Significance
There remains a need for improved treatments for rheumatoid arthritis (RA). Also beneficial for disease management would be agents that allow the real-time, noninvasive monitoring of the RA microenvironment. This report details an approach that appears promising in terms of addressing both needs. Specifically, we show that encapsulation of both Prussian blue and tumor necrosis factor-α/interleukin-6 silenced small interfering RNAs within biomimetic macrophage membrane vesicles provides nanoparticles that exert a therapeutic effect against rheumatoid arthritis as judged from in vitro tests and in vivo mouse model studies. Near-infrared photoacoustic imaging allowed the targeting behavior of the nanoparticles to be followed in real time, as well as an evaluation of their therapeutic efficacy without the need for invasive procedures.
Keywords: biomimetic nanoparticles, photoacoustic, rheumatoid arthritis, targeted imaging, small interfering RNA
Abstract
The high level of reactive oxygen species (ROS) in the rheumatoid arthritis (RA) microenvironment (RAM) and its persistent inflammatory nature can promote damage to joints, bones, and the synovium. Targeting strategies that integrate effective RAM regulation with imaging-based monitoring could lead to improvements in the diagnosis and treatment of RA. Here, we report the combined use of small interfering RNAs (siRNAsT/I) and Prussian blue nanoparticles (PBNPs) to silence the expression of proinflammatory cytokines TNF-α/IL-6 and scavenge the ROS associated with RAM. To enhance the in vitro and in vivo biological stability, biocompatibility, and targeting capability of the siRNAsT/I and PBNPs, macrophage membrane vesicles were used to prepare biomimetic nanoparticles, M@P-siRNAsT/I. The resulting constructs were found to suppress tumor necrosis factor-α/interleukin-6 expression and overcome the hypoxic nature of RAM, thus alleviating RA-induced joint damage in a mouse model. The M@P-siRNAsT/I of this study could be monitored via near-infrared photoacoustic (PA) imaging. Moreover, multispectral PA imaging without the need for labeling permitted the real-time evaluation of M@P-siRNAsT/I as a putative RA treatment. Clinical microcomputed tomography and histological analysis confirmed the effectiveness of the treatment. We thus suggest that macrophage-biomimetic M@P-siRNAsT/I and their analogs assisted by PA imaging could provide a new strategy for RA diagnosis, treatment, and monitoring.
Rheumatoid arthritis (RA) is a chronic autoimmune disease that directly impacts joints. Symptoms include swelling and deformation due to inflammatory damage to bone, cartilage, and synovium. RA can also lead to cardiovascular, pulmonary, psychological, and skeletal disease (1). While the exact etiology of RA is unknown, its pathogenesis in joints includes the high-expression of multiple proinflammatory cytokines, overproduction of reactive oxygen species (ROS), and a hypoxic state leading to a chronic inflammatory environment referred to as the RA microenvironment (RAM) (2–4). Traditional anti-rheumatic therapeutics includes nonsteroidal anti-inflammatory drugs, disease-modifying anti-rheumatic, and glucocorticoids. More recently, the suppression of proinflammatory cytokines, tumor necrosis factor-α (TNF-α) and interleukin 6 (IL-6) by means of biological agents, such as infliximab and tocilizumab, has been demonstrated for RA patients that failed to respond well to conventional anti-rheumatic drugs (5–8). These treatment modalities highlight the importance of targeting proinflammatory factors for RA therapy (9, 10). Unfortunately, the biological agents used to date have disadvantages (e.g., high cost, adverse side effects [e.g., infection], and poor target specificity) that have limited their wider application (11–14). In addition, it is now appreciated that to treat the uncontrolled progression of RA, determinants other than the proinflammatory factors specific to the RAM need to be targeted. These include hypoxia and ROS (15–18).
