Abstract
Introduction
Considering the importance of ceftazidime/avibactam (CAZ/AVI) and polymyxin B (PMB) in treating carbapenem-resistant Klebsiella pneumoniae (CRKP) infection, it is essential to evaluate the efficacy and safety of these agents and provide appropriate medical advice to clinical specialists.
Methods
We conducted a retrospective cohort study in two Chinese tertiary hospitals for critically ill patients with CRKP infection who received at least 24-h CAZ/AVI-based or PMB-based treatment. A binary logistic model and a Cox proportional hazards regression model were constructed to analyze variables that could potentially affect 30-day microbiological eradication and all-cause mortality, respectively.
Results
From January 2019 to December 2021, 164 eligible patients were divided into CAZ/AVI and PMB cohorts. A notably lower 30-day mortality rate (35.4% vs 69.5%, P < 0.001) and a higher 30-day microbiological eradication rate (80.5% vs 32.9%, P < 0.001) were observed for patients receiving CAZ/AVI-based treatment, compared with cases in the PMB group. A longer antimicrobial treatment duration (> 7 days) could also significantly decrease the mortality rate and increase the microbiological eradication rate. Female patients had a higher survival rate than male patients. Age over 65 years, sepsis, continuous renal replacement therapy, and organ transplantation were identified as negative factors for survival. In the subgroup analysis, CAZ/AVI combined with tigecycline or amikacin could effectively lower mortality. According to safety evaluation results, potential elevation of hepatic enzymes was associated with CAZ/AVI-based treatment, while renal impairment was probably related to PMB-based treatment.
Conclusions
CAZ/AVI was more effective than PMB in treating CRKP-infected patients. Tigecycline and amikacin were proven to be beneficial as concomitant agents in combination with CAZ/AVI. A treatment period lasting over 7 days was recommended. Hepatoxicity of CAZ/AVI and nephrotoxicity of PMB should be monitored carefully. Further well-designed studies should be performed to verify our conclusion.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00682-0.
Keywords: Ceftazidime/avibactam, Carbapenem-resistant Klebsiella pneumoniae, Polymyxin B, Critically ill patients, Mortality, Microbiological eradication, Safety, Combination therapy
Key Summary Points
| Both ceftazidime/avibactam and polymyxin B were considered as the first-line agents against carbapenem-resistant Klebsiella pneumoniae infection. |
| Ceftazidime/avibactam is more advantageous than polymyxin B when treating carbapenem-resistant K. pneumoniae-infected patients in decreasing both 30-day mortality and increasing 30-day microbiological eradication rate. |
| Tigecycline or amikacin might be two potentially effective combined agents in ceftazidime/avibactam-based therapeutic regimens. |
| Carbapenem-resistant K. pneumoniae-infected patients might benefit from a longer antimicrobial treatment duration (> 7 days), but the optimal antimicrobial duration should be individualized according to the different sources and severity of infection. |
| Hepatoxicity of ceftazidime/avibactam and nephrotoxicity of polymyxin B should be emphasized in routine carbapenem-resistant K. pneumoniae therapies. |
Introduction
In the last few decades, carbapenem-resistant Enterobacteriaceae (CREs) have been regarded as one of the fatal medical threats to public health based on the World Health Organization priority list of antibiotic-resistant bacteria, which could cause a variety of intractable infections, such as pneumonia, bloodstream infections, and urinary tract infections [1, 2]. Carbapenem-resistant Klebsiella pneumoniae (CRKP), the most common pathogen amidst the various strains of CREs, is considered as a nationwide clinical therapeutic challenge in China [3, 4]. According to the corresponding statistics from the China Antimicrobial Surveillance Network, rapidly increasing incidence and prevalence rates of CRKP infection have been observed from 2.9% in 2005 to 25% in 2021 [5]. K. pneumoniae carbapenemase (KPC) is the most clinically common carbapenemase in CRKP strains in China [6–8].
Only a few available antimicrobial agents show adequate clinical efficacy in treating CRKP infection because of its multidrug resistance, such as aminoglycosides, carbapenems (only used with high dose and prolonged infusion time), tigecycline, and fosfomycin. It is crucial to determine whether combinations of the aforementioned drugs show adequate synergies to achieve bactericidal effects against CRKP [2, 9, 10].
Moreover, some novel antimicrobial agents have been developed for the sake of overcoming the treating dilemma of CRKP infection in recent years. It is acknowledged that ceftazidime/avibactam (CAZ/AVI) and polymyxins [polymyxin B (PMB) & colistin] reveal their own antibacterial effects as the effective agents against CRKP infection [2, 9, 10]. As far as we know, clinical studies about comparing clinical efficacy between CAZ/AVI and polymyxin-based therapeutic regimens are still rare [5, 11]. Consequently, it is worthwhile conducting several further clinical investigations to provide sufficient evidence for making guidelines on treatment of CRKP infection with CAZ/AVI and PMB-based therapeutic regimens.
In a previous study, we found that using a combination treatment scheme of CAZ/AVI with carbapenems, fosfomycin, or tigecycline could significantly decrease the mortality of critically ill patients with CRKP infection [12]. However, PMB-based treatment regimen were not evaluated. Hence, the current study compares the clinical efficacy and safety of treating CRKP infection in critical ill patients between CAZ/AVI-based and PMB-based regimen.
Methods
Study Design and Participants
This retrospective cohort study was performed at two tertiary hospitals in China and is based upon the ethical standards of the Declaration of Helsinki 1964 and its later amendments or comparable ethical standards. Our study was approved by the Institutional Review Board of Huashan Hospital Affiliated to Fudan University and Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine. All complete data were extracted respectively from the electronic medical record information system in each hospital without direct interaction with the enrolled participants.
Adult patients (age 18 years or over) who were admitted to the intensive care unit (ICU) from January 2019 to October 2021 and received at least one dose of CZA/AVI or PMB for empirical or definitive treatment with verified CRKP infections (based on microbiological culture test) and documented susceptibility testing results were enrolled in our study. The exclusion criteria were as follows: (1) patients who received CAZ/AVI-based or PMB-based treatment for less than 24 h or died within this period; and (2) patients with missing data.
Antibiotic Dosing Regimens
CAZ/AVI-based therapy was considered as an antimicrobial treatment with CAZ/AVI and any other antibiotics except for PMB. Correspondingly, PMB-based therapy was classified as an antimicrobial treatment with PMB and any other antibiotics except for CAZ/AVI. Combination therapy was defined as using any other anti-CRKP agents together with CAZ/AVI or PMB at the onset of CAZ/AVI or PMB treatment, respectively. The selection of CAZ/AVI-based and PMB-based therapy as well as concomitant antibiotics and their duration was at the discretion of the clinicians.
