Abstract
Broadly neutralizing antibodies (bNAbs), when administered through passive immunization, are protective against HIV-1 infection. Current HIV-1 vaccine strategies are aimed at guiding the immune system to make bNAbs by mimicking their development during infection. Somatic hypermutation of the variable region of bNAbs is known to be crucial for the development of bNAbs. More recently, however, studies have shown how class-switch recombination resulting in the generation of different antibody isotypes may serve as an additional mechanism through which antibodies can gain neutralization breadth and potency. In this review, we discuss the importance of different antibody isotypes for HIV-1 neutralization breadth and potency and how this information can be leveraged to improve passive and active immunization against HIV-1.
Keywords: Neutralization, IgG3 hinge, IgA CH1, class-switching, isotype, HIV-1 vaccine design
Broadly neutralizing antibodies are crucial for HIV prevention
HIV-1 continues to be a major public health emergency, with 1.5 million new infections in 2020 (UNIAIDS; https://www.unaids.org/en/resources/fact-sheet). The pursuit of an HIV-1 vaccine, however, remains elusive due to the highly variable nature of the virus envelope glycoprotein, which enables the virus to evade the immune system, and our inability to clear the infection naturally [1]. However, ~25% HIV-infected individuals develop antibody responses capable of neutralizing (see glossary) many viral variants, and these are termed broadly neutralizing antibodies (bNAbs, see glossary) [1]. The ability of bNAbs to prevent HIV-1 infection has been confirmed in animal models and in the HVTN 703/704 Antibody Mediated Prevention (AMP) human trial, where the infusion of the bNAb VRC01 prevented infection by bNAb-sensitive viruses [2–5]. The success of this trial has re-invigorated the traditional vaccine design pipeline, but also placed additional emphasis on the use of passively administered antibodies for HIV-1 prevention.
HIV-1 is a highly diverse virus both globally and within the host creating a quasispecies of genetically complex viral populations during infection [6]. Several HIV-1 vaccine strategies aim to elicit bNAbs by designing immunogens based on viral variants that drove their development during infection [7]. This strategy relies on our understanding of the natural developmental pathway taken by bNAbs during infection, including the viral variants that shaped their development. This knowledge has been generated through virus antibody co-evolution studies (see glossary). Co-evolution studies of antibody lineages and HIV-1 quasispecies have shown how increased somatic hypermutation (SHM, see glossary) within individual antibody lineages enables the neutralization of new viral variants within the quasispecies and in some cases neutralization breadth of globally circulating strains [8–14]. Co-evolutionary studies have largely focused on changes within the variable region (see glossary) and tested the function of intermediate antibodies identified within lineages as IgG1 subtypes, regardless of their native isotype, which is often unknown as sequencing approaches fail to capture enough of the constant region (see glossary). However, there is increasing evidence that changes in the constant region can also influence the neutralization capacity of antibodies [15–21]. This review will discuss the underappreciated role of the constant region in mediating HIV-1 neutralization, and how this may be leveraged for active and passive immunization strategies in the future.
Class-switching recombination is a key component of immune responses
The advent of bulk B-cell receptor repertoire sequencing has enabled the exploration of the role of class-switch recombination (CSR, see glossary) in antibody repertoires in healthy individuals as well as various disease states. CSR occurs in B-cells that have been activated and undergone SHM, followed by clonal expansion and further SHM [22]. CSR results in the expression of antibodies as different isotypes (IgG3, IgG1 and IgA1 for example). In healthy individuals, there is evidence of IGHV (see glossary) gene bias toward certain isotypes and a preference for switches to IgA1 or IgG1 [22–24]. However, certain disease states can alter IGHV gene usage and CSR events to favour certain isotypes [22,24,25]. In HIV-1 and other viral infections, for example, there is an increase in CSR to IgG3 compared to uninfected individuals [25,26]. CSR occurs in a directional manner dependent on the order in which the genes, which encode the isotypes, occur on the IGH locus on chromosome 14 [23,27]. This irreversible nature of gene excision that occurs during class-switching enables us to infer the order of recombination events, which has proven useful in determining the contribution of isotype diversity within antibody lineages, described in more detail below.
Antibody isotype diversity is linked to neutralization breadth
A key approach to HIV-1 vaccine design has been studies of bNAb donors, and efforts to define the factors that drive bNAb development in some donors but not others. Such studies have shown that antigenic drivers such as duration of infection, high viral load, and viral diversity contribute to the development of breadth [8,28–32]. However, host factors have also been shown to support bNAb development. These include markers of germinal center activity such as Tfh frequency, levels of CXCL13, and activation-induced cytidine deaminase (AID) [31–33]. These host factors likely support SHM, but also provide early insights into the potential role of the constant region in bNAb maturation. In a cohort study comparing bNAb donors with individuals who failed to develop breadth, increased Fc effector function (see glossary) and IgG subtype diversity were shown to be associated with breadth [33]. Similarly, Lofano et al showed enhanced Fc effector function and better Fc receptor binding to complement receptors in individuals who acquired breadth [34]. Together, these studies indicated that mechanistic links occur between Fc (see glossary) and Fab (see glossary)-mediated activities in the development of breadth, raising the question of whether this could be exploited for vaccine design.
The constant region of antibodies modulates neutralization breadth and potency
Monoclonal anti-HIV-1 antibodies (mAbs) have also been shown to exhibit variable functions when expressed as different isotypes. These antibodies have largely been studied as IgG1 subtypes, regardless of their native isotype, which is often unknown. Isotype is well known to alter Fc function, through the variable interactions of different constant regions with Fc receptors on innate immune cells. For example, IgG3 subtypes are able to mediate better Fc effector functions compared to other IgG subtypes, and therefore changing an HIV-1 mAb from an IgG1 to an IgG3 isotype tends to improve Fc effector function [35]. Antibody-dependent cellular phagocytosis (ADCP, see glossary) and antibody-dependent cellular trogocytosis (ADCT, see glossary) are enhanced in IgG3 versions of CAP256-VRC26, VRC01, and 447-52D bNAbs [16,17,36]. VRC01, 10E8, 3BNC117, and other bNAbs also show enhanced antibody-dependent cellular cytotoxicity (ADCC, see glossary) as IgG3 versions, though this was not true of all mAbs tested [17].
However, what was perhaps less expected were differences in the neutralization of HIV-1 when comparing IgG3 and IgG1 versions of antibodies [15–17,21,37,38]. For example, in a comparison of 15 different bNAbs engineered as IgG3 or IgG1 versions and tested against 11 HIV-1 strains, PGT151, 35022, PGT145, and CAP255.G3 showed 3 – 60 fold enhanced neutralization as IgG3 versions compared to IgG1 versions, whereas 10E8v4 had 3 – 4 fold enhanced neutralization as an IgG1 version [17].
