Abstract
Introduction
Early onset sepsis (EOS) remains a potentially fatal newborn condition, especially in very preterm infants. Data on the pathogen distribution and antibiotic susceptibility patterns of EOS among very preterm infants are scarce but essential for the choice of empirical antibiotic administration. We sought to assess the epidemiologic characteristics and antibiotic susceptibility patterns of pathogens causing EOS among a cohort of very preterm infants in China.
Methods
This prospective, observational study included a cohort of infants born at a gestational age (GA) less than 32 weeks of 32 newborn intensive care units (NICUs) in China between January 1, 2018 and December 31, 2020. EOS was defined by isolation of pathogenic species from blood culture within 72 h of birth.
Results
A total of 108 EOS cases (18.4 per 1000 admissions) were identified among 5865 very preterm infants. Incidence of EOS increased with the decrease of GA and birthweight. Escherichia coli (n = 44, 40.7%) was the most common pathogen, followed by Klebsiella spp. (n = 10, 9.3%). The distribution and proportion of pathogenic bacteria varied significantly by GA. E. coli and Klebsiella spp. showed high resistance to ampicillin and third-generation cephalosporins, while they showed good susceptibility to carbapenem antibiotics and piperacillin–tazobactam.
Conclusion
Our data demonstrated that pathogens causing neonatal EOS showed high rates of resistance to ampicillin and third-generation cephalosporins. This raised questions about the best empirical antibiotic choice for preterm infants suspected of having EOS in low- and middle-income countries (LMICs).
Keywords: Infants, Premature, Early-onset neonatal sepsis, Pathogen, Antimicrobial resistance
Key Summary Points
| Very preterm infants are at much greater risk for early-onset sepsis (EOS) than more mature infants. |
| E. coli and Klebsiella spp. were the predominant organisms, and both showed high resistance rates to ampicillin and third-generation cephalosporins. |
| Empiric treatment should be based on regional EOS pathogen distribution and resistance rates. |
| This study highlights the need for improved EOS treatment guidance in LMICs. |
Introduction
Neonatal sepsis is a major cause of morbidity and mortality and is a global health concern, especially in very preterm infant [1, 2]. Neonatal sepsis is a systemic infection and can be classified into two subgroups, early onset sepsis (EOS) and late-onset sepsis (LOS). EOS is most consistently defined as an infection occurring within 72 h after birth caused by bacterial pathogens transmitted vertically from mother to infant before or during delivery [3, 4]. Very preterm infants (gestational age less than 32 weeks) are at high risk of developing EOS, because of their compromised immunity. Survivors of EOS are at increased risk for adverse neurodevelopmental outcomes including cerebral palsy, hearing loss, visual impairment, and cognitive delays [5]. The high incidence and the alarming complications of EOS underscore the need to understand the epidemiological characteristics of EOS in very preterm infants [6].
Accurately diagnosing EOS among very premature infants is challenging because of non-specific clinical symptoms and the difficulty of evaluating infection markers for EOS in the early stage. Antibiotics are initiated in the majority of very preterm infants and frequently continued despite sterile blood culture [7–10]. The selection of empirical antibiotics varies among countries and regions. Ampicillin in combination with gentamicin is recommended for the management of clinical neonatal sepsis by the World Health Organization (WHO) [11]. However, the Burden of Antibiotic Resistance in Neonates from Developing Societies (BARNARDS) study indicated that in low-income and middle-income countries (LMICs) the use of ampicillin–gentamicin as empirical antibiotics for neonatal sepsis should be reviewed, and potentially replaced with ceftazidime–amikacin [12]. The selection of empirical antibiotics should be based on the common pathogens and related drug susceptibility in each region. The high incidence of sepsis and alarming degree of antimicrobial resistance among pathogens in EOS in neonates underscore the necessity to understand the pathogenesis of early-onset sepsis and to choose the optimum empirical antibiotics.
It is therefore essential to monitor the epidemiology of neonatal EOS regionally to guide the selection of empirical antibiotics. There is a paucity of contemporary, neonatal-specific pathogen distribution and antibiotic susceptibility data for EOS in the northeast of China. The present study aimed to report data for incidence, profile of organisms, and antimicrobial resistance from a prospective cohort study involving 32 newborn intensive care units (NICUs) in China.
Methods
Study Population
This is a prospective, multicenter, observational cohort study conducted from January 1, 2018 to December 31, 2020. Thirty-two NICUs participated in this study. All infants born at less than 32 weeks of gestation and admitted to NICUs participated in this study within 3 days after birth were included in this study. All infants were followed until death or discharge from the participating NICUs. The studies involving human participants were reviewed and approved by Ethics Committee of Shandong Provincial Hospital affiliated to Shandong First Medical University and Shandong University (LCYJ: NO.2019-132). The ethics committees of all 32 participating hospitals approved the study and allowed data sharing. This study was conducted in accordance with the Declaration of Helsinki.