As for the dose regimen of CAZ/AVI, a 2.5-g fixed dose was administered every 8 h with a 2-h infusion time. Dose adjustment was in accordance with patients’ creatinine clearance (CrCl) level. Patients who underwent continuous renal replacement therapy (CRRT) received a standard dosing regimen regardless of the different modes of CRRT [13].
In the PMB-based therapy group, patients received a loading dose of 2.0–2.5 mg/kg/day and maintenance doses of 1.25–1.5 mg/kg/day every 12 h. Both loading dose and maintenance dose were calculated on the basis of total body weight (TBW) and administered with at least 1-h infusion time. No renal function-based dose adjustment was performed in our study, even if patients were receiving CRRT [14].
Study Objectives and Variables
The 30-day mortality rate was classified as primary outcome in the current study, and the 30-day microbiological eradication rate was evaluated to compare the clinical efficacy between CAZ/AVI- and PMB-based therapy. Microbiological eradication was determined as the disappearance of CRKP from all subsequent cultures.
Variables that were recorded in our study included age, sex, TBW, site of infection (defined in line with the Centers for Disease Control and Prevention (CDC) criteria [15]); polymicrobial infections; Sequential Organ Failure Assessment (SOFA) [16] and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores at the onset of CAZ/AVI-based or PMB-based treatment [17]; sepsis (identified by SOFA scores ≥ 2 [16]) when starting CAZ/AVI-based or PMB-based therapy; CrCl (calculated by Cockcroft–Gault formula [18]) at the beginning of CAZ/AVI-based or PMB-based therapy; CRRT or extracorporeal membrane oxygenation (ECMO) within the duration of CAZ/AVI-based or PMB-based therapy; length of ICU stay before starting antimicrobial therapy; combination therapy and concomitant antibiotic treatment; concomitant use of vasoactive drugs or mechanical ventilation by the start of CAZ/AVI-based or PMB-based therapy; Charlson comorbidity index (CCI) score [19] and comorbidities at admission; CAZ/AVI-based or PMB-based treatment duration.
Microbiology
All pathogen isolation and antimicrobial susceptibility tests, except for CAZ/AVI, were performed by the Vitek 2 Compact system (bioMérieux, Inc.). The susceptibility of CAZ/AVI was determined by the disk-diffusion method (Kirby–Bauer method); the diameter of inhibition zone > 21 mm and < 20 mm meant susceptibility and resistance, respectively. The Clinical and Laboratory Standards Institute (CLSI) criteria 2020 were utilized as the evaluation standard of breakpoints to interpret all antibiotic susceptibility testing results. In addition, carbapenem resistance was defined as a minimum inhibitory concentration (MIC) of imipenem or meropenem of 4 mg/L or over [20].
Statistical Analysis
All statistical analyses were performed with SPSS software (version 26.0, IBM Corp, Armonk, NY, USA). The Shapiro–Wilk test was carried out to validate the normality of the distribution of each variable. As for the categorical variables, Pearson’s chi-square test or Fisher’s exact test was utilized for data analysis and calculation of P values. Student’s t test or Mann–Whitney U test was applied to analyze continuous variables and calculate P values. To set up a multivariate regression analysis model for investigating the potential risk factors for 30-day microbiological eradication, each variable was evaluated by univariate analysis at first. Variables with P values ≤ 0.10 were added in the binary logistic regression analysis. The Kaplan–Meier method was chosen to achieve the survival analysis. Any variable with P value ≤ 0.10 was involved in a Cox proportional hazards regression model with a forward stepwise selection for analyzing 30-day mortality, while only variables with P values ≤ 0.10 remained in this model. The differences of variables between CAZ/AVI group and PMB group were compared in advance. Variables with P values ≤ 0.20 were included in both binary and Cox proportional hazards regression analysis, with the purpose of adjusting for confounding by indication. Covariates with P values ≤ 0.10 were kept in the models. Furthermore, a propensity score for the CAZ/AVI group was calculated by a logistic regression model covering the aforementioned variables with P values ≤ 0.20 and included in these two regression models. The plot of log [−log(survival)] versus log(time) was utilized to evaluate the proportional hazard assumption graphically. The collinearity between covariates was also checked. Tests for interactions were not performed. All tests were two-tailed, and P values ≤ 0.05 were considered statistically significant.
Results
Comparison of Efficacy Between CAZ/AVI-Based and PMB-Based Therapeutic Regimen
From January 2019 to December 2021, 164 eligible patients were included in our study (Fig. 1); 128 patients were admitted to Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and 36 were hospitalized in Huashan Hospital Affiliated to Fudan University. Their mean age was 65.4 ± 14.9 years and 60.4% of patients were over 65 years of age. In total 105 patients (64.0%) were female. The most common primary infection site in these patients was the respiratory tract (56.1%). About 15% and 36% patients suffered from polymicrobial infection and sepsis, respectively. Cardiovascular disease (51.2%) and respiratory disease (45.1%) were two major types of comorbidities. Nearly three-quarter (74.4%) of patients were treated with a CAZ/AVI-based or PMB-based therapeutic regimen for a period of at least 7 days. The antimicrobial susceptibility test results of K. pneumoniae isolates are listed in Supplementary Table S1. The K. pneumoniae isolates were highly susceptible to CAZ/AVI, colistin, and tigecycline, while amikacin showed suboptimal antibacterial activities against these isolates. It is worthwhile mentioning that none of the patients with CAZ/AVI resistance were prescribed CAZ/AVI.
Fig. 1.
Study design
All patients were divided into two groups according to CAZ/AVI-based or PMB-based therapeutic regimen. The number of patients in each group was equal (n = 82). The demographic and clinical characteristics of the patients (Table 1) indicated that there were only minor differences between the two groups. However, the proportion of patients with sepsis in the PMB cohort was significantly inferior to the CAZ/AVI cohort (22.0% vs 50%, P < 0.001); and patients in the CAZ/AVI group had a significantly longer antimicrobial treatment duration than the other group (14 days vs 9 days, P = 0.001).
Table 1.
