Abstract
Introduction
Evaluation of cirrhosis appears to be easily overlooked in the clinic for the HBsAg-negative (hepatitis B surface antigen-negative) and HBcAb-positive (hepatitis B core antibody-positive) population. Herein, we determine the prevalence of cirrhosis/advanced fibrosis among HBsAg-negative/HBcAb-positive US adults.
Methods
Data came from the National Health and Nutrition Examination Survey (NHANES) 2001–2018. A total of 3115 HBsAg-negative/HBcAb-positive US adults were enrolled in this study. We assessed cirrhosis by using the Fibrosis-4 (FIB-4) and aspartate aminotransferase to platelet ratio index (APRI) score.
Results
Out of 50,201 NHANES adults, 45,087 were tested for HBcAb/HBsAg, of whom 3115 met the inclusion criteria (HBsAg-negative/HBcAb-positive with available data for FIB-4/APRI). The weighted proportion of HBsAg-negative/HBcAb-positive among US adults was 4.46% (95% CI 4.17–4.75%), affecting 9.87 million US adults. According to the results of the FIB-4, the weighted prevalence of cirrhosis/advanced fibrosis among HBsAg-negative/HBcAb-positive US adults was 3.76% (95% CI 2.80–4.72%), which corresponds to 371,112 (95% CI 276,360–465,864) HBsAg-negative/HBcAb-positive American adults who had already developed cirrhosis. Among those, cirrhosis/advanced fibrosis in the HBsAb-negative (hepatitis B surface antibody) group (6.28%, 95% CI 4.10–8.45%) was significantly higher than in the HBsAb-positive group (3.08%, 95% CI 2.07–4.08%). Results were similar when APRI was used.
Conclusion
According to the FIB-4, 3.76% of HBsAg-negative and HBcAb-positive US adults had cirrhosis/advanced fibrosis, much higher than in the general population of the USA. Our data highlight the importance of cirrhosis screening in the HBsAg-negative/HBcAb-positive population to prevent advanced liver disease, especially in those who are HBsAb-negative.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00680-2.
Keywords: Hepatitis B, Hepatitis B surface antigen, Liver cirrhosis, Prevalence
Key Summary Points
| Why carry out this study? |
| Evaluation of cirrhosis/fibrosis appears to be easily overlooked in the clinic for the HBsAg-negative/HBcAb-positive population. |
| This study determines the prevalence of cirrhosis/advanced fibrosis among HBsAg-negative/HBcAb-positive US adults given the lack of such reliable and generalizable data. |
| What was learned from the study? |
| According to the FIB-4, 3.76% of HBsAg-negative and HBcAb-positive US adults had cirrhosis/advanced fibrosis, much higher than in the general population of the USA. |
| Our data highlights the importance of cirrhosis/fibrosis screening in the HBsAg-negative/HBcAb-positive population to prevent advanced liver disease, especially among those who are HBsAb-negative. |
Introduction
Hepatitis B virus (HBV) infection is still a major public health issue in the world, impacting 290 million people [1]. Loss of hepatitis B surface antigen (HBsAg) is now considered the best therapeutic outcome for hepatitis B infection, termed as “functional cure” or “resolved hepatitis B” [2]. Before the onset of cirrhosis, HBsAg loss is related to a low risk of cirrhosis or hepatocellular cancer (HCC) [3, 4]. However, patients with HBV infection who have achieved HBsAg loss but with coexisting cirrhosis are still at a higher risk of HCC and are usually associated with poor outcomes [5–8].
Although current guidelines consider HBsAg loss to be a safe endpoint for stopping acute or chronic hepatitis B treatment, the presence of cirrhosis can have a significant impact on clinical decision-making [1, 2]. The evaluation of cirrhosis is an important part of the care of patients with HBV infection, but it appears to be easily overlooked in the clinic for the HBsAg-negative/HBcAb-positive population. Early screening, diagnosis, and intervention could benefit this population who have achieved HBsAg loss but have coexisting cirrhosis.
From a public health perspective, determining the disease burden of cirrhosis among the HBsAg-negative/HBcAb-positive population is critical to guiding healthcare resource planning. Nevertheless, there is a lack of reliable and generalizable data on the prevalence of cirrhosis among HBsAg-negative/HBcAb-positive US adults. To address this knowledge gap, we determined the prevalence and predictors of cirrhosis/advanced fibrosis in HBsAg-negative/HBcAb-positive US residents based on population-based data that is generalizable to entire US households.
Methods
Data Source and Study Population
Data came from the National Health and Nutrition Examination Survey (NHANES) 2001–2018. The National Center for Health Statistics’ NHANES is a program that tracks the health and nutritional status of adults and children in the USA throughout time [9]. NHANES collected nationally representative health-related data on the US population using a complicated, multistage probability sampling procedure [9]. Before participating in the NHANES, all individuals gave written informed consent. The study was conducted in accordance with the principles of the Helsinki Declaration. The approval of the study from the National Center of Health and Statistics Research ethics review board was waived because the research relied upon publicly used, de-identified secondary data.