Date Collection and Quality Management
Detailed maternal intrapartum and newborn information were abstracted from the Sino-northern Neonatal Network (SNN) database. SNN is a large, comprehensive administrative database of preterm inpatients with birthweight (BW) less than 1500 g or gestational age (GA) less than 32 weeks from tertiary hospitals in northern China. Maternal and neonatal information were prospectively recorded into the database by a local neonatologist of 32 NICUs. The aforementioned data sets were collected and transmitted by trained research staff utilizing a standardized manual of operations from each site to the coordinating center in Jinan, Shandong Province, China, and was audited by senior physicians. Maternal data included mother’s age, delivery type, use of antenatal steroid, use of intrapartum antibiotics, rupture of the membranes (ROM) (18 h or more) before delivery. Neonate data included gestational age, birthweight, sex, multiple birth (twin and above), small for gestational age, 1-min and 5-min Apgar score, and fetal tachycardia.
Study Definitions
EOS was defined by the presence of clinical symptoms and a positive culture from blood obtained within 72 h of birth. Gestational age was determined by obstetric estimates. Neonatal necrotizing enterocolitis (NEC) was defined as stage 2 or higher according to the system of Bell et al. [13]. Bronchopulmonary dysplasia (BPD) was defined as mechanical ventilation or oxygen dependency at 36 weeks of postmenstrual age or discharge [14]. Intraventricular hemorrhage (IVH) was defined as stage 3 or higher according to Papile criteria [15]. Retinopathy of prematurity (ROP) was defined as stage3 or higher according to the International Classification of ROP [16]. Small for gestational age (SGA) was defined as birthweight below the 10th percentile for gestational age on a Fenton growth chart. Extrauterine growth retardation (EUGR) was defined according to the 2013 Fenton growth curve, the weight of neonates with gestational age of 36 weeks at discharge or corrected was lower than the 10th percentile of the corresponding gestational age [17].
The definition of contaminant was based on the following three criteria and was similar to in our previous study [18]: (1) isolates which were usually considered as contaminants; (2) coagulase-negative staphylococci (CoNS), when the blood samples were collected there was no peripheral or central catheter; (3) cultures that grew more than one organism.
Multidrug-resistant (MDR) bacteria are defined as non-susceptible to at least one agent in three or more antimicrobial categories [19].
EOS incidence was expressed as the rate of EOS per 1000 infants admitted to the participating NICUs. All-cause mortality was defined as a proportion of neonates deceased among admitted neonates.
Identification and Antimicrobial Susceptibilities Testing
Blood cultures were performed from infants presenting with clinical signs of infection according to clinicians. At least 0.5 ml of blood sample were collected and delivered to the microbiology laboratory within 2 h of collection. Each microbiology laboratory performed organism identification and antimicrobial susceptibility testing (AST). Antimicrobial susceptibilities were classified as susceptible or resistant (intermediate or resistant) based on microbiology reports. Resistance rate was calculated as the number of resistant pathogens/number of pathogens tested.
Statistical Analysis
Descriptive analysis was used to characterize the study population and the pathogen distribution. Numerical data are presented as mean and standard deviation (SD) or median and interquartile range (IQR) as appropriate and compared using the Student’s t test. Categorical data are presented as percentage and analyzed by χ2. P value of less than 0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 25.0 (SPSS Inc, Chicago, IL).
Results
Maternal and Neonatal Characteristics
A total of 5865 infants at a GA less than 32 weeks were admitted during the study. The clinical characteristics including maternal and neonatal information are shown in Table 1. Infants with EOS intended to have a lower GA, birthweight, and Apgar scores for 1 min and 5 min than did infants without EOS. Mothers of EOS infants were more likely to have a maternal history of rupture of membranes at least 18 h before delivery. The rate of antenatal steroid use in the EOS group was 85.1%, which was higher than in infants without EOS. Infants with EOS showed significantly higher rates of all-cause mortality and major morbidities, including BPD, IVH, and EUGR.
Table 1.