Differences in neutralization have also been observed between IgG and IgA isotypes [15,18–20,39,40]. For example, HIV-1 bNAb 2F5 showed ~8-fold enhanced neutralization as an IgA2 version compared to IgG1 and the HIV-1-directed CAP88-CH06 mAb showed 3 – 149 fold enhanced neutralization as an IgA1 version compared to IgG1 [15,18]. These data provide substantial evidence that the constant regions of HIV-1-specific mAbs can influence their Fc effector function and neutralization activity. However, it appears that the effect of isotype on neutralization is variable, in an epitope- (see glossary) or virus-specific manner. Additional research with more monoclonal antibodies tested against large panels of heterologous viruses may provide insights into generalizable features, if these exist.
Role of class-switching in antibody lineage responses to HIV-1
CSR clearly plays a role in bulk immune responses to disease or infection. However, the role of CSR in the development of neutralization within individual lineages has only recently been investigated. This is relevant when using the developmental process of specific lineages as a basis for a vaccine, as is happening in the HIV-1 field. Recently, three independent studies have traced the evolution of individual anti-HIV-1 antibody lineages involving IgG and IgA clonal relatives [15,19,41]. CSR within these lineages involved direct switches from IgM to IgG or IgA as well as sequential switches from IgM to IgG and then further from IgG to IgA [15,19,41].
Within the CAP88-CH06 lineage of neutralizing antibodies, the identification of V-region identical clonal relatives as different IgG and IgA subtypes allowed the fine-mapping of the CSR events during infection [15]. In this longitudinal study, next generation sequencing combined with phenotypic characterisation of members of the antibody lineage allowed the tracing of both SHM and CSR concurrently. In this lineage, the least mutated antibody showed enhanced neutralization of early viral variants as an IgG3 version compared to IgG1 and IgA1 versions, supporting a role for the constant region in neutralization [15]. Increased somatic hypermutation within IgG3 lineage members enabled the neutralization of new viral variants during infection, consistent with traditional co-evolution studies involving single isotypes (Figure 1a). However, early CSR events from IgG3 to IgG1 antibodies resulted in reduced or no neutralization of new viral variants and eventually lead to the termination of those sub-lineages [15]. Thus, like SHM [42], some CSR events can have negative effects on antibody function and result in aborted evolution (Figure 1b). Also like SHM, some CSR events can be beneficial and in the case of CAP88-CH06, CSR to an IgA1 isotype improved the neutralization of new viral variants compared to IgG1 [15]. This suggests that the loss of neutralization because of CSR can be restored by a further CSR, termed “switch redemption” (see glossary) (Figure 1c). CSR to isotypes with improved neutralization enables the lineage to accumulate additional SHM as that isotype and thereby increased neutralization of new viral variants. Therefore, like SHM, CSR can serve as an additional mechanism through which antibody lineages can counter viral escape from emerging HIV-1 viral variants. This data forms the basis of the model for the role of CSR in lineage affinity maturation shown in Figure 1 and is consistent with what has been observed in other anti-HIV-1 lineages involving IgG and IgA isotypes.
Figure 1: Role of class-switching in antibody maturation and neutralization breadth.
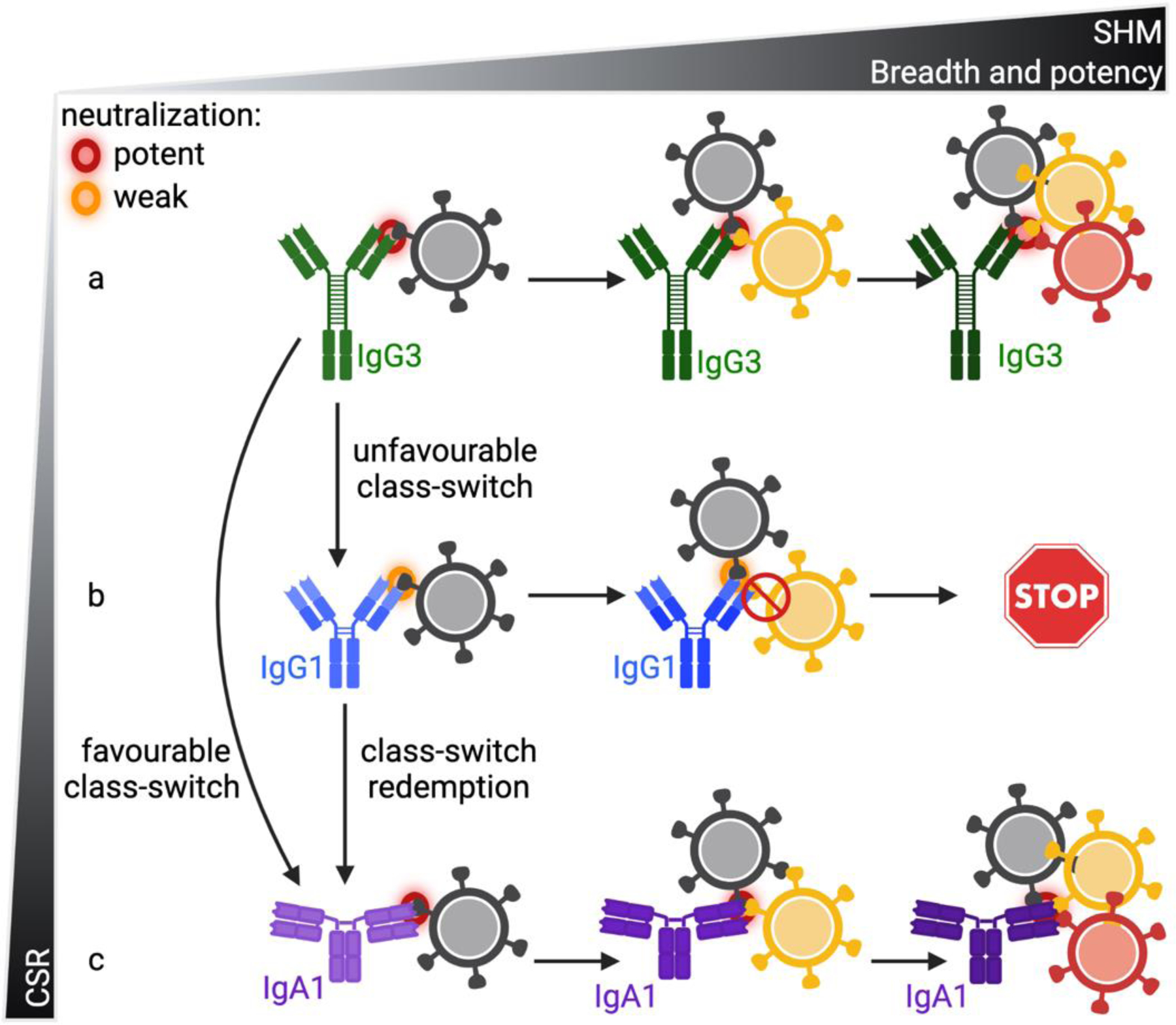
Schematic representation of the role of CSR in antibody maturation to acquire improved neutralization. (A) The traditional arms race involving increasing SHM which enables the neutralization of new viral variants and neutralization breadth. (B) CSR involving subtypes with reduced activity against circulating viral variants resulting in the termination of that sub-lineage (C) CSR involving favourable subtypes which can either restore a previous detrimental switch termed “switch redemption” or have enhanced activity compared to a previous subtype.
