Abstract
Objectives:
To investigate risk factors and disease burden in pediatric acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP).
Methods:
Data were obtained from INternational Study group of Pediatric Pancreatitis: In search for a cuRE-2 (INSPPIRE-2), the largest multi-center prospective cohort study in pediatric patients with ARP or CP.
Results:
Of 689 children, 365 had ARP (53%), 324 CP (47%). CP was more commonly associated with female sex, younger age at first acute pancreatitis (AP) attack, Asian race, family history of CP, lower BMI%, genetic and obstructive factors, PRSS1 mutations and pancreas divisum. CFTR mutations, toxic-metabolic factors, medication use, hypertriglyceridemia, Crohn disease were more common in children with ARP. Constant or frequent abdominal pain, emergency room (ER) visits, hospitalizations, medical, endoscopic or surgical therapies were significantly more common in CP, episodic pain in ARP. 33.1% of children with CP had exocrine pancreatic insufficiency (EPI), 8.7% had diabetes mellitus. Compared to boys, girls were more likely to report pain impacting socialization and school, medical therapies, cholecystectomy, but no increased opioid use. There was no difference in race, ethnicity, age at first AP episode, age at CP diagnosis, duration of disease, risk factors, prevalence EPI or diabetes between boys and girls. Multivariate analysis revealed that family history of CP, constant pain, obstructive risk factors were predictors of CP.
Conclusions:
Children with family history of CP, constant pain or obstructive risk factors should raise suspicion for CP.
Keywords: acute recurrent pancreatitis, chronic pancreatitis, children, diabetes, exocrine pancreatic insufficiency, pancreatitis, child, obstructive, pain, PRSS1, race, CFTR
Introduction
Acute pancreatitis (AP) is increasingly recognized in children, with an incidence approaching that of adults (1, 2). Most children with pancreatitis have a single episode that resolves without complications, but some develop recurrent attacks (defined as acute recurrent pancreatitis or ARP) (3, 4) and may progress to chronic pancreatitis (CP) (2, 5, 6). Chronic pancreatitis (CP), in which children have evidence of irreversible pancreatic damage on imaging (7), is estimated to have an incidence of ~2 per 100,000 children per year (2, 5). Although ARP and CP represent uncommon conditions in childhood, they are associated with high disease burden and healthcare costs. Children with ARP or CP experience frequent abdominal pain, emergency room visits, and hospitalizations (8, 9). The disease burden is higher in CP compared with ARP with more emergency room visits, hospitalizations, missed school days, medical, endoscopic and surgical interventions (8).
Genetic risk factors predispose children to CP or rapid progression from ARP to CP (3, 10–14). Children with cationic trypsinogen (PRSS1) variants are more likely to present with CP, develop early-onset CP and progress to CP faster than children without PRSS1 variants (8, 15, 16). Serine protease inhibitor Kazal type 1 (SPINK1) or chymotrypsin-C (CTRC) c.180TT variants are commonly associated with CP diagnosis (8, 17); CTRC and carboxypeptidase A1 (CPA1) associate with early-onset CP (8, 15, 17); CPA1 with early onset CP (18), Carboxylester lipase (CEL) variants are found in children and young adults with CP and CEL-Hybrid (19, 20) variants associated with increased CP risk in adults.
It is not known whether patient demographics and particularly sex or race-based differences play a role in pediatric pancreatitis and disease progression. None of the well-known pancreatitis-associated genes are inherited on the X-chromosome. Studies in adult CP with predominantly environmental risk factors, largely have not demonstrated gender differences (21–23). However, in these studies, women were less likely to have alcohol as the cause of their CP compared to males (24). In multivariate analysis, female sex is found as a risk factor for post-endoscopic retrograde pancreatography (ERCP) pancreatitis for unclear reasons (25, 26). Interestingly, pediatric pancreatitis studies have noted female predominance in a large Polish cohort in children even with autosomal mutations (27, 28). Female preponderance was also observed in INSPPIRE (International Study Group of Pediatric Pancreatitis: In Search for a CuRE) (8, 15, 29–33), a multinational cross-sectional study of children with ARP or CP. In adult populations, African Americans are more likely to develop CP, or suffer from complications of CP with etiologies predominantly related to alcohol and tobacco (34, 35).
The aim of this study was to evaluate the newly developed INSPPIRE-2 cohort and examine the risk factors and disease burden associated with pediatric ARP and CP. Knowing the cohort-specific differences may help understand the risk factors for disease onset and progression and possibly identify targets for therapy.