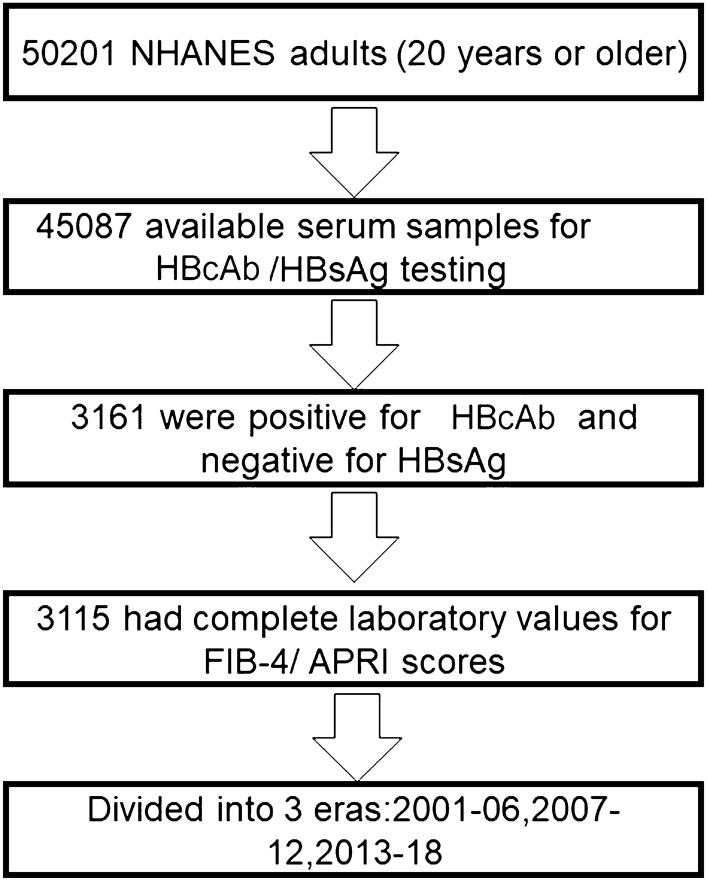
In this analysis, we included HBsAg-negative/HBcAb-positive participants from the continuous NHANES 2001–2018. HBsAg-negative and HBcAb-positive subjects were enrolled, with the HBsAb positive or negative. Only those who were aged 20 years or older at the time of the baseline survey were included. Subjects lacking laboratory data for Fibrosis-4 score/APRI calculation, including age, AST (aspartate aminotransferase), ALT (alanine aminotransferase), and PLT (platelet count) [10], were excluded. As a result, 3115 persons were left in our cohort for analysis (Fig. 1).
Fig. 1.
Flow diagram of subject selection
Definitions of Advanced Fibrosis/Cirrhosis
We assessed liver fibrosis using the Fibrosis-4 score and APRI score; these scores were calculated for participants with AST, ALT, and platelet count data from baseline laboratory data [10]. Descriptions of laboratory methodology, quality assurance, and monitoring were available through NHANES laboratory method manuals.
The formula for the FIB-4 score is (age × AST level)/(platelet count × ALT1/2) [10], and the threshold used to indicate significant fibrosis was > 3.25. With a FIB-4 threshold of 3.25, the sensitivity and specificity were 16.2% and 95.2% for significant fibrosis (Ishak stages F3–F6) for chronic hepatitis B (CHB) [11]. The formula for APRI score is (AST level/AST upper limit of normal) × 100/platelet count [12], and the threshold used to indicate advanced fibrosis was > 1.0; with an APRI threshold of 1.0, the sensitivity and specificity values were 50.0% and 83.0% for advanced fibrosis (Ishak stages F4–F6) for CHB [11].
Information on covariates was available through baseline questionnaires, including age, race/ethnicity, sex, family income-to-poverty ratio, education level, smoking status, body mass index, drinking status, self-reported baseline history of diabetes, hypertension, and liver condition.
Data Analysis
NHANES used a complex hierarchical survey design to ensure accurate projections of the non-institutionalized civilian households in the USA, taking into account the oversampling and unresponsiveness of certain populations to interviews and medical examinations [9]. All estimates in this analysis accounted for complex survey designs and sampling weights (including clusters, strata, and corresponding weights) of NHANES.
Adjusted prevalence estimates of advanced fibrosis/cirrhosis were calculated for NHANES 2001–2018. The trend analysis was performed by combining data from three 6-year periods: 2001–2006, 2007–2012, and 2013–2018. Univariate and multivariate logistic regression analyses were used to estimate odds ratios relating various characteristics to advanced fibrosis/cirrhosis. All variables that had P < 0.10 in stepwise forward logistic regression, or those that were clinically relevant, were included in the model. A two-tailed P value of less than 0.05 was considered to be statistically significant; all statistical analyses were performed using SAS 9.4, which allows appropriate use of the NHANES survey weights to project the results of the analysis to the noninstitutionalized, general population of the USA.
Results
In the NHANES 2001–2018, 50,201 adults aged 20 years or older participated in the examination and laboratory testing. Of those, 45,087(89.8%) had available serum samples for HBcAb/HBsAg testing, of whom 3161 (7.01%) were positive for HBcAb and negative for HBsAg. Out of the 3161 HBsAg-negative/HBcAb-positive adults, 3115 adults (98.5%) had complete laboratory values for calculating FIB-4/APRI scores.
The weighted proportion of HBsAg-negative/HBcAb-positive US adults was 4.46% (95% CI 4.17–4.75%), affecting 9.87 million adults. The prevalence of HBsAg-negative/HBcAb-positive in US adults was 5.19% (95% CI 4.61–5.77) in NHANES 2001–2006, 4.15% (95% CI 3.63–4.67) in NHANES 2007–2012, and 4.27% (95% CI 3.73–4.81) in NHANES 2012–2018, which corresponded to 10.85 million (95% CI 9.81–11.89 million), 9.11 million (95% CI 8.07–10.15 million), and 10.00 million (95% CI 8.84–11.16 million) HBsAg-negative and HBcAb-positive Americans, for NHANES 2001–2006, 2007–2012, and 2012–2018, respectively (Table 1).
Table 1.
Prevalence and number in HBsAg-negative and HBcAb-positive US adults, 2001–2018 (weighted)
| NHANES 2001–2018 (Total) |
NHANES 2001–2006 (Era 1) |
NHANES 2007–2012 (Era 2) |
NHANES 2013–2018 (Era 3) |
|
|---|---|---|---|---|
| Number of US adults, in millions (95% CI) | 220.82 (212.88–228.76) | 208.98 (194.94–223.02) | 219.36 (204.9–233.82) | 234.13 (221.34–246.86) |
| Prevalence of HBsAg-negative and HBcAb-positive, % (95% CI) | 4.46 (4.17–4.75) | 5.19 (4.69–5.68) | 4.15 (3.67–4.62) | 4.27 (3.77–4.76) |
| Number of HBsAg-negative and HBcAb-positive adults, in millions (95% CI) | 9.87 (9.23–10.50) | 10.85 (9.81–11.89) | 9.11 (8.07–10.15) | 10.00 (8.84–11.16) |
All estimates accounted for complex survey designs and sampling weights of NHANES
Table 2 depicts the characteristics of HBsAg-negative/HBcAb-positive US adults. The mean age of survey participants increased from 51.6 to 56.3 over time. The proportion of women and non-Hispanic whites did not change. Over time, the proportion of survey participants with low income, drinking, hypertension, and diabetes increased, whereas those with smoking decreased. The proportion of survey participants with advanced fibrosis/cirrhosis holds steady over time (Supplementary Material Table S1). According to the results of the Fibrosis-4 score, 3.76% (95% CI 2.80–4.72%) of HBsAg-negative/HBcAb-positive populations had cirrhosis from 2001–2018, corresponding to 371,112 (95% CI 276,360–465,864) HBsAg-negative/HBcAb-positive American adults with coexisting cirrhosis. In the most recent era (2013–2018), the cirrhosis prevalence among the HBsAg-negative/HBcAb-positive population was 3.32% (1.63–5.02%), representing 328,680 (95% CI 161,370–496,980) Americans.