Small interfering RNAs (siRNAs) are synthetic RNA constructs designed to inhibit gene expression via a sequence-specific pairing with mRNA (19, 20). A notable example is Patisiran, which was approved for the treatment of hereditary transthyretin amyloidosis. In many respects, this approval marked the beginning of a new era for siRNA-based therapeutics (21, 22). Moreover, recent reports have demonstrated siRNA as effective treatments methods in inflammation-based disease models (e.g., collagen induced arthritis [CIA]) (23). In comparison to biological agents, siRNA have inherent advantages as the interference of mRNA translation directly effects the regulation of downstream protein expression (24–26). On the other hand, several barriers have limited the wide-spread clinical application of siRNA. Naked and unmodified siRNA often 1) displays unsatisfactory stability, and 2) can give rise to off-target effects (21, 26, 27). To address these latter concerns, efforts have been made to develop drug delivery systems (DDS), such as lipids, lipid-like materials (lipidoids), polymers, peptides, inorganic nanoparticles, etc., that are effective for siRNAs (28–31). However, efforts are increasingly being devoted to the use of cell-biomimetic vesicles (CBVs) as DDS (30, 32, 33).
CBVs have a natural advantage as drug delivery systems because they are derived from endogenous cell membranes, have inherent biocompatibility, and give rise to minimal immunogenicity. Macrophage membrane vesicles (MMVs), a class of CBVs, were recently used to deliver the immunosuppressor tacrolimus to the inflamed joints of an RA mouse model (34) and were reported as being able to “actively find the door” needed to target these lesions (35, 36). We were thus keen to explore whether MMVs could be used as a DDS to deliver a siRNA to RAM as a potential RA treatment.
In this work, TNF-α and IL-6 silenced siRNAs (siRNAsT/I) were synthesized to target RA joints and effect an anti-inflammatory response in the RAM. The United States Food and Drug Administration (FDA)-approved Prussian blue nanoparticles (PBNPs) were prepared and used to mimic peroxidase, catalase, and superoxide dismutase activity (37, 38). MMVs were employed as the carriers (Fig. 1). The near-infrared (NIR) absorbance of PBNPs afforded a strong photoacoustic (PA) signal that permitted the noninvasive real-time evaluation of the targeting properties of the associated macrophage-biomimetic nanoparticles (M@P-siRNAsT/I). As detailed below, M@P-siRNAsT/I were found to suppress the proinflammatory factors and ameliorate RAM hypoxia in vitro and in vivo as compared to PBNPs and siRNAsT/I alone. This resulted in a statistically significant alleviation of RA-based symptoms. PA imaging (39–41) allowed the M@P-siRNAsT/I and the blood oxygenation of RAM to be detected noninvasively in vivo. Micro-computed tomography (CT) and histopathology provided support for the proposed therapeutic effect.
Fig. 1.
Basic schematic showing how the M@P-siRNAsT/I of the present study could function as a PA imaging-guided RA therapeutic.
Results and Discussion
Preparation and Characterization of M@P-siRNAsT/I.
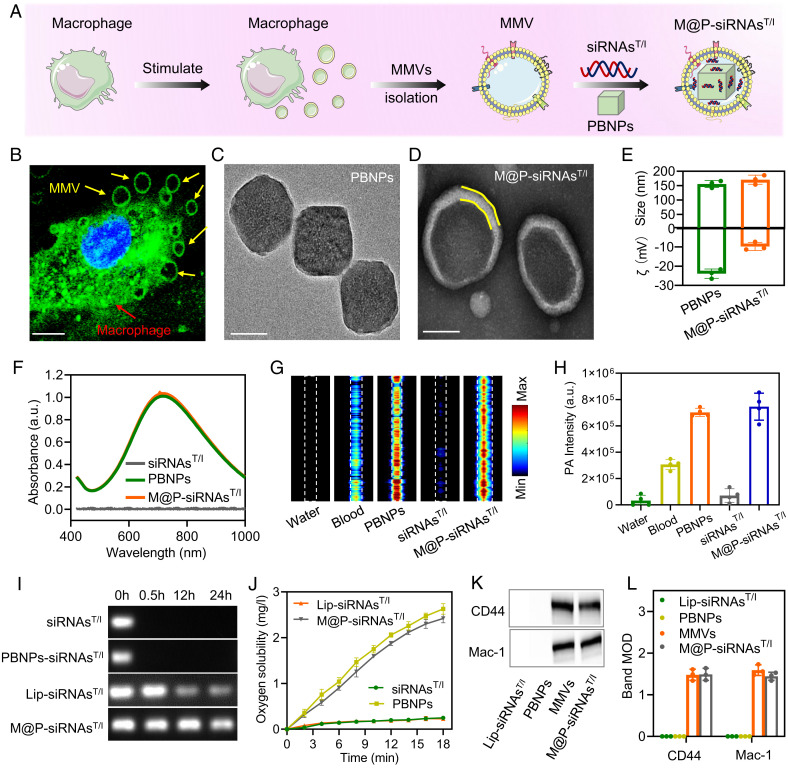