Methods
Children with ARP or CP enrolled in INternational Study Group of Pediatric Pancreatitis: In search for a cuRE (INSPPIRE)-2 registry were eligible for the analysis. INSPPIRE-2 study includes 22 tertiary centers from 4 countries with data available on the clinical presentation, risk factors, diagnosis and management of children with ARP or CP, now funded by Consortium for the Study of Chronic Pancreatitis, Diabetes and Pancreatic Cancer (CPDPC), a NIH U01 funded effort (36). Children who met the diagnostic criteria for ARP or CP were enrolled into the study. Briefly acute pancreatitis diagnosis required 2 out of 3 criteria: (1) abdominal pain suggestive of, or compatible with AP; (2) serum amylase and /or lipase activity at least 3 times greater than the upper limit of normal; (3) imaging findings characteristic of, or compatible with AP. ARP definition required: (1) at least 2 distinct episodes of AP along with complete resolution of pain (≥ 1 month pain-free interval between the diagnoses of AP); or complete normalization of serum amylase and lipase before the subsequent episode of AP is diagnosed, along with complete resolution of pain symptoms, irrespective of a specific time interval between AP episodes. The definition of CP in children required irreversible changes in the pancreas with or without abdominal pain, OR with EPI, OR with diabetes (36). All centers have obtained local Institutional Review Board approval or the equivalent for their country prior to enrolling subjects. Consent was obtained from the parents of participants less than 18 years and directly from participants 18 years or older. Children gave assent at the age specified by the local institutional review board. This report is a cross-sectional analysis of the baseline enrollment of the cohort, beginning July 1, 2017. Inclusion and exclusion criteria of INSPPIRE-2 were previously reported (36).
Data were entered into the U01 CPDPC’s Central Data Management Center CDMC’s Integrated Information Management System (IIMS) database. Disease burden was determined by asking the subject if they had experienced abdominal pain associated with pancreatitis within the past year, severity and pattern of the pain, number of visits to the ER and number of hospitalizations for pain, pain interference in several life domains, and medications specifically used for pain. Subjects rated pain severity using a 0–10 numeric scale with accompanying Wong-Baker Faces scale (37, 38). The impact of pain on 6 domains was queried on a 5-point Likert scale (39). The pain impact median score was calculated using the average of all six pain impact questions in those who answered at least 4 items. The opioid use and frequency were classified into two categories based on subject-reported current medication lists and dose frequency: 1) Infrequent opioid use was defined as “a few times a month,” “once a month,” or “less than once a month;” 2) Frequent with opioid use was defined as “daily” or “a few times a week. Pancreas divisum was documented on magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) and/or endoscopic retrograde cholangiopancreatography (ERCP) results as recorded on the physician questionnaire.
Patients’ demographics, risk factors, disease burden and treatments outcomes were summarized through descriptive statistics by cohorts and gender. Fisher’s exact test was used to assess the association between categorical variables. Two-sample t-test was used to compare continuous variables between groups. Logistic regression was used to study the association between binary endpoints and predictor variables. For the multivariate analysis, we screened the candidate covariates using univariate logistic regression at the significance level of 0.05, and then applied stepwise model selection procedure with the significance level of 0.05 to build the multivariate inferential model. As “sex” and “pancreas divisum” were of particular interest, they were directly included in the multivariate model without performing variable selection. Results were expressed as mean ± SD.
Results
Patient Characteristics
Demographics of the entire cohort is shown in Table 1. Of 689 subjects in INSPPIRE-2, 365 had ARP (53%), 324 had CP (47%). Compared to ARP, children with CP were significantly more likely to be of female sex (50.5% female vs 42.7% male, p=0.046) (Table S1), with lower BMI percentiles (61.8±32.3 vs 55.9±32.9, p=0.007) and younger at the time of first AP attack (9.28±4.31 vs 8.35±4.18, p=0.006). The cohort was predominantly White; there were fewer children of White and African American race compared to Asians in CP (p<0.05). The disease duration (time between the first AP episode and the date of ARP or CP diagnosis) was short in both groups. There was no difference between the sexes in age at enrollment, age at first AP, age at diagnosis of CP, ethnicity, race, and BMI% (Table S1). Figure S1 shows that the distribution of sex favored females in both ARP and CP cohorts without a clear age-related trend. In 205 girls, age of menarche was available. In 89, ARP began before menarche (43.4%); in 98 ARP began after menarche (47.8%) and in 18 in the same year as menarche (8.8%).
Table 1:
Demographics of the INSPPIRE-2 cohort
| All (n=689) N (%) | ARP (n=365) N (%) | CP (n=324) N (%) | p-value | |
|---|---|---|---|---|
| Gender (Female) | 380 (55.2 %) | 188 (51.5 %) | 192 (59.3 %) | 0.046 |
|
| ||||
| Age at enrollment | 12.1±4.0 | 11.9±4.1 | 12.3±3.8 | 0.153 |
|
| ||||
| Age at first AP | 8.9±4.3 | 9.3±4.3 | 8.4±4.2 | 0.006 |
|
| ||||
| Age at CP | NA | NA | 10.1±4.2 | NA |
|
| ||||
| Disease duration (years) | 1.2±1.8 | 0.94±1.2 | 1.6±2.2 | 0.45 |
|
| ||||
| Ethnicity (Hispanic) | 170 (24.7 %) | 99 (27.1 %) | 71 (21.9 %) | 0.132 |
|
| ||||
| Race | 0.049 | |||
| White | 505 (73.3 %) | 272 (74.5 %) | 233 (71.9 %) | |
| African American | 36 (5.2%) | 22 (6.0 %) | 14 (4.3 %) | |
| Asian | 32 (4.6 %) | 10 (2.7 %) | 22 (6.8 %) | |
| Other | 116 (16.9%) | 61 (16.8%) | 55 (17.0%) | |
|
| ||||
| BMI % | (n=678) 59.1±32.7 | (n=361) 61.8±32.3 | (n=317) 55.9±32.9 | 0.007 |
Values are represented as Mean ± SD. Statistically significant differences are shown in bold.