Table 2.
Characteristics of HBsAg-negative and HBcAb-positive US adults, NHANES, 2001–2018 (weighted)
| Characteristics | NHANES 2001–18 (Total) |
NHANES 2001–2006 (Era 1) |
NHANES 2007–2012 (Era 2) |
NHANES 2013–2018 (Era 3) |
|---|---|---|---|---|
| Weighted number, in millions (95% CI) | 9.87 (9.24–10.50) | 10.75 (9.70–11.80) | 8.97 (7.94–10.01) | 9.90 (8.82–11.08) |
| Sex, % female (95% CI) | 44.31 (41.79–46.83) | 43.44 (39.00–47.87) | 48.27 (44.02–52.51) | 41.67 (37.13–46.21) |
| Age, years, mean (95% CI) | 53.67 (52.98–54.36) | 51.60 (50.34–52.86) | 53.18 (52.06–54.30) | 56.37 (55.24–57.50) |
| BMI, kg/m2, mean (95% CI) | 27.75 (27.40–28.10) | 27.17 (26.66–27.68) | 28.05 (27.32–28.78) | 28.29 (27.68–28.90) |
| Ethnicity, % (95% CI) | ||||
| Non-Hispanic white | 40.30 (37.15–43.44) | 46.24 (41.74–50.74) | 35.29 (29.10–41.48) | 38.38 (32.19–44.56) |
| Non-Hispanic black | 25.07 (22.75–27.40) | 25.36 (21.30–29.41) | 25.70 (21.95–29.46) | 24.20 (19.81–28.59) |
| Others | 34.62 (31.79–37.44) | 28.39 (23.76–33.02) | 39.00 (33.42–44.57) | 37.41 (32.55–42.27) |
| Education, % (95% CI) | ||||
| Less than high school | 26.57 (24.40–28.73) | 27.76 (24.88–30.65) | 26.25 (22.38–30.12) | 25.56 (20.98–30.13) |
| High school or above | 73.42 (71.26–75.59) | 72.23 (69.34–75.11) | 73.74 (69.87–77.61) | 74.43 (69.86–79.01) |
| Income-to-poverty ratio level, % (95% CI) | ||||
| 0–1.0 | 21.50 (19.26–23.74) | 18.37 (15.37–21.37) | 22.12 (18.66–25.58) | 24.46 (19.23–29.70) |
| > 1.0 | 78.49 (76.25–80.73) | 81.62 (78.62–84.62) | 77.87 (74.41–81.33) | 75.53 (70.29–80.76) |
| Alcohol drinking, % (95% CI) | ||||
| Non-drinker | 28.33 (26.03–30.62) | 30.10 (26.05–34.16) | 29.98 (25.46–34.49) | 25.06 (21.57–28.54) |
| Drinker | 71.66 (69.37–73.96) | 69.89 (65.83–73.94) | 70.05 (65.50–74.59) | 74.93 (71.45–78.42) |
| Excessive alcohol drinkinga | ||||
| Non-drinker | 31.60 (29.13–34.07) | 30.10 (26.05–34.16) | 29.98 (25.43–34.52) | 35.45 (31.45–40.27) |
| Low to moderate drinker | 58.67 (56.13–61.21) | 59.40 (54.82–63.97) | 60.23 (56.61–63.85) | 50.56 (50.56–60.79) |
| Heavy drinker | 9.71 (8.16–11.26) | 10.49 (8.49–12.48) | 9.78 (6.48–13.09) | 8.45 (5.40–11.51) |
| Smoking status, % (95% CI) | ||||
| Never smoker | 47.69 (45.25–50.13) | 42.02 (38.20–45.83) | 50.81 (46.14–55.48) | 51.01 (46.58–55.44) |
| Former smoker | 25.15 (23.36–26.95) | 24.69 (21.43–27.95) | 24.87 (21.62–28.11) | 25.9 (22.99–28.84) |
| Current smoker | 27.14 (24.62–19.66) | 33.28 (29.03–37.52) | 24.31 (19.89–28.73) | 23.06 (18.65–27.46) |
| Liver disease, % (95% CI) | 12.05 (10.26–13.84) | 11.28 (8.19–14.36) | 12.11 (8.69–15.53) | 12.84 (9.86–15.81) |
| Chronic hepatitis C,b % (95% CI) | 9.56 (7.96–11.17) | 15.51 (12.05–18.97) | 7.92 (5.07–10.78) | 15.51 (12.05–18.97) |
| Diabetes, % (95% CI) | 12.22 (10.94–13.49) | 10.78 (8.38–13.17) | 12.52 (10.11–14.93) | 13.51 (11.56–15.45) |
| Hypertension, % (95% CI) | 30.86 (28.74–32.97) | 25.76 (22.60–28.93) | 29.55 (25.69–33.41) | 37.57 (33.34–41.80) |
| HBsAb-negative, % (95% CI) | 21.48 (19.56–23.41) | 18.52 (15.47–21.57) | 23.34 (20.24–26.45) | 23.02 (19.01–27.03) |
| AST, U/L, mean (95% CI) | 28.08 (27.14–29.02) | 28.15 (26.71–29.58) | 30.33 (28.29–32.37) | 25.96 (24.43–27.48) |
| ALT, U/L, mean (95% CI) | 27.52 (26.55–28.49) | 28.66 (26.68–30.64) | 28.77 (27.25–30.28) | 25.16 (23.65–26.66) |
| Platelets, × 103/μL, mean (95% CI) | 243.90 (240.35–247.44) | 261.45 (255.06–267.84) | 236.84 (230.39–243.30) | 231.22 (225.95–236.50) |
| FIB-4 score, mean (95% CI) | 1.37 (1.31–1.43) | 1.23 (1.13–1.34) | 1.47 (1.33–1.61) | 1.43 (1.36–1.50) |
Participants had complete laboratory values for calculating FIB-4 scores (age ≥ 20)
All estimates accounted for complex survey designs and sampling weights of NHANES
aLow to moderate drinker defined as drinking < 1 drink/day in women and < 2 drinks/day in men, and heavy drinker defined as ≥ 1 drink/day in women and ≥ 2 drinks/day in men
bChronic hepatitis C is defined as hepatitis C virus RNA positive
Among HBsAg-negative/HBcAb-positive adults, adjusted cirrhosis prevalence rates were higher for those aged 60 and above versus those aged 40–59 years; for those on lower income versus higher income; for those less educated versus highly educated; for those with liver disease/chronic hepatitis C/diabetes versus those without; and for those who were male versus female (Table 3). The prevalence of cirrhosis was 3.76% (95% CI 2.80–4.72%) for HBsAg-negative/HBcAb-positive adults, versus 6.28% (95% CI 4.10–8.45%) for those with HBsAb negative. Results were similar when APRI was used (Supplementary Material Table S2). When multivariate analysis by regression was used to determine factors associated with advanced fibrosis or cirrhosis, gender, age, race, education, drinking, income, diabetes, liver condition, and HBsAb were significant factors (Table 4).