Characteristics of patients receiving CAZ/AVI-based and PMB-based therapeutic regimens
| Variable | CAZ/AVI (n = 82) | PMB (n = 82) | P value |
|---|---|---|---|
| Age, years | 63.2 ± 17.0 | 67.5 ± 12.3 | 0.173 |
| Sex (female) | 56 (68.3) | 49 (59.8) | 0.255 |
| TBW, kg | 65.2 ± 13.8 | 64.1 ± 13.2 | 0.586 |
| Primary site of infection | |||
| Primary bloodstream infection | 10 (12.2) | 12 (14.6) | 0.647 |
| Respiratory infection | 38 (46.3) | 54 (65.9) | 0.012 |
| Abdominal infection | 17 (20.7) | 12 (14.6) | 0.306 |
| Urinary tract infection | 12 (14.6) | 3 (3.7) | 0.027 |
| Other infections | 5 (6.1) | 1 (1.2) | 0.210 |
| Sepsis | 41 (50) | 18 (22.0) | < 0.001 |
| Polymicrobial infection | 16 (19.5) | 9 (11.0) | 0.128 |
| APACHE II score (antimicrobial treatment onset) | 16.5 (14–19) | 16.5 (14–19) | 0.895 |
| CrCl, mL/min | 76.7 (46.4–119.8) | 56.8 (39.0–95.1) | 0.039 |
| CRRT | 11 (13.4) | 21 (25.6) | 0.049 |
| ECMO | 1 (1.2) | 1 (1.2) | 1.000 |
| Length of ICU stay before starting antimicrobial therapy, days | 23 (11.8–53.3) | 35.5 (21.5–54.8) | 0.017 |
| Vasoactive drugs | 46 (56.1) | 42 (51.2) | 0.531 |
| Mechanical ventilation | 54 (65.9) | 51 (62.2) | 0.625 |
| Comorbidities | |||
| Cardiovascular disease | 28 (34.1) | 56 (68.3) | < 0.001 |
| Respiratory disease | 38 (46.3) | 36 (43.9) | 0.754 |
| Central nervous system disease | 17 (20.7) | 21 (25.6) | 0.459 |
| Autoimmune disease | 9 (11.0) | 6 (7.3) | 0.416 |
| Liver disease | 25 (30.5) | 19 (23.2) | 0.290 |
| Renal insufficiency | 22 (26.8) | 30 (36.6) | 0.179 |
| Diabetes | 18 (22.0) | 21 (25.6) | 0.582 |
| Organ transplantation | 10 (12.2) | 5 (6.1) | 0.176 |
| Neoplasia | 25 (30.5) | 10 (12.2) | 0.004 |
| CCI score | 4 (3–6) | 5 (3.8–6) | 0.223 |
| Antimicrobial treatment duration, days | 14 (10–14) | 9 (6–14.3) | 0.001 |
| Antimicrobial combination therapy | 49 (59.8) | 60 (73.2) | 0.069 |
All data are exhibited as number (%), mean ± standard deviation (SD), or median (interquartile range)
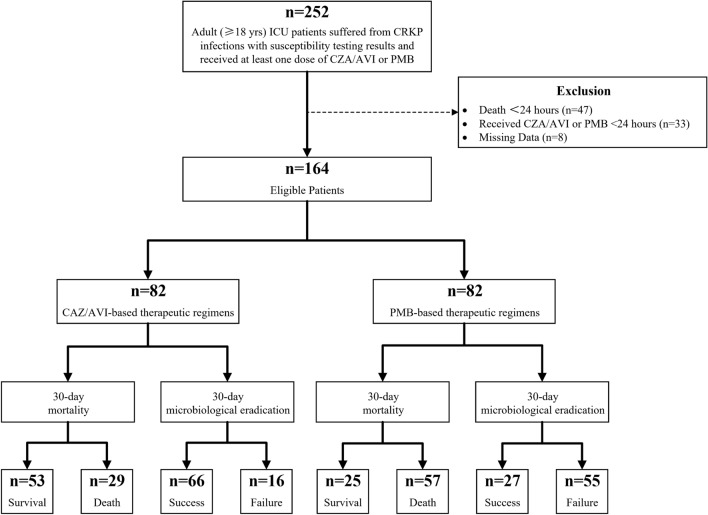
There were 59.8% and 73.2% of cases receiving antimicrobial combination therapy in the CAZ/AVI and PMB cohorts, respectively. Detailed antimicrobial therapy information is listed in Supplementary Table S2. Carbapenems, tigecycline, amikacin, and fosfomycin were the main concomitant drugs of combination therapy in each group. Intragroup data analysis indicated that combination therapy was superior to monotherapy in the CAZ/AVI cohort because of its higher 30-day microbiological eradication and lower 30-day mortality rate (Fig. 2). However, there was virtually no difference in mortality between combination therapy and monotherapy in the PMB cohort, while monotherapy even had a higher microbiological eradication rate than combination therapy.
Fig. 2.
Comparison of 30-day mortality and microbiological eradication rate between CAZ/AVI combination therapy and monotherapy
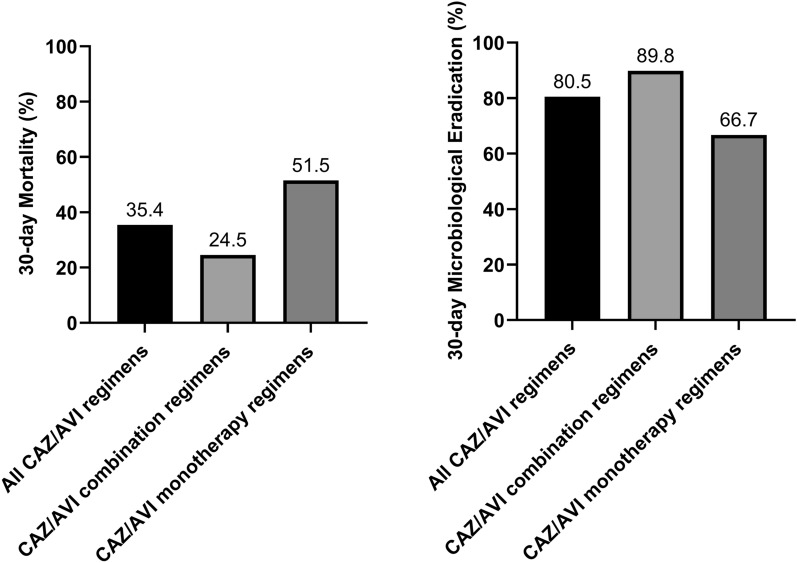
The microbiological eradication rates of the two cohorts are compared in Fig. 3. The 14-day microbiological eradication rate in the CAZ/AVI group was significantly higher than that in the PMB group (51.2% vs 26.8%, P = 0.001). The 30-day microbiological eradication rate was 80.5% for patients treated with CAZ/AVI-based therapy, and 32.9% for patients in the PMB cohort (P < 0.001).
Fig. 3.
Comparison of microbiological eradication rate at 7, 14, 21, and 30 days between CAZ/AVI and PMB cohorts
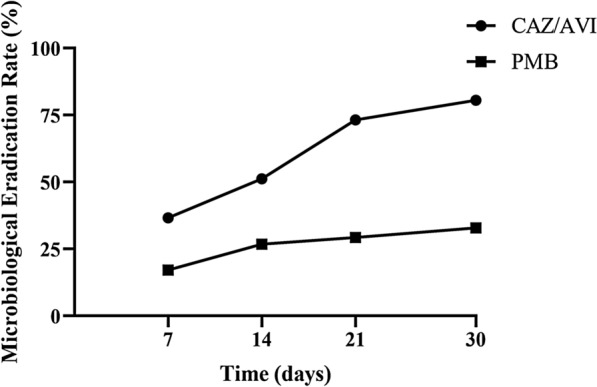
According to the result of survival analysis (Fig. 4), the 30-day all-cause mortality rate in the CAZ/AVI group was significantly lower than that in the PMB group (35.4% vs 69.5%, log-rank, P < 0.001). The mortality rate for patients receiving CAZ/AVI-based and PMB-based therapeutic regimen was 14.2/1000 patient-days and 42.6/1000 patient-days (P < 0.001), respectively.
