Abstract
Objective:
There are four conditions caused by Kaposi sarcoma herpesvirus (KSHV): Kaposi sarcoma (KS), KSHV-associated multicentric Castleman disease (MCD), primary effusion lymphoma (PEL), and KSHV inflammatory cytokine syndrome (KICS). These KSHV-associated disorders (KADs) often occur in people with HIV and can lead to multiorgan dysfunction requiring admission to the intensive care unit (ICU). However, little is known about patient outcomes in this setting.
Methods:
A retrospective study of patients with KADs admitted to the ICU between 2010–2021 was conducted, examining KAD admission diagnoses, HIV characteristics, selected cytokine profiles, and ICU interventions. Primary outcomes were 60-day and median overall survival from ICU admission to death from any cause.
Results:
Forty-seven patients (all but one with HIV coinfection) were included. At ICU admission, 44 patients (94%) were on antiretroviral therapy with a median CD4 count of 88 cells/μL and HIV viral load of 23 copies/ml. The most common presentation was respiratory failure alone (19%) or with hypotension (17%). Twenty-two (47%) patients had presumed KICS (+/− KS) at admission and an additional KAD was diagnosed in 36% of these patients. IL-6 levels did not vary across KAD subtype. Twenty-one (47%) patients received KAD-directed therapy in the ICU. 60-day survival was 70% and median overall survival was 9 months.
Conclusion:
The majority of patients with HIV and KADs admitted to the ICU had well-controlled HIV. Additional KAD were diagnosed during ICU admission in a proportion of patients who presented with presumed KICS. Critical illness did not preclude a subset of patients from receiving KAD-directed therapy in the ICU.
Keywords: Kaposi sarcoma, multicentric Castleman disease, primary effusion lymphoma, HIV, Kaposi sarcoma herpesvirus, human herpesvirus 8, critical care
INTRODUCTION
Kaposi sarcoma herpesvirus (KSHV), or human herpesvirus 8 (HHV-8), is the causative agent in Kaposi sarcoma (KS), primary effusion lymphoma (PEL), KSHV-associated multicentric Castleman disease (MCD), and KSHV inflammatory cytokine syndrome (KICS) [1]. These KSHV-associated diseases (KADs) can present concurrently and can lead to multi-organ dysfunction and mortality [2–4]. KADs most commonly occur in people with HIV (PWH) and have specific diagnostic criteria (Supplemental Table 1). KS, a vascular, multicentric tumor, usually manifests as cutaneous lesions, but can involve the visceral organs, most commonly the gastrointestinal and respiratory tracts; given the vascularity of KS lesions, severe visceral KS can cause life-threatening bleeding [2]. PEL, an aggressive B cell non-Hodgkin lymphoma, usually presents with malignant cavitary effusions in the pleura, peritoneum, or pericardium and can lead to cardiorespiratory compromise [3]. MCD, a B cell lymphoproliferative disorder, is associated with inflammatory signs and symptoms, including pancytopenia, hypotension, edema, and lymphadenopathy [5]. It is characterized by relapsing-remitting flares that are generally fatal if untreated [4]. The most recently described KAD, KICS, a diagnosis of exclusion, is characterized by inflammatory symptoms similar to MCD without a histologic diagnosis of MCD [6] or PEL. KICS, MCD, and PEL are all associated with elevated inflammatory cytokines, such as IL-6 and IL-10 [5–7].
The seroprevalence of KSHV infection, which is necessary for KADs, has substantial global variation. Prevalence is highest in sub-Saharan Africa, as high as 95% in parts of Uganda [8], compared to less than 10% in the United States (U.S.) general population [9], though prevalence is much higher in men who have sex with men (MSM) [10]. Immune dysregulation, most commonly HIV, is the major risk factor for development of KADs [11–13]. However, despite advances in antiretroviral therapy (ART), KADs persist as a significant challenge for PWH. KS remains a common tumor in this population with over 900 cases per year in the U.S. [14] and increasing incidence among Black men in the southern U.S. [15] despite decreases elsewhere [16]. MCD, which can occur with KS and/or PEL, is underdiagnosed and is associated with the development of non-Hodgkin lymphoma [5, 11]. PEL, a rare condition accounting for 1–4% of HIV-related lymphomas [11], has worse overall survival compared with other HIV-associated lymphomas and currently has no standard treatment [3].
Widespread use of ART has changed the indications for admission to the intensive care unit (ICU) for PWH. Previously, ICU-level care was required for malnutrition and opportunistic infections (OIs) in young adults, which carried estimated ICU mortality rates of 70–90%. In the ART era, ICU care for PWH is more commonly required for complications of chronic diseases in older adults [17]. ICU mortality among PWH in the ART era is reported to be 16–37%, which is similar to patients without HIV [18]. This range in mortality is associated with access to care, awareness of HIV status, and ART adherence. Respiratory failure is the most common cause of ICU admission among PWH; among those with untreated HIV, these presentations (prior to the COVID-19 pandemic) were due to Pneumocystis pneumonia (PCP), whereas PWH on ART presented with chronic obstructive pulmonary disease, cardiogenic pulmonary edema, and bacterial pneumonia, similar to the general population [19, 20]. Malignancies such as pulmonary KS and PEL remain important causes of respiratory failure requiring ICU admission [21].
In addition to the psychosocial barriers and stigma that this population faces, PWH and cancer are significantly less likely to be offered appropriate cancer treatment [22], making them susceptible to severe disease manifestations requiring ICU-level care. However, little is known about the admissions and outcomes of patients with KADs who require ICU-level care in the current ART era, as existing literature is largely limited to case reports [23, 24]. As patients with symptomatic KADs and HIV can deteriorate rapidly requiring ICU admission, better characterization is needed for optimal, equitable care of these patients. Here, we present the admission characteristics, ICU management, and outcomes of patients with KADs who were admitted to the ICU.
METHODS
Patient selection and study design
We conducted a retrospective review of a well-annotated cohort of patients from the HIV and AIDS Malignancy Branch at the National Cancer Institute (NCI). Patients are referred to our hospital from outside medical providers with a diagnosis of KAD. The inclusion criteria for these analyses were patients with KADs, with or without HIV, who were admitted to the National Institutes of Health (NIH) Clinical Center ICU from February 2010- June 2021. Eligible patients were enrolled on natural history protocols for sample collection and management of KADs (NCT01419561, NCT00092222, NCT00006518, NCT00923065). These protocols were approved by the NCI Institutional Review Board (IRB) and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent. In cases where patients had altered mental status, a bioethics consult was conducted, and the legally authorized representative consented to standard of care (NCT00923065) as approved by the NCI IRB.