Table 3.
Prevalence of the cirrhosis by selected characteristics in HBsAg-negative and HBcAb-positive US adults, NHANES, 2001–18
| Characteristics | NHANES 2001–2018 (Total) | NHANES 2001–2006 (Era 1) | NHANES 2007–2012 (Era 2) | NHANES 2013–2018 (Era 3) |
|---|---|---|---|---|
| Adjusted prevalence % (95% confidence interval) | Adjusted prevalence % (95% confidence interval) | Adjusted prevalence % (95% confidence interval) | Adjusted prevalence % (95% confidence interval) | |
| Overall | 3.76 (2.80–4.72) | 3.07 (1.70–4.44) | 5.08 (3.04–7.13) | 3.32 (1.63–5.02) |
| Sex | ||||
| Male | 4.56 (3.08–6.03) | 3.96 (1.97–5.94) | 7.06 (3.10–11.02) | 3.18 (1.15–5.22) |
| Female | 2.77 (1.71–3.82) | 1.91 (0.08–3.74) | 2.97 (1.60–4.34) | 3.52 (1.20–5.85) |
| Age groups (years) | ||||
| 20–39 | 0.26 (− 0.11 to 0.46) | 0 (0–0) | 0.49 (− 0.53 to 1.52) | 0.44 (− 0.46 to 1.36) |
| 40–59 | 3.69 (2.04–5.35) | 3.68 (1.20–6.16) | 5.46 (1.23–9.68) | 1.89 (0.44–3.33) |
| 60 and above | 5.77 (4.06–7.49) | 4.45 (2.56–6.34) | 7.09 (3.95–10.24) | 5.75 (2.49–9.02) |
| BMI | ||||
| < 25 | 3.79 (2.18–5.40) | 3.08 (.63–5.53) | 3.54 (1.24–5.84) | 5.60 (1.07–10.12) |
| 25–30 | 4.56 (2.56–6.56) | 3.27 (0.78–5.77) | 9.24 (3.93–14.54) | 1.07 (− 0.19 to 2.34) |
| > 30 | 2.43 (1.21–3.66) | 2.81 (0.09–5.53) | 2.83 (1.02–4.65) | 1.39 (− 0.04 to 2.84) |
| Ethnicity | ||||
| Non-Hispanic white | 4.62 (2.50–6.74) | 3.86 (1.18–6.55) | 7.04 (1.52–12.56) | 3.60 (0.17–7.02) |
| Non-Hispanic black | 3.92 (2.83–5.01) | 3.00 (1.56–4.44) | 4.38 (2.44–6.33) | 4.53 (2.15–6.91) |
| Others | 2.65 (1.60–3.70) | 1.82 (0.32–3.33) | 3.78 (1.40–6.15) | 2.27 (0.91–3.63) |
| Education | ||||
| Less than high school | 5.98 (4.35–7.61) | 5.32 (3.20–7.44) | 9.25 (5.14–13.35) | 3.72 (1.28–6.17) |
| High school or above | 2.97 (1.85–4.09) | 2.21 (0.51–3.90) | 3.61 (1.36–5.86) | 3.19 (1.20–5.19) |
| Income-to-poverty ratio level | ||||
| 0–1.0 | 5.07 (3.10–7.04) | 3.36 (0.69–6.02) | 5.20 (2.75–7.65) | 6.41 (2.00–10.83) |
| > 1.0 | 3.54 (2.38–4.69) | 3.02 (1.36–4.69) | 5.40 (2.75–8.06) | 2.40 (0.58–4.21) |
| Alcohol drinking | ||||
| Non-drinker | 1.99 (1.25–3.91) | 0.31 (− 0.32 to 0.95) | 3.17 (1.50–4.83) | 2.95 (1.19–4.70) |
| Drinker | 4.20 (2.83–5.57) | 4.33 (2.24–6.42) | 5.57 (2.29–8.85) | 2.98 (0.97–4.98) |
| Excessive alcohol drinkinga | ||||
| Non-drinker | 1.99 (1.25–2.74) | 0.31 (− 0.32 to 0.95) | 3.17 (1.50–4.83) | 2.95 (1.17–4.72) |
| Low to moderate drinker | 4.29 (2.62–5.95) | 4.19 (1.89–6.49) | 4.95 (1.66–8.23) | 3.58 (0.11–7.05) |
| Heavy drinker | 6.86 (2.71–11.01) | 5.13 (1.15–9.11) | 9.45 (− 1.71 to 20.63) | 6.46 (1.32–11.60) |
| Smoking status | ||||
| Never smoker | 2.72 (1.5–3.91) | 1.79 (0.68–2.91) | 4.69 (1.37–8.02) | 1.77 (0.78–2.75) |
| Former smoker | 4.00 (2.32–5.69) | 5.22 (1.24–9.29) | 3.20 (1.23–5.16) | 3.45 (1.22–5.69) |
| Current smoker | 5.40 (3.05–7.74) | 3.10 (0.68–5.52) | 7.85 (2.63–13.09) | 6.64 (1.26–12.02) |
| Liver disease | ||||
| No | 3.07 (2.09–4.05) | 2.46 (1.22–3.69) | 4.27 (2.05–6.49) | 2.65 (0.90–4.40) |
| Yes | 8.83 (5.21–12.45) | 7.87 (1.50–14.24) | 10.99 (3.36–18.62) | 7.91 (2.74–13.07) |
