Significance
Eating and drinking both activate a subset of accumbal neurons but it was unknown whether same or different neurons represent distinct need states. We set out to study the state-coding principles of hunger and thirst in the nucleus accumbens (NAc) using two-photon calcium imaging of neural activity in awake mice during feeding and drinking. We find that highly overlapping sets of individual D1 and D2 neurons respond similarly to food and water with specific subsets showing distinct temporal activity patterns throughout the consummatory phase. Modulating D1 and D2 neurons elicited analogous effects on both behavioral programs. These data suggest a general role of NAc to regulate instinctive behaviors, likely by modulating motivation, and further indicate that need-specific representations and prioritizations are encoded elsewhere.
Keywords: need states, motivation, two-photon calcium imaging, feeding behavior, reward
Abstract
The nucleus accumbens (NAc) is a canonical reward center that regulates feeding and drinking but it is not known whether these behaviors are mediated by same or different neurons. We employed two-photon calcium imaging in awake, behaving mice and found that during the appetitive phase, both hunger and thirst are sensed by a nearly identical population of individual D1 and D2 neurons in the NAc that respond monophasically to food cues in fasted animals and water cues in dehydrated animals. During the consummatory phase, we identified three distinct neuronal clusters that are temporally correlated with action initiation, consumption, and cessation shared by feeding and drinking. These dynamic clusters also show a nearly complete overlap of individual D1 neurons and extensive overlap among D2 neurons. Modulating D1 and D2 neural activities revealed analogous effects on feeding versus drinking behaviors. In aggregate, these data show that a highly overlapping set of D1 and D2 neurons in NAc detect food and water reward and elicit concordant responses to hunger and thirst. These studies establish a general role of this mesolimbic pathway in mediating instinctive behaviors by controlling motivation-associated variables rather than conferring behavioral specificity.
Organisms maintain homeostatic control of energy and fluid balance by sensing need states and in turn initiating behaviors that enable them to find, and then consume, food and water. The ability of an animal to successfully execute these behaviors depends on their ability to parse various interoceptive and exteroceptive signals and respond appropriately. Once engaged, these goal-driven behaviors begin with an appetitive, or sensory, phase (locating food and water) followed by a consummatory phase (ingestion of food and water) (1). These behaviors are controlled by several brain centers and circuits, and the appropriate prioritization and fulfillment of hunger or thirst by the brain is essential for homeostasis (2). However, while separate hypothalamic nodes convey hunger and thirst, these two need states also elicit potent responses in reward centers in the brain, in particular in the nucleus accumbens (NAc) (3). In NAc, both food and water activate subsets of neurons that have been shown to invigorate the behavior by transmitting positive or negative valence (4). Furthermore, operant-based tasks examining food- or water-seeking behavior have suggested that dopamine signaling in NAc is critical for both goal-directed behaviors and for reinforcement learning (5, 6). This dopamine signal is parsed by two predominant neuronal cell types within the NAc expressing the excitatory dopamine D1-receptor or the inhibitory D2-receptor, and these neurons have been suggested to exert opposing influences on goal-directed behavior (7). It is unknown whether dopamine transmission itself fully explains the antagonistic functions between these two cell types.
It is also unknown whether there are dedicated pathways in NAc encoding food and water rewards or if the same neurons are activated in response to each. This distinction is important because the former would suggest a role in behavior selection while the latter would be more consistent with a role in motivation. Unraveling the role of D1 and D2 in different need states has been limited by the inability to follow the activity patterns of individual D1 or D2 neurons in the NAc serially while an animal is engaged in different goal-directed behaviors. Thus, deconstructing the dynamics of NAc neural ensembles in awake, behaving animals serially could reveal the underlying coding principles in NAc governing goal-directed behaviors required for coordinating these two homeostatic needs.
Here, we report the use of in vivo imaging to follow the activity pattern among hundreds of individual D1 and D2 neurons simultaneously in the NAc in response to food vs. water. We also performed functional studies to establish the role of these neurons to regulate seeking and consumption for both food and water in hungry and dehydrated animals. We find that individual D1 and D2 neurons show activation in response to both hunger and thirst during the appetitive phase. We also find that highly overlapping D1 and D2 neurons are activated during consumption, but in this case, the neurons segregate into at least three different temporal clusters corresponding to initiation, reward consumption and behavioral cessation. These data together with functional studies modulating D1 and D2 neural activity reveal a general role of D1 and D2 NAc neurons to elicit a coordinated neuronal response to these two need states.
Results
Neural Substrates of Feeding and Drinking in NAc.
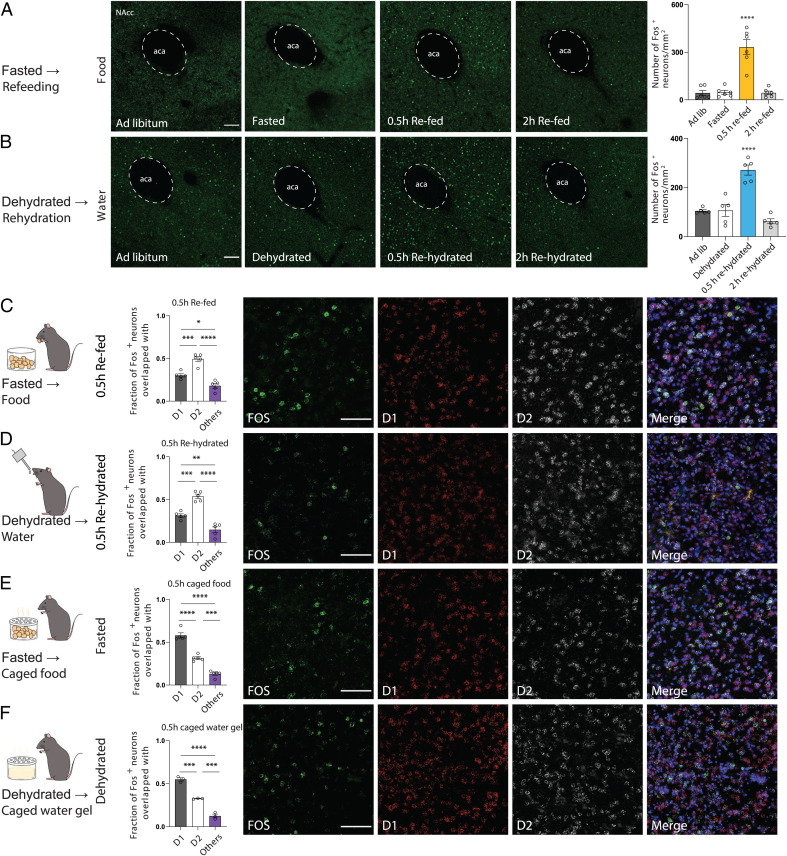
Multiple lines of evidence have shown that mesolimbic dopamine system activity is a key brain circuit regulating multiple goal-directed behaviors. However, while dopamine release in NAc is associated with both food and water consumption (8), it is not known whether the same or distinct neural ensembles in NAc respond to these two different need states. To address this question, we began by comparing the expression pattern of c-Fos in NAc using immunohistochemistry (IHC) under four different conditions: ad libitum access to food and water, after an overnight fast, and refeeding after a fast for 30 min or 2 h. We observed increased c-Fos expression in the NAc core (NAcc), but not the shell, after 30 min after refeeding that did not persist at 2 h (Fig. 1 A and B and SI Appendix, Fig. 1 A–C). We next performed the analogous experiment by providing water after fluid deprivation and found a similar pattern of increased c-Fos expression in the NAcc, but not shell, 30 min after rehydration (Fig. 1 A and B and SI Appendix, Fig. 1 A–C). In both cases, c-Fos expression returned to baseline 2 h after refeeding or rehydration (Fig. 1 A and B and SI Appendix, Fig. 1B).
