Abstract
Background/Objective
The study of autoantibody isotypes in autoimmune diseases has been useful for identifying clinically relevant endotypes. Here, we studied the prevalence and clinical significance of different isotypes and IgG subclasses of anti-peptidylarginine deiminase 4 (PAD4) autoantibodies in individuals with rheumatoid arthritis (RA).
Methods
Anti-PAD4 antibody types were determined by ELISA in 196 RA subjects and 64 healthy controls. We investigated associations of anti-PAD4 antibodies and clinical outcomes. Relevant features were confirmed using an independent RA cohort.
Results
Anti-PAD4 IgG1, IgG2, IgG3, IgG4, IgA, and IgE antibodies were more frequent in patients with RA than healthy controls (P<0.001 for all). Anti-PAD4 IgG1, IgG3 and IgE were associated with distinct features. Anti-PAD4 IgG1 was predictive of progressive radiographic joint damage (OR 4.88, P=0.005), especially in RA patients without baseline joint damage (40% vs. 0%, P=0.003) or in those negative for anti-CCP and/or rheumatoid factor (OR 32, P=0.009). IgG1 was also associated with higher levels of C-reactive protein (P=0.006) and IL-6 (P=0.021). RA patients with anti-PAD4 IgG3 had higher baseline joint damage (mean SHS 13 vs. 7, P=0.046), while those with anti-PAD4 IgE had higher DAS28 scores (mean 4.0 vs. 3.5, P=0.025), more frequent rheumatoid nodules (31% vs. 16%, P=0.025) and interstitial lung disease (glass ground opacification) (24% vs. 9%, P=0.014). Joint damage associations of anti-PAD4 IgG1 antibodies were corroborated in an independent RA cohort.
Conclusion
Anti-PAD4 IgG1, IgG3 and IgE antibodies identify discrete disease subsets in RA, suggesting that heavy chain usage drives distinct effector mechanisms of anti-PAD4 antibodies in RA.
Keywords: Anti-PAD4 antibodies, rheumatoid arthritis, isotypes, radiographic progression, disease activity
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by synovial joint inflammation leading to cartilage destruction and subchondral bone erosion (1). RA is a clinically heterogeneous disease with variable disease courses among patients. While several individual factors may interplay to determine disease progression and severity (2), autoantibodies, such as anti-citrullinated protein antibodies (ACPA), are clinically useful tools to define endotypes of RA with distinct clinical features and prognoses (3). Peptidylarginine deiminase 4 (PAD4), a key enzyme in the pathogenesis of RA, catalyzes the calcium-dependent citrullination of proteins, producing the main antigenic targets of ACPA in RA (4).
Antibodies targeting PAD4 are found in 24%–45% of RA patients (5–8). These autoantibodies are associated with a subset of RA characterized by more severe joint damage, faster progression of joint erosions and interstitial lung disease (ILD) (5–7, 9). These phenotypes have been principally attributed to a subset of anti-PAD4 antibodies that are cross-reactive to PAD3 (anti-PAD3/4) (5, 6, 10). Although, the exact mechanism by which anti-PAD3/4 may result in a more severe disease course is unknown, this subset of anti-PAD4 antibodies increases the calcium sensitivity of PAD4 and enhances the production of citrullinated antigens (6). Nonetheless, since anti-PAD3/4 are only found in 32–43% of anti-PAD4 positive RA patients (5, 6), they do not entirely explain the clinical manifestations attributed to anti-PAD4. This suggests that additional types of anti-PAD4 antibodies may exist, which may further identify unique RA endotypes.
Immunoglobulins (Ig) exert their effects via two principal regions. The variable region determines the antigen specificity and the constant region of the heavy chain (IgH) defines the effector functions of the antibody (e.g., complement activation, cell activation, placental transport) (11). Ig are classified into five major isotypes in humans according to their IgH constant region: IgA, IgM, IgE, IgD, and IgG. IgG is further subclassified into IgG1, IgG2, IgG3 and IgG4 (11, 12). The distinct Ig types have different tissue distribution, Fc receptor affinities, and complement activation capacity (13). Therefore, different isotypes of antibodies against the same autoantigen may inform about distinct underlying pathogenic mechanisms, resulting in different clinical manifestations. Such studies have proven useful in systemic lupus erythematosus to identify subsets of patients who have distinct clinical outcomes and pathogenic mechanisms (14, 15).
Currently, it is known that PAD4 elicits IgG1 and IgG3 responses in RA patients (16), but IgA, IgM and IgE responses have not been studied. Furthermore, there is no information regarding the association of different anti-PAD4 isotypes/subclasses and clinical manifestations in RA. Given that the anti-PAD4 autoantibody system is known to associate with RA outcomes, we studied the prevalence of anti-PAD4 IgA, IgE, IgM and IgG subclasses and defined their association with clinical features in RA patients from two independent cohorts.
PATIENTS AND METHODS
Study subjects.
We studied sera from 196 RA subjects enrolled in the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE RA) cohort, as previously described (6, 17, 18). Briefly, all RA subjects were classified per American College of Rheumatology 1987 revised criteria (19). Single-view anterior-posterior X-rays of the hands and postero-anterior X-rays of the feet were obtained at study enrollment (baseline) and at a follow up visit, which occurred an average of 39 ± 4 months after the baseline visit. Radiographs were scored using the van der Heijde modification of the Sharp method (SHS) to quantify joint damage (20). All 196 subjects had radiographic evaluation at baseline, and 152 had follow up radiographs as well. RA disease activity was calculated using the Disease Activity Score for 28 joints (DAS28) with C-reactive protein (CRP) (21). The Stanford Health Assessment Questionnaire (HAQ) was used to assess disability (22). Current and past treatment were determined by examiner-administered questionnaires. Participants (n=176) underwent multidetector row computed tomography (CT) of the chest at the baseline visit, and the presence and extent of ILD was scored as previously described (23). The healthy control group was composed by 64 healthy volunteers, recruited from the general population of Johns Hopkins. All participants gave their written informed consent before participating in the study procedures. The study was approved by the Institutional Review Board of Johns Hopkins, and all study procedures were conducted under the declaration of Helsinki.
As a confirmation cohort, we further tested serum samples from 135 subjects enrolled in the Consortium for the Longitudinal Evaluation of African-Americans with Early Rheumatoid Arthritis (CLEAR) registry. The methods and procedures of the CLEAR-I and CLEAR-II registries have been described previously (24) and all CLEAR participants have been extensively characterized genetically (25).
Determination of anti-PAD4 antibody isotypes and IgG subclasses.