For patients’ initial ICU admission, we reviewed KADs present on admission, clinical presentation, HIV and KSHV characteristics, and ICU interventions. Per KICS criteria [6], patients with KSHV-associated inflammation were classified as having “confirmed KICS” if they had undergone workup for MCD and PEL with none found prior to ICU admission. Patients with KSHV-associated inflammation in whom MCD and PEL had not been excluded were classified as “provisional KICS”. The types of chemotherapy and biologics administered in the ICU for KADs were also described. To evaluate multi-organ dysfunction, we calculated sequential organ failure assessment (SOFA) scores, incorporating measures of oxygenation, mental status, vasopressor requirements, platelet count, bilirubin, and creatinine on a scale from 0–24, with 24 representing the most severe organ dysfunction [25]. A SOFA score was calculated in each patient on the day of ICU admission and at ICU discharge or death in the ICU, as appropriate. Additionally, for those who received KAD-directed therapy in the ICU, a SOFA score was calculated on the day before first ICU KAD treatment and the day after the final ICU KAD treatment.
We also examined KADs diagnosed during the ICU admission. Patients’ presenting KADs and KADs on ICU discharge or death were visualized using a Sankey diagram (constructed in R studio using networkD3 package [26]). For this diagram, patients with both confirmed and provisional KICS were classified as KICS.
Cytokine, viral, and other assays
When possible, serum samples were collected within 10 days of ICU admission and discharge or death and stored at −70° C. We analyzed serum inflammatory cytokine profiles from samples stored at −70° C. Serum levels of human interleukins [(IL-) IL-2, IL-4, IL-6, IL-1β, IL-8, IL-10, IL-12p70, IL-13)], inducible protein-10 (IP-10), interferon gamma (IFN-γ), and tumor necrosis factor alpha and (TNF-α), cytokines previously found to be associated with KADs, were evaluated using the Mesoscale Discovery (MSD) Proinflammatory Panel 1 human kit (Rockville, MD). Peripheral blood mononuclear cells (PBMC) KSHV viral load (VL) was quantified via quantitative real-time polymerase chain reaction (PCR) using primers from KSHV K6 gene region as previously described [27]. PBMC VLs were quantified using a human endogenous retrovirus 3 (ERV-3) assay [28] and were expressed as copies per million cell equivalents.
Statistical analysis
The primary outcome was 60-day survival and median overall survival (OS) from ICU admission to death from any cause with follow-up through October 1st, 2021. Survival was evaluated using Kaplan-Meier method curves and 2-sided log-rank tests to determine statistical differences between curves. Where available, changes in levels of cytokines collected within 10 days of admission and discharge or death timepoints were evaluated using the Wilcoxon sign rank test. Where available for both time points, the difference between cytokine concentrations at discharge and admission was determined and a comparison was made between patients who survived beyond 60 days from ICU admission and those who died within 60 days of the ICU admission. Cytokine concentrations between 2 separate KAD diagnosis was also analyzed using Wilcoxon rank sum test. In view of the number of tests, only p values of p<0.005 were considered statistically significant, while p values of 0.005 to <0.05 were considered a strong trend. Due to the exploratory nature of these analyses and the small sample size of rare conditions, no other formal correction was made for multiple comparisons. Univariate analyses of selected prognostic factors were performed using the Cox proportional hazard model. Statistical analyses were completed in STATA [29] and R Studio [30].
RESULTS
Patient characteristics
47 patients (44 cisgender male, 3 cisgender female) with KADs were admitted to the ICU at the NIH Clinical Center ICU between 2010–2021 (Table 1). The median (IQR) age was 38 years (31 – 51). Fifty-five percent of patients were non-Hispanic Black, 34% were non-Hispanic White and 6% were Hispanic/Latinx. Ten patients (21%) had documented chronic comorbid cardiovascular, pulmonary, or renal conditions. All but one patient (98%) had HIV co-infection. Median (IQR) time from HIV diagnosis to ICU admission was 70 months (9 – 132), and median duration of ART was 12.5 months (7–83). Five patients had HIV diagnosed less than 3 months prior to ICU admission. Forty-four patients (95%) were on ART at the time of ICU admission. Median (IQR) CD4 count was 88 copies/μL (38.5 – 222.5) and median (IQR) HIV VL was 23 copies/mL (20 – 95). Where appropriate, patients were on opportunistic infection (OI) prophylaxis per HHS guidelines [31]. Seven patients (15%) had one KAD present on admission, 36 patients (77%) had 2 KADs (20 with KS and KICS), and 4 patients (9%) had KS, MCD, and PEL. Median (IQR) KSHV VL was 885 copies/106 PBMCs (0–247728). Median (IQR) SOFA score was 6 (1 – 9). Thirty-three patients (70%) had received KAD-directed therapy prior to ICU admission. Treatments included single-agent chemotherapy, immunotherapy, or combination chemotherapy (including experimental therapy as part of a clinical trial) for PEL.
Table 1:
Characteristics of patients with KSHV-associated diseases (KAD) admitted to the ICU at the NIH Clinical Center between 2010–2021
| All patients | Alive at 60 days | Died within 60 days | |
|---|---|---|---|
| N | 47 | 33 | 14 |
| Median Age (IQR), years | 38 (31 – 51) | 42 (30 – 52) | 37 (32 – 50) |
| Categorical ages, n (%) | |||
| 20–30 | 11 (23) | 9 (27) | 2 (14) |
| 31–40 | 13 (28) | 7 (21) | 6 (43) |
