Significance
A variety of cellular functions are driven by actin, which undergoes cyclic transitions between the monomeric G-form and the filamentous F-form. To gain insights into actin dynamics, the mechanism by which the energy is supplied by the ATP hydrolysis reaction in the F-form actin must be elucidated. This has been hampered by the lack of actin filament structures at atomic resolutions. Here, we have crystallized actin molecules trapped in the F-form without forming filaments, and based upon these structures we determined the reaction path by quantum mechanics calculations. The results are consistent with previous biochemical data. Remarkably, the hydrolysis reaction mechanism is essentially identical to those of motor proteins, while the process of Pi release is distinct.
Keywords: actin, ATP hydrolysis, protein crystallography, QM/MM simulation
Abstract
The major cytoskeleton protein actin undergoes cyclic transitions between the monomeric G-form and the filamentous F-form, which drive organelle transport and cell motility. This mechanical work is driven by the ATPase activity at the catalytic site in the F-form. For deeper understanding of the actin cellular functions, the reaction mechanism must be elucidated. Here, we show that a single actin molecule is trapped in the F-form by fragmin domain-1 binding and present their crystal structures in the ATP analog-, ADP-Pi-, and ADP-bound forms, at 1.15-Å resolutions. The G-to-F conformational transition shifts the side chains of Gln137 and His161, which relocate four water molecules including W1 (attacking water) and W2 (helping water) to facilitate the hydrolysis. By applying quantum mechanics/molecular mechanics calculations to the structures, we have revealed a consistent and comprehensive reaction path of ATP hydrolysis by the F-form actin. The reaction path consists of four steps: 1) W1 and W2 rotations; 2) PG–O3B bond cleavage; 3) four concomitant events: W1–PO3− formation, OH− and proton cleavage, nucleophilic attack by the OH− against PG, and the abstracted proton transfer; and 4) proton relocation that stabilizes the ADP-Pi–bound F-form actin. The mechanism explains the slow rate of ATP hydrolysis by actin and the irreversibility of the hydrolysis reaction. While the catalytic strategy of actin ATP hydrolysis is essentially the same as those of motor proteins like myosin, the process after the hydrolysis is distinct and discussed in terms of Pi release, F-form destabilization, and global conformational changes.
Actin, a major cytoskeletal protein, is an ATP-binding protein that exists in monomeric (G-actin) and filamentous (F-actin) forms. A large variety of cellular functions are driven by the cyclic processes of actin molecule assembly (polymerization) and disassembly (depolymerization). Actin ATP hydrolysis, which was originally discovered without knowing its biological significance (1), energetically drives the cyclic assembly–disassembly (2). Therefore, the elucidation of the mechanism of ATP hydrolysis is crucially important for our understanding of the cellular functions of actin.
The actin molecule comprises two major domains, the outer domain (OD) and the inner domain (ID). An ATP molecule with a divalent cation binds in the cleft between the OD and ID (3). Upon incorporation into the actin filament, the actin molecule undergoes a conformational transition (4). In the monomeric G-form, the OD is twisted by about 20° relative to the ID, whereas in the filamentous F-form the two domains are almost flat.
The entire cycle of actin assembly–disassembly proceeds in five sequential processes (below, each nucleotide tightly binds Mg2+, which is not explicitly indicated for simplicity. -G and -F indicate G-form and F-form actin, respectively):
-
1)
ATP-G → ATP-F: The conformational transition, which is associated with the polymerization of the ATP-bound G-form actin.
-
2)
ATP-F → ADP-Pi-F: The ATP hydrolysis reaction, triggered by process 1 with a rate constant of 0.3 s−1 (5), which is considerably slower than other ATPases [e.g., myosin (20 to 200 s−1) (6)]. The slow rate which allows further elongation, and the hydrolysis reaction occurs in the interior of the filament. The reaction is irreversible (7).
-
3)
ADP-Pi-F → ADP-F: The Pi release, which occurs at an extremely slow rate with actin molecules in the interior of the filament [0.003 s−1 (8) or 0.007 s−1 (9)] but is much faster with molecules around barbed ends [>2 s−1 (10) or 1.8 s−1 (9)]. The Pi release is reversible. ADP-F has a modest affinity for Pi with a dissociation constant of 1 mM (9, 10), which is far below the cytosolic concentration, whereas ADP-G hardly binds Pi (∼60 mM) (10).
-
4)
ADP-F → ADP-G: The depolymerization releases actin subunits at the ends of the actin filament.
-
5)
ADP-G → ATP-G: The bound nucleotide exchanges from ADP for ATP, which occurs only in G-actin.
The cyclic reaction of barbed end assembly and the pointed end disassembly, occurring in actin alone at the critical concentration of monomers using ATP as an energy source, is called tread-milling (11). In the cytosol, where the actin concentration is far beyond the critical concentration for polymerization, cooperative behaviors of regulatory proteins accelerate the spontaneous tread-milling and/or facilitate filament turnover by promoting multiple reactions involving nucleation, capping, and severing, to drive fast cell migration (12, 13). Recent progress, particularly in kinetic analyses of single actin filaments, has revealed the contribution of the barbed end disassembly to monomer recycling (14, 15). Pi release dramatically alters the assembly properties of actin filaments in two ways: 1) by promoting spontaneous depolymerization and, more importantly, 2) by making the ADP-actin subunits more attractive to proteins that facilitate filament disassembly such as ADF/cofilin. Therefore, for comprehensively understanding of the actin cycle of assembly–disassembly, the mechanism and significance of the Pi release are of crucially important. This should be based upon our knowledge of the actin ATP hydrolysis process and the properties of ADP-Pi-F.
Studies of the actin ATP hydrolysis mechanism have been hampered, mainly due to the lack of F-form actin structures at a sufficient resolution for identifying water molecules, which are the keys to the reaction. The crystal structure of the G-form actin is not suitable (3, 16), since the G-form actin is not ATP hydrolysis–competent (17). The cryogenic electron microscopy (cryo-EM) structures of the actin filament at over 3.1-Å resolutions (18–20) are also insufficient, because the details of the water molecules in the nucleoside binding cleft, particularly those surrounding the phosphate moiety, are missing. The actin filament has not been crystallized, because filaments with a fixed length have never been prepared. Previously, the ATP hydrolysis mechanism of F-form actin was studied by employing metadynamics simulations (21). However, the details of the reaction mechanism have remained obscure, since the simulations were based upon structural data that lacked water molecules (4).
We now show that, in a 1:1 complex of the actin-binding protein fragmin domain-1 (F1) and actin (referred to as the F1A complex), the actin molecule is trapped in the F-form. This is the first monomeric actin structure in the F-form among the more than 260 actin structures deposited to date in the Protein Data Bank (PDB) (22). Fragmin is a member of the villin-gelsolin protein superfamily (23) and was first isolated from the slime mold Physarum polycephalum (24). Like gelsolin, fragmin severs F-actin and caps the barbed end in a Ca2+-dependent manner (24–26). Fragmin consists of three tandemly linked domains (F1, F2, F3) that are homologous to the N-terminal half of gelsolin (G1, G2, G3). G1 also forms a 1:1 complex with actin. However, in the G1–actin complex, the actin is in the G-form (27).
The F1A complex provided 1.15-Å resolution structures of F-form actin binding AMPPNP, ADP-Pi, or ADP, with a magnesium ion at the catalytic site. Based on the high precision of the pre- and posthydrolysis structures, we performed quantum mechanics/molecular mechanics (QM/MM) calculations (28), which revealed the comprehensive reaction mechanism of the actin ATP hydrolysis. The results provide mechanistic answers to key questions regarding the cycle of actin state transitions: how the G-to-F conformational transition triggers ATP hydrolysis, why the ATP hydrolysis rate is much slower than those of other ATPases, why the hydrolysis is irreversible, why the Pi release is so slow, and why F-form actin is destabilized after the Pi-release.
Our results demonstrate that, while some properties are quantitatively different, the overall catalytic strategy of actin ATP hydrolysis (up until the formation of ADP-Pi-F) is almost identical to those of P-loop type-motor proteins, such as F1-ATPase (29), kinesin (30), and myosin (31–33), despite the substantial differences in the catalytic site structures. In contrast to the common ATP hydrolysis strategies, the processes after the hydrolysis are distinct in actin: The hydrolysis does not cause any overall conformational changes; therefore, the Pi is not promptly released and the ADP-Pi F-form actin structure is readily available. These observations have led us to propose hypotheses about how ATP hydrolysis and Pi release are related in general, and how Pi is released by actin in particular.