Fig. 1.
c-Fos labeling identifies NAc neurons are activated by both food and water in a state-dependent manner. (A) Quantification of c-Fos protein expression in fed, fasted, and refed animals (above images, n = 6 mice per group for refeeding assay, one-way ANOVA, with Tukey’s multiple comparisons). (B) Quantification of c-Fos expression in hydrated, dehydrated and rehydrated animals (bottom images, n = 5 mice per group for rehydration assay, one-way ANOVA, with Tukey’s multiple comparisons). (C–F) Coexpression of D1 (Drd1) and D2 (Drd2) receptor mRNA with c-Fos mRNA in NAc after refeeding and rehydration assay, caged food, and caged water gel assay (Left). Representative images (Right) showing colocalization of neurons expressing c-Fos (green), Drd1 (red) and Drd2 (white); DAPI, blue. (Scale bar, right images: 100 μm.) (C and D) Quantification of percentages of colocalization between c-Fos, Drd1, and Drd2 mRNA-expressing neurons (n = 5 sections from three mice for both refeeding and rehydration assays, one-way ANOVA, with Tukey’s multiple comparisons). (E and F) Quantification of percentages of colocalization between c-Fos-, Drd1-, and Drd2-expressing neurons (n = 5 sections from three mice for caged food assay, n = 3 sections from three mice for caged water gel assay, one-way ANOVA, with Tukey’s multiple comparisons). All error bars represent mean ± SEM. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next used in situ hybridization to colocalize c-Fos mRNA induced with Drd1 (D1) or Drd2 (D2) expressing neurons after refeeding or rehydration. We found extensive colocalization of c-Fos in both D1 and D2 neurons 30 min after refeeding (Fig. 1C and SI Appendix, Fig. 1D), with ∼60% of c-Fos-positive neurons expressing D2 and ∼30% expressing D1. A similar pattern was seen after rehydration, with ∼60% c-Fos-positive neurons expressing D2 and ∼30% expressing D1 (Fig. 1D and SI Appendix, Fig. 1D). Only a small number of c-Fos-positive cells (∼10%) failed to express either D1 or D2. To further explore the identity of the remaining (D1R and D2R negative) c-Fos-positive cells, we performed ISH for c-Fos mRNA together with mRNA for choline acetyltransferase (ChAT)—a genetic marker for cholinergic interneurons which are also present in low numbers in the NAc. However, we found that only 2.3% of c-Fos-positive neurons were also ChAT-positive (SI Appendix, Fig. 2 A and B). This suggests that the great majority of neurons that respond to a food or water reward express either D1R or D2R while a small subset of cholinergic and other cell types also showing cFos activation.
We next tested whether sensory cues or consumption were responsible for activation of D1 or D2 neurons in the NAc by exposing fasted or dehydrated animals to caged food or water that they could not consume. This protocol enabled animals to perceive appetitive sensory cues associated with food or water while preventing consumption. While the total number of neurons that were activated was similar to those in prior experiments, we found that the proportion of D1 and D2 neurons responding to either stimulus was altered, with ∼60% c-Fos-positive neurons now expressing D1 and ∼30% of neurons expressing D2 (Fig. 1 E and F and SI Appendix, Fig. 1E). These data suggest that D1 neurons can sense both hunger and thirst and may play a key role in the sensory phase while also raising the possibility that D2 neurons may play a more prominent role during the consummatory phase.
Shared D1 and D2 Neuronal Ensembles in NAc Control Feeding and Drinking.
While similar numbers of D1 and D2 neurons showed c-Fos expression after providing a food or water reward to fasted or dehydrated animals, only ∼30% of the neurons showed biochemical activation, and thus, this approach did not enable us to determine whether the same or different neurons were activated by the two stimuli. We thus set out to determine how individual D1 and D2 neurons differentially respond to food vs. water using in vivo two-photon Ca2+ imaging via an implanted GRIN lens in head-fixed mice (SI Appendix, Fig. 3A). The use of this imaging approach enabled us to record from a large number of the same D1 or D2 neurons over the course of several days by first providing hungry animals with food and then providing thirsty animals with water.
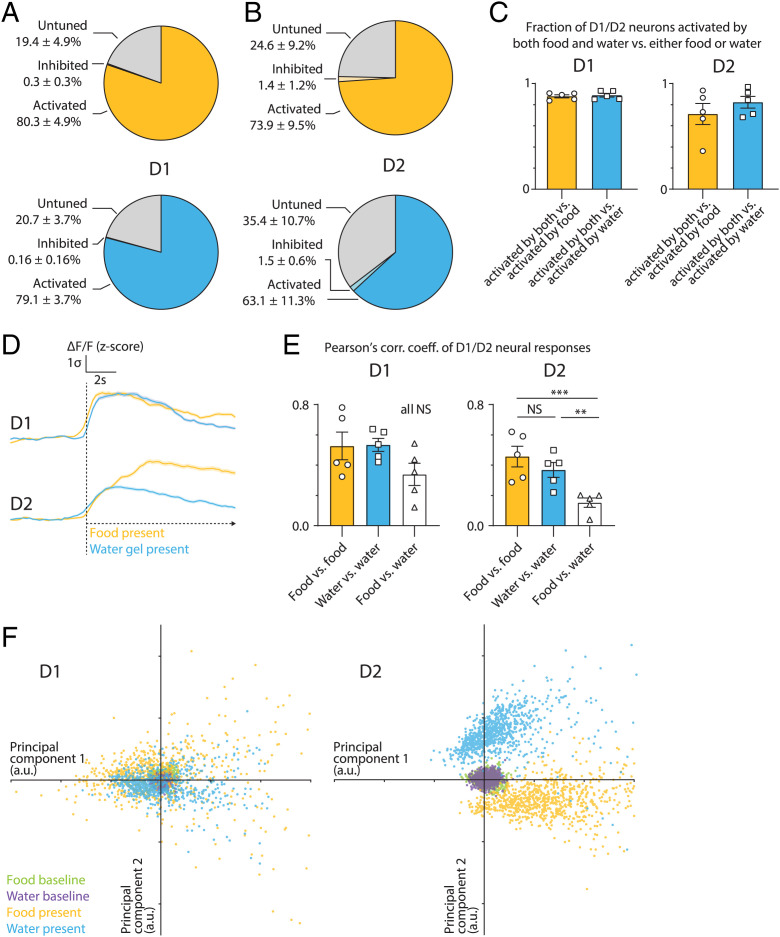
We stereotaxically injected AAV1-hsyn1-flex-GCaMP6s into either NAcc of D1-Cre or D2-Cre mice. We then compared the Ca2+ signals in D1 and D2 neurons in separate groups of animals during the sensory phase by placing food beyond the animals’ reach after a 24-h fast. Thus, animals were able to process exteroceptive cues associated with each stimulus but could not consume it. Similarly, we studied the sensory response to water deprivation by placing a water gel beyond the animals reach. We began by quantitating the relative proportion of D1 and D2 neurons that showed Ca2+ signals after exposure to food or water cues. This study revealed that ∼80% of the D1 and D2 neurons sampled were activated by food or water (Fig. 2 A and B), with 80.3% of D1 neurons (693 neurons sampled) being activated and 79.1% of D2 neurons (900 neurons sampled) being activated by both. This represents a dramatically greater percentage of D1 and D2 neurons showing activation as assessed with in vivo imaging compared to that evident by IHC for c-Fos expression.