Anti-PAD4 antibody isotypes and IgG subclasses were determined using an enzyme linked immunosorbent assay (ELISA). Briefly, Nunc-Maxisorp plates (Sigma) were coated overnight with recombinant PAD4 in 0.1M sodium carbonate buffer pH 9.6 PBS. Different concentrations of recombinant PAD4 were used to detect the different antibody subsets, as follows: IgA & IgM: 1μg/ml; IgG1 & IgG3: 2μg/ml; and IgG2, IgG4 & IgE: 3ug/ml. Plates were blocked with 2% BSA in phosphate buffered saline (PBS) for 1 hour at room temperature (RT). Sera were diluted in 1% BSA/0.05% Tween-20 in PBS and incubated at room temperature for 2 hrs. in antigen-coated wells and wells without antigen for background subtraction (Dilution factor for sera: IgE: 1/10, IgG2 & IgG4: 1/50, IgG1 & IgG3: 1/100, IgA & IgM: 1/250). HRP-conjugated anti-human IgG1 (ThermoFisher, cat:MH1715, dilution factor: 1/5000), IgG2 (ThermoFisher, cat: MH1722, dilution factor: 1/2500), IgG3 (ThermoFisher, cat: 05–3620, dilution factor: 1/5000), IgG4 (ThermoFisher, cat: MH1742, dilution factor: 1/2500), IgA (ThermoFisher, cat: PA1–74395, dilution factor: 1/5000), IgE (ThermoFisher, cat: SA5–10306, dilution factor: 1/2000), and IgM (ThermoFisher, cat: 05–4920, dilution factor: 1/10000) were used as secondary antibodies diluted in 1% BSA/0.05% Tween-20 in PBS. Anti-PAD4 antibody arbitrary units (AU/mL) were calculated for each background corrected sample using a serial dilution of a human sera with high levels of each anti-PAD4 antibody subtype. We determined the cut-off value for anti-PAD4 antibody positivity using the 95-percentile of antibody levels in healthy controls.
Statistical analysis.
We compared the serum levels and positivity of the different anti-PAD4 antibodies between RA subjects and healthy controls using Student’s T and chi-square tests, respectively. We calculated the intersections between the different anti-PAD4 isotypes present in each RA subject using UpSet function in the ComplexHeatmap package (26) for Bioconductor (release 3.12). The co-occurrence of anti-PAD4 isotypes was represented in a chord diagram generated with the circlize package for Bioconductor (release 0.4.12.1004) (27). The significance of the isotypes’ co-occurrence was tested using the Jaccard/Tanimoto test implemented in the R package jaccard (version 0.10) (28). To evaluate patient characteristics according to the presence of each anti-PAD4 isotype, student’s t-tests were used for group-wise comparisons of normally distributed continuous variables; the Kruskal-Wallis test was used for group-wise comparisons of non-normally distributed variables; and the chi-squared or two-sided Fisher’s exact tests were use as appropriate for group-wise comparisons of categorical variables.
The significance of the comparisons of patient characteristics according the anti-PAD4 isotypes presence was summarized in a heatmap using the −log2 P value. We explored the independent association of anti-PAD4 Ig level with radiographic progression (any progression and progression≥ 4 units/year) using multivariable logistic regression, adjusting for covariates associated with the outcomes of interest and anti-PAD4 Ig level at the P < 0.20 level in univariate modeling. The Likelihood Ratio Test was used to exclude non-contributory covariates from the models. We modeled differences in radiographic progression according to anti-PAD4 Ig status in subgroups defined by other RA-associated autoantibodies (i.e. RA and anti-CCP) and baseline radiographic erosion status (SHS = 0 vs. > 0). STATA/SE version 16 was used and a two-tailed alpha = 0.05 was used throughout.
RESULTS
Different isotypes and subclasses of antibodies to PAD4 are prevalent in RA.