| 41–50 | 11 (23) | 8 (24) | 3 (21) |
| 51–60 | 9 (19) | 6 (18) | 3 (21) |
| 61–65 | 0 (0) | 0 (0) | 0 (0) |
| 65+ | 3 (6) | 3 (9) | 0 (0) |
| Sex, n (%) | |||
| Cisgender Female | 3 (6) | 1 (3) | 2 (14) |
| Cisgender Male | 44 (94) | 32 (97) | 12 (86) |
| Race n (%) | |||
| White | 16 (34) | 11 (33) | 5 (36) |
| Black | 26 (55) | 20 (61) | 6 (43) |
| Hispanic | 3 (6) | 1 (3) | 2 (14) |
| Other | 2 (4) | 1 (3) | 1 (7) |
| KAD present on admission, n (%) | |||
| KS only * | 3 (6) | 3 (9) | 0 (0) |
| MCD +/− KS | 10 (21) | 8 (24) | 2 (14) |
| MCD only | 1 (2) | 0 (0) | 1 (7) |
| KS + MCD | 9 (19) | 8 (24) | 1 (7) |
| KICS +/− KS | 22 (47) | 17 (52) | 5 (36) |
| KICS only | 2 (4) | 1 (3) | 1 (7) |
| Confirmed** | 1 (2) | 1 (3) | 0 (0) |
| Provisional | 1 (2) | 0 (0) | 1 (7) |
| KS + KICS | 20 (43) | 16 (48) | 4 (29) |
| Confirmed** | 11 (23) | 8 (24) | 3 (21) |
| Provisional | 9 (19) | 8 (24) | 1 (7) |
| PEL +/− KS +/− MCD | 12 (26) | 5 (15) | 7 (50) |
| PEL only | 1 (2) | 0 (0) | 1 (7) |
| KS + PEL | 7 (15) | 5 (15) | 2 (14) |
| KS + MCD + PEL | 4 (9) | 0 (0) | 4 (29) |
| HIV characteristics | |||
| Median HIV duration (IQR), months | 70 (9 – 132) | 83 (13.5 – 150.5) | 12.5 (8.5 – 76) |
| Median CD4 T cell count (IQR), cells/μL | 88 (38.5 – 222.5) | 84 (38 – 272) | 101.5 (44.5 – 176.8) |
| CD4 T cell count > 200 cells/μL at admission, n (%) | 12 (26) | 9 (27) | 3 (21) |
| Median HIV viral load (IQR), copies/ml | 23 (20 – 95) | 48 (20 – 247) | 20 (20 – 39.25) |
| HIV VL < 50 copies/mL at admission, n (%) |
29 (62) | 18 (55) | 11 (79) |
| On ART, n (%) | 44 (94) | 31 (94) | 13 (93) |
| Median duration of ART (IQR), months | 12.5 (7 – 83) | 21.5 (7 – 93) | 9 (7 – 13) |
| ART stopped during admission, n (%) | 7 (15) | 4 (12) | 3 (21) |
| Reasons for ICU admission, n (%) | |||
| Respiratory failure | 26 (55) | 17 (52) | 9 (64) |
| Alone | 9 (19) | 7 (21) | 2 (14) |
| + Hypotension | 8 (17) | 3 (9) | 5 (36) |
| + Fever | 2 (4) | 2 (6) | 0 (0) |
| + Cardiac condition | 1 (2) | 1 (3) | 0 (0) |
| + Altered mental status (AMS) | 2 (4) | 1 (3) | 1 (7) |
| + Fever and AMS | 3 (6) | 3 (9) | 0 (0) |
| + Fever, hypotension, and AMS | 1 (2) | 0 (0) | 1 (7) |
| Hypotension | 10 (21) | 6 (18) | 4 (29) |
| Alone | 5 (11) | 2 (6) | 3 (21) |
| + Fever | 2 (4) | 1 (3) | 1 (7) |
| + Cardiac condition | 1 (2) | 1 (3) | 0 (0) |
| + Hemorrhage | 2 (4) | 2 (6) | 0 (0) |
| Cardiac Condition | 4 (9) | 4 (12) | 0 (0) |
| Alone | 2 (4) | 2 (6) | 0 (0) |
| + Fever | 1 (2) | 1 (3) | 0 (0) |
| + Fever and AMS | 1 (2) | 1 (3) | 0 (0) |
| Hemorrhage | 2 (4) | 2 (6) | 0 (0) |
| Altered mental status | 2 (4) | 1 (3) | 1 (7) |
| Planned admission for/after procedure | 3 (6) | 2 (6) | 1 (7) |
| Median SOFA score at admission (IQR) | 6 (1–9) | 6 (1–9) | 7 (5–11.25) |
| ICU interventions, n (%) | |||
| Intubation | 16 (34) | 7 (21) | 9 (64) |
| CRRT/dialysis/CVVH | 13 (28) | 8 (24) | 5 (36) |
| Vasopressors | 24 (51) | 14 (42) | 10 (71) |
| >1 intervention as above | 19 (40) | 9 (27) | 10 (71) |
| ICU chemotherapy/KAD therapy | 20 (43) | 13 (39) | 7 (50) |
| KSHV viral load levels | |||
| Entry KSHV VL median (IQR), copies/106 PBMCs | 885 (0–247728) | 933 (0–294091) | 885 (253.5–110417) |
| Discharge KSHV VL median (IQR), copies/106 PBMCs | 0 (0–3193) | 0 (0–2094) | 507 (0–4296) |
Of patients with KS as their only KAD, 1 was admitted for observed ketamine infusion for pain management, 1 for airway compromise from a concomitant diffuse large B cell lymphoma (DLBCL) mass, and 1 for GI hemorrhage secondary to visceral KS.
Patients had “confirmed KICS” if they had already undergone workup for MCD and PEL, with none found, prior to ICU admission. Patients had “provisional KICS” if they had KSHV-associated inflammation but had not previously undergone MCD and PEL workup.
ICU admission and interventions
The median (range) ICU length of stay was 7 days (1–61). The most common presentation was respiratory failure with (17%) or without (19%) hypotension. During ICU admission, 51% of patients required vasopressor support, 34% required mechanical ventilation, 28% required renal replacement therapy (intermittent hemodialysis (HD) (n=4) and continuous veno-venous hemofiltration (CVVH) (n=9)), and 40% required more than one intervention (Table 1). Twenty-nine patients (62%) received broad-spectrum antibiotics, and 27 patients (57%) received directed antibiotics, antivirals, or antifungals for a variety of opportunistic (as defined by HIV.gov [31]) and non-opportunistic infections (Supplemental Table 2). Anti-infective agents were administered alongside KAD-directed therapy for 15 patients (32%). Antiretroviral therapy was paused during admission for 7 patients due to inability to take oral medication (n=3), acute renal failure (n=3), and concern for drug-induced pancreatitis (n=1). It was restarted in all but 1 patient, who died in the ICU.
Twenty-two patients (47%) were admitted to the ICU with inflammatory symptoms and elevated KSHV VL suggestive of KICS. Of these, 12 patients had confirmed KICS and 10 patients provisionally met KICS criteria contingent on MCD and PEL exclusion. Additional evaluation in the ICU included pathologic examination of a biopsied lymph node, cytologic examination of pleural or peritoneal fluid after thoracentesis or paracentesis, respectively, and exclusion of infectious etiology. Following this assessment, 2 of the 10 patients with provisional KICS retained a KICS diagnosis, 4 were diagnosed with MCD, 3 were diagnosed with PEL, and 1 was diagnosed with MCD and PEL (Figure 1).
Figure. 1. KSHV-associated diseases (KADs) or combination of KADs at ICU entry (left) and ICU discharge or death (right).
