Abstract
Anterior cruciate ligament (ACL) injury initiates a biochemical cascade thought to contribute to the onset and progression of posttraumatic osteoarthritis (PTOA). Interleukin-1ß (IL-1ß), IL-6, and C-telopeptide fragments of type II collagen (CTX-II) are implicated in joint inflammation and cartilage degradation following ACL injury; however, their association with pain is still being explored. The purpose of this study was to evaluate the associations between synovial fluid concentrations of IL-1ß, IL-6, and CTX-II with pain following ACL injury and reconstruction. We hypothesized that greater IL-1ß, IL-6, and CTX-II would correlate with greater Pain Visual Analogue Scale (VAS) scores. This was a secondary analysis of 23 patients (mean age=18.4 y, BMI=27.4, 13 Females/10 Males) with acute ACL tears who participated in a pilot randomized trial. Synovial fluid and VAS scores were collected on the day of initial presentation, at ACL reconstruction, and 1- and 4-weeks after surgery. Synovial fluid concentrations of IL-1ß, IL-6, and CTX-II were assessed using enzyme linked immunoabsorbent assays (ELISA), and repeated measures correlations were used to assess the relationships between pain and synovial IL-1ß, IL-6, or CTX-II after ACL injury and reconstruction. Pain was positively correlated with synovial fluid IL-6 concentrations (r=0.52, p<0.001); however, pain was inversely correlated with CTX-II (r= −0.39, p=0.002). IL-1ß had no significant correlation with pain.
Keywords: anterior cruciate ligament, knee, cartilage, pain, biomarker
Introduction
Post-traumatic osteoarthritis (PTOA) develops following anterior cruciate ligament (ACL) injuries, and the incidence of PTOA following ACL injury is as high as 87%.1 Because knee injuries commonly occur in young, active patients, the onset of PTOA is often earlier than idiopathic OA.1 Those with a history of joint trauma are diagnosed with OA 10 years earlier than those without a history of joint injury2, indicating that PTOA can be an especially debilitating disease with the potential to affect younger patients earlier in life.
OA was previously thought to be a mechanical “wear and tear” disease, but inflammation may play a key role in the pathogenesis of knee OA and its associated pain.3 Several of the inflammatory mediators that have been implicated as potentially contributing to OA pathogenesis include interleukins and tumor necrosis factors (TNF).4 As part of the inflammatory response following acute ACL injury, intra-articular levels of pro-inflammatory cytokines interleukin-1ß (IL-1ß) and IL-6 increase. The initial acute phase of inflammation after an injury is followed by a subacute phase, which can last more than three months following initial injury.5,6 When inflammation does not resolve, the joint enters a low grade chronic inflammatory state that may last months to years and is characterized by elevated levels of pro-inflammatory cytokines, proteolytic enzymes, markers of tissue injury, and complement components.2 This prolonged inflammatory response may play a role in precipitating cartilage injury.6,7 One marker of joint degradation, specifically of the cartilage extracellular matrix, is C-telopeptide of type II collagen (CTX-II).8,9 CTX-II levels increase acutely after joint injury and can remain elevated more than 2 years post-injury.2,10 Taken together, these results show that joint injuries can lead to a prolonged inflammatory environment in the joint, which is associated with cartilage degradation, and therefore, likely play a significant role in the progression to PTOA.2
While IL-1ß, IL-6, and CTX-II are elevated following injury, their associations with perioperative and/or OA pain are still being explored. Recent studies have had contradicting results; some studies have shown positive correlations between synovial fluid IL-1ß and IL-6 with various pain scores, while others show no association.11–13 The relationship between CTX-II and pain is also unclear. The purpose of this study is to evaluate the associations between perioperative pain following ACL injury and reconstruction with synovial fluid concentrations of IL-1ß, IL-6 and CTX-II. We hypothesized that greater synovial fluid IL-1ß, IL-6 and CTX-II would correlate with greater Pain Visual Analogue Scale (VAS) scores.
Methods
This prospective study is a secondary analysis (Level of evidence: III) of skeletally mature patients with acute ACL tears who consented to participate in an IRB-approved pilot randomized trial (NCT03429140)14. Isolated ACL injury was determined via clinical exam and once identified, patients were screened for enrollment in the study. Inclusion criteria included: patients between the ages of 14 and 32 with no history of previous traumatic ipsilateral knee injury, no clinical evidence of posterior cruciate ligament injury, and no more than grade 1 medial or lateral collateral ligament injury. Exclusion criteria included: injury occurring more than 12 days prior to enrollment, previous ipsilateral knee surgery, or a history of any inflammatory disease.
In the original pilot study, patients were randomized to receive either an intraarticular hyaluronate injection or saline placebo one week post reconstruction. It has been previously reported that IL-6, IL-1β, and CTX-II did not differ between the hyaluronate and placebo groups14, and we further demonstrate in the current study that perioperative pain also did not differ between groups (p=0.48). As such, data was collapsed across groups for the current analyses.