2. Results
2.1. Crystal Structures of F-Form Actin.
Like gelsolin domain-1 (G1) (27), fragmin domain-1 (F1) (residues 1 to 160) forms a 1:1 complex with actin. The crystal structures of the F1A complex at 1.15-Å resolution (Fig. 1 A and B) show that F1 binds to the hydrophobic cleft of actin via the calcium ion, which binds to the intermolecular site formed in the interface between F1 and actin (SI Appendix, Fig. S1A), like G1 in the G1–actin complex (27). However, except for the D-loop, which is missing as in most monomeric actin crystal structures, the main chain conformation of actin in F1A is nearly identical to that of F-actin obtained by cryo-EM, with a Cα root-mean-square deviation (RMSD) = 0.37 Å between the actin molecules in ADP-Pi–bound F1A and the ADP-Pi–bound F-actin molecule (6DJN), the highest-resolution F-actin structure to date (20) (Fig. 1C). Therefore, the actin molecule in F1A adopts the F-form conformation but not the G-form conformation as in the G1–actin complex (27). Compared with G1, the F1 domain has a fragmin-specific 32-amino-acid N-terminal extension (referred to as FNEX: Fragmin N-terminal EXtension). FNEX binds to the backside of actin between the ID and OD, with Asn13 (F1) forming multiple hydrogen bonds with the actin Pro-rich loop residues (108 to 112) (SI Appendix, Fig. S1 B and C). The crystal structure of actin in complex with F115-160, an N-terminally truncated version of F1 that lacks Asn13, shows that actin adopts the G-form, thus demonstrating that the interactions between the FNEX of F1 and the Pro-rich loop of actin play a key role in stabilizing the strained F-form conformation (SI Appendix, Fig. S1 D–F). The F1A complex is formed in the presence of Ca2+, and three cations are identified in the F1A complex structure. One fortifies the F1 actin interactions, by binding to the intermolecular Ca2+-binding site formed between F1 and actin (Type-1 Ca2+-binding site) (27). The second cation (Ca2+) binds to the intra-F1 Ca2+-binding site (Type-2 Ca2+-binding site), possibly stabilizing the F1 conformation (SI Appendix, Fig. S1A). The third cation (Ca2+), which was bound to the nucleotide binding site (catalytic site) of actin after the complex formation, was replaced by a Mg2+ ion, the major cation in the cytosol (34), by adding Mg2+ and EGTA to the F1A solution (see SI Appendix, SI Materials and Methods for details).
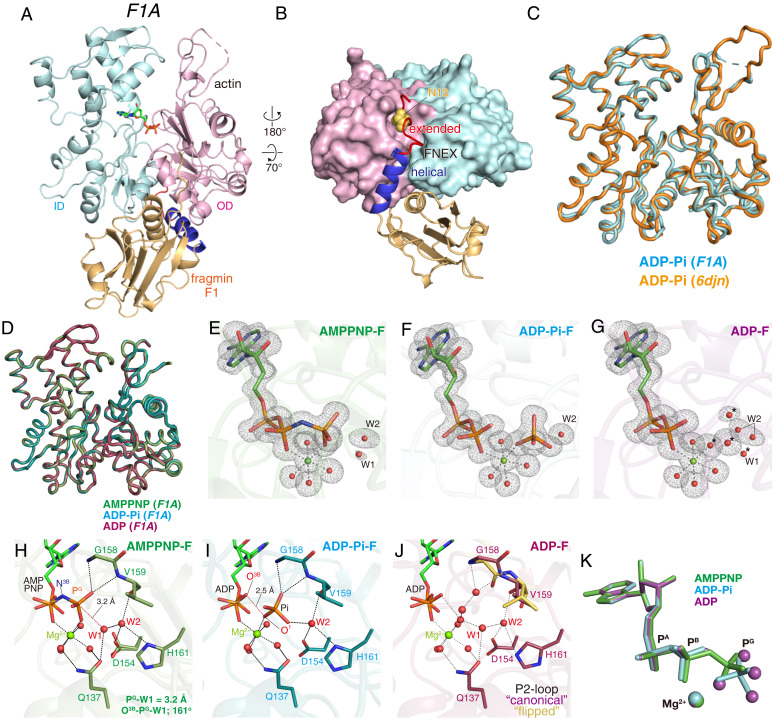
Fig. 1.
Crystal structures of F1A. (A and B) Overall structure of F1A: front (A) and bottom (B) views. The actin ID (cyan), OD (magenta), fragmin F1 core (light brown), helical region (blue), and extended region (red) of the FNEX segment of F1 are shown. F1 consists of the FNEX segment (residues 1 to 32) and the core (residues 33 to 160). FNEX is further divided into the extended (residues 7 to 20) and helical (residues 21 to 32) regions, and no electron densities were assignable to residues 1 to 6 (see SI Appendix, Fig. S3D for a schematic representation of specific regions of F1). In B, Asn13 (F1) is depicted by yellow spheres (see SI Appendix, Fig. S1 B and C for more details). (C) The crystal structure of ADP-Pi-F within the F1A complex (cyan), superimposed with the ADP-Pi–bound F-actin cryo-EM structure (orange) (6DJN). The main chain RMSD is 0.37 Å. (D) Superimposed main chain folds of actin within F1A crystals, AMPPNP-F (green), ADP-Pi-F (cyan) and ADP-F (violet). The main chain RMSDs among the three structures are less than 0.13 Å. (E–G) Comparisons of the conformations of actin-bound nucleotides with Mg2+ and water molecules coordinated to Mg2+, W1 (lytic water) and W2 (helping water), and four water molecules replacing Pi in ADP-F (marked by asterisks). (E) AMPPNP-F, (F) ADP-Pi-F, and (G) ADP-F, each within the F1A complex. Meshes represent the Fo − Fc omit map contoured at 3.5 σ. (H–J) Structures of the nucleotide binding sites of (H) AMPPNP-F (dark green), (I) ADP-Pi-F (teal), and (J) ADP-F (light pink). Nucleotides, Mg2+, key residues, and water molecules are shown. The P2-loop residues adopting “flipped” conformations (Gly158 and Val159) are colored yellow in J. (K) The nucleotides (stick models) with Mg2+ (balls) are superimposed: AMPPNP in AMPPNP-F (green), ADP-Pi in ADP-Pi-F (cyan), and ADP in ADP-F (violet). The four violet balls are four water molecules in the structure of ADP-F and replace the four oxygen atoms of Pi in the ADP-Pi-F structure.
We have obtained crystal structures of three species of the F1A complex, all at 1.15-Å resolutions, containing F-form actin in either the Mg-AMPPNP bound form (AMPPNP-F) as the prehydrolysis structure, the Mg-ADP-Pi–bound form (ADP-Pi-F) as the posthydrolysis structure, or the Mg-ADP–bound form (ADP-F) as the post-Pi release structure (Fig. 1 H–J). AMPPNP (β,γ-imidoadenosine 5′-triphosphate), with a nitrogen atom replacing the oxygen atom bridging PB and PG, was used as a slowly hydrolyzed ATP analog (35) (see also SI Appendix, SI Discussion). Among these F1A structures, the main chain conformations of actin are essentially identical (Cα RMSD = 0.11 Å between ADP-Pi-F and ADP-F), indicating that no global conformational alterations are associated with either ATP hydrolysis or Pi release (Fig. 1D), albeit the positions of side-chain atoms and water molecules trapped within the catalytic site are distinct. In each F-form structure, clear electron densities are observed for nucleotides, cations, and water molecules, allowing us to unambiguously determine the catalytic site structures in the key phases of the cycle of actin state transitions (Fig. 1 E–G). The orientations and configurations of the nucleotides are almost completely conserved among the three structures (Fig. 1K).
When we used ATP-G-actin to form the F1A complex, the final structure contained ADP at the catalytic site, indicating that the ATP was hydrolyzed during the crystal growth. Small but not negligible electron densities were observed in the PG position of ATP, which were assignable to Pi with very low occupancy. Therefore, to obtain the ADP-F structure, the crystallization solution was completely deprived of Pi enzymatically (see SI Appendix, SI Materials and Methods for details). The ADP-Pi-F structure with full Pi occupancy was obtained by forming the F1A complex by mixing F1 with ATP-bound G-actin and crystallizing it with inorganic phosphate (0.1 M Na2HPO4) in the crystallization drop.