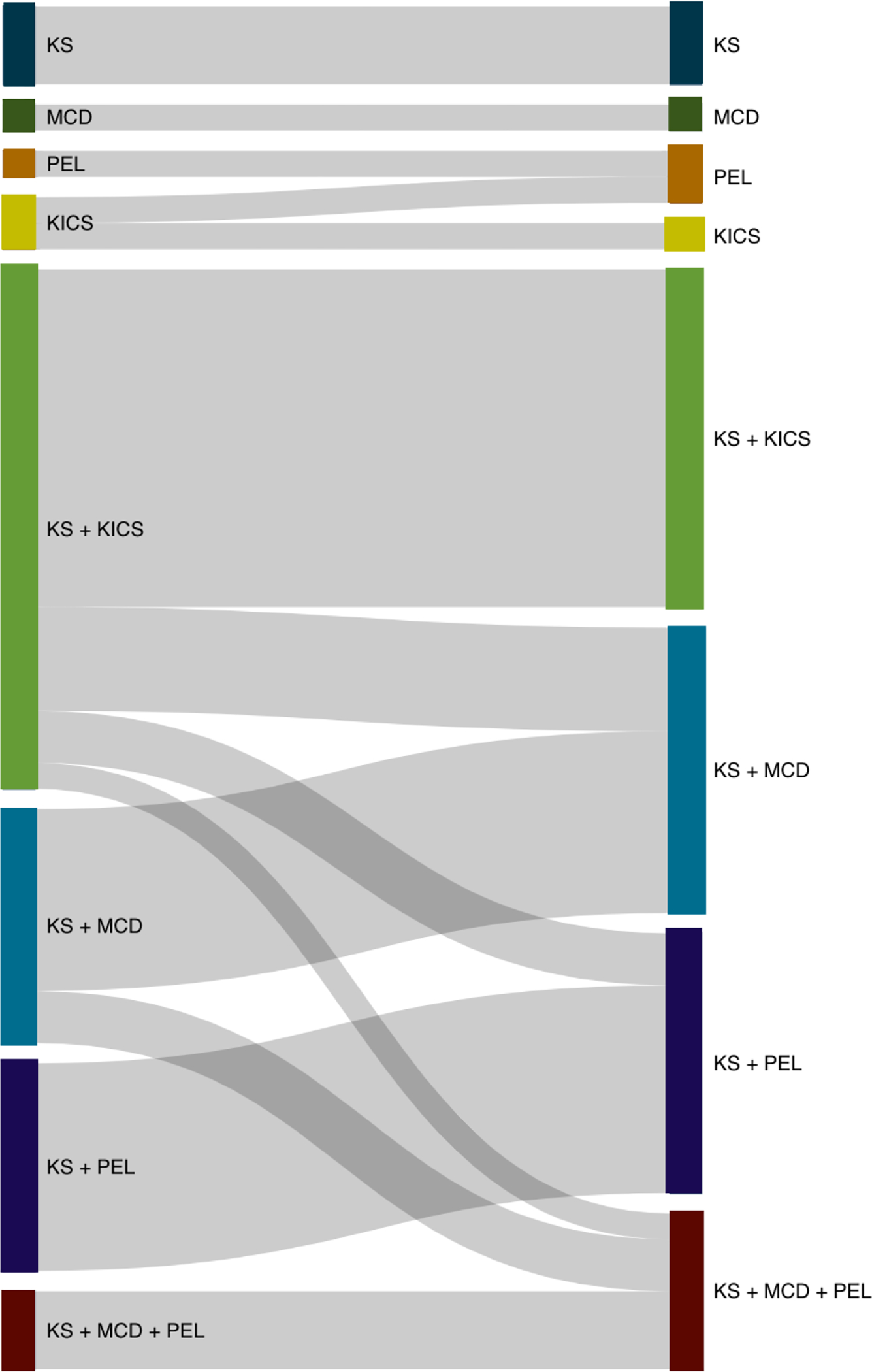
Note: The width of each line is proportional to the number of patients who underwent each transition. Both confirmed and provisional KICS were classified as “KICS” for the purpose of this diagram at ICU entry (left)
KAD therapy (chemotherapy, monoclonal antibodies, and/or immunomodulatory agents) was administered in the ICU to 20 patients (43%). Median SOFA scores decreased after therapy for patients with KICS and MCD, but not for patients with PEL (Supplemental Table 3). Median (IQR) time from admission to initiation of KAD therapy was 2 days (1–3). Three patients experienced complications related to KAD-directed therapy. One patient developed respiratory distress following rituximab infusion and recovered with administration of bronchodilators, steroids, diuretics for pulmonary edema, and supplemental oxygen. Two patients developed neutropenic fever; both initially recovered with antibiotics, though 1 patient ultimately died in the ICU due to disease progression.
Median (range) SOFA score was 6 (0 – 16) at ICU admission and 3 (0 – 19) at discharge or death. Nine patients in this cohort had an initial ICU admission SOFA score of ≥ 10, which predicts mortality of 50% [32]. Five of these patients presented with KICS (2 provisional), 3 of whom had diagnoses of MCD (1 patient) or PEL (2 patients) made in the ICU. The remaining 4 presented with MCD (2 patients), PEL (1 patient), or MCD and PEL (1 patient). Six of the 9 patients with SOFA scores ≥ 10 at the time of ICU admission received KAD-directed therapy in the ICU, and 4 of those 6 survived to ICU discharge. Three of these patients are actively being followed up and have achieved survival longer than 6 months.
Cytokines and viral changes
Serum cytokine concentrations were measured in 29 patients at admission and 18 patients (of whom 11 subsequently died) at the time of ICU discharge or death. At admission, IFN-γ, TNF-α, IL4, IL6, IL8 and IL10 were elevated as compared to established normal ranges from healthy volunteers (Supplemental Table 4). Median IL10 levels decreased significantly from admission to discharge or death among all patients (16.13 pg/ml to 5.40 pg/ml p=0.005, Supplemental Table 4). On assessing differences in serum inflammatory cytokine profiles by KAD on ICU admission, IL-6 levels were not different across these conditions. IL-10 levels appeared to trend higher in patients with PEL+/−KS+/−MCD as compared to confirmed KICS+/−KS (p = 0.03; Figure 2, Supplemental Table 5). Conversely, there was a trend toward increased IL-8 levels among patients with KICS+/−KS as compared to those with PEL+/−KS+/−MCD (p=0.03, Figure 2, Supplemental Table 5). There was no change in the KSHV VL levels between KAD. The median KSHV VL was 885 copies/106 PBMCs (n=39, IQR (0 – 247728)) at ICU admission. There was a strong trend for decreased KSHV VL at discharge with an undetectable VL (P=0.008). There was no significant change in the median CD4+ T cell count from admission to discharge (median at admission 88 cells/μL vs. 53 cells/μL at discharge, P=0.90) or HIV VL.
Figure. 2. Differences in selected inflammatory cytokine levels at entry between KSHV-associated diseases.