Arthrocentesis and collection of Pain Visual Analogue Scale (VAS) scores were performed on the day of initial presentation (mean 6 days post-injury), pre-operatively on the day of ACL reconstruction prior to the patient receiving any medication or anesthesia, and 1- and 4-weeks after surgery (Table 1). Joint aspiration was performed through a superolateral suprapatellar approach with local cutaneous anesthesia. Synovial fluid samples were immediately centrifuged at 3,500 rpm for 10 minutes. The supernatant was collected, aliquoted and stored at −80°C. Synovial fluid IL-1ß (Meso Scale Discovery), IL-6 (Meso Scale Discovery), and CTX-II (Immunodiagnostic Systems) were assessed using commercially available enzyme linked immunoabsorbant assays. Assays were completed per the manufacturer’s instructions and were run in duplicate. Any samples outside of the limits of detection or quantification were rerun. For all plates, intra-assay coefficients of variance were < 9.5. All samples were assessed on a single plate to avoid issues with inter-assay variation (ie 1 plate each IL-6, IL-1β, and CTX-II).
Table 1.
Description of study visits and arthrocentesis success
| Visit | Study tasks performed | Successful Arthrocentesis |
|---|---|---|
| Preoperative enrollment | Informed consent, arthrocentesis and VAS scores | 21/23 (91.3%) |
| Day of Surgery | Arthrocentesis and VAS scores (collected preoperatively) | 18/23 (78.3%) |
| 1 week postoperative | Arthrocentesis and VAS scores | 21/23 (91.3%) |
| 4 weeks postoperative | Arthrocentesis and VAS scores | 19/23 (82.6%) |
VAS=Visual Analog Scale
Statistical Analyses
The change in pain over the four study time points was assessed with a repeated measures analysis of variance (ANOVA), with Bonferroni post hoc analyses used to identify pairwise differences. Biomarker concentrations were not normally distributed, and were log transformed for analysis. Using the methods described by Bakdash and Marusich15, repeated measures correlations were used to assess the relationships between pain and synovial fluid IL-1ß, IL-6, or CTX-II after ACL injury and reconstruction. Analyses were performed in R using the rmcorr package16 and an α-level of p ≤ 0.05 was considered statistically significant.
Results
ACL reconstructions were performed by 3 experienced, board-certified sports medicine surgeons. All procedures were performed arthroscopically, with 19 treated with a bone-patellar tendon-bone autograft and 4 treated with a hamstring autograft. Synovial fluid samples from 23 patients were included in the study. Demographic information can be found in Table 2. Concomitant meniscus injuries are presented in Table 3. Of the 23 patients, only 3 had no meniscus pathology; 8 had isolated medial meniscus tears, 5 had isolated lateral meniscus tears, and 7 patients had both medial and lateral meniscus tears. All meniscal repairs and partial meniscectomies were performed arthroscopically. No full thickness articular cartilage lesions were noted at the time of surgery. Synovial fluid was successfully collected in 79 of 92 possible aspirations (86%), with most dry aspirations occurring on the day of surgery (n=5) or the 1-month postoperative follow-up (n=4, Table 1).
Table 2.
Demographic information of subjects
| Variable | Frequency |
|---|---|
|
| |
| Male | 10 |
| Female | 13 |
|
| |
| Mean ± SD | |
|
| |
| Age | 18.4± 2.6 y |
| BMI | 27.4 ± 4.9 kg/m2 |
Table 3.
Concomitant meniscus injuries and treatment
| Medial Meniscus | Lateral Meniscus | |
|---|---|---|
| Not Injured | 8 (34.8%) | 11 (47.8%) |
| Meniscus Repair | 13 (56.5%) | 9 (39.1%) |
| Partial Meniscectomy | 2 (8.7%) | 3 (13.0%) |
Pain at initial presentation was 54.7 ± 28.3 and significantly improved by the day of surgery (28.3 ± 5.1, p<0.001). Pain then significantly increased 1 week after surgery (55.7 ± 5.8, p=0.001), and again demonstrated a significant decrease 4 weeks postoperatively (27.0 ± 5.4, p<0.001). Pain was positively correlated with synovial fluid IL-6 concentrations (r = 0.52 [95%CI: 0.30 to 0.69], p = 0.00003, Figure 1); however, pain was inversely correlated with CTX-II (r = −0.39 [95%CI: −0.60 to −0.14], p = 0.003, Figure 2). IL-1ß was not significantly correlated with pain (r = −0.09 [95%CI: −0.35 to 0.19], p = 0.53, Figure 3).