Maternal and neonatal characteristics and outcomes of preterm infants enrolled in this study
| Variables | EOS n = 108 |
Non-EOS n = 5757 |
P value |
|---|---|---|---|
| Neonatal characteristics | |||
| Birthweight, g (median, IQR) | 1300 (1100, 1540) | 1370 (1170, 1580) | 0.029 |
| GA, week (median, IQR) | 29 (28, 30) | 30 (29, 31) | 0.001 |
| Apgar 1 min score, mean (SD) | 6.59 (2.48) | 7.15 (2.30) | 0.001 |
| Apgar 5 min score, mean (SD) | 7.81 (1.88) | 8.15 (1.81) | 0.009 |
| Female, n (%) | 50 (46.3) | 2261 (51.4) | 0.164 |
| SGA, n (%) | 5 (4.6) | 518 (9.0) | 0.126 |
| Fetal tachycardia, n (%) | 4 (3.7) | 356 (6.2) | 0.415 |
| Multiple birth, n (%) | 29 (26.9) | 1358 (23.6) | 0.425 |
| Maternal characteristics | |||
| Mother’s age, mean (SD) | 32 (4.46) | 31 (8.01) | 0.494 |
| Caesarean section, n (%) | 71 (65.6) | 3995 (69.4) | 0.402 |
| Antenatal steroid, n (%) | 92 (85.1) | 4024 (69.9) | 0.000 |
| Antepartum antibiotic, n (%) | 48 (44.4) | 2245 (39.0) | 0.274 |
| Rupture of membrane ≥ 18 h, n (%) | 26 (24.0) | 720 (12.5) | 0.001 |
| Outcome of infants | |||
| Mortality, n (%) | 18 (16.7) | 524 (9.1) | 0.011 |
| EUGR, n (%) | 48 (44.4) | 1877 (32.6) | 0.013 |
| BPD, n (%) | 24 (22.2) | 524 (9.1) | 0.000 |
| IVH, n (%) | 6 (5.5) | 86 (1.5) | 0.007 |
| NEC, n (%) | 1 (0.9) | 86 (1.5) | 1.000 |
| ROP, n (%) | 3 (2.8) | 391 (6.8) | 0.119 |
GA gestational age, SGA small for gestational age, EUGR extrauterine growth retardation, BPD bronchopulmonary dysplasia, IVH intraventricular hemorrhage, NEC necrotizing enterocolitis, ROP retinopathy of prematurity
Incidence of EOS
Excluding 28 contaminants, 108 cases of EOS were identified, yielding an incidence of 18.4 cases per 1000 admissions. The incidence rate of EOS increased significantly with decreasing of GA or birthweight (Table 2). The incidence of EOS was highest among infants with a GA of 23–28 weeks (35.8%) and lowest among those with GA of 31–32 weeks (13.5%). The incidence of EOS also increased with the decreasing of birthweight, from 16.3% in the infants with birthweight greater than 1500 g to 23.3% in neonates with birthweight less than 1000 g. The incidence of EOS caused by E. coli also significantly increased with the decreasing of GA, while the trend was not distinct with the decreasing of birthweight. EOS caused by GBS was highest in the neonates with GA of 31–32 weeks and infants with birthweight more than 1500 g. Incidence of EOS caused by Klebsiella spp. showed no significant difference by GA and birthweight in preterm infants.
Table 2.
Incidence rate per 1000 admissions of EOS among preterm infants in China
| ALL infants (n = 5865) |
All EOS (n = 108) |
EOS caused by E. coli (n = 44) |
EOS caused by GBS (n = 8) |
EOS caused by Klebsiella (n = 10) |
|
|---|---|---|---|---|---|
| By GA, weeks | |||||
| 23+0–28+0, n (‰) | 838 | 30 (35.80) | 16 (19.09) | 1 (1.19) | 1 (1.19) |
| 28+1–30+0 | 1841 | 35 (19.01) | 15 (8.15) | 2 (1.09) | 5 (2.71) |
| 30+1–32+0 | 3186 | 43 (13.50) | 13 (4.08) | 5 (1.57) | 4 (1.25) |
| By birthweight (g) | |||||
| < 1000 | 861 | 20 (23.23) | 5 (5.81) | 1 (1.16) | 1 (1.16) |
| 1000–1500 | 3286 | 60 (18.26) | 30 (9.13) | 2 (0.61) | 6 (1.83) |
| > 1500 | 1718 | 28 (16.30) | 9 (5.24) | 5 (2.91) | 3 (1.75) |
Pathogen Distribution of EOS
The distribution of pathogens causing EOS is shown in Table 3. The majority of bacterial isolates were Gram-negative pathogens, which accounted for 63.9% of all the pathogens. Gram-positive organisms were identified in 38 of 108 infants. One case had infection with fungal organisms.
Table 3.
Pathogen distribution of EOS of preterm infants with GA less than 32 weeks
| Organism | No. (%) |
|---|---|
| Gram-positive | 38 (35.2) |
| Group B streptococci (GBS) | 8 (7.4) |
| Enterococcus spp. | 7 (6.5) |
| Coagulase-negative staphylococci | 7 (6.5) |
| Listeria monocytogenes | 6 (5.6) |
| Staphylococcus aureus | 5 (4.6) |
| Viridans streptococci | 3 (2.8) |
| Other Gram-positive cocci | 2 (1.9) |
| Gram-negative | 69 (63.9) |
| Escherichia coli | 44 (40.7) |
| Klebsiella spp. | 10 (9.3) |
| Enterobacter spp. | 7 (6.5) |
| Serratia marcescens | 5 (4.6) |
| Acinetobacter spp. | 1 (0.9) |
| Other Gram-negative bacilli | 2 (1.9) |
| Fungi | |
| Candida albicans | 1 (0.9) |
| Total | 108 |
E. coli was the leading cause of EOS in infants with GA less than 32 weeks, accounting for 40.7% (44/108). Klebsiella spp. accounted for 9.3% of EOS cases (10/108). Other Gram-negative bacteria included Enterobacter spp. (n = 7, 6.5%) and Serratia marcescens (n = 5, 4.6%). EOS caused by fungi (1, 0.9%) was relatively rare.