2.2. ATP Hydrolysis Activity of Actin in the F1A Complex.
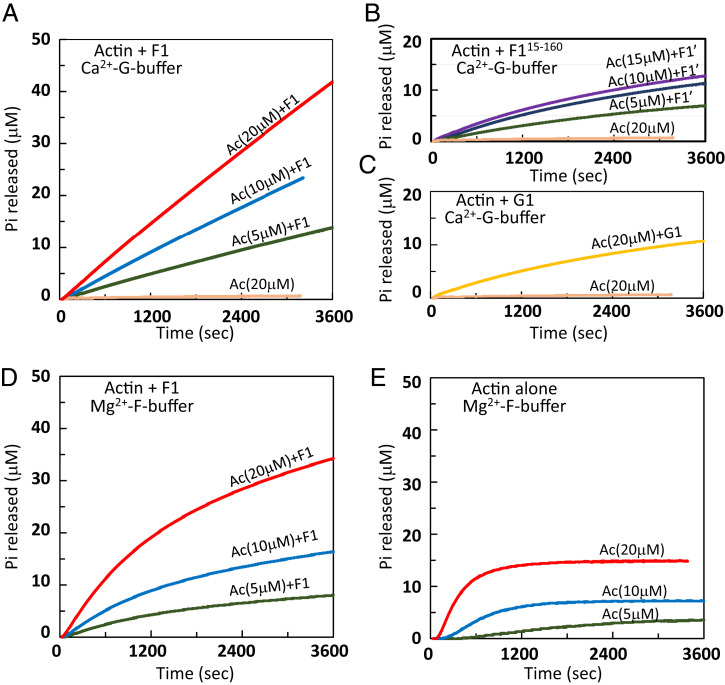
Given that F1 constrains actin in the F-form conformation in the crystal, we performed biochemical assays to examine the effect of F1 on actin, particularly to test whether F1 can promote the ATPase activity of actin in solution. First, we investigated F1 binding to actin by isothermal titration calorimetry (ITC) (SI Appendix, Fig. S2A). F1 bound actin at a stoichiometry of 0.956 ± 0.015, consistent with the 1:1 binding in the crystal, and the affinity was beyond the sensitivity of the ITC apparatus (<1 nM). Similarly, gelsolin G1 was reportedly binds actin with an extremely high affinity (∼5 pM) (36). We next examined the F1 activated ATPase activity of actin by measuring the Pi release. F1A, formed under nonpolymerizing conditions, released Pi at a constant rate (∼0.0006 s−1) during our measurements (1 h) (Fig. 2A), whereas actin alone did not. This clearly demonstrates that without polymerization F1 can induce ATP hydrolysis in actin, probably by shifting the actin conformation to the F-form. Compared to F1A, about ∼25% of Pi was released from actin in the presence of F115-160 or gelsolin G1 (Fig. 2 B and C), although neither has been found complexed with an F-form actin in crystals (SI Appendix, Fig. S1D) (27). This may be explained by actin transiently adopting the F-form conformation in solution when complexed with F115-160 or G1 (see SI Appendix, SI Discussion for details). The same trend was observed under the polymerizing conditions (Fig. 2D). The increment of Pi plateaued in the F-actin control because subunits in the filament interior cannot exchange the bound nucleotide (Fig. 2E). Interestingly, under both polymerizing and nonpolymerizing conditions, the Pi released from F1A did not reach a plateau even after it exceeded the actin concentration, indicating that a large fraction of actin underwent multiple rounds of hydrolysis, requiring the nucleotide exchange. Using ε-ATP as an indicator, we confirmed that actin exchanges the bound nucleotide in the presence of F1 (SI Appendix, Fig. S2B). To determine when hydrolysis occurs in F1A, we measured the total amount of ADP (i.e., released from actin and remaining bound). We found that ADP was not produced immediately after the complex formation but increased slowly, requiring at least 30 min for all actin to complete the first hydrolysis (SI Appendix, Fig. S2C), suggesting that in solution the actin molecule in F1A does not always adopt the hydrolysis-competent, F-form conformation. We crystallized F1A under conditions similar to F115-160A (SI Appendix, Fig. S3 A and B). In this crystal, actin adopts the G-form and the extended region of FNEX is detached from actin (SI Appendix, Fig. S3C), suggesting that this region acts as a switch for the G-to-F transition (SI Appendix, Fig. S3).
Fig. 2.
Time courses of Pi release from actin in the F1A complex. (A) The Pi release time courses from the F1A complex (actin concentrations of 20 µM [red], 10 µM [blue], and 5 µM [green]) or from G-actin alone (20 µM; wheat) in the nonpolymerizing Ca2+-G-buffer, measured with an EnzChek phosphate assay kit. The Ca2+-ATP-G-actin and F1 were mixed at t = 0, at a molar ratio of 1:1.2 to ensure complete F1-bound actin. (B and C) The same as A, but F1 was replaced by an N-terminally truncated F115-160 (B) or by gelsolin segment 1 (G1) (C). Neither promotes the G-to-F conformational transition of actin. In B and C, the curves are color-coded according to the actin concentrations: 15 µM (purple), 10 µM (blue), 5 µM (green), or 20 µM of G-actin alone (wheat) and 20 µM of G1A complex (orange). Note that F115-160 and G1 moderately enhance the ATPase activity of actin, even though neither was found in complexed with F-form actin in the crystal (SI Appendix, Fig. S1F and ref. 27). (D) Pi release time courses from the F1A complex in the polymerizing Mg2+-F-buffer. Each curve is color-coded according to the actin concentrations as in A. Note that in D the Pi release rate decreased gradually as compared to the time courses in A, which is not fully accounted for at present. (E) Time courses of Pi released from polymerizing actin. Each curve is color-coded according to the actin concentrations as in A.
2.3. How Does the G-to-F Transition Trigger ATP Hydrolysis?
The global conformation of the actin molecule changes from the G-form to F-form upon polymerization (4). Cryo-EM structural analyses have revealed that the G-to-F conformational transition occurs prior to ATP hydrolysis and Gln137 and His161 repositioning in the catalytic site (19, 20), which must be accompanied by the relocation of water molecules, but hardly observable due to the limited map resolution. In the EM maps, while a density protrusion from the NE2 atom of His161 in the AMPPNP-F-actin was suggested as a water molecule (W2, see below), no other water molecules were identified (20).
The present crystal structures of F-form actin within the F1A complex allowed us to determine how the G-to-F transition triggers the rearrangement of water molecules, which play critical roles in ATP hydrolysis, in the catalytic site. A comparison between the Mg-AMPPNP–bound F-form actin in the F1A complex and the Mg-ATP–bound G-actin (PDB ID code: 4B1Y) (37) revealed that the G-to-F transition has limited consequences with regard to the catalytic site structure, since the nucleotides are in nearly identical configurations with their phosphate moieties clamped by two loops emerging from the OD (P1-loop: residues 13 to 16) and the ID (P2-loop: 156 to 159) (Fig. 3 and SI Appendix, Fig. S4). As described previously (19, 20), the transition induces the structural changes of essentially two residues in the vicinity of PG. First, Gln137, a key residue in hydrolysis (16, 38) that hydrogen bonds with a putative lytic water (W1), moves 1.1 Å closer to PG due to a shift of the hinge helix (residues 137 to 145) linking the two major domains (Fig. 3). The second residue is His161 (Fig. 3 and SI Appendix, Fig. S4). In ATP-G, the His161 side chain is located between Pro109 of the Pro-rich loop (OD: residues 107 to 113) and Arg177 (ID). The G-to-F transition brings the Pro-rich loop and Arg177 closer to each other, and multiple hydrogen bonds are formed between them (SI Appendix, Fig. S4). To avoid the predicted steric clashes, the imidazole ring of His161 flips toward the nucleotide, excluding two water molecules (W3 and W4) (19) and forming a hydrogen bond with another water molecule (W2).
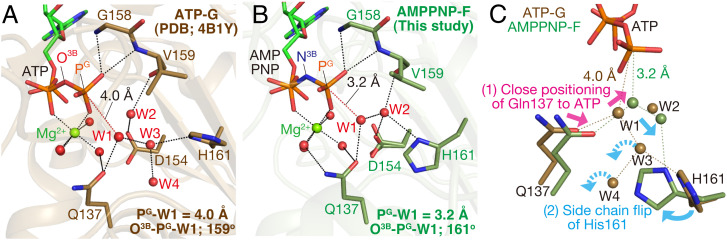
Fig. 3.
Relocations of side chains and water molecules upon the G-to-F transition. Structures of the nucleotide binding sites of (A) ATP-G-actin [brown, PDB ID code: 4B1Y (37)] and (B) AMPPNP-F (dark green). Nucleotides, Mg2+, key residues, and water molecules are shown. The distances between the lytic water (W1) and PG are indicated in A and B. Note that a hydrogen bond is formed between W1 and W3 in A and is replaced by one between W1 and W2 in B. B is the same as Fig. 1H. (C) Comparison of the catalytic site structures between (A) (brown) and (B) (dark green), with nucleotides aligned. The G-to-F transition shifts Gln137, flips the side chain of His161, and relocates the water molecules in the vicinity of ATP (see SI Appendix, Fig. S4 for more details).
These elaborate structural rearrangements have two important consequences concerning the position and environment of W1. First, while the W1-PG-O3B angle is almost unchanged (159° to 161°), W1 moves closer to PG, from 4.0 Å to 3.2 Å, and becomes capable of attacking PG (Fig. 3C). Second, the water molecule that hydrogen-bonds with W1 switches from W3 to W2 and is apparently stabilized by hydrogen bonds with the side chains of Asp154 and His161 and the main chain carbonyl of Val159. W2 and the side chain of Gln137 are thus suitably positioned to facilitate the deprotonation of W1 during the nucleophilic attack (ref. 39 and see below). Notably, although the occupancy of W2 is full, its position is unstable. According to the electron density maps, W2 appears to fluctuate between O1G (of AMPPNP) and His161, partially hindering the near-in-line positioning of W1 (SI Appendix, Fig. S5 A and C). Perhaps in response to the fluctuations of W2, Asp154, which hydrogen-bonds with W2, adopts two conformations (SI Appendix, Fig. S5A). The W2 fluctuations may partly explain the relatively slow rate of actin-mediated ATP hydrolysis (0.3 s−1) (5), as compared with other highly efficient ATPases (see SI Appendix, Fig. S5 A and C for details). In myosin and F1-ATPase, W1 is held near PG via hydrogen bonds with at least two polypeptide residues (32). In contrast, W1 only interacts with Gln137 and W2 in AMPPNP-F, and thus its positioning relies largely on W2 (Fig. 3B).
2.4. The ADP-Pi-F Structure.
In the F1A complex, actin hydrolyzes ATP and releases Pi not only in solution (Fig. 2) but also in the crystallization process (section 2.1). Therefore, to obtain the ADP-Pi-F structure, we crystallized the ATP-bound F1A complex (F1A-ATP) in the presence of inorganic phosphate. This ADP-Pi-F crystal structure does not necessarily represent the structure just after hydrolysis, since it is reconstituted from ADP-bound F-form actin and Pi acquired from the solvent. Such Pi uptake has not been reported for the G-form actin crystals, suggesting that the nucleotide binding site of F-form actin is highly preferable for the Pi binding. This is consistent with the biochemical results that Pi has much higher affinity for F-actin than G-actin (10).
