendments (CLIA) for clinical coreceptor quantification. Tropism was determined at 1--3 time points for each subject; time points were: 1) WIHS Visit 1; 2) WIHS visit preceding first cART visit; 3) WIHS visit following ≥18 months of continuous cART and virologic failure. X4 strains at ≥1 time point counted as detection of X4 tropism.
Statistical Analyses.
Longitudinal CD4 counts were modeled using mixed effects models. We also fit regression splines to individual CD4 trajectories and overlaid a linear mixed effects model stratified by coreceptor type to look at overall trends. These models were performed using SAS version 9.3. counts over time.
Kaplan-Meier curves stratified by coreceptor usage were employed to model time to AIDS death or a new AIDS-defining illness (ADI). Participants were also categorized by the number of visits on cART and groups were compared using the Log-rank Test. Categorical data were assessed using Fisher’s Exact Test. The Kruskal-Wallis Test was used for continuous data.
Logistic regression analysis and Cox proportional hazards models were used for binary outcomes and time to event data respectively. Regression models controlled for maximal viral load and CD4 nadir and were performed using mortality due to AIDS as the outcome.
Primary outcomes for survival analyses were participants’ time from date of the first WIHS visit to AIDS death or incident ADI. Ascertainment and classification of deaths in participants have been published [25], with deaths due to ADI and infection classified as AIDS deaths in this analysis. ADI were self-reported and classified as incident as previously described [26].
RESULTS
We studied 529 women with HIV-1; (See Table, Supplemental Digital Content 1, describing participants' demographic, virologic, and immunologic characteristics.) The cohort’s racial and ethnic profile (61% African American, 30% Hispanic, 6% White, and 3% Other) is representative of WIHS and women diagnosed with HIV-1 infection in the US in 2016 [23,27,28].
We categorized participants into three groups according to the reported number of semiannual visits on cART after initiation: Group 1: ≤3 visits on cART, 74% of these participants reporting no cART, called “little or no cART,” Group 2: ≥4 visits and <70% of visits on cART, called “intermittent cART,” and Group 3) ≥70% of visits on cART, “consistent cART.” To determine virologic efficacy, we ascertained the number and percentage of visits with viral suppression (HIV-1 RNA load <80 copies/mL) for each subject over time.
Table 1 presents participants' characteristics in relation to tropism and cART. Thirty percent of participants took little cART; 18% took intermittent cART; and 52%, consistent cART. Notably, the consistent cART participants had the highest prevalence of X4-strains (47%)(P=0.01). The little cART group had higher HIV-1 loads (P=0.0028) and lower CD4 counts (P=0.002) at baseline than the other groups.
Table 1.
Participants’ characteristics, morbidity, and mortality in relation to tropism and cART
| A. Virologic, immunologic, and cART-related profiles | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Participants with Little or No cART ≤3 visits on cART |
Participants with Intermittent cART ≥4 and <70% of visits on cART |
Participants with Consistent cART ≥70% of visits on cART |
P Value | |||
| Number and percentage (%) of participants in each cART category | 160 (30%) | 97 (18%) | 272 (52%) | ||||
| Number and percentage of participants with X4 strains a | 58 (36%) | 30 (31%) | 127 (47%) | 0.01b | |||
| Median HIV-1 load at WIHS entry, (log10 copies/mL) [Interquartile Range (IQR)] | 4.63 [1.21] | 4.28 [0.97 IQR] | 4.30 [1.16 IQR] | 0.0028c | |||
| Median CD4 cell count at WIHS entry, cells/mm,3 [IQR] | 215 [311 IQR] | 387 [252 IQR] | 311 [242 IQR] | 0.002d | |||
| Median CD4+/CD8 ratio at WIHS entry, [IQR] | 0.30 [0.39 IQR] | 0.47 [0.33 IQR] | 0.40 [0.32 IQR] | <0.0001e | |||
| Participants who ever achieved complete viral suppression on cART, [Viral load (VL) <80 copies/mL], Number (%) | 19 (12%) | 72 (74%) | 251 (92%) | <0.0001f | |||
| Mean number of visits on cART with complete viral suppression, (VL<80) [Standard Deviation (SD)] | 1.2 [0.8 SD] | 5.6 [5.7 SD] | 11.9 [8.9 SD] | <0.0019g | |||
| Mortality due to AIDS stratified by cART group, Number (%) | 77 (48%) | 22 (23%) | 30 (11%) | <0. 001h | |||
| B. Morbidity and mortality in relation to tropism and cART | |||||||
| Participants with Little or no cART |
Participants with Intermittent cART |
Participants with Consistent cART |
P value | ||||
| X4a | R5i | X4 | R5 | X4 | R5 | ||
| Mortality due to AIDS, stratified by cART group and tropism, Number (%) | 36(62%) | 41(40%) | 7(23%) | 15(22%) | 9(7%) | 21(14%) | 0.0088 j, NSk, NS |
| Participants who progressed to a new AIDS- defining illness or AIDS death, stratified by cART group and tropism, Number (%) | 49(85%) | 70(69%) | 20(67%) | 43(64%) | 74(58%) | 87(60%) | 0.037 l, NS, NS |
cART, combination antiretroviral therapy; X4, HIV-1 strains using the CXCR4 coreceptor; R5, HIV-1 strains using the CCR5 coreceptor; WIHS, Women’s Interagency HIV Study.