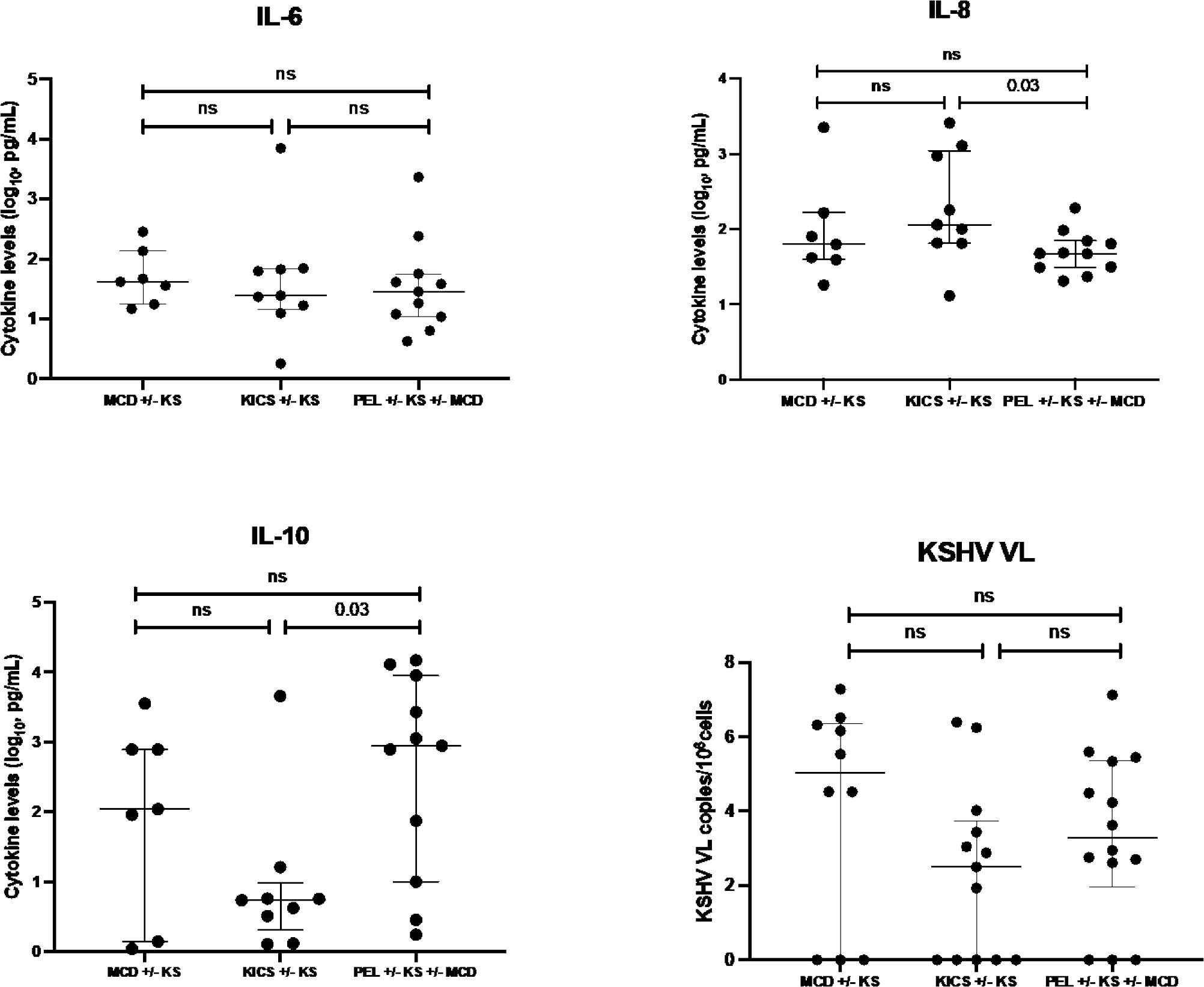
Note: Diagnoses are the final diagnoses inclusive of any workup during ICU admission
Survival outcomes and prognostic factors
ICU survival for all patients with KADs was 72%, and 60-day survival was 70%. Median OS was 9 months from initial ICU admission. Eleven patients (23%) required ICU readmission. Only a diagnosis of PEL+/−KS+/−MCD trended toward a significant impact on survival at 60 days (HR 3.37, 95% CI 0.99–11.55, Table 2), but was not associated with worse OS following ICU admission. There was no difference between survival outcomes between patients who had KADs – MCD+/−KS, KICS+/−KS, or PEL+/−KS+/−MCD (Figure 3). As expected, ICU interventions required for multiorgan dysfunction, such as the need for vasopressors or mechanical ventilation, trended toward a significant association with worse OS [(vasopressors: HR 2.23, 95% CI 1.02–4.88), ventilation: HR 2.99 (95% CI 1.29–6.97), respectively]. However, receipt of KAD-directed therapy in the ICU was not significantly associated with 60-day survival (HR 3.22, 95% CI 0.85, 12.14, P=0.85) or OS (HR 1.54, 95% CI 0.71, 3.32, P=0.27, Table 2). Neither cytokines, KSHV VL, nor HIV VL were prognostic for survival.
Table 2:
Hazard ratios and 95% confidence intervals for selected variables showing association with 60-day and overall survival
| Variable | Hazard ratio for 60-day survival (95% confidence interval) | P | Hazard ratio for overall survival (95% confidence interval) | P |
|---|---|---|---|---|
| Patient characteristics at ICU entry | ||||
| Age (above vs. below median) | 0.83 (0.25, 2.71) | 0.75 | 0.89 (0.42, 1.89) | 0.76 |
| CD4 count (<100 vs. ≥100) | 1.35 (0.41, 4.42) | 0.62 | 0.77 (0.34, 1.72) | 0.52 |
| HIV VL (<25 vs. ≥25) | 0.37 (0.10, 1.39) | 0.14 | 1.00 (0.47, 2.15) | 0.99 |
| SOFA score (<6 vs. ≥6) | 1.73 (0.51, 5.91) | 0.38 | 1.43 (0.66, 3.09) | 0.37 |
| KSHV VL (above vs. below median) | 1.82 (0.48, 6.89) | 0.37 | 1.44 (0.66, 3.16) | 0.37 |
| KAD diagnoses at ICU discharge or death | ||||
| MCD +/− KS | 0.28 (0.04, 2.15) | 0.21 | 0.59 (0.22, 1.57) | 0.29 |
| KICS +/− KS | 0.82 (0.22, 3.09) | 0.77 | 1.35 (0.59, 3.08) | 0.47 |
| PEL +/− MCD +/− KS | 3.37 (0.99, 11.55) | 0.05 | 2.03 (0.95, 4.35) | 0.07 |
| ICU Interventions | ||||
| Vasopressors | 3.25 (0.86, 12.25) | 0.08 | 2.23 (1.02, 4.88) | 0.05 |
| Mechanical ventilation | 4.95 (1.44, 16.96) | 0.01 | 2.99 (1.29, 6.97) | 0.01 |
| Chemotherapy or other for KAD | 3.22 (0.85, 12.14) | 0.85 | 1.54 (0.71, 3.32) | 0.27 |
| Cytokines at admission to ICU – (above vs. below median) | ||||
| Interferon-γ, pg/mL | 1.29 (0.34, 4.85) | 0.71 | 1.65 (0.68, 4.02) | 0.27 |
| IL-10, pg/mL | 1.33 (0.35, 5.03) | 0.67 | 1.14 (0.51, 2.56) | 0.75 |
| IL-12, pg/mL | 0.82 (0.24, 2.82) | 0.76 | 1.06 (0.47, 2.37) | 0.90 |
| IL-13, pg/mL | 0.40 (0.12, 1.32) | 0.13 | 0.67 (0.31, 1.43) | 0.30 |
| IL-1β, pg/mL | 0.65 (0.20, 2.12) | 0.47 | 0.77 (0.36, 1.71) | 0.54 |
| IL-2, pg/mL* | 1 (NA) | NA | 1 (NA) | NA |
| IL-4, pg/mL | 1.30 (0.35, 4.91) | 0.70 | 1.43 (0.62, 3.28) | 0.40 |
| IL-6, pg/mL | 1.33 (0.35, 5.03) | 0.67 | 1.79 (0.76, 4.22) | 0.19 |
| IL-8, pg/mL | 0.71 (0.21, 2.41) | 0.58 | 0.54 (0.25, 1.15) | 0.11 |
| TNF-α, pg/mL | 0.74 (0.22, 2.53) | 0.63 | 0.84 (0.38, 1.85) | 0.66 |
No values below median
Figure. 3. Overall survival for patients with KSHV-associated diseases admitted to the ICU.