Fig. 4.
Survival curves of critically ill patients with CRKP infection receiving CAZ/AVI-based and PMB-based therapeutic regimens
Risk Factors for 30-Day All-Cause Mortality in Critically Ill Patients with CRKP Infection
As the primary outcome of the current study, the 30-day all-cause mortality was analyzed among all critically ill patients with CRKP infection. These patients were divided into survival and death groups based on their 30-day survival status. Table 2 lists the demographic and clinical characteristics for grouped patients. The 30-day mortality rate was 52.4% (78/164) for all eligible patients.
Table 2.
Potential risk factors for 30-day mortality in patients receiving CAZ/AVI-based or PMB-based therapeutic regimens
| Variable | 30-day mortality | P value | |
|---|---|---|---|
| Survival (n = 78) | Death (n = 86) | ||
| Age (> 65 years) | 40 (51.3) | 59 (68.6) | 0.024 |
| Sex (female) | 56 (71.8) | 49 (57.0) | 0.048 |
| TBW, kg | 64.9 ± 13.6 | 64.4 ± 13.5 | 0.792 |
| Primary site of infection | |||
| Primary bloodstream infection | 11 (14.1) | 11 (12.8) | 0.806 |
| Respiratory infection | 41 (52.6) | 51 (59.3) | 0.385 |
| Abdominal infection | 11 (14.1) | 18 (20.9) | 0.252 |
| Urinary tract infection | 10 (12.8) | 5 (5.8) | 0.120 |
| Other infections | 5 (6.4) | 1 (1.2) | 0.103 |
| Sepsis | 26 (33.3) | 33 (38.4) | 0.502 |
| Polymicrobial infection | 15 (19.2) | 10 (11.6) | 0.176 |
| APACHE II score (antimicrobial treatment onset) | 15 (14–19) | 17 (14.8–19) | 0.072 |
| CrCl, mL/min | 83.6 (49.2–116.4) | 55.8 (33.5–96.9) | 0.007 |
| CRRT | 9 (11.5) | 23 (26.7) | 0.013 |
| ECMO | 1 (1.3) | 1 (1.2) | 1.000 |
| Length of ICU stay before starting antimicrobial therapy, days | 30 (15–63.8) | 29.5 (18–52.3) | 0.678 |
| Vasoactive drugs | 38 (48.7) | 50 (58.1) | 0.227 |
| Mechanical ventilation | 47 (60.3) | 58 (67.4) | 0.338 |
| Comorbidities | |||
| Cardiovascular disease | 32 (41.0) | 52 (60.5) | 0.013 |
| Respiratory disease | 33 (42.3) | 41 (47.7) | 0.490 |
| Central nervous system disease | 21 (26.9) | 17 (19.8) | 0.278 |
| Autoimmune disease | 6 (7.7) | 9 (10.5) | 0.538 |
| Liver disease | 21 (26.9) | 23 (26.7) | 0.979 |
| Renal insufficiency | 22 (28.2) | 30 (34.9) | 0.359 |
| Diabetes | 19 (24.4) | 20 (23.3) | 0.868 |
| Organ transplantation | 1 (1.3) | 14 (16.3) | 0.001 |
| Neoplasia | 22 (28.2) | 13 (15.1) | 0.041 |
| CCI score | 4 (3–6) | 5 (4–6) | 0.206 |
| Antimicrobial treatment duration (> 7 days) | 71 (91.0) | 51 (59.3) | < 0.001 |
| CAZ/AVI-based therapeutic regimens | 53 (67.9) | 29 (33.7) | < 0.001 |
| Antimicrobial combination therapy | 56 (71.8) | 53 (61.6) | 0.168 |
All data are exhibited as number (%), mean ± SD, or median (interquartile range)
The results of Cox proportional hazards regression analysis are listed in Table 3. Female gender, receiving antimicrobial treatment for a duration of over 7 days, or employing CAZ/AVI therapeutic regimen were significantly associated with lower 30-day mortality rates for critically ill patients with CRKP infection. On the contrary, a higher age (> 65 years), application of CRRT, concurrent sepsis, and comorbidity of organ transplantation were identified as independently negative factors for patients’ 30-day survival. The propensity score adjustment had not changed the consequences of this Cox regression model.
Table 3.
Cox proportional hazards regression analysis of potential risk factors for 30-day mortality
| Variable | Without/with propensity score adjustmenta | ||
|---|---|---|---|
| HR | 95% CI | P value | |
| Age (> 65 years) | 2.038 | 1.263–3.286 | 0.004 |
| CRRT | 1.786 | 1.087–2.932 | 0.022 |
| Sepsis | 1.868 | 1.127–3.097 | 0.015 |
| Organ transplantation | 4.660 | 2.390–9.088 | < 0.001 |
| Sex (female) | 0.628 | 0.397–0.995 | 0.047 |
| Antimicrobial treatment duration (> 7 days) | 0.171 | 0.104–0.281 | < 0.001 |
| CAZ/AVI-based therapeutic regimens | 0.391 | 0.236–0.648 | < 0.001 |
HR hazard ratio, CI confidence interval
aThe propensity score that was included in the Cox-proportional hazards regression model showed no significant alteration to the results of other variables (P = 0.475)
Subgroup Analysis to Find Out CAZ/AVI-Based Therapeutic Schemes
Taking the data of Table 3 into consideration, we could make an initial deduction that CAZ/AVI-based therapy would probably be conducive to lower mortality rate, compared to PMB-based therapy. Thus, further subgroup analysis was conducted to find out appropriate CAZ/AVI-based therapy schemes for further investigation. As a result, CAZ/AVI-base therapeutic regimen could be beneficial to reduce the 30-day mortality rate significantly when tigecycline (P = 0.037) or amikacin (P = 0.026) was prescribed as another concomitant agent with CAZ/AVI (Table 4).
Table 4.
