Abstract
Objective:
Low dose computed tomography (LDCT) became the standard method for lung cancer (LC) screening in 2013. However, it is unclear whether there are differences in survival rates based on sex and whether the differences depend on screening status. We aimed to evaluate the LC survival rates between females and males based on screening.
Material and methods:
This retrospective cohort study examined data from the Boston LC Study (BLCS) between 2013–2021. LC screening depends on patients’ demographics (age and smoking history) to determine whether a person is a high-risk individual and, therefore, undergo LDCT. Descriptive statistics were calculated for race, age, histology, smoking history, stage, and treatment. These variables’ distributions were compared between sex and screening status using t-test and chi-square, respectively. Cox proportional hazards model and Kaplan-Meier curves were used to compare survival between sex and screening. Propensity score matching was applied to account for selection bias in screening when evaluating the association between screening and stage.
Results:
A total of 1,216 LC patients were identified with a screening incidence of 9.4%, among whom 56% were female. Unscreened males had 1.59 times higher risk of mortality than unscreened females (P=.0002) and had a worse 5-year survival (male 50%; 95%CI, 0.38,0.6 vs. female 70%; 95%CI,0.62,0.76). In contrast, there were no significant differences in survival between sexes among screened. In a balanced cohort of screened and unscreened, the odds of being diagnosed at late stages for females and smokers were 1.33 and 2.51 times that of males and nonsmokers; however, there were no statistical significance.
Conclusion:
Unscreened females had a lower risk of mortality and better survival than males, while among the screened population, there was no difference in the overall survival. These observations demonstrate the influence of sex on survival prognosis in LC when screening is not performed.
Keywords: Lung cancer, LDCT, screening, mortality, survival, sex disparities
1. BACKGROUND
Women are underrepresented in lung cancer (LC) screening studies, raising concerns regarding the generalizability of screening, criteria accuracy, and its overall applicability to women [1–5, 25]. Specifically, the National Lung Screening Trial (NLST) demonstrated that screening with low dose computed tomography (LDCT) in high-risk populations reduces lung cancer mortality by 20% while having a non-equivalent sex representation (females 41% vs. males 59%) [1,2]. A Korean study based on nationwide chest radiography screening showed that females had an earlier lung cancer stage, higher surgical resectability, and more prolonged survival. However, only 28% of the participants were female [1,3]. A Dutch-Belgian Lung Cancer Screening (NELSON) trial indicated that screening for lung cancer reduced mortality by 61% in women and 26% in men, yet a lack of female participation continues to be present (16%) [1,4, 25]. The Veterans Health Administration undertook the most extensive study to date, but only 3.7% of participants who underwent lung cancer screening were women [5]. In these studies, sex imbalance has severely hampered the generalizability and interpretability of the results in the context of lung cancer screening.
Over the last four decades, the incidence of lung cancer decreased by 35% in men but increased by 87% in women [1], which might be driven by the increasing rate of smoking among females [6]. While in the past, women were less likely to smoke than men, smoking rates have recently become more comparable [7]. LC mortality in women has also risen by more than 600% since 1950[1]. Recently, the LC incidence and mortality in both sexes has declined due to the implementation of LDCT [25]. However, it continues to be the leading cause of cancer death [9, 25].
Officially in 2013, the US Preventive Services Task Force (USPSTF) recommended annual screening for lung cancer with LDCT to high-risk individuals (B recommendation) [8]. The USPSTF defines a high-risk individual as someone between the ages of 50 to 80 with a 20 pack-year smoking history or who has quit within the last 15 years [9]. Studies have shown that generally, younger women with non-small cell lung cancer have a better prognosis than men [7,10]. As a result, women are more likely to be excluded from the current USPSTF LC screening guidelines and, therefore, less likely to have access to early detection diagnoses that could potentially save their lives. Despite females not having equal access to lung cancer screening, previous literature has shown that women have better survival after lung cancer treatment when compared to men [10–12]. However, much remains unknown about the association of sex with lung cancer screening, diagnosis, treatment, and survival. The increase in LC incidence and mortality in women relative to men continues to be a public health concern. Therefore, it is relevant to study lung cancer in women, from early detection to survival, to better understand how to reduce the onset of lung cancer. The current study evaluated the Boston Lung Cancer Study (BLCS) database to assess differences in survival rates between men and women dependent upon screening status. Additionally, equal representation between sex and screening was implemented to take into account the lack of distribution among these groups.