Participants with X4 strains detected at one or more time point.
Fisher’s exact test. The percentage of women with detectable X4 strains in the group with consistent use of cART was higher than expected by chance.
Kruskal-Wallis test. The median HIV-1 RNA load at WIHS entry of participants reporting little or no cART significantly exceeded those of the participants in the two other cART groups.
Kruskal-Wallis test. The median CD4 cell count at WIHS entry differed among the three cART groups, with the lowest counts in the subjects with little or no cART, and the highest in those reporting intermittent cART.
Kruskal-Wallis test. The median CD4/CD8 cell ratio at WIHS entry differed among the three cART groups, with the lowest median ratio in the subjects with little or no cART and the highest in subjects reporting intermittent cART.
Fisher’s exact test. The percentage of subjects who achieved complete viral suppression (VL<80) at one or more visits differed among the three cART groups, with the smallest percentage occurring in the participants with little or no cART and the largest in those with consistent cART.
Wilcoxon rank sum and Fisher’s exact test. The mean number of visits on cART with complete viral suppression differed among the three cART groups, with the smallest number of visits with VL <80 in the participants with little or no cART and the largest in those with consistent cART.
Fisher’s exact test. Mortality rates due to AIDS in the three cART groups differed, with the highest rate in the group with little or no cART and the lowest in those with consistent cART. Outcomes for survival analyses were participants’ time from date of the first WIHS visit to AIDS death.
Participants with R5 strains detected exclusively.
Fisher’s exact test. P values refer to comparisons of rates of death due to AIDS in participants with X4 strains as compared to those with R5 strains exclusively. In those with little or no cART, participants with X4 strains had a higher mortality rate than those with R5 strains exclusively. The mortality rates in the two other cART groups did not differ when stratified by tropism.
NS, not significant.
Fisher’s exact test. P values refer to comparisons of rates of death due to AIDS and new AIDS-defining illnesses, including designated cancers, in participants with X4 strains as compared to those with R5 strains exclusively. In those with little or no cART, participants with X4 strains had significantly higher rates of AIDS death or new AIDS-defining illnesses than those with R5 strains exclusively. The rates of AIDS deaths and new ADI in the two other cART groups did not differ when stratified by tropism.
Primary outcomes for survival analyses were participants’ time from date of the first WIHS visit to AIDS death or incident ADI.
Viral suppression was strongly correlated with the number of visits on cART. Only 12% of women with little cART ever had complete viral suppression, compared to 92% with consistent therapy and 74% with intermittent cART (P<0.0001).
X4-viruses were detected, exclusively or in a mixture with R5-variants, in 39% of participants. We compared the CD4 trajectories of participants with X4-variants to those with R5-viruses exclusively, controlling for viral load. Although CD4 counts rose in both groups, participants with X4-strains displayed significantly diminished CD4 counts throughout follow-up (P=0.026). (See Figure, Supplemental Digital Content 2, displaying trajectories of participants' CD4 cell counts over time, stratified by tropism).