Figure 1:
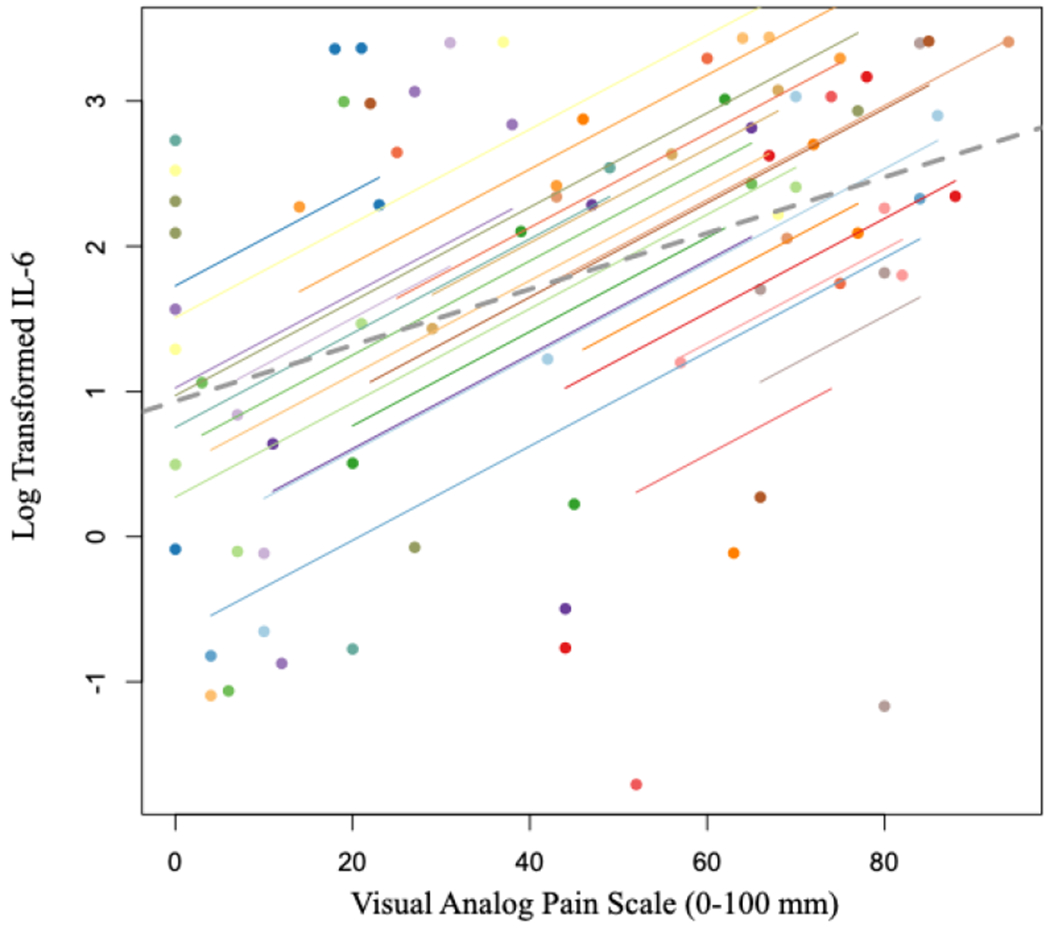
Scatterplot of log transformed IL-6 values and VAS Pain Scores. Participants’ data and corresponding lines are shown in different colors, and the dashed grey line represents the regression line if each participant’s data was averaged across the four time points.
Figure 2:
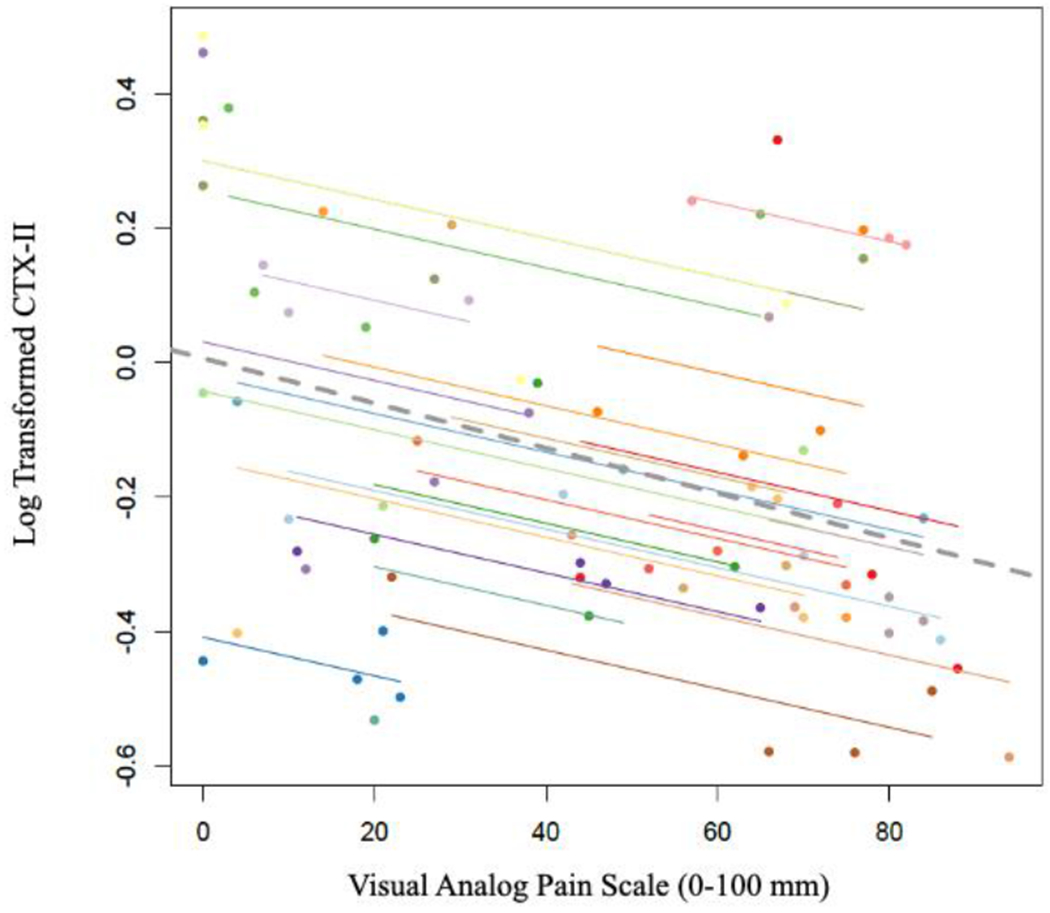
Scatterplot of log transformed CTX-II values and VAS Pain Scores. Participants’ data and corresponding lines are shown in different colors, and the dashed grey line represents the regression line if each participant’s data was averaged across the four time points
Figure 3:
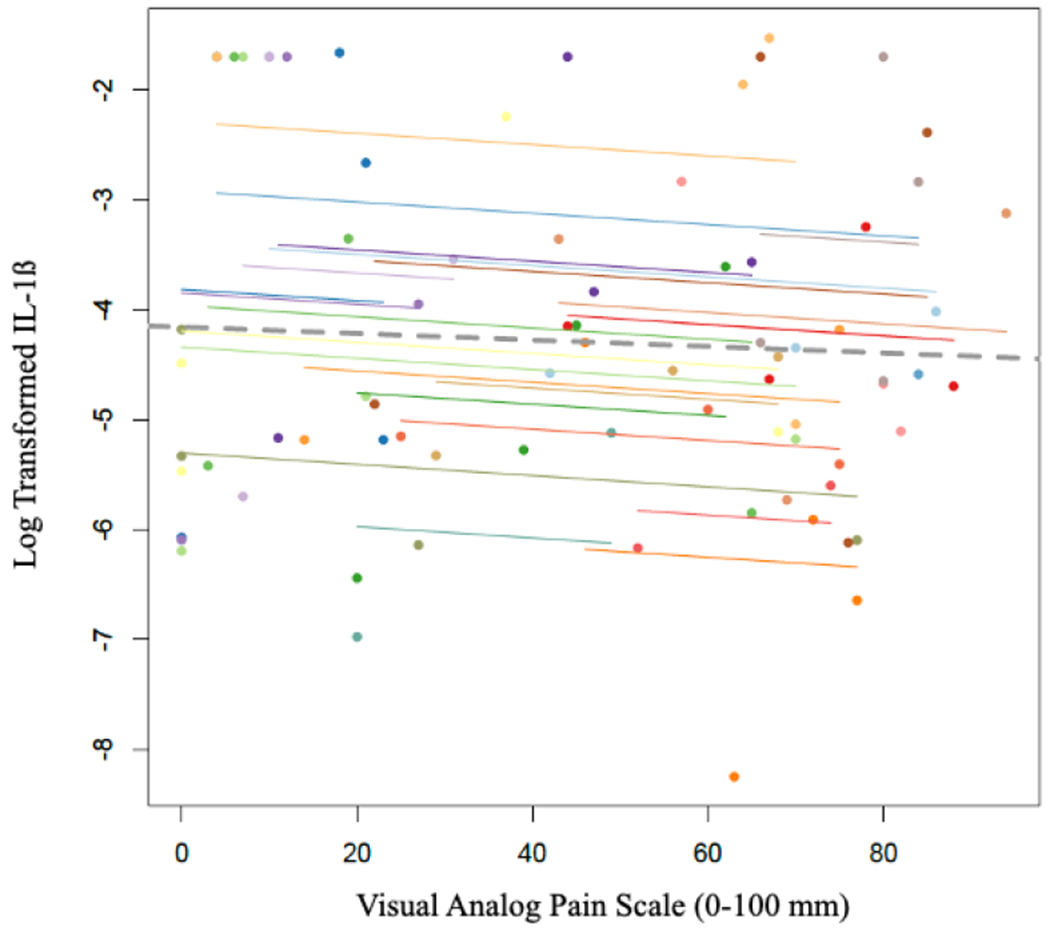
Scatterplot of log transformed IL-1ß values and VAS Pain Scores. Participants’ data and corresponding lines are shown in different colors, and the dashed grey line represents the regression line if each participant’s data was averaged across the four time points.
Discussion
The primary findings of this study were that patient reported pain was significantly and positively correlated with synovial fluid IL-6, and pain was negatively correlated with CTX-II. These results suggest that IL-6 may play a role in the pain signaling pathways following ACL injury and subsequent reconstruction. A more novel finding is that increased early cartilage breakdown was present despite being associated with less pain.