While the overall actin conformation in ADP-Pi-F is almost identical to that in AMPPNP-F (Cα RMSD = 0.1 Å) (Fig. 1D), the hydrogen bond networks within the nucleotide binding cleft of ADP-Pi-F are substantially better defined than those in AMPPNP-F. In ADP-Pi-F, Pi adopts an inverted configuration as its O4(G), originated from W1, is directed toward His161 (Fig. 1 I and K). Consequently, W2, which fluctuates in AMPPNP-F, is now settled at a specific position. This is due to the stable hydrogen bonds formed with Pi and the surrounding residues, Val159, His161, and Asp154. Particularly, the dual conformations of Asp154 observed in the AMPPNP‐F structure are not observed in ADP‐Pi‐F (SI Appendix, Fig. S5B). Another remarkable hydrogen bond (2.53 Å) is between ADP(O3B) and Pi(O1(G)). This tight hydrogen bond is characteristic of the ADP-Pi-F structure and direct evidence that the Pi is the acceptor of the proton derived from W1 during the hydrolysis reaction, as confirmed by our QM/MM analysis (see below).
2.5. The Path of the F-Form Actin ATP Hydrolysis Reaction Consists of Four Steps.
With high-resolution crystal structures of the F1A complex at 1.15-Å resolution, we employed combined quantum and classical (QM/MM) calculations to elucidate the reaction path of the F-form actin ATP hydrolysis. Here the active site is treated by quantum mechanics, otherwise atoms are treated by force field for the efficient evaluation of the potential energy surface. The reaction energy profile can be obtained by searching the minimum energy pathway for chemical reactions on the QM/MM potential energy surface, on which we can see which step is the bottleneck of the reaction and how the active-site residues contribute to it. While no experimental techniques are currently suited for this purpose, computational QM/MM studies have provided true insights into ATP hydrolysis mechanisms (33). By using both the pre- and posthydrolysis crystal structures and comparing the reaction pathway with those of other ATPases, we expect to obtain a comprehensive and robust solution for the reaction mechanism.
In our QM/MM calculations, the experimentally obtained AMPPNP-F structure (SI Appendix, Fig. S6B), which is also referred to as Rxtal (R for reactant), was geometry-optimized and used as the prehydrolysis structure, Rxtal-based-R, without major modifications, except that the nitrogen atom bridging PB and PG was replaced with an oxygen atom (Fig. 4A). This is based on our assumption that AMPPNP-F is a good analog of ATP-F for the hydrolysis reaction. In the same manner, the experimentally obtained ADP-Pi-F (SI Appendix, Fig. S6C), which is also referred to as Pxtal (P for product), was geometry optimized and used as the posthydrolysis structure Pxtal-based-P, without major modifications (Fig. 4B). Here we assumed that ADP-Pi-F is identical, whether it is reconstituted or formed through the hydrolysis (see SI Appendix, SI Discussion for more details about the assumptions). QM/MM calculations can be performed based on either Rxtal or Pxtal (Fig. 4C). In other words, the energy level difference, the height of the energy barrier, and the atomic positional shifts for each intermediate (local minima of the potential energy surface, abbreviated as IM) and transition state (saddle points of the potential energy surface, TS) can be calculated successively, starting from either Rxtal or Pxtal. Note that this has nothing to do with the reversibility of the reaction: Under natural conditions, the hydrolysis reaction is irreversible (7); therefore, the backward reaction does not proceed (SI Appendix, SI Discussion).
Fig. 4.
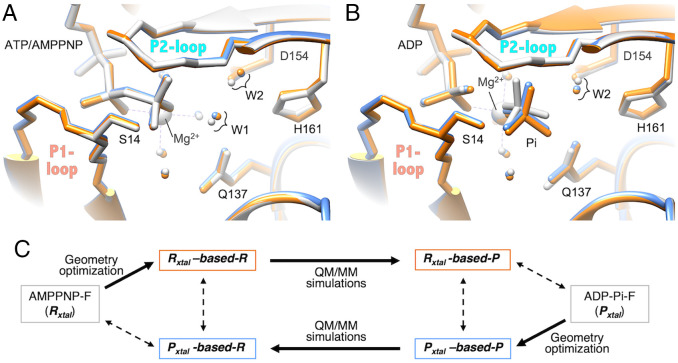
Comparisons between the crystal structures (experimental) and the structures obtained by the QM/MM simulations (calculated). (A) Three structures of state R (reactant) are compared: the crystal structure Rxtal (AMPPNP-F) (gray), Rxtal-based-R (orange), which is the geometry optimized Rxtal, and Pxtal-based-R (blue), which is the QM/MM simulated based on Pxtal. (B) Three structures of state P (product) are compared: the crystal structure Pxtal (ADP-Pi-F) (gray), Pxtal-based-P (blue), which is the geometry-optimized Pxtal, and Rxtal-based-P (orange), which is the QM/MM simulated based on Rxtal. In A and B, small balls indicate the positions of oxygen atoms of selected water molecules, while large balls are Mg2+, color-coded in the same manner as for the peptides and nucleotides. The dashed lines centered at Mg2+ indicate the coordinate bonds between Mg2+ ion and oxygen atoms. (C) Keys for the structures compared in A and in B. Note that, in the natural actin, the hydrolysis reaction is irreversible (7); therefore, the backward reaction, from state P to state R, does not proceed.
The QM/MM calculations provided a plausible reaction path via several intermediates (IM) and transition states (TS) (Fig. 5A). Based on the energy profile (Fig. 5G), we divided the entire reaction path into four steps:
Fig. 5.
The ATP hydrolysis reaction path of F-form actin, simulated by QM/MM calculations. (A) The reaction scheme of ATP hydrolysis in the F-form actin from reactant (R) to product (P) state via intermediate (IM) and transition (ST) states. (B) The molecular structure of PO3−, W1, and W2 at the rate-limiting TS4. The proton transfers from W1 to W2 in concert with the other from W2 to PO3−. (C–E) Atomic positions in the catalytic site and some interatomic distances in the states R (C), IM3 (D), and P (E), calculated based on the structure of Pxtal. In B–E, phosphorus atoms (P), oxygen atoms (O), protons (H+), and magnesium ions (Mg2+) are colored orange, red, gray, and light green, respectively. (F) The three structures in C–E are superimposed, where state R, IM3, and P are colored orange, magenta, and blue, respectively. Note that all of the atoms, except for PG, W1, and W2, remain at almost the same positions. (G) Potential energy profiles along the reaction path indicated in A. The profile calculated based on the structure of Rxtal, and the other calculated based on the structure of Pxtal, are indicated in orange and blue, respectively.
Step 1: The initial hydrogen-bond rearrangement (HBR-1): from state R to IM2. In this step, only W1 and W2 change their orientations. Particularly, W1 is rotated so the lone-pair electrons are directed toward PG of ATP. IM2 is at an energy level of 5 kcal/mol and less stable relative to state R, because the hydrogen bond between ATP and W1 is disrupted. If this step were omitted, then the hydrolysis reaction should not proceed, because without the preceding reorientation of W1, the W1–PO3− complex should not be formed (SI Appendix, Fig. S8).
Step 2: The P–O bond dissociation (POD): from IM2 to IM3. The PG–O3B bond is cleaved, producing PO3− (metaphosphate). This reduces the W1–PG distance to 1.9 Å, allowing the formation of the W1–PO3− complex in the IM3 state (Fig. 5D).
Step 3: The proton transfer (PT): from IM3 to IM4. Within the W1–PO3− complex, W1 is disrupted to produce OH− and a proton. This OH− nucleophilically attacks PG, producing HPO42−. The proton dissociated from W1 is transferred to W2, in concert with another proton transfer from W2 to HPO42−, forming H2PO4− in IM4 (Fig. 5B). In this step, the disruption of W1, the nucleophilic attack of OH−, the proton abstraction, and the proton transfer occur concomitantly. The corresponding transition state (TS4) forms the highest energy barrier in the entire reaction, so the proton transfer is the rate-limiting step of the hydrolysis. The height of the barrier is in the range from 13 to 17 kcal/mol, consistent with the experimental rate of 0.3 s−1 for the F-actin-catalyzed hydrolysis (5) that corresponds to a barrier height of 18 kcal/mol, based on the transition state theory.
Step 4: The final hydrogen-bond rearrangements (HBR-2): from IM4 to state P. In IM4, H2PO4− forms a hydrogen bond network with its surrounding groups, such as Mg2+, W2, and the side chain of Gln137. In step 4, these hydrogen bonds are rearranged to form a tight hydrogen bond with ADP, through shifts of the transferred proton within H2PO4− (Fig. 5E), stabilizing structure P. More details of the hydrogen-bond rearrangement are indicated in SI Appendix, Fig. S7. This rearrangement is associated with energy decreases from state R by −8 kcal/mol for the reaction calculated based on Rxtal and −12 kcal/mol for that based on Pxtal (Fig. 5G).