2. MATERIALS AND METHODS
2.1. Boston Lung Cancer Study
The BLCS is a cancer epidemiology cohort of 12,000 lung cancer cases enrolled at Massachusetts General Hospital (MGH) and Dana-Farber Cancer Institute (DFCI) since 1992. The BLCS gathered information on demographics, smoking, occupation, diet, pathology, imaging, treatments, oncogenic (somatic driver) mutation status, and bio-samples data. In 2013, MGH started using low-dose computed tomography (LDCT) to screen for lung cancer in high-risk individuals [13]. From then until 2021, the individuals who had data on the variables mentioned in section 2.2 (age, sex, race, smoking status, stage, histology, treatment, screening, and survival), date of birth, date of death, and date of the last visit were included in the study.
2.2. Study design and subject population
This retrospective cohort study examined data from the Boston Lung Cancer Study (BLCS) database between 2013 to 2021. The data included demographics such as age, sex, and race. The covariates evaluated included smoking status, stage, histology (adenocarcinoma, squamous, other), treatment, screening, and survival. Smokers were those who smoked more than 100 cigarettes in their lives [14].
2.3. Statistical Analysis
The primary objective of the study was to compare the overall survival rates between females and males based on screening status. The secondary endpoint was to evaluate the probability of detecting early stages through lung cancer screening in a balanced cohort of screened and unscreened patients. The balanced cohort was defined as subjects matched on sex, race, age, and smoking status between screened and unscreened groups.
The study population was categorized into four groups defined by sex and screening status (female screened, female unscreened, male screened, and male unscreened). Descriptive statistics were used to describe demographic variables (race and age), clinicopathological characteristics, smoking history, screening, and TNM stage frequency were also compared across these four groups. The Pearson chi-square test and t-test were applied to compare the distributions for categorical variables and continuous variables, respectively.
In the primary analysis, univariate Cox proportional hazards regression models were applied to estimate the hazard ratio (HRa) with 95% confidence intervals (CI) and p-value for each of the covariates. The following covariates were used: sex, sex based on screening, screening, age, smoking status, and stage. Stage was categorized as early-stage (1A, 1B, 2A, 2B), local advanced (3A), and late-stage (3B, 4, extensive) based on treatment guidelines [10]. Survival time was calculated from the date of diagnosis until the date of last visit or date of death, whichever occurred first. Additionally, multivariable models (HRb) were computed to adjust for confounders. Survival was further compared across the four groups defined by sex and screening status by fitting Kaplan-Meier survival curves to estimate standardized survival proportions for men and women from the date of diagnosis to the date of the last visit [10, 15–18].
In the secondary analysis, propensity score models were developed to estimate the probability of screening based on demographics (sex, race, age, and smoking status) for each individual, and 1:1 propensity score matching was used to create a balanced cohort of screened and unscreened subjects. As screening was not randomized, this method sought to adjust for sampling bias by matching subjects for comparison based on their likelihood of being screened and, therefore, create a pseudo-randomized setting. Current multivariable ordinal logistic regression, adjusted for the covariates, was applied to model the odds of being diagnosed at a later stage comparing the screened vs. unscreened groups [19–21].
A P value of <.05 was considered statistically significant. All statistical analyses were performed by using R studio version 1.3.1093.
3. RESULTS
3.1. Study Cohort
A total of 2,839 patients with lung cancer were identified from the BLCS database, all of whom had complete data for sex, date of birth, date of death, and date of the last visit. From these, 1,216 participants who were diagnosed with lung cancer between 2013 to 2021 were included in the analysis. The overall screening prevalence was 9.4%. In both groups (screened vs. unscreened), the female population was the most predominant (56%). (Figure 1)
Figure 1.
