Significance
Neandertals’ diets are a topic of continued debate, especially since their disappearance has been frequently attributed to their subsistence strategy. There is no clear consensus on how variable their diets were in time and space. Isotope studies have helped quantify meat consumption in Neandertals, but usually rely on nitrogen isotope analyses of collagen, a protein rarely preserved in samples older than 50 ka. Moreover, collagen extraction for isotope analyses is rarely successful in Iberian skeletal material. Here, we employ zinc isotope analysis of dental enamel of a Neandertal and associated fauna (Gabasa, Spain), which can be applied to contexts >50 ka. This proxy confirms a high level of carnivory in an Iberian Neandertal.
Keywords: carnivory, Middle Paleolithic, zinc isotope ratios, Iberian Neandertals, hominin
Abstract
The characterization of Neandertals’ diets has mostly relied on nitrogen isotope analyses of bone and tooth collagen. However, few nitrogen isotope data have been recovered from bones or teeth from Iberia due to poor collagen preservation at Paleolithic sites in the region. Zinc isotopes have been shown to be a reliable method for reconstructing trophic levels in the absence of organic matter preservation. Here, we present the results of zinc (Zn), strontium (Sr), carbon (C), and oxygen (O) isotope and trace element ratio analysis measured in dental enamel on a Pleistocene food web in Gabasa, Spain, to characterize the diet and ecology of a Middle Paleolithic Neandertal individual. Based on the extremely low δ66Zn value observed in the Neandertal’s tooth enamel, our results support the interpretation of Neandertals as carnivores as already suggested by δ15N isotope values of specimens from other regions. Further work could help identify if such isotopic peculiarities (lowest δ66Zn and highest δ15N of the food web) are due to a metabolic and/or dietary specificity of the Neandertals.
Over the last 30 years, analyses of nitrogen isotopes in collagen (δ15Ncollagen) have provided direct evidence for Neandertal diets across Europe and Asia. These studies all indicate a carnivorous (1–12), or at least a meat-heavy, diet for European Neandertals. However, one peculiarity of Neandertal δ15Ncollagen remains the subject of an ongoing debate. From the one Siberian and eight western European sites, where both Neandertal and associated fauna have been analyzed, nitrogen isotope ratios in Neandertal collagen are systematically higher than that of other carnivores (3, 6–8, 10, 11, 13, 14). An explanation for such elevated values could be the consumption of herbivores, such as mammoths, which themselves exhibit elevated δ15N values due to the consumption of plants growing on arid soils (1, 2, 7). While mammoth remains are usually scarce at Neandertal fossil localities, they were nonetheless occasionally consumed, as suggested by remains with cut marks and other human butchery signatures (15). The absence of mammoth remains at Middle Paleolithic sites could be a result of 1) Neandertals chose to leave large bone elements at the kill site and transport other edible carcass products, mainly meat, back to the habitation site (15), or 2) mammoths were not frequently consumed, and the δ15N peculiarity consequently reflects the consumption of other resources enriched in 15N.
Alongside this δ15N peculiarity, one major obstacle to our knowledge of Neandertals’ subsistence patterns is that the preservation of organic matter limits the application of collagen-bound nitrogen isotope analysis to fossil specimens. Collagen degrades over time at a varying speed depending on climatic and environmental conditions (16). The oldest hominin specimen in which bone protein is preserved is that of Scladina (Belgium), which dates to 90,000 cal BP (calibrated years before the present) (17), but most studied specimens are younger than 50,000 cal BP (1–3, 6–8, 10–13, 18). Furthermore, these specimens are only from sites in northwestern and central Europe and Siberia, where climatic conditions favored collagen preservation. As a result, the variability of Neandertals’ diet over time and between regions may not accurately be reflected by the currently available isotope data. In Iberia, where the latest surviving Neandertals have been discovered (19, 20), collagen was successfully extracted for only one site (21). Therefore, our knowledge of Iberian Neandertal diets mostly relies on zooarcheological and dental calculus data, which show some inconsistencies (21–25). For instance, similar to other western European sites, zooarcheological studies emphasize the consumption of terrestrial mammals and birds (21). In contrast, analysis of dental calculus for microremains and ancient DNA metagenomic analysis (26–28) highlight the frequent consumption of plants and mushrooms. Indeed, Weyrich et al. (26) even suggest that Neandertals at El Sidrón (Fig. 1) rarely consumed meat but often ate mushrooms, which would also result in elevated δ15N values (29). The consumption of marine foods is also attested for coastal Neandertals, but its frequency cannot be truly assessed in the absence of isotope studies (21, 23–25, 30). Finally, cannibalism has been documented at two Iberian sites (El Sidrón and Zafarraya) (22, 31) (Fig. 1), though such practices appear limited and most likely occurred only during periods of nutritional stress (32). Therefore, it is challenging to confirm the homogeneity of Neandertals’ diets across time and space, calling into question a direct link between their subsistence strategy and disappearance.
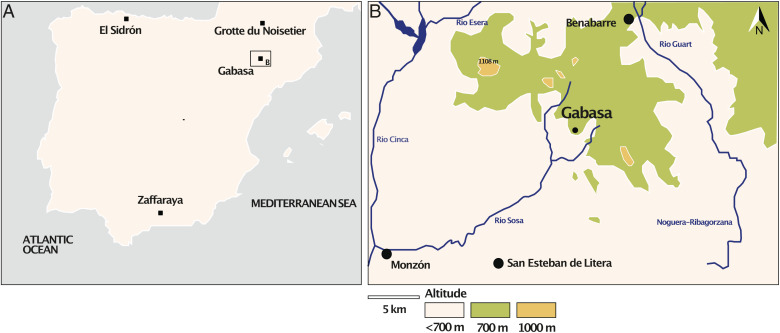
Fig. 1.
(A) Location of the Gabasa site as well as other Neandertal sites mentioned in the text. (B) Detailed map of the Gabasa region. San Estaban de Litera and Benabarre are nearby modern cities.
This study aims to investigate if the Zn isotope proxy could help elucidate the dietary behaviors of Neandertals and the source of their δ15N peculiarity, specifically by studying a Late Pleistocene Iberian food web where the presence of mammoth has not been documented (33). The development of Zn isotope analysis (66Zn/64Zn, expressed as δ66Zn) has proven that trophic level information can be retrieved from mammalian tooth enamel (δ66Znenamel) (34, 35), including fossil samples from Pleistocene food webs where organic matter is typically not preserved (36, 37). Previous studies have demonstrated that δ66Znenamel decreases by ca. 0.30 to 0.60 ‰ with each step in archeological and modern food webs (34–38) and that δ66Zn values associated with breastfeeding are higher than postweaning-associated values (39). While the main source of variation of δ66Znenamel values is diet, local geology can also likely influence the isotope ratio of a given animal (36, 39). To date, three modern assemblages from Koobi Fora (Kenya), Kruger Park, and the western Cape (South Africa) (40), a few animals from a historical site (Rennes, France) (41), and three Late Pleistocene sites (Tam Hay Marklot, Nam Lot, and Tam Pa Ling, Laos) (36, 37) are the only terrestrial food webs for which Zn isotope data in teeth and/or bones have been published (SI Appendix, Fig. S14). In the modern Koobi Fora savannah food web, δ66Znenamel differences have been observed between browsers and grazers (35), but this pattern was not seen in any of the three Pleistocene Asian forest food webs (36, 37). Among modern and historical human populations, historically documented diets relying on plants are associated with higher δ66Zn values than those that include a substantial quantity of animal products (41–44). Zinc isotopes of ancient hominins have been analyzed only in one Pleistocene Homo sapiens individual (37) from Southeast Asia, but not yet in any Neandertal specimen.