Fig. 2.
Sensory cues activate highly overlapping sets of NAccD1 and NAccD2 neurons. (A and B) Percentages of NAccD1 and NAccD2 neurons responding to the presence of food or water in the hungry or thirsty state (n = 5 D1:GCaMP6s and n = 5 D2:GCaMP6s mice). (C) Overlap percentages of neurons activated by the presence of food compared to neurons activated by the presence of the water gel. (D) Average NAccD1 and NAccD2 neural responses by sensory stimuli from food in hungry mice and from water gel in thirsty mice. (E) Average PCC of NAccD1 and NAccD2 neural responses to the presence of food and water (n = 5 D1:GCaMP6s and n = 5 D2:GCaMP6s mice, PCCs were averaged across three trials per condition per mouse, one-way ANOVA, with Tukey’s multiple comparisons). (F) Population activity vectors in principal component space for D1-Cre (n = 693 neurons from five mice averaged across three trials) and D2-Cre (n = 900 neurons from five mice averaged across three trials) mice. AU, arbitrary unit. All error bars represent mean ± SEM. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We then assessed the extent of overlap of D1 neurons that are activated by hunger vs. thirst by serially measuring neural activity in the same mouse first after overnight fasting and then exposing them to food cue, then followed by overnight dehydration and then exposing them to a water cue. In control studies, water-deprived (or food-deprived) animals were water- (or food-) deprived a second time to establish interassay variability; these different groups are referred to as food:water, water:water, and food:food below. We found that 88% of D1 neurons that were activated by exposing fasted mice to a food cue also responded to the water cue after overnight dehydration (Fig. 2C). Moreover, the population-averaged neural activity traces of all D1 neurons for hunger and thirst were indistinguishable from the overlap between repeated trials in hungry mice (i.e., food:food) and thirsty mice (i.e., water:water) (Fig. 4D). Consistent with this, principal component analysis (PCA) of the responses of individual neurons identified two principal components with highly intermingled neural responses to food and water (Fig. 2F). We quantified the across-trial similarity and consistency of neuronal responses by evaluating the Pearson correlation coefficient (PCC) between pairs of single-trial mean activation vectors (see Materials and Methods): the PCCs across trials of food vs. food, water vs. water, food vs. water, and water vs. food were indistinguishable (Fig. 2E). Thus, the same ensembles of D1 neurons within the NAc respond to sensory cues associated with both food and water and these neurons represent the vast majority of the cells in this region.
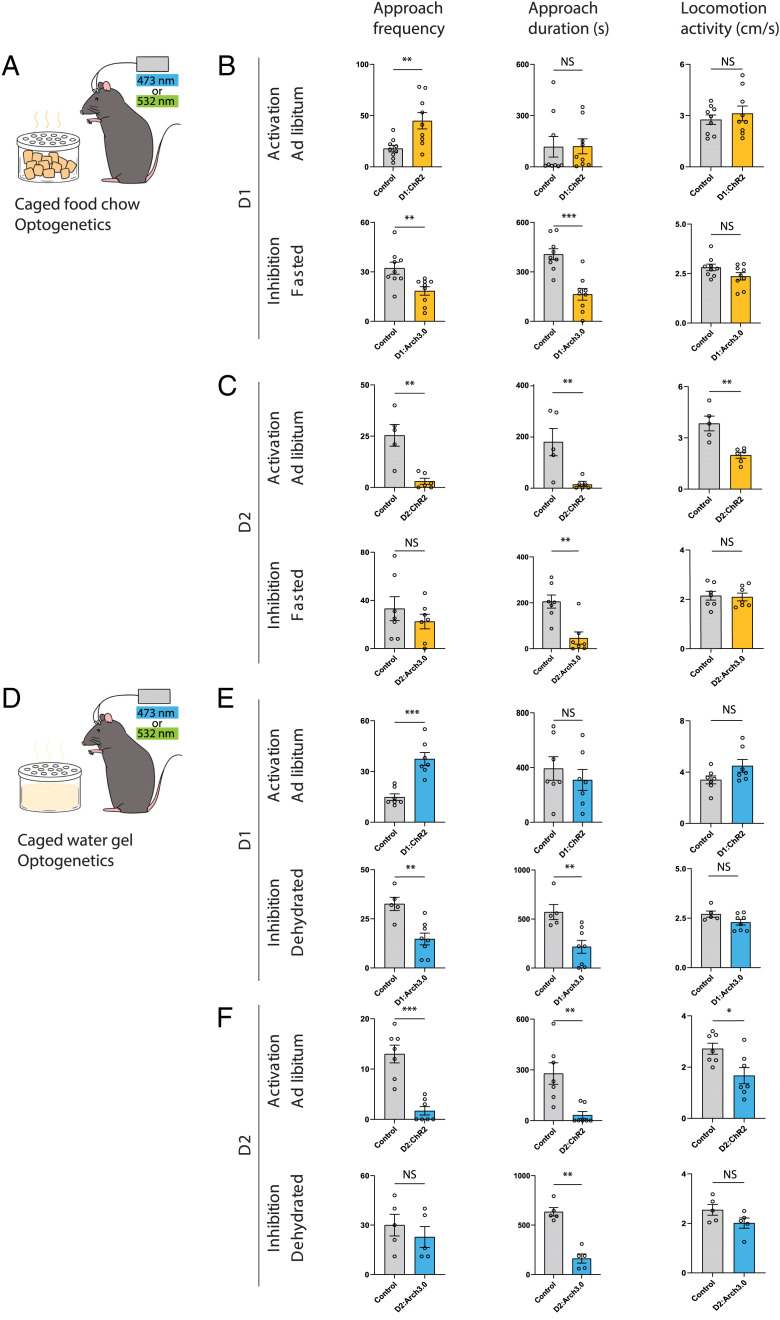
Fig. 4.