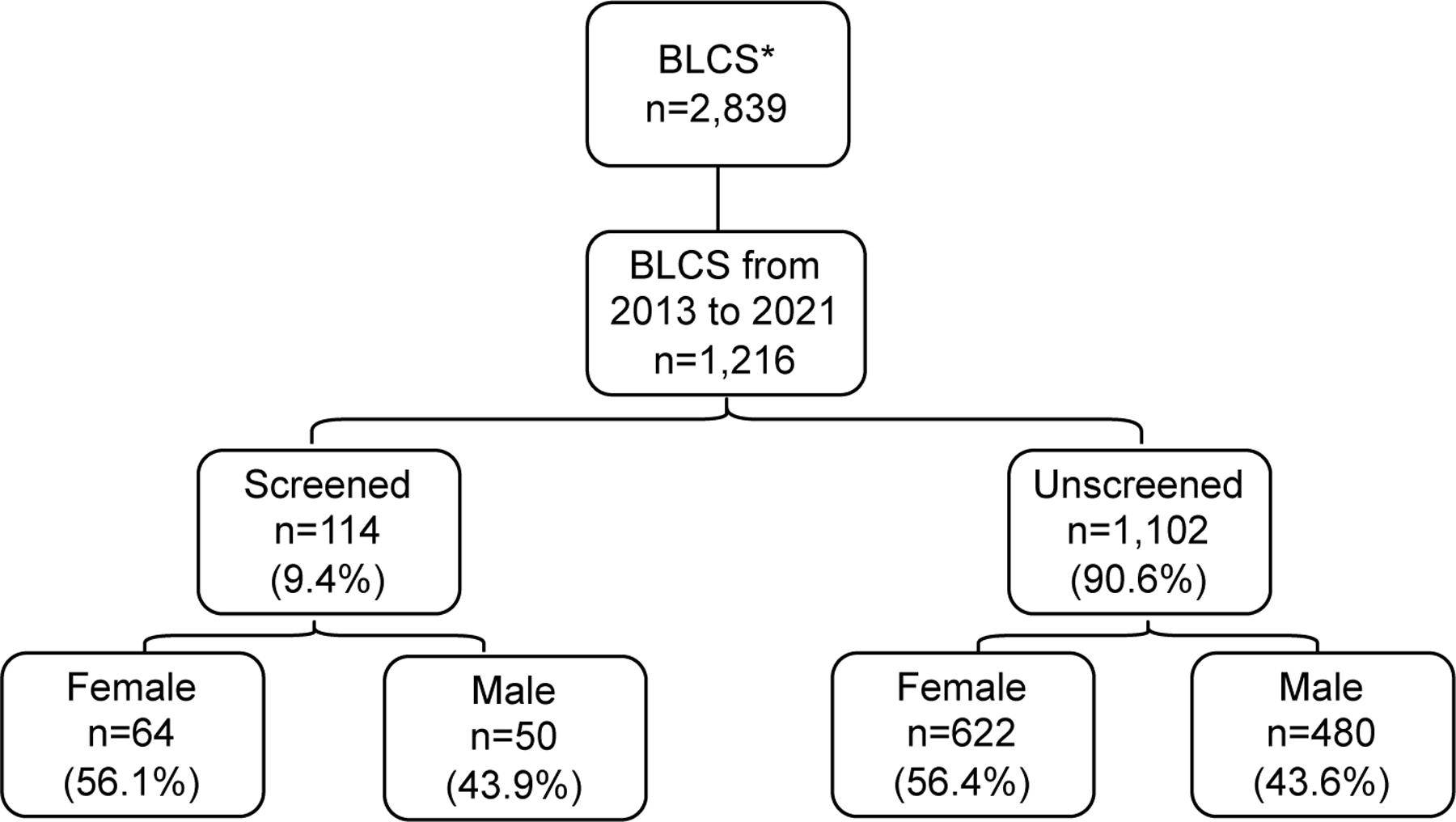
Study cohort
*Boston Lung Cancer Study (BLCS)
There were a total of 631 patients eligible to undergo LDCT according to the USPSTF eligibility criteria for lung cancer screening, but only 65 subjects were screened. Of the 441 patients with lung cancer who did not meet eligibility criteria 7 were screened. (Table A)
3.2. Demographic data
Table 1 showed the comparison between females screened vs. females unscreened and males screened vs. males unscreened. The mean age at the time of diagnosis was not significantly different between sex and screening status. The mean age among the four groups did not differ (females 66–67 years and males 67–68 years). Although the mean age at the time of death had a greater difference between females not screened and screened (68 years vs. 71 years), it was not statistically significant (P=.23).
Table 1.
Demographic characteristics of the study population from 2013 to 2021 (n=1,216)
| Female | Male | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Not screened (n= 622) |
Screened (n= 64) |
P value | Not screened (n=480) |
Screened (n=50) |
P value | |
| Age | 66.71 (10.32) | 67.94 (6.17) | .16 | 67.32 (10.83) | 68.15 | .54 |
| (6.2) | ||||||
| Race | .002 | .98 | ||||
| White/Caucasian | 587 (94.4) | 59 (93.7) | 443(92.5) | 44 (91.7) | ||
| Black / African American | 11 (1.8) | 2 (3.2) | 6 (1.3) | 1 (2.1) | ||
| Hispanic or Latino | 2 (0.3) | - | 2 (0.4) | - | ||
| Asian | 11 (1.8) | - | 18 (3.8) | 3 (6.2) | ||
| Native American | - | 2 (3.2) | 2 (0.4) | - | ||
| Other | 2 (0.3) | - | 2 (0.4) | - | ||
| Unknown/Declined | 9 (1.4) | - | 6 (1.25) | - | ||
| Smokers | 482 (80.33) | 60(93.8) | .022 | 397 (85.4) | 45 (100) | .01 |
| Stage | <.0001 | .005 | ||||
| 1A | 237 (38.1) | 45 (70.3) | 131(27.3) | 29 (58) | ||
| 1B | 72 (11.6) | 3 (4.7) | 69 (14.4) | 7 (14.6) | ||
| 2A | 15 (2.4) | 3 (4.7) | 21 (4.4) | 2 (4.2) | ||
| 2B | 34 (5.5) | 5 (7.8) | 41 (8.5) | 5 (10.4) | ||
| 3A | 64 (10.3) | 5 (7.8) | 59 (12.3) | 3 (6.2) | ||
| 3B | 17 (2.7) | 1 (1.6) | 16 (3.3) | 1 (2.2) | ||
| 4 | 152 (24.4) | 1 (1.6) | 119 (24.8) | - | ||
| Extensive | 21 (3.4) | - | 14 (2.9) | 1 (2.1) | ||
| Limited | 10 (1.6) | 1 (1.6) | 10 (2.1) | - | ||
| Histologic cell type | .63 | .82 | ||||
| Adenocarcinoma | 431 (70.8) | 51 (81) | 288 (60.8) | 34 (69.4) | ||
| Squamous cells carcinoma | 78 (12.8) | 5 (7.9) | 109 (23) | 8 (16.3) | ||
| Large cell carcinoma | 6 (1) | 1 (1.6) | 6 (1.3) | - | ||
| Small cell carcinoma | 31 (5.1) | 1 (1.6) | 24 (5.1) | 1 (2.1) | ||
| Bronchioalveolar carcinoma | 1 (0.2) | - | 1 (0.2) | - | ||
| Mixed type | 12 (2) | - | 8 (1.7) | 2 (4.1) | ||
| More than one primary | 4 (0.7) | 1 (1.6) | 4 (0.8) | - | ||
| NSCLC | 23 (3.8) | 1 (1.6) | 27 (5.7) | 1 (2) | ||
| Not classified | 4 (0.7) | 1 (1.6) | 1 (0.2) | 3 (6.1) | ||
| Treatment | ||||||
| Surgery | 374 (60.1) | 57 (89.1) | < .0001 | 265 (55.2) | 40 (80) | .0013 |