How does isotype structure impact neutralization breadth and potency?
Antibody isotypes differ in sequence and structure and therefore the mechanism by which they influence neutralization differs. IgG1, IgG3, and IgA1 isotypes differentially impact HIV-1 neutralization as detailed above. The potential mechanisms for increased neutralization are described in more detail below.
IgG3
The most defining feature of the IgG3 isotype is the extended hinge region (see glossary), which can vary between 32 – 62 amino acids, depending on the immunoglobulin germline IGHG3 (see glossary) allele [43] (Figure 2a). Hinge swap, hinge extension, and F(ab’)2 (see glossary) experiments have shown that longer hinge lengths can improve antibody function including neutralization and ADCP [16,17,36,44,45]. IgG3 antibodies are the most flexible IgG subtype, showing increased flexibility across the Fab-Fab and Fab-Fc regions [46] (Figure 2b).
Figure 2: Key features of IgG3 and IgA1 antibodies that mediate improved neutralization breadth and potency.
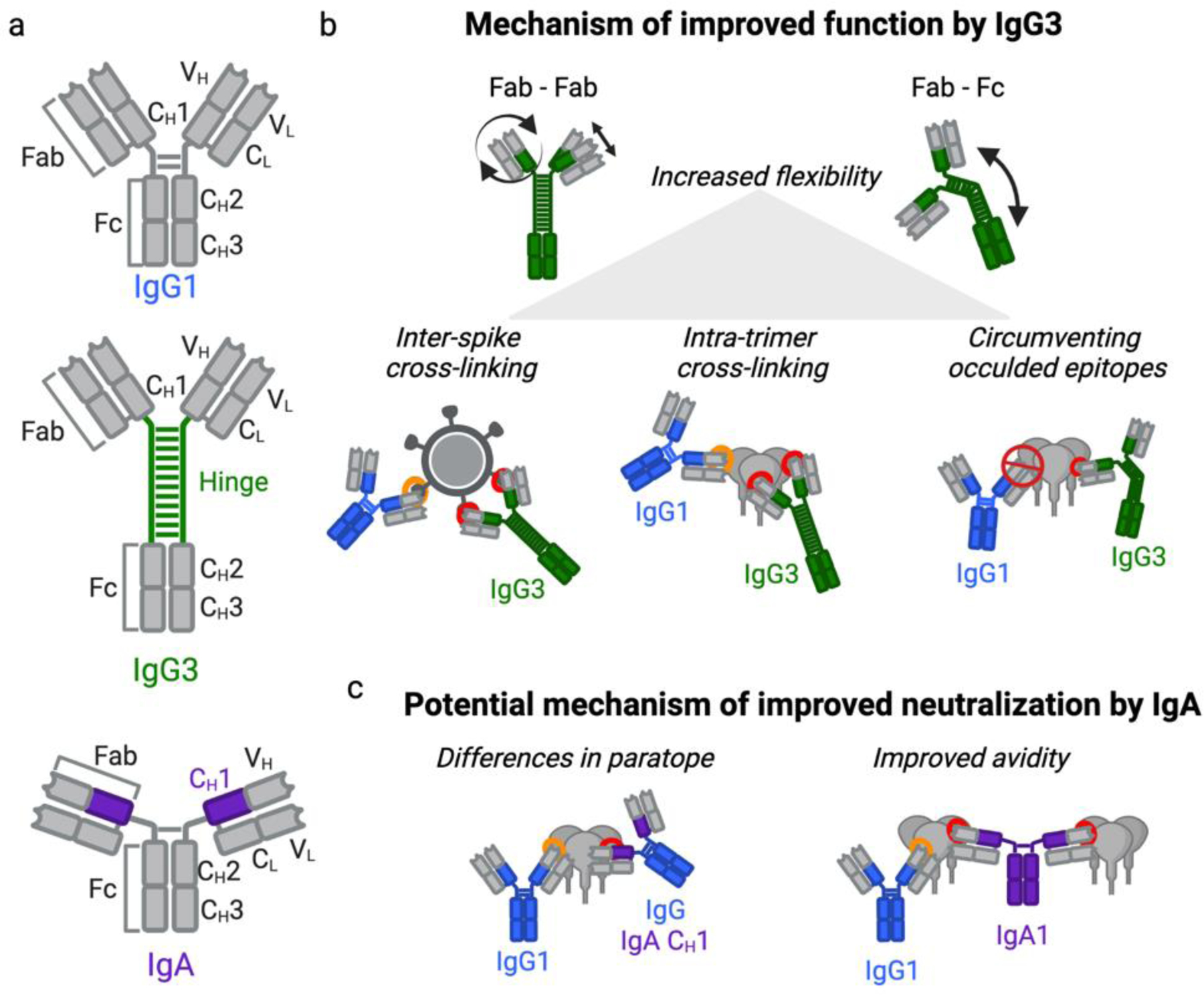
(A) Schematic of IgG1, IgG3, and IgA1 antibodies showing different antibody regions including the heavy and light chain variable regions (VH and VL), the three heavy chain constant regions (CH1 - CH3), the light chain constant region (CL), and the hinge region that make up the Fab and Fc portions. Highlighted in green and purple are the key features for IgG3 and IgA1 that mediate improved neutralization, respectively. (B) Mechanisms through which IgG3 mediates increased neutralization breadth, potency, and ADCP compared to IgG1. (C) The potential mechanisms through which IgA improves neutralization breadth and potency compared to IgG1.