Hazard ratio of CAZ/AVI-based therapeutic regimens and 30-day mortality according to the subgroup analysis
| Subgroupa | n | HR | 95% CI | P value |
|---|---|---|---|---|
| CAZ/AVI + carbapenemb | 17 | 0.585 | 0.325–1.053 | 0.074 |
| CAZ/AVI + tigecycline | 11 | 0.267 | 0.077–0.921 | 0.037 |
| CAZ/AVI + amikacin | 11 | 0.105 | 0.014–0.766 | 0.026 |
| CAZ/AVI + fosfomycin | 7 | 0.299 | 0.077–1.160 | 0.081 |
| CAZ/AVI monotherapy | 33 | 0.591 | 0.332–1.054 | 0.075 |
HR hazard ratio, CI confidence interval
aAdjusted for age (> 65 years), CRRT, sepsis, organ transplantation, sex (female), antimicrobial treatment duration (> 7 days)
bFourteen patients received meropenem and three patients received imipenem
Risk Factors for 30-day Microbiologic Eradication in Critically Ill Patients with CRKP Infection
The microbiological eradication rate at 30 days was 80.5% for the CAZ/AVI group and 32.9% for the PMB group. In our study, univariate and multivariable analyses were applied to ascertain potential risk factors for 30-day microbiological eradication. All enrolled participants were classified into success and failure groups, which depended on their 30-day bacterial eradication status. Table 5 summarizes the details of demographic and clinical characteristics for patients in these two groups. Variables with P values ≤ 0.10, including sex (female), comorbidity of cardiovascular disease and neoplasia, antimicrobial treatment duration (> 7 days), and CAZ/AVI-based therapeutic regimen were chosen in the next step of multivariate analysis. After forward stepwise selection of covariates and adjustment of the propensity score, the eventual results of binary logistic regression analysis showed that the antimicrobial treatment duration of more than 7 days (odds ratio, 4.375; 95% confidence interval, 1.824–10.496; P < 0.001) and CAZ/AVI-based therapeutic regimen (odds ratio, 6.392; 95% confidence interval, 3.037–13.457; P < 0.001) were the only two independent factors relating to a lower rate of 30-day microbiological eradication (Table 6). What is more, the propensity score had not made any significant alteration to the results of the other variables in the binary logistic regression model.
Table 5.
Potential risk factors for 30-day microbiological eradication in patients receiving CAZ/AVI- or PMB-based therapeutic regimens
| Variable | 30-day microbiological eradication | P value | |
|---|---|---|---|
| Success (n = 93) | Failure (n = 71) | ||
| Age, years | 64.4 ± 16.7 | 66.7 ± 12.2 | 0.328 |
| Sex (female) | 65 (69.9) | 40 (56.3) | 0.073 |
| TBW, kg | 64.8 ± 14.0 | 64.5 ± 12.9 | 0.887 |
| Primary site of infection | |||
| Primary bloodstream infection | 13 (14.0) | 9 (12.7) | 0.808 |
| Respiratory infection | 50 (53.8) | 42 (59.2) | 0.491 |
| Abdominal infection | 14 (15.1) | 15 (21.1) | 0.312 |
| Urinary tract infection | 11 (11.8) | 4 (5.6) | 0.173 |
| Other infections | 5 (5.4) | 1 (1.4) | 0.236 |
| Sepsis | 37 (39.8) | 22 (31.0) | 0.245 |
| Polymicrobial infection | 13 (14.0) | 12 (16.9) | 0.606 |
| APACHE II score (antimicrobial treatment onset) | 16 (14–19) | 17 (14–19) | 0.975 |
| CrCl, mL/min | 67.0 (41.1–110.5) | 61.5 (41.0–121.5) | 0.828 |
| CRRT | 15 (16.1) | 17 (23.9) | 0.194 |
| ECMO | 1 (1.1) | 1 (1.4) | 1.000 |
| Length of ICU stay before starting antimicrobial therapy, days | 30 (15–61.5) | 33 (20–50) | 0.960 |
| Vasoactive drugs | 49 (52.7) | 39 (54.9) | 0.775 |
| Mechanical ventilation | 59 (63.4) | 46 (64.8) | 0.859 |
| Comorbidities | |||
| Cardiovascular disease | 40 (43.0) | 44 (62.0) | 0.016 |
| Respiratory disease | 43 (46.2) | 31 (43.7) | 0.743 |
| Central nervous system disease | 21 (22.6) | 17 (23.9) | 0.838 |
| Autoimmune disease | 7 (7.5) | 8 (11.3) | 0.410 |
| Liver disease | 24 (25.8) | 20 (28.2) | 0.735 |
| Renal insufficiency | 29 (31.2) | 23 (32.4) | 0.869 |
| Diabetes | 26 (28.0) | 13 (18.3) | 0.150 |
| Organ transplantation | 9 (9.7) | 6 (8.5) | 0.787 |
| Neoplasia | 25 (26.9) | 10 (14.1) | 0.047 |
| CCI score | 5 (3–6) | 4 (3–6) | 0.860 |
| Antimicrobial treatment duration (> 7 days) | 83 (89.2) | 39 (54.9) | < 0.001 |
| CAZ/AVI-based therapeutic regimens | 66 (71.0) | 16 (22.5) | < 0.001 |
| Antimicrobial combination therapy | 63 (67.7) | 46 (64.8) | 0.691 |
All data are exhibited as number (%), mean ± SD, or median (interquartile range)
Table 6.
Binary logistic regression analysis of potential risk factors for 30-day microbiological eradication
| Variable | Without/with propensity score adjustmenta | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Antimicrobial treatment duration (> 7 days) | 4.375 | 1.824–10.496 | < 0.001 |
| CAZ/AVI-based therapeutic regimens | 6.392 | 3.037–13.457 | < 0.001 |
OR odds ratio, CI confidence interval
aThe propensity score that was included in the binary logistic regression model showed no significant alteration to the results of other variables (P = 0.393)
Safety Evaluation Between CAZ/AVI-Based and PMB-Based Therapeutic Regimen
The clinical safety of CAZ/AVI-based and PMB-based therapy was evaluated by laboratory parameters in three aspects (Supplementary Table S3), namely liver function [alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil)], kidney function [CrCl, blood urea nitrogen (BUN)], and coagulation function [activated partial thromboplastin time (APTT), prothrombin time (PT), fibrinogen (Fib)]. Significantly elevated ALT and AST were observed after CAZ/AVI-based treatment. Differences in the CrCl and BUN values before and after treatment in the PMB group were all statistically significant. No significant alteration was identified for all three coagulation parameters in both cohorts.
Adverse events (AEs) data was also collected in the current study. Diarrhea was the main AE recorded in both cohorts. There were 17.1% (14/82) of patients suffering from diarrhea during the CZA/AVI treatment period, while 12.2% (10/82) of cases had diarrhea in the PMB group (P = 0.377). In the CAZ/AVI group, abnormal elevations (more than three times the upper reference limit and 150% increase from baseline) of ALT or AST levels were found in 4.9% (4/82) patients. Acute kidney failure (AKI) was found in 8.5% (7/82) of patients after using PMB-based therapy. In addition, it must be stressed that seven and two patients developed acute kidney injury and Clostridium difficile infection (CDI) when they received PMB-based therapeutic regimes, respectively, while only one patient had CDI in the CAZ/AVI group.