| Chronic hepatitis Cb | ||||
| No | 2.11 (1.35–2.87) | 2.22 (0.61–3.83) | 2.49 (1.31–3.66) | 1.47 (0.70–2.32) |
| Yes | 15.86 (9.36–22.36) | 18.24 (4.70–31.77) | 18.49 (5.25–31.73) | 12.96 (3.72–22.20) |
| Diabetes | ||||
| No | 3.33 (2.34–4.32) | 3.10 (1.64–4.55) | 4.31 (1.85–6.76) | 2.70 (1.43–3.97) |
| Yes | 6.86 (3.40–10.33) | 2.81 (− 0.62 to 6.25) | 10.50 (4.44–16.56) | 7.33 (0.08–14.57) |
| Hypertension | ||||
| No | 2.98 (1.88–4.04) | 3.29 (1.40–5.01) | 4.00 (1.37–6.63) | 1.65 (0.67–2.63) |
| Yes | 5.52 (3.83–7.20) | 2.67 (1.12–4.21) | 7.67 (5.21–10.12) | 6.11 (2.49–9.72) |
| HBsAb negative | ||||
| No | 3.08 (2.07–4.08) | 2.51 (1.21–3.82) | 4.23 (1.78–6.69) | 2.68 (1.08–4.28) |
| Yes | 6.28 (4.10–8.45) | 5.51 (134–9.68) | 7.87 (4.44–11.30) | 5.48 (1.60–9.37) |
Cirrhosis defined as Fibrosis-4 score > 3.25
All estimates accounted for complex survey designs and sampling weights of NHANES
aLow to moderate drinker defined as drinking < 1 drink/day in women and < 2 drinks/day in men, and heavy drinker defined as ≥ 1 drink/day in women and ≥ 2 drinks/day in men
bChronic hepatitis C is defined as hepatitis C virus RNA positive
Table4.
Factors associated with cirrhosis among HBsAg-negative and HBcAb-positive US adults, NHANES 2001–18
| Characteristics | Univariate analysis | Multivariable analysis |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| HBsAb (reference = positive) | 2.10 (1.72–2.58) | 1.45 (1.44–1.46) |
| Age | 1.03 (0.99–1.09) | 1.07 (1.09–1.08) |
| Sex (reference = female) | 1.67 (0.26–10.71) | 2.38 (2.36–2.40) |
| Race/ethnicity (reference = NH white) | ||
| NH Black | 0.84 (0.61–1.15) | 1.39 (1.37–1.40) |
| Other | 0.56 (0.20–1.51) | 0.87 (0.87–0.88) |
| BMI (reference = 0–25) | ||
| 25–30 | 1.21 (1.16–1.26) | 0.80 (0.80–0.81) |
| > 30 | 0.63 (0.13–2.98) | 0.80 (0.79–0.80) |
| Education (reference = high school or above) | 2.07 (0.83–5.13) | 1.88 (1.86–1.89) |
| Income-to-poverty ratio level (reference ≥ 1) | 1.45 (1.04–2.02) | 1.40 (1.38–1.41) |
| Alcohol drinking: (reference = non-drinker) | 2.15 (1.23–3.75) | 1.82 (1.80–1.83) |
| Smoking status (reference = never smoker) | ||
| Former smoker | 1.49 (0.94–2.36) | 0.76 (0.76–077) |
| Current smoker | 2.03 (0.83–4.96) | 1.36 (1.35–1.38) |
| Liver disease (reference = no) | 3.05 (1.76–5.29) | 2.91 (2.89–2.94) |
| Diabetes (reference = no) | 2.13 (1.48–3.06) | 1.03 (1.02–1.04) |
| Hypertension (reference = no) | 1.89 (1.00–3.58) | 1.21 (1.20–1.22) |
Table 5 depicts the characteristics of HBsAg-negative/HBcAb-positive participants according to hepatitis B surface antibody status. The following characteristics were more frequent among HBsAg-negative/HBcAb-positive adults who were HBsAb negative versus HBsAb positive: being older (55.68 vs 53.12, respectively), drinking (75.75% vs. 70.56%, respectively), being on low income (24.15% vs. 20.77%, respectively), being less educated (32.81% vs. 24.85%, respectively), having diabetes (14.82% vs. 11.45%, respectively), having hypertension (36.98% vs. 29.18% respectively), and having a liver condition (18.24% vs. 10.29%, respectively).
Table 5.