This flexibility has been linked to increased antibody avidity through the bivalent binding (see glossary) of antibodies to multiple antigen sites on a pathogen’s surface, including viruses and bacteria, often resulting in increased neutralization [47–50] (Figure 2b). While viruses such as SARS-CoV-2, Influenza A, Dengue, and Hepatitis B have many spike proteins on their surface, HIV-1 has a low density of sparsely distributed Env (see glossary) spike proteins. This may limit inter-spike cross-linking (see glossary) which is commonly observed in high density viruses [47]. Furthermore, improved antibody avidity through intra-trimeric cross-linking (see glossary) of the two Fab arms to the same Env trimer has been linked to improved neutralization, particularly within bi-specific bNAbs involving 3BNC117 and 10–1074 [47,48,50] (Figure 2b).
Another possible mechanism for improved neutralization of IgG3 over IgG1 subtypes involves flexibility within the Fab-Fc region. HIV-1 Env trimers are highly glycosylated resulting in the occlusion of certain epitopes [51]. In a comparison of CAP88-CH06 as IgG3 and IgG1 versions, the introduction of a glycan into the viral epitope resulted in reduced or no neutralization activity of the IgG1 subtype compared to IgG3 [15]. It is possible therefore that the increased flexibility in IgG3 antibodies circumvents glycans that might otherwise occlude the epitope, consistent with a previous suggestion that the increased hinge length and reach of Fab arms allow IgG3 antibodies to probe into less exposed antigens [35] (Figure 2b).
IgA
Early studies using 2F5 suggested that the improved neutralization of the IgA2 version over IgG1 was a result of differences in the CH1 region [18]. The CH1 has also recently been implicated in 7 – 10 fold enhanced IgA1 neutralization using chimeric antibodies within the CAP88-CH06 lineage [45] and within mAbs isolated from highly-(HIV)-exposed but persistently seronegative individuals [20], compared to mAbs containing IgG1 CH1. These data implicate the CH1 region, rather than the hinge region as seen with IgG3, as the mechanism for improved neutralization of IgA antibodies (Figure 2a).
Direct comparisons of the Fab (VH-CH1) region between related antibodies with either an IgG1 or IgA1 CH1 revealed a more rigid IgA1 Fab with a ~2.5 fold tighter VH-CH1 interaction and ~5° difference in elbow angle compared to IgG1 [52]. It has been suggested that the differences in the Fab region of IgA compared to IgG1 may exert subtle allosteric effects on the antigen binding site and therefore impact binding affinity, thus providing possible mechanism for enhanced neutralization of IgA1 and IgA2 over IgG1 (Figure 2c) [18,20,52–54].
We have previously shown that IgA1 and IgA2 may also have different neutralization profiles, with an IgA1 version of an HIV antibody showing 4-fold enhanced neutralization over the same antibody expressed as IgA2 [15]. Electron microscopy and X-ray neutron scattering of IgA1 in solution have revealed a greater average Fab to Fab distance compared to IgA2 and IgG, allowing for a more extended Y-shaped structure with the Fabs positioned further away from the Fc [52–54]. IgA1 and IgA2 differ most significantly within the hinge region, where IgA1 contains a longer hinge region (~20 amino acids), because of an 8 amino acid repeat region and high levels of O-linked glycosylation, which is absent in IgA2 (~ 6 amino acid hinge) [53,54]. Biolayer interferometry binding comparisons of an HIV-1 antibody (CAP88-CH06) as either an IgA1 or IgG1, suggested increased binding avidity in the IgA1 version compared to IgG1 [15]. This data along with the extended Y-shape of IgA1 suggests a possible mechanism for improved neutralization of IgG1, though this mechanism may not apply to IgA2 (Figure 2c). We note that these comparisons involve monomeric IgA1 rather than dimeric IgA1. Thus, it remains unclear whether dimeric IgA1 would have enhanced neutralization over IgG1 or even monomeric IgA1, though dimeric IgA1 would mostly likely have increased avidity (see Box 1).
Box 1: Harnessing isotype for improved passive and active immunization.
The antibody mediated protection (AMP) trial of VRC01 provided the proof of concept that broadly neutralizing antibodies could be used to protect against HIV-1 infection [3]. There are ~16 bNAbs within the clinical trial pipeline for HIV-1 prevention, all of which are IgG1 [76,77].
Many of these have undergone modifications to improve half-life (~70 days) with the introduction of the “LS” mutations involving M428L and N434S which improves binding of the antibodies to the Fc neonatal receptor (FcRn) [77,78]. Concerns over IgG3 half-life have limited the development of this isotype as a therapeutic or for prevention. However, a single amino acid change R435H within IgG3 results in a comparable half-life to IgG1 [79]. In addition, H435 is found naturally in at least five IgG3 alleles (IGHG3*17, IGHG3*18, IGHG3*19, IGHG3*22, and IGHG3*23, (www.imgt.org)). These and other IgG3 alleles may also benefit from the LS mutation, though this remains to be determined. In addition to the LS mutations, others have been identified for improved half-life of IgG antibodies under different pH conditions, YTE, LSF, C6A-66 or C6A-78 for example [78]. All of which could be explored using an IgG3 isotype to leverage the enhanced function observed within IgG3 over IgG1. IgA antibodies, which lack FcRn binding, are also associated with short half-lives [80]. Attempts at introducing FcRn binding into IgA antibodies using albumin-binding domain (ABD) coupling or IgG/IgA hybrid antibodies, where the CH2 – CH3 of IgA is swapped out for the IgG CH2 – CH3, can improve serum half-life [80]. Furthermore, IgA half-lives can be improved with modifications of glycosylation patterns on the antibodies reducing asialoglycoprotein receptor (ASGPR) clearance [80]. Leveraging the best properties of these isotypes, IgG1 half-life, IgG3 hinge flexibility and IgA CH1 may result in better therapeutics and prevention tools.
Several studies have shown that isotype diversity improves responses to HIV-1. How this can be exploited in an active vaccination setting, however, requires more investigation. For example, the use of certain adjuvants (see “Fine-tuning class-switching in vaccination using different adjuvants”) can promote class-switching, which may result in a more poly-functional response to immunization. The impact of increased isotype diversity may however be immunogen dependent. Furthermore, whether some adjuvants preferentially drive one isotype over another is an under-explored area of research. To address both questions, head-to-head clinical trials would need to specifically evaluate the impact of traditional and next-generation adjuvants on isotype diversity and/or skewing and assess overall impact on prevention.