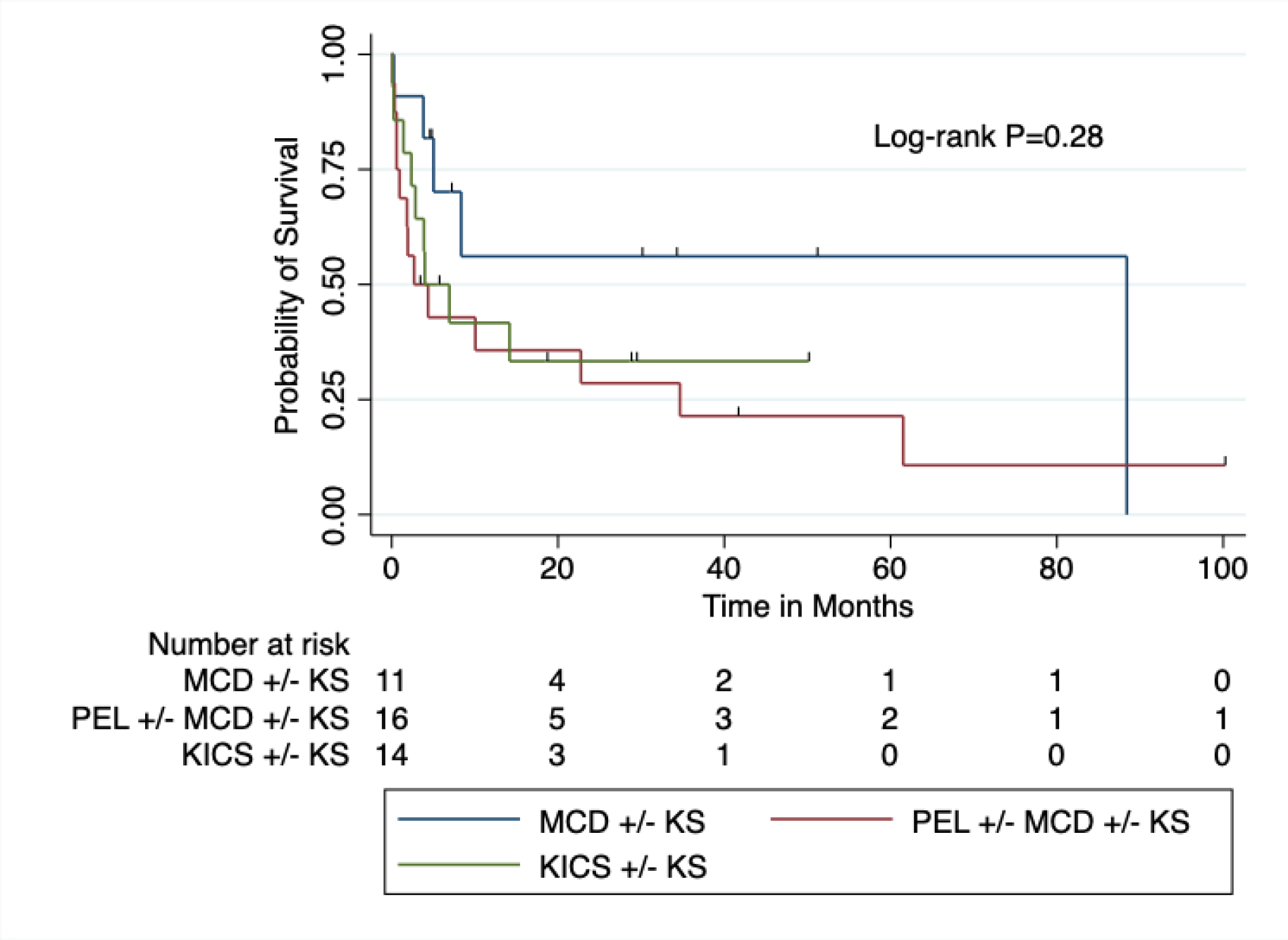
Note: Diagnoses are the final diagnoses inclusive of any workup during ICU admission
Autopsies were performed on 9 patients. KAD was the primary cause of death in 3 patients (2 with pulmonary KS and 1 with PEL). An additional 4 patients had causes of death likely related to their KAD (pleural effusions, pleuritis, diffuse alveolar damage, and disseminated intravascular coagulation). Invasive fungal infection was the primary cause of death in 2 patients. Clinical progression of KAD resulting in multi-organ failure was the presumed cause of death in 15 of 38 (39%) patients who did not undergo autopsy.
DISCUSSION
This retrospective analysis is the first to study patients with KADs who were admitted to the ICU. Among those with HIV, the majority of patients were on ART with well-controlled HIV but low CD4+ T cell counts at the time of ICU admission. While KS is the most common KAD in the U.S. and worldwide, most patients in this cohort who required ICU-level care had more than one KAD. In fact, 47% of patients had presumed or confirmed KICS at ICU entry, and MCD and/or PEL was diagnosed in the ICU in 36% of these patients. Therefore, in our cohort of patients with KAD in the ICU, additional workup—including imaging, lymph node biopsies, and flow cytometry of effusions—provided additional diagnoses of underlying KAD and guided treatment options.
Patients with PEL had increased risk of mortality in the first 60 days of their ICU admission but beyond this, a diagnosis of PEL did not impact overall survival. Consistent with other studies published from our group [3, 5, 7], IL6 and IL10 were elevated at ICU admission among a substantial subset of patients within the cohort. Though none of the cytokine levels were of prognostic value, the measurement of these cytokines in combination with KSHV-VL levels may aid critical care and infectious disease teams in identifying KAD as a potential cause of multiorgan dysfunction in PWH in the ICU, especially when patients present with a sepsis-like clinical picture but no identifiable source of infection.