In the group of Gram-positive infections, group B streptococci (GBS) were responsible for 7.4% (8/108) of pathogens in preterm infants with EOS. The following two Gram-positive pathogens were Enterococcus spp. (n = 7, 6.5%) and coagulase-negative staphylococci (n = 7, 6.5%).
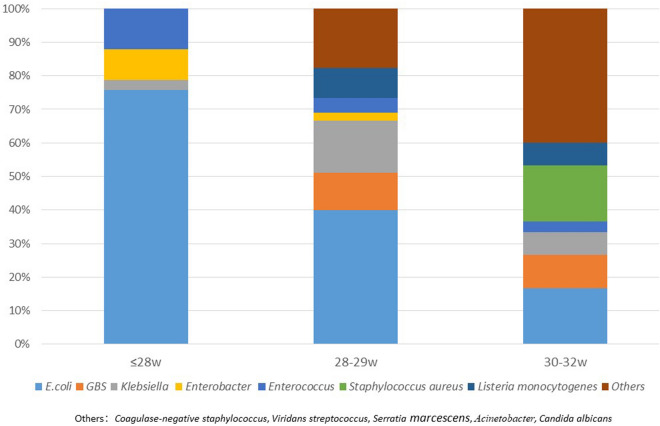
Figure 1 presents the pathogen distribution according to GA. The types of pathogens causing EOS increased with increasing GA. E. coli infection accounted for 75.6% in the group of infants with GA ≤ 28 weeks, while in infants with GA > 30 weeks, the percentage reduced to 16.7%. GBS infection was not found in infants with GA ≤ 28 weeks and the proportion increased with increasing GA.
Fig. 1.
Pathogen distribution and proportion in different GA groups of preterm infants with GA less than 32 weeks
Antibiotic Resistance Pattern
The antibiotic resistance of E. coli and Klebsiella species to different antibiotics is shown in Table 4. Within the β-lactam antibiotics, E. coli demonstrated maximum susceptibility to meropenem and imipenem and piperacillin-tazobactam while showing high resistance to ampicillin, ceftriaxone, cefotaxime. Klebsiella species showed high resistance to ampicillin, cefotaxime, and ceftriaxone, while they showed high susceptibility to imipenem, meropenem, and piperacillin–tazobactam. Among non-β-lactam antibiotics, E. coli showed high resistance to ciprofloxacin and ofloxacin, and high susceptibility to amikacin. All the Klebsiella isolates were susceptible to aminoglycosides. Twenty of 33 (60.6%) E. coli isolates were mutidrug-resistant bacteria according to our predefined definition; 42.9% of Klebsiella spp. were multidrug resistant.
Table 4.
Antimicrobial resistance patterns from the main Gram-negative bacteria of neonates with sepsis, 2018–2020
| Antibiotics |
E. coli n = 44 |
% (95% CI) |
Klebsiella n = 10 |
% (95% CI) |
|---|---|---|---|---|
| Piperacillin–tazobactam | 2/40 | 5.0 (1.4–16.5) | 3/10 | 30.0 (10.8–60.3) |
| Cefotaxime | 22/44 | 50.0 (35.8–64.2) | 6/8 | 75.0 (40.9–92.8) |
| Ceftriaxone | 29/40 | 72.5 (57.1–83.9) | 8/10 | 80.0 (49.0–94.3) |
| Ceftazidime | 13/40 | 32.5 (20.1–47.9) | 4/10 | 40.0 (16.8–68.7) |
| Imipenem | 0/39 | 0 (0–8.9) | 1/10 | 10.0 (1.8–40.4) |
| Meropenem | 0/37 | 0 (0–9.4) | 1/10 | 10.0 (1.8–40.4) |
| Ertapenem | 0/32 | 0 (0–10.7) | 0/10 | 0 (0–27.8) |
| Ampicillin | 35/44 | 79.5 (65.5–88.5) | 9/10 | 90.0 (59.6–98.2) |
| Aztreonam | 15/35 | 42.9 (27.9–59.1) | 3/10 | 30.0 (10.8–60.3) |
| Amikacin | 4/44 | 9.1 (3.6–21.2) | 0/10 | 0 (0–27.8) |
| Gentamicin | 15/44 | 34.1 (21.9–48.9) | 4/10 | 40.0 (16.8–68.7) |
| Tobramycin | 22/44 | 50.0 (34.6–65.4) | 4/8 | 50.0 (21.5–78.5) |
| Ciprofloxacin | 23/44 | 52.3 (36.9–67.6) | 0/10 | 0 (0–27.8) |
| Ofloxacin | 17/40 | 42.5 (26.5–58.5) | 0/10 | 0 (0–27.8) |
The antibiotic resistance of GBS and Enterococcus spp. are listed in Table 5. GBS demonstrated maximum susceptibility to linezolid, vancomycin, and amikacin, while showing high resistance to erythromycin and ofloxacin. Enterococcus spp., they showed 100% resistance to erythromycin, 71.4% resistance to ciprofloxacin, 57.1% resistance to ofloxacin, and 100% susceptibility to linezolid and vancomycin. All six Listeria isolates were susceptible to ampicillin, erythromycin, linezolid, and vancomycin.