Because of the long-term difference in CD4 trajectories, we asked whether X4-tropism may predispose to immunologic nonresponder status [29-31], defined here as CD4 counts remaining <500 cells/mm3 despite viral load <80 copies/mL for ≥5 consecutive years on cART. Fourteen immunologic nonresponders were identified, and eleven (79%) had X4-viruses, suggesting an association of X4-tropism and incomplete CD4 recovery (P=0.018 by Fisher’s Exact Test). CD4 cell nadirs in the nonresponders did not differ significantly by tropism.
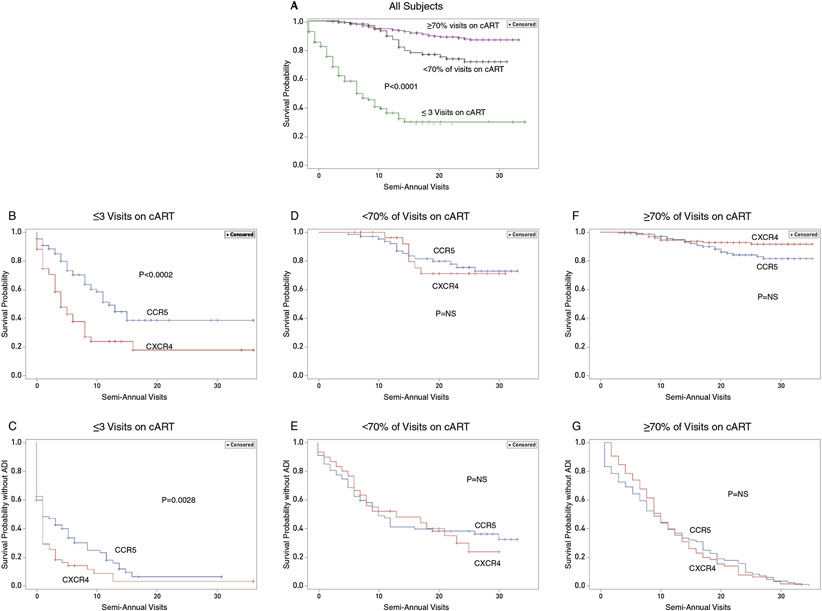
Next, we analyzed the relationship of tropism to clinical outcomes during cART. Kaplan-Meier curves stratified by tropism were employed to model time to AIDS death or a new AIDS-defining illness (ADI). For participants with little cART, there was a markedly accelerated mortality rate for participants with X4-viruses (P=0.0002) (Fig 1B). If ADI or death was the outcome, the result was similar (P=0.0028) (Fig 1C). By contrast, curves depicting time to mortality in intermittent and consistent cART groups showed no difference based upon tropism (NS) (Fig 1D, F), nor was there a difference if the outcome encompassed ADI or mortality rates. (NS)(Fig 1E,G). These results support the idea that cART mitigates or eliminates the deleterious effects of X4-viruses.
Figure 1. Kaplan-Meier curves of the time until death due to AIDS or a new AIDS defining illness (ADI) and the relationship to HIV-1 tropism.
A. Survival time until death due to AIDS for all 529 participants, stratified by number of reported visits on cART. These Kaplan-Meier curves show a significant difference in survival among the three groups defined by number of visits of cART. (P<0.0001 by the log-rank test).
B. Survival time until death due to AIDS in participants with little or no cART (≤3 visits on cART) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan-Meier curves show decreased survival in subjects with X4 strains as compared to participants harboring R5 strains exclusively (P=0.0002 by the log-rank test).
C. Time until progression to a new AIDS-defining illness or death due to AIDS among participants with little or no cART (≤3 visits on cART) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan-Meier curves showed more rapid disease progression or death due to AIDS for those with X4 strains as compared to participants harboring R5 strains exclusively (P=0.0028 by the log-rank test).
D. Survival time until death due to AIDS in participants with intermittent cART (<70% of visits on cART after initiation of therapy) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan-Meier curves were not significantly different between the two coreceptor groups and hence did not demonstrate a difference in mortality rates associated with HIV-1 tropism. [Nonsignificant (NS) by the log-rank test].
E. Time until progression to a new AIDS-defining illness or death due to AIDS in participants taking intermittent cART (<70% of visits on cART after initiation of therapy) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan-Meier curves were not significantly different between the two coreceptor groups and hence did not demonstrate a difference in the time to clinical disease progression or AIDS death associated with tropism (NS).