Thus, while IgG3 and IgA1 show enhanced neutralization over IgG1, they accomplish this through different mechanisms. IgG3 likely leverages its improved flexibility to block more of the antigen while IgA1 relies on conformational changes within the Fab.
Fine-tuning class-switching in vaccination using different adjuvants
Though adjuvants have been used for many years, the molecular mechanism by which they enhance immune responses within vaccines has largely been unknown. Much research has recently gone into understanding these mechanisms and comparing various adjuvants within the same vaccine to further tailor the responses, particularly with regard to Fc effector functions [55,56]. The most well-known and widely used adjuvant is alum, which induces antibody and CD4+ T helper cell responses [55]. Studies that have compared various doses of alum or direct comparisons with other adjuvants (pIC:LC, MF59, ISCOM, adjuvant nanoemulsion (ANE) with toll-like receptor (TLR) 4 or 7 and alum with TLR4/TLR7) have shown differences in titers of mucosal and serum IgG and IgA antibodies [57–63]. Differences within IgG subtypes have also been observed across different adjuvants, with cationic liposome-based adjuvant (CAF01), squalene-based oil-in-water emulsion (SE), monophosphoryl lipid A (MPL), and MF59 showing differences in IgG1 and IgG2c titers [58,60,63] and glucopyranosyl lipid A – SE (GLA-SE) resulting in improved IgG3 and IgG1 titers [61]. The mechanism through which adjuvants induce class-switching may differ. For example, the induction of CSR using CD4 knock-out mice showed that alum is dependent on CD4+ T helper cells but MF59 is not [60]. Regardless of the mechanism, these data suggest the possibility that the use of adjuvants that elicit diverse isotypes, as well as increased SHM could be beneficial in strategies to improve HIV-1 neutralization breadth in a vaccine setting (see Box 1).
Concluding Remarks:
Although this is an emerging field with limited data, there is strong evidence that indicates that the constant region of antibodies influences antibody responses to pathogens through Fc effector functions as well as through neutralization. The mechanisms through which isotypes mediate function differ. Furthermore, changes in antibody function (Fc effector function and neutralization) have been shown to be dependent on both the antibody and viral epitope, and how generalisable these findings are, requires further data. Nonetheless, the utilization of CSR to induce isotype diversity may improve vaccine efficacy aimed at inducing bNAbs, as well as the engineering of monoclonal antibodies for passive immunisation or treatment against certain viral epitopes. However, some key questions remain unanswered, as detailed below, also see Outstanding Questions.
Outstanding questions.
Though there is some evidence for the importance of antibody glycosylation on neutralization, many questions remain. For example, does Fab glycosylation affect HIV-1 neutralization breadth and potency? Do additional O-linked glycans in dimeric IgA1 influence neutralization compared to monomeric IgA1? Do N-linked glycans have different effects on neutralization compared to O-linked glycans? Does the type of glycan present impact the neutralization breadth and potency of HIV-1?
Allelic diversity within IgG3 antibodies is known to affect neutralization. However, little is known about the genetic diversity of IgA, particularly in regions where HIV-1 infection is high. Are there novel constant region gene alleles in underrepresented populations? If so, do the novel alleles affect HIV-1 neutralization breadth and potency?
IgG3 has increased Fc polyfunctionality and both IgG3 and IgA1 have enhanced neutralization in vitro compared to IgG1. However, would this enhanced in vitro activity translate into better protection against HIV-1 infection? Therefore, head-to-head studies comparing IgG3, IgA1 and IgG1 would need to be conducted in animal models and eventually human trials to compare the influence of these functions on protection against infection.
Next-generation adjuvants have been associated with differences in titers between isotypes both in mucosal and serum antibodies. Can adjuvants be used to induce CSR enabling increased isotype diversity? Would increased isotype diversity result in increased neutralization breadth and potency upon immunization?
Does isotype allelic variation influence antibody function?
Allelic variations of IgG3, particularly those involving different hinge lengths, have been shown to influence HIV-1 neutralization [16]. Constant region genes, particularly IGHG3, have high levels of genetic diversity involving individual variation and population-specific variation [64–66]. For example, high levels of genetic diversity have been described in IGHV genes of South African individuals, a region highly affected by HIV-1 infection, and thus likely involves IGHC (see glossary) genes as well [67]. However, many populations especially on the African continent, remain poorly represented in these types of studies [68]. The need to capture global diversity will require further exploration of genetic diversity in constant region genes. Furthermore, the genetic diversity of IGHA genes, encoding IgA1 and IgA2 antibodies, is poorly understood. In addition to the need for increased genomic studies, there is also a need to define the role of allelic variants on antibody function and on the half-lives of antibodies, which is in most cases is unknown. Future studies are needed to expand our understanding of constant region gene diversity, particularly in underrepresented populations, and to assess the impact of this diversity on antibody function.
What role does antibody glycosylation play in HIV-1 neutralization?
Apart from sequence and structure, another way in which the antibodies vary between isotypes is through the type and pattern of glycosylation. For example, both IgG and IgA antibodies contain N-linked glycosylation sites which differ in number and composition, with IgA having more sites and high levels of heterogeneity compared to IgG [43,69]. In the dimeric form, IgA antibodies have additional N-linked oligosaccharides associated with the J chain and secretory components [69]. IgA1 and to a lesser extent IgG antibodies also have O-linked glycosylation sites [69–71]. The role of these glycans in stabilizing the quaternary structure of the Fc and induction of different effector functions has been well established [72]. Different disease states including HIV-1 infection alter the glycosylation profiles of antibodies which in turn can affect antibody function [72]. For example, the neutralization of HIV-1 by the IgG1 monoclonal antibody F240 is dependent on the glycosylation of the mAb [38]. While there is some evidence to suggest the influence of glycosylation on neutralization, much remains unclear. For example, how do the different Fc glycosylation profiles affect neutralization, and do N-linked glycans have different effects on neutralization compared to O-linked glycans? Furthermore, approximately 10–20% of the Fab region of antibodies contain N-linked glycosylation sites within the binding region [43] and the role of these in antibody neutralization is also unknown.