| Chemotherapy | 227 (36.5) | 7 (11.1) | < .0001 | 201 (41.9) | 7 (14) | <.0001 |
| Radiotherapy | 120 (19.3) | 5 (7.9) | < .0001 | 108 (22.5) | 9 (18) | .58 |
| Deaths | 111 (17.8) | 6 (9.3) | .12 | 132 (27.5) | 4 (8) | .005 |
Data are reported as No. (%) in categorical variables and mean (SD) for continuous variables; percentages may not addup to 100 due to rounding and missing values in treatment, histologic cell type, race, smoking history and stage. Age was regarded as the mean age at the moment of diagnosis.
Smoking status was significantly different between females not screened vs. females screened and males not screened vs. screened (P= .022, P=.01). The majority of the study population was Caucasian (~95%), followed by Asian (~3%) and Black/African American (~2%).
Adenocarcinoma was the histological cell type most recurrent in screened and not screened participants (>60%). Nevertheless, the screened groups and females had the highest frequency of this histologic subtype (>69%). In addition, squamous cell carcinoma was the second most common subtype, primarily presented in males in both groups.
Although Stage 1A was the most frequent stage diagnosed in the four groups, the percentage of diagnosis in the screened population was higher than the non-screened. Additionally, Stage IV (NSCLC) and extensive stage (SCLC) were more common in unscreened participants. (Table 1)
Of the 1,216 participants, there was a statistically significant difference in treatment between females not screened vs. screened and males not screened vs. screened (surgery P<.0001, P=.0013; chemotherapy P<.0001, P<.0001). The unscreened groups had a higher mortality percentage, predominantly in men (females 17.8% and males 27.5%). (Table 1)
3.3. Survival analysis
Table 2 shows the mortality risk by identifying the hazard ratio for each covariate (screening, sex, sex based on screening, age, smoking status and stage). Those who were screened had a 45% lower risk of mortality than those who were unscreened and the effect was marginally significant (P=0.07); but after adjusting for age, smoking status, sex, and stage, this risk increased to 1.14 times as that for the unscreened and was highly non-significant (P=.79). Therefore, age, smoking status, sex, and stage were strong confounders for screening. Overall, males had 1.59 times the risk of mortality as females, and after adjustment of covariates, the risk increased to 1.62; however, among those screened, males’ mortality risk was only 0.5 and 0.57 as females’, without and with covariate adjustment, respectively, though both were non-significant. Smoking and late stages were associated with higher mortality without covariate adjustment (smoker P=.014, HR=1.62; local advanced P<.0001, HR=2.82; late-stage P<.0001, HR=6.41) and with covariate adjustment (smoker P=.0013, HR=1.89; local advanced P<.0001, HR=2.91; late-stage P<.0001, HR=7.73).
Table 2.