F. Survival time after initiating cART until death due to AIDS in participants with consistent cART (≥70% of visits on cART after initiation) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan Meier curves were not significantly different between the two coreceptor groups and hence did not demonstrate a difference in mortality rates associated with HIV-1 tropism (NS by the log-rank test).
G. Time after initiating cART until progression to a new AIDS-defining illness or death due to AIDS in participants with consistent ART (<70% of visits on cART after initiation of therapy) stratified by coreceptor type (CCR5 or CXCR4). The Kaplan-Meier curves were not significantly different between the two coreceptor groups and hence did not demonstrate a difference in the time to clinical disease progression or AIDS death associated with tropism (NS by the log-rank test).
We also analyzed the relationship of tropism to clinical outcomes using Fisher’s Exact Test, (full results in Table 1A&B). Mortality rates for women with X4- versus R5-variants, respectively, were: little cART: 62% vs 40% (P=0.0088); intermittent cART: 23% vs 22% (NS); and consistent cART: 7% vs 14% (NS); these results also support the idea of mitigation by cART.
Logistic regression analysis of all participants found that women who had <10 visits with complete HIV-1 suppression on cART were >3 times [OR 3.342 (1.952-5.72)] more likely to experience AIDS mortality than those who had ≥10 visits with viral suppression (See Table, Supplemental Digital Content 3, showing Logistic Regression analysis of relation of AIDS mortality to cART). Higher CD4 nadir was associated with lower likelihood of mortality due to AIDS [22,31]. Notably, for women achieving HIV-1 suppression for ≥10 continuous or noncontinuous semi-annual visits, the difference in mortality between participants who ever had detectable X4-strains and those with R5-virus exclusively was no longer significant after controlling for maximum viral load and CD4 nadir.
These findings show that complete HIV-1 suppression for ≥10 visits (5 years) mitigated the deleterious effect of X4-strains on mortality.
DISCUSSION
This study demonstrates that long-term cART greatly mitigates the deleterious effect of CXCR4(X4)-tropic HIV-1 on morbidity and mortality. A body of literature documents that X4-tropism heralds CD4 depletion and disease progression, even in those receiving cART; most individuals studied, however, were antiretroviral-naïve or received cART for only a few years [3-6,8-10,12-14,16-21]. We updated these findings by studying women followed up to 18 years. Multiple analyses point to a role for both long-term cART and viral suppression in mitigating X4-tropism’s effects (Table 1B, Fig 1, Supplementary Table 2.)
Logistic regression found that participants who had <10 visits with complete HIV-1 suppression on cART were >3 times [OR 3.342 (1.952-5.72)] more likely to experience AIDS death than those who had ≥10 visits with suppression (Supplemental Digital Content 3). Furthermore, logistic regression revealed that HIV-1 suppression for ≥10 semiannual visits (≥5 years total) mitigated the effect of X4-tropic virus on mortality, controlling for maximal viral load and CD4 nadir.
In this study, participants taking consistent cART had the highest prevalence of X4-variants among cART groups; they also achieved the most visits with complete viral suppression and the lowest morbidity and mortality. Data presented here suggest that mitigation of X4-tropism’s effects provides a partial explanation for the success of cART, even conferring mitigation on those taking intermittent cART. Morbidity and mortality rates, however, were lowest in participants who achieved the most visits with viral suppression, underscoring the importance of HIV-1 suppression.
Mechanisms that mitigate X4-viruses’ effect are likely to be complex because CD4 count, tropism, and cART are intertwined [5,8-10,13-16,18,19,32]. cART inhibits replication of all viral strains, generally leading to increased CD4 counts and reversal of HIV-1 disease [19,20,33]. In addition, multiple previous studies revealed that during treatment, cART preferentially suppresses X4-tropic viruses in peripheral blood and X4-proviral variants [34-38]. Mechanisms for preferential X4-virus suppression are likely to stem from differences in cellular targets for HIV-1 [35,39-41].
A small group of immunologic nonresponders was identified. They displayed a significant association between X4-tropism and nonresponder status, suggesting that X4-tropism may play a role in incomplete CD4 cell recovery. These data suggest that a larger study of this question would be worthwhile.