Implications for passive immunization
Several studies have shown that manipulation of the constant region of antibodies can impact function in laboratory assays. These findings have implications for the selection of suitable antibody subtypes for passive immunization, in two ways. Firstly, increased potency of neutralization likely translates into lower therapeutic doses. Lower therapeutic doses make passive immunization approaches more cost-effective (requiring smaller volumes) and more practical (enabling antibodies to be administered subcutaneously rather than intravenously) [73]. Secondly, the increased Fc effector function of IgG3 versions of mAbs not only recruits additional immune cells to clear the pathogen but may also enhance the clearance of previously infected cells [56,74]. Clearance of infected cells is clearly of great relevance in the context of treatment. However, is also important in prevention studies, where for example the early administration of mAbs to infant macaques enabled the clearance of local viral foci, limited viral dissemination, and prevented the establishment of viral reservoirs [75]. Future studies should perform head-to-head comparisons of antibodies of varying isotypes with different functionality to evaluate the benefits of enhanced polyfunctionality (see Clinician’s corner). Although such studies have been limited by concerns over shorter half-lives, exploring the undescribed allelic diversity mentioned above may yield IgG3 variants with half-lives similar to IgG1, IGHG3*17 for example (see Box 1) [16].
Clinician’s Corner.
The constant region of antibodies, which determines isotype, plays a larger role in antibody function than just mediating Fc effector function. Changes in antibody isotype can affect antibody affinity and avidity which is important for neutralization breadth and potency.
Antibodies can be engineered to have enhanced potency and Fc effector function. Potent antibodies require reduced dosages and are more practical leading to subcutaneous instead of intravenous administration when used in passive immunization or therapeutics.
Antibodies with improved Fc effector function could enhance overall immune responses in both treatment and prevention and aid in the clearance of previously infected cells.
During HIV-1 infection antibody lineages directed towards different HIV-1 Env epitopes showed evidence of CSR to IgG and IgA isotypes. This natural phenomenon could potentially be mimicked with the use of adjuvants in active immunization strategies. The induction of class-switch recombination events through vaccine adjuvants would improve isotype diversity and therefore potentially enhance immune responses.
Will CSR enhance neutralization breadth and potency in active vaccination?
It is now clear that the introduction of key amino acid residues into IgG1 isotypes through SHM may not be enough to reach the desired level of neutralization breadth and potency. Substantial data shows that isotypes other than IgG1 may have enhanced capacity to neutralize. Thus, vaccine strategies that focused solely on V-region SHM and viral variants that drive certain SHM [7] may be limited in neutralization breadth. The use of specific adjuvants which promote CSR in combination with sequential viral variants may enhance the overall immune response not only through enhanced Fc effector function but also through improved neutralization breadth and potency. Future studies would need to assess the ability of different adjuvant strategies to induce CSR and whether having increased isotype diversity would further enhance neutralization breadth and potency (see Clinician’s Corner and Box 1).
Understanding the developmental pathways of bNAb lineages has provided a roadmap for vaccine strategies aimed at eliciting the same types of responses. In this review, we have shown that antibody isotypes influence neutralization breadth and potency through different mechanisms. The use of CSR within vaccine design may either complement the current strategy involving V-region SHM or represent an alternative approach. Furthermore, monoclonal antibodies used as therapeutics or in passive immunization could be enhanced by altering the constant region enabling improved effector function and potency.
Highlights.
Class-switch recombination, which involves maintaining the antigen-binding variable region and swapping out the constant region of antibodies, enables diversity in immune responses to disease.
Antibody isotype diversity is associated with increased neutralization breadth towards pathogens such as HIV-1.
The constant region of antibodies, previously thought to only mediate Fc effector function, can also influence the neutralization breadth and potency of HIV-1-directed antibodies.
Within HIV-1 directed antibody lineages, class-switch recombination represents another mechanism through which antibodies can counter viral immune escape.
Antibody IgG3 and IgA1 isotypes mediate enhanced function through different mechanisms. IgG3 relies on increased flexibility, while IgA1 depends on conformational changes within the paratope.
Acknowledgments
C.S, S.I.R and P.L.M are supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number U01AI136677. S.I.R and P.L.M. are supported by the South African Research Chairs Initiative of the Department of Science and Innovation (DSI) and the National Research Foundation (NRF) (Grant No 98341), the SA Medical Research Council SHIP program and the Centre for the AIDS program of research (CAPRISA). S.I.R is a L’Oreal/UNESCO Women in Science South Africa Young Talents awardee. Related research by the authors was supported by the Poliomyelitis Research Foundation and the Columbia University-Southern African Fogarty AIDS International Training and Research Program (AITRP).
Glossary
- ADCC
antibody-dependent cellular cytotoxicity refers to the ability of antibodies to mediate cell killing
- ADCP
antibody-dependent cellular phagocytosis refers to the ability of antibodies to mediate cell engulfment
- ADCT
antibody-dependent cellular trogocytosis refers to the ability of antibodies to mediate membrane nibbling
- Bivalent binding
refers to the binding of both Fab regions of an antibody to an antigen
- bNAbs
broadly neutralizing antibodies are antibodies that can neutralize a wide range of globally circulating HIV-1 strains
- Breadth
refers to the ability of antibodies to neutralize multiple global HIV-1 strains
- Co-evolutionary studies
refers to studies of the changes and development of antibodies and viral quasispecies during infection
- Constant region
refers to the region of the antibody that is encoded by constant region genes and determines antibody isotype
- Class-switch recombination (CSR)
involves the changing of one antibody isotype (eg: IgM, IgD, IgG, IgE and IgA) to another.
- Env
refers to the HIV-1 envelope protein to which antibodies bind
- Epitope(s)
refers to the region on an antigen (eg: the virus) that antibodies bind
- Fab
refers to the fragment antigen portion of antibodies which are responsible for binding to the antigen
- F(ab’)2
refers to part of an antibody including both Fab arms and the hinge region
- Fc
refers to the fragment crystallizable portion of antibodies that is encoded by constant region genes and responsible for inducing effector functions
- Fc effector function
refers to the ability of antibodies to recruit and mediate innate immune cell functions such as ADCP, ADCC and ADCT.
- Hinge
the hinge region is a part of an antibody that sits between the Fab and the Fc portions and differs between isotypes
- IGHC
refers to the immunoglobulin gene heavy chain constant region gene which encodes the constant region of the heavy chain of antibodies that determines an antibodies isotype
- IGHG3
refers to the immunoglobulin gene heavy chain constant gamma 3 gene located on chromosome 14 that encodes the IgG3 antibody constant region.
- IGHV
refers to the immunoglobulin gene heavy chain variable region gene located on chromosome 14 that encodes the variable region of the heavy chain, which makes up the largest part of the of the Fab
- Inter-spike cross-linking
refers to the ability of antibodies to bind using both Fab arms to different spike or Env proteins on the same virus
- Intra-trimeric cross-linking
refers to the ability of antibodies to bind using both Fab arms to different monomers of the same spike or trimeric Env protein.