Median time from HIV diagnosis and ART initiation to ICU admission was 70 months and 12.5 months, respectively. Well-controlled HIV and CD4+ T cell count of less than 100 copies/μL may be reflective of poor immune reconstitution and it is unclear whether this is due to prior therapy or whether this is coincidental as many KAD diagnoses present at lower CD4+ T cell counts. Outcomes in our cohort parallel those reported in HIV patients requiring critical care writ large in the ART era, which show that factors such as malignancy and chronic disease are significantly associated with ICU mortality, while factors related to HIV status have no independent association [18, 33, 34]. CD4 count has been found to be a significant predictor of mortality in some studies [33–36], but not others [37]. Similar to non-HIV infected ICU patients, the need to address multiorgan dysfunction—including the need for mechanical ventilation [19, 33, 35, 36], vasopressors [33, 38], and renal replacement therapy [36]—have a significant association with worse ICU mortality in PWH. These findings were echoed in our cohort, in which requiring vasopressors and mechanical ventilation were significantly associated with mortality. However, these observations likely reflect the severity of multi-organ dysfunction rather than the intervention itself as causing death. Further, ICU mortality in this cohort (28%) is very similar to estimate ICU mortality for patients with and without HIV (16–37%) [18]. Thus, the presence of multi-organ dysfunction in patients with KADs should not preclude critical care physicians, infectious disease specialists, or oncologists from supporting this population through critical illness.
In addition to critical care support, 43% of patients received cancer treatment, and 32% received both KAD therapy and anti-infective agents. While receiving KAD-directed therapy had no impact on survival, this may have been due to the sample size or administration of KAD therapy prior to ICU admission. However, it is important to consider cases where this intervention may have impacted survival. For example, among a small subset of severely ill patients with SOFA scores ≥ 10, 6 these patients received KAD therapy, and 2 of these patients attained stable remission with ICU and KAD treatment with no long-term physical sequelae of ICU admission. This cohort included young patients with few chronic medical comorbidities outside of KADs and HIV. Though it may not be possible to derive a generalizable algorithm for KAD treatment, these analyses demonstrate that in patients for whom critical illness is primarily driven by KADs, aggressive KAD-directed therapy may be appropriate and, in some cases, lifesaving.
These findings focus on a single major referral center for patients with severe manifestations of KADs or who require further workup following initial assessment by their outside medical provider. Therefore, these cases and data may be limited in generalizability and referral bias. Additional limitations include the heterogenous nature of these conditions and their treatments, resulting in a small sample size. Our data is not adjusted for multiple comparisons, favoring a more stringent cutoff for statistical significance given the exploratory nature of these analyses. We did not adjust models for other medical comorbidities and only a subset of patients had samples for cytokine profiling collected at ICU admission and discharge. Cytokine analysis was also limited by potential confounding factors (such as administration of corticosteroids) that may have contributed to decreasing cytokine levels. Although a proportion of patients required ICU readmission, these findings only consider the characteristics of the initial ICU admission. Finally, as KAD can present concurrently and often have overlapping features, there may be some degree of misclassification bias in patients with KSHV-association inflammation who may have undiagnosed MCD or PEL. Despite these limitations, a notable strength of this study is the well-annotated nature of the cohort and the longitudinal analyses.
In summary, patients with KADs and HIV continue to present to a critical care setting with multiorgan dysfunction and the majority have more than one underlying KAD. Despite the critical illness of this cohort, KAD-directed therapy with a multidisciplinary approach was feasible, did not appear to negatively impact survival, and in a small number of patients, this supportive treatment resulted in remission from KAD diagnoses.
Supplementary Material
Funding Sources:
This work was supported by the Intramural Research Program of the NIH, National Cancer Institute. This work has also been funded in part with federal funds from the National Cancer Institute, NIH, under Contract Nos. HHSN261201500003I and 75N91019D00024. Support was also provided by the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, and the Colgate-Palmolive Company.
Disclosure of Conflicts of Interest and Source of Funding:
R. Ramaswami, T.S. Uldrick, K. Lurain, and R. Yarchoan report receiving research support from Celgene/Bristol Myers Squibb through a CRADA with the NCI. R. Ramaswami, T.S. Uldrick, K. Lurain, and R. Yarchoan report receiving drug for a clinical trial from Merck through a CRADA with the NCI; R. Ramaswami, K. Lurain, and R. Yarchoan report receiving drug for a clinical trial from EMD Serono. T.S. Uldrick reports receiving other commercial research support from Roche through a CTA with Fred Hutchinson Cancer Research Center. T.S. Uldrick, R. Yarchoan, and D. Whitby are co-inventors on US Patent 10,001,483 entitled “Methods for the treatment of Kaposi’s sarcoma or KSHV-induced lymphoma using immunomodulatory compounds and uses of biomarkers.” R. Yarchoan is also a coinventor on patents on a peptide vaccine for HIV and on the treatment of Kaposi sarcoma with IL12, and an immediate family member of R. Yarchoan is a co-inventor on patents related to internalization of target receptors, on KSHV viral IL-6, and on the use of calreticulin and calreticulin fragments to inhibit angiogenesis. All rights, title, and interest to these patents have been or should by law be assigned to the U.S. Department of Health and Human Services; the government conveys a portion of the royalties it receives to its employee inventors under the Federal Technology Transfer Act of 1986 (P.L. 99–502). No potential conflicts of interest were disclosed by the other authors.