Characteristics of HBsAg-negative and HBcAb-positive participants according to HBsAb status (2001–2018)
| Characteristics | HBsAb (+) | HBsAb (−) | P valuea |
|---|---|---|---|
| Weighted number, in millions (95% CI) | 7.75 (7.17–8.32) | 2.12 (1.91–2.32) | < 0.0001 |
| Sex, % female (95% CI) | 45.43 (42.32–48.55) | 40.16 (34.94–45.39) | 0.1141 |
| Age, years, mean (95% CI) | 53.12 (52.41–53.82) | 55.68 (54.24–57.12) | 0.009 |
| BMI, kg/m2, mean (95% CI) | 27.59 (27.20–27.97) | 28.36 (27.68–29.04) | 0.0308 |
| Ethnicity, % (95% CI) | |||
| Non-Hispanic white | 41.14 (37.81–44.48) | 37.29 (31.73–42.86) | 0.2259 |
| Non-Hispanic black | 24.39 (21.81–26.96) | 27.64 (24.15–31.13) | |
| Others | 34.45 (31.43–37.48) | 35.05 (30.33–39.77) | |
| Smoking status, % (95% CI) | |||
| Never smoker | 49.36 (46.67–52.05) | 41.58 (36.86–46.30) | 0.0145 |
| Former smoker | 24.68 (22.57–26.79) | 26.87 (22.74–31.00) | |
| Current smoker | 25.95 (23.32–28.58) | 31.53 (26.53–36.54) | |
| Alcohol drinking, % non-drinker (95% CI) | 29.44 (26.91–31.97) | 24.25 (20.06–28.45) | 0.0331 |
| Education, % high school or above (95% CI) | 75.15 (72.95–77.36) | 67.19 (62.53–71.84) | 0.0007 |
| Income-to-poverty ratio level, % > 1.0 (95% CI) | 79.23 (76.71–81.75) | 75.85 (71.76–79.93) | 0.1367 |
| Liver disease, % (95% CI) | 10.29 (8.37–12.21) | 18.42 (13.88–22.95) | 0.0003 |
| Diabetes, % (95% CI) | 11.45 (9.98–12.92) | 14.82 (12.11–17.53) | 0.0269 |
| Hypertension, % (95% CI) | 29.18 (26.80–31.55) | 36.98 (32.04–41.92) | 0.0049 |
| AST, U/L, mean (95% CI) | 27.32 (26.28–28.36) | 30.88 (28.76–33.01) | 0.0036 |
| ALT, U/L, mean (95% CI) | 26.56 (25.57–27.55) | 31.06 (28.03–34.09) | 0.0076 |
| Platelets, × 103/μL, mean (95% CI) | 246.41 (242.41–250.42) | 234.55 (227.90–241.25) | 0.0021 |
| FIB-4 score, mean (95% CI) | 1.32 (1.25–1.38) | 1.56 (1.45–1.68) | 0.0002 |
All estimates accounted for complex survey designs and sampling weights of NHANES
aFor categorical variables, P value was calculated by the Rao–Scott χ2 test, which is a design-adjusted version of the Pearson χ2 test. For continuous variables, t tests adjusting for sampling weights were used to calculate P values
To verify the robustness of our results, we compared the prevalence of cirrhosis using different definitions (cirrhosis defined by liver stiffness > 13.4 kPa, APRI > 1.0, or FIB-4 > 3.25) in NHANES 2017–2018 (liver stiffness data was only available in NHANES 2017–2018). Based on previous reports, with an LSM (liver stiffness measurement) threshold of 13.4 kPa, the sensitivity and specificity values were 79.0% and 92.0% for cirrhosis (F4) in chronic hepatitis B [13]. Owing to the higher sensitivity of LSM, the prevalence of cirrhosis defined by LSM was higher than that of APRI and FIB-4 in NHANES 2017–2018 (Supplementary Material Table S3), which suggested that we may have underestimated the prevalence of cirrhosis in the HBsAg-negative/HBcAb-positive population to some extent.
Awareness and therapy for HBV are important factors affecting liver fibrosis, but the data is only available in NHANES 2013–2018 (Supplementary Material Tables S4 and S5). Only 8.84% of participants were aware of hepatitis B virus infection and 1.68% of participants ever received treatment for hepatitis B. Participants aware or unaware of HBV infection had similar rates of cirrhosis (3.54% versus 3.29%). Cirrhosis defined by FIB-4 did not occur in patients ever treated for hepatitis B (0/10).
Hepatic steatosis may be associated with cirrhotic risk, regardless of the status of hepatitis B. The controlled attenuation parameter (CAP) is a non-invasive method for the detection of hepatic steatosis based on transient elastography [14]. We summarized the prevalence of cirrhosis by CAP in HBsAg-negative/HBcAb-positive adults in NHANES 2017–2018. Participants with a higher CAP have a higher prevalence of cirrhosis/advanced fibrosis versus those with a normal CAP (Supplementary material Table S6).
Discussion
HBsAg loss is considered a “resolution” or “functional cure” of hepatitis B, and to a considerable extent, this leads to neglect in the health management of the population who were positive for HBcAb and negative for HBsAg. Accurate data on the prevalence of cirrhosis is essential for developing treatment options and optimizing resource allocation for the HBsAg-negative/HBcAb-positive population. Using NHANES data, we report in this work that the overall prevalence of HBsAg-negative/HBcAb-positive has remained relatively stable over time. The proportion of the HBsAg-negative/HBcAb-positive population with coexisting cirrhosis is still at a certain level, especially among those who are HBsAb-negative.
To the best of our knowledge, this is the first study to determine the prevalence of cirrhosis among the HBsAg-negative/HBcAb-positive population based on the nationwide US population. Despite multifaceted efforts to reduce HBV infection and the related disease burden, there has been no significant change in the HBsAg-negative/HBcAb-positive population in the USA over time. At the same time, the proportion of those with cirrhosis among the HBsAg-negative/HBcAb-positive population also remained at a relatively stable level for a long time.
The prevalence of cirrhosis in the general US population was approximately 0.27%, as reported in the previous literature [15]. Unexpectedly, the prevalence of cirrhosis was much higher among HBsAg-negative/HBcAb-positive US adults. This high prevalence is worrisome.