- Isotype
refers to the type of constant regions encoded by antibodies (eg: IgM, IgD, IgE, IgG and IgA)
- Neutralization/Neutralizing
refers to the ability of antibodies to block infection
- Somatic hypermutation (SHM)
is the accumulation of mutations within the variable region of antibodies that lead to increased affinity to an antigen.
- Variable region
refers to the largest part of the antibody Fab region which is encoded by the V, D and J genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stamatatos L et al. (2009) Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat. Med 15, 866–870 [DOI] [PubMed] [Google Scholar]
- 2.Julg B et al. (2017) Protective Efficacy of Broadly Neutralizing Antibodies with Incomplete Neutralization Activity against Simian-Human Immunodeficiency Virus in Rhesus Monkeys. J. Virol 91, e01187–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corey L et al. (2021) Two Randomized Trials of Neutralizing Antibodies to Prevent HIV-1 Acquisition. N. Engl. J. Med 384, 1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautam R et al. (2016) A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moldt B et al. (2012) Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci 109, 18921–18925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth RP et al. (2012) The origin of genetic diversity in HIV-1. Virus Res. 169, 415–429 [DOI] [PubMed] [Google Scholar]
- 7.Burton DR (2019) Advancing an HIV vaccine; advancing vaccinology. Nat. Rev. Immunol 19, 77–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore PL et al. (2015) Virological features associated with the development of broadly neutralizing antibodies to HIV-1. Trends Microbiol. 23, 204–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhiman JN et al. (2015) Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat. Med 21, 1332–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doria-Rose NA et al. (2014) Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 509, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liao HX et al. (2013) Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496, 469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soto C et al. (2016) Developmental pathway of the MPER-directed HIV-1-neutralizing antibody 10E8. PLoS One 11, e0157409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu X et al. (2015) Maturation and diversity of the VRC01-antibody lineage over 15 years of chronic HIV-1 infection. Cell 161, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doria-Rose NA et al. (2016) New Member of the V1V2-Directed CAP256-VRC26 Lineage That Shows Increased Breadth and Exceptional Potency. J. Virol 90, 76–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheepers C et al. (2020) Antibody Isotype Switching as a Mechanism to Counter HIV Neutralization Escape. Cell Rep. 33, 108430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson SI et al. (2019) IgG3 enhances neutralization potency and Fc effector function of an HIV V2-specific broadly neutralizing antibody. PLOS Pathog. 15, e1008064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson SI et al. (2021) HIV Broadly Neutralizing Antibodies Expressed as IgG3 Preserve Neutralization Potency and Show Improved Fc Effector Function. Front. Immunol 12, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tudor D et al. (2012) Isotype modulates epitope specificity, affinity, and antiviral activities of anti-HIV-1 human broadly neutralizing 2F5 antibody. Proc. Natl. Acad. Sci 109, 12680–12685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jia M et al. (2020) VSV-Displayed HIV-1 Envelope Identifies Broadly Neutralizing Antibodies Class-Switched to IgG and IgA. Cell Host Microbe DOI: 10.1016/j.chom.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khamassi M et al. (2020) The CH1α domain of mucosal gp41 IgA contributes to antibody specificity and antiviral functions in HIV-1 highly exposed Sero-Negative individuals. PLOS Pathog. 16, e1009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Onodera T et al. (2021) A SARS-CoV-2 antibody broadly neutralizes SARS-related coronaviruses and variants by coordinated recognition of a virus-vulnerable site. Immunity 54, 2385–2398.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrova VN et al. (2018) Combined influence of B-cell receptor rearrangement and somatic hypermutation on B-cell class-switch fate in health and in chronic lymphocytic leukemia. Front. Immunol 9, 1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horns F et al. (2016) Lineage tracing of human B cells reveals the in vivo landscape of human antibody class switching. Elife 5, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashford-Rogers RJM et al. (2019) Analysis of the B cell receptor repertoire in six immune-mediated diseases. Nature 574, 122–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotagiri P et al. (2022) B cell receptor repertoire kinetics after SARS-CoV-2 infection and vaccination. Cell Rep. 38, 110393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kardava L and Moir S (2019) B-cell abnormalities in HIV-1 infection: Roles for IgG3 and T-bet. Curr. Opin. HIV AIDS 14, 240–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghraichy M et al. (2021) Different b cell subpopulations show distinct patterns in their igh repertoire metrics. Elife 10, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray ES et al. (2011) The Neutralization Breadth of HIV-1 Develops Incrementally over Four Years and Is Associated with CD4+ T Cell Decline and High Viral Load during Acute Infection. J. Virol 85, 4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hraber P et al. (2014) Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28, 163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landais E and Moore PL (2018) Development of broadly neutralizing antibodies in HIV - 1 infected elite neutralizers. Retrovirology 15, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbaraman H et al. (2018) Broadly neutralizing antibodies: What is needed to move from a rare event in HIV-1 infection to vaccine efficacy? Retrovirology 15, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landais E and Sok D (2021) Nature or nurture: Factors that influence bnAb development. Cell Host Microbe 29, 540–542 [DOI] [PubMed] [Google Scholar]
- 33.Richardson SI et al. (2018) HIV-specific Fc effector function early in infection predicts the development of broadly neutralizing antibodies. PLoS Pathog. 14, 1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lofano G et al. (2018) Antigen-specific antibody Fc glycosylation enhances humoral immunity via the recruitment of complement. Sci. Immunol 3, eaat7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damelang T et al. (2019) Role of IgG3 in Infectious Diseases. Trends Immunol. 40, 197–211 [DOI] [PubMed] [Google Scholar]
- 36.Chu TH et al. (2020) Hinge length contributes to the phagocytic activity of HIV-specific IgG1 and IgG3 antibodies. PLOS Pathog. 16, e1008083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cavacini LA et al. (1995) Influence of heavy chain constant regions on antigen binding and HIV-1 neutralization by a human monoclonal antibody. J. Immunol 155, 3638–3644 [PubMed] [Google Scholar]
- 38.Miranda LR et al. (2007) The Neutralization properties of a HIV-specific antibody are markedly altered by glycosylation events outside the antigen-binding domain. J. Immunol 178, 7132–7138 [DOI] [PubMed] [Google Scholar]