The preparation of the M@P-siRNAsT/I of this study is summarized in Fig. 2A. Briefly, RAW246.7 macrophages were first stimulated to secrete microvesicles (MMVs) using cytochalasin B. MMVs were seen around the macrophages (Fig. 2B). The protein profiles of MMVs matched the profile of the macrophages as inferred from SDS–polyacrylamide gel electrophoresis (SI Appendix, Fig. S1); this is as would be expected for MMVs derived from macrophages. Separately, FDA-approved PBNPs were synthesized using a literature protocol (37, 42). Element mapping and transmission electron microscope (TEM) analyses were used to confirm the successful preparation of PBNPs (Fig. 2C and SI Appendix, Fig. S2). Using an Avanti mini extruder, the MMVs were then used to encapsulate a mixture of PBNPs and TNF-α/IL-6 dual-silenced siRNAs (siRNAsT/I). TEM images show a distinct core-shell structure for the resulting constructs, M@P-siRNAsT/I, with an average diameter of 170.7 ± 16 nm, which is larger than that of the PBNPs (155.7 ± 12.3 nm) making up the core (Fig. 2 D and E). Negligible changes in diameter were seen over the course of 8 d in PBS solution at room temperature, which was considered an augury of physiology stability (SI Appendix, Fig. S3).
Fig. 2.
Synthesis and characterization of M@P-siRNAsT/I. (A) Preparation of M@P-siRNAsT/I. (B) Fluorescence image of macrophages showing the production of macrophage-derived microvesicles (MMVs) induced by cytochalasin B. (Scale bar: 5 μm.) (C and D) TEM images of PBNPs and M@P-siRNAsT/I, respectively. (Scale bar: 100 nm.) (E) Average size and zeta potential of PBNPs and M@P-siRNAsT/I. (F) Absorbance spectra of siRNAsT/I, PBNPs, and M@P-siRNAsT/I, respectively. (G and H) PA images and statistical results of water, fresh mouse blood, siRNAsT/I, PBNPs, and M@P-siRNAsT/I, respectively. (I) Electrophoretic test of siRNAsT/I, PBNP/siRNAsT/I mixture, Lip-siRNAsT/I, and M@P-siRNAsT/I in serum before and after 24 h, respectively. (J) The oxygen solubility levels for H2O2 solutions containing siRNAsT/I, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I incubated at room temperature, respectively. (K and L) Western blots and protein content for two characteristic macrophage membrane markers (CD44 and Mac-1) seen for MMVs, Lip-siRNAsT/I, PBNPs, and M@P-siRNAsT/I, respectively.
The M@P-siRNAsT/I displayed strong near-infrared (NIR) absorbance at 720 nm similar to literature reports (Fig. 2F) (37). Photoacoustic (PA) signals were measured using our custom-built PA imaging system (43). M@P-siRNAsT/I and PBNPs displayed strong PA signals, higher than water or fresh mouse blood (Fig. 2 G and H). No photo-instability issues were seen for the M@P-siRNAsT/I after subjecting them to 4,000 pulses of laser irradiation (SI Appendix, Fig. S4). As reported in the literature, siRNA is prone to degradation by ribonucleases in serum (44, 45). This proved true in our hands. Both naked siRNAsT/I and a mixture of PBNP and siRNAsT/I displayed obvious signs of degradation after incubating for 24 h in serum. In contrast, little degradation was seen for the M@P-siRNAsT/I under these conditions. By way of reference, commercial Lipofectamine-loaded siRNAsT/I (Lip-siRNAsT/I) showed slight degradation (Fig. 2I). Given the instability of naked siRNAsT/I, Lip-siRNAsT/I was used as a semistable control in the in vitro and in vivo portions of the present study.