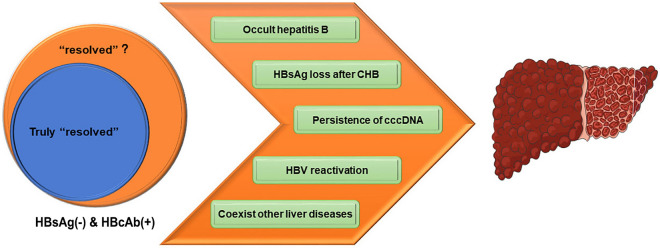
The high prevalence of cirrhosis in the HBsAg-negative/HBcAb-positive population may be explained by the following factors (Fig. 2). Firstly, even with HBsAg loss, some patients still develop liver cancer and cirrhosis. It has been reported in recent literature based on liver biopsy that positive HBcAb is associated with cirrhosis and possibly HCC and cirrhotic complications in patients with NFALD (non-alcoholic fatty liver disease) [16]. The population who was positive for HBcAb and negative for HBsAg should not be simply interpreted as “past infection” or “resolved infection”. Such groups seem to be heterogeneous. It should be considered that for patients with chronic hepatitis B and with HBsAg loss, cirrhosis occurs during the HBsAg carrying period, will also lead to a poor prognosis, and should not be regarded as the real “resolution” of hepatitis B. Health management after HBsAg loss is still very important for those populations. In addition, as HBV cannot be completely eradicated because of the persistence of covalently closed circular DNA (cccDNA) and integrated HBV DNA, another important consideration is occult HBV or HBV reactivation [17, 18]. Occasionally, occult HBV or HBV reactivation may be mild or even asymptomatic, which results in occult HBV or HBV reactivation being easily neglected in clinical practice. Studies have demonstrated a higher risk of cirrhosis or HCC in people with occult HBV compared to HBsAg-negative individuals and those with no occult HBV [19]. In addition, numerous studies have shown that the reactivation of HBV can accelerate the progress of the disease in those with negative HBsAg in the setting of immunocompromised conditions [20, 21]. An alternative explanation for the high prevalence of cirrhosis may be related to high-risk behaviors (e.g., intravenous drug use, casual sex, etc.) and related conditions (e.g., HCV, illicit drug-related complications, etc.) in those HBsAg-negative/HBcAb-positive individuals. The coexistence of liver conditions such as chronic hepatitis C or NAFLD would increase the risk of liver fibrosis.
Fig. 2.
Factors may contribute to the cirrhosis risk in HBsAg-negative/HBcAb-positive population
HBsAg-negative/HBcAb-positive patients with coexisting cirrhosis should be urgently sought, to provide appropriate and individualized care. Of note, this condition (HBsAg-negative/HBcAb-positive) may not be a major driver of cirrhosis. Such groups seem to be heterogeneous, and interventions for cirrhosis should be cautious and individualized in these populations.
Given the large population base of HBsAg-negative/HBcAb-positive people (about 10 million in the USA), screening the entire HBsAg-negative/HBcAb-positive population for cirrhosis/advanced fibrosis is unlikely to be cost-effective. “Semi-targeted” approaches based on minority demographic characteristics readily available from electronic health records (such as age, sex, and race/ethnicity) may enhance screening and diagnosis [22]. For this purpose, using multivariate analysis by regression to determine factors associated with cirrhosis among the HBsAg-negative and HBcAb-positive population, we found that gender, age, race, education, comorbidities, income, and HBsAb were significant factors. Screening for cirrhosis/fibrosis seems to focus more on men, older individuals, those with lower education levels, the poor, HBsAb-negative individuals, and those with other comorbidities.
As a common indicator of hepatitis B and immune status screening, we pay special attention to the role of HBsAb. As part of humoral immunity, HBsAb seroconversion means stable control of the virus by the body. Although some studies suggest that HBsAb seroconversion does not affect the prognosis once HBsAg loss is achieved [4, 23], more and more studies have revealed that HBsAb is associated with the durability of nucleoside analog- or pegylated-interferon-induced HBsAg loss [24–26]. Moreover, HBsAb-negative patients seem to be more frequently associated with occult HBV/HBV reactivation [16, 27–29]. The disparity in cirrhosis prevalence between HBsAb-negative and HBsAb-positive populations in our data supports that among HBsAb-negative populations, HBsAg-negative/HBcAb-positive individuals need more attention and rigorous monitoring.
The advantages of this study lie in its large sample size and national representativeness.
This study has several limitations. First, this study lacks data on treatment and regular monitoring, which may affect fibrosis progression. Secondly, large-scale epidemiological studies using liver biopsies are impractical, and we have to rely on some surrogate tests, but APRI and FIB-4 scores can be impacted by variables other than cirrhosis and may fluctuate during follow-up. In addition, occult hepatitis B infection (OBI) refers to a condition where replication-competent HBV DNA is present in the liver, with or without HBV DNA in the blood, in individuals with serum HBsAg negativity assessed by currently available assays [30]. It is generally believed that OBI has a higher prevalence of cirrhosis and may require more rigorous surveillance. Unfortunately, it is difficult to evaluate HBV DNA in the liver in practice in large-scale epidemiological studies, and HBV DNA data both in the liver and blood are not available in NHANES. This limitation makes it difficult to reasonably assess the prevalence of cirrhosis/advanced fibrosis in OBI and non-OBI subjects in our study. Finally, as a result of the low sensitivity of FIB-4 and APRI in the diagnosis of cirrhosis, we may have underestimated the prevalence of cirrhosis to some extent.
Conclusion
In summary, according to FIB-4 results, 3.76% of HBsAg-negative/HBcAb-positive adults had cirrhosis, much higher than the general population in the USA. There are wide variations in the prevalence of cirrhosis by gender, age, race, education, comorbidities, and HBsAb. Our data highlight the importance of cirrhosis/fibrosis screening in the HBsAg-negative/HBcAb-positive population to prevent advanced liver disease, especially in those who are HBsAb-negative.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank all NHANES participants.
Funding
No funding or sponsorship was received for publication of this article. The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Study concept and design: Shuai-Wen Huang, Chen Chen. Acquisition of data: Shuai-Wen Huang, Chen Chen, Hong-Yan Kong, Jia-Quan Huang. Analysis and interpretation of data: Shuai-Wen Huang, Chen Chen, Hong-Yan Kong, Jia-Quan Huang. Drafting of the manuscript: Shuai-Wen Huang, Jia-Quan Huang, Chen Chen, Hong-Yan Kong. Study supervision: Hong-Yan Kong, Jia-Quan Huang.
Disclosures
All authors declare that there is no conflict of interest regarding the publication of this article.
Compliance with Ethics Guidelines
The study was conducted in accordance with the principles of the Helsinki Declaration. The approval of the study from the National Center of Health and Statistics Research ethics review board was waived because the research relied on publicly used, de-identified secondary data.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shuai-Wen Huang, Email: 395044541@qq.com.
Chen Chen, Email: 1154877187@qq.com.
Hong-Yan Kong, Email: 734470068@qq.com.
Jia-Quan Huang, Email: huangjiaquan21@aliyun.com.