This current study contributes significantly to our understanding of Iberian Neandertal diets by providing information on their trophic ecology for a region where traditional nitrogen isotope analyses are unfeasible due to the poor preservation of organic matter. We use the Zn isotopic tool as well as other mobility, ecological, and dietary proxies applied on tooth enamel from hominin and animal remains from the cave site Cueva de los Moros 1 (Gabasa, Pyrenees, Spain; Fig. 1). The site, excavated in the 1980s, is very well documented [for stratigraphic context, see Montes and Utrilla (45) and SI Appendix, Section S1]. All remains come from layers e, f, and g of a single stratigraphic layer directly above layer h dated to 143 ± 43 ka. Numerous carnivore remains were recovered along with Neandertal remains (layers e and f), allowing for comparison of the different meat-eating taxa. Coexisting herbivores from three different types of environmental contexts are homogeneously represented in layers e, f, and g: mountains (Iberian ibex [Capra pyrenaica], chamois [Rupicapra rupicapra]), forest (cervids including red deer [Cervus elaphus]), and open environments (horses [Equus ferus], European wild asses [Equus hydruntinus]).
Sample Collection
Species, Sample, and Proxy Selection.
We analyzed the tooth enamel of 65 samples belonging to 43 different teeth of 12 taxa for δ66Znenamel (Fig. 2 and SI Appendix, Table S15), 42 samples belonging to 39 different teeth for δ13Cenamel and δ18Oenamel (SI Appendix, Table S19), 23 samples belonging to 18 different teeth for 87Sr/86Sr (SI Appendix, Table S7), and 40 samples for trace element ratios (SI Appendix, Table S11). We sampled 12 to 50 mg of tooth enamel (1 to 20 mg for Zn isotopes) of five carnivore taxa (lynx [Lynx spelaea], wolf [Canis lupus], fox [Vulpes vulpes], dhole [Cuon alpinus], cave hyena [Crocuta spelaea]), one omnivore ( cave bear [Ursus spelaeus]), and at least six herbivore species (Iberian ibex [C. pyrenaica], chamois [R. rupicapra], red deer [C. elaphus], rabbit [Oryctolagus cuniculus], horse [E. ferus], European wild ass [E. hydruntinus]), as well as one of the four Neandertal dental remains recovered from the site. Hyenas (C. spelaea) are treated as a separate dietary group (bone-eating carnivores) based on the results of the Koobi Fora and Nam Lot food webs (35, 37).
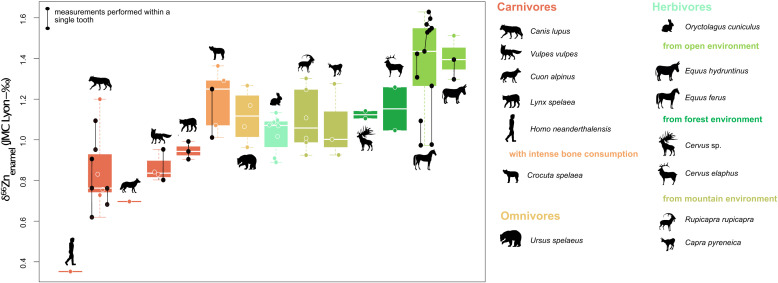
Fig. 2.
Enamel δ66Zn values per mammalian taxon for different dietary groups from Gabasa, Spain given as boxplots. Note that subsamples taken from one single tooth reflect differences in dietary 66Zn values. See text for detailed discussion about the low Neandertal δ66Zn value.
Sampling Strategy Related to Breastfeeding.
The single sampled Neandertal tooth is a first molar (M1; Ga1.Rev.303) (46). Previous studies based on tooth wear and trace elements suggest that Neandertals were likely weaned between the ages of 1 and 2.5 y old (47–50), thus possibly overlapping with the crown’s formation period for this tooth. To minimize destructive analyses, we sampled an enamel fragment from the lower part of the crown, next to the cervical margin. This part of the tooth probably formed after weaning or at least when maternal milk consumption could have been strongly reduced (51). The specimen was heavily worn, making it difficult to evaluate the enamel formation period of the part that we sampled. Still, we were able to evaluate trophic spacing between species while taking into account the influence of maternal milk consumption, which triggers higher δ66Zn values in human M1 (39). To do so, we analyzed δ66Zenamel on different types of nonhominin teeth (dp4, C, P2, P3, P4, M1, M2, and M3; SI Appendix, Table S4) formed at different times during ontogeny and obtained values associated with breastfeeding and/or solid food consumption for all species analyzed from Gabasa. In addition, we analyzed Sr/Ca and Ba/Ca ratios that also show variation between tooth enamel mineralized in utero and during pre- or postweaning periods (52).
Sampling Strategy Related to Neandertal Mobility and Ecology.
Other factors such as diagenesis, geographical origin, environmental context, or metabolism can be a source of Zn isotope variability (35, 36, 39, 53). In recent studies, linear mixing models highlighted a possible link between Zn and Sr isotope ratios in teeth, which has been interpreted as resulting from the influence of the local geology on the Zn isotope ratios of its associated food webs (36, 37, 39). To control for this potential bias, we analyzed strontium isotope ratios of Gabasa tooth enamel (87Sr/86Srenamel) for samples with sufficient material (SI Appendix, Section S5). We also collected Sr isotope data from rocks, soils, plants, and teeth to assess the regional bedrock isotopic data (87Sr/86Srbedrock) and identify whether some animals could have been derived from areas proximal to Gabasa with different geology (SI Appendix, Table S18). We hypothesize that the Neandertal consumed local food sources, whereby its 87Sr/86Sr should fall within the ranges observed both for the local bedrock and assumed sympatric animals.
We estimated carbon and oxygen isotope compositions (δ13Cenamel and δ18Oenamel, respectively) of the Neandertal and associated fauna. Notably, δ13Cenamel and δ18Oenamel values can offer insights into ecological proxies, such as the environment from which the Neandertal individual obtained its prey (i.e., open arid vs. closed mesic habitats), and possibly reveal information about which types of prey were consumed (54–56). Moreover, carbon and oxygen isotope data allow us to compare the food web of Gabasa with other food webs where Neandertals have been discovered. For one horse tooth, five samples were taken serially along the growth axis of the tooth to evaluate potential seasonality and intratooth variability effects over time on Sr and Zn isotope ratios.
Sampling Strategy to Assess the Degree of Chemical Tooth Alteration.
Although it has been shown that dental enamel preserves biogenic Zn isotope signatures very well in Pleistocene or even older samples (36, 57), we independently confirmed that Zn isotope variability is not related to diagenetic processes. For this purpose, we analyzed trace elements that are abundant in soils but not in tooth enamel (Fe, Al, Mn) and hence are indicative of postmortem trace element uptake (SI Appendix, Section S3). Additionally, we examined the carbonate content of dental enamel to eliminate samples with obvious diagenetic alteration (>10 wt% CO3) (SI Appendix, Section S3). To capture the influence of diagenesis on isotope ratios, we also performed measurements on dentin samples from two wolves for Sr, C, and O isotopes and trace elements and one wild ass for Zn isotopes. Finally, four roots of teeth which enamel was sampled (one chamois, two foxes, and one lynx) were used to attempt collagen extraction (SI Appendix, Tables S9 and S10), using a high extraction–yield protocol adapted for radiocarbon dating (58, 59) in the hope of getting additional trophic level information and possibly permitting high-precision radiocarbon dating.
Results
Datasets, methods, and additional discussions are given in detail in SI Appendix.
Zinc Isotope Data.