To determine the prevalence of distinct anti-PAD4 antibodies in RA, we assayed sera from 196 RA subjects in the ESCAPE RA cohort and 64 healthy controls for IgG1, IgG2, IgG3, IgG4, IgA, IgE and IgM antibodies against PAD4. Serum levels of anti-PAD4 isotypes and IgG subclasses were significantly higher in RA sera compared to controls, with the exception of IgG4 and IgM (Figure 1A). The most frequent anti-PAD4 antibody subset in RA was IgG1 (28.6%, n=56), followed by IgG3 (25.5%, n=50), IgG4 (25.5 %, n=50), IgE (25%, n=49), IgG2 (21.4%, n=42), IgA (20.9%, n=41), and IgM (9.2%, n=18) (Figure 1B and Supplementary Table 1). Each anti-PAD4 antibody was detected in 4.6% (3/65) of healthy controls, with different individuals being positive for different subtypes (Figure 1B).
Figure 1.
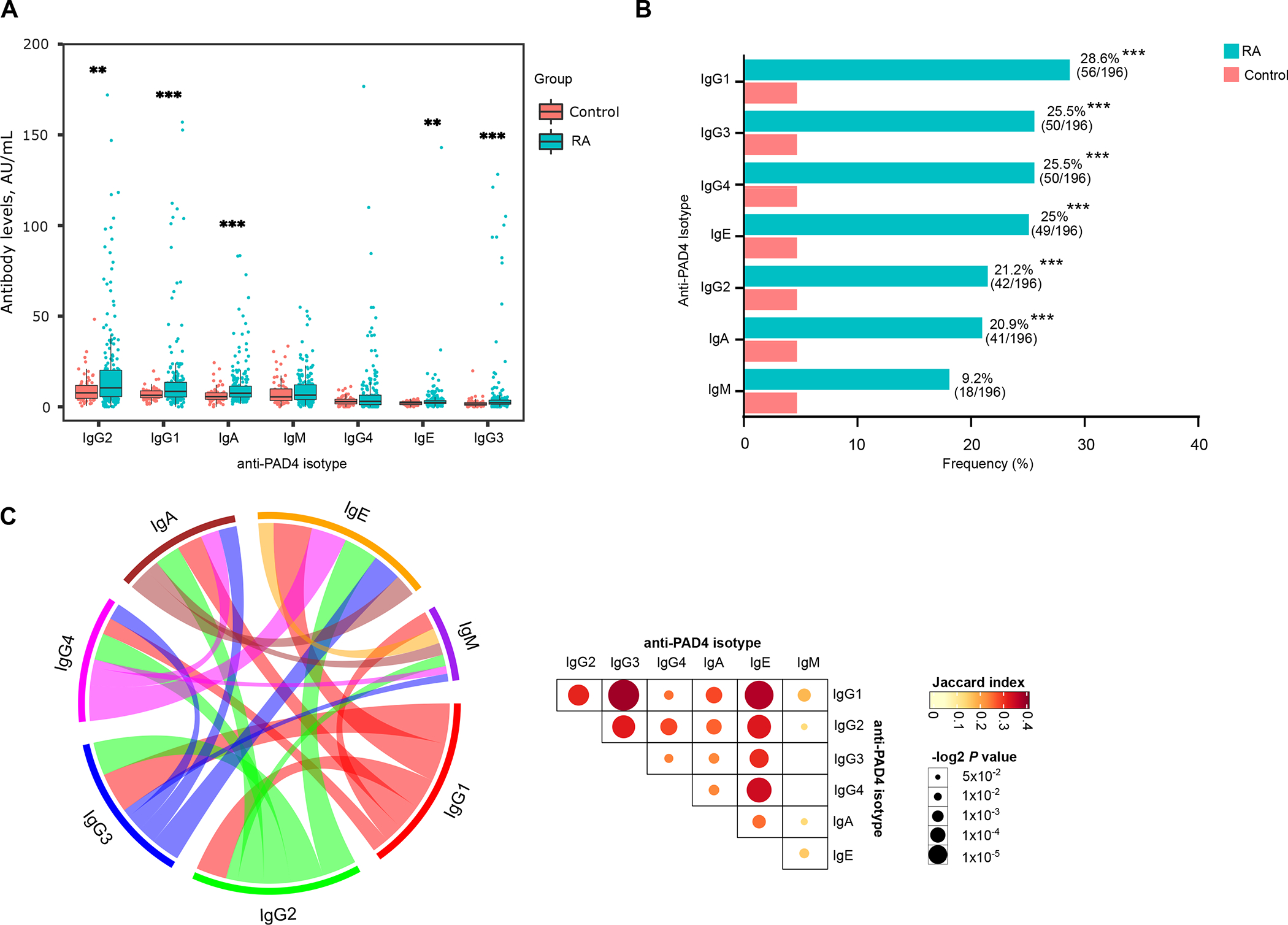
RA serum is enriched in different anti-PAD4 antibody types. A, Serum levels of anti-PAD4 antibody isotypes and IgG subclasses from 196 RA subjects and 64 healthy controls. Comparisons were done using Student’s τ test. *** P < 0.001 and ** P < 0.01. B, Frequency of anti-PAD4 antibody isotypes and IgG subclasses in patients with RA and healthy controls. P values were obtained with Fisher’s exact test, *** P < 0.001. C, Left panel, chord diagram showing the co-occurrence of anti-PAD4 antibody isotypes in RA subjects. The different colors represent each anti-PAD4 isotype and their respective link to other isotypes. Line thickness is proportional to the Jaccard index, as shown in the matrix on the right. Right panel, Jaccard index matrix, showing the similarity between anti-PAD4 isotype pair combinations. Color intensity represents the Jaccard’s similarity coefficient, and circle size represents the −log2 P value.
Considering all isotypes and IgG subclasses, 66% (129/196) of RA subjects were positive for any anti-PAD4 isotype. Of these, 59% (76/129) were positive for more than one antibody type, and 41% (53/129) were positive for a single antibody (Supplementary Figure 1A). We further analyzed the co-occurrence of anti-PAD4 antibodies (Figure 1C and Supplementary Figure 1B) in RA by computing the different possible combinations. Combinations comprised of pairs of different types of anti-PAD4 antibodies were the most frequent (Supplementary Figure 1B). The highest co-occurrences were between anti-PAD4 IgG1 and IgG3 (Jaccard index = 0.377, P < 0.001), IgG1-IgE (Jaccard index = 0.364, P < 0.001), and IgE-IgG4 (Jaccard index = 0.338, P < 0.001) (Figure 1C and Supplementary Table 2). Importantly, IgM had the lowest co-occurrence with other antibody types (Jaccard index, 0.115–0.175) (Figure 1C and Supplementary Table 2).
Anti-PAD4 antibody isotypes and IgG subclasses associate with distinct clinical subsets in RA.
The summary of demographic and clinical characteristics of ESCAPE RA subjects according to anti-PAD4 status is shown in Figure 2A and Supplementary Tables 3 and 4. RA subjects positive for anti-PAD4 antibody isotypes, except IgG4 and IgM, had a longer disease duration than anti-PAD4 negative individuals (Figure 2A and Supplemental table 3 and 4). White subjects were less likely to be positive for anti-PAD4 IgG1, IgA or IgE (Supplemental tables 3 and 4). None of the anti-PAD4 antibodies were associated with HLA-DRB1 ‘SE’ alleles or smoking (Figure 2 and Supplementary Tables 3 and 4).
Figure 2.
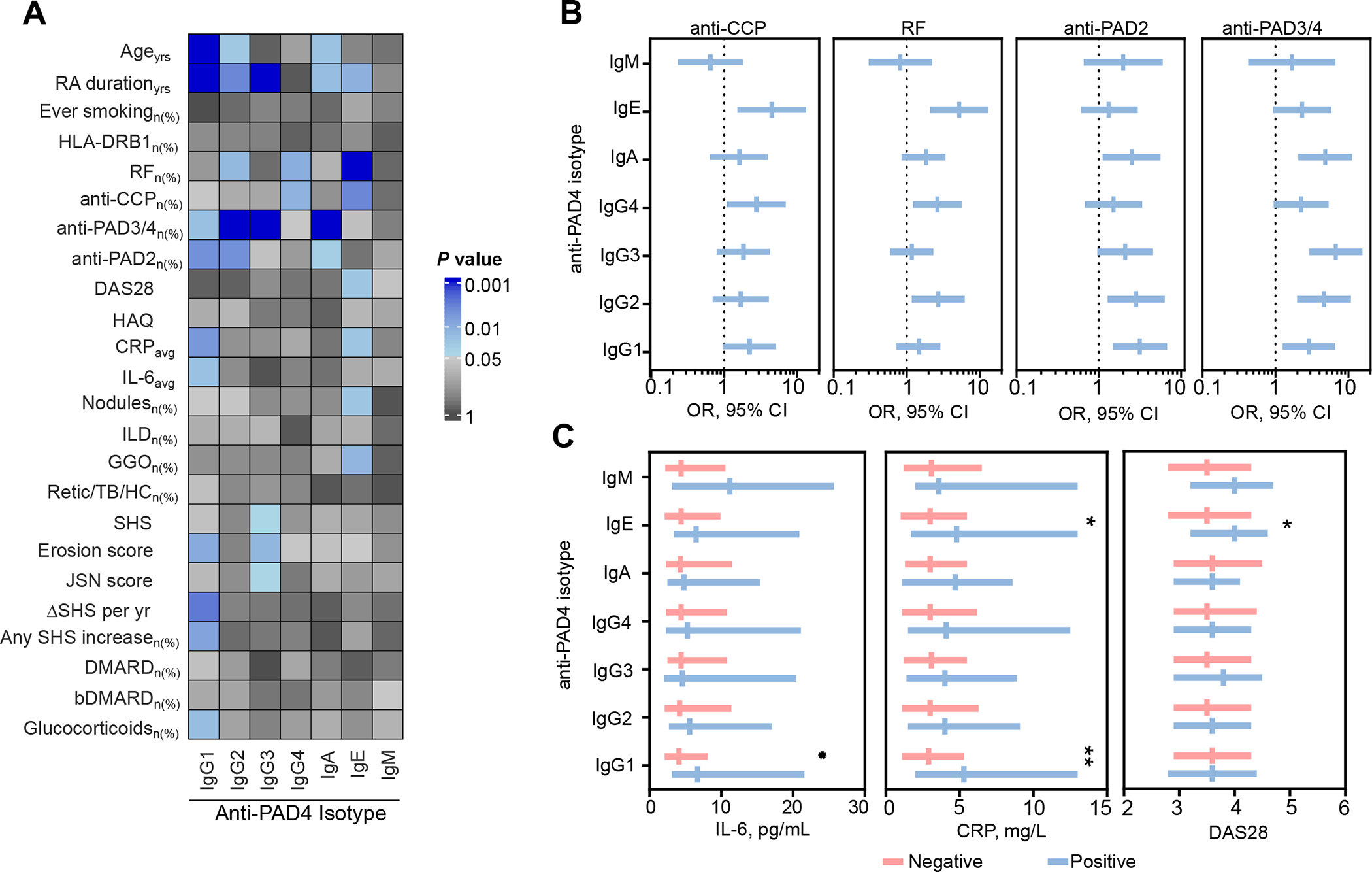
Clinical associations between the anti-PAD4 antibody subsets in RA. A, Heat map showing the demographic and clinical associations with anti-PAD4 antibody types in RA (n=196). The color scale represents −log2 P value, obtained from the comparison of the corresponding anti-PAD4 isotype positive vs. negative, using Student’s T test or χ2 as appropriate. In the color scale, blue represents the associations with a P value < 0.05. All significant associations, represent variables positively associated with the indicated anti-PAD4 isotype. B, Associations between anti-PAD4 antibody subsets with other known serological biomarkers. Bars represent OR with 95% CI. C, Comparison of serum IL-6, CRP and DAS28 between RA patients negative