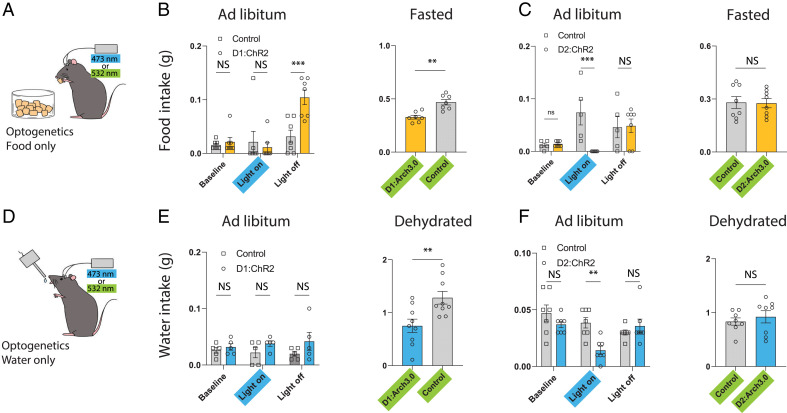
Modulation of NAccD1 and NAccD2 neurons has complementary effects on goal-seeking behaviors, irrespective of the specific need states. (A) Schematic of caged food assay with optogenetic apparatus. (B) Activating NAccD1 neurons significantly increases approaches to caged food chow (n = 9 mice per group, P = 0.0058, two-tailed Mann–Whitney U test). Silencing NAccD1 neurons in hungry mice decreases caged food visits and duration (n = 9 mice per group, two-tailed Mann–Whitney U test). Neither manipulation affects total locomotor activity. (C) Activating NAccD2 neurons significantly down-regulates caged-food seeking behaviors in ad libitum mice (n = 5 mice per group, two-tailed Mann–Whitney U test). Silencing NAccD2 neurons decreases time spent proximity to caged food in hungry mice (n = 7 mice per group, two-tailed Mann–Whitney U test). Activating D2 neurons also decreases total locomotor activity (D) Schematic of caged water gel assay with optogenetic apparatus. (E) Activating NAccD1 neurons significantly increases approaches to the caged water gel (n = 7 mice per group, two-tailed Mann–Whitney U test). Activating D2 neurons also decreased locomotor activity. Silencing NAccD1 neurons in thirsty mice decreases caged water gel visits and duration spent on caged water gel (n = 5, 8 for control, D1:Arch3.0 group; two-tailed Mann–Whitney U test). (F) Activating NAccD2 neurons down-regulates approach frequency to a caged water gel (n = 7 mice per group, two-tailed Mann–Whitney U test). Silencing NAccD2 neurons decreases time spent in proximity to a caged water gel in thirsty mice (n = 5 mice per group, two-tailed Mann–Whitney U test). Box plots show mean (+), median, quartiles (boxes), and range (whiskers). NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
We next studied D2 neurons in the same manner and found that 71% out of a total of 900 D2 neurons that were activated by food cues were also activated by placing the animals in proximity to a water gel (without allowing them to consume it). Similarly, 82% of D2 neurons that showed activation after providing water subsequently responded to food (see “overlapping neurons,” Fig. 2C). However, while a very highly overlapping population of D2 neurons were clearly activated by both food and water, we did note that the population-averaged neural activity traces of D2 neurons showed a significantly lower amplitude in response to the water gel compared to the same neurons that also responded to food (Fig. 2D). Thus, while the qualitative response was the same, in contrast to the data for D1 neurons, projecting the individual neuronal activity vectors onto their first two principal components reveals distinct clusters of neural responses that reflect this quantitative difference in D2 neural responses (Fig. 2F). Consistent with this, we observed that PCCs of mean activation vectors between pairs of same-condition trials (food:food or water:water) are significantly higher than across conditions (Fig. 2E). We conclude that, despite a highly significant qualitative overlap across D2 neurons that respond to food and water, there is a difference in the intensity of the neural responses between food and water cues for this neuronal population.
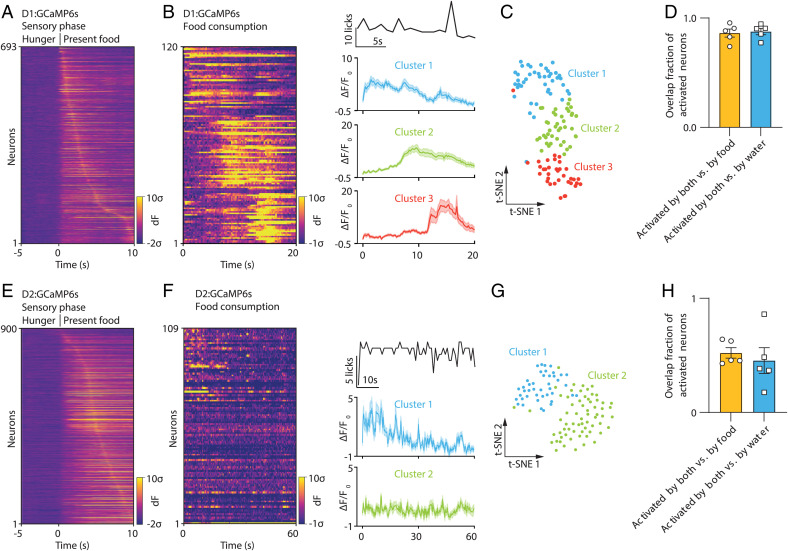
We next examined the response of individual D1 and D2 neurons in fasted or dehydrated animals during the consummatory phase by placing food or water delivery ports in proximity and allowing the animals to ingest it. Here again, we observed extensive overlap among the D1 neurons that respond to food and water during the consummatory phase. However, in contrast to the monophasic activation of individual D1 and D2 neurons in the sensory phase (Fig. 3 A and E), consumption led to distinct patterns of activation of D1 and D2 neurons over time. These clusters were defined by inspection of activity heatmaps for individual neurons after sorting by k-means clustering and by nonlinear embedding into a two-dimensional space using the t-distributed stochastic neighbor embedding (t-SNE) algorithm (Fig. 3 B and C and SI Appendix, Fig. 3 B and C). Based on these analyses, D1 neurons can be grouped into three neural clusters: Cluster 1 neurons are activated at the onset of consumption and their activity diminishes after 10 s; Cluster 2 neurons are active continuously during consumption and their activity diminishes only when consumption stops; and Cluster 3 neurons are not activated during consumption and instead show Ca2+ signals only after consumption ceases. As mentioned, there was a nearly complete overlap between the response of D1 neurons in all three clusters to both food and water consumption; of the 534 neurons that were sampled, 86% that were activated by food were also activated by water while 88% of those activated by water were also activated by food (Fig. 3D).
Fig. 3.
Highly overlapping NAccD1 and NAccD2 neurons respond similarly to food vs. water during consummatory phase. (A) Heatmap of all D1 neuronal responses to food presentation, n = 693 neurons. (B) Heatmap of D1 neuronal responses to food consumption from one example mouse and averaged neural traces from k-means clustering, n = 120 neurons. (C) t-SNE map of D1 neuronal states labeled by clustering from the example mouse. (D) Percentage of D1 neurons activated by both vs. by food or water, n = 5 mice. (E) Heatmap of all D2 neuronal responses to water gel presentation, n = 900 neurons. (F) Heatmap of D2 neuronal responses to water consumption from one example mouse and averaged neural traces from k-means clustering, n = 109 neurons. (G) t-SNE map of D2 neuronal states labeled by clustering for visualization. (H) Percentage of D2 neurons activated by both vs. by food or water, n = 5 mice.
In contrast to the multiple clusters of D1 neurons activated during consumption, the population of D2 neurons that previously responded to sensory cues associated with food and water segregated into two groups during consumption: one that was transiently activated when ingestion began, and another that remains inactive at baseline and thereafter (Fig. 3 F and G). This latter group thus appears to be active only during the sensory phase. However, while there was a statistically significant overlap among the 575 D2 neurons responding to both food and water, the corresponding fractions were much smaller than seen during the sensory phase with only 52% of food-activated neurons being activated by water and 45% of water-activated neurons being activated by food. Fig. 3H). Thus, even though the same population of D2 neurons respond to food and water during the sensory phase (albeit with different amplitudes), subsets of D2 neurons respond differentially to food and water during the consummatory phase.