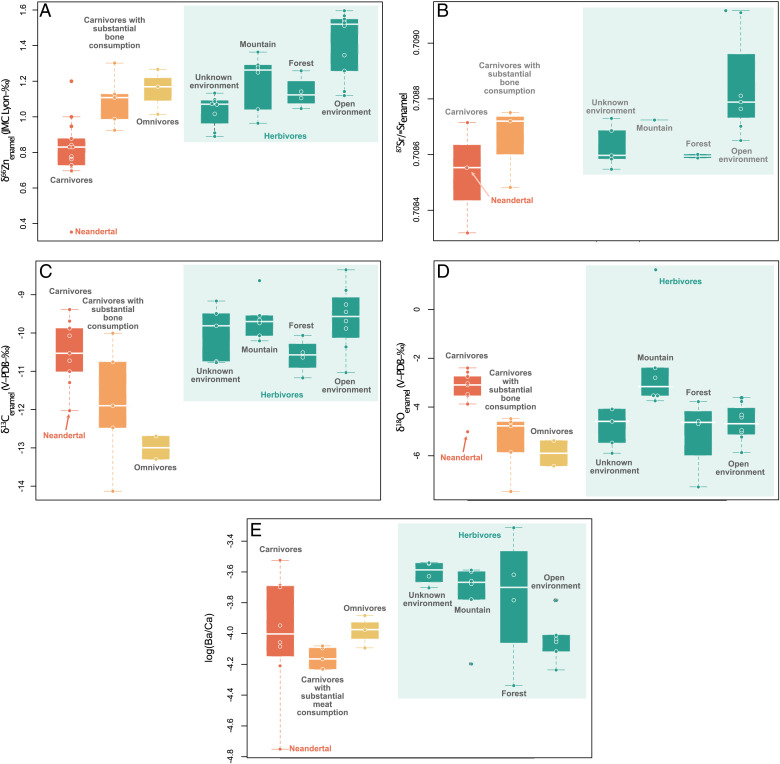
Zinc isotope values of Gabasa mammalian tooth enamel range from 0.35 to 1.63 ‰ (n = 65) and are mainly influenced by the dietary group of the animals (Figs. 2 and 3) (Kruskal–Wallis χ2 = 19.496, P = 0.0002159 and χ2 = 18.298, P = 0.0003818; samples with elevated Al, Mn, and Fe content are excluded), which are assigned based on those used in other δ66Znenamel isotope studies and zooarcheological data (dietary groups: carnivores, bone-eating carnivores, omnivores, and herbivores) (35, 60, 61). Animal values from different archeological layers fall in the same range (SI Appendix, Section S4). As previously observed for modern, historical, and Late Pleistocene tooth enamel, there is no correlation between Zn isotope values and Zn concentration data (35, 36, 39, 41, 42). This is an excellent indicator to assess the preservation of biogenic Zn isotope signatures (62). On average, large herbivores have higher Zn isotope ratios (δ66Znenamel = 1.24 ± 0.17 ‰ 1σ; n = 16) than carnivores (δ66Znenamel = 0.92 ± 0.18 ‰ 1σ; n = 16; 0.85 ± 0.14 ‰ if hyenas and the Neandertal are excluded). The Neandertal tooth exhibits the lowest δ66Znenamel value (0.35 ± 0.00 ‰), substantially lower (Δ66ZnNeandertal-carnivores = 0.57 ‰) than that of the lowest carnivore’s value. Mirroring patterns in the modern Koobi Fora (East Africa) food web, the difference between herbivore and carnivore values is even more pronounced when hyenas are excluded, as bone consumption is thought to induce higher δ66Zn values (35). The omnivorous cave bears (δ66Znenamel = 1.15 ± 0.13 ‰ 1σ; n = 3) exhibit Zn isotope ratios closer to those of large herbivores and rabbits rather than the carnivores’, similar to a pattern often observed in nitrogen isotopes (63–66). The herbivores show the highest δ66Zn values (0.96 to 1.60 ‰; nmeasurements = 40 from ntooth = 27 teeth) with grazers (European wild asses and horses, δ66Zn = 1.35 ± 0.18 ‰ 1σ; ntooth = 6) exhibiting the highest values, another similarity to observations in modern food webs (35, 40).
Fig. 3.
Average dental enamel isotope and trace element ratios for the different dietary categories at Gabasa, Spain. (A) δ66Znenamel. (B) 87Sr/86Sr. (C) δ13Cenamel. (D) δ18Oenamel. (E) log(Ba/Ca). Omnivores correspond to one single taxon (cave bears) as well as carnivores with significant bone consumption (cave hyenas).
Strontium Isotope Data.
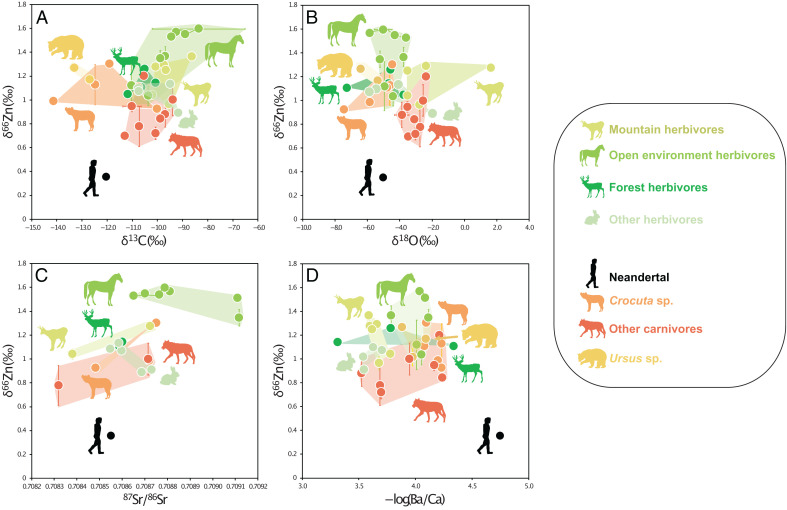
The 87Sr/86Srenamel range from 0.70834 to 0.70916 (n = 24). Strontium and Zn isotope ratios show no correlation, and the Neandertal individual shows an 87Sr/86Srenamel overlapping with those of deer and rabbits (Fig. 4A). Strontium isotope ratios from dental enamel and dentin are compatible with the values expected for the region of Gabasa (SI Appendix, Sections S1 and S5). As dentin is likely to be affected by diagenetic alteration and Sr exchange with the soil, these values might reflect soil values and can thus be an indicator of the local bioavailable Sr isotope composition at Gabasa (SI Appendix, Section S3).
Fig. 4.
Zinc isotope values (δ66Zn, average dental enamel values per tooth) cross-plotted versus (A) carbon (δ13C), (B) oxygen (δ18O), and (C) strontium (87Sr/86Sr) isotope composition as well as (D) Ba/Ca ratios measured in faunal and Neandertal tooth enamel at Gabasa, Spain. Each point corresponds to a single tooth, except for the horse tooth, for which five samples were taken (samples are connected with a line). Error bars correspond to the SD between analytical duplicates for C and O isotopes (0.02 to 0.36 ‰; one sample with higher SD has been removed) and for Zn (analytical duplicates: 0.00 to 0.04 ‰; SD for separate samples taken from a single tooth: 0.04 to 0.21 ‰). SEs for strontium isotope ratios are 0.000003 to 0.000010 and are too small to display.
Carbon and Oxygen Isotope Data.
δ13Cenamel data range from −14.13 to −8.35 ‰ (n = 44), and δ18Oenamel data range from −7.46 to 1.64 ‰ (n = 43). Mountain herbivores (chamois and Iberian ibex) have distinctly higher δ13Cenamel and δ18Oenamel compared with other herbivores, which might be due to water evaporation on mountain slopes and consumption of plants from more arid contexts (Fig. 3 and SI Appendix, Section S6). The Neandertal’s δ13Cenamel and δ18Oenamel values overlap with those of cave animals (Fig. 4 A and B and SI Appendix, Fig. S12). Five carbon (δ13C) and oxygen (δ18O) values were possibly impacted by diagenetic carbonate, and were excluded for ecological interpretation (mostly rabbits; SI Appendix, Sections S3 and S6).
Trace Element Ratios (Ba/Ca and Sr/Ca).
Trace element ratios are mostly discussed in SI Appendix, Sections S3 and S7. The −log(Ba/Ca) ranges from 3.25 to 4.75 (n = 40) and −log(Sr/Ca) from 2.77 to 3.62 (n = 40). The two ratios measured in dental enamel do not correlate. As expected (67), mixed feeders exhibit lower Sr/Ca than grazers, but carnivores (especially hyenas) show Sr/Ca ratios overlapping with those of grazers (SI Appendix, Fig. S13). We report the highest Sr/Ca and Ba/Ca ratios for mountain and forest animals and the lowest Ba/Ca for the Neandertal individual. Here, these ratios do not correlate with tooth developmental stages (i.e., ontogenetic time of crown mineralization).