Lung cancer mortality risk (n=1,216)
| HRa | 95%CI | P value | HRb | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Screened (vs. unscreened) | 0.55 | 0.29, 1.04 | .07 | 1.14 | 0.41, 3.13 | .79 |
| Male (vs. female) | 1.59 | 1.25, 2.04 | .0002 | 1.62 | 1.25, 2.08 | .00022 |
| Male screened (vs female screened) | 0.5 | 0.14, 1.84 | .3 | 0.57 | 0.12, 2.58 | .46 |
| Male unscreened (vs female unscreened) | 1.97 | 0.54, 7.16 | .3 | 1.6 | 0.35, 7.31 | .54 |
| Age | 1.005 | 0.99, 1.02 | .38 | 1.02 | 1.01, 1.04 | .00036 |
| Smokers | 1.62 | 1.1, 2.37 | .014 | 1.89 | 1.28, 2.79 | .0013 |
| Local advanced | 2.82 | 1.84, 4.34 | <.0001 | 2.91 | 1.89, 4.49 | <.0001 |
| Late stage | 6.41 | 4.77, 8.62 | <.0001 | 7.73 | 5.65, 10.59 | <.0001 |
Hazard ratios (HRs) with P-value and 95% confidence intervals (CI) were calculated using the univariate Cox proportional hazard model.
HR were adjusted for sex, age, smoking history and stage.
The stages were classified as early-stage (1A, 1B, 2A, 2B), local advanced (3A), and late-stage (3B, 4, extensive).
Kaplan Meier survival estimates showed that females’ 5-year survival rate is about 0.70 (or 70%) [95% CI=0.62, 0.76] for unscreened and 0.75 (or 75%) [95% CI=0.57, 1.00] for screened, and there were no significant differences in survival between these two groups (Figure 2. A). On the other hand, the probability of survival at year 5 was approximately 0.55 (or 55%) [95% CI=0.38, 0.60] for males unscreened and 0.875 (or 87.5%) [95% CI=0.73, 1.00] for males screened, suggesting a better outcome for the male screened group in contrast to the male unscreened group (Figure 2. B). Among the screened population, the comparison between males vs. females did not suggest a significant difference (Figure 2. C). However, Figure 2. D did suggest better survival for women compared to men among the unscreened group.
Figure 2.
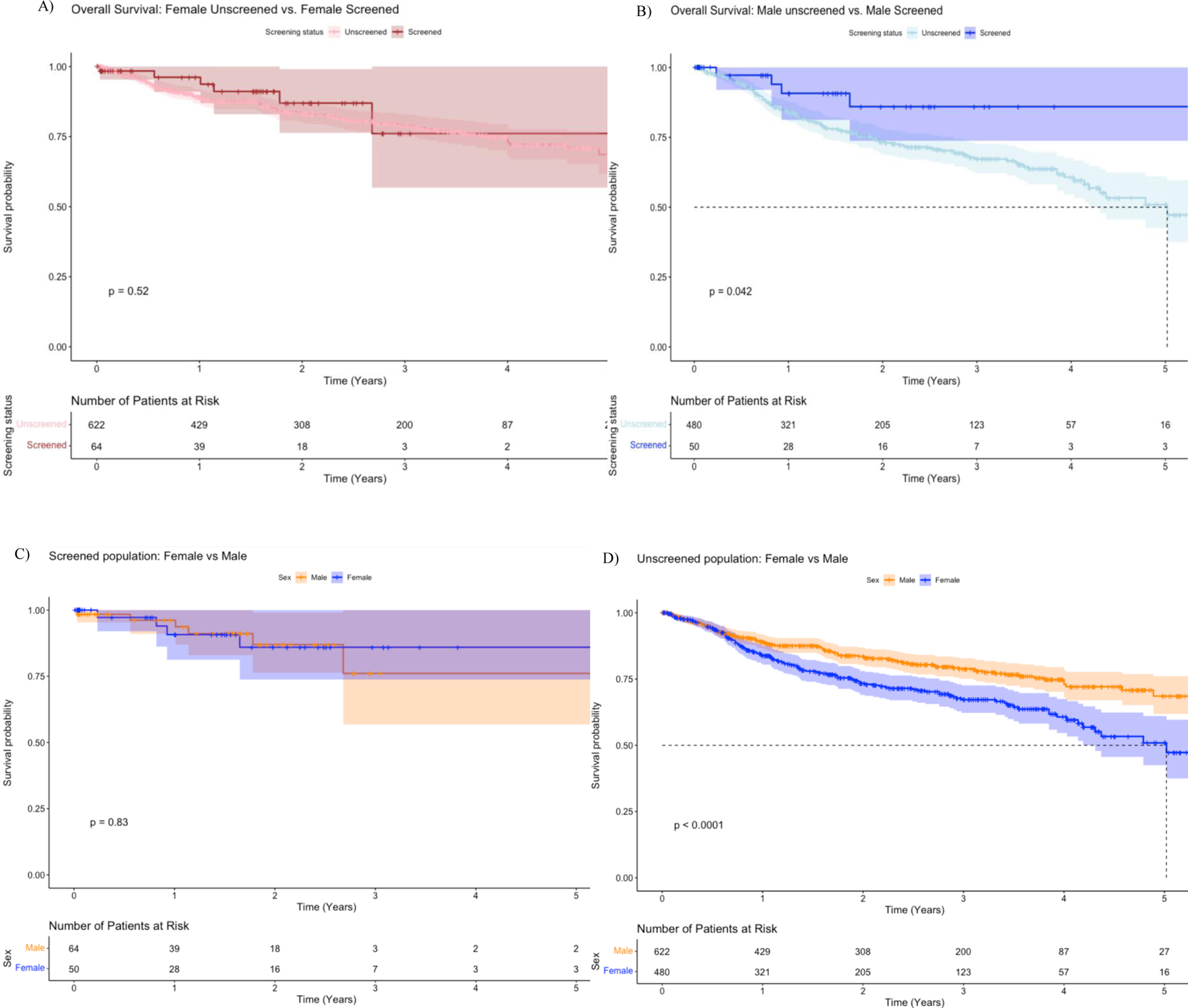
Overall survival between each sex group compared to their screening status.