Causal Control of Feeding and Drinking by D1 and D2 Neuronal Activity.
These comparisons of D1 and D2 neural activity revealed distinct cell-type-specific patterns of activation for each during both the sensory and consummatory phases, suggesting that D1 and D2 neurons may serve different functions to regulate feeding and drinking behavior. We assessed this directly using optogenetics to modulate NAc D1 or D2 neuron activity during food seeking (i.e., the appetitive or sensory phase) by providing caged food or caged water that could not be consumed, and during the consummatory phase by then making food or water available. We activated D1 or D2 neurons by bilaterally injecting AAV5-EF1a-DIO-ChR2-YFP into the NAcc of D1-Cre and D2-Cre mice. We inhibited the neurons using AAV5-EF1a-DIO-Arch3.0-YFP and the effect of optogenetic activation and inhibition on the approach to, and then consumption of, food and water was monitored. Goal approach was measured by counting the number of times mice enter the caged-goal zone (defined as within a ∼10 cm diameter as quantified by automated video tracking). We found that optogenetic activation of D1 neurons in ad libitum fed mice increased the frequency of approaches toward either caged food or caged water gel without altering the amount of time spent in the vicinity of the caged zone and without affecting the total overall amount of locomotor activity (Fig. 4 B and E and SI Appendix, Fig. 4 A and B; control vs. D1:ChR2, frequency: 17.8 ± 3.2, 44.9 ± 8.0, P < 0.01; duration: 117.5 ± 60.4, 120.0 ± 43.9, P = 0.42; velocity: 2.8 ± 0.3, 3.1 ± 0.4, P = 0.67). Conversely, silencing D1 neurons in hungry or thirsty mice decreased the number of approaches to the caged zone relative to control mice, again without altering total locomotor activity (Fig. 4 B and E and SI Appendix, Fig. 4 A and B; caged food assay: control vs. D1:Arch3.0, frequency: 32.2 ± 3.7, 18.4 ± 2.6, P < 0.01; duration: 407.0 ± 33.3, 163.8 ± 35.4, P < 0.001; velocity: 2.8 ± 0.2, 2.4 ± 0.2, P = 0.19).
In contrast, optogenetic activation of D2 neurons in ad libitum fed mice decreased the frequency of approaches to the caged food or water and decreased the time spent in the vicinity of the zone (Fig. 4 C and F and SI Appendix, Fig. 4 C and D; caged food assay: control vs. D2:ChR2, frequency: 25.4 ± 5.3, 3 ± 1.5, P < 0.01; duration: 178.3 ± 53.2, 13.3 ± 8.5, P < 0.01; velocity: 3.8 ± 0.4, 2.0 ± 0.2, P < 0.01). Silencing D2 neurons in hungry or thirsty mice did not affect the frequency of approaches or locomotor activity, but decreased the time spent in the caged zone (Fig. 4 C and F and SI Appendix, Fig. 4 C and D; caged food assay: control vs. D2:Arch3.0, frequency: 33.1 ± 10.0, 22.4 ± 6.1, P = 0.55; duration: 205.4 ± 28.6, 46.2 ± 26.5, P < 0.01; velocity: 2.1 ± 0.2, 2.1 ± 0.2, P = 0.71).
Taken together, these data show that D1 neurons are both necessary and sufficient to drive movement toward food and water, independent of consumption, while D2 neurons exert an opposite effect by decreasing the number of approaches. The data further suggest that D2 neuron activation leads to the cessation of movement after the food and water has been located, which would thus act to promote the behavioral transition toward consumption.
We next evaluated the effect of modulating NAc D1 and D2 neurons during consumption. We found that optogenetic activation of D1 neurons did not affect food or water intake in fed, hydrated animals at baseline (Fig. 5 A, B, D, and E), whereas silencing D1 neurons in hungry or dehydrated mice acutely decreased food and water intake (Fig. 5 B and E). However, we did observe a moderate and sustained increase in food intake 20-min post activation of D1 neurons in fed mice, although we did not see an analogous response after D1 activation in thirsty animals (Fig. 5 B and E), raising the possibility that the neural circuits downstream of D1 neurons elicit differential effects on feeding vs. drinking (9–12). In contrast, activation of D2 neurons in ad libitum mice reduced both food and water consumption (Fig. 5 C and F), while silencing D2 neurons in hungry or thirsty mice had no effect (Fig. 5 C and F). The aggregate data for these experiments are summarized in SI Appendix, Fig. 5 G and H. Overall these studies show that D1 and D2 neurons exert complementary effects both during the appetitive and consummatory phases of eating and drinking behaviors, and modulating these neurons has nearly identical effects on the response to food and water.
Fig. 5.
Modulation of NAccD1 and NAccD2 neurons reciprocally regulates consumption, irrespective of the specific need states. (A) Schematic of food consumption assay with optogenetic apparatus. (B) Activating NAccD1 neurons in ad libitum mice does not affect food intake. However, there was a significant increase of food but not water consumption 20-min post-activation (n = 7 mice per group; blue box, laser on; two-way ANOVA, with Sidak’s multiple comparisons). Silencing NAccD1 neurons in hungry mice decreases food consumption (n = 7 mice per group; green box, laser on; two-tailed Student’s t tests). (C) Activating NAccD2 neurons decreases food intake in ad libitum mice (n = 5, 7 for control and D2:ChR2 group; blue box, laser on; two-way ANOVA, with Sidak’s multiple comparisons). Silencing NAccD2 neurons does not affect food intake in hungry mice (n = 8 mice per group; green box, laser on; two-tailed Student’s t tests). (D) Schematic of water consumption assay with optogenetic apparatus. (E) Activating NAccD1 neurons does not affect water consumption, during or after stimulation (n = 5 mice per group; blue box, laser on; two-way ANOVA, with Sidak’s multiple comparisons). Silencing NAccD1 neurons decreases water consumption in thirsty mice (n = 9 mice per group; green box, laser on; two-tailed Student’s t tests). (F) Activating NAccD2 neurons decreases water consumption (n = 7 mice per group; blue box, laser on; two-way ANOVA, with Sidak’s multiple comparisons), whereas silencing them doesn’t affect water consumption (n = 8 mice per group; green box, laser on; two-tailed Student’s t tests). All error bars represent mean ± SEM. NS, not significant, *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
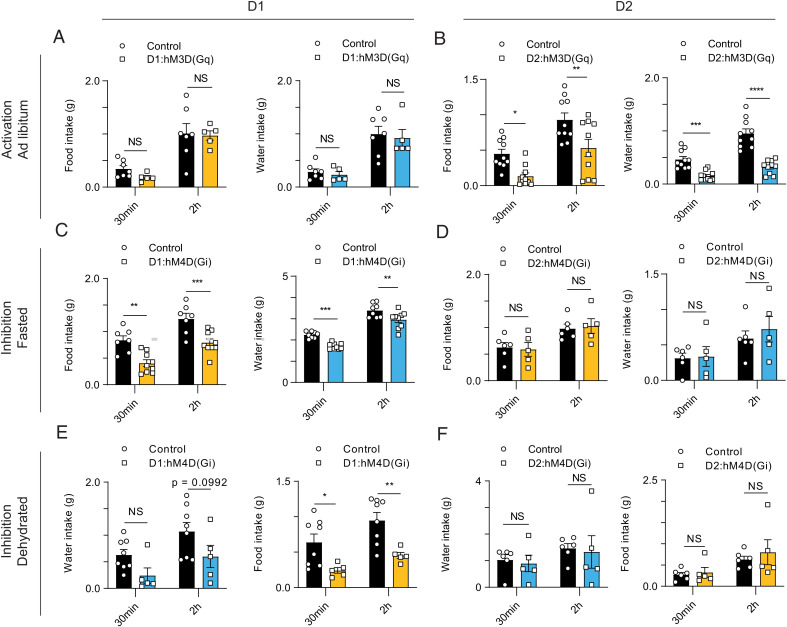
Finally, because optogenetics is most suitable for assessing the effects of acute changes neural activity, we used chemogenetics to chronically modulate D1 and D2 neural activity in mice provided food and/or water in their home cages. D1 and D2 neurons were activated by injecting AAV5-hsyn1-DIO-hM3D(Gq)-mCherry and were silenced after injection of AAV5-hsyn1-DIO-hM4D(Gi)-mCherry, and in both cases the effect of clozapine N-oxide (CNO) (which activates both types of DREADDs) and saline were compared. As an additional control, animals injected with AAV5-hsyn1-DIO-mcherry were treated with CNO. Chemogenetic activation of D1 neurons under these conditions did not affect food or water intake, while activation of D2 neurons reduced both (Fig. 6 A and B and SI Appendix, Fig. 5 A and D). Chemogenetic silencing recapitulated the effect seen in the optogenetic assays, in that silencing D1 neurons reduced the amount consumed, while silencing D2 neurons did not affect consumption (Fig. 6 C–F and SI Appendix, Fig. 5 B, C, E, and F). Therefore, here again modulating D1 or D2 neurons exerts similar effects on food and water consumption in ad libitum vs. fasting vs. dehydrated states. Furthermore, in these assays, we consistently observed that the amount of food and water consumption are linked, suggesting D1 and D2 neurons might also play a coregulatory role to coordinate feeding and drinking (Fig. 6 A–F).