AP: acute pancreatitis; ARP: acute recurrent pancreatitis; CP: chronic pancreatitis; BMI: body mass index; Statistically significant differences are shown in bold.
Risk factors
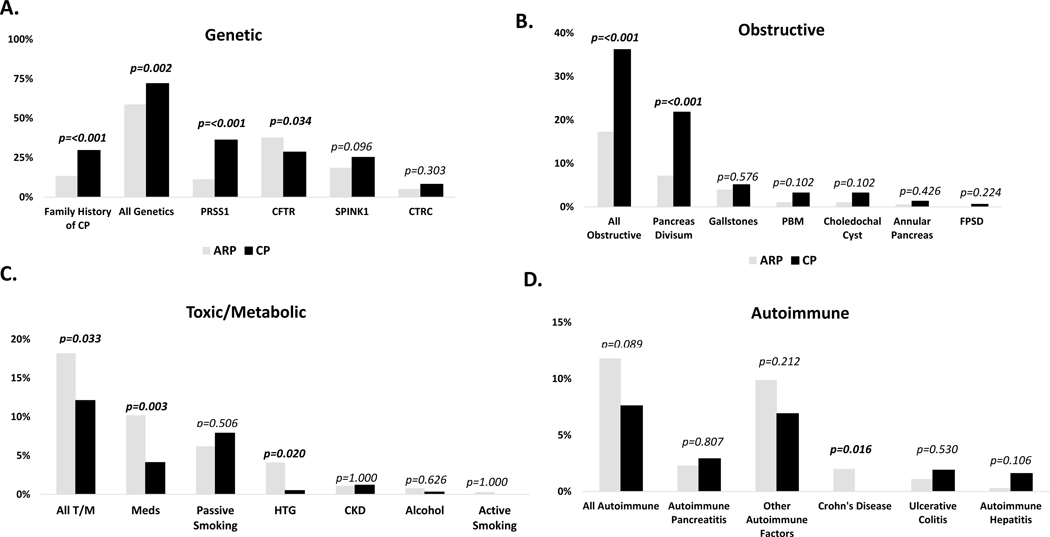
Risk factors were divided into 5 categories: family history of CP, genetic (PRSS1, SPINK1, CFTR, CTRC), obstructive, toxic/metabolic, or autoimmune risk factors (Figure 1). The most common risk factors for development of ARP or CP in the entire cohort were genetic (65.6%) (Figure 1A). Family history of CP and pancreatitis-related gene mutations were more frequently found in CP compared to ARP (13.5% vs 29.6%; p <0.001; 72.2% vs 58.8%, p=0.002 respectively). The most common gene mutation identified in ARP was CFTR (37.8% vs 28.6%, p=0.034), and the most common gene mutation identified in CP was PRSS1 (36.2% vs 11.3%, p<0.001). The second and third most common risk factors were: obstructive (26.2%) and toxic/metabolic (15.3%) (Figure 1B and C). Obstructive factors were more common in CP (36.2% vs 17.3%, p<0.001); whereas toxic metabolic factors were more frequent in ARP (18.2% vs 12.1%, p=0.03). Pancreas divisum (PD) was the most common obstructive risk factor in CP (21.6% vs 6%, p<0.001) whereas medications and hypertriglyceridemia were more frequent in ARP (10.2% vs 4.1%, p=0.003; 4.1% vs 0.5% p=0.02 respectively). Autoimmune factors were less common (Figure 1D). Although rare, Crohn’s disease was more commonly reported in ARP (2% vs 0%; p=0.016). Alcohol use and cigarette smoking were negligible. There were no statistically significant differences between males and females in any of the risk factors (Table S2).
Figure 1. Risk factors in INSPPIRE-2 Cohort.
A. Genetic; B. Obstructive; C. Toxic-Metabolic (T/M); D. Autoimmune. ARP (gray): acute recurrent pancreatitis; CP (black): chronic pancreatitis; PBM: Pancreaticobiliary malunion; FPSD: Functional pancreatic sphincter dysfunction; HTG: hypertriglyceridemia; CKD: chronic kidney disease; Meds: medication use; CFTR: cystic fibrosis transmembrane conductance regulator; PRSS1: cationic trypsinogen; CTRC: chymotrypsin C; SPINK1: serine protease inhibitor Kazal-type 1. P values are shown (statistically significant differences are shown in bold).