and positive for anti-PAD4 antibody subsets. Bars represent the median and IQR range. Yrs, years; SE, shared epitope; PAD, peptidylarginine deiminase; DAS28 disease activity score on 28 joints. HAQ, health assessment questionnaire; CRPavg, average study c-reactive protein; IL-6, interleukin-6; ILD, interstitial lung disease; GGO, ground glass opacification; retic/TB/HC, reticulation/traction bronchiectasis/honeycombing; SHS, Sharp/van der Heijde Score; JSN, joint space narrowing; DMARD, disease-modifying antirheumatic drugs; bDMARD, biological DMARD. *P < 0.05, **P < 0.01, ***P < 0.001.
Anti-PAD4 IgG2, IgG4 and IgE were associated with classic ‘seropositive’ RA (Figure 2A, and Supplementary Tables 3 and 4). Subjects positive for any of these isotypes had a higher frequency of RF compared with patients without these antibody types, and IgG4 or IgE-positive patients also had a higher frequency of anti-CCP antibodies. Importantly, subjects positive for anti-PAD4 IgA, IgG1, IgG2, or IgG3 were more likely to be positive for antibodies targeting other PAD isoenzymes (Figure 2A, and Supplementary Tables 3 and 4). Among these, IgA, IgG1, or IgG2 positive patients were more likely positive for anti-PAD2 antibodies, and those with anti-PAD4 IgA, IgG1, IgG2, or IgG3 had a higher frequency of anti-PAD3/4.
Anti-PAD4 IgE, IgG1, and IgG3 were unique among anti-PAD4 antibody types in their association with distinct clinical features of RA. Anti-PAD4 IgE positivity was associated with a higher DAS28 [4.0 (3.2–4.6) vs. 3.5 (2.8–4.3), P = 0.025], average study CRP [4.8 (1.7–13.0) vs. 3.0 (1.0–5.5), P = 0.025], and extra-articular manifestations (EAM) of RA such as radiographic evidence of ground glass opacification (GGO) in the lung [24% (10/42) vs. 9% (12/134), P = 0.014] and rheumatoid nodules [31% (14/49) vs. 16% (21/1460), P = 0.025] (Figure 2 and Supplementary Table 4). Anti-PAD4 IgG1 was associated with a higher average study CRP [5.3 (2.0–13.0) vs. 2.9 (1.1–5.3), P = 0.006], and IL-6 [6.7 (3.1–21.6) vs. 4.1 (2.1–8.1), P = 0.021] and more frequent use of glucocorticoids [52% (29/56) vs. 34% (47/140), P = 0.018] (Figure 2A and 2C).
Anti-PAD4 IgG1 positive patients had more erosive disease, higher erosion scores [5 (1–26) vs. 2 (0–8), P = 0.010], and were more prone to radiographic progression, as 73% had an increase in SHS over the course of the study, versus 49% of anti-PAD4 IgG1 negative patients (P = 0.008). Importantly, patients with anti-PAD4 IgG1 also showed a faster rate of radiographic progression than those who were anti-PAD4 IgG1 negative, with an increase in SHS per/year [1.1 (0–4.2) vs. 0 (0–1.4), respectively (P = 0.003)] (Figure 2A and Table 3). Like anti-PAD4 IgG1, anti-PAD4 IgG3 was also associated with an increased burden of joint damage. Anti-PAD4 IgG3 positive patients had higher SHS [13 (2–85) vs. 7 (0–27), P = 0.046], erosion score [6 (0–31) vs. 2 (0–8), P = 0.015], and joint space narrowing score [10 (0–54) vs. 4 (0–19), P = 0.045] (Figure 2A and Supplementary Table 3) when compared to negative subjects. Neither anti-PAD4 IgG2, IG4, IgA nor IgM antibodies were associated with clinically relevant outcomes (Figure 2A and Supplementary Tables 3 and 4).
Anti-PAD4 IgG1 antibodies are predictive of radiographic progression in RA.
To analyze the predictive value of anti-PAD4 IgG subclass antibodies and possible covariates, we classified ESCAPE RA patients into two groups: 1) subjects with any radiographic progression (n= 85), or 2) subjects with no radiographic progression (n = 68). Among the anti-PAD4 IgG subclass antibodies, only IgG1 antibodies were significantly associated with any radiographic progression (Table 1). Other variables associated with radiographic progression were disease duration, anti-PAD3/4, HLA-DRB1 SE alleles, swollen joint count, HAQ, CRP, IL-6, rheumatoid nodules, pain, adiponectin levels, and baseline SHS (Table 1).
Table 1.