(A)Overall survival of 622 females unscreened (pink line) and 64 females screened (red line). (B) Overall survival of 480 males unscreened (light blue line) and 50 males screened (dark blue line). (C) Overall survival among the screened population of 64 females (orange line) and 50 males (dark blue line). (D) Overall survival among the unscreened group of 622 females (orange line) and 480 males (dark blue line).
3.4. Propensity Score Matching
Propensity score matching was used when assessing the association between screening and stage of diagnosis. The matching criteria were detailed in Table B. The match produced an appropriate number of unscreened participants who matched the screened participants, and there was a substantial reduction in bias associated with the match. Table B showed that the screened and matched groups were not statistically different on any of the matching covariates.
3.5. Ordinal Logistic Regression Discharge Models
A proportional odds model was used to study the association of screening with stage (a 3-level ordinal variable). As shown in Table 3, the odds of being diagnosed at a later stage (stage 1 vs stage 2,3 / stage 1,2 vs stage 3) for screened patients was only 0.2 times that of individuals who were not screened (P<.0001). In other words, those who were screened had a higher probability of being diagnosed at an earlier stage. Furthermore, there was no statistically significant difference in the odds of being diagnosed at a later stage between sexes. Race and age did not have an association with the stage (OR=0.9; 95% CI=0.32, 2.7) (OR=0.98; 95% CI=0.94, 1.02).
Table 3.
Odds ordinal logistic regression with the match data according to their propensity score (n=210).
| OR* | 95% CI | P value | |
|---|---|---|---|
| Screening (vs. Unscreened) | 0.2 | 0.11, 0.36 | <.0001 |
| Age | 0.98 | 0.94, 1.02 | .33 |
| Female (vs. Male) | 1.33 | 0.73, 2.43` | .35 |
| White (vs. other races) | 0.9 | 0.32, 2.7 | .85 |
| Smoking | 2.51 | 0.51, 18.81 | .29 |
| Stage 1|Stage 2 (Intercept −0.93) | 0.39 | - | .63 |
| Stage 2|Stage more 3 (Intercept −0.34) | 0.71 | - | .86 |
Odds ratio (OR) were calculated (late stage vs. early stage).
4. DISCUSSION
This is the first study to our knowledge to evaluate LC survival rates between females and males based on screening status and the association between screening and stage in a balanced cohort. Among the 1,216 patients assessed, the screening incidence was 9.4%. When compared to the national rate (6%), the proportion is high but when compared to its state, Massachusetts (MA), the rate was low (9% vs. 18%). This was expected since the data was retrieved from only two hospitals. Although MA has the highest rate of LC screening in high-risk adults among all states [23], nationwide screening is critical for prevention as LC remains the leading cause of cancer death worldwide [1].
Similar to prior studies, the current study demonstrated a better prognosis for women than men [7,9], highlighting our first objective. Unscreened women, from the diagnosis date to the last visit date, showed more prolonged survival than unscreened men. However, when screened women were compared to screened men, there were no differences in survival probability. These findings could suggest that screening, regardless of sex, is effective to reduce sex disparities in lung cancer survival. To support this observation, future research should continue examining sex disparities with a larger sample size to include higher incidences of death. Furthermore, when women were compared to their screening status, there was no difference between the unscreened group and screened group. This might be due to the limited sample size of the screened group. Due to the novelty of LC screening, uptake is low, and the system has not updated all the codes for screening, exposing the study to selection bias. Accounting for this, propensity score matching (PSM) was implemented. Furthermore, the large number of participants with LC who were not screened gave adequate power to the study to obtain statistical significance. Further research should use a larger sample size to strengthen the understanding of survival rates in women based on screening status.