Correlations and Comparisons between Different Dietary Proxies.
Zn isotopes do not correlate with Sr/Ca or Ba/Ca ratios (Fig. 4 and SI Appendix, Sections S3 and S4). Equids have higher δ66Zn, δ13C, and 87Sr/86Sr and lower Ba/Ca than the rest of the herbivores (Fig. 4). When performing a principal-component analysis (PCA) based on five proxies (δ66Zn, δ13C, δ18O, Sr/Ca, and Ba/Ca), the PC1 places the Neandertal among cave animals (hyenas and bears). This first component is mostly based on δ13C, δ18O, and Ba/Ca values. Strangely, it is precisely when cave animals are removed that a positive correlation appears between δ13C and δ66Zn (R2 = 0.41, P = 0.00009). This correlation could be due to a combination of a trophic level effect and higher δ13C and δ66Zn values among grazers. No clear trend has been observed between δ66Zn values and the type of tooth sampled (SI Appendix, Section S4), but it should be noted that this could result from the low sample size per tooth group and different timing of weaning and dental development between species.
Collagen Extraction.
The collagen extraction performed on the dentin from four teeth was unsuccessful, and not enough collagen was extracted for δ15Ncollagen and/or radiocarbon analyses. This failure is not surprising for samples dating back to about 150 ka (62) (SI Appendix, Section S2).
Discussion
Insights into the Diet and Ecology of Iberian Pleistocene Mammals from Zn Isotopes in Tooth Enamel.
Our study shows similar trends to those previously reported in modern and fossil food webs: Herbivores exhibit higher δ66Zn than carnivores (Δ66Znherbivores-carnivores = +0.31 ‰ and +0.38 ‰ excluding hyenas). The trophic level depletion is similar to that of the modern food web of Koobi Fora, Kenya (dental enamel values: +0.40 ‰ hyenas included, and +0.44‰ excluding hyenas), or the marine mammal food webs of Arctic Canada (+0.32 ‰ on average in bones) (34, 38) but lower than that of the Laotian fossil sites (+0.63 ‰ hyenas excluded). Grazers (horses and European wild asses) tend to show higher δ66Zn than browsers, as observed in the savannah of Koobi Fora (35) but not in the tropical rain forests of Tam Hay Marklot and Nam Lot (36, 37). Hyenas have more elevated δ66Znenamel values than other carnivores (Fig. 2), as systematically observed in other sites (35, 37, 40). These higher values are likely due to bone consumption (35), although hyenas have more extended breastfeeding time than other carnivores (68) and milk would also similarly drive δ66Znenamel up. However, high Sr/Ca and low Ba/Ca ratios observed in the different teeth of all hyenas support the interpretation of the signature of solid food consumption and consequently of bones as a likely driving factor behind their elevated δ66Znenamel compared with other sympatric carnivores (SI Appendix, Fig. S13 and Table S4). Cave bears exhibit δ66Znenamel overlapping with rabbits, deer, and chamois (Fig. 2), which confirms the frequent observations based on low δ15Ncollagen, indicating they were mostly herbivorous (12, 63, 66).
The δ66Znenamel values of the entire food web of Gabasa are the most elevated values ever measured in dental enamel [mean values about 0.2 ‰ higher than in the modern food web of Koobi Fora and 0.7 ‰ higher than in the Late Pleistocene food webs of Marklot, Nam Lot, and Tam Pa Ling (35, 36, 41, 42); SI Appendix, Section S4 and Fig. S14]. This is probably not due to the geology because limestones, known to show the highest δ66Zn (69), are present at both Gabasa and the Laotian sites. The Gabasa food web’s uniqueness comes from its environmental context as it is the coldest terrestrial biotope ever studied for faunal δ66Zn. Pollen data suggest an arid and cold climate (70) with periods characterized by steppe vegetation and some forest patches mainly composed of conifers (45, 70, 71). Carbon and oxygen isotopes exhibit patterns similar to those observed for mammals from various Middle Paleolithic western European sites (54, 55, 72) and are compatible with midmountain mosaic landscape (SI Appendix, Sections S1 and S6). Tree leaves usually exhibit relatively low δ66Zn and were probably scarce in Gabasa, as opposed to the forest of Tam Hay Marklot and the fynbos of the western Cape (40), where the lowest δ66Zn in herbivore teeth was measured.
The Diet of a Neandertal at Gabasa.
Our results demonstrate that the Neandertal individual from Gabasa shows a Zn isotope signature of a top-level carnivore, similar to that observed for nitrogen isotopes for other sites with Neandertal occupation. Carbon and oxygen isotope and trace element data suggest that the individual inhabited the local area around the cave (SI Appendix, Sections S6 and S7). Of all the animal taxa analyzed in Gabasa, the Neandertal specimen easily exhibits the lowest Zn isotope ratio. As the Sr isotope ratio of the Neandertal tooth enamel is similar to those of other animals (Fig. 4), the low δ66Znenamel value is unlikely the result of a different geographic origin (and consequently different bedrock and isotope baseline) of this individual but reflects diet instead. Previous work on animal food webs documented a trophic spacing for δ66Znenamel of 0.3 to 0.6 ‰ (34–36, 38). In Gabasa, the average difference between carnivores and herbivores is 0.31 ‰, but the Neandertal shows a δ66Zn value 0.57 ‰ lower than that of the carnivores—0.50 ‰ if hyenas are excluded—and 0.85 ‰ lower than the herbivores, which would be about three trophic levels higher than that of the herbivores if we consider a trophic spacing of 0.31 ‰. This mirrors dietary reconstructions based on nitrogen isotopes from individuals from other European sites such as Goyet (18) (Belgium), Jonzac (13) (France), or Les Cottés (7) (France), where Neandertals exhibit higher δ15Ncollagen values than sympatric carnivores.
In order to explain the low δ66Znenamel value of the Neandertal tooth, we here consider 1) a signature of breastfeeding, 2) specific diets (the consumption of either light Zn-bearing foods or the absence of consumption of heavy Zn-bearing foods), and 3) a metabolic origin.
The breastfeeding signature hypothesis can be ruled out because it leads to high δ66Znenamel. In a previous study, teeth formed during the breastfeeding period of two human populations from very different geographical and archeological contexts exhibit average δ66Znenamel values that are 0.3 ‰ higher than the teeth whose dental enamel forms after weaning (39). Furthermore, the low Ba/Ca of the Neandertal dental enamel suggests that it was formed after the breastfeeding period (49, 52, 73) (SI Appendix, Table S20). Moreover, the region of the tooth sampled was very close to the tooth’s cervical margin because of tooth wear, which usually corresponds to formation ages of 2 to 3 y old (74). The turnover of Zn in the body occurs over the course of just a few months, and weaning age (or at least an age with substantial consumption of solid food) among Neandertals is assumed to be around 1 y old (49). Even if milk consumption extended to the period from which enamel was sampled from the tooth, it is unlikely that it was a major source of Zn in the diet at this stage. Therefore, the value observed in the Neandertal tooth enamel is likely a reflection of an adult diet. An early weaning age also prevails in recent humans and has also been documented with Zn isotopes in a Late Pleistocene H. sapiens (37), strongly contrasting with the conditions observed among apes, Australopithecus (75), and earlier representatives of the genus Homo (76). This adaptation results from the necessity for the breastfeeding mother to share the energetic burden of her child’s large brain with other adults able to contribute to solid food. It is central to the human pattern of “cooperative breeding” and has critical social and behavioral implications.
Some dietary explanations for the low δ66Znenamel of the Neandertal individual involving light Zn-bearing foods are also excluded:
-
1)
Adult vs. juvenile prey: In principle, if [as in humans (39)] milk consumption leads to higher δ66Zn, preferential consumption of postweaning juveniles or adults might lead to lower δ66Zn. However, this explanation seems unlikely for two reasons. First, such an isotopic disparity has not been observed in herbivore teeth in relationship to weaning in this study or elsewhere (36), so the age of the prey does not appear to matter. Second, Neandertals targeted younger deer and horses (61) (SI Appendix, Section S1) while other carnivores targeted adult ibexes. As such, if prey age mattered, Neandertal Zn isotope compositions should be biased toward higher δ66Zn, not lower. Although we cannot rule out the possibility that the formation period of the Neandertal tooth is asynchronous with the site’s period of occupancy, there is currently no evidence to support this.