References
- 1.Yarchoan R, Uldrick TS. HIV-Associated Cancers and Related Diseases. New England Journal of Medicine 2018; 378(11):1029–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramaswami R, Lurain K, Yarchoan R Oncologic Treatment of HIV-associated Kaposi Sarcoma 40 Years on. Journal of Clinical Oncology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lurain K, Polizzotto MN, Aleman K, Bhutani M, Wyvill KM, Gonçalves PH, et al. Viral, immunologic, and clinical features of primary effusion lymphoma. Blood 2019; 133(16):1753–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower M, Newsom-Davis T, Naresh K, Merchant S, Lee B, Gazzard B, et al. Clinical Features and Outcome in HIV-Associated Multicentric Castleman’s Disease. Journal of Clinical Oncology 2011; 29(18):2481–2486. [DOI] [PubMed] [Google Scholar]
- 5.Ramaswami R, Lurain K, Polizzotto MN, Ekwede I, Waldon K, Steinberg SM, et al. Characteristics and outcomes of KSHV-associated multicentric Castleman disease with or without other KSHV diseases. Blood Advances 2021; 5(6):1660–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Marshall V, Wang V, et al. Clinical Features and Outcomes of Patients With Symptomatic Kaposi Sarcoma Herpesvirus (KSHV)-associated Inflammation: Prospective Characterization of KSHV Inflammatory Cytokine Syndrome (KICS). Clinical Infectious Diseases 2016; 62(6):730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polizzotto MN, Uldrick TS, Wang V, Aleman K, Wyvill KM, Marshall V, et al. Human and viral interleukin-6 and other cytokines in Kaposi sarcoma herpesvirus-associated multicentric Castleman disease. Blood 2013; 122(26):4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton R, Labo N, Wakeham K, Miley W, Asiki G, Johnston WT, et al. Kaposi Sarcoma-Associated Herpesvirus in a Rural Ugandan Cohort, 1992–2008. J Infect Dis 2018; 217(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis 2007; 196(2):199–207. [DOI] [PubMed] [Google Scholar]
- 10.Gonçalves PH, Uldrick TS, Yarchoan R. HIV-associated Kaposi sarcoma and related diseases. AIDS 2017; 31(14):1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhutani M, Polizzotto MN, Uldrick TS, Yarchoan R. Kaposi Sarcoma–Associated Herpesvirus-Associated Malignancies: Epidemiology, Pathogenesis, and Advances in Treatment. Seminars in Oncology 2015; 42(2):223–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goncalves PH, Ziegelbauer J, Uldrick TS, Yarchoan R. Kaposi sarcoma herpesvirus-associated cancers and related diseases. Current Opinion in HIV and AIDS 2017; 12(1):47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryan Pantanowitz L, Casper C, Bruce Stebbing J. HIV/AIDS: Epidemiology, Pathophysiology, and Treatment of Kaposi Sarcoma–Associated Herpesvirus Disease: Kaposi Sarcoma, Primary Effusion Lymphoma, and Multicentric Castleman Disease. Clinical Infectious Diseases 2008; 47(9):1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robbins HA, Pfeiffer RM, Shiels MS, Li J, Hall HI, Engels EA. Excess Cancers Among HIV-Infected People in the United States. JNCI: Journal of the National Cancer Institute 2015; 107(4):dju503–dju503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Royse KE, El Chaer F, Amirian ES, Hartman C, Krown SE, Uldrick TS, et al. Disparities in Kaposi sarcoma incidence and survival in the United States: 2000–2013. PLoS One 2017; 12(8):e0182750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Collaboration on HIV and Cancer. Highly Active Antiretroviral Therapy and Incidence of Cancer in Human Immunodeficiency Virus-Infected Adults. JNCI: Journal of the National Cancer Institute 2000; 92(22):1823–1830. [DOI] [PubMed] [Google Scholar]
- 17.Masur H Management of Patients with HIV in the Intensive Care Unit. Proceedings of the American Thoracic Society 2006; 3(1):96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azoulay É, De Castro N, Barbier F. Critically Ill Patients With HIV. Chest 2020; 157(2):293–309. [DOI] [PubMed] [Google Scholar]
- 19.Powell K, Davis JL, Morris AM, Chi A, Bensley MR, Huang L. Survival for Patients With HIV Admitted to the ICU Continues to Improve in the Current Era of Combination Antiretroviral Therapy. Chest 2009; 135(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbier F, Coquet I, Legriel S, Pavie J, Darmon M, Mayaux J, et al. Etiologies and outcome of acute respiratory failure in HIV-infected patients. Intensive Care Medicine 2009; 35(10):1678–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganesan A, Masur H. Critical Care of Persons Infected with the Human Immunodeficiency Virus. Clinics in Chest Medicine 2013; 34(2):307–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suneja G, Shiels MS, Angulo R, Copeland GE, Gonsalves L, Hakenewerth AM, et al. Cancer Treatment Disparities in HIV-Infected Individuals in the United States. Journal of Clinical Oncology 2014; 32(22):2344–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim H, Abu Ghanimeh M, Al-Saheli ZI, Naffouj S. Life-threatening Gastrointestinal Bleeding Secondary to Kaposi’s Sarcoma of the Duodenum. Cureus 2020; 12(3):7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns RH, Doyle T, Lipman MC, Cwynarski K, Cleverley JR, Isaacson PG, et al. Successful treatment of HIV-associated multicentric Castleman’s disease and multiple organ failure with rituximab and supportive care: a case report. Journal of Medical Case Reports 2010; 4(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Medicine 1996; 22(7):707–710. [DOI] [PubMed] [Google Scholar]
- 26.Allaire JJ, Gandrud C, Russell K, Yetman CJ. networkD3: D3 Javascript Network Graphs from R. In: R package version 04; 2017. [Google Scholar]
- 27.Uldrick TS, Wang V, O’Mahony D, Aleman K, Wyvill KM, Marshall V, et al. An interleukin-6-related systemic inflammatory syndrome in patients co-infected with Kaposi sarcoma-associated herpesvirus and HIV but without Multicentric Castleman disease. Clin Infect Dis 2010; 51(3):350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods 2001; 91(2):109–117. [DOI] [PubMed] [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 17. In. College Station, TX: StataCorp LLC; 2021. [Google Scholar]
- 30.R Core Team. R: A Language and Environment for Statistical Computing. In: R Foundation for Statistical Computing; 2021. [Google Scholar]
- 31.Kaplan JE, Benson C, Holmes KK, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep 2009; 58(Rr-4):1–207; quiz CE201–204. [PubMed] [Google Scholar]
- 32.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. Jama 2001; 286(14):1754–1758. [DOI] [PubMed] [Google Scholar]
- 33.Maphula R, Laher A, Richards G. Patterns of presentation and survival of HIV-infected patients admitted to a tertiary-level intensive care unit. HIV Medicine 2020; 21(5):334–341. [DOI] [PubMed] [Google Scholar]
- 34.Vidal-Cortés P, Álvarez-Rocha LA, Fernández-Ugidos P, Pérez-Veloso MA, Suárez-Paul IM, Virgós-Pedreira A, et al. Epidemiology and outcome of HIV-infected patients admitted to the ICU in the current highly active antiretroviral therapy era. Medicina Intensiva 2020; 44(5):283–293. [DOI] [PubMed] [Google Scholar]
- 35.Dickson SJ, Batson S, Copas AJ, Edwards SG, Singer M, Miller RF. Survival of HIV-infected patients in the intensive care unit in the era of highly active antiretroviral therapy. Thorax 2007; 62(11):964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adlakha A, Pavlou M, Walker DA, Copas AJ, Dufty N, Batson S, et al. Survival of HIV-infected patients admitted to the intensive care unit in the era of highly active antiretroviral therapy. International Journal of STD & AIDS 2011; 22(9):498–504. [DOI] [PubMed] [Google Scholar]
- 37.Coquet I, Pavie J, Palmer P, Barbier F, Legriel S, Mayaux J, et al. Survival trends in critically ill HIV-infected patients in the highly active antiretroviral therapy era. Crit Care 2010; 14(3):R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morquin D, Le Moing V, Mura T, Makinson A, Klouche K, Jonquet O, et al. Short- and long-term outcomes of HIV-infected patients admitted to the intensive care unit: impact of antiretroviral therapy and immunovirological status. Annals of Intensive Care 2012; 2(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