Early PTOA has been described as a “silent killer” of the knee joint17 with progressive, irreversible cartilage degradation occurring in the years following injury but often with little or no pain. The current results further support this concept as there was a negative association between CTX-II and pain. There may be additional temporal differences in pain signaling pathways across the spectrum of PTOA. Synovial IL-6 has been associated with pain early after ACL injury and reconstruction but has not consistently correlated with pain for those with moderate to severe knee OA.7,11,12 Conversely, synovial CTX-II did not correlate with pain early after ACL injury or reconstruction. However, urinary CTX-II (uCTX-II) was predictive of pain progression for those with mild to moderate knee OA and was also demonstrated to be increasingly elevated for those with short-term and longer-term OA pain.18,19
IL-6 and Pain
Our findings are congruent with those of Gupta et al. which found a positive correlation between pre-operative IL-6 synovial fluid concentrations and postoperative VAS pain scores at one, two, six and twelve months after ACL reconstruction.20 Since IL-6 was only collected preoperatively in this previous study, the preoperative inflammatory state of the joint may affect postoperative pain20. The results of the present study add to this understanding by demonstrating that the inflammatory environment of the joint and intra-articular IL-6 levels may contribute to pain both prior to and following surgery. After ACL injury, synovial fluid IL-6 concentrations have demonstrated a more than 10-fold increase during the acute phase post-injury (0-48 hours) and then decreased in the days to months following injury.7 Although IL-6 levels decreased relative to the acute phase, they remained elevated in the chronic phase (3 months or more after injury or surgery) compared to reported low normal levels.7,21 In a study by Watt et al., higher pre-operative synovial fluid IL-6 was associated with a lower (worse) Knee Injury and Osteoarthritis Outcome Score 4 (KOOS4) measured at baseline.21 Another study of patients with acute knee injuries found a significant negative correlation (p=0.017) between synovial fluid IL-6 and KOOS4 scores at 2 years following surgery.22 The pain subdomain of the KOOS4 score (KOOS pain) highly correlated with overall KOOS4 scores at 2 years,22 indicating a likely positive correlation between IL-6 and pain, agreeing with the results of the present study. However, Watt et al. also showed that at 3 months, IL-6 was associated with a greater improvement in KOOS4. 21 This demonstrates that the relationship between IL-6 and pain at different time points is not fully understood. Different pathways may be involved with pain signaling at more prolonged follow-ups. Synovial IL-6 has also been reported to continue to be elevated 5 years after ACL reconstruction when compared to healthy control subjects;23,24 however, synovial fluid IL-6 concentrations 2 years after ACL injury do not correlate with patient-reported outcomes at 5 years.25 When taken with the current results, this suggests that different pain signaling pathways are involved over the time course of PTOA progression.
The relationship between synovial fluid IL-6 and VAS pain scores observed in the current study indicates that IL-6 may play a role in pain signaling following joint injury, which has been observed in other studies. IL-6 and soluble IL-6 receptor (sIL-6R) were injected into rat knee joints, and action potentials of afferent fibers supplying the knee joint were then recorded in response to innocuous and noxious stimuli (rotation of the tibia against the femur).26 Injection of IL-6 alone and IL-6 plus sIL-6R both led to sensitization of unmyelinated C fibers within 1 hour.26 Sensitization of these fibers has two implications in pain production and perception: 1) sensitization lowers the threshold of high-threshold nociceptors to mechanical stimulation, such that previously innocuous stimuli can then cause pain and 2) increases the responsiveness of low-threshold fibers26,27. Low-threshold fibers produce low-discharge rates in response to innocuous stimuli and higher discharge rates in response to noxious stimuli.26,27 These results demonstrate that mechanical stimulation in the presence of IL-6 produces pain and leads to mechanical hypersensitivity, which may be a responsible pain mechanism when loading the knee following ACL reconstruction.
CTX-II and Pain
Another finding of the present study was that synovial fluid CTX-II levels were inversely correlated with VAS pain scores, disproving our hypothesis. This finding suggests that CTX-II, unlike IL-6, is likely not involved in the joint pain signaling pathway early after ACL reconstruction. Previous studies investigating pain and uCTX-II have had varying results. uCTX-II is a breakdown product of articular cartilage that is excreted in the urine.28 Many studies measure uCTX-II in lieu of serum or synovial CTX-II because it less invasive to obtain urine samples.29 Although it is not a direct measure of the intra-articular environment, uCTX-II is accepted as a measure of cartilage breakdown and may therefore have diagnostic and prognostic value for patients with OA.28,29
The inverse relationship between synovial fluid CTX-II and joint pain observed in the present study indicates that, while inflammation may contribute to joint pain, early cartilage degradation following joint trauma may be painless. Placed in the context of early PTOA progression, this observation makes sense. Cartilage itself is avascular and aneural30, so its early destruction may not directly lead to pain. Thus, PTOA joint pain likely arises from other joint structures, such as the synovium, bone and surrounding soft tissue.30 For older individuals with radiographic OA, uCTX-II has been found to be predictive of pain progression and is increasingly elevated for those with no pain, short-term pain (<15 days) and longer-term OA pain (> 15 days).18,19 These differences indicate that more studies investigating CTX-II and pain are needed to establish the relationship and the role of CTX-II on pain production and perception at the different stages of disease progression.