Burden of Disease
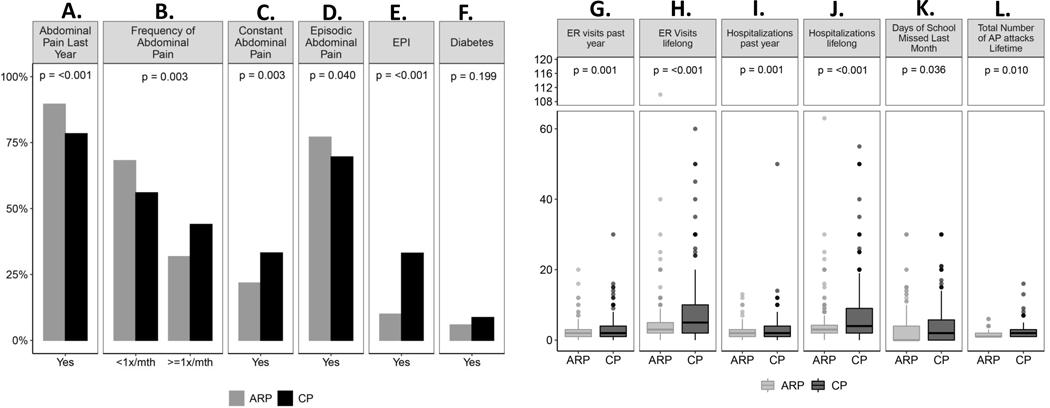
Pancreatitis-related abdominal pain was a major symptom in 84.3% of children with ARP or CP within the last year and more common in ARP (89.6% vs 78.4%; p<0.001) (Figure 2A). Children with CP reported pain more frequently (at least once a month, 44%) whereas those with ARP reported pain less than once a month (68.2%) (p=0.003) (Figure 2B). Constant pain was more common in CP compared to ARP (21.8% vs 33.2%, p=0.003) while episodic pain was more common in ARP (77.1% vs 69.6%, p=0.04) (Figure 2C, D). There was no difference in pain intensity, pain impact on daily life including enjoyment, concentration, activities, recreation, school participation and overall pain impact score between ARP and CP cohorts.
Figure 2. Disease Burden in INSPPIRE-2 Cohort.
Children with A. abdominal pain previous year; B. Frequency of abdominal pain (at least once a month vs less than once a month); C. Constant abdominal pain; D. Episodic abdominal pain; E. Exocrine pancreatic insufficiency (EPI); F. Diabetes mellitus; G. Number of emergency room (ER) visits past year; H. Number of ER visits lifelong; I. Number of hospitalizations past year; J. Number of hospitalizations lifelong; K. Number of days of school missed last month; L. Number of acute pancreatitis (AP) attacks lifetime. ARP (gray): acute recurrent pancreatitis; CP (black)
Exocrine pancreatic insufficiency was more frequently present in subjects with CP (33.1%) than ARP (10%) (p <0.001) as was diabetes mellitus; CP (8.7%) vs ARP (5.9%) (p=0.199) (Figure 2E, F). The number of emergency room (ER) visits and hospitalizations were significantly greater in patients with CP compared to ARP (Figure 2G–J). Likewise, number of AP attacks was higher in CP compared to ARP, with significantly more school days missed in CP (Figure 2K, L). In ARP group, 5 children had Type 1 diabetes mellitus (T1DM) and another 4 had undergone TPIAT; in CP 4 children had T1DM and 59 had undergone TPIAT; 2 children who had undergone TPIAT also had T1DM. Children with TPIAT or T1DM were not included in EPI and diabetes analysis of ARP and CP cohorts.
Females and males equally reported having pain within the previous year (Table S3). There was a trend for more frequent pain in females, but the difference was not statistically significant. There was no difference in overall pain impact scores, episodic or constant pain frequency, episodic or constant pain scores. Pain interference with daily functioning in enjoyment, concentration, activities, and recreation were also not different between females and males. Females reported more pain interference with school and socialization compared to males (p=0.015 and p=0.048, respectively). Health care utilization were also not different between males and females, with similar rates of ER visits, hospitalizations and missed school days. There was also no difference between sexes in the frequency of EPI or diabetes.
Treatments
Table S4 summarizes all treatments utilized for children with ARP or CP. Children with CP were more likely to receive medical (p<0.001), endoscopic (p<0.001), and surgical therapies (p<0.001) when compared with those with ARP. ~78% of all patients were on some form of medication; vitamins/antioxidants, pancreatic enzymes or pain medications were most utilized. Opioids were used infrequently with no significant difference between ARP and CP. Endoscopic retrograde cholangiopancreatography (ERCP) was performed more than 6 times more often in children with CP (72.7%) than those with ARP (11.6%) (p<0.001). Pancreatic sphincterotomy and pancreatic duct stent placement were the most common interventions. Cholecystectomy and total pancreatectomy with islet auto transplant (TPIAT) were the two most frequently performed surgical procedures, again in CP (p<0.001 for both). All other procedures were utilized infrequently with lateral pancreaticojejunostomy performed 14 times more often in CP than ARP (p<0.001).
Females used pancreatitis-related medications and pain medications more frequently than males (p=0.006 and p=0.003, respectively) (Table S5), with no increased use of opioids. There was also an increase in the usage of pancreatic enzymes and steroids in females (p=0.028 for both). There was no difference between sexes in the number of endoscopic procedures or surgical therapies, except cholecystectomy was more commonly done in females (19.7% vs 13.2%; p=0.023).
Imaging Findings
Table S6 summarizes results of all imaging studies in all participants at the time of enrollment. Acute inflammatory findings (focal acute pancreatitis, inflammatory changes, pancreatic gland enlargement, peripancreatic inflammation/fat stranding) were seen in both cohorts. Pancreatic calcifications, ductal stones, abnormal side branches were used to validated CP, thus not found in ARP. PD stricture/obstruction, irregularity, dilatation were most commonly found in CP. These findings alone are not diagnostic of CP unless they are persistent on imaging (36).