Characteristics of RA patients according to radiographic progression
| None (n=68) |
Any (n=85) |
P | |
|---|---|---|---|
|
|
|||
| Age, years, mean ± SD | 58 ± 8 | 60 ± 8 | 0.080 |
| Male gender, n (%) | 32 (47) | 25 (29) | 0.025 |
| White, n (%) | 62 (91) | 72 (85) | 0.23 |
| BMI | 28.0 ± 4.3 | 28.2 ± 5.5 | 0.88 |
| Total fat (DXA) | 29.2 ± 8.3 | 29.7 ± 11.4 | 0.73 |
| Total lean (DXA) | 47.4 ± 12.2 | 43.9 ± 10.2 | 0.057 |
| Ever smoking, n (%) | 38 (56) | 48 (56) | 0.94 |
| RA duration, years | 6 (2.5–14.5) | 12 (7–19) | <0.001 |
| RF seropositivity > 40 units, n (%) | 39 (57) | 53 (62) | 0.53 |
| Anti-CCP seropositivity > 20 units, n (%) | 48 (72) | 65 (76) | 0.50 |
| RF or anti-CCP seropositivity, n (%) | 51 (76) | 66 (78) | 0.82 |
| Anti-CCP units among seropositive | 145 (89–170) | 142 (89–174) | 0.89 |
| Anti-PAD2 positive, n (%) | 17 (25) | 13 (16) | 0.15 |
| Anti-PAD3/4 positive, n (%) | 3 (4) | 12 (14) | 0.045 |
| Any HLA-DRB1 Shared Epitope Alleles | 42 (62) | 65 (78) | 0.026 |
| DAS28, median (IQR) | 3.3 (2.8–4.2) | 3.7 (3.1–4.5) | 0.044 |
| Average DAS | 3.0 (2.4–4.0) | 3.4 (2.8–4.1) | 0.022 |
| Pain (100mm VAS) | 13 (5–24) | 25 (12–47) | <0.001 |
| Swollen joint count | 6 (2–9) | 7 (5–10) | 0.038 |
| Tender joint count | 6 (2–12) | 7 (3–13) | 0.39 |
| HAQ score (0 – 3) | 0.38 (0–0.94) | 1.0 (0.50–1.38) | <0.001 |
| CRP, mg/L | 1.6 (0.7–4.4) | 3.0 (1.5–8.2) | 0.006 |
| Average CRP, mg/L | 1.8 (0.8–4.3) | 4.6 (1.7–8.7) | <0.001 |
| IL-6, pg/mL | 2.4 (1.3–5.5) | 4.5 (2.0–8.5) | 0.008 |
| Average IL-6, pg/mL | 3.4 (1.9–6.4) | 5.6 (3.2–21.3) | 0.001 |
| Nodules, n (%) | 6 (9) | 17 (21) | 0.045 |
| Non-biologic DMARDs, n (%) | 58 (85) | 74 (87) | 0.82 |
| Biologic DMARDs, n (%) | 31 (46) | 37 (44) | 0.80 |
| Glucocorticoids, n (%) | 24 (35) | 31 (36) | 0.88 |
| Cumulative prednisone, grams, | 2.8 (0–7.8) | 3.5 (0–10.0) | 0.54 |
| Number of prior DMARDs, n (%) | 1 (0–2) | 0 (1–3) | 0.22 |
| Baseline SHS>0, n (%) | 41 (60) | 73 (86) | <0.001 |
| anti-PAD4-IgG1, n (%) | 11 (16) | 30 (35) | 0.008 |
| anti-PAD4-IgG2, n (%) | 14 (21) | 15 (18) | 0.64 |
| anti-PAD4-IgG3, n (%) | 19 (28) | 20 (24) | 0.53 |
| anti-PAD4-IgG4, n (%) | 14 (21) | 22 (26) | 0.44 |
| Number of anti-PAD4 isotypes | 1 (0–2) | 1 (0–2) | 0.26 |
SD, standard deviation; BMI, body mass index; RA, rheumatoid arthritis; RF, rheumatoid factor; anti-CCP, anti-cyclic citrullinated peptide antibodies; PAD, peptidylarginine deiminase; HLA, Human leukocyte antigen; SE, shared epitope; DAS28, disease activity score based on 28 joint count. VAS, visual analogue scale; HAQ, health assessment questionnaire; CRP, c-reactive protein; IL-6, interleulkin-6; DMARDs, disease-modifying antirheumatic drugs; bDMARDs, biologic disease-modifying antirheumatic drugs; ILD, interstitial lung disease; SHS, Sharp/van der Heijde score; JSN, joint space narrowing. Continuous variables are represented as median (IQR) unless indicated otherwise.
In the univariate analysis, anti-PAD4 IgG1 positive subjects were more likely to have any increase in SHS and an increase in SHS ≥ 4 units/year in comparison to anti-PAD IgG1 negative individuals [73% (n =30) vs. 49% (n = 55), respectively; P = 0.008; and 29% (n = 12) vs. 6% (n = 7), respectively; P < 0.001] (Figure 3A and B and Supplementary Table 3). After adjustment for baseline SHS, pain, IL-6, and gender, subjects positive for anti-PAD4 IgG1 were 2.83-times (P = 0.009) more likely to have any radiographic progression and 6.21-times more likely to have an increase in SHS of ≥ 4 Units/year (Table 2). Importantly, anti-PAD4 IgG1 alone was predictive of any radiographic progression (OR 2.93, P = 0.036), and an increase in SHS of ≥ 4 Units/year (OR 4.88, P = 0.005) (Table 2). Among RA patients who were negative for anti-CCP and/or RF, the odds of radiographic progression among anti-PAD4 IgG1 positive individuals was more than 32-fold higher (P = 0.009) than those negative for anti-PAD4 IgG1 (Figure 3C). In contrast, anti-PAD4 IgG1 did not predict radiographic progression among individuals positive for RF and/or anti-CCP (OR = 1.76, P = 0.31) (Figure 3C).
Figure 3.