Worldwide, 52% of individuals with HIV-1 are women, as are 23% of persons with HIV-1 in the US [28,42]. Most studies of HIV-1 pathogenesis and disease progression, however, have focused predominantly on men [10,14,18-21]. There are numerous ethical and scientific reasons to include women in HIV-1 research (27,42-63). Biological differences between the sexes influence HIV-1 disease progression [27,52-62], as do socioeconomic, demographic, and cultural disparities [28,42,63]. Women with HIV-1 often experience gender inequality and sexual violence [42,63]. In the US, 61% of woman with HIV-1 are Black, and the rate of new infections is 15-fold higher in Black compared to white women [28]. Genetic diversity is relevant to HIV-1 infection. The CCR5 Δ32 heterozygous state, which confers partial resistance to HIV-1 infection in women, is found primarily in whites [2,27,64,65]. A WIHS study found that Black women taking cART experienced more adverse HIV-1 outcomes than whites, adjusting for confounders; these data suggest a possible role for genetic variability in clinical outcomes [23]. To optimize cART, it will be important to perform more studies examining the interplay between HIV-1 pathogenesis and treatment in diverse gender and racial groups.
Supplementary Material
Acknowledgements.
We thank the women in the WIHS cohort for their long-term, devoted participation in the study, the Wadsworth Center Applied Genomics Technologies Core for HIV sequence analysis, Chad Carpenter for work on the digital figures and tables, and Dr. Monica Parker for scientific administration.
Funding.
Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS) Bronx and Brooklyn sites, now the MACS/WIHS Combined Cohort Study (MWCCS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Bronx CRS (Kathryn Anastos, David Hanna and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Topper), U01-HL146193; The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR).
This work was also supported by awards from NIAID (R01-AI-52015) and Health Research, Incorporated to BW and NIAID support of the University of California, Los Angeles (UCLA) Center for AIDS Research (AI-028697) and the UCLA AIDS Institute to CR.
Footnotes
Conflicts of Interest and Source of Funding
BW, HB and CR are co-inventors of seven patented technologies for the genotypic determination of HIV-1 coreceptor usage. The patents are owned by Health Research, Incorporated, the research foundation for the New York State Department of Health (NYSDOH), and are licensed to Quest Diagnostics. In this study, tropism determinations were performed at the Wadsworth Center of the NYSDOH by coauthors of this paper.
The remaining authors have declared no conflicts of interest and none of the authors has financial relationships with commercial entities relevant to this manuscript.
Presentations at Meetings. Part of the information in this article was presented at the 9th International AIDS Society Conference on HIV Science, 23-26 July 2017, Paris, France, Abstract MOPEB0239; the 9th International Workshop on HIV & Women, 2-3 March 2019, Seattle, Washington, Abstract 51; and AIDS 2020, the 23rd International AIDS Conference, 6-10 July 2020 Virtual Conference, Abstract PEA0053.
REFERENCES
- 1.Feng Y, Broder CC, Kennedy PE, Berger EA, HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G-protein coupled receptor. Science 1996;272:872–877. [DOI] [PubMed] [Google Scholar]
- 2.Samson M, Libert F, Doranz BJ, Rucker J, Liesnard C, Farber CM et al. Resistance to HIV-1 infection in Caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature 1996; 382:722–725. [DOI] [PubMed] [Google Scholar]
- 3.Ray N, Doms RW. HIV-1 coreceptors and their inhibitors. Curr Topics Microbiol Immunol 2006;303:97–120. [DOI] [PubMed] [Google Scholar]
- 4.Bjorndal A, Deng H, Jansson M, Fiore JR, Colognesi C, Karlsson A et al. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J Virol 1997; 71:7478–7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol 1999;73:10489–10502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng HK et al. In vivo evolution of HIV-1 coreceptor usage and sensitivity to chemokine-mediated suppression. Nat Med 1997; 3:1259–1265. [DOI] [PubMed] [Google Scholar]
- 7.Freel SA, Fiscus SA, Pilcher CD, Menezes P, Giner J, Patrick E et al. Envelope diversity, coreceptor usage and syncytium inducing phenotype of HIV-1 variants in saliva and blood during primary infections. AIDS 2003;17:2025–2033. [DOI] [PubMed] [Google Scholar]