Multivariate regression analysis
Seven candidate covariates were significant at 0.05 and identified using the stepwise selection model: sex, pancreas divisum, family history of CP, constant pain, obstructive factors, EPI, and surgical therapies (Table 2). Using the multivariate inferential model, females were 1.77 times more likely to have CP than males, while holding other covariates constant. Multivariate analysis did not identify sex a risk factor for CP. Family history of CP, presence of constant pain, obstructive factors were predictive of CP. History of surgical therapies, EPI were associated with CP, while holding other covariates constant.
Table 2.
Multiple regression analysis predicting chronic pancreatitis
| Independent Variable | Odds Ratio | (95% CI) | p-value |
|---|---|---|---|
| Sex-Female | 1.770 | (0.943, 3.322) | 0.076 |
| Pancreas Divisum | 2.231 | (0.573, 8.693) | 0.248 |
| Family history of chronic pancreatitis | 3.477 | (1.580, 7.648) | 0.002 |
| Constant Pain | 2.331 | (1.124, 4.833) | 0.023 |
| Obstructive Factor | 4.685 | (1.778, 12.345) | 0.002 |
| Exocrine pancreatic insufficiency | 7.197 | (3.205, 16.160) | <0.001 |
| Surgical therapies | 3.313 | (1.561, 7.030) | 0.002 |
Statistically significant differences are shown in bold.
DISCUSSION
This study represents the largest series of pediatric acute recurrent or chronic pancreatitis. Univariate analysis has shown CP to be more commonly associated with female sex, Asian race, younger age at the first AP attack, family history of CP, lower BMI%, genetic and obstructive factors, PRSS1 mutations and pancreas divisum. In contrast, CFTR mutations, Crohn disease, toxic-metabolic factors, medication use, and hypertriglyceridemia were more common in children with ARP. CP compared to ARP was associated with more constant and frequent pain, exocrine pancreatic insufficiency, diabetes mellitus, higher disease burden and health care utilization. Finally, in the multivariate models, we identified that family history of CP, constant pain, and obstructive factors were predictors of CP.
The study summarizes the work of INSPPIRE-2, now under the umbrella of CPDPC, which is an extension and expansion of NIDDK R21-funded INSPPIRE cohort built to better phenotype and characterize a multi-center registry of children with ARP or CP (36, 40). We observed an association only with female sex and CP. Of note, female predominance has been observed in other pediatric studies. For example, in a Polish cohort of 276 children with CP due to most genetic causes, 53% were female (28). Similarly, previous reports from INSPPIRE have noted a female predominance in pediatric CP (8, 15, 29–33), but no statistical significance between ARP and CP (15). Our observation may be due to a recruitment bias of girls presenting more commonly for CP than ARP. It is also possible that larger sample sizes will be better able to delineate sex differences in the pancreatic disease process.
As previously observed, most of the INSPPIRE-2 cohort were White (8, 29, 33) with slight preponderance of Asians in the CP cohort and fewer African American children in ARP cohort. CP predisposing mutations have been reported in children of Asian race and may be explaining these differences (41, 42). INSPPIRE-2 cohort does not have the environmental influences (i.e. alcohol and smoking) observed in adult African Americans with CP (34, 35). Nevertheless, it is difficult to make meaningful comparisons due to small numbers of non-White populations in INSPPIRE-2.
Compared to the previous report from INSPPIRE (33), the frequency of EPI and diabetes was slightly higher in this cohort, probably because of improved characterization of these parameters in INSPPIRE-2 (36). The diagnosis of EPI continues to be challenging. CPDPC has ongoing efforts to better delineate EPI and diabetes mellitus in both pediatric and adult cohorts. Of note, EPI and diabetes were also observed in children with ARP. There could be several reasons for this. One is some children with ARP had type 1 diabetes mellitus and may have coexisting exocrine pancreatic disease as previously described (43, 44). Another possibility is the difficulty in identifying EPI. Despite recent advances in the pancreas field, the diagnosis of EPI remains challenging without an ideal exocrine pancreatic function test (45). Diagnosis of EPI is mostly based on clinical findings and fecal elastase testing. If fecal elastase is obtained during an acute pancreatitis attack, it may be falsely low. A study limitation is that the diagnostic criteria for ARP and CP are mostly based on imaging findings, with poor agreement among radiologists (46). There is a potential for misclassification as early-stage CP may not be detected on imaging studies. Going forward, more standardized pancreatic imaging criteria are needed for early diagnosis of CP.
Pancreas divisum was significantly associated with CP in the univariate analysis of the INSPPIRE-2 cohort, but not predictive of CP in multivariate analysis. We previously reported that PD was a risk factor for ARP and CP and found an odd ratio of ~2 for pancreas divisum in children with CP compared to ARP, but without a statistically significant difference (8). Longitudinal follow-up of patients with PD and ARP is needed to better define the role of PD in the progression to CP and the role of interventions in this process.