-
2)
Cannibalism or hypercarnivorism: Cannibalism or carnivore meat consumption could be associated with low Zn isotope ratios, but the absence of cut marks on hominin and carnivore bones rules out this hypothesis.
-
3)
Food with unusually low δ66Zn values: Archeological evidence for some isotopically light foods that Neandertals might have specifically targeted may be missing [e.g., animal livers (53, 77, 78), birds, mushrooms, fruits, and leaves (79–81), insects (82, 83), or aquatic resources (43)]. However, δ66Znenamel of the consumers of those foods has been measured and is not associated with lower values than that of sympatric carnivores, both in Gabasa (e.g., lynx are known to consume liver, deer consume leaves, while foxes, bears, and rabbits have been known to consume mushrooms) and other food webs [apes and tapirs for insects and fruits (36)].
-
4)
Aquatic foods: The consumption of aquatic resources has been demonstrated for some Iberian Neandertals, though not at Gabasa (23, 25, 30). Still, the consumption of common trout (Salmo trutta) has been suggested for a Mousterian site at the Grotte du Noisetier (84), located in the French Pyrenees (Fig. 1). The trophic level of this species could potentially explain low δ66Znenamel values, but would imply that the main source of Zn in the diet comes from freshwater fish. Trout Zn content is about 10 times less than that of liver or muscle from mammals (85), requiring Neandertals to have consumed trout almost exclusively to shift their δ66Znenamel to the observed low value. As no fishbone or other evidence for aquatic resource use was found in the Gabasa region, we can disregard this hypothesis as unlikely.
-
5)
Food processing: Elevated δ15N values among Neandertals have been suggested to result from food processing such as fermentation or cooking. However, Zn fractionates only at a temperature above ∼900 °C (86), which is not reached in open fires, and even if fractionation did occur, it would enrich the Neandertal food in heavy Zn isotopes (86). Biotic fractionation during meat processing is not documented, but could happen within muscle tissues if the reactions were incomplete during fermentation (87) or other curing processes. However, this would only influence the final δ66Zn of the consumed food if both reagents and products of the curing process would not be contained in the final food product.
A combination of dietary practices might explain the low δ66Znenamel value of the Neandertal. Zinc isotopes are highly fractionated in mammalian tissues (77, 78, 88, 89), and different mammalian species have different isotope compositions. Thus, a low δ66Zn value might reflect consumption of muscle and liver (low δ66Zn) from deer and rabbits (lower δ66Zn than other herbivores; Fig. 3) (also supported by δ13C and δ18O for which Neandertals exhibit values close to these animals; Figs. 3 and 4 and SI Appendix, Fig. S10), while excluding bones and blood (high δ66Zn). Although cut marks suggest Neandertals hunted deer and horses (61, 90), rabbits are the most abundant taxon of the site (SI Appendix, Fig. S2), and some rabbit bones show cut marks (SI Appendix, Fig. S3). Disarticulation of rabbits requires minimal use of tools (61), so they could have been heavily consumed without physical evidence. Temporal asynchrony between cave deposits and tooth growth might explain a discrepancy between zooarcheological and isotopic dietary conclusions if the deposits represent a period when Neandertals preferentially ate horses and deer, whereas the portion of the tooth corresponds to a period when rabbits were preferentially eaten. Higher δ66Zn among other sympatric carnivores hunting the same species as Neandertal (SI Appendix, Table S3) (61, 91) might partially reflect bone consumption: Hyenas tend to have elevated δ66Znenamel values, likely due to bone consumption (35, 37). Red foxes and wolves gnaw on bones [although they ingest much less bone than hyenas (92)], while lynxes partially digest the bones of rabbits, which make up 80 to 100% of their typical diets (91). In addition, all these carnivores may consume substantial blood, possibly helping to explain higher δ66Zn values than the Neandertal.
Metabolic specificity (different diet–tissue isotopic offsets) for Neandertals compared with other animals might also explain the unusually low δ66Zn value and possibly the higher δ15Ncollagen values that have been measured elsewhere. It is indeed striking how similarly the patterns between δ66Znenamel and δ15Ncollagen mirror each other: In both proxies, Neandertals typically exhibit isotope ratios that would appear to imply a higher trophic level than sympatric carnivores (1, 3–5, 7, 11, 63). Although such distinct compositions have been interpreted to reflect aquatic food resources for some Paleolithic humans (11), compound-specific isotope analysis of amino acids shows that anatomically modern humans from Buran Kaya (Crimea) relied on terrestrial resources, even though they exhibit the highest δ15Ncollagen known for the Paleolithic (93). These authors suggest preferential consumption of mammoths (absent from Gabasa), but a metabolic origin might yield similarly high δ15Ncollagen values. We acknowledge that some studies show minimal offsets in δ15Ncollagen between humans and sympatric herbivores (94). However, the trophic spacing between the Paleolithic and Neolithic periods did not decrease to the extent expected for a transition from hunter-gatherer to a more cereal-based diet (95). Furthermore, for Zn isotopes, a medieval population with expected high meat and marine fish consumption shows strikingly lower δ66Znenamel values than for sympatric herbivores (0.6 ‰ lower) and for a dog, cat, and pig (0.3 ‰ lower), while δ15Ncollagen values were strikingly higher than those of the herbivores (4 ‰) and dog, cat, and pig (1 ‰) (41). These observations closely resemble patterns for Neandertals at Gabasa (for Zn isotopes) and elsewhere (for N isotopes), and suggest a metabolic effect. Controlled feeding experiments on animals with an omnivorous diet are necessary to assess this further. The reason for this metabolic difference remains unknown, but it should be noted that the Neandertal is the most carnivorous species among primates, and the only meat eater not from the order Carnivora within the Gabasa faunal assemblage. Therefore, Neandertals may have had some metabolic peculiarities compared with other carnivores and primates regarding adaptations of their gastrointestinal tract and their dietary preferences (96).
Concluding Remarks
Zinc isotope analysis of tooth enamel can successfully characterize past animal and human diets in the absence of collagen preservation for δ15N analysis. Whichever method is used to characterize Neandertals’ diets (δ66Znenamel and δ15Ncollagen), isotope values represent an extreme in carnivore trophic levels, and Iberian Neandertals appear to be no exception. Meat consumption is supported by both the zooarcheological data at Gabasa and δ66Znenamel analysis. Furthermore, the low Zn isotope ratio observed in the single measured Gabasa Neandertal specimen suggests that this individual might have had a distinct diet compared with other carnivores (possibly avoiding the consumption of bones and blood) and/or may not have fractionated Zn isotopes like other sympatric carnivores. Our successful analysis of Zn isotopes from a Pleistocene hominintooth paves the road for a refined understanding of Neandertal diet. Further work should focus on understanding whether such unusual compositions result from specific diets or metabolic fractionation in these extinct hominins.
Materials and Methods
Materials.
Details of the context of Gabasa cave deposits, geology, dating, zooarcheology, paleoenvironment, and sampling strategy are available in SI Appendix. The material used in this study is associated with the permit “Resolution” by the Gobierno de Aragon on 10 February 2016 and 31 August 2018.
Methods.