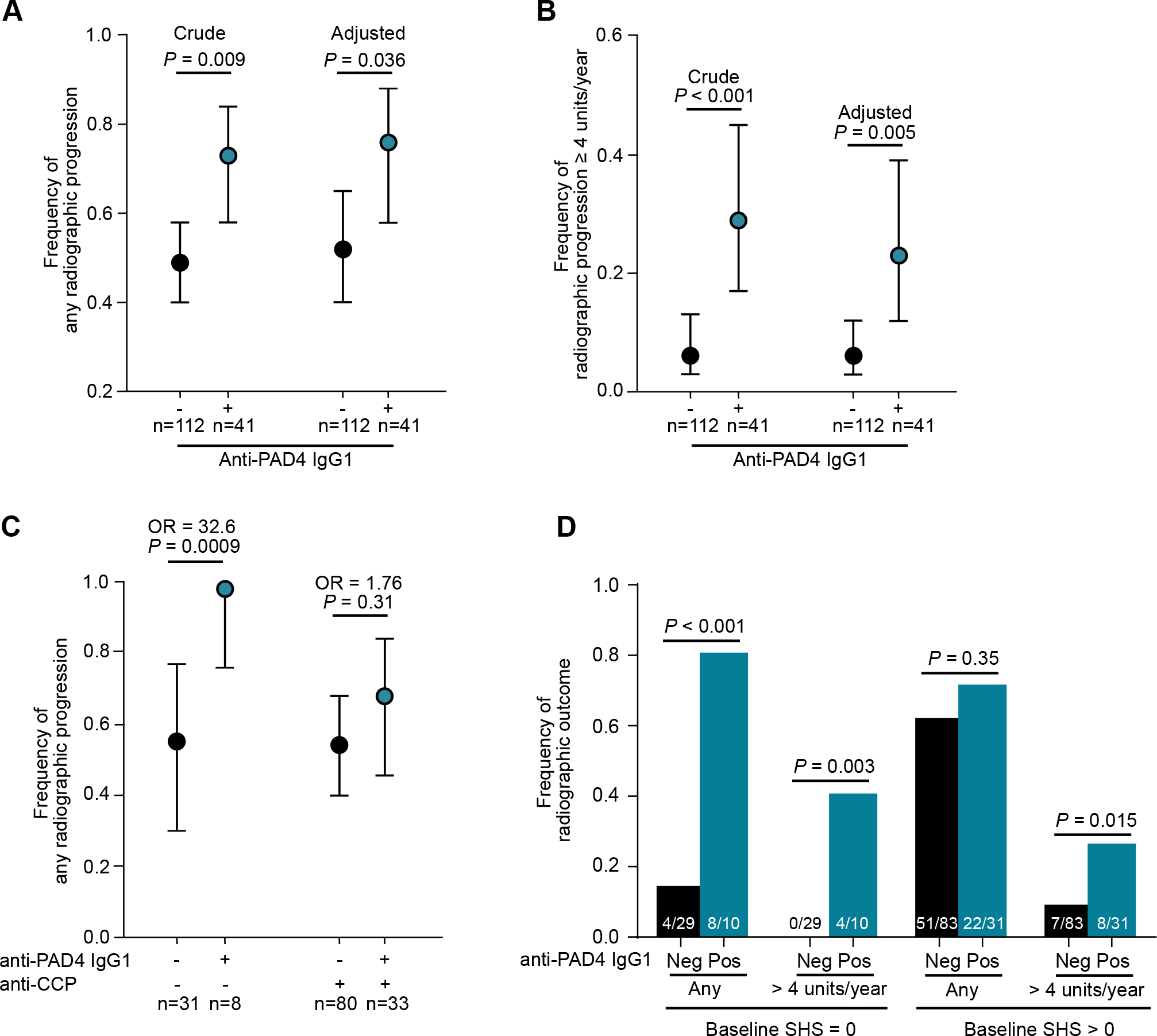
Radiographic progression by anti-PAD4 IgG1 status. A and B, Crude and adjusted frequency of any increase in SHS (A). Frequency was adjusted for gender, anti-PAD2 positivity, average IL-6, baseline pain and baseline SHS >0. Frequency of radiographic progression of SHS ≥ 4 units/year (B). Frequency was adjusted for average IL-6. C, Frequency of any radiographic progression according to anti-PAD4 IgG1 and anti-CCP and/or RF status. The OR and 95% CI are shown. OR were adjusted for gender, anti-PAD2, average IL-6, baseline pain, and baseline SHS >0. D, Frequency of any radiographic progression according to anti-PAD4 IgG1 status and baseline SHS. Disease duration, anti-PAD3/4 antibodies, HLA-DRB1, DAS-28, Swollen joint count, HAQ score, and rheumatoid nodules were not significant in multivariate analyses. Average CRP was not co-modelled with average IL-6 because they are collinear.
Table 2.
Multivariable predictors of any radiographic progression and progression ≥ 4 SHS Units/Year
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Any radiographic progression | ≥ 4 SHS Units/Year | |||||||||
|
|
||||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 3 | ||||||
| OR | P | OR | P | OR | P | OR | P | OR | P | |
|
|
|
|||||||||
| anti-PAD4-IgG1 | 2.83 | 0.009 | 2.93 | 0.036 | 6.21 | <0.001 | 5.39 | 0.006 | 4.88 | 0.005 |
| Male vs female | 0.40 | 0.034 | 0.48 | 0.30 | ||||||
| anti-PAD2 | 0.26 | 0.023 | 0.34 | 0.19 | ||||||
| Average IL-6 | 1.06 | 0.010 | 1.04 | 0.002 | 1.03 | 0.004 | ||||
| Baseline pain | 1.03 | 0.006 | 1.01 | 0.52 | ||||||
| Baseline SHS>0 | 4.97 | 0.001 | 2.10 | 0.37 | ||||||
|
|
|
|||||||||
| AUC (95% CI) | 0.596 (0.528–0.663) | 0.817 (0.749–0.887) | 0.708 (0.591–0.824) | 0.868 (0.795–0.929) | 0.851 (0.777–0.915) | |||||
SHS, Sharp/van der Heijde score; OD, odds ratio; PAD, peptidylarginine deiminase; IL-6, interleukin-6; AUC, area under the curve.
Anti-PAD4 IgG1 was also associated with incident radiographic progression, with 80% of anti-PAD4 IgG1 positive patients with baseline SHS = 0 having any radiographic progression during follow-up compared to only 13% of anti-PAD4 IgG1 negative individuals (P < 0.001) (Figure 3D). Anti-PAD4 IgG1 patients with baseline SHS = 0 were also more likely to have an increase in SHS ≥ 4 compared to anti-PAD4 IgG1 negative patients (40% vs. 0%, respectively; P = 0.003) (Figure 3D). In RA patients with a baseline SHS > 0, anti-PAD4 IgG1 was associated with an increase in SHS of ≥ 4 units/year (25.8% vs. 8.4%, P = 0.015), but not with radiographic progression (Figure 3D).
To confirm the association between anti-PAD4 IgG1 and radiographic progression and define the reproducibility of this finding in a demographically distinct cohort, we tested additional serum samples from African American subjects enrolled in the CLEAR-I (n = 41) and CLEAR-II cohorts (n=94) (Supplementary table 5). Of these, 28.9% (39/135) were positive for anti-PAD4 IgG1. Similar to the ESCAPE RA cohort, anti-PAD4 IgG1 positive subjects in the CLEAR cohorts had a longer disease duration compared to anti-PAD4 IgG1 negative individuals [median (IQR) 11.25 yrs (1.9 – 24.3) vs. 3.6 (1.2 – 16.7), P = 0.010]. Importantly, anti-PAD4 IgG1 was associated with significantly increased joint space narrowing (JSN) [median (IQR), 54 (0–76) vs. 2 (0–47), P = 0.028]. In order to address whether anti-PAD4 IgG1 was predictive of more severe radiographic damage, we classified RA subjects according to tertile of SHS, erosion and JSN scores. As observed in ESCAPE, anti-PAD4 IgG1 positive subjects in the CLEAR cohorts were 2.4 to 2.7-times more likely to have radiographic damage scores on the highest tertile for SHS, erosion and JSN scores (Supplementary table 6).