Fig. 6.
Chemogenetic modulation of NAccD1 and NAccD2 neurons coregulates food and water consumption. (A) Chemogenetic activation of D1 neurons does not regulate food or water consumption in a physiological ad libitum state. Both groups of animals receive CNO injections. (n = 7, five mice per group; two-way ANOVA, with Sidak’s multiple comparisons). (B) Chemogenetic activation of D2 neurons reduces food and water consumption in a physiological ad libitum state (n = 10 mice per group; two-way ANOVA, with Sidak’s multiple comparisons). Both groups of animals receive CNO injections. (C) Chemogenetic silencing of D1 neurons reduces food and water consumption in fasted animals (n = 7,10 mice per group; two-way ANOVA, with Sidak’s multiple comparisons). Both groups of animals receive CNO injections. (D) Chemogenetic silencing of D2 neurons does not affect food and water consumption in fasted animals (n = 5 mice per group; two-way ANOVA, with Sidak’s multiple comparisons). Both groups of animals receive CNO injections. (E) Chemogenetic silencing of D1 neurons reduces food and water consumption in water-deprived animals (n = 8, five mice per group; two-way ANOVA, with Sidak’s multiple comparisons). Both groups of animals receive CNO injections. (F) Chemogenetic silencing of D2 neurons does not affect food and water consumption in water-deprived animals (n = 5 mice per group; two-way ANOVA, with Sidak’s multiple comparisons). Both groups of animals receive CNO injections.
Overall, by showing similar effects of neural modulation on food and water seeking and consumption, these functional data are consistent with the c-Fos and imaging data. These studies also show that D1 and D2 neurons play distinct and complementary roles in mediating different aspects of the response to a need state after food or water deprivation (SI Appendix, Fig. 5 G and H).
Discussion
Food and water deprivation lead to goal directed behaviors that serve to alleviate the need state. The mesolimbic dopamine system is known to play a key role in conveying reward and coordinates several motivated behaviors including the response to food and water deprivation. However, it was not known whether overlapping or distinct neural ensembles in this pathway alleviate hunger by eating vs. alleviating thirst by drinking. We therefore set out to establish the coding properties of the nucleus accumbens. Because our initial studies using cFos revealed that neurons in NAc core but not the shell were activated by food and water, we focused on the NAc core, a major target of mesolimbic dopamine neurons. More than 90% of NAcc is composed of neurons expressing either the D1 or D2 dopamine receptor and we then used in vivo neural imaging to study both populations. We found that a nearly identical set of D1 and a highly overlapping set of D2 neurons are activated during food and water reward. We also tested the effect of modulating the activity of these neurons on food vs. water intake and here again nearly identical effects on reward seeking and consumption were observed. Functional studies also revealed complementary roles between D1 and D2 neurons for both behaviors, with each population serving specialized subfunctions including reward seeking, consumption, and behavioral cessation.
Previously it was unclear whether or not different subsets of NAc neurons process different rewarding stimuli. Thus, our data show that neurons in NAc, and associated mesolimbic pathways, function as a common pathway for natural rewards rather than being parsed into heterogeneous subpopulations representing different internal states and their associated sensory stimuli. Moreover, our finding nearly 80% of the neurons are activated also suggests that there may be significant overlap with other rewarding stimuli such as sucrose, drugs of abuse such as cocaine and opioids, though this will need to be tested directly, currently underway. The extensive overlap we observe also suggests that these neurons may play a more general role to control motivation and that the regulation of distinct downstream behavioral programs specific for each motor behavior is encoded elsewhere. By establishing common roles for NAc D1 and D2 neurons in regulating responses to these need states, these results may have important implications for understanding the common neural substrates of need states and the neural representations of reward.
While these studies of neural activity and function reported here show conclusively that two different natural need states (and their associated goals: food and water) engage a highly overlapping set of D1 neurons in NAc in a nearly identical qualitative and quantitative manner except there are some subtle differences particularly for D2 neurons. Thus, while food and water activated a highly overlapping set of D2 neurons, we observed different quantitative effects on their level of activation, with a greater response to food than water in D2 neurons during the appetitive phase. In addition, the dynamics of the D2 neuron responses were homogeneous between food and water though albeit with lesser overlap than was the case for D1 neurons during the consummatory phase. Thus, despite the fact that food and water deprivation are each sensed by different neural circuits in hypothalamus (see below), sensory inputs corresponding to each ultimately converge at the level of the same NAc neurons. Together, these data are consistent with the possibility that responses of NAc neurons likely regulate the strength of an animal’s motivation rather than showing specificity for specific stimuli. These findings and the functional data presented here further establish the generic role for D1 and D2 neurons in the NAc in modulating these motivated behaviors.
The present work exclusively examined male mice because the extant literatures upon which our experiments are based have focused virtually exclusively on males (13). Our data now set the stage to extend the findings to females. For basic drives such as feeding and drinking, we would not anticipate major differences between sexes with respect to the effects observed. Consistent with this, human fMRI studies indicate that valence coding (e.g., reward vs. punishment) is not different between sexes across several key brain regions including NAc (14). Thus, we do not anticipate valence coding for fundamental need states (hunger and thirst) to vary significantly due to sex.