Discussion
Nowadays, among the available antimicrobial agents against CRKP infection, novel β-lactam/β-lactamase inhibitors (BL/BLIs) are the mainstay of effective pharmacotherapeutic schemes for CRKP-infected patients. It is recommended that CAZ/AVI is the preferred therapeutic agent against multiple sources of CRKP infection, according to the clinical guidance from Infectious Diseases Society of America (IDSA) [21]. In China, there were few effective drugs against CRKP, while CAZ/AVI was the only novel BL/BLI approved in clinical use, and PMB was also utilized in recent years as well.
Several in vitro and in vivo studies have discussed the effectiveness of CAZ/AVI and PMB, which proved that these two agents were both reliable treatment options for patients with CRKP infections [22–25]. Fang et al. drew the conclusion that CAZ/AVI-based therapy was more effective than PMB-based therapy in treating CRKP infection by implementing a retrospective analysis to compare the efficacy between these two therapies [5]. Nevertheless, suitable therapeutic combined agents with CAZ/AVI remain unclear. Further safety evaluation of CAZ/AVI and PMB should also be performed. It is reasonable for us to design a novel clinical trial to make a comprehensive analysis about effectiveness and safety of CAZ/AVI-based and PMB-based therapeutic schemes.
In this study, we evaluated the 30-day all-cause mortality as primary outcome of clinical efficacy between these two therapies. Patients with a higher age (> 65 years), suffering from sepsis, receiving CRRT during antimicrobial treatment, or having comorbidity of organ transplantation had a significantly higher risk of death.
CRRT is a negative factor for survival in our study, which is contrary to our knowledge that CRRT is widely used in critically ill patients since it plays a crucial role in elimination of inflammatory mediators and continuous control of hemodynamic and electrolytical stability in vivo. This phenomenon might have possible causes in two respects. On the one hand, surviving patients have better renal function than patients in the dead group according to the comparison of CrCl, which is related to a lower demand on CRRT. On the other hand, some clinical studies indicate that CRRT might not be beneficial to effectively lower mortality for infected patients or those with sepsis [26, 27]. Regarding the controversy over CRRT in the current study, we should put too great an emphasis on kidney function to exclude the interference of utilizing CRRT in our further studies.
As for the treatment duration, we advocate that more than a 7-day antimicrobial treatment period might have a positive correlation with survival rate for CRKP-infected patients. Zhou et al.’s research revealed that a short duration of antimicrobial therapy from 4 to 9 days would significantly increase the mortality, which provided a strongly support for our result [28]. However, we could not ignore the fact that patients may die within the 7-day duration of antimicrobial therapy. In the current study, the majority of patients (87.6%) received antimicrobial treatment for over 7 days, which implied that our conclusion might be convincing but still requires further verification. Moreover, it is acknowledged that appropriate treatment duration of infection is influenced by multiple factors, such as various sources of infection, severity of infection, immune status, and general response to therapy [21, 29, 30]. Our conclusion on treatment duration might be too general to play an important role in clinical practice. Individualized therapy duration with different types of CRKP infection should be investigated case by case.
A gender-dependent difference also exists in our study. This is evidently important for patients with infection and sepsis, which is attributed to sex hormones specifically. Female patients with sepsis may have a survival benefit in comparison with male patients owing to the salutary effects of estrogen on releasing cytokines which could improve the positive immune response and restoring organ function after sepsis. The immunosuppressive role of testosterone is also associated with the higher mortality rate for male patients with infection [31–33]. It is meaningful to investigate the mortality risk by using clinically accurate preclinical models that reflect sex differences in our further research.
CAZ/AVI-based therapy was proved to be apparently effective in treating patients with CRKP in the current study, not only improving survival rate but also increasing bacterial clearance rate, compared with PMB-based therapy. Quite a few studies demonstrate the great value of CAZ/AVI in treating CRKP infection. CAZ/AVI therapy was more clinically advantageous than other antibiotics to decrease 30-day mortality for patients with CRKP infection, according to Gu et al.’s study [34]. Chen et al. analyzed CRKP-infected patients after liver transplantation retrospectively and summarized that no matter whether CAZ/AVI-based combination therapy or monotherapy was used, promising clinical efficacy and safety were revealed in treating severe CRKP infections [35].
It is worth noting that CAZ/AVI resistance was observed in very few CRKP strains in our study, although none of the patients with CAZ/AVI-resistant CRKP infection were prescribed CAZ/AVI. Shields et al. found that both pneumonia and prior use of CRRT were risk factors for the development of CAZ/AVI resistance, which could possibly induce microbiological failure or mortality [36]. Hence, potential risk of clinical failure should be a concern when clinicians prescribe CAZ/AVI as empirical therapy to treat CRKP-infected patients.
According to the result from subgroup analysis, we have recognized that CAZ/AVI combination therapies with tigecycline or amikacin showed notable differences in lowering 30-day mortality, compared to other therapeutic schemes. Tigecycline was identified as a notably effective combined agent in our previous study [12]. Ojdana et al. undertook one in vitro research study to explore the synergy of antibiotics combination against CRKP. They found that a combination with CAZ/AVI and tigecycline was capable of exerting synergistic effects against CRKP [37]. Another in vitro time-kill experiment demonstrated that combinations of CZA/AVI with both tigecycline and amikacin exhibited better antimicrobial effects than monotherapy [38]. These two drugs could enhance the therapeutic efficiency against CRKP in terms of Chen et al.’s study [25]. Nevertheless, we should point out that better therapeutic outcome was observed when using tigecycline and amikacin to treat pneumonia and urinary tract infection, in view of the pharmacokinetic/pharmacodynamic (PK/PD) characteristics of these two antibiotics, respectively. Since a fraction of patients suffered from the two aforementioned types of infection in the CAZ/AVI group (46.3% with respiratory infection and 14.1% with urinary tract infection), one needs to confirm if tigecycline and amikacin show sufficient clinical efficacy in other types of infection.
The results from our study also showed that patients who received CAZ/AVI-based antimicrobial therapy would have a significantly higher probability of CRKP clearance than those receiving PMB-based antimicrobial therapy in vivo, which was consistent with the conclusion of Fang et al.’s article [5].
The safety analysis of corresponding laboratory parameters and AEs was conducted to verify the safety of these two therapeutic schemes as well. Generally speaking, we could conclude that safety could be ensured if patients receive CAZ/AVI or PMB therapeutic regimens since diarrhea was the most common AE during the treatment period in both cohorts and no severe AE was observed in the present study. A large study evaluating the safety of CAZ/AVI with the pooled data from seven phase II and III clinical studies elaborated that the incidence of CAZ/AVI-induced diarrhea varied from 3.1% to 15.4%, which was similar to our result [39].
Significant augmentation of AST and ALT values was found during the CAZ/AVI treatment period in our study, which is consistent with statistical data from Cheng et al.’s study [39]. This could be attributed to ceftazidime-induced transient elevations in hepatic enzymes [40–42]. We should attach great importance to monitoring when using CAZ/AVI-based therapeutic treatment, especially in combination with drugs having verified hepatoxicity.