The inverse relationship between pain and synovial fluid CTX-II may also be interpreted as early cartilage breakdown potentially being greater for those with less pain in the perioperative period. Future prospective longitudinal studies are necessary to determine if increased pain may in fact play a protective role, whereas reduced pain may be associated with increased weightbearing thereby increasing cartilage degradation in the early post-injury or postoperative periods. To date, little is also known about the optimal joint loading in the immediate post-injury and/or postoperative periods. Recent animal models of PTOA suggest that unloading the limb early after injury via tail suspension can be protective against early PTOA changes31; however, clinically, the opposite has been reported. In a cross-sectional study of patients one month after ACL reconstruction, Wellsandt et al. reported that relative underloading of the operative limb was associated with greater cartilage composition changes on MRI.32 Similarly, there is debate in the literature as to whether under- vs. over-loading of the operative knee 6-12 months after ACL reconstruction is associated with serum and imaging biomarkers of early cartilage degradation.33–35 The potential interplay between pain, biological factors, biomechanical factors and cartilage degradation requires additional study but offers the potential for novel multimodal treatment algorithms to optimize joint health throughout the first year after ACL injury.
IL-1ß and Pain
Though our study did not show any significant relationship between synovial fluid IL-1ß and VAS pain scores in the perioperative period for ACL reconstruction, previous studies have shown that IL-1ß may play a role in the pathophysiology of idiopathic OA pain. In a study investigating the effects of IL-1ß and TNFα on rabbit articular chondrocytes, IL-1ß was found to stimulate prostaglandin E2 (PGE2) production.36 A synergistic effect was observed when TNFα was added with IL-1ß.36 This is substantial as PGE2 is believed to be a major contributor to inflammatory pain in arthritis.37 The current results suggest that IL-1ß is not a predominant factor in early post-injury or postoperative pain signaling. Future studies are necessary to determine if different pain signaling pathways are involved over the time course of PTOA progression, as well as whether pain pathways differ between PTOA and idiopathic OA.
Limitations
Our study with a relatively small sample size was not without limitation. First and foremost, pain is undoubtedly multifactorial and may be influenced by surgical technique, graft type, presence and magnitude of subchondral bone marrow edema, and multimodal perioperative pain protocols, as well as by psychosocial factors and each individual’s personal experiences. The present study assessed one specific area and evaluated the potential associations of synovial fluid cytokine concentrations and early evidence of type II collagen breakdown with pain. The present study examined inflammatory and chondrodegenerative markers up to 4 weeks post-operatively, and so it is unknown if markers remain elevated and contribute to pain long-term following ACL reconstruction. Only acute ACL injuries were included in the present study (within 12 days of injury), so inflammatory and chondrodegenerative markers and pain may differ in subacute or chronic ACL injuries. Similarly, the study involved a younger patient population; thus, the results may not be generalizable to older ACL-injured patient populations. Additionally, including other biomarkers may have led to a greater understanding of the role that inflammatory and chondrodegenerative biomarkers may play in joint injury and subsequent pain. Finally, since synovial fluid biomarkers were investigated in this study, the results may be biased as the patients with persistent effusions would be potentially overrepresented when compared to those without effusions leading to dry aspirations. Fortunately, synovial fluid samples were collected for the majority of patients at all 4 time points. However, the results may not be generalizable to ACL reconstruction patients without effusions, who may have different amounts of inflammation and cartilage breakdown that was not able to be measured in the present study.
Conclusion
Synovial fluid IL-6 concentrations positively correlated with pain after ACL injury and reconstruction; however, pain was inversely correlated with the synovial biomarker of type II collagen breakdown, CTX-II. PTOA has been described as a “silent killer” and these results suggest that there may be pathway differences in early PTOA that are not primarily pain driven, but still lead to progressive cartilage loss despite minimal symptoms.
Statement of Clinical Relevance:
PTOA has been described as a “silent killer” and these results suggest that early PTOA may have pro-inflammatory pathways that are not primarily associated with pain but still lead to progressive cartilage loss.