Since the underrepresentation of women in lung cancer research has been apparent [1,5], the current study used matched group analysis that resulted in an even distribution to allow for a more accurate interpretation of results, as indicated in the second objective. Although our balanced cohort did not show statistical significance in demonstrating that females and smokers were more likely to be in later stages than males and nonsmokers, this trend is important to consider due to the difficulties women face in the screening process. It has been shown that young women are more predisposed than men to develop LC and yet, are unable to meet the age requirement to get screened. Therefore, it is not surprising to see that women were diagnosed in later stages, as they are not able to get screened early on [7,9]. Future research should evaluate the significance of this observation in a larger sample size. Moreover, in previous research, lung cancer was found to be sex-specific, women showing greater rates of adenocarcinoma and men having higher rates of squamous cell carcinoma [7, 9]. However, our study observed adenocarcinoma as the most prevalent subtype across all the groups regardless of sex. These findings raise uncertainty over whether LC is sex-specific or whether an unbalanced cohort in past studies skewed the results to a particular sex group.
Across all four groups, the most common stage of LC was IA. However, in the screening group, the percentage of diagnoses was higher than in the non-screened group. This was expected as the goal of screening is to detect LC in its early stages. Additionally, stage IV and extensive stage were more common in non-screened subjects. This was expected since the majority of individuals with LC who do not undergo thorough screening are diagnosed later [24]. Since the current study used a database from only two hospitals, the diversity of the sample was limited. Results showed a low proportion of Latin, Black, and Asian patients. Future research should use a larger and more diverse sample to acknowledge the potential impact race has on cancer type and stage.
5. CONCLUSION
Overall, our study findings supported that screening has an association with lower lung cancer mortality. Age, smoking status, and lung cancer stage were shown to be strong effect modifiers for screening. Although unscreened females had a lower risk of mortality than unscreened males, among the screened population, there was no difference in the overall survival rate. These observations demonstrate the influence that sex has on survival prognosis in LC when screening is not performed.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Janice Weinberg and Professor Stacey Hess Pino for proofreading my Capstone project, on which this study was based. We also thank Ms. Andrea Shafer for research study support and all of the study participants.
FUNDING
This study was supported by NIH (NCI) grant # U01CA209414.
Footnotes
The manuscript, including related data, figures, and tables, has not been published previously, and it is not under consideration elsewhere.
REFERENCES
- [1].Randhawa S, Sferra S, Das C, Kaiser L, Ma G and Erkmen C, 2020. Examining Gender Differences in Lung Cancer Screening. Journal of Community Health, 45(5), pp.1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Aberle DR, Adams AM, Berg CD, et al. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine, 365(5), 395–409. [PubMed: 21714641] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koo HJ, Choi CM, Park S, et al. (2019). Chest radiography surveillance for lung cancer: Results from a National Health Insurance database in South Korea. Lung Cancer, 128, 120–126. [PubMed: 30642443] [DOI] [PubMed] [Google Scholar]
- [4].Dawson Q (2020). NELSON trial: Reduced lung-cancer mortality with volume CT screening. Lancet Respiratory Medicine, 8(3), 236. [PubMed: 32035508] [DOI] [PubMed] [Google Scholar]
- [5].Kinsinger LS, Anderson C, & Kim J (2017). Implementation of lung cancer screening in the veterans’ health administration. JAMA Internal Medicine, 177(3), 399–406. [PubMed: 28135352] [DOI] [PubMed] [Google Scholar]
- [6].Egleston B, Meireles S, Flieder D and Clapper M, 2009. Population-Based Trends in Lung Cancer Incidence in Women. Seminars in Oncology, [online] 36(6), pp.506–515. Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2846780/pdf/nihms167042.pdf> [Accessed 17 April 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stapelfeld C, Dammann C and Maser E, 2019. Sex‐specificity in lung cancer risk. International Journal of Cancer, 146(9), pp.2376–2382. [DOI] [PubMed] [Google Scholar]
- [8].Szabo P, 2021. Lung cancer screening with low dose computed tomography. Nursing, 51(6), pp.65–67. [DOI] [PubMed] [Google Scholar]