This difference between the two profiles could solely be attributed to the structural bias from the original structures. In the present calculations, the heavy atoms of the MM region are fixed at their crystal structure coordinates throughout each calculation, since the MM relaxation was expected to have a minor impact on the energy profile according to our precursory calculations shown in SI Appendix, Table S3. However, the coordinates may vary by minute extents as the reaction proceeds. Therefore, while in state R and the following states the calculated energy levels, as well as the level changes based on AMPPNP-F, may be close to the actual levels, whereas in state P and the preceding states the calculated energies may be overestimated. The pair of energy profiles (Fig. 5G) show that the model-dependent bias is small, at most 5 kcal/mol, indicating that the simulated reaction that is supposed to proceed in the QM region is consistent with the fixed structures of the MM region. Moreover, the Rxtal-based-P structure (the QM/MM simulated structure of state P based on the structure Rxtal) is very close to Pxtal, the experimentally obtained structure of ADP-Pi-F (Fig. 4B). This suggests that the simulated reaction path is robust and the crystal structures of AMPPNP-F and ADP-Pi-F are good models for the prehydrolysis state R and the posthydrolysis state P, respectively (see SI Appendix, SI Discussion).
In the present study, we confirmed the idea that the induced polarizations of the peptide groups in the nucleotide surrounding loops, i.e., P-loop of motor proteins or P1- and P2-loops of F-form actin, contribute significantly to stabilize PO3− (31) (SI Appendix, Fig. S9). Therefore, to include the contributions by the induced polarizations properly, these atoms were treated by QM, as proposed for the peptide groups of the P-loop in the analysis of the myosin ATP hydrolysis reaction (31). In myosin, kinesin, and F1-ATPase, the negative charges of the ATP4- induce polarizations of the peptide groups in the P-loop. The induced polarizations modify the charge distributions of the polarized groups, strengthening their interactions with the phosphate moiety of ATP (33). In the F-form actin, a pair of phosphate binding loops, the P1-loop of OD and the P2-loop of ID, play identical roles to those of the P-loop in myosin.
Remarkably, the catalytic strategy of the F-form actin is essentially the same as those of some P-loop type motor proteins, F1-ATPase (29), kinesin (30) and myosin (32, 33), despite the fact that the biological function of ATP hydrolysis and the structure of the catalytic site are distinct from those of the motor proteins (see section 3.2. for more details).
The ATP hydrolysis mechanisms of the F-form actin presented here are dissimilar from those reported previously (21). While the previous work suggested that three residues, Gln137, Asp154, and His161, are candidates for catalytic residues, the present work indicates that these residues are not directly involved in the chemical bond formation and dissociation, with minimal conformational changes (Fig. 5F). The main role played by these residues is likely to be the stabilization of W1 and W2 at appropriate positions (SI Appendix, Fig. S5). The previous analysis concluded that the proton transfer path consists of more than three water molecules plus Asp154, but our results have demonstrated that the proton transfer can proceed with only two water molecules (W1 and W2) with a reasonable barrier height. More importantly, the present work has revealed the details of the reaction path, including the structures and energy levels of the IMs and the energy barriers heights of the individual TSs connecting IMs (Fig. 5G). This was made possible by the precise structural information of the water molecules which play pivotal roles in the ATP hydrolysis reaction.
2.6. Structural Changes Associated with the Pi Release.
The Pi release from the stable ADP-Pi-F state enhances the spontaneous monomer dissociation at either end of an actin filament (10). In addition, ADP-F has higher affinity for ADF/cofilin (40–42), and this interaction eventually switches the filament to the disassembly phase (14). Therefore, it is crucial to capture the structural changes in actin induced by the Pi release. However, the main chain conformations of three species, AMPPNP-F, ADP-Pi-F, and ADP-F, are essentially identical (Cα RMSD = 0.11 Å between ADP-Pi-F and ADP-F), indicating that global conformational alterations are not associated with the Pi release (Fig. 1D) and confirming the results obtained from the cryo-EM structures (19, 20).
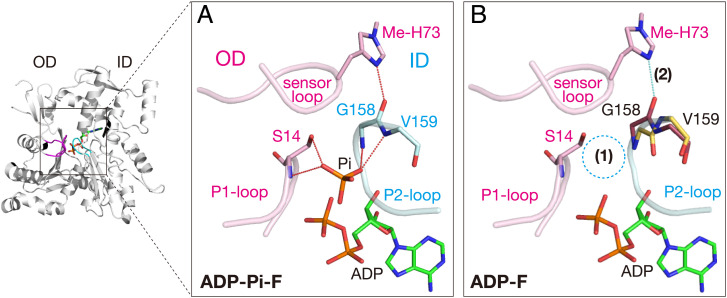
A careful comparison of the catalytic sites revealed two changes that obviously weaken the ID/OD contact (Fig. 6). First, the Pi in ADP-Pi-F is replaced by four water molecules in ADP-F. Second, in ADP-F, two residues of the P2-loop, Gly158 and Val159, adopt two conformations (Fig. 6B and SI Appendix, Fig. S10). In the first conformer, with an occupancy of 0.55, the carbonyl oxygen of Gly158 (ID) forms a hydrogen bond with 3-methyl histidine-73 (Me-His73) (OD) on the sensor loop (residues 71 to 76), as in the other nucleotide states (Fig. 6B and SI Appendix, Fig. S10). In the second conformer, this interdomain hydrogen bond is disrupted due to a 180° flip of the carbonyl oxygen of Gly158. As compared with the first conformer, this excludes one of the four water molecules replacing the oxygen atoms of Pi. This peptide flip appears to be energetically unfavorable, because it abolishes the interdomain hydrogen bond between Gly158 and Me-His73, and the adjacent residue, Val159, is forced to adopt an unfavorable Ramachandran φ angle (+45.4°) (SI Appendix, Fig. S10). This implies that an attractive force at the “PG-site” could hold Pi near the PB of ADP, which in turn pulls the carbonyl oxygen of Gly158 strongly toward the catalytic site after the Pi release.
Fig. 6.
Diminished interactions between two major domains caused by Pi release. (A) In ADP-Pi–bound F-form actin, the interactions between two major domains, OD (magenta) and ID (cyan), are composed of the P1-loop (OD)/P2-loop (ID) interactions mediated by Pi and the sensor loop (OD)/P2-loop (ID) interactions mediated by the hydrogen bond (dotted cyan line) between Gly158 and Me-His73. (B) The ADP-bound F-form actin structure indicates that Pi release has two consequences, indicated as (1) loss of Pi and (2) hydrogen bond disruption. Both diminish the interdomain interactions, and thereby promote the F-to-G back transition (see SI Appendix, Fig. S10 for more details).
The F1A structures demonstrated that the interactions between the two major domains are weakened at the active site upon Pi release, which can in turn increase the fluctuations of the whole actin molecule and affect the conformation of the intrasubunit binding site, including the D-loop. Our computational analyses support this notion. Regardless of the binding of the F1 domain including FNEX, the strained F-form conformation is less stably maintained by the ADP-F-form actin, as compared with the ATP-F-form (SI Appendix, Fig. S11). (The Pi-release-associated structural changes suggested by cryo-EM studies are considered in section 3.4.)
2.7. Why Is the ATP Hydrolysis Rate of F-Form Actin Slow?
The F-actin ATP hydrolysis rate is about 0.3 s−1 (5), which is much slower than those of other ATPases, such as myosin (20 to 200 s−1) (6). The association rate of ATP-G-actin to the filament barbed end is over 1,000 s−1 [at a monomer concentration of 100 μM (43)], clearly indicating the significantly delayed hydrolysis after polymerization (i.e., the G-to-F conformational transition). The uncoupling between the conformation transition and the hydrolysis may be beneficial for the actin dynamics, because it could prevent opportunistic hydrolysis when G-actin eventually adopts the F-form in solution. The slow rate of the F-form actin ATP hydrolysis may be mechanistically accounted for by the instability of W2 (SI Appendix, Fig. S5) and the small‐membered and distorted proton transfer ring.
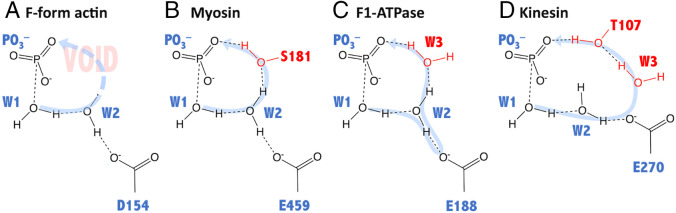
While the hydrolysis strategies are essentially the same in actin and some P-loop type motor proteins as described above, certain mechanistic properties of the proton transfer ring are distinct in actin. In the IM3 state of actin, the number of proton transfer ring mediator for the PT step is just three, W1, W2, and metaphosphate (Figs. 5 A and 7A). Moreover, the proton transfer ring is distorted: Between W2 and PO3−, the OW2–H…O2G angle is substantially bent, and the OW2–O2G distance is 3.10 Å (Fig. 5D), and both are disadvantageous for proton transfer. In contrast, highly efficient ATPases like myosin and F1-ATPase have four mediators: with Ser181 in myosin (32) (Fig. 7B) and W3 in F1-ATPase (29) (Fig. 7C). In myosin, when Ser181 is omitted from the proton transfer ring, making it three-membered as in F-form actin, the barrier height of the proton transfer increases (32). In kinesin, two additional groups, one water molecule and Thr107, participate in the proton relay (30) (Fig. 7D). With the aid of two additional proton mediators, the PT step is energetically less demanding, and thus the highest energy barrier of the entire reaction path shifts from the PT to the POD step (30).