- 8.Koot M, Keet IP, Vos AH, de Goede RE, Roos MT, Coutinho RA et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4 cell depletion and progression in AIDS. Ann Intern Med 1993; 118:681–688. [DOI] [PubMed] [Google Scholar]
- 9.Connor RI, Sheridan KE, Ceradini D, Choe S, Landau NR. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med 1997;185:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaak H, van’t Wout AB, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA(+) CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4 cell decline. Proc Natl Acad Sci USA 2000; 97:1269–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou S, Bednar MM, Sturdevant CB, Hauser BM, Swanstrom R. Deep sequencing of the HIV-1 env gene reveals discrete X4 lineages and linkage disequilibrium between X4 and R5 viruses in the V1/V2 and V3 variable regions. J Virol 2016;90:7142–7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreisberg JF, Kwa D, Schramm B, Trautner V, Connor R, Schuitemaker H et al. Cytopathicity of human immunodeficiency virus type 1 primary isolates depends on coreceptor usage and not patient disease status. J Virol 2001;75:8842–8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jekle A, Keppler OT, De Clercq E, Schols D, Weinstein M, Goldsmith MA. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J Virol 2003;77:5846–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shepherd JC, Jacobson LP, Qiao W, Jamieson BD, Phair JP, Piazza P et al. Emergence and persistence of CXCR4-tropic HIV-1 in a population of men from the multicenter AIDS cohort study. J Infect Dis 2008;198:1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin N, Gonzalez OA, Registre L, Becerril C, Etemad B, Lu H, et al. Humoral immune pressure selects for HIV-1 CXC-chemokine receptor 4-using variants. EbioMedicine 2016;8:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brumme ZL, Dong W, Yip B, Wynhoven b, Hoffman NG, Swanstrom R, et al. Clinical and immunological impact of HIV envelope V3 sequence variation after starting initial triple antiretroviral therapy. AIDS. 2004;18:F1–F9. [DOI] [PubMed] [Google Scholar]
- 17.Brumme ZL, Goodrich J, Mayer HB, Brumme CJ, Henrick BM, Wynhoven B, et al. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naïve individuals. J Infect Dis 2005;192:466–474. [DOI] [PubMed] [Google Scholar]
- 18.Daar ES, Kesler KL, Petropoulos CJ, Huang W, Bates M, Lail AE, et al. Baseline HIV type 1 coreceptor predicts disease progression. Clin Infect Dis 2007;45:643–649. [DOI] [PubMed] [Google Scholar]
- 19.Weiser B, Philpott S, Klimkait T, Burgisser P, Gorgievski M, Perrin L, et al. HIV-1 coreceptor usage and CXCR4-specific viral load predict clinical disease progression during combination antiretroviral therapy. AIDS 2008;22:469–79. [DOI] [PubMed] [Google Scholar]
- 20.Waters L, Mandalia S, Randell P, Wildfire A, Gazzard B, Moyle G. The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to first antiretroviral therapy regimen. Clin Infect Dis 2008;46:1617–1623. [DOI] [PubMed] [Google Scholar]
- 21.Goetz MB, Leduc R, Kostman JR, Labriola AR, Lie y, Weidler J, et al. Relationship between HIV coreceptor tropism and disease progression in persons with untreated chronic HIV infection. J Acquir Immune Defic Syndr. 2009;50:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the Gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013; 8:e8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy K, Hoover DR, Shi Q, Cohen M, Gandhi M, Golub ET, et al. Association of self-reported race with AIDS death in continuous HAART users in a cohort of HIV-infected women in the United States. AIDS 2013; 27:2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DHHS/Henry J. Kaiser Family Foundation Panel on clinical practices for the treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents; November 3, 2008. revision. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf.
- 25.French AL, Gawel SH, Hershow R, Benning L, Hessol NA, Levine AM, et al. Trends in mortality and causes of death among women with HIV in the US: a 10-year study. J Acquir Immune Defic Syndr 2009;51:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 1992;41:1–19. [PubMed] [Google Scholar]
- 27.Philpott S, Weiser B, Tarwater PM, Vermund SH, Kleeberger CA, Gange SJ, et al. CC chemokine receptor 5 genotype and susceptibility to transmission of human immunodeficiency virus type 1 in women. J Infect Dis 2003;187:569–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. HIV in Women. Available at:https://www.cdc.gov/hiv/group/gender/women/index.html. Accessed 25 February 2019.