The finding that both populations respond to food and water suggests that they represent a point of convergence for signals from other populations that directly sense either need state. Hunger is encoded in part by neurons expressing agouti-related peptide (AgRP) in arcuate nucleus (ARC), while thirst is sensed by nitric-oxide synthase expressing neurons in the subfornical organ (SFO) (15, 16). While each cell type responds to distinct interoceptive signals, these neurons share common features. First, activation of each transmits negative valence (13). Second, activation of each elicits similar behavioral programs leading to the seeking and consumption of food or water (10, 11, 13). Third, the elevated neural activity associated with each need state is rapidly quenched by the availability of food or water (13, 17–20). These data suggest that these regions conveying food and water deprivation each relay onto the same population in NAcc. Recent studies indicate several indirect projections from ARC or SFO possibly via insular cortex, ventral tegmental area, or lateral amygdala (21–23). While distinct pathways lead to food or water intake, there is also evidence that these behaviors are interrelated as it has been observed that feeding and drinking are highly associated during meals in humans (3), raising the possibility that there may be neural mechanisms that coregulate and link feeding and drinking behaviors. The nearly identical responses of NAcc neurons to both need states and their similar roles in its satisfactions also raises the possibility that neurons in NAcc may also play a role in the well-established interrelationship between food and water consumption and the motor actions associated with each (24). However, further studies will be required to confirm this possibility.
Previous studies have shown that sensory signals activate ventral tegmental area dopamine neurons to mediate reward and that this activation occurs both before and after the consumption of sucrose as well as in response to other learned stimuli that are associated with goal acquisition (4, 6, 25–27). While our study examined neural dynamics of dopamine-receptor-expressing neurons in NAc during the sensory vs. oral phase, a recent study suggests that gastrointestinal (GI) infusions of nutrients vs. water activate distinct subsets of dopaminergic neurons in the VTA (22). This raises the possibility that oral phase and gastrointestinal phases engage distinct circuit elements in response to food vs. water. The NAcc integrates dopamine signals, and our data thus indicate that the specific feeding and drinking behaviors modulated by VTA dopamine neurons in response to gastrointestinal stimuli are likely relayed by sites outside of the NAc. The aggregate data thus suggest that VTA dopamine neurons regulate behavior-specific outputs via these other sites, while the NAc encodes the general motivational component. Thus, future studies mapping the inputs and outputs of NAc D1 vs. D2 neurons will be necessary to understand how distinct types of interoceptive information is integrated and in turn modulates the intensity of an animal’s behavior while also enabling selection of the appropriate action. The findings and approach we report here now also make it possible to compare distinct consummatory behaviors in future studies that characterize individual neural responses in NAc and associated projection neurons during GI infusions of distinct stimuli such as salts, nutrients and water. Such studies would allow us to compare these responses to those associated with anticipatory representations of food vs. water in the oral phase reported here.
Our study also shows that individual D1 and D2 neurons show distinct patterns of temporal activation suggesting that there are coordinated subtypes of neurons whose activity is correlated with specific actions throughout the consummatory phase. it will now be important to modulate the activity of distinct subsets of NAc neurons that show different patterns of activation, especially during the consummatory phase, to assess whether they elicit distinct responses. Recent advances now make it possible to modulate neural activity based on their pattern of activation (28). Thus, in order to further correlate these specific subpopulations with their downstream functions, additional studies modulating neural activity via spatially restricted delivery of light, in the form of multiphoton targeted optogenetics will be necessary, currently underway (29).
Previous studies of the effects of drugs of abuse have suggested that D1 vs. D2 neurons in NAc play “push-pull” roles in the pathogenesis of addiction (30, 31). Activation of D1 neurons promote rewarding effects and addiction induced by drugs of abuse, while activation of D2 neurons induces aversion (7). While previous studies have also found that D1 neurons located in NAc shell curb feeding via direct projections to the lateral hypothalamus (9, 32–34), a canonical region controlling feeding behavior, the identity of individual neurons, especially in NAc core, that sense hunger and thirst was nebulous. In the present study, we demonstrate that opto- or chemogenetic silencing of D1 neurons suppresses food or water intake in fasted or water-deprived mice.
The present results also demonstrate that a binary role for D2 neurons to induce aversion is an oversimplification. Our studies of food and water processing show that D1 and D2 neuron each causally regulate different aspects of goal-directed behaviors during the appetitive and consummatory phases for both rewards. We also found that D2 neurons are transiently activated in response to food and water and eventually act to suppress food and water intake. However, there is less overlap between D2 neurons responding to food vs. water during the consummatory phase compared to the sensory phase. Thus we cannot exclude the possibility that some nonoverlapping D2 neurons elicit different effects on food vs. water consumption. These data suggest that D2 neurons may play an important role in tightly regulating motor outputs, enabling shifts in behavior toward those required for consumption, rather than approach, while also potentially signaling satiety. In addition, D2 neurons are activated by sensory cues also suggesting a role in salience. These results are consistent with previous studies showing that D2 neurons have an important role in promoting motivated behavior (35–37). Together, our findings suggest that D2 neurons play a broader role than simply promoting aversion, with subsets of D2 neurons and their distinct outputs possibly being critical for promoting and fine-tuning motivated behavior as is also suggested by the different quantitative responses to the sensory cues associated with food and water.
In summary, our results provide direct evidence that food and water elicit highly overlapping patterns of activation in NAc and that modulating the activity of D1 and D2 neurons there has similar effects on the appetitive and consummatory phases for both natural rewards. This suggests that these neurons play an important role in coregulating feeding and drinking perhaps by modulating the general level of motivation. These results also provide a template for further studies of cell-type-specific ensemble activity in the NAc to determine how these systems are modulated in response to other stimuli such as reward prediction errors, in response to drugs of abuse and in various pathologic conditions.
Materials and Methods
Experimental Model and Mouse Strains.
Experimental protocols were approved by the Rockefeller University IACUC, according to the NIH Guide for the Care and Use of Laboratory Animals. Details are included in the SI Appendix.
Viral Vectors.
The details of AAV viruses described in the paper are included in the SI Appendix.
Stereotaxic Surgery.
Mice were injected viruses, implanted optic fibers, or implanted GRIN lens following details included in the SI Appendix.
In Vivo Two-Photon Imaging Paradigm.
On experiment day, fasted or dehydrated mice went through a 3-min baseline recording, followed by 3 μL water or liquid food dispensed by the lickometer spout at the beginning of the consumption trial. After the trial started, the lickometer spout dispensed 3 μL water or liquid food upon each lick from mice. Details are included in the SI Appendix.
Optogenetic Modulations.
Photostimulation protocols are included in the SI Appendix.
Chemogenetic Modulations.
Mice from both groups received intraperitoneal (i.p.) injections of 1 mg/kg clozapine N-oxide (CNO) 20 min prior to the access to food and water. On a separate control session, mice from both groups received i.p. injections of PBS 20 min prior to the access to food and water.
Caged Object Assay.
Mice behaviors are tracked and evaluated by Ethovision 9.0 (Noldus). Details are included in the SI Appendix.
Feeding and Drinking Behaviors.