Fig. 7.
Comparison of the proton transfer rings among various ATP hydrolysis proteins. The proton transfer rings and the constituting mediators of the ATP hydrolysis reactions of (A) F-form actin, compared with some motor proteins, (B) myosin (32), (C) F1-ATPase (29), and (D) kinesin (30). Note that in F-form actin the mediators are W1 and W2, whereas the other three have additional mediators (indicated in red). Moreover, F1-ATPase and kinesin have multistep proton transfer strategies via the transient protonation of the glutamate side chains. The curved arrows in light blue indicate the directions and paths of proton transfer, and the dashed lines are hydrogen bonds.
2.8. Why Is the ATP Hydrolysis of F-Form Actin Irreversible?
Another distinct feature of the actin ATP hydrolysis is the large stabilization energy: the potential energy difference of state P (ADP-Pi-F) relative to state R (ATP-F). In the F-form actin, the stabilization energy is −8 to −12 kcal/mol, which is much larger than those of F1-ATPase and myosin, −3 and −2 kcal/mol, respectively. This larger negative stabilization energy accounts for the irreversibility of the actin ATP hydrolysis reaction (7), in contrast to the reversible ATP hydrolyses by F1-ATPase and myosin, because the energy barrier for the reverse reaction (ADP + Pi → ATP) is the sum of the stabilization energy and the height of the highest energy barrier for the forward reaction.
Why is the ADP-Pi-F stabilization energy so large? We propose three reasons. First, a tight hydrogen bond is formed between ADP and Pi and fortified by the nearby Mg2+ ion coordinated to ADP and Pi. The natural population analysis indicated that the strongest noncovalent interaction involving hydrogen atoms in the catalytic site of ADP-Pi-F lies in the hydrogen bond between ADP and Pi [O3B(ADP)–O1(G)(Pi)]), with a Wiberg bond index (44) of 0.15 for O3B…H that is at least 50% higher than those for other hydrogen bonds. In addition, the bond index of 0.53 for the H–O1(G) of Pi, which donates the hydrogen in the bond, is the lowest among those for all covalent bonds in the catalytic site, suggesting the significant weakness of the H–O1(G) bond. While similar hydrogen bonds (with distances of 2.45 to 2.55 Å measured in the electron density maps) have been reported in the catalytic sites of ADP(GDP)-Pi-bound ATP(GTP) hydrolyzing proteins (see section 3.3), the characteristic of the F-form actin is that the hydrogen-bond donating O1(G) atom of Pi is coordinated to the Mg2+ ion (Fig. 5 A and E). The vicinal positive charge of Mg2+ weakens the proton affinity of Pi, enhancing the relevant hydrogen bond and shortening its distance. Second, the two hydrogen bonds between the P2-loop and ADP-Pi, O2G and NV159 and O3B and ND157, are substantially shorter in state P than in state R, according to the distances obtained from either the crystal structures (experimental) or the QM/MM simulations (SI Appendix, Table S2). Accordingly, these hydrogen bonds are strengthened by the atomic rearrangements associated with the hydrolysis. Overall, in ADP-Pi-F, Pi is tightly anchored to the catalytic site by multiple hydrogen bonds formed between ADP, the P2-loop, and Ser14 of the P1-loop. Third, the hydrolysis does not induce a global conformational change of the protein, therefore, not disrupting the multiple hydrogen bonds, unlike the majority of ATP hydrolyzing proteins. The large stabilization energy of ADP-Pi-F may also partly account for the slow Pi release rate (see below).
3. Discussion
Many characteristic properties of ATP hydrolysis by F-form actin have been mechanistically accounted for in the present study:
-
1)
The G-to-F conformational transition shifts Gln137 and His161, rearranging two water molecules, W1 and W2, to positions required for the nucleophilic attack of PG (Fig. 3 and SI Appendix, Fig. S4).
-
2)
The ATP hydrolysis reaction path has been specified (Fig. 5).
-
3)
The slow rate of ATP hydrolysis is mainly due to the small and distorted proton transfer ring (Fig. 7A) and partly due to the positional instability of W2 (SI Appendix, Fig. S5).
-
4)
The irreversibility of the ATP hydrolysis is due to the large negative value of the energy level of the product (ADP-Pi-F) relative to that of the reactant (ATP-F) (Fig. 5G).
-
5)
No overall conformational change of the main chain of actin is associated with the Pi release, as reported previously based on the cryo-EM structures.
-
6)
Upon Pi dissociation, the contacts between the ID and OD diminish due to the loss of Pi and the flipping of the main chain peptide bonds of the P2-loop (Fig. 6 and SI Appendix, Fig. S10), presumably destabilizing the F-form.
3.1. Mechanisms of ATP Hydrolysis by F-Form Actin.
The actin ATP hydrolysis reaction path deciphered in the present study is consistent and comprehensive and therefore robust and highly plausible. It is consistent because the reaction mechanism agrees well with the previously reported biochemical results (the hydrolysis rate and the irreversibility of the reaction) and the structural data presented here. It is comprehensive because the reaction mechanism from the prehydrolysis state through to the posthydrolysis state has been revealed.
While no experimental method that could directly prove/disprove the obtained reaction path is presently available, the consistency between the simulated structures and experimentally obtained structures is particularly remarkable and shall be described in three aspects as follows. First, the prehydrolysis structure Rxtal-based-R and the posthydrolysis structure Pxtal-based-P were obtained by geometric optimization of the crystal structures Rxtal (AMPPNP-F structure) and Pxtal (ADP-Pi-F), respectively, with only minute positional shifts of atoms (Fig. 4). Second, structures obtained by simulations, Rxtal-based-P (the structure of state P simulated based on the structure Rxtal) and Pxtal-based-R (the simulated structure of state R based on Pxtal), are almost identical to the corresponding experimentally obtained structures, Pxtal and Rxtal, respectively (Fig. 4 A and B). Third, the energy profiles of the forward and reverse reactions are similar (Fig. 5G), indicating that the reaction path is free from model bias and therefore self-consistent.
In the present work, the QM/MM simulations were based on three assumptions. First, the F-form actin within the F1A complex should be a good model for the F-actin molecule within the actin filament. Second, AMPPNP-F should be the prehydrolysis structure. Third, ADP-Pi-F reconstituted from ADP-bound actin and Pi should be the posthydrolysis structure. Since the obtained actin ATP hydrolysis reaction path is highly plausible, we suggest that all three assumptions should be correct. In SI Appendix, SI Discussion possible limitations and consequences are discussed for each assumption.
3.2. Common Catalytic Strategy Shared by Actin and Some Motor Proteins.
As described in section 2.5, the catalytic strategy of F-form actin is essentially the same as those of some P-loop type motor proteins, F1-ATPase (29), kinesin (30), and myosin (32, 33), although the catalytic site structure of actin is dissimilar to those of the motor proteins. The essential characteristics of the common catalytic strategy are as follows. The PG–O3B bond is cleaved prior to the nucleophilic attack of W1 on PG, producing metaphosphate PO3−. PO3− then forms the W1–PO3− complex, where four events occur concomitantly: the OW1–H bond cleavage, the nucleophilic attack producing HPO42−, the proton dissociation, and the proton transfer producing H2PO4−. In this manner, the two highest energy barriers along the entire reaction, the PG–O3B bond and OW1–H bond cleavages, are overcome sequentially, allowing the entire reaction to proceed. Indeed, if the two cleavage events occurred concurrently, then the two energy barriers would be merged into a single insurmountable barrier (33).
The sequential strategy is made possible by a number of assisting mechanisms with the F-form actin, as in the motor proteins. The shift of a negative charge from PG to PB is facilitated by strengthening the ND157–O3B hydrogen bond, which favors the PG–O3B bond cleavage (SI Appendix, Table S2). PO3− is stabilized by hydrogen bonds with the P1- and P2-loops (Fig. 5D and SI Appendix, Fig. S9). The complex formation of W1–PO3− is made possible by the reorientation of W1 (in the HBR-1 step) (SI Appendix, Fig. S8). The OW1–H bond is polarized by W2 and Oε1(Gln137), which facilitates the bond cleavage (SI Appendix, Fig. S5A).
It is worth noting that the overall architecture of the catalytic site of F-form actin is distinct from those of the motor proteins. In the motor proteins, the P-loop binds ATP by clamping its phosphate moiety, and on the other side the switch-1 and switch-2 loops work as a lid to control the opening/closure of the active site. In the F-form actin, the cavity for the phosphate moiety of ATP is lined with the P1- and P2-loops, which tightly bind the ATP through hydrogen bonds without the lid-like movement observed in the switch-1 and switch-2 loops, suggesting the absence of a mechanism to trigger conformational changes in the protein upon the completion of ATP hydrolysis (for this mechanism in GTPase RhoA; see figures 4 E and F in ref. 45). Moreover, while in the motor proteins the proton transfer ring consists of four or more atoms (Fig. 7 B–D), in the F-form actin only three atoms (Fig. 7A). Altogether, the catalytic site structure of the F-form actin is much simpler than those of the motor proteins and may fulfill the minimum requirement for ATP hydrolysis.
The present work with the F-form actin has confirmed and strengthened the sequential catalytic strategy of ATP hydrolysis originally proposed by studies of motor proteins. This is mainly because the structural data of F-form actin, on which the present QM/MM analyses are based, have much higher quality and are more complete, compared with the structural data used in the analyses of motor proteins (for more details, see SI Appendix, SI Discussion).