DISCUSSION
This study explored the clinical associations of different isotypes and IgG subclasses of anti-PAD4 antibodies in patients with RA. We found that the humoral response against PAD4 in RA is characterized by the usage of diverse IgH constant regions linked to different immune effector functions. Despite great overlap among different anti-PAD4 antibodies, we observed that anti-PAD4 IgG1, IgG3 and IgE were associated with specific disease subsets. Interestingly, IgG subclasses known to have higher capacity to activate complement (i.e., IgG1 and IgG3)(13), identified anti-PAD4 positive patients with the worst joint damage burden. Importantly, anti-PAD4 IgG1 was strongly associated with rapid disease progression and higher serum IL-6. Moreover, IgE anti-PAD4 antibodies were associated with a subset of RA patients characterized by higher frequency of RF and anti-CCP, higher DAS-28, higher CRP, and EAM (GGO and rheumatoid nodules). Together, these findings support the notion that different isotypes and IgG subclasses from a single autoantibody specificity are useful for identifying distinct disease subsets in RA.
Importantly, we found that anti-PAD4 IgG1 was more predictive of radiographic progression than the most commonly used serological clinical indicators (i.e., RF and anti-CCP antibodies) and was independent of treatment or RA duration. Furthermore, anti-PAD4 IgG1 was associated with the inflammatory response in RA, since this subset of patients has higher IL-6 and CRP than anti-PAD4 IgG1 negative individuals. Interestingly, this inflammatory response seems to be clinically silent as there was no association with other components of the DAS28 (i.e., tender and swollen joint counts). It is intriguing that anti-PAD4 IgG1 was most strikingly associated with erosive damage and progression of erosive disease among RA patients who were seronegative for RF and/or anti-CCP. In addition, since anti-PAD4 IgG1 was strongly associated with incident radiographic progression among those with no erosive disease at baseline, it may be clinically useful for identifying a susceptible RA subgroup that would ordinarily not be identified as ‘at risk’ for radiographic progression with current predictors (i.e. seropositivity and baseline erosions) (29, 30). We also note that this association appears to be consistent across racial/ethnic groups, as the ESCAPE cohort is predominantly White, while the confirmation cohort (subjects from the CLEAR Registry) was African American.
Although anti-PAD4 IgE was not associated with articular damage, it was linked to a RA subset characterized by a higher frequency of EAM, which in turn are associated with worse disease outcomes. Rheumatoid nodules are associated with a small but significantly higher risk for cardiovascular events and mortality in RA along with ILD (31–33). Furthermore, the association with higher disease activity, RF and anti-CCP antibodies suggest that indeed, anti-PAD4 IgE identifies a RA subset with a higher inflammatory milieu prone to develop EAM.
Although we identified anti-PAD4 antibody types associated with different clinical features in RA, our study has some limitations. We only evaluated the levels of IL-6 in RA serum, but analysis of other cytokines may help decipher additional molecular mechanisms associated with anti-PAD4 isotypes. In addition, a larger longitudinal cohort study is needed to confirm the association of anti-PAD4 IgG1 with radiographic progression, especially in seronegative RA, since this group was small in our cohort. Also, we do not have follow-up data for lung tomography to evaluate the prognosis/evolution of anti-PAD4 IgE positive RA with GGO or ILD.
Mechanistically, the diverse array of class-switch recombination events leading to the development of anti-PAD4 antibodies with different constant regions suggests that these antibodies are generated in distinct immune microenvironments, not limited to the joints, but likely including mucosal-associated lymphoid tissues, such as the airways and the gut. This notion is consistent with the recent finding that anti-PAD4 antibodies are present in the sputum of patients with RA (34). Moreover, the finding that some anti-PAD4 types, in particular IgG1 and IgE, are associated with radiographic progression and lung damage, respectively, suggest that these antibodies have mechanistic properties that promote target-tissue damage in RA. In summary, these data suggest that anti-PAD4 IgG1, IgG3 and IgE are promising mechanistic biomarkers associated with unique disease outcomes in patients with RA.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Dr. Joan Bathon (Columbia University) for providing access to serum samples from the ESCAPE RA cohort participants.
Grant support:
Funding for this project was provided by the Jerome L. Greene Foundation, National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) at the National Institutes of Health (NIH) [grants R01-AR-050026, R01 AR069569, R01 AR079404 and R21 AR079891], and by MedImmune, formerly the global biologics R&D arm of AstraZeneca. The content of this paper is solely the responsibility of the author and do not represent the official views of the NIAMS or the NIH.
Footnotes
Conflict of interest: ED, JTG, and FA are authors on licensed patent no. 8,975,033, entitled “Human autoantibodies specific for PAD3 which are cross-reactive with PAD4 and their use in the diagnosis and treatment of rheumatoid arthritis and related diseases” and ED and FA are authors on provisional patent no. 62/481,158 entitled “Anti-PAD2 antibody for treating and evaluating rheumatoid arthritis”. FA received a grant from Medimmune and Bristol-Myers Squibb, and consulting fees, speaking fees, and/or honoraria from Celgene, Advise Connect Inspire, and Vivo Ventures, outside of this submitted work. ED has received a grant from Pfizer, Celgene, and Bristol-Myers Squibb and personal fees from Celgene, outside of this submitted work. GPS is an employee and shareholder of AstraZeneca. JS is a shareholder of Goldfinch Bio and an employee of Mellitus, LLC. MID received a grant from Horizon Pharma USA, Inc.