- [9].Uspreventiveservicestaskforce.org. 2021. Recommendation: Lung Cancer: Screening | United States Preventive Services Taskforce [online] Available at: <https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening> [Accessed 27 June 2021]. [Google Scholar]
- [10].Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, & Lambe M (2019). Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLOS ONE, 14(6), e0219206. 10.1371/journal.pone.0219206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Samet JM, Avila-Tang E, Boffetta P, Hannan LM, Olivo-Marston S, Thun MJ, & Rudin CM (2009). Lung Cancer in Never Smokers: Clinical Epidemiology and Environmental Risk Factors. Clinical Cancer Research, 15(18), 5626–5645. 10.1158/1078-0432.ccr-09-0376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Radkiewicz C, Johansson AL, Dickman PW, Lambe M, & Edgren G (2017). Sex differences in cancer risk and survival: A Swedish cohort study. European Journal of Cancer, 84, 130–140. 10.1016/j.ejca.2017.07.01 [DOI] [PubMed] [Google Scholar]
- [13].Epi.grants.cancer.gov. 2022. Boston Lung Cancer Survival Cohort | EGRP/DCCPS/NCI/NIH [online] Available at: <https://epi.grants.cancer.gov/cohort-consortium/members/blcs.html> [Accessed 22 February 2022]. [Google Scholar]
- [14].Centers for Disease Control and Prevention. 2022. Adult Tobacco Use Information, Glossary [online] Available at: <https://www.cdc.gov/nchs/nhis/tobacco/tobacco_glossary.htm> [Accessed 19 April 2022]. [Google Scholar]
- [15].Ouellette D, Desbiens G, Emond C, & Beauchamp G (1998). Lung cancer in women compared with men: stage, treatment, and survival. The Annals of Thoracic Surgery, 66(4), 1140–1143. 10.1016/s0003-4975(98)00557-8 [DOI] [PubMed] [Google Scholar]
- [16].Sakurai H, Asamura H, Goya T, Eguchi K, Nakanishi Y, Sawabata N, Okumura M, Miyaoka E, & Fujii Y (2010). Survival Differences by Gender for Resected Non-small Cell Lung Cancer: A Retrospective Analysis of 12,509 Cases in a Japanese Lung Cancer Registry Study. Journal of Thoracic Oncology, 5(10), 1594–1601. 10.1097/jto.0b013e3181f1923b [DOI] [PubMed] [Google Scholar]
- [17].Lambert PC, & Royston P (2009). Further Development of Flexible Parametric Models for Survival Analysis. The Stata Journal: Promoting Communications on Statistics and Stata, 9(2), 265–290. 10.1177/1536867x0900900206 [DOI] [Google Scholar]
- [18].Sjölander A (2016). Regression standardization with the R package stdReg. European Journal of Epidemiology, 31(6), 563–574. 10.1007/s10654-016-0157-3. [DOI] [PubMed] [Google Scholar]
- [19].Sacco P, Unick G, Zanjani F and Camlin E, 2015. Hospital Outcomes in Major Depression Among Older Adults: Differences by Alcohol Comorbidity. Journal of Dual Diagnosis, 11(1), pp.83–92. Available at: Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4718396/pdf/nihms751028.pdf> [Accesed 15 February 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Brookhart M, Wyss R, Layton J and Stürmer T, 2013. Propensity Score Methods for Confounding Control in Nonexperimental Research. Circulation: Cardiovascular Quality and Outcomes, [online] 6(5), pp.604–611. Available at: < [Accessed 15 February 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhao Q, Luo J, Su Y, Zhang Y, Tu G and Luo Z, 2021. Propensity score matching with R: conventional methods and new features. Annals of Translational Medicine, [online] 9(9), pp.812-812. Available at: <https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8246231/pdf/atm-09-09-812.pdf> [Accessed 15 February 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Richards T, Soman A, Thomas C, VanFrank B, Henley J, Gallaway M and Richardson L, 2020. Screening for Lung Cancer — 10 States, 2017. Centers for Disease Control and Prevention, [online] 69(8), pp.201–206. Available at: <https://www.cdc.gov/mmwr/volumes/69/wr/pdfs/mm6908a1-H.pdf> [Accessed 03 August 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lung.org. 2022. State of Lung Cancer | Massachusetts [online] Available at: <https://www.lung.org/research/state-of-lung-cancer/states/massachusetts> [Accessed 30 March 2022]. [Google Scholar]
- [24].Jonas D, Reuland D, Reddy S, Nagle M, Clark S, Weber R, Enyioha C, Malo T, Brenner A, Armstrong C, Coker-Schwimmer M, Middleton J, Voisin C and Harris R, 2021. Screening for Lung Cancer with Low-Dose Computed Tomography. JAMA, 325(10), p.971. [DOI] [PubMed] [Google Scholar]
- [25].de Koning H, van der Aalst C, de Jong P, Scholten E, Nackaerts K, & Heuvelmans M et al. (2020). Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. New England Journal Of Medicine, 382(6), 503–513. doi: 10.1056/nejmoa1911793 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