Collectively, the present work strengthens the idea that the sequential strategy is shared by the ATP hydrolyzing proteins. This common strategy may also be adopted by some GTP binding proteins, like the small GTPases and the heterodimeric G proteins, which have catalytic site structures similar to those of the P-loop type motor proteins (46).
3.3. Pi Release and Conformational Change of the Protein.
In the present work, two observations reflect the distinct characteristics of the F-form actin. First, the global conformation of the ADP-bound F-form actin molecule is identical to that of the ATP-bound form. While this confirms the previous cryo-EM observations (19, 20), it contrasts with many other ATPase and GTPase proteins (the NTPase proteins), where the global conformations of the NTP-bound and NDP-bound forms substantially differ. Second, the crystal structures of ADP-Pi–bound F-form actin have been readily obtained, while among other NTPase proteins the crystal structures of NDP-Pi–bound forms are rarely available (see below). These two observations have led us to propose the following three hypotheses:
The first hypothesis is that the NTPase should undergo a process of three sequential steps: NTP hydrolysis → global conformational change of the protein → Pi release. The completion of the NTP hydrolysis triggers an unknown mechanism for the protein conformational change, which then induces the Pi release. While this sequence of events has never been clearly understood, it is consistent with previous results showing that artificial preventions of conformational changes create NDP-Pi–bound structures, as in the small GTPase Arl2 (1KSH), whose conformational change was prevented by complex formation with another protein (47), Rab11a (1QIX), by the Gln70Leu point mutation (48), and RhoA (5C4M), by crystal lattice restrictions (45). In each structure, GDP-Mg2+-Pi adopted a similar configuration to our ADP-Pi-F structure, in which GDP and Pi are linked together through both the coordinate bonds via Mg2+ and the short hydrogen bond, with distances of 2.45 to 2.55 Å.
The second hypothesis is that the F-form actin should not have any specialized mechanism for triggering a global conformational change of the protein. This appears to be consistent with the simple structure of its catalytic site, as described in section 3.2.
The third hypothesis is that while the Pi release should generally be driven by the protein conformational change in the NTPase, with the F-form actin it should be driven by structural fluctuations controlled by the manner of interactions with contacting molecules: tighter and/or more balanced interactions diminish the fluctuations of the actin molecule. This hypothesis is consistent with, but not proven by, the experimental results that suggested two rate constants for the Pi release from the actin filament: the slow ∼0.006 s−1 for the Pi release from the interior of the actin filament and the fast ∼2 s−1 for the Pi release from the actin molecule at the barbed end (9, 10).
3.4. How Do Some Actin Binding Proteins Preferentially Bind to ADP-F Rather Than ADP-Pi-F or ATP-F?
Many actin binding proteins, including profilin, ADF/cofilin, and capping protein, play crucial roles in regulating the assembly–disassembly cycle of actin, as described in the Introduction. The regulations by ADF/cofilin, for example, are initiated by preferential binding to ADP-F, rather than ADP-Pi-F or ATP-F. The loss of the PG moiety from the catalytic site must alter the properties of actin, resulting in the enhanced actin affinity of ADF/cofilin. What properties are altered within actin upon Pi release?
Cryo-EM studies on F-actin structures described structural changes of the F-form actin molecule in association with Pi release. 1) The D-loop conformation is variable in nucleotide- (19, 20) or actin-stabilizing-agent- (19) dependent manners. The deformation at the C terminus of the D-loop in ADP-F-actin may explain its preference for binding cofilin (20). 2) A flip of Ser14 in the P1-loop upon Pi release, associated with the side chain rotations of Me-His73 and Arg177, was implied (20). 3) The local structure in the C-terminal region, including Cys374, changes (19, 20). In our F1A structures, the electron densities of the D-loop are missing due to the absence of a binding partner, and the structures of these residues, Ser14, Me-His73, Arg177, and Cys374, are all identical regardless of the nucleotide states (Me-His73 has dual conformations in ADP-F; see SI Appendix, Fig. S10). The lack of structural changes in the F1A crystal structures does not necessarily preclude the structural changes in the filament. This is because actin structural changes within F1A crystals may be suppressed by constraints imposed by the crystal lattice and/or the F1 binding. Conversely, the structural changes interpreted from the EM density maps may suffer from misinterpretation due to insufficient spatial resolutions. We have concentrated on analyzing the changes in the atomic arrangements and hydrogen bond networks in the catalytic site, which are directly influenced by the Pi dissociation. This is because, on the one hand, the changes in the vicinity of Pi could be less influenced by the external constraints, and on the other, the Pi-mediated hydrogen bonds between the two major domains should form the basis for the ADP-Pi-F stability (see sections 2.4 and 2.8).
Collectively, the mechanism for the differential actin affinities remains to be elucidated. As our fourth hypothesis, we suggest that the preferential affinities of some actin binding proteins to ADP-F should be due to differential structural fluctuations of F-form actin, as proposed for the preferential binding of cofilin to ADP-bound actin filaments (49). This idea is consistent with the present finding that the link between two major domains, the OD and ID, is weakened upon Pi release from the nucleotide binding cleft. It is also consistent with the finding that, diminished subunit-subunit interactions at barbed ends accelerates the Pi release by 200-fold, compared with the Pi release rate from actin in the filament interior (9, 10).
3.5. The F1A Complex as a Tool for Studying the Pi Release Mechanism.
In this work, we have introduced the F1A complex, which shows the first isolated actin molecule that performs ATP hydrolysis, Pi release, and nucleotide exchange while actin is in a dynamic equilibrium between the G- and F-forms. The conformation transition is not driven by actin assembly–disassembly but by interactions with F1. We propose that the complex should be suitable for studies of elementary processes of actin, including Pi release. Actin is unique among NTP hydrolysis proteins in that its global conformation is not altered upon hydrolysis completion, and the NDP-Pi–bound structure is stable and available. In other words, for analyses of the Pi release process, the initial and final structures are available. Additionally, the F1A complex is monodispersed with a total chain-weight of 61 kDa, and thus amenable to spectroscopic measurements such as NMR.
4. How to Name Atoms in the Phosphate Moieties of ATP and AMPPNP
In this report, we adopted IUPAC Recommendations 2016 (50), with some modifications, so that each atom of AMPPNP is named in the same manner as in ATP, and each atom keeps the same name before and after bond cleavage, as indicated in Fig. 5 C–E.
5. Materials and Methods
The fragmin proteins (F1: 1 to 160, F115-160: 15 to 160) from the plasmodia of P. polycephalum were prepared using the Escherichia coli expression system. ATP-G-actin was prepared from chicken skeletal muscle acetone powder. The F1A complexes were prepared by mixing the Ca2+-activated F1 proteins and actin at a 1.2:1 molar ratio. Diffraction datasets were collected at BL2S1 (Aichi SR). Data collection and refinement statistics are summarized in SI Appendix, Table S1. Details of preparation methods for proteins and protein crystals, methods for diffraction data acquisition, structure determination, and structure refinement, as well as biochemical assays, QM/MM, and molecular dynamics simulations are provided in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Gettemans (Ghent University) for the kind gift of the fragmin complementary DNA; S. Fischer (Heidelberg University) for valuable advice at the early stage of our QM/MM simulations; Atsushi Nakagawa and Kota Nagano (Osaka University) for ITC measurements; and Yasunori Saitoh, Michihiro Suga, and Jian-Ren Shen (Okayama University) for HPLC measurements. The X-ray diffraction data sets were collected at beamline BL2S1 (Aichi SR, Japan) under the proposals 2016N6009-10, 6013, 2017N6007, 2018N5002, 5004, and 6005. We thank the beamline staff for their technical assistance. The QM/MM computations were performed using the Research Center for Computational Science, Okazaki, Japan. This work was supported by Japan Society for the Promotion of Science grants KAKENHI 26251017 (to Y.M. and A.N.), 16K17708 and 20K06522 (to S.T.), 17J08102 (to Y.K.), 20H05883 (to Y.T.), 17K07373 (to T.O., I.F., and S.T.), and 21H00394 and 19K12217 (to R.K.) and by Japan Agency for Medical Research and Development grant JP21am0101111 (to M.O.). Support to Y.M. from Takeda Science Foundation, Daiko Foundation, Toyota Riken, and Actin Research Association is acknowledged. Encouragement and advice from the late Sadashi Hatano and the late Fumio Oosawa are gratefully acknowledged.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. L.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2122641119/-/DCSupplemental.
Data, Materials, and Software Availability
X-ray crystallography data have been deposited in Protein Data Bank (7W4Z, 7W50, 7W51, 7W52, 7YNE). All other study data are included in the article and/or SI Appendix.