- 39.Astronomo RD et al. (2016) Neutralization takes precedence over IgG or IgA isotype-related functions in mucosal HIV-1 antibody-mediated protection. EBioMedicine 14, 97–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheeseman HM et al. (2017) Broadly Neutralizing Antibodies Display Potential for Prevention of HIV-1 Infection of Mucosal Tissue Superior to That of Nonneutralizing Antibodies Downloaded from. 91, 1762–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lorin V et al. (2022) Epitope convergence of broadly HIV-1 neutralizing IgA and IgG antibody lineages in a viremic controller. J. Exp. Med 219, e20212045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sacks D et al. (2019) Somatic hypermutation to counter a globally rare viral immunotype drove off-track antibodies in the CAP256-VRC26 HIV-1 V2-directed bNAb lineage. PLOS Pathog. 15, e1008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidarsson G et al. (2014) IgG subclasses and allotypes: from structure to effector functions. Front. Immunol 5, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scharf O et al. (2001) Immunoglobulin G3 from Polyclonal Human Immunodeficiency Virus (HIV) Immune Globulin Is More Potent than Other Subclasses in Neutralizing HIV Type 1. J. Virol 75, 6558 LP–6565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moyo-Gwete T et al. (2022) Enhanced neutralization potency of an identical HIV neutralizing antibody expressed as a. bioRxiv DOI: https://www.biorxiv.org/content/10.1101/2022.08.17.504233v1 [DOI] [PMC free article] [PubMed]
- 46.Chu TH et al. (2021) Coming together at the hinges: Therapeutic prospects of IgG3. MAbs 13, e1882028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Einav T et al. (2019) Harnessing Avidity: Quantifying the Entropic and Energetic Effects of Linker Length and Rigidity for Multivalent Binding of Antibodies to HIV-1. Cell Syst. 9, 466–474.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galimidi RP et al. (2015) Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell 160, 433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giuntini S et al. (2016) Human IgG1, IgG3, and IgG3 hinge-truncated mutants show different protection capabilities against meningococci depending on the target antigen and epitope specificity. Clin. Vaccine Immunol 23, 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bournazos S et al. (2016) Bispecific Anti-HIV-1 Antibodies with Enhanced Breadth and Potency. Cell 165, 1609–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu C et al. (2019) Rationally designed carbohydrate-occluded epitopes elicit HIV-1 Env-specific antibodies. Nat. Commun 10, 948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Correa A et al. (2013) Structure of a human IgA1 Fab fragment at 1.55 Å resolution: potential effect of the constant domains on antigen-affinity modulation. Acta Crystallogr. Sect. D Biol. Crystallogr 69, 388–397 [DOI] [PubMed] [Google Scholar]
- 53.de Sousa-Pereira P and Woof JM (2019) IgA: Structure, Function, and Developability. Antibodies 8, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pan S et al. (2021) 3D Structures of IgA, IgM, and Components. Int. J. Mol. Sci 22, 12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pulendran B et al. (2021) Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov 20, 454–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Richardson SI and Moore PL (2021) Targeting Fc effector function in vaccine design. Expert Opin. Ther. Targets 25, 467–477 [DOI] [PubMed] [Google Scholar]
- 57.Francica JR et al. (2017) Innate transcriptional effects by adjuvants on the magnitude, quality, and durability of HIV envelope responses in NHPs. Blood Adv. 1, 2329–2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedersen GK et al. (2020) Vaccine Adjuvants Differentially Affect Kinetics of Antibody and Germinal Center Responses. Front. Immunol 11, 579761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vaccari M et al. (2016) Adjuvant-dependent innate and adaptive immune signatures of risk of SIVmac251 acquisition. Nat. Med 22, 762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ko E-J et al. (2016) Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4 + T Cells. J. Virol 90, 6976–6988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coler RN et al. (2018) The TLR-4 agonist adjuvant, GLA-SE, improves magnitude and quality of immune responses elicited by the ID93 tuberculosis vaccine: first-in-human trial. npj Vaccines 3, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schifanella L et al. (2019) ALVAC-HIV B/C candidate HIV vaccine efficacy dependent on neutralization profile of challenge virus and adjuvant dose and type. PLOS Pathog. 15, e1008121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko E-J et al. (2017) Roles of Aluminum Hydroxide and Monophosphoryl Lipid A Adjuvants in Overcoming CD4 + T Cell Deficiency To Induce Isotype-Switched IgG Antibody Responses and Protection by T-Dependent Influenza Vaccine. J. Immunol 198, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Watson CT et al. (2017) The Individual and Population Genetics of Antibody Immunity. Trends Immunol. 38, 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bashirova AA et al. (2021) Population-specific diversity of the immunoglobulin constant heavy G chain (IGHG) genes. Genes Immun. 22, 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calonga-Solís V et al. (2019) Unveiling the Diversity of Immunoglobulin Heavy Constant Gamma (IGHG) Gene Segments in Brazilian Populations Reveals 28 Novel Alleles and Evidence of Gene Conversion and Natural Selection. Front. Immunol 10, 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scheepers C et al. (2015) Ability to develop broadly neutralizing HIV-1 antibodies is not restricted by the germline Ig gene repertoire. J. Immunol 194, 4371–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peng K et al. (2021) Diversity in immunogenomics: the value and the challenge. Nat. Methods 18, 588–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woof JM and Russell MW (2011) Structure and function relationships in IgA. Mucosal Immunol. 4, 590–597 [DOI] [PubMed] [Google Scholar]
- 70.Plomp R et al. (2015) Hinge-region O-glycosylation of human immunoglobulin G3 (IgG3). Mol. Cell. Proteomics 14, 1373–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed AA et al. (2014) Structural characterization of anti-inflammatory Immunoglobulin G Fc proteins. J Mol Biol 426, 3166–3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Irvine EB and Alter G (2020) Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 30, 241–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mahomed S et al. (2021) Clinical Trials of Broadly Neutralizing Monoclonal Antibodies for Human Immunodeficiency Virus Prevention: A Review. J. Infect. Dis 223, 370–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu C et al. (2016) Enhanced clearance of HIV-1–infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science (80-.) 352, 1001–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hessell AJ et al. (2016) Early short-term treatment with neutralizing human monoclonal antibodies halts SHIV infection in infant macaques. Nat. Med 22, 362–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Miner MD et al. (2021) Broadly neutralizing monoclonal antibodies for HIV prevention. J. Int. AIDS Soc 2021, 25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gruell H and Klein F (2018) Antibody-mediated prevention and treatment of HIV-1 infection. Retrovirology 15, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saunders KO (2019) Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front. Immunol 10, 1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stapleton NM et al. (2011) Competition for FcRn-mediated transport gives rise to short half-life of human IgG3 and offers therapeutic potential. Nat. Commun DOI: 10.1038/ncomms1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Tetering G et al. (2020) Fc Engineering Strategies to Advance IgA Antibodies as Therapeutic Agents. Antibodies 9, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]