Tooth enamel fragments and powders were sampled at the Department of Human Evolution of the Max Planck Institute for Evolutionary Anthropology (MPI-EVA) using a drill with a diamond saw and at the Géosciences Environnement Toulouse of the Observatoire Midi Pyrénées (GET/OMP) using a MicroMill. For Zn isotope analyses, 1- to 20-mg enamel fragments were dissolved in 1 mL 1 N HCl. Samples were cleaned with double-distilled H2O in an ultrasonic bath and dried at 50 °C. Zinc was then extracted by column chromatography using the modified protocol of Moynier et al. (97) described by Jaouen et al. (34). The δ66Znenamel analyses were conducted on three different multicollector inductively- coupled plasma mass spectrometers (MC-ICP-MS) instruments: a Nu 500 (Ecole Normale Supérieure), a Neptune (MPI-EVA), and a Neptune Plus (GET/OMP) using a Cu doping technique (SI Appendix, Section S4). The δ66Znenamel values were corrected to take into account the offset (+0.27 ‰) existing between our in-house standard, bracketing standard (AA-MPI, which stands for Alfa Aesar- Max Planck Institute), and JMC-Lyon solution. Zinc isotope data are thus reported toward the JMC-Lyon values, and this correction does not impact the relative offsets or our interpretations. Strontium isotope analyses were conducted on 5 to 20 mg of enamel powder, after performing a purification step based on a modified protocol of Deniel and Pin (98) described by Richards et al. (13), using a Triton Plus thermal ionization mass spectrometer (TIMS) and a Neptune Plus at GET/OMP Toulouse. O and C isotope data were obtained from untreated tooth enamel powder (<100 µg) using the “cold trap” method with a Thermo Scientific Delta V IRMS in the Vonhof laboratory at the Max Planck Institute for Chemistry (MPIC) after Vonhof et al. (99). The reference materials NIST SRM 1400 (for Zn), NIST SRM 1486 (for Sr), IAEA-603, NBS18, NIST SRM 120c, and three internal house standards—a carbonate standard (VICS) and a tooth enamel structural carbonate (AG-Lox) for C and O and a dentin sample (AZE) for Zn—were analyzed in the same runs as the samples and gave consistent results for each laboratory and compared with previous studies (SI Appendix, Tables S3–S5). Trace element analyses were conducted at the GET using an ICap (Thermo Fisher) triple quadrupole mass spectrometer (TQ-ICP-MS). Finally, collagen was extracted from four tooth roots for δ15N analyses using the protocol of Talamo and Richards (58). All trace elements and isotope results are available in SI Appendix.
Supplementary Material
Acknowledgments
This study was funded by Deutsche Forschungsgemeinschaft Project PALEODIET (378496604), European Research Council (ERC) Project ARCHEIS (Grant 803676), ERC Project VERTEBRATE HERBIVORY (Grant 681450), and the Max Planck Society. G.M.S. received funding from the European Union’s Horizon Europe Research and Innovation Programme under Marie Skłodowska–Curie Grant Agreement 101027850. We thank Maria Alonso Lescún from the Museum of Huesca for her help during the collection sampling, Adeline Le Cabec for her help in understanding of tooth development, Sven Steinbrenner for technical help, Mary Kita for her help in the improvement of the figures, Jeremy McCormack for helpful discussions, and Hubert Vonhof for access to the stable isotope lab at MPIC Mainz. D.C.S.G. acknowledges funding by the Generalitat Valenciana (CIDEGENT/2019/061), and L.M. and P.U. from the Ministerio de Ciencia e Innovación (MICINN) (PID2020-116598GB-I00 GAPS AND DATES. DINAMICAS CULTURALES EN LA PREHISTORIA DE LA CUENCA DEL EBRO). We are grateful to the two reviewers who helped to significantly improve the manuscript.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.P.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2109315119/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Bocherens H., Drucker D. G., Billiou D., Patou-Mathis M., Vandermeersch B., Isotopic evidence for diet and subsistence pattern of the Saint-Césaire I Neanderthal: Review and use of a multi-source mixing model. J. Hum. Evol. 49, 71–87 (2005). [DOI] [PubMed] [Google Scholar]
- 2.Bocherens H., “Neanderthal dietary habits: Review of the isotopic evidence” in The Evolution of Hominin Diets, J.-J. Hublin, M. P. Richards, Eds. (Springer, 2009), pp. 241–250. [Google Scholar]
- 3.Bocherens H., et al. , New isotopic evidence for dietary habits of Neandertals from Belgium. J. Hum. Evol. 40, 497–505 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Wißing C., et al. , Isotopic evidence for dietary ecology of late Neandertals in north-western Europe. Quat. Int. 411, 327–345 (2016). [Google Scholar]
- 5.Richards M. P., et al. , Neanderthal diet at Vindija and Neanderthal predation: The evidence from stable isotopes. Proc. Natl. Acad. Sci. U.S.A. 97, 7663–7666 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards M. P., Schmitz R. W., Isotope evidence for the diet of the Neanderthal type specimen. Antiquity 82, 553–559 (2008). [Google Scholar]
- 7.Jaouen K., et al. , Exceptionally high δ15N values in collagen single amino acids confirm Neandertals as high-trophic level carnivores. Proc. Natl. Acad. Sci. U.S.A. 116, 4928–4933 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welker F., et al. , Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc. Natl. Acad. Sci. U.S.A. 113, 11162–11167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocherens H., et al. , Isotopic biogeochemistry (13C,15N) of fossil vertebrate collagen: Application to the study of a past food web including Neandertal man. J. Hum. Evol. 20, 481–492 (1991). [Google Scholar]
- 10.Naito Y. I., et al. , Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. J. Hum. Evol. 93, 82–90 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Richards M. P., Trinkaus E., Out of Africa: Modern human origins special feature: Isotopic evidence for the diets of European Neanderthals and early modern humans. Proc. Natl. Acad. Sci. U.S.A. 106, 16034–16039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bocherens H., et al. , Palaeoenvironmental and palaeodietary implications of isotopic biogeochemistry of Last Interglacial Neanderthal and mammal bones in Scladina Cave (Belgium). J. Archaeol. Sci. 26, 599–607 (1999). [Google Scholar]
- 13.Richards M. P., et al. , Isotopic dietary analysis of a Neanderthal and associated fauna from the site of Jonzac (Charente-Maritime), France. J. Hum. Evol. 55, 179–185 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Salazar-García D. C., et al. , Dietary evidence from Central Asian Neanderthals: A combined isotope and plant microremains approach at Chagyrskaya Cave (Altai, Russia). J. Hum. Evol. 156, 102985 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Smith G. M., Neanderthal megafaunal exploitation in western Europe and its dietary implications: A contextual reassessment of La Cotte de St Brelade (Jersey). J. Hum. Evol. 78, 181–201 (2015). [DOI] [PubMed] [Google Scholar]
- 16.van Klinken G. J., Bone collagen quality indicators for palaeodietary and radiocarbon measurements. J. Archaeol. Sci. 26, 687–695 (1999). [Google Scholar]
- 17.Pirson S., Toussaint M., Bonjean D., Di Modica K., “Spy and Scladina caves: A Neandertal’s story” in Landscapes and Landforms of Belgium and Luxembourg, A. Demoulin, Ed. (Springer, 2018), pp. 357–383. [Google Scholar]
- 18.Wißing C., et al. , Stable isotopes reveal patterns of diet and mobility in the last Neandertals and first modern humans in Europe. Sci. Rep. 9, 4433 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jennings R., Finlayson C., Fa D., Finlayson G., Southern Iberia as a refuge for the last Neanderthal populations. J. Biogeogr. 38, 1873–1885 (2011). [Google Scholar]
- 20.Galván B., et al. , New evidence of early Neanderthal disappearance in the Iberian Peninsula. J. Hum. Evol. 75, 16–27 (2014). [DOI] [PubMed] [Google Scholar]
- 21.Salazar-García D. C., et al. , Neanderthal diets in central and southeastern Mediterranean Iberia. Quat. Int. 318, 3–18 (2013). [Google Scholar]