Table 5.
Selected antimicrobial resistance patterns from the main Gram-positive bacteria of neonates with sepsis, 2018–2020
| Antibiotics | GBS n = 8 |
% (95% CI) |
Enterococcus n = 7 |
% (95% CI) |
|---|---|---|---|---|
| Cefotaxime | 5/8 | 62.5 (30.5–86.3) | – | – |
| Ceftriaxone | 6/8 | 75.0 (40.9–92.85) | – | – |
| Ampicillin | 1/8 | 12.5 (2.2–47.1) | 3/7 (42.9) | 42.9 (15.8–79.8) |
| Amikacin | 0/8 | 0 (0–32.4) | – | |
| Erythromycin | 6/8 | 75.0 (40.1–92.9) | 7/7 | 100 (64.6–100) |
| Gentamicin | – | – | 0/8 | 0 (0–32.4) |
| Ciprofloxacin | 6/8 | 75.0 (40.1–92.9) | 5/7 | 71.4 (35.9–91.8) |
| Ofloxacin | 4/8 | 50.0 (21.5–78.5) | 4/7 | 57.1 (25.0–84.2) |
| Linezolid | 0/8 | 0 (0–32.4) | 0/7 | 0 (0–35.4) |
| Vancomycin | 0/8 | 0 (0–32.4) | 0/7 | 0 (0–35.4) |
Discussion
In this real-world study, we record a high incidence of EOS (18.4 per 1000 admissions) among infants with GA less than 32 weeks in 32 NICUs in northern China. The incidence rate of EOS increased with the decreasing of GA and birthweight. E. coli and Klebsiella spp. emerged as the predominant causative organisms. Most pathogens causing EOS showed an alarming degree of antimicrobial resistance, especially to ampicillin and third-generation cephalosporins. The results of this study have important implications for clinicians who must choose empirical antibiotics for very preterm infants.
Incidence rates of EOS vary between countries. The NeonIN study from 30 neonatal units in the UK reported an EOS incidence of 5.6 per 1000 neonate admissions [20]. The rate in Canada was 6.2–6.8/1000 admissions [21]. The rate of EOS reported in Korea among preterm infants less than 32 weeks was 4.1% [22]. A higher EOS rate was reported in a large study among preterm infants conducted in China with 14 cases per 1000 admissions of infants less than 33 weeks’ gestation [23]. The current incidence of EOS among infants born at less than 32 weeks of gestation in this prospective study was 18.4 per 1000 admissions. It is worth noting that for this study, the cohort of infants (all preterm infants less than 32 weeks) may be a contributing factor to a higher reported EOS incidence. We have demonstrated that the incidence of EOS was inversely related to GA, which was consistent with previous studies [24, 25].
The pathogen distribution of EOS informs clinicians choice of empirical antibiotics therapy. The pathogen distribution of EOS among different countries was significantly different. The most common pathogens that cause neonatal EOS in four US states from 2005 to 2014 were GBS (36%) and E. coli (25%) [26]. In particular, current reports have shown that there was a marked reduction in EOS caused by GBS, but an increase in E. coli neonatal sepsis [25, 27]. In our previous study, the most frequent pathogens causing EOS were E. coli and GBS in all neonates admitted into 25 NICUs in our multicenter study [18]. While in this study, for infants with GA less than 32 weeks, the predominant organisms were E. coli and Klebsiella spp. Repots have shown that in most LMICs, Klebsiella spp. and E. coli are the most frequent causative organisms [28, 29].
Klebsiella spp., as Gram-negative bacteria, are traditionally considered opportunistic or nosocomial pathogens [30]. In this study, Klebsiella spp. caused 9.3% EOS in very preterm infants. This could possibly be due to very early horizontal transmission in the delivery room or vertical transmission from maternal genital tract colonized with these pathogens after unhygienic personal and obstetric practice [31, 32]. Further investigations are warranted to identify these infections caused by Gram-negative bacteria and subsequently develop targeted prevention strategies. In addition, clinicians should consider empirically treating neonates suspected of EOS empirically with antibiotics predicted to be effective against Klebsiella or Gram-negative pathogens.
E. coli (40.7%) emerged as the most common isolate in our cohort, which was consistent with findings from LMICs [28, 33]. GBS were isolated rarely in this study. Findings from a few studies from other LMICs also showed similar results [28, 34]. The probably reason may due to limitation in detection methodology, and GBS screening was not widespread in these LMICs [35].