It is inevitable to discuss the controversy of the predominant AE of PMB, namely PMB-associated nephrotoxicity [43]. Although PMB showed adequate efficacy against CRKP, it is not highly recommended for the treatment CRKP infections, because of its nephrotoxicity [21]. Polymyxin-associated acute kidney injury (AKI) has a high incidence ranging from 10% to 60%, which is mainly ascribed to receipt of concomitant nephrotoxic agents and selection of inappropriate dose regimens [14, 44, 45]. One must be cautious of PMB-induced AKI, while a significant decline of CrCl and BUN was found during PMB treatment duration.
The current study achieves both clinical efficacy and safety comparison between CAZ/AVI-based and PMB-based therapeutic regimen in critically ill CRKP-infected patients for the first time. We have tried our utmost to control the potential for indication bias in this study. On the one hand, variables which related to the potential difference between CAZ/AVI-based and PMB-based treatment were all evaluated in the multivariate model. On the other hand, the propensity scores were calculated and incorporated into regression analysis, which did not alter any variable in the final multivariate and Cox regression models. In summary, we maintain that our study is convincing because the indication bias could barely affect our investigation result.
The present study had some limitations. First of all, our investigation was a retrospective observational cohort study with insufficient participants, which could not exclude the indication biases. More well-designed clinical trials with a larger number of eligible patients should be performed to validate our conclusion in the future. Secondly, genotypic identification of carbapenemases for all clinical isolates of CRKP was not performed in the present study because we lacked the essential equipment and experimental reagents. Thirdly, therapeutic drug monitoring (TDM) was not utilized to evaluate PMB serum concentration, which might cause treatment failure or increase the risk of AKI due to subtherapeutic or excessive dose, respectively. Last but not the least, in order to lower 30-day mortality, appropriate antimicrobial therapy should be initiated within 24 h after the collection of microbiological cultures [46]. However, the exact time to appropriate antimicrobial therapy for each patient was not collected in our study. We should include this variable in our future investigation.
Conclusions
Our study showed that CZA/AVI-based therapeutic regimen was superior to PMB-based therapeutic regimen in reducing all-cause mortality and increasing the microbiological eradication rate for critically ill patients with CRKP infection. Tigecycline and amikacin might be two effective combined agents in CAZ/AVI therapy. A longer antimicrobial treatment duration (> 7 days) might be a potentially protective factor for treating CRKP-infected patients, while optimal antimicrobial duration should be individualized according to the different sources and severity of infection. Considering the severity and occurrence of AEs, clinical safety could be guaranteed for those who receive CAZ/AVI or PMB therapeutic regimens in general. However, clinicians and pharmacists should still pay more attention to the hepatoxicity of CAZ/AVI and nephrotoxicity of PMB when treating CRKP infection. Further large-scale prospective studies should be designed to explore the efficacy and safety between these two agents thoroughly.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Authors’ Contributions
Guanhao Zheng, Jiaqi Cai, Juan He and Xiaolan Bian conceived and designed this study. Guanhao Zheng, Liang Zhang, Dayu Chen, Linyu Wang, Yusi Qiu, Han Deng and Hao Bai collected the information in the case, and contributed to the acquisition, analysis, and interpretation of the data. Guanhao Zheng, Jiaqi Cai, Liang Zhang, Juan He and Xiaolan Bian wrote and revised the manuscript. All authors read and approved the final manuscript.
Disclosures
Guanhao Zheng, Jiaqi Cai, Liang Zhang, Dayu Chen, Linyu Wang, Yusi Qiu, Han Deng, Hao Bai, Xiaolan Bian and Juan He have nothing to disclose.
Compliance with Ethics Guidelines
This study was approved by Huashan Hospital Affiliated to Fudan University and Ruijin Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and has been performed in accordance with the ethical standards laid down in the Declaration of Helsinki of 1964 and its later amendments.
Data Availability
All data generated or analyzed during this study are included in this published article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guanhao Zheng and Jiaqi Cai contributed equally to this work and share first authorship.
Contributor Information
Xiaolan Bian, Email: bxl70029@hotmail.com.
Juan He, Email: hejuanwin@126.com.
References
- 1.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3. [DOI] [PubMed] [Google Scholar]
- 2.Morrill HJ, Pogue JM, Kaye KS, LaPlante KL. Treatment options for carbapenem-resistant Enterobacteriaceae infections. Open Forum Infect Dis. 2015;2(2):ofv050. [DOI] [PMC free article] [PubMed]
- 3.Zhang Y, Wang Q, Yin Y, et al. Epidemiology of carbapenem-resistant Enterobacteriaceae infections: report from the China CRE Network. Antimicrob Agents Chemother. 2018;62(2):e01882-17. [DOI] [PMC free article] [PubMed]
- 4.van Duin D, Arias CA, Komarow L, et al. Molecular and clinical epidemiology of carbapenem-resistant enterobacterales in the USA (CRACKLE-2): a prospective cohort study. Lancet Infect Dis. 2020;20(6):731–741. doi: 10.1016/S1473-3099(19)30755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang J, Li H, Zhang M, et al. Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol. 2021;12:780940. doi: 10.3389/fphar.2021.780940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Jiang X, Yang T, et al. Genomic epidemiology of carbapenemase-producing Klebsiella pneumoniae in China. Genom Proteom Bioinform. 2022. 10.1016/j.gpb.2022.02.005. [DOI] [PMC free article] [PubMed]
- 7.Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi: 10.3389/fcimb.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang WX, Chen HY, Chen C, et al. Resistance phenotype and molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae isolates in Shanghai. Microb Drug Resist (Larchmont, NY) 2021;27(10):1312–1318. doi: 10.1089/mdr.2020.0390. [DOI] [PubMed] [Google Scholar]
- 9.Doi Y. Treatment options for carbapenem-resistant gram-negative bacterial infections. Clin Infect Dis. 2019;69(Suppl 7):S565–75. [DOI] [PMC free article] [PubMed]
- 10.Tompkins K, van Duin D. Treatment for carbapenem-resistant Enterobacterales infections: recent advances and future directions. Eur J Clin Microbiol Infect Dis. 2021;40(10):2053–2068. doi: 10.1007/s10096-021-04296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883-17. [DOI] [PMC free article] [PubMed]