- 22.Rosas A., et al. , Paleobiology and comparative morphology of a late Neandertal sample from El Sidron, Asturias, Spain. Proc. Natl. Acad. Sci. U.S.A. 103, 19266–19271 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zilhão J., et al. , Last Interglacial Iberian Neandertals as fisher-hunter-gatherers. Science 367, eaaz7943 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Nabais M., Zilhão J., The consumption of tortoise among Last Interglacial Iberian Neanderthals. Quat. Sci. Rev. 217, 225–246 (2019). [Google Scholar]
- 25.Cortés-Sánchez M., et al. , Earliest known use of marine resources by Neanderthals. PLoS One 6, e24026 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weyrich L. S., et al. , Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature 544, 357–361 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Power R. C., et al. , Dental calculus indicates widespread plant use within the stable Neanderthal dietary niche. J. Hum. Evol. 119, 27–41 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Hardy K., et al. , Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften 99, 617–626 (2012). [DOI] [PubMed] [Google Scholar]
- 29.O’Regan H. J., Lamb A. L., Wilkinson D. M., The missing mushrooms: Searching for fungi in ancient human dietary analysis. J. Archaeol. Sci. 75, 139–143 (2016). [Google Scholar]
- 30.Stringer C. B., et al. , Neanderthal exploitation of marine mammals in Gibraltar. Proc. Natl. Acad. Sci. U.S.A. 105, 14319–14324 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michel V., Delanghe-Sabatier D., Bard E., Barroso Ruiz C., U-series, ESR and 14C studies of the fossil remains from the Mousterian levels of Zafarraya Cave (Spain): A revised chronology of Neandertal presence. Quat. Geochronol. 15, 20–33 (2013). [Google Scholar]
- 32.Yustos M., de los Terreros J. Y. S., Cannibalism in the Neanderthal world: An exhaustive revision. J. Taphon. 13, 33–52 (2015). [Google Scholar]
- 33.Álvarez-Lao D. J., García N., Chronological distribution of Pleistocene cold-adapted large mammal faunas in the Iberian Peninsula. Quat. Int. 212, 120–128 (2010). [Google Scholar]
- 34.Jaouen K., Szpak P., Richards M. P., Zinc isotope ratios as indicators of diet and trophic level in Arctic marine mammals. PLoS One 11, e0152299 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaouen K., Beasley M., Schoeninger M., Hublin J.-J., Richards M. P., Zinc isotope ratios of bones and teeth as new dietary indicators: Results from a modern food web (Koobi Fora, Kenya). Sci. Rep. 6, 26281 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bourgon N., et al. , Zinc isotopes in Late Pleistocene fossil teeth from a Southeast Asian cave setting preserve paleodietary information. Proc. Natl. Acad. Sci. U.S.A. 117, 4675–4681 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bourgon N., et al. , Trophic ecology of a Late Pleistocene early modern human from tropical Southeast Asia inferred from zinc isotopes. J. Hum. Evol. 161, 103075 (2021). [DOI] [PubMed] [Google Scholar]
- 38.McCormack J., et al. , Zinc isotopes from archaeological bones provide reliable tropic level information for marine mammals. Commun. Biol. 4, 683 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaouen K., et al. , Zinc isotope variations in archeological human teeth (Lapa do Santo, Brazil) reveal dietary transitions in childhood and no contamination from gloves. PLoS One 15, e0232379 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaouen K., Pons M.-L., Balter V., Iron, copper and zinc isotopic fractionation up mammal trophic chains. Earth Planet. Sci. Lett. 374, 164–172 (2013). [Google Scholar]
- 41.Jaouen K., et al. , Tracing intensive fish and meat consumption using Zn isotope ratios: Evidence from a historical Breton population (Rennes, France). Sci. Rep. 8, 5077 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jaouen K., Herrscher E., Balter V., Copper and zinc isotope ratios in human bone and enamel. Am. J. Phys. Anthropol. 162, 491–500 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Costas-Rodríguez M., Van Heghe L., Vanhaecke F., Evidence for a possible dietary effect on the isotopic composition of Zn in blood via isotopic analysis of food products by multi-collector ICP-mass spectrometry. Metallomics 6, 139–146 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Van Heghe L., Engström E., Rodushkin I., Cloquet C., Vanhaecke F., Isotopic analysis of the metabolically relevant transition metals Cu, Fe and Zn in human blood from vegetarians and omnivores using multi-collector ICP-mass spectrometry. J. Anal. At. Spectrom. 27, 1327–1334 (2012). [Google Scholar]
- 45.Montes L., Utrilla P., “The cave of Los Moros-1 at Gabasa (Huesca)” in Pleistocene and Holocene Hunters-Gatherers in Iberia and the Gibraltar Strait. The Current Archaeological Record, R. Sala Ramos, Ed. (Universidad de Burgos-Fundación Atapuerca, 2014), pp. 181–188. [Google Scholar]
- 46.Lorenzo J. I., Montes L., Restes Néandertaliens de la Grotte de “Los Moros de Gabasa”(Huesca, Espagne). Trabalhos de Arqueologia 17, 77–86 (2001). [Google Scholar]
- 47.Skinner M., Developmental stress in immature hominines from Late Pleistocene Eurasia: Evidence from enamel hypoplasia. J. Archaeol. Sci. 23, 833–852 (1996). [Google Scholar]
- 48.Pettitt P. B., Neanderthal lifecycles: Developmental and social phases in the lives of the last archaics. World Archaeol. 31, 351–366 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Austin C., et al. , Barium distributions in teeth reveal early-life dietary transitions in primates. Nature 498, 216–219 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith T. M., et al. , Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc. Natl. Acad. Sci. U.S.A. 107, 20923–20928 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Macchiarelli R., et al. , How Neanderthal molar teeth grew. Nature 444, 748–751 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Tsutaya T., Yoneda M., Reconstruction of breastfeeding and weaning practices using stable isotope and trace element analyses: A review. Am. J. Phys. Anthropol. 156 (suppl. 59), 2–21 (2015). [DOI] [PubMed] [Google Scholar]
- 53.Jaouen K., et al. , Dynamic homeostasis modeling of Zn isotope ratios in the human body. Metallomics 11, 1049–1059 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Bocherens H., et al. , Direct isotopic evidence for subsistence variability in Middle Pleistocene Neanderthals (Payre, southeastern France). Quat. Sci. Rev. 154, 226–236 (2016). [Google Scholar]
- 55.Ecker M., et al. , Middle Pleistocene ecology and Neanderthal subsistence: Insights from stable isotope analyses in Payre (Ardèche, southeastern France). J. Hum. Evol. 65, 363–373 (2013). [DOI] [PubMed] [Google Scholar]
- 56.Stevens R. E., Hedges R. E. M., Carbon and nitrogen stable isotope analysis of northwest European horse bone and tooth collagen, 40,000 BP–present: Palaeoclimatic interpretations. Quat. Sci. Rev. 23, 977–991 (2004). [Google Scholar]
- 57.McCormack J., et al. , Trophic position of Otodus megalodon and great white sharks through time revealed by zinc isotopes. Nat. Commun. 13, 2980 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Talamo S., Richards M., A comparison of bone pretreatment methods for AMS dating of samples >30,000 BP. Radiocarbon 53, 443–449 (2011). [Google Scholar]
- 59.Talamo S., Fewlass H., Maria R., Jaouen K., “Here we go again”: The inspection of collagen extraction protocols for 14C dating and palaeodietary analysis. Sci. Technol. Archaeol. Res. 7, 62–77 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blasco M. F., In the pursuit of game: The Mousterian cave site of Gabasa 1 in the Spanish Pyrenees. J. Anthropol. Res. 53, 177–217 (1997). [Google Scholar]
- 61.Blasco Sancho F., Hombres, Fieras y Presas: Estudio Arqueozoológico y Tafonómico del Yacimiento del Paleolítico Medio de la Cueva de Gabasa 1 (Universidad de Zaragoza, Huesca, Spain, 1995). [Google Scholar]