- 29.Kelley CF, Kitchen CMR, Hunt PW, Rodriquez B, Hecht FM, Kitahata M, et al. Incomplete peripheral CD4 cell count restoration in HIV-infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 2009;48:787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore RD, Keruly JC. CD4+ cell count 6 years after commencement of highly active antiretrovial therapy in persons with sustained virologic suppression. Clin Infect Dis 2007;44:441–446. [DOI] [PubMed] [Google Scholar]
- 31.Engsig FN, Zangerie R, Katsarou O, Dabis F, Reiss P, Gill J, et al. Long-term mortality in HIV-positive individuals virally suppressed for >3 years with incomplete CD4+ recovery. Clin Infect Dis 2014; 58:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitchen CMR, Philpott S, Burger H, Weiser B, Anastos K, Suchard MA. Evolution of HIV-1 coreceptor usage during antiretroviral therapy: a Bayesian approach. J Virol 2004; 78: 11296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anastos K, Barron Y, Cohen MH, Greenblatt RM, Minkoff H, Levine A, et al. The prognostic importance of changes in CD4+ cell count and HIV-1 RNA level in women after initiating highly active antiretroviral therapy. Ann Intern Med. 2004;140:256–264. [DOI] [PubMed] [Google Scholar]
- 34.Equils O, Garratty E, Wei LS, Plaeger S, Tapia M, Deville J, et al. Recovery of replication-competent virus from CD4 T cell reservoirs and change in coreceptor use in human immunodeficiency virus type 1-infected children responding to highly active antiretroviral therapy. J Infect Dis 2000;182:751–757. [DOI] [PubMed] [Google Scholar]
- 35.Philpott S, Weiser B, Anastos K, Kitchen CM, Robison E, Meyer WA, et al. Preferential suppression of CXCR4-specific strains of HIV-1 by antiviral therapy. J Clin Invest 2001;107:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skrabal K, Trouplin V, Labrosse B, Obry V, Damond F, Hance AJ, et al. Impact of antiretroviral treatment on the tropism of HIV-1 plasma virus populations. AIDS 2003;17:809–814. [DOI] [PubMed] [Google Scholar]
- 37.Jiao Y, Wang P, Zhang H, Zhang T, Zhang Y, Zhu H, et al. HIV-1 co-receptor usage based on V3 loop sequence analysis: preferential suppression of CXCR4 virus post HAART? Immunol Invest 2011; 40:597–613. [DOI] [PubMed] [Google Scholar]
- 38.Bader J, Däumer M, Schöni-Affolter F, Boni J, Gorgievski-Hrisoho, Martinetti G, et al. Therapeutic immune recovery and reduction of CXCR4-tropic HIV-1. Clin Infect Dis 2017;64:295–300. [DOI] [PubMed] [Google Scholar]
- 39.Roche M, Tumpach C, Symons J, Gartner M, Anderson JL, Khoury G et al. CXCR4-using HIV strains predominate in naïve and central memory CD4+ T cells in people living with HIV on antiretroviral therapy: implications for how latency is established and maintained. J Virol 2019; 10.1128/JVI.01736-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro RM, Hazenberg MD, Perelson AS, Davenport MP. Naïve and memory cell turnover as drivers of CCR5-to-CXCR4 tropism switch in human immunodeficiency virus type 1: implications for therapy. J Virol 2006;80:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weinberger AD, Perelson AS. Persistence and emergence of X4 virus in HIV infection. Math Biosci Eng 2011;8:605–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.UN WOMEN. Facts and figures: HIV and AIDS and factors that fuel HIV. unwomen.org Accessed 21 December 2019. [Google Scholar]
- 43.Kemal KS, Foley B, Burger H, Anastos K, Minkoff H, Kitchen C, et al. HIV-1 in genital tract and plasma of women: compartmentalization of viral sequences, coreceptor usage, and glycoslyation. Proc Natl Acad Sci USA 2003:100:12972–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chohan B, Lavreys L, Rainwater SM, Overbaugh J. Evidence for frequent reinfection with human immunodeficiency virus type 1 of a different subtype. J Virol 2005;79:10701–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Philpott S, Burger H, Tsoukas C, Foley B, Anastos K, Kitchen C et al. Human immunodeficiency virus type 1 genomic RNA sequences in the female genital tract and blood: compartmentalization and intrapatient recombination. J Virol 2005; 79:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blish CA, McClelland RS, Richardson BA, Jaoko W, Mandaliya K, Baeten JM, et al. Genital inflammation predicts HIV-1 shedding independent of plasma viral load and systemic inflammation. J Acquir Immune Defic Syndr 2012;61:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McClelland