Emergence of antimicrobial resistance has become a global concern. Both the inappropriate overuse of broad-spectrum antibiotics and the inadequacy of antibiotics stewardship led to the increased rate of antimicrobial resistance. Ampicillin resistance among E. coli EOS isolates now exceeds 70% [36]. The Centers for Disease Control and Prevention (CDC) and National Institute of Child Health and Human Development (NICHD) studies found 8–10% of EOS E. coli isolates to be resistant to both ampicillin and gentamicin [26, 37], a combination frequently used empirically for EOS. A single-center study about the changes in antimicrobial resistance of E. coli in eastern China has shown that E. coli isolates from preterm infants had a significantly higher rate of resistance to ampicillin, and the overall resistance of E. coli to third-generation cephalosporins increased from 14.3% to 46.7% in a decade [38]. In particular, E. coli strains causing EOS exhibit a higher resistance to ampicillin, gentamicin, and third-generation cephalosporins in preterm infants than in term infants [29, 38]. In this study, in the infants with GA less than 32 weeks, the resistance rate of E. coli to ampicillin was 81.8%, similar to the rate in developed countries. Resistance rates of E. coli to third-generation cephalosporins ranged from 32.5% to 72.5%. E. coli showed good susceptibility to piperacillin–tazobactam. Klebsiella spp., which were the second most common cause of EOS in very preterm infants, also showed high resistance to ampicillin and third-generation cephalosporins. Resistance in E. coli and Klebsiella spp. is usually acquired via plasmid-mediated extended-spectrum beta-lactamase (ESBL) production [39]. EOS caused by ESBL-producing multidrug-resistant pathogen are resistant to beta-lactams, including third-generation cephalosporins [40]. The very high rates of ESBL production led to a substantial use of carbapenem antibiotics, resulting in the emergence of resistance to carbapenems [41]. Data obtained from China showed that the rate of carbapenem resistance in clinical E. coli and Klebsiella strains was around 0.6–3.6% and 1.2–18.9%, respectively [42]. The development of rapid diagnostic tests to identify pathogens and their antimicrobial drug susceptibility may therefore prevent unnecessary use of broad-spectrum antimicrobial drugs.
The strength of this study was that it was a real-world study. Detailed maternal and newborn information were prospectively collected from multiple centers covering 32 NICUs in China. This study explored pathogen distribution and the antibiotic resistance pattern in infants with GA less than 32 weeks. Our data raise questions about the empirical use of ampicillin and third-generation cephalosporins for preterm infants with EOS in LMICs. It is of great significance for clinicians who must choose effective antibiotics for neonates suspected of having EOS while awaiting blood culture results to reduce the abuse of antibiotics.
There were some limitations of this study. EOS was defined by positive cultures. Because of the widespread use of intrapartum antibiotics, some infants with true, but culture-negative infection would not have been identified in this study. This was a limitation of all studies that base EOS rates on newborn blood culture results. Another limitation is that the study does not look at the regional differences in incidence and resistance patterns between regions in China.
Conclusion
E. coli and Klebsiella spp. were the most common pathogens of EOS of infants with GA less than 32 weeks in China. The incidence of EOS increased with the decreasing of GA or birthweight. E. coli and Klebsiella spp. showed high resistance to ampicillin and third-generation cephalosporins, which were commonly used for EOS in preterm infants. Our data raise the question about the use of empirical antibiotics for preterm infants with EOS in LMICs. It was suggested that for preterm infants with EOS, empirical antibiotics should be selected according to the local pathogen distribution and antibiotic sensitivity data.
Acknowledgements
We sincerely appreciate all the clinical medical experts, epidemiological experts, statistics experts, and study participants of the Sino-northern neonatal network (SNN) and the CARE-Preterm cohort study group for their contributions to data collection and quality control, research design and data analysis.
Funding
The research was funded by 2021 Shandong Medical Association Clinical Research Fund - Qilu Special Project (YXH2022DZX0200X). The journal’s Rapid Service Fee was funded by the authors.
Author Contributions
Yonghui Yu designed the study and revised the manuscript content. Other authors collected and submitted the data into Shandong Neonatal Network database. Hongyan Ji collected and analyzed the data, and drafted and revised the manuscript. All authors contributed to the article and approved the submitted version.
Disclosures
Hongyan Ji, Yonghui Yu, Lei Huang, Yan Kou, Xin Liu, Shina Li, Yongfeng Zhang, Zhongliang Li, Xuemei Sun, Jing Wang, Kun Yang, Liying Zhou, Yao Luo, Guoying, Zhao, Zhenying Yang, Xiao Zhang, Xiujie Cui, Jing Li, Ying Wang, Jing Shi, Weibing Chen, Yanying Ma, Peng Zhao, Riming Zhao, Kun Zhou, Binghui Li, Renxia Zhu, Yanling Gao, Zhiyuan Zhou, Huan Li, Jinlan Dou, Haiyan Li, Changliang Zhao, Bingjin Zhang, Xiaokang Wang have nothing to disclose.