Children with CP sustain significant disease burden reporting constant and frequent pain, acute pancreatitis attacks, multiple hospitalizations and ER visits, undergoing medical, endoscopic and surgical therapies as reported previously (8, 29). Despite the pain impacting the social life and school performance and higher pain medication use in girls, we found no increase in opioid use between sexes. In fact, the overall opioid use was much lower in this cohort than previously reported (47). In general, children utilize much less opioids than adults with CP (33), and the data reported here may reflect a down trend in the opioid prescriptions for pancreatic pain. Nevertheless, EPI and diabetes were similar in both sexes, thus the disease did not seem to be advancing faster in females compared to males.
Our observation of higher BMI percentile associated with pediatric ARP is consistent with our previous observations (48) and may suggest that obesity delays the progression from ARP to CP. Of note, we also observed a few children with Crohn’s disease in ARP and none in CP. Pancreatitis may be an extra-intestinal manifestation of inflammatory bowel disease but may also be caused by medications or autoimmunity (49). We also noted that children with CFTR mutations were more likely to have ARP. Because INSPPIRE-2 study only enrolls children with ARP or CP, the cohort may include children with pancreatic-sufficient CF (CF-PS) or CFTR-related disorder (CFTR-RD) (50, 51). In children with CF-PS, pancreatic imaging may be variable, with fatty infiltration and pancreatic atrophy, none of which are at the present diagnostic criteria for pediatric CP (52). This longitudinal prospective study has the potential to determine whether these children with ARP will progress to CP.
One important study limitation is the absence of complete and comprehensive gene testing. The genetic testing was clinician-directed and entered to the database as available. It also only involved 4 most commonly seen genes (CFTR, PRSS1, SPINK1, CTRC). Within INSPPIRE-2, efforts are ongoing to extend the gene testing to involve newly isolated genes such as CASR, CEL, CLDN2, CPA1, CTRC, GGT1, PRSS1–2, PRSS3, SBDS, SLC26A9, and UBR1 (36). It is possible that currently unknown genetic or epigenetic factor(s) play a role in sex differences in children with ARP and CP. The contribution of genetic risk factors to PD leading to CP will also require a complete genotyping of the cohort.
In summary, this is the largest pediatric study to date that examines a prospectively enrolled cohort of children with acute recurrent or chronic pancreatitis. The report highlights that children with chronic abdominal pain and family history of chronic pancreatitis, obstructive factors should raise suspicion for CP. Presence of family history emphasizes the importance of genetic mutations in pediatric ARP and CP; obstructive factors may be contributing to acinar cell damage by increasing pancreatic ductal pressure.
Supplementary Material
What is Known
Sex distribution is similar between acute recurrent pancreatitis (ARP) and chronic pancreatitis (CP).
Pancreatitis is more common in white populations; race distribution is similar across ARP and CP.
Genetic mutations are common in ARP and CP; PRSS1 and SPINK1 mutations predominate in CP.
Obstructive and toxic/metabolic risk factors do not differ between ARP or CP.
~25% of children with CP have exocrine pancreatic insufficiency, ~5% have diabetes mellitus.
What Is New
In INSPPIRE-2, CP is associated with female sex and Asian race.
PRSS1 variants and obstructive risk factors are more common in CP; CFTR variants and toxic/metabolic risk factors predominate in ARP.
~1/3rd of children with CP have exocrine pancreatic insufficiency, ~9% have diabetes mellitus.
CP should be suspected in children with constant pain, obstructive risk factors and family history of CP.
Funding:
Research reported in this publication was supported by National Institute of Diabetes and Digestive and Kidney Diseases and National Cancer Institute of the National Institutes of Health under award numbers 2U01 DK108334, 2U01 DK108328, R01 DK118752. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Mark Lowe is on the Board of Directors of the National Pancreas Association and receives royalties from Millipore Inc and UpToDate. Dr. Tanja Gonska received a research grant from Vertex Pharmaceuticals, and she is a consultant for Cystic Fibrosis Foundation (CFF). Dr. Aliye Uc is a member of American Board of Pediatrics, Subboard of Pediatric Gastroenterology, Associate Editor of Pancreatology and consultant for CFF. Dr. Schwarzenberg is a consultant for UpToDate, Nestle, Abbvie and the Cystic Fibrosis Foundation, she has a grant from Gilead. Dr. Veronique Morinville is an Associate Editor for JPGN Reports.
Footnotes
Conflicts of Interest: The other authors declare no conflicts of interest.