- 62.Jaouen K., What is our toolbox of analytical chemistry for exploring ancient hominin diets in the absence of organic preservation? Quat. Sci. Rev. 197, 307–318 (2018). [Google Scholar]
- 63.Naito Y. I., et al. , Evidence for herbivorous cave bears (Ursus spelaeus) in Goyet Cave, Belgium: Implications for palaeodietary reconstruction of fossil bears using amino acid δ15N approaches. J. Quat. Sci. 31, 598–606 (2016). [Google Scholar]
- 64.Münzel S. C., et al. , Behavioural ecology of Late Pleistocene bears (Ursus spelaeus, Ursus ingressus): Insight from stable isotopes (C, N, O) and tooth microwear. Quat. Int. 339, 148–163 (2014). [Google Scholar]
- 65.Bocherens H., Fizet M., Mariotti A., Diet, physiology and ecology of fossil mammals as inferred from stable carbon and nitrogen isotope biogeochemistry: Implications for Pleistocene bears. Palaeogeogr. Palaeoclimatol. Palaeoecol. 107, 213–225 (1994). [Google Scholar]
- 66.Richards M. P., et al. , Isotopic evidence for omnivory among European cave bears: Late Pleistocene Ursus spelaeus from the Peştera cu Oase, Romania. Proc. Natl. Acad. Sci. U.S.A. 105, 600–604 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sponheimer M., de Ruiter D., Lee-Thorp J., Späth A., Sr/Ca and early hominin diets revisited: New data from modern and fossil tooth enamel. J. Hum. Evol. 48, 147–156 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Binder W. J., Van Valkenburgh B., Development of bite strength and feeding behaviour in juvenile spotted hyenas (Crocuta crocuta). J. Zool. 252, 273–283 (2000). [Google Scholar]
- 69.Moynier F., Vance D., Fujii T., Savage P., The isotope geochemistry of zinc and copper. Rev. Mineral. Geochem. 82, 543–600 (2017). [Google Scholar]
- 70.González-Sampériz P., Montes L., Utrilla P., Pollen in hyena coprolites from Gabasa Cave (northern Spain). Rev. Palaeobot. Palynol. 126, 7–15 (2003). [Google Scholar]
- 71.Utrilla P., Montes L., Fernanda B., Torres Pérez-Hidalgo T. J., Ortiz Menéndez J. E., La cueva de Gabasa revisada 15 años después: Un cubil para las hienas y un cazadero para los Neandertales. Zona Arqueológica 13, 376–389 (2010). [Google Scholar]
- 72.Bocherens H., Fogel M. L., Tuross N., Zeder M., Trophic structure and climatic information from isotopic signatures in Pleistocene cave fauna of southern England. J. Archaeol. Sci. 22, 327–340 (1995). [Google Scholar]
- 73.Li Q., et al. , Spatially-resolved Ca isotopic and trace element variations in human deciduous teeth record diet and physiological change. Environ. Archaeol. 10.1080/14614103.2020.1758988 (2020). [DOI] [Google Scholar]
- 74.Guatelli‐Steinberg D., Recent studies of dental development in Neandertals: Implications for Neandertal life histories. Evol. Anthropol. 18, 9–20 (2009). [Google Scholar]
- 75.Joannes-Boyau R., et al. , Elemental signatures of Australopithecus africanus teeth reveal seasonal dietary stress. Nature 572, 112–115 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dean M. C., Smith B. H., “Growth and development of the Nariokotome youth, KNM-WT 15000” in The First Humans—Origin and Early Evolution of the Genus Homo, F. E. Grine, J. G. Fleagle, R. E. Leakey, Eds. (Springer, 2009), pp. 101–120. [Google Scholar]
- 77.Moynier F., Fujii T., Shaw A. S., Le Borgne M., Heterogeneous distribution of natural zinc isotopes in mice. Metallomics 5, 693–699 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Balter V., et al. , Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics 5, 1470–1482 (2013). [DOI] [PubMed] [Google Scholar]
- 79.Viers J., et al. , Evidence of Zn isotopic fractionation in a soil–plant system of a pristine tropical watershed (Nsimi, Cameroon). Chem. Geol. 239, 124–137 (2007). [Google Scholar]
- 80.Cloquet C., Carignan J., Lehmann M. F., Vanhaecke F., Variation in the isotopic composition of zinc in the natural environment and the use of zinc isotopes in biogeosciences: A review. Anal. Bioanal. Chem. 390, 451–463 (2008). [DOI] [PubMed] [Google Scholar]
- 81.Caldelas C., Weiss D. J., Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 411, 17–46 (2017). [Google Scholar]
- 82.Evans R. D., Wang W., Evans H. E., Georg R. B., Variation in Zn, C, and N isotope ratios in three stream insects. Facets 1, 205–216 (2016). [Google Scholar]
- 83.Nitzsche K. N., et al. , Magnesium and zinc stable isotopes as a new tool to understand Mg and Zn sources in stream food webs. Ecosphere 11, e03197 (2020). [Google Scholar]
- 84.Mourre V., et al. , “Exploitation du milieu montagnard dans le Moustérien final: La Grotte du Noisetier à Fréchet-Aure (Pyrénées Centrales Françaises)” in Proceedings of the XV World Congress UISPP (Lisbon, 4e9 September 2006). Mountain Environments in Prehistoric Europe: Settlement and Mobility Strategies, S. Grimaldi, Th. Perrin, J. Guilaine, Eds. (Archaeopress, Oxford, UK, 2008). pp. 1–10
- 85.Scherz H., Kirchhoff E., Trace elements in foods: Zinc contents of raw foods—A comparison of data originating from different geographical regions of the world. J. Food Compos. Anal. 19, 420–433 (2006). [Google Scholar]
- 86.Monge G., et al. , Earliest evidence of pollution by heavy metals in archaeological sites. Sci. Rep. 5, 14252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coutaud A., Meheut M., Viers J., Rols J.-L., Pokrovsky O. S., Zn isotope fractionation during interaction with phototrophic biofilm. Chem. Geol. 390, 46–60 (2014). [Google Scholar]
- 88.Mahan B., Moynier F., Jørgensen A. L., Habekost M., Siebert J., Examining the homeostatic distribution of metals and Zn isotopes in Göttingen minipigs. Metallomics 10, 1264–1281 (2018). [DOI] [PubMed] [Google Scholar]
- 89.Jaouen K., Balter V., Menopause effect on blood Fe and Cu isotope compositions. Am. J. Phys. Anthropol. 153, 280–285 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Blasco F., Montes L., Utrilla P., “Deux modèles de stratégie occupationelle dans le Moustérien tardif de la vallée de l’Ebre: Les grottes de Peña Miel et Gabasa” in The Last Neandertals, the First Anatomically Modern Humans: A Tale about the Human Diversity, E. Carbonell, M. Vaquero, Eds. (Universitat Rovira i Virgili, Tarragona, Spain, 1996), pp. 385–434. [Google Scholar]
- 91.Lloveras L., Moreno-García M., Nadal J., Taphonomic analysis of leporid remains obtained from modern Iberian lynx (Lynx pardinus) scats. J. Archaeol. Sci. 35, 1–13 (2008). [Google Scholar]
- 92.Haynes G., A guide for differentiating mammalian Carnivore taxa responsible for gnaw damage to herbivore limb bones. Paleobiology 9, 164–172 (1983). [Google Scholar]
- 93.Drucker D. G., et al. , Isotopic analyses suggest mammoth and plant in the diet of the oldest anatomically modern humans from far southeast Europe. Sci. Rep. 7, 6833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lösch S., Grupe G., Peters J., Stable isotopes and dietary adaptations in humans and animals at pre-pottery Neolithic Nevalli Cori, southeast Anatolia. Am. J. Phys. Anthropol. 131, 181–193 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Hedges R. E. M., Reynard L. M., Nitrogen isotopes and the trophic level of humans in archaeology. J. Archaeol. Sci. 34, 1240–1251 (2007). [Google Scholar]
- 96.Langer P., Clauss M., Morphological adaptation of the eutherian gastrointestinal tract to diet. Vertebr. Zool. 68, 237–252 (2018). [Google Scholar]
- 97.Moynier F., Albarède F., Herzog G. F., Isotopic composition of zinc, copper, and iron in lunar samples. Geochim. Cosmochim. Acta 70, 6103–6117 (2006). [Google Scholar]
- 98.Deniel C., Pin C., Single-stage method for the simultaneous isolation of lead and strontium from silicate samples for isotopic measurements. Anal. Chim. Acta 426, 95–103 (2001). [Google Scholar]
- 99.Vonhof H. B., et al. , High-precision stable isotope analysis of <5 μg CaCO3 samples by continuous-flow mass spectrometry. Rapid Commun. Mass Spectrom. 34, e8878 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.