Compliance with Ethics Guidelines
The Institutional Review Board of Shandong Provincial Hospital Affiliated to Shandong First Medical University approved this project (LCYJ: NO.2019-132). The independent ethics committee of each participating hospital approved this study (Shandong Provincial Maternity and Child Health Care Hospital, The First Affiliated Hospital of Shandong First Medical University, Jinan Maternity and Child Health Care Hospital, Yantai Yuhuangding Hospital, Affiliated Hospital of Weifang Medical University, WeiFang Maternal and Child Health Hospital, Linyi People's Hospital, Women and Children’s Healthcare Hospital of Linyi, Taian City Central Hospital, Liaocheng People's Hospital, Binzhou Medical University Hospital, Taian Maternal and Child health Care Hospital, Dongying People’s Hospital, Dongying, The Second Affiliated Hospital of Shandong First Medical University, Jinan Central Hospital, The Second People’s Hospital of Liaocheng, Liaocheng, People's Hospital of Rizhao, Jinan Second Maternal and Child Health Care Hospital, Central People’s Hospital of Tengzhou, Juxian Peoples Hospital, Liaocheng Dongchangfu Maternal and Child Health Hospital, Zibo Maternal and Child Health Hospital, Dezhou Peoples Hospital, Heze Municipal Hospital, Hebei Petro China Central Hospital, Weifang Yidu Central Hospital, Yantai Yantaishan Hospital, Baogang Third Hospital of Hongci Group, Shengli Oilfield Central Hospital). All authors have signed written informed consent and approved the submission of this version of the manuscript and take full responsibility for the manuscript. The legal guardian of all participants signed an informed consent form that their data could be used for various clinical studies.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the sustainable development goals. The Lancet. 2016;388(10063):3027–3035. doi: 10.1016/S0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wynn JL, Wong HR, Shanley TP, Bizzarro MJ, Saiman L, Polin RA. Time for a neonatal-specific consensus definition for sepsis. Pediatr Crit Care Med. 2014;15(6):523–528. doi: 10.1097/PCC.0000000000000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polin RA, Committee on F, Newborn. Management of neonates with suspected or proven early-onset bacterial sepsis. Pediatrics. 2012;129(5):1006–15. [DOI] [PubMed]
- 5.Klinger G, Levy I, Sirota L, et al. Outcome of early-onset sepsis in a national cohort of very low birth weight infants. Pediatrics. 2010;125(4):e736–e740. doi: 10.1542/peds.2009-2017. [DOI] [PubMed] [Google Scholar]
- 6.Milton R, Gillespie D, Dyer C, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health. 2022;10(5):e661–e672. doi: 10.1016/S2214-109X(22)00043-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135(5):826–833. doi: 10.1542/peds.2014-3409. [DOI] [PubMed] [Google Scholar]
- 8.Karen M, Puopolo M, Sagori M. Identification of extremely premature infants at low risk for early-onset sepsis. Pediatrics. 2017;140:5. doi: 10.1542/peds.2017-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg RG, Chowdhury D, Hansen NI, et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res. 2019;85(7):994–1000. doi: 10.1038/s41390-019-0300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flannery DD, Ross RK, Mukhopadhyay S, Tribble AC, Puopolo KM, Gerber JS. Temporal trends and center variation in early antibiotic use among premature infants. JAMA Netw Open. 2018;1(1):e180164. doi: 10.1001/jamanetworkopen.2018.0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Guidelines Approved by the Guidelines Review Committee. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. Geneva: World Health Organization; 2013. [PubMed]
- 12.Thomson KM, Dyer C, Liu F, et al. Effects of antibiotic resistance, drug target attainment, bacterial pathogenicity and virulence, and antibiotic access and affordability on outcomes in neonatal sepsis: an international microbiology and drug evaluation prospective substudy (BARNARDS) Lancet Infect Dis. 2021;21(12):1677–1688. doi: 10.1016/S1473-3099(21)00050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis. Therapeutic decisions based upon clinical staging. Ann Surg. 1978;187(1):1–7. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 15.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 16.International Committee for the Classification of Retinopathy of Prematurity. The international classification of retinopathy of prematurity revisited. Arch Ophthalmol. 2005;123(7):991–9. [DOI] [PubMed]
- 17.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111(5 Pt 1):986–990. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Fang Z, Yu Y, et al. Pathogens distribution and antimicrobial resistance in bloodstream infections in twenty-five neonatal intensive care units in China, 2017–2019. Antimicrob Resist Infect Control. 2021;10(1):121. doi: 10.1186/s13756-021-00989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 20.Cailes B, Kortsalioudaki C, Buttery J, et al. Epidemiology of UK neonatal infections: the neonIN infection surveillance network. Arch Dis Child Fetal Neonatal Ed. 2018;103(6):F547–f553. doi: 10.1136/archdischild-2017-313203. [DOI] [PubMed] [Google Scholar]