References
- 1.Morinville VD, Barmada MM, Lowe ME Increasing incidence of acute pancreatitis at an American pediatric tertiary care center: is greater awareness among physicians responsible? Pancreas 2010;39(1):5–8. [DOI] [PubMed] [Google Scholar]
- 2.Sellers ZM, MacIsaac D, Yu H, et al. Nationwide Trends in Acute and Chronic Pancreatitis Among Privately Insured Children and Non-elderly Adults in the United States, 2007–2014. Gastroenterology 2018;155:469–478.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poddar U, Yachha SK, Borkar V, et al. A Report of 320 Cases of Childhood Pancreatitis: Increasing Incidence, Etiologic Categorization, Dynamics, Severity Assessment, and Outcome. Pancreas 2017;46(1):110–115. [DOI] [PubMed] [Google Scholar]
- 4.Sweeny KF, Lin TK, Nathan JD, et al. Rapid Progression of Acute Pancreatitis to Acute Recurrent Pancreatitis in Children. J Pediatr Gastroenterol Nutr 2019;68(1):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pant C, Sferra TJ Emergency Department Visits and Hospitalizations in Children With Chronic Pancreatitis in the United States. J Pediatr Gastroenterol Nutr 2015;61(5):568–570. [DOI] [PubMed] [Google Scholar]
- 6.Poddar U, Yachha SK, Borkar V, et al. Is acute recurrent pancreatitis in children a precursor of chronic pancreatitis? A long-term follow-up study of 93 cases. Dig Liver Dis 2017;49(7):796–801. [DOI] [PubMed] [Google Scholar]
- 7.Morinville VD, Husain SZ, Bai H, et al. Definitions of pediatric pancreatitis and survey of present clinical practices. J Pediatr Gastroenterol Nutr 2012;55(3):261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar S, Ooi CY, Werlin S, et al. Risk Factors Associated With Pediatric Acute Recurrent and Chronic Pancreatitis: Lessons From INSPPIRE. JAMA Pediatr 2016;170(6):562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015;166(4):890–6 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-El-Haija M, Valencia CA, Hornung L, et al. Genetic variants in acute, acute recurrent and chronic pancreatitis affect the progression of disease in children. Pancreatology 2019;19(4):535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poddar U, Yachha SK, Mathias A, et al. Genetic predisposition and its impact on natural history of idiopathic acute and acute recurrent pancreatitis in children. Dig Liver Dis 2015;47(8):709–714. [DOI] [PubMed] [Google Scholar]
- 12.Poddar U, Yachha SK, Borkar V, et al. Clinical profile and treatment outcome of chronic pancreatitis in children: a long-term follow-up study of 156 cases. Scand J Gastroenterol 2017;52(6–7):773–778. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Sun XT, Weng XL, et al. Comprehensive screening for PRSS1, SPINK1, CFTR, CTRC and CLDN2 gene mutations in Chinese paediatric patients with idiopathic chronic pancreatitis: a cohort study. BMJ Open 2013;3(9):e003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Y, Yuan W, Yu B, et al. Targeted Gene Next-Generation Sequencing in Chinese Children with Chronic Pancreatitis and Acute Recurrent Pancreatitis. J Pediatr 2017;19:158–63 e3. [DOI] [PubMed] [Google Scholar]
- 15.Giefer MJ, Lowe ME, Werlin SL, et al. Early-Onset Acute Recurrent and Chronic Pancreatitis Is Associated with PRSS1 or CTRC Gene Mutations. J Pediatr 2017;186:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu QY, Abu-El-Haija M, Husain SZ, et al. Risk Factors for Rapid Progression From Acute Recurrent to Chronic Pancreatitis in Children: Report From INSPPIRE. J Pediatr Gastroenterol Nutr 2019;69(2):206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabarczyk AM, Oracz G, Wertheim-Tysarowska K, et al. Chymotrypsinogen C Genetic Variants, Including c.180TT, Are Strongly Associated With Chronic Pancreatitis in Pediatric Patients. J Pediatr Gastroenterol Nutr 2017;65(6):652–657. [DOI] [PubMed] [Google Scholar]
- 18.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet 2013;45(10):1216–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fjeld K, Weiss FU, Lasher D, et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet 2015;47(5):518–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ragvin A, Fjeld K, Weiss FU, et al. The number of tandem repeats in the carboxyl-ester lipase (CEL) gene as a risk factor in alcoholic and idiopathic chronic pancreatitis. Pancreatology 2013;13(1):29–32. [DOI] [PubMed] [Google Scholar]
- 21.Conwell DL, Banks PA, Sandhu BS, et al. Validation of Demographics, Etiology, and Risk Factors for Chronic Pancreatitis in the USA: A Report of the North American Pancreas Study (NAPS) Group. Dig Dis Sci 2017;62(8):2133–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav D, Timmons L, Benson JT, et al. Incidence, prevalence, and survival of chronic pancreatitis: a population-based study. Am J Gastroenterol 2011;106(12):2192–2199. [DOI] [PubMed] [Google Scholar]
- 23.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8(4–5):520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romagnuolo J, Talluri J, Kennard E, et al. Clinical Profile, Etiology, and Treatment of Chronic Pancreatitis in North American Women: Analysis of a Large Multicenter Cohort. Pancreas 2016;45(7):934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hookey LC, RioTinto R, Delhaye M, et al. Risk factors for pancreatitis after pancreatic sphincterotomy: a review of 572 cases. Endoscopy. 2006;38(7):670–676. [DOI] [PubMed] [Google Scholar]
- 26.Freeman ML, DiSario JA, Nelson DB, et al. Risk factors for post-ERCP pancreatitis: a prospective, multicenter study. Gastrointest. Endosc 2001;54(4):425–434. [DOI] [PubMed] [Google Scholar]