RS, Lingappa JR, Srinivasan S, Kinuthia J, John-Stewart GC, Jaoko W et al. Evaluation of the association between the concentrations of key vaginal bacteria and the increased risk of HIV acquisition in African women from five cohorts: a nested case-control study. Lancet Infect Dis. 2018;18:554–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eastment MC, McClelland RS. Vaginal microbiota and susceptibility to HIV. AIDS 2018;32:687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yegorov S, Joag V, Galiwango RM, Good SV, Mpendo J, Tannich E, et al. Schistomona mansoni treatment reduces HIV entry into cervical CD4+ T cells and induces IFN-1 pathways. Nat Commun 2019. May 24 10.1038/s41467-019-09900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Joag V, Obila O, Gajer P, Scott MC, Dizzell S, Humphrys M et al. Impact of standard bacterial vaginosis treatment on the genital microbiota, immune milieu, and ex vivo human immunodeficiency virus susceptibility. Clin Infect Dis 2019;68:1675–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson JAE, De Paris K, Ramirez C, Edmonds A, Mollan KR, Bay CP et al. Female genital tract shedding of HIV-1 is rare in women with suppressed HIV-1 in plasma. AIDS 2020;34:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anastos K, Gange SJ, Lau B, Weiser B, Detels R, Giorgi JV et al. The association of race and gender with HIV-1 RNA levels and immunologic progression. J Acquir Immune Defic Syndr Hum Retrovirol, 2000;24:218–226. [DOI] [PubMed] [Google Scholar]
- 53.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, Kulkarni S, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009; 15:955–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andany N, Raboud JM, Walmsley S, Diong C, Rourke SB, Rueda S, et al. Ethnicity and gender differences in lipodystrophy of HIV-positive individuals taking antiretroviral therapy in Ontario, Canada. HIV Clin Trials 2011: 12: 89–103. [DOI] [PubMed] [Google Scholar]
- 55.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis 2014; 209:S86–S92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang OO, Cumberland WG, Escobar R, Liao D, Chew KW. Demographics and natural history of HIV-1-infected spontaneous controllers of viremia. AIDS. 2017; 31:1091–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raghavan A, Rimmelin DE, Fitch KV, Zanni MV. Sex differences in select non-communicable HIV-associated comorbidities: Exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep 2017; 14:220–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goulder P, Deeks SG. HIV control: Is getting there the same as staying there? PLoS Pathog. 2018. Nov; 14(11): e1007222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scully EP, Gandhi M, Johnston R, Hoh R, Lockhart A, Dobrowolski C, et al. Sex-based differences in human immunodeficiency virus type 1 reservoir activity and residual immune activation. J Infect Dis 2019; 219:1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leddy AM, Roque A, Sheira LA, Frongillo EA, Landay AL, Adedimeji AA, et al. Food insecurity is associated with inflammation among women living with HIV. J Infect Dis 2019; 219: 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zanni MV, Awadalla M, Toribio M, Robinson J, Stone LA, Cagliero D et al. , Immune correlates of diffuse myocardial fibrosis and diastolic dysfunction among aging women with Human Immunodefiency Virus. J Infect Dis 2019; doi: 10.1093/infdis/jiz184, published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moyayedi Y, Walmsley S. Heart failure with preserved ejection fraction in women living with HIV: Another inflammatory comorbidity? J Infect Dis 2019; doi.org/ 10.1093/infdis/jiz185, published online ahead of print. [DOI] [PubMed] [Google Scholar]
- 63.Aziz M, and Smith K. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis 2011; 52:S231–S237. [DOI] [PubMed] [Google Scholar]
- 64.Michael NL, Chang G, Louie LG, Mascola JR, Dondero D, Birx DL, et al. The role of viral phenotype and CCR-5 gene defects in HIV-1 transmission and disease progression. Nat Med 1997; 3:338–340. [DOI] [PubMed] [Google Scholar]
- 65.Laurichesse JJ, Persoz A, Theodorou I, Rouzioux C, Delfraissy JF, Meyer L. Improved virological response to highly active antiretrovial therapy in HIV-1-infected patients carrying the CCR5 Δ32 deletion. HIV Medicine 2007; 8:213–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.