- 21.Sgro M, Shah PS, Campbell D, et al. Early-onset neonatal sepsis: rate and organism pattern between 2003 and 2008. J Perinatol. 2011;31(12):794–798. doi: 10.1038/jp.2011.40. [DOI] [PubMed] [Google Scholar]
- 22.Lee SM, Chang M, Kim KS. Blood culture proven early onset sepsis and late onset sepsis in very-low-birth-weight infants in Korea. J Korean Med Sci. 2015;30(Suppl 1):S67–74. doi: 10.3346/jkms.2015.30.S1.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang S, Hong L, Gai J, et al. Early-onset sepsis among preterm neonates in China, 2015 to 2018. Pediatr Infect Dis J. 2019;38(12):1236–1241. doi: 10.1097/INF.0000000000002492. [DOI] [PubMed] [Google Scholar]
- 24.Gagliardi L, Rusconi F, Bellu R, Zanini R, Italian Neonatal N. Association of maternal hypertension and chorioamnionitis with preterm outcomes. Pediatrics. 2014;134(1):e154–e161. doi: 10.1542/peds.2013-3898. [DOI] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen N, Fanaroff AA, et al. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347(4):240–247. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 26.Schrag SJ, Farley MM, Petit S, et al. Epidemiology of invasive early-onset neonatal sepsis, 2005 to 2014. Pediatrics. 2016;138:6. doi: 10.1542/peds.2016-2013. [DOI] [PubMed] [Google Scholar]
- 27.Moore MR, Schrag SJ, Schuchat A. Effects of intrapartum antimicrobial prophylaxis for prevention of group-B-streptococcal disease on the incidence and ecology of early-onset neonatal sepsis. Lancet Infect Dis. 2003;3(4):201–213. doi: 10.1016/S1473-3099(03)00577-2. [DOI] [PubMed] [Google Scholar]
- 28.Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Glob Health. 2016;4(10):e752–e760. [DOI] [PubMed]
- 29.Glikman D, Curiel N, Glatman-Freedman A, et al. Nationwide epidemiology of early-onset sepsis in Israel 2010–2015, time to re-evaluate empiric treatment. Acta Paediatr. 2019;108(12):2192–2198. doi: 10.1111/apa.14889. [DOI] [PubMed] [Google Scholar]
- 30.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy S, Viswanathan R, Singh A, Das P, Basu S. Gut colonization by multidrug-resistant and carbapenem-resistant Acinetobacter baumannii in neonates. Eur J Clin Microbiol Infect Dis. 2010;29(12):1495–1500. doi: 10.1007/s10096-010-1030-z. [DOI] [PubMed] [Google Scholar]
- 32.Kamath S, Mallaya S, Shenoy S. Nosocomial infections in neonatal intensive care units: profile, risk factor assessment and antibiogram. Indian J Pediatr. 2010;77(1):37–39. doi: 10.1007/s12098-010-0005-5. [DOI] [PubMed] [Google Scholar]
- 33.Sands K, Carvalho MJ, Portal E, et al. Characterization of antimicrobial-resistant Gram-negative bacteria that cause neonatal sepsis in seven low- and middle-income countries. Nat Microbiol. 2021;6(4):512–523. doi: 10.1038/s41564-021-00870-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Popescu CR, Cavanagh MMM, Tembo B, et al. Neonatal sepsis in low-income countries: epidemiology, diagnosis and prevention. Expert Rev Anti Infect Ther. 2020;18(5):443–452. doi: 10.1080/14787210.2020.1732818. [DOI] [PubMed] [Google Scholar]
- 35.Dagnew AF, Cunnington MC, Dube Q, et al. Variation in reported neonatal group B streptococcal disease incidence in developing countries. Clin Infect Dis. 2012;55(1):91–102. doi: 10.1093/cid/cis395. [DOI] [PubMed] [Google Scholar]
- 36.Wattal C, Kler N, Oberoi JK, Fursule A, Kumar A, Thakur A. Neonatal sepsis: mortality and morbidity in neonatal sepsis due to multidrug-resistant (MDR) organisms: part 1. Indian J Pediatr. 2020;87(2):117–121. doi: 10.1007/s12098-019-03106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stoll BJ, Puopolo KM, Hansen NI, et al. Early-onset neonatal sepsis 2015 to 2017, the rise of Escherichia coli, and the need for novel prevention strategies. JAMA Pediatr. 2020;174(7):e200593. doi: 10.1001/jamapediatrics.2020.0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu M, Jin Y, Duan Y, He M, Lin Z, Lin J. Multi-drug resistant escherichia coli causing early-onset neonatal sepsis—a single center experience from China. Infect Drug Resist. 2019;12:3695–3702. doi: 10.2147/IDR.S229799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin J, Wu C, Ou Q, et al. Nasal colonization of Staphylococcus aureus colonal complex 5: prevalence, influencing factors, and phenotypic and molecular characteristics in pregnant Chinese women. Am J Infect Control. 2017;45(10):1106–1110. doi: 10.1016/j.ajic.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Padmini N, Ajilda AAK, Sivakumar N, Selvakumar G. Extended spectrum β-lactamase producing Escherichia coli and Klebsiella pneumoniae: critical tools for antibiotic resistance pattern. J Basic Microbiol. 2017;57(6):460–470. doi: 10.1002/jobm.201700008. [DOI] [PubMed] [Google Scholar]
- 41.Friedman ND, Carmeli Y, Walton AL, Schwaber MJ. Carbapenem-resistant enterobacteriaceae: a strategic roadmap for infection control. Infect Control Hosp Epidemiol. 2017;38(5):580–594. doi: 10.1017/ice.2017.42. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Liu L, Zhou H, et al. Nationwide surveillance of clinical carbapenem-resistant Enterobacteriaceae (CRE) strains in China. EBioMedicine. 2017;19:98–106. doi: 10.1016/j.ebiom.2017.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.