References
- 1.European Association for the Study of the Liver EASL Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;2017(67):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vittal A, Sharma D, Hu A, et al. Systematic review with meta-analysis: the impact of functional cure on clinical outcomes in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2022;55:8–25. doi: 10.1111/apt.16659. [DOI] [PubMed] [Google Scholar]
- 4.Freeland C, Racho R, Kamischke M, et al. Cure everyone and vaccinate the rest: the patient perspective on future hepatitis B treatment. J Viral Hepat. 2021;28(11):1539–1544. doi: 10.1111/jvh.13592. [DOI] [PubMed] [Google Scholar]
- 5.Simonetti J, Bulkow L, McMahon BJ, et al. Clearance of hepatitis B surface antigen and risk of hepatocellular carcinoma in a cohort chronically infected with hepatitis B virus. Hepatology. 2010;51:1531–1537. doi: 10.1002/hep.23464. [DOI] [PubMed] [Google Scholar]
- 6.Morgan TR, Redeker AG, Yamada S, et al. HBsAg clearance in chronic active hepatitis B. A possible cause of cryptogenic cirrhosis. Dig Dis Sci. 1986;31:700–704. doi: 10.1007/BF01296446. [DOI] [PubMed] [Google Scholar]
- 7.Tong MJ, Nguyen MO, Tong LT, et al. Development of hepatocellular carcinoma after seroclearance of hepatitis B surface antigen. Clin Gastroenterol Hepatol. 2009;7:889–893. doi: 10.1016/j.cgh.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Peng J-W, Lin G-N, Xiao J-J, et al. Hepatitis B virus reactivation in hepatocellular carcinoma patients undergoing transcatheter arterial chemoembolization therapy. Asia-Pacific J Clin Oncol. 2012;8:356–361. doi: 10.1111/j.1743-7563.2012.01534.x. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. About the National Health and Nutrition Examination Survey. 2022. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
- 10.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 11.Xiao G, Yang J, Yan L. Comparison of diagnostic accuracy of aspartate aminotransferase to Platelet Ratio Index and Fibrosis-4 Index for detecting liver fibrosis in adult patients with chronic hepatitis B virus infection: a systemic review and meta-analysis. Hepatology. 2015;61:292–302. doi: 10.1002/hep.27382. [DOI] [PubMed] [Google Scholar]
- 12.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 13.Chan HL, Wong GL, Choi PC, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepat. 2009;16(1):36–44. doi: 10.1111/j.1365-2893.2008.01037.x. [DOI] [PubMed] [Google Scholar]
- 14.Sasso M, Miette V, Sandrin L, Beaugrand M. The controlled attenuation parameter (CAP): a novel tool for the non-invasive evaluation of steatosis using Fibroscan. Clin Res Hepatol Gastroenterol. 2012;36:13–20. doi: 10.1016/j.clinre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Scaglione S, Kliethermes S, Cao GC, et al. The epidemiology of cirrhosis in the United States a population-based study. J Clin Gastroenterol. 2015;49:690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 16.Chan TT, Chan WK, Wong GL, et al. Positive hepatitis B core antibody is associated with cirrhosis and hepatocellular carcinoma in nonalcoholic fatty liver disease. Am J Gastroenterol. 2020;115(6):867–875. doi: 10.14309/ajg.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 17.Raimondo G, Pollicino T, Cacciola I, et al. Occult hepatitis B virus infection. J Hepatol. 2007;46:160–170. doi: 10.1016/j.jhep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Ji DZ, Pang XY, Shen DT, et al. Global prevalence of occult hepatitis B: a systematic review and meta-analysis. J Viral Hepat. 2022 doi: 10.1111/jvh.13660. [DOI] [PubMed] [Google Scholar]
- 19.Mak L-Y, Wong DK-H, Pollicino T, et al. Occult hepatitis B infection and hepatocellular carcinoma: epidemiology, virology, hepatocarcinogenesis and clinical significance. J Hepatol. 2020;73:952–964. doi: 10.1016/j.jhep.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 20.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamitsukasa H, Iri M, Tanaka A, Nagashima S, et al. Spontaneous reactivation of hepatitis B virus (HBV) infection in patients with resolved or occult HBV infection. J Med Virol. 2015;87:589–600. doi: 10.1002/jmv.24115. [DOI] [PubMed] [Google Scholar]
- 22.Ramrakhiani NS, Chen VL, Le M, et al. Optimizing hepatitis B virus screening in the United States using a simple demographics-based model. Hepatology. 2022;75:430–437. doi: 10.1002/hep.32142. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Yang HI, Lee MH, et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut. 2014;63:1648–1657. doi: 10.1136/gutjnl-2013-305785. [DOI] [PubMed] [Google Scholar]
- 24.Kim GA, Lim YS, An J, et al. HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut. 2014;63:1325–1332. doi: 10.1136/gutjnl-2013-305517. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, Wu D, Wang P, et al. End-of-treatment HBcrAg and HBsAb levels identify durable functional cure after Peg-IFN-based therapy in patients with CHB. J Hepatol. 2022;77(1):42–54. [DOI] [PubMed]
- 26.Wu YL, Liu YL, Lu JF, et al. Durability of interferon-induced hepatitis B surface antigen seroclearance. Clin Gastroenterol Hepatol. 2020;18:514–516. doi: 10.1016/j.cgh.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Wong GLH, Wong VWS, Yuen BWY, et al. Risk of hepatitis B surface antigen seroreversion after corticosteroid treatment in patients with previous hepatitis B virus exposure. J Hepatol. 2020;72:57–66. doi: 10.1016/j.jhep.2019.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Poola S, Sanaka S, Sewell K, et al. Hepatitis B surface antibody titres and hepatitis B reactivation with direct-acting antiviral therapy for hepatitis C. J Viral Hepat. 2021;28(2):373–382. doi: 10.1111/jvh.13421. [DOI] [PubMed] [Google Scholar]
- 29.Nishida T, Matsubara T, Yakushijin T, et al. Prediction and clinical implications of HBV reactivation in lymphoma patients with resolved HBV infection: focus on anti-HBs and anti-HBc antibody titers. Hep Intl. 2019;13:407–415. doi: 10.1007/s12072-019-09966-z. [DOI] [PubMed] [Google Scholar]
- 30.Raimondo G, Locarnini S, Pollicino T, et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J Hepatol. 2019;71(2):397–408. doi: 10.1016/j.jhep.2019.03.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.