References
- 1.Arend WP, Firestein GS. Pre-rheumatoid arthritis: predisposition and transition to clinical synovitis. Nature reviews Rheumatology. 2012;8(10):573–86. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Ikari K, Yano K, Inoue E, Toyama Y, Taniguchi A, et al. PADI4 and HLA-DRB1 are genetic risks for radiographic progression in RA patients, independent of ACPA status: results from the IORRA cohort study. PloS one. 2013;8(4):e61045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malmstrom V, Catrina AI, Klareskog L. The immunopathogenesis of seropositive rheumatoid arthritis: from triggering to targeting. Nature reviews Immunology. 2017;17(1):60–75. [DOI] [PubMed] [Google Scholar]
- 4.Curran AM, Naik P, Giles JT, Darrah E. PAD enzymes in rheumatoid arthritis: pathogenic effectors and autoimmune targets. Nature reviews Rheumatology. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Millan I, Darrah E, Westfall AO, Mikuls TR, Reynolds RJ, Danila MI, et al. Association of anti-peptidyl arginine deiminase antibodies with radiographic severity of rheumatoid arthritis in African Americans. Arthritis research & therapy. 2016;18(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darrah E, Giles JT, Ols ML, Bull HG, Andrade F, Rosen A. Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Science translational medicine. 2013;5(186):186ra65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J, Zhao Y, He J, Jia R, Li Z. Prevalence and significance of anti-peptidylarginine deiminase 4 antibodies in rheumatoid arthritis. The Journal of rheumatology. 2008;35(6):969–74. [PubMed] [Google Scholar]
- 8.Guderud K, Maehlen MT, Nordang GBN, Viken MK, Andreassen BK, Molberg O, et al. Lack of Association among Peptidyl Arginine Deiminase Type 4 Autoantibodies, PADI4 Polymorphisms, and Clinical Characteristics in Rheumatoid Arthritis. The Journal of rheumatology. 2018;45(9):1211–9. [DOI] [PubMed] [Google Scholar]
- 9.Halvorsen EH, Haavardsholm EA, Pollmann S, Boonen A, van der Heijde D, Kvien TK, et al. Serum IgG antibodies to peptidylarginine deiminase 4 predict radiographic progression in patients with rheumatoid arthritis treated with tumour necrosis factor-alpha blocking agents. Annals of the rheumatic diseases. 2009;68(2):249–52. [DOI] [PubMed] [Google Scholar]
- 10.Seaman A, Darrah E, Infantino M, Meacci F, Manfredi M, Benucci M, et al. Anti-peptidyl-arginine deaminase 3 (PAD3) antibodies as a promising marker to measure joint damage in patients with rheumatoid arthritis. Autoimmunity reviews. 2016;15(7):776–80. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder HW, Jr., Cavacini L. Structure and function of immunoglobulins. The Journal of allergy and clinical immunology. 2010;125(2 Suppl 2):S41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hjelholt A, Christiansen G, Sorensen US, Birkelund S. IgG subclass profiles in normal human sera of antibodies specific to five kinds of microbial antigens. Pathogens and disease. 2013;67(3):206–13. [DOI] [PubMed] [Google Scholar]
- 13.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henault J, Riggs JM, Karnell JL, Liarski VM, Li J, Shirinian L, et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat Immunol. 2016;17(2):196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dema B, Charles N. Autoantibodies in SLE: Specificities, Isotypes and Receptors. Antibodies. 2016;5(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Li J. Predominance of IgG1 and IgG3 subclasses of autoantibodies to peptidylarginine deiminase 4 in rheumatoid arthritis. Clinical rheumatology. 2011;30(4):563–7. [DOI] [PubMed] [Google Scholar]
- 17.Giles JT, Ling SM, Ferrucci L, Bartlett SJ, Andersen RE, Towns M, et al. Abnormal body composition phenotypes in older rheumatoid arthritis patients: association with disease characteristics and pharmacotherapies. Arthritis Rheum. 2008;59(6):807–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Darrah E, Giles JT, Davis RL, Naik P, Wang H, Konig MF, et al. Autoantibodies to Peptidylarginine Deiminase 2 Are Associated With Less Severe Disease in Rheumatoid Arthritis. Front Immunol. 2018;9:2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis and rheumatism. 1988;31(3):315–24. [DOI] [PubMed] [Google Scholar]
- 20.van der Heijde D How to read radiographs according to the Sharp/van der Heijde method. The Journal of rheumatology. 2000;27(1):261–3. [PubMed] [Google Scholar]
- 21.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis and rheumatism. 1995;38(1):44–8. [DOI] [PubMed] [Google Scholar]
- 22.Wolfe F A reappraisal of HAQ disability in rheumatoid arthritis. Arthritis and rheumatism. 2000;43(12):2751–61. [DOI] [PubMed] [Google Scholar]
- 23.Giles JT, Darrah E, Danoff S, Johnson C, Andrade F, Rosen A, et al. Association of cross-reactive antibodies targeting peptidyl-arginine deiminase 3 and 4 with rheumatoid arthritis-associated interstitial lung disease. PloS one. 2014;9(6):e98794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bridges SL, Causey ZL, Burgos PI, Huynh BQ, Hughes LB, Danila MI, et al. Radiographic severity of rheumatoid arthritis in African Americans: results from a multicenter observational study. Arthritis Care Res (Hoboken). 2010;62(5):624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laufer VA, Tiwari HK, Reynolds RJ, Danila MI, Wang J, Edberg JC, et al. Genetic influences on susceptibility to rheumatoid arthritis in African-Americans. Hum Mol Genet. 2019;28(5):858–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–9. [DOI] [PubMed] [Google Scholar]
- 27.Gu Z, Gu L, Eils R, Schlesner M, Brors B. circlize Implements and enhances circular visualization in R. Bioinformatics. 2014;30(19):2811–2. [DOI] [PubMed] [Google Scholar]
- 28.Chung NC, Miasojedow B, Startek M, Gambin A. Jaccard/Tanimoto similarity test and estimation methods for biological presence-absence data. BMC Bioinformatics. 2019;20(Suppl 15):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiozawa K, Kawasaki Y, Yamane T, Yoshihara R, Tanaka Y, Uto K, et al. Anticitrullinated protein antibody, but not its titer, is a predictor of radiographic progression and disease activity in rheumatoid arthritis. The Journal of rheumatology. 2012;39(4):694–700. [DOI] [PubMed] [Google Scholar]
- 30.Guillemin F, Gerard N, van Leeuwen M, Smedstad LM, Kvien TK, van den Heuvel W, et al. Prognostic factors for joint destruction in rheumatoid arthritis: a prospective longitudinal study of 318 patients. The Journal of rheumatology. 2003;30(12):2585–9. [PubMed] [Google Scholar]
- 31.Kaushik P, Solomon DH, Greenberg JD, Anderson JT, Reed G, Pala O, et al. Subcutaneous nodules are associated with cardiovascular events in patients with rheumatoid arthritis: results from a large US registry. Clinical rheumatology. 2015;34(10):1697–704. [DOI] [PubMed] [Google Scholar]
- 32.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. The Journal of rheumatology. 2002;29(1):62–7. [PubMed] [Google Scholar]
- 33.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis and rheumatism. 2010;62(6):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demoruelle MK, Wang H, Davis RL, Visser A, Hoang J, Norris JM, et al. Anti-peptidylarginine deiminase-4 antibodies at mucosal sites can activate peptidylarginine deiminase-4 enzyme activity in rheumatoid arthritis. Arthritis Res Ther. 2021;23(1):163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