Acknowledgements:
(1) Funding was provided in part by the University of Kentucky College of Medicine MVP Grant; NIH National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR001998 (2) Disclosures: Austin Stone (3C-Allosource, 3C-Smith & Nephew, 5-Allosource, 5-Flexion Therapeutics, 9-AOSSM, 9-AANA), Christian Lattermann (3B-Samumed, Vericel, Joint Restoration Foundation; 8-Cartilage, J Sports Physiology, The Knee, OJSM; 9-Biologic Association, International Cartilage Repair Society), Cale Jacobs (5-Flexion Therapeutics, 5-Smith & Nephew, 8-VJSM)
References
- 1.Wang LJ, Zeng N, Yan ZP, Li JT, Ni GX. Post-traumatic osteoarthritis following ACL injury. Arthritis Research and Therapy. 2020;22(1):1–8. doi: 10.1186/s13075-020-02156-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khella CM, Asgarian R, Horvath JM, Rolauffs B, Hart ML. An evidence-based systematic review of human knee post-traumatic osteoarthritis (Ptoa): Timeline of clinical presentation and disease markers, comparison of knee joint ptoa models and early disease implications. International Journal of Molecular Sciences. 2021;22(4):1–48. doi: 10.3390/ijms22041996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Li Z, Li Y, Hu X, Zhang Y, Fan P. Profiling of inflammatory mediators in the synovial fluid related to pain in knee osteoarthritis. BMC Musculoskeletal Disorders. 2020;21(1):1–10. doi: 10.1186/s12891-020-3120-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Mi-Na, Liu Lu, Zhao Luo-Peng, Yuan Fang, Fu Yuan-Bo, BL Xiao-Bai Xu. Research of inflammatory factors and signaling pathways in knee osteoarthritis. Zhongguo Gu Shang. 2020;33(4):388–392. [DOI] [PubMed] [Google Scholar]
- 5.Elsaid KA; Fleming BC; Oksendahl HL; Machan JT; Fadale PD; Hulstyn MJ; Shalvoy R; Jay G Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheumatol. 2008;58:1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso B, Bravo B, Mediavilla L, et al. Osteoarthritis-related biomarkers profile in chronic anterior cruciate ligament injured knee. Knee. 2020;27(1):51–60. doi: 10.1016/j.knee.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 7.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. Journal of Orthopaedic Research. 2013;31(2):315–321. doi: 10.1002/jor.22208 [DOI] [PubMed] [Google Scholar]
- 8.Chmielewski TL, Trumble TN, Joseph AM, et al. Urinary CTX-II concentrations are elevated and associated with knee pain and function in subjects with ACL reconstruction. Osteoarthritis and Cartilage. 2012;20(11):1294–1301. doi: 10.1016/j.joca.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin Bai; Li Y. Combined detection of serum CTX-II and COMP concentrations in osteoarthritis model rabbits: an effective technique for early diagnosis and estimation of disease severity. J Orthop Surg Res. 2016;11:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lattermann C, Jacobs CA, Bunnell MP, et al. Logistical challenges and design considerations for studies using acute anterior cruciate ligament injury as a potential model for early posttraumatic osteoarthritis. Journal of Orthopaedic Research. 2017;35(3):641–650. doi: 10.1002/jor.23329 [DOI] [PubMed] [Google Scholar]
- 11.Orita S, Koshi T, Mitsuka T, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskeletal Disorders. 2011;12:2–9. doi: 10.1186/1471-2474-12-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung YY, Huebner JL, Haaland B, Wong SBS, Kraus VB. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthritis and Cartilage. 2017;25(9):1420–1427. doi: 10.1016/j.joca.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 13.Bas S, Finckh A, Puskas GJ, et al. Adipokines correlate with pain in lower limb osteoarthritis: Different associations in hip and knee. International Orthopaedics. 2014;38(12):2577–2583. doi: 10.1007/s00264-014-2416-9 [DOI] [PubMed] [Google Scholar]
- 14.Hunt ER, Jacobs CA, Conley CEW, Ireland ML, Johnson DL, Lattermann C. Anterior cruciate ligament reconstruction reinitiates an inflammatory and chondrodegenerative process in the knee joint. Journal of Orthopaedic Research. 2021;39(6):1281–1288. doi: 10.1002/jor.24783 [DOI] [PubMed] [Google Scholar]
- 15.Bakdash Jonathan Z; Marusich LR. Repeated measures correlation. Front Psychol. 2017;7(8):456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Repeated measures correlation. https://cran.r-project.org/web/packages/rmcorr/
- 17.Lattermann C, Jacobs CA, Proffitt Bunnell M, et al. A Multicenter Study of Early Anti- inflammatory Treatment in Patients with Acute Anterior Cruciate Ligament Tear. American Journal of Sports Medicine. 2017;45(2):325–333. doi: 10.1177/0363546516666818 [DOI] [PubMed] [Google Scholar]