- 27.Oracz G, Kolodziejczyk E, Sobczynska-Tomaszewska A, et al. The clinical course of hereditary pancreatitis in children - A comprehensive analysis of 41 cases. Pancreatology 2016;16(4):535–541. [DOI] [PubMed] [Google Scholar]
- 28.Wejnarska K, Kolodziejczyk E, Wertheim-Tysarowska K, et al. The Etiology and Clinical Course of Chronic Pancreatitis in Children With Early Onset of the Disease. J Pediatr Gastroenterol Nutr 2016;63(6):665–670. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzenberg SJ, Bellin M, Husain SZ, et al. Pediatric chronic pancreatitis is associated with genetic risk factors and substantial disease burden. J Pediatr 2015;166(4):890-896 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-El-Haija M, Lowe M, Barth B, et al. Pediatric chronic pancreatitis without prior acute or acute recurrent pancreatitis: A report from the INSPPIRE consortium. Pancreatology 2020;20(4):781–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ting J, Wilson L, Schwarzenberg SJ, et al. Direct Costs of Acute Recurrent and Chronic Pancreatitis in Children in the INSPPIRE Registry. J Pediatr Gastroenterol Nutr 2016;62(3):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troendle DM, Fishman DS, Barth BA, et al. Therapeutic Endoscopic Retrograde Cholangiopancreatography in Pediatric Patients With Acute Recurrent and Chronic Pancreatitis: Data From the INSPPIRE (INternational Study group of Pediatric Pancreatitis: In search for a cuRE) Study. Pancreas 2017;46(6):764–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwarzenberg SJ, Uc A, Zimmerman B, et al. Chronic Pancreatitis: Pediatric and Adult Cohorts Show Similarities in Disease Progress Despite Different Risk Factors. J Pediatr Gastroenterol Nutr 2019;68(4):566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowenfels AB, Maisonneuve P, Grover H, et al. Racial factors and the risk of chronic pancreatitis. Am J Gastroenterol 1999;94(3):790–794. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111(10):1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uc A, Perito ER, Pohl JF, et al. INternational Study Group of Pediatric Pancreatitis: In Search for a CuRE Cohort Study: Design and Rationale for INSPPIRE 2 From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018;47(10):1222–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wong DL, Baker CM Pain in children: comparison of assessment scales. Pediatr Nurs 1988;14(1):9–17. [PubMed] [Google Scholar]
- 38.Keck JF, Gerkensmeyer JE, Joyce BA, et al. Reliability and validity of the Faces and Word Descriptor Scales to measure procedural pain. J Pediatr Nurs 1996;11(6):368–374. [DOI] [PubMed] [Google Scholar]
- 39.Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain 2010;150(1):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morinville VD, Lowe ME, Ahuja M, et al. Design and implementation of INSPPIRE. J Pediatr Gastroenterol Nutr 2014;59(3):360–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho SM, Shin S, Lee KA PRSS1, SPINK1, CFTR, and CTRC Pathogenic Variants in Korean Patients With Idiopathic Pancreatitis. Ann Lab Med 2016;36(6):555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nabi Z, Talukdar R, Venkata R, et al. Genetic Evaluation of Children with Idiopathic Recurrent Acute Pancreatitis. Dig Dis Sci 2020;65(10):3000–3005. [DOI] [PubMed] [Google Scholar]
- 43.Ludvigsson J No acute pancreatitis but reduced exocrine pancreatic function at diagnosis of type 1 diabetes in children. Pediatr Diabetes 2019;20(7):915–919. [DOI] [PubMed] [Google Scholar]
- 44.Mohapatra S, Majumder S, Smyrk TC, et al. Diabetes Mellitus Is Associated With an Exocrine Pancreatopathy: Conclusions From a Review of Literature. Pancreas 2016;45(8):1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taylor CJ, Chen K, Horvath K, et al. ESPGHAN and NASPGHAN Report on the Assessment of Exocrine Pancreatic Function and Pancreatitis in Children. J Pediatr Gastroenterol Nutr 2015;61(1):144–153. [DOI] [PubMed] [Google Scholar]
- 46.Trout AT, Abu-El-Haija M, Anupindi SA, et al. Interobserver Agreement for CT and MRI Findings of Chronic Pancreatitis in Children: A Multicenter Ancillary Study Under the INSPPIRE Consortium. AJR Am J Roentgenol 2022;219(2):303–313.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perito ER, Palermo TM, Pohl JF, et al. Factors Associated With Frequent Opioid Use in Children With Acute Recurrent and Chronic Pancreatitis. J Pediatr Gastroenterol Nutr 2020;70(1):106–114.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uc A, Zimmerman MB, Wilschanski M, et al. Impact of Obesity on Pediatric Acute Recurrent and Chronic Pancreatitis. Pancreas 2018;47(8):967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rogler G, Singh A, Kavanaugh A, et al. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021;161(4):1118–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ooi CY, Dorfman R, Cipolli M, et al. Type of CFTR mutation determines risk of pancreatitis in patients with cystic fibrosis. Gastroenterology 2011;140(1):153–161. [DOI] [PubMed] [Google Scholar]
- 51.Baldwin C, Zerofsky M, Sathe M, et al. Acute Recurrent and Chronic Pancreatitis as Initial Manifestations of Cystic Fibrosis and Cystic Fibrosis Transmembrane Conductance Regulator-Related Disorders. Pancreas 2019;48(7):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feigelson J, Pecau Y, Poquet M, et al. Imaging changes in the pancreas in cystic fibrosis: a retrospective evaluation of 55 cases seen over a period of 9 years. J Pediatr Gastroenterol Nutr 2000;30(2):145–151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.