References
- 1.Straub F. B., Feuer G., Adenosinetriphosphate the functional group of actin. Biochim. Biophys. Acta 4, 45–470 (1950). [PubMed] [Google Scholar]
- 2.Korn E. D., Carlier M. F., Pantaloni D., Actin polymerization and ATP hydrolysis. Science 238, 638–644 (1987). [DOI] [PubMed] [Google Scholar]
- 3.Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C., Atomic structure of the actin:DNase I complex. Nature 347, 37–44 (1990). [DOI] [PubMed] [Google Scholar]
- 4.Oda T., Iwasa M., Aihara T., Maéda Y., Narita A., The nature of the globular- to fibrous-actin transition. Nature 457, 441–445 (2009). [DOI] [PubMed] [Google Scholar]
- 5.Blanchoin L., Pollard T. D., Hydrolysis of ATP by polymerized actin depends on the bound divalent cation but not profilin. Biochemistry 41, 597–602 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Geeves M. A., Fedorov R., Manstein D. J., Molecular mechanism of actomyosin-based motility. Cell. Mol. Life Sci. 62, 1462–1477 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlier M. F., et al. , The hydrolysis of ATP that accompanies actin polymerization is essentially irreversible. FEBS Lett. 235, 211–214 (1988). [DOI] [PubMed] [Google Scholar]
- 8.Carlier M. F., Pantaloni D., Direct evidence for ADP-Pi-F-actin as the major intermediate in ATP-actin polymerization. Rate of dissociation of Pi from actin filaments. Biochemistry 25, 7789–7792 (1986). [DOI] [PubMed] [Google Scholar]
- 9.Jégou A., et al. , Individual actin filaments in a microfluidic flow reveal the mechanism of ATP hydrolysis and give insight into the properties of profilin. PLoS Biol. 9, e1001161 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara I., Vavylonis D., Pollard T. D., Polymerization kinetics of ADP- and ADP-Pi-actin determined by fluorescence microscopy. Proc. Natl. Acad. Sci. U.S.A. 104, 8827–8832 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegner A., Head to tail polymerization of actin. J. Mol. Biol. 108, 139–150 (1976). [DOI] [PubMed] [Google Scholar]
- 12.Pantaloni D., Le Clainche C., Carlier M. F., Mechanism of actin-based motility. Science 292, 1502–1506 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Pollard T. D., Borisy G. G., Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Wioland H., et al. , ADF/Cofilin accelerates actin dynamics by severing filaments and promoting their depolymerization at both ends. Curr. Biol. 27, 1956–1967.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lappalainen P., Kotila T., Jégou A., Romet-Lemonne G., Biochemical and mechanical regulation of actin dynamics. Nat. Rev. Mol. Cell Biol., 10.1038/s41580-022-00508-4 (2022). [DOI] [PubMed] [Google Scholar]
- 16.Vorobiev S., et al. , The structure of nonvertebrate actin: implications for the ATP hydrolytic mechanism. Proc. Natl. Acad. Sci. U.S.A. 100, 5760–5765 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rould M. A., Wan Q., Joel P. B., Lowey S., Trybus K. M., Crystal structures of expressed non-polymerizable monomeric actin in the ADP and ATP states. J. Biol. Chem. 281, 31909–31919 (2006). [DOI] [PubMed] [Google Scholar]
- 18.von der Ecken J., et al. , Structure of the F-actin-tropomyosin complex. Nature 519, 114–117 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merino F., et al. , Structural transitions of F-actin upon ATP hydrolysis at near-atomic resolution revealed by cryo-EM. Nat. Struct. Mol. Biol. 25, 528–537 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Chou S. Z., Pollard T. D., Mechanism of actin polymerization revealed by cryo-EM structures of actin filaments with three different bound nucleotides. Proc. Natl. Acad. Sci. U.S.A. 116, 4265–4274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCullagh M., Saunders M. G., Voth G. A., Unraveling the mystery of ATP hydrolysis in actin filaments. J. Am. Chem. Soc. 136, 13053–13058 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oda T., Takeda S., Narita A., Maéda Y., Structural polymorphism of actin. J. Mol. Biol. 431, 3217–3228 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Khurana S., George S. P., Regulation of cell structure and function by actin-binding proteins: villin’s perspective. FEBS Lett. 582, 2128–2139 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasegawa T., Takahashi S., Hayashi H., Hatano S., Fragmin: A calcium ion sensitive regulatory factor on the formation of actin filaments. Biochemistry 19, 2677–2683 (1980). [DOI] [PubMed] [Google Scholar]
- 25.Hinssen H., An actin-modulating protein from Physarum polycephalum. I. Isolation and purification. Eur. J. Cell Biol. 23, 225–233 (1981). [PubMed] [Google Scholar]
- 26.Gettemans J., De Ville Y., Waelkens E., Vandekerckhove J., The actin-binding properties of the Physarum actin-fragmin complex. Regulation by calcium, phospholipids, and phosphorylation. J. Biol. Chem. 270, 2644–2651 (1995). [DOI] [PubMed] [Google Scholar]
- 27.McLaughlin P. J., Gooch J. T., Mannherz H. G., Weeds A. G., Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature 364, 685–692 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Warshel A., Levitt M., Theoretical studies of enzymic reactions: Dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J. Mol. Biol. 103, 227–249 (1976). [DOI] [PubMed] [Google Scholar]
- 29.Hayashi S., et al. , Molecular mechanism of ATP hydrolysis in F1-ATPase revealed by molecular simulations and single-molecule observations. J. Am. Chem. Soc. 134, 8447–8454 (2012). [DOI] [PubMed] [Google Scholar]
- 30.McGrath M. J., Kuo I. F., Hayashi S., Takada S., Adenosine triphosphate hydrolysis mechanism in kinesin studied by combined quantum-mechanical/molecular-mechanical metadynamics simulations. J. Am. Chem. Soc. 135, 8908–8919 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Kiani F. A., Fischer S., Stabilization of the ADP/metaphosphate intermediate during ATP hydrolysis in pre-power stroke myosin: quantitative anatomy of an enzyme. J. Biol. Chem. 288, 35569–35580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kiani F. A., Fischer S., Catalytic strategy used by the myosin motor to hydrolyze ATP. Proc. Natl. Acad. Sci. U.S.A. 111, E2947–E2956 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiani F. A., Fischer S., Comparing the catalytic strategy of ATP hydrolysis in biomolecular motors. Phys. Chem. Chem. Phys. 18, 20219–20233 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Kitazawa T., Shuman H., Somlyo A. P., Calcium and magnesium binding to thin and thick filaments in skinned muscle fibres: Electron probe analysis. J. Muscle Res. Cell Motil. 3, 437–454 (1982). [DOI] [PubMed] [Google Scholar]
- 35.Yount R. G., Babcock D., Ballantyne W., Ojala D., Adenylyl imidodiphosphate, an adenosine triphosphate analog containing a P–N–P linkage. Biochemistry 10, 2484–2489 (1971). [DOI] [PubMed] [Google Scholar]
- 36.Bryan J., Gelsolin has three actin-binding sites. J. Cell Biol. 106, 1553–1562 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mouilleron S., Wiezlak M., O’Reilly N., Treisman R., McDonald N. Q., Structures of the Phactr1 RPEL domain and RPEL motif complexes with G-actin reveal the molecular basis for actin binding cooperativity. Structure 20, 1960–1970 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Iwasa M., Maeda K., Narita A., Maéda Y., Oda T., Dual roles of Gln137 of actin revealed by recombinant human cardiac muscle alpha-actin mutants. J. Biol. Chem. 283, 21045–21053 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheidig A. J., Burmester C., Goody R. S., The pre-hydrolysis state of p21(ras) in complex with GTP: new insights into the role of water molecules in the GTP hydrolysis reaction of ras-like proteins. Structure 7, 1311–1324 (1999). [DOI] [PubMed] [Google Scholar]
- 40.Maciver S. K., Zot H. G., Pollard T. D., Characterization of actin filament severing by actophorin from Acanthamoeba castellanii. J. Cell Biol. 115, 1611–1620 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlier M. F., et al. , Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 136, 1307–1322 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanchoin L., Pollard T. D., Mechanism of interaction of Acanthamoeba actophorin (ADF/Cofilin) with actin filaments. J. Biol. Chem. 274, 15538–15546 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Pollard T. D., Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J. Cell Biol. 103, 2747–2754 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiberg K. B., Application of the Pople-Santry-Segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24, 1083–1096 (1968). [Google Scholar]
- 45.R. W. Molt, Jr, Pellegrini E., Jin Y., A GAP-GTPase-GDP-Pi intermediate crystal structure analyzed by DFT shows GTP hydrolysis involves serial proton transfers. Chemistry 25, 8484–8488 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saraste M., Sibbald P. R., Wittinghofer A., The P-loop–a common motif in ATP- and GTP-binding proteins. Trends Biochem. Sci. 15, 430–434 (1990). [DOI] [PubMed] [Google Scholar]
- 47.Hanzal-Bayer M., Renault L., Roversi P., Wittinghofer A., Hillig R. C., The complex of Arl2-GTP and PDE delta: From structure to function. EMBO J. 21, 2095–2106 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pasqualato S., Cherfils J., Crystallographic evidence for substrate-assisted GTP hydrolysis by a small GTP binding protein. Structure 13, 533–540 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Tanaka K., et al. , Structural basis for cofilin binding and actin filament disassembly. Nat. Commun. 9, 1860 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blackburn G. M., et al. , How to name atoms in phosphates, polyphosphates, their derivatives and mimics, and transition state analogues for enzyme-catalysed phosphoryl transfer reactions (IUPAC Recommendations 2016). Pure Appl. Chem. 89, 653–679 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
X-ray crystallography data have been deposited in Protein Data Bank (7W4Z, 7W50, 7W51, 7W52, 7YNE). All other study data are included in the article and/or SI Appendix.