- 12.Zheng G, Zhang J, Wang B, et al. Ceftazidime-avibactam in combination with in vitro non-susceptible antimicrobials versus ceftazidime-avibactam in monotherapy in critically ill patients with carbapenem-resistant Klebsiella pneumoniae infection: a retrospective cohort study. Infect Dis Ther. 2021;10(3):1699–1713. doi: 10.1007/s40121-021-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L, Li X, Xia Y, et al. Recommendation of antimicrobial dosing optimization during continuous renal replacement therapy. Front Pharmacol. 2020;11:786. doi: 10.3389/fphar.2020.00786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji BT, Pogue JM, Zavascki AP, et al. International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP) Pharmacotherapy. 2019;39(1):10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Codjoe FS, Donkor ES. Carbapenem resistance: a review. Med Sci (Basel). 2017;6(1):1. [DOI] [PMC free article] [PubMed]
- 21.Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ. Infectious Diseases Society of America guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-E), carbapenem-resistant enterobacterales (CRE), and Pseudomonas aeruginosa with difficult-to-treat resistance (DTR-P. aeruginosa). Clin Infect Dis. 2021;72(7):e169–83. [DOI] [PubMed]
- 22.King M, Heil E, Kuriakose S, et al. Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2017;61(7):e00449-17. [DOI] [PMC free article] [PubMed]
- 23.Liang Q, Huang M, Xu Z. Early use of polymyxin B reduces the mortality of carbapenem-resistant Klebsiella pneumoniae bloodstream infection. Braz J Infect Dis. 2019;23(1):60–65. doi: 10.1016/j.bjid.2018.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou SY, Wu D, Feng XH. Polymyxin monotherapy versus polymyxin-based combination therapy against carbapenem-resistant Klebsiella pneumoniae: a systematic review and meta-analysis. J Glob Antimicrob Resist. 2020;23:197–202. doi: 10.1016/j.jgar.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Yang Y, Yao H, et al. Prediction of prognosis in adult patients with carbapenem-resistant Klebsiella pneumoniae infection. Front Cell Infect Microbiol. 2021;11:818308. doi: 10.3389/fcimb.2021.818308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z, Zhang L, Xu F, Han D, Lyu J. The association between continuous renal replacement therapy as treatment for sepsis-associated acute kidney injury and trend of lactate trajectory as risk factor of 28-day mortality in intensive care units. BMC Emerg Med. 2022;22(1):32. doi: 10.1186/s12873-022-00589-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ren Y, Zhang L, Xu F, et al. Risk factor analysis and nomogram for predicting in-hospital mortality in ICU patients with sepsis and lung infection. BMC Pulm Med. 2022;22(1):17. doi: 10.1186/s12890-021-01809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou C, Jin L, Wang Q, et al. Bloodstream infections caused by carbapenem-resistant enterobacterales: risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infect Drug Resist. 2021;14:731–742. doi: 10.2147/IDR.S294282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Busch LM, Kadri SS. Antimicrobial treatment duration in sepsis and serious infections. J Infect Dis. 2020;222(Suppl 2):S142–S155. doi: 10.1093/infdis/jiaa247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Waele JJ, Martin-Loeches I. Optimal duration of antibiotic treatment in Gram-negative infections. Curr Opin Infect Dis. 2018;31(6):606–611. doi: 10.1097/QCO.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 31.Beery TA. Sex differences in infection and sepsis. Crit Care Nurs Clin N Am. 2003;15(1):55–62. doi: 10.1016/S0899-5885(02)00028-X. [DOI] [PubMed] [Google Scholar]
- 32.Bösch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Mil Med Res. 2018;5(1):35. doi: 10.1186/s40779-018-0182-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang MQ, Macala KF, Fox-Robichaud A, Mendelson AA, Lalu MM. Sex- and gender-dependent differences in clinical and preclinical sepsis. Shock. 2021;56(2):178–187. doi: 10.1097/SHK.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 34.Gu J, Xu J, Zuo TT, Chen YB. Ceftazidime-avibactam in the treatment of infections from carbapenem-resistant Klebsiella pneumoniae: ceftazidime-avibactam against CR-KP infections. J Glob Antimicrob Resist. 2021;26:20–25. doi: 10.1016/j.jgar.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 35.Chen F, Zhong H, Yang T, et al. Ceftazidime-avibactam as salvage treatment for infections due to carbapenem-resistant Klebsiella pneumoniae in liver transplantation recipients. Infect Drug Resist. 2021;14:5603–5612. doi: 10.2147/IDR.S342163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shields RK, Nguyen MH, Chen L, Press EG, Kreiswirth BN, Clancy CJ. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother. 2018;62(5):e02497-17. [DOI] [PMC free article] [PubMed]
- 37.Ojdana D, Gutowska A, Sacha P, Majewski P, Wieczorek P, Tryniszewska E. Activity of ceftazidime-avibactam alone and in combination with ertapenem, fosfomycin, and tigecycline against carbapenemase-producing Klebsiella pneumoniae. Microb Drug Resist. 2019;25(9):1357–1364. doi: 10.1089/mdr.2018.0234. [DOI] [PubMed] [Google Scholar]
- 38.Wang F, Zhou Q, Yang X, Bai Y, Cui J. Evaluation of ceftazidime/avibactam alone and in combination with amikacin, colistin and tigecycline against Klebsiella pneumoniae carbapenemase-producing K. pneumoniae by in vitro time-kill experiment. PLoS One. 2021;16(10):e0258426. [DOI] [PMC free article] [PubMed]
- 39.Cheng K, Newell P, Chow JW, et al. Safety profile of ceftazidime-avibactam: pooled data from the adult phase II and phase III clinical trial programme. Drug Saf. 2020;43(8):751–766. doi: 10.1007/s40264-020-00934-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrade RJ, Tulkens PM. Hepatic safety of antibiotics used in primary care. J Antimicrob Chemother. 2011;66(7):1431–1446. doi: 10.1093/jac/dkr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo YM, Ge FL, Song HB, et al. Relative risk analysis of liver-related adverse drug reactions in children based on China's national spontaneous reporting system. J Pediatr. 2021;234:85–91. doi: 10.1016/j.jpeds.2021.03.044. [DOI] [PubMed] [Google Scholar]
- 42.Shah T, Joslyn JA, Lai J. Ceftazidime induced liver injury. BMJ Case Rep. 2021;14(12):e246571. [DOI] [PMC free article] [PubMed]
- 43.Truong CB, Durham SH, Qian J. Comparisons of adverse event reporting for colistin versus polymyxin B using the US Food and Drug Administration Adverse Event Reporting System (FAERS) Expert Opin Drug Saf. 2021;20(5):603–609. doi: 10.1080/14740338.2021.1890024. [DOI] [PubMed] [Google Scholar]
- 44.Oliota AF, Penteado ST, Tonin FS, Fernandez-Llimos F, Sanches AC. Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn Microbiol Infect Dis. 2019;94(1):41–49. doi: 10.1016/j.diagmicrobio.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Rigatto MH, Behle TF, Falci DR, et al. Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J Antimicrob Chemother. 2015;70(5):1552–1557. doi: 10.1093/jac/dku561. [DOI] [PubMed] [Google Scholar]
- 46.Falcone M, Bassetti M, Tiseo G, et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care. 2020;24(1):29. doi: 10.1186/s13054-020-2742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.