For optogenetic stimulation, mice were acclimated to a new clean cage for 5 min before experiments started. Mice were then provided free access to either food or water. For ChR2 stimulation in ad libitum fed mice, the baseline food or water intake was measured for 20 min, followed by 20 min of photostimulation, followed by 20 min with laser off. For Arch3.0 stimulation in fasted or dehydrated mice, mice directly received constant laser stimulation for 20 min.
Histology.
Brains were dissected, fixed, stained, imaged, and analyzed following details included in the SI Appendix.
Data Analysis.
Behavior data were analyzed using the Ethovision ×9 software (Noldus). For two-photon imaging experiments, behavioral data, and imaging data were analyzed using the Suite2p pipeline (38) and custom Python scripts. Details are included in the SI Appendix.
Statistics and Reproducibility.
We conducted statistical analyses using GraphPad Prism 9.0. Throughout the paper, values are reported as mean ± SEM (error bar or shaded area). The statistical models used for imaging data analysis as described above were carried out using the scikit-learn Python package (39). No statistical methods were used to predetermine sample size. Details are included in the SI Appendix.
Supplementary Material
Acknowledgments
We thank Kristina Hedbacker and Estefania Azevedo for advice on behavioral and molecular experiments, as well as illustrations. We thank Inna Piscitello for help with manuscript submission. We thank Brandon Chen and Francisca Traub for advice on GRIN lens implantation surgeries. We thank James Petrillo at the Precision Instrumentation Facility, Bio-Imaging Resource Center at Rockefeller University. We thank Xinyuan Zhang for illustrations. We thank Zuohang Wu for discussions. B.T. acknowledges support from the David Rockefeller Fellowship. J.M.F acknowledges support from JPB foundation. T.N. has been supported by a Kavli Fellowship at The Rockefeller University. C.J.B. was supported by a Postdoctoral Fellowship from the National Sciences and Engineering Research Council of Canada. This work was supported through funding from the NIH (5U01NS115530, 1RF1NS110501, 1RF1NS113251 to A.V. and P01DA047233 to E.J.N.), the National Science Foundation (DBI-1707408 to A.V.) and the Kavli Foundation (to A.V.).
Footnotes
Reviewers: T.L.H., Yale University; and C.A.M., University of Pittsburgh School of Medicine.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2211688119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Craig W., Appetites and aversions as-constituents of instincts. Proc. Natl. Acad. Sci. U.S.A. 3, 685–688 (1917). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burnett C. J., et al. , Need-based prioritization of behavior. eLife 8, e44527 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKiernan F., Hollis J. H., McCabe G. P., Mattes R. D., Thirst-drinking, hunger-eating; tight coupling? J. Am. Diet. Assoc. 109, 486–490 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohebi A., et al. , Dissociable dopamine dynamics for learning and motivation. Nature 570, 65–70 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips P. E., Stuber G. D., Heien M. L., Wightman R. M., Carelli R. M., Subsecond dopamine release promotes cocaine seeking. Nature 422, 614–618 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Roitman M. F., Stuber G. D., Phillips P. E., Wightman R. M., Carelli R. M., Dopamine operates as a subsecond modulator of food seeking. J. Neurosci. 24, 1265–1271 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Self D. W., Barnhart W. J., Lehman D. A., Nestler E. J., Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science 271, 1586–1589 (1996). [DOI] [PubMed] [Google Scholar]
- 8.Yoshida M., et al. , Eating and drinking cause increased dopamine release in the nucleus accumbens and ventral tegmental area in the rat: Measurement by in vivo microdialysis. Neurosci. Lett. 139, 73–76 (1992). [DOI] [PubMed] [Google Scholar]
- 9.O’Connor E. C., et al. , Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 88, 553–564 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Chen Y., Lin Y. C., Zimmerman C. A., Essner R. A., Knight Z. A., Hunger neurons drive feeding through a sustained, positive reinforcement signal. eLife 5, e18640 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leib D. E., et al. , The forebrain thirst circuit drives drinking through negative reinforcement. Neuron 96, 1272–1281.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., et al. , Sustained NPY signaling enables AgRP neurons to drive feeding. eLife 8, e46348 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betley J. N., et al. , Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521, 180–185 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warthen K. G., et al. , Sex differences in the human reward system: Convergent behavioral, autonomic and neural evidence. Soc. Cogn. Affect. Neurosci. 15, 789–801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atasoy D., Betley J. N., Su H. H., Sternson S. M., Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oka Y., Ye M., Zuker C. S., Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature 520, 349–352 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berrios J., et al. , Food cue regulation of AGRP hunger neurons guides learning. Nature 595, 695–700 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y., Lin Y. C., Kuo T. W., Knight Z. A., Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 160, 829–841 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandelblat-Cerf Y., et al. , Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4, e07122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zimmerman C. A., et al. , Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature 537, 680–684 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livneh Y., et al. , Estimation of current and future physiological states in insular cortex. Neuron 105, 1094–1111.e10 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grove J. C. R., et al. , Dopamine subsystems that track internal states. Nature 608, 374–380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burgess C. R., et al. , Hunger-dependent enhancement of food cue responses in mouse postrhinal cortex and lateral amygdala. Neuron 91, 1154–1169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gong R., Xu S., Hermundstad A., Yu Y., Sternson S. M., Hindbrain double-negative feedback mediates palatability-guided food and water consumption. Cell 182, 1589–1605 e1522 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Engelhard B., et al. , Specialized coding of sensory, motor and cognitive variables in VTA dopamine neurons. Nature 570, 509–513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun F., et al. , A genetically encoded fluorescent sensor enables rapid and specific detection of dopamine in flies, fish, and mice. Cell 174, 481–496.e19 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azevedo E. P., et al. , A role of Drd2 hippocampal neurons in context-dependent food intake. Neuron 102, 873–886.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Yang W., Carrillo-Reid L., Bando Y., Peterka D. S., Yuste R., Simultaneous two-photon imaging and two-photon optogenetics of cortical circuits in three dimensions. eLife 7, e32671 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrasfalvy B. K., Zemelman B. V., Tang J., Vaziri A., Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. U.S.A. 107, 11981–11986 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calipari E. S., et al. , In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc. Natl. Acad. Sci. U.S.A. 113, 2726–2731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo M. K., et al. , Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bond C. W., et al. , Medial nucleus accumbens projections to the ventral tegmental area control food consumption. J. Neurosci. 40, 4727–4738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thoeni S., Loureiro M., O’Connor E. C., Lüscher C., Depression of accumbal to lateral hypothalamic synapses gates overeating. Neuron 107, 158–172.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo Y. J., et al. , Nucleus accumbens controls wakefulness by a subpopulation of neurons expressing dopamine D1 receptors. Nat. Commun. 9, 1576 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Self D. W., Stein L., Receptor subtypes in opioid and stimulant reward. Pharmacol. Toxicol. 70, 87–94 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Soares-Cunha C., et al. , Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat. Commun. 7, 11829 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole S. L., Robinson M. J. F., Berridge K. C., Optogenetic self-stimulation in the nucleus accumbens: D1 reward versus D2 ambivalence. PLoS One 13, e0207694 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pachitariu M., et al. , Suite2p: Beyond 10,000 neurons with standard two-photon microscopy. bioRxiv [Preprint] (2017). 10.1101/061507. Accessed 20 July 2017.
- 39.Pedregosa F., et al. , Scikit-learn: Machine learning in python. J. Mach. Learn. Res. 12, 2825–2830 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.