- 18.Kraus VB, Collins JE, Hargrove D, et al. Predictive validity of biochemical biomarkers in knee osteoarthritis: Data from the FNIH OA Biomarkers Consortium. Annals of the Rheumatic Diseases. 2017;76(1):186–195. doi: 10.1136/annrheumdis-2016-209252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.García-Alvarado FJ, González-Martínez M del R, Jaramillo-Rodríguez Y, Delgado- Aguirre HA. Increased Urinary Concentration of C-Terminal Telopeptide of Type II Collagen and Pain by Radiographic Grade in Women with Knee Osteoarthritis in Northeastern Mexico: A Cross-Sectional Study. BioResearch Open Access. 2020;9(1):7–12. doi: 10.1089/biores.2019.0003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta R, Khatri S, Malhotra A, Bachhal V, Masih GD, Kaur J. Pre-operative Joint Inflammation has no Bearing on Outcome of Arthroscopic Anterior Cruciate Ligament Reconstruction at 1-Year Follow-Up; a Prospective Study. Indian Journal of Orthopaedics. 2021;55(2):360–367. doi: 10.1007/s43465-020-00150-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watt FE, Paterson E, Freidin A, et al. Acute Molecular Changes in Synovial Fluid Following Human Knee Injury: Association With Early Clinical Outcomes. Arthritis and Rheumatology. 2016;68(9). doi: 10.1002/art.39677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garriga C, Goff M, Paterson E, et al. Clinical and molecular associations with outcomes at 2 years after acute knee injury: a longitudinal study in the Knee Injury Cohort at the Kennedy (KICK). The Lancet Rheumatology. 2021;3(9). doi: 10.1016/S2665-9913(21)00116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsson S, Struglics A, Lohmander LS, Frobell R. Surgical reconstruction of ruptured anterior cruciate ligament prolongs trauma-induced increase of inflammatory cytokines in synovial fluid: an exploratory analysis in the KANON trial. Osteoarthritis and Cartilage. 2017;25(9):1443–1451. doi: 10.1016/j.joca.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 24.Struglics A, Larsson S, Kumahashi N, Frobell R, Lohmander LS. Changes in cytokines and aggrecan ARGS neoepitope in synovial fluid and serum and in C-terminal crosslinking telopeptide of type II collagen and N-terminal crosslinking telopeptide of type i collagen in urine over five years after anterior cruciate ligament rupture: An exploratory analysis in the knee anterior cruciate ligament, nonsurgical versus surgical treatment trial. Arthritis and Rheumatology. 2015;67(7). doi: 10.1002/art.39146 [DOI] [PubMed] [Google Scholar]
- 25.Struglics A, Turkiewicz A, Larsson S, et al. Molecular and imaging biomarkers of local inflammation at 2 years after anterior cruciate ligament injury do not associate with patient reported outcomes at 5 years. Osteoarthritis and Cartilage. 2020;28(3). doi: 10.1016/j.joca.2019.12.010 [DOI] [PubMed] [Google Scholar]
- 26.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: An inflammatory mechanism of joint pain. Arthritis and Rheumatism. 2007;56(1):351–359. doi: 10.1002/art.22282 [DOI] [PubMed] [Google Scholar]
- 27.Schaible HG. Basic mechanisms of deep somatic pain. In: McMahon SB; Koltzenburg M, ed. Wall andMelzack’s Textbook of Pain. 5th ed. Churchill Livingstone; 2005:621–633. [Google Scholar]
- 28.Klocke R, Levasseur K, Kitas GD, Smith JP, Hirsch G. Cartilage turnover and intra-articular corticosteroid injections in knee osteoarthritis. Rheumatology International. 2018;38(3):455–459. doi: 10.1007/s00296-018-3988-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chmielewski TL, Trumble TN, Joseph AM, et al. Urinary CTX-II concentrations are elevated and associated with knee pain and function in subjects with ACL reconstruction. Osteoarthritis and Cartilage. 2012;20(11):1294–1301. doi: 10.1016/j.joca.2012.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and central pain processing. Rheumatology. 2011;50(12):2157–2165. doi: 10.1093/rheumatology/ker283 [DOI] [PubMed] [Google Scholar]
- 31.Hsia AW, Jbeily EH, Mendez ME, et al. Post-traumatic osteoarthritis progression is diminished by early mechanical unloading and anti-inflammatory treatment in mice. Osteoarthritis and Cartilage. 2021;29(12). doi: 10.1016/j.joca.2021.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wellsandt E, Kallman T, Golightly Y, et al. Knee joint unloading and daily physical activity associate with cartilage T2 relaxation times 1 month after ACL injury. Journal of Orthopaedic Research. 2022;40(1). doi: 10.1002/jor.25034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjornsen E, Schwartz TA, Lisee C, et al. Loading during midstance of gait is associated with magnetic resonance imaging of cartilage composition following anterior cruciate ligament reconstruction. Cartilage . 2022;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luc-Harkey BA, Franz JR, Hackney AC, Blackburn JT, Padua DA, Pietrosimone B. Lesser lower extremity mechanical loading associates with a greater increase in serum cartilage oligomeric matrix protein following walking in individuals with anterior cruciate ligament reconstruction. Clinical Biomechanics. 2018;60. doi: 10.1016/j.clinbiomech.2018.09.024 [DOI] [PubMed] [Google Scholar]
- 35.Shimizu T, Samaan MA, Tanaka MS, et al. Abnormal Biomechanics at 6 Months Are Associated With Cartilage Degeneration at 3 Years After Anterior Cruciate Ligament Reconstruction. Arthroscopy - Journal of Arthroscopic and Related Surgery. 2019;35(2). doi: 10.1016/j.arthro.2018.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berenbaum F, Jacques C, Thomas G, Corvol MT, Béréziat G, Masliah J. Synergistic effect of interleukin-1β and tumor necrosis factor a on PGE2 production by articular chondrocytes does not involve PLA2 stimulation. Experimental Cell Research. 1996;222(2):379–384. doi: 10.1006/excr.1996.0047 [DOI] [PubMed] [Google Scholar]
- 37.Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527(2):440–447. doi: 10.1016/j.gene.2013.05.069 [DOI] [PMC free article] [PubMed] [Google Scholar]