As noted above, high-concentrations of ROS, such as H2O2, as well as hypoxia, are hallmarks of the RAM and are thought to contribute to RA joint damage (16). On the other hand, PBNPs have been reported as being effective ROS scavengers that display multi-enzyme–like activity, including that of peroxidase (POD), catalase (CAT), and superoxide dismutase (SOD) (37). We thus tested whether M@P-siRNAsT/I would promote the conversion of H2O2 to oxygen. As shown in Fig. 2J, this proved to be the case, as evidenced by the consumption of H2O2 and the production of oxygen (SI Appendix, Fig. S5). We thus hypothesized M@P-siRNAsT/I could increase oxygenation levels in the RAM. Last, Western blot analyses of the macrophage membrane marker proteins CD44 and Mac-1 confirmed M@P-siRNAsT/I had membrane function analogous to the macrophages from which they were derived. Specifically, M@P-siRNAsT/I gave rise to similar marker protein expressions as the constituent MMVs (Fig. 2 K and L). We thus suggest that the M@P-siRNAsT/I maintain key macrophage functions, making them attractive for use as a DDS in vivo.
In Vitro and In Vivo Evaluation of M@P-siRNAsT/I for Targeting Inflamed Cells and Tissues.
Based on the above results, we hypothesized that the M@P-siRNAsT/I of this study would display targeting properties similar to those of macrophages in vitro and in vivo. To trace the fate of M@P-siRNAsT/I in vitro, a fluorescent dye (Cy5) was used to label the siRNAsT/I. Fluorescence spectral studies were used to confirm that siRNAsT/I, Lip-siRNAsT/I, and M@P-siRNAsT/I could all be successfully labeled using Cy5 (SI Appendix, Figs. S6 and S7). We next evaluated whether M@P-siRNAsT/I targeted inflamed vessels cells. For this study, mouse cerebral microvascular endothelial cells (bEnd.3) were treated with IL-1β (a proinflammatory cytokine) to induce inflammation, followed by incubation with M@P-siRNAsT/I; untreated bEnd.3 cells and Lip@P-siRNAsT/I (commercial Lipofectamine loaded mixture of PBNPs and siRNAsT/I served as controls. As shown in Fig. 3A, the binding of M@P-siRNAsT/I (pink) to IL-1β-treated bEnd.3 cells was found to be higher than that of untreated bEnd.3 cells and Lip@P-siRNAsT/I, respectively. This observation is attributed to the increased expression of adhesion molecules and stronger molecular interactions between M@P-siRNAsT/I and bEnd.3 cells resulting from the expression of P-selectin and ICAM-1 in bEnd.3 cells. The expression of P-selectin (Fig. 3 A, Top, green and Fig. 3B) and ICAM-1 (Fig. 3 A, Bottom, green and Fig. 3C) was found to be up-regulated in the IL-1β-treated bEnd.3 cells. In accord with our design expectations, M@P-siRNAsT/I was found to bind to P-selectin and ICAM-1 more effectively than Lip@P-siRNAsT/I (Fig. 3 A–C), an effect ascribed to the molecular interactions between CD44 and Mac-1 on the M@P-siRNAsT/I surface with P-selectin and ICAM-1, respectively.
Fig. 3.
Evaluation of M@P-siRNAsT/I targeting using established in vitro and in vivo inflammation models. (A) Representative fluorescence images of two nanoparticles under study (Lip@P-siRNAsT/I and M@P-siRNAsT/I, pink), bEnd.3 cells (blue), and P-selectin/ICAM-1 (green) in the untreated and IL-1β-pretreated groups. (Scale bar: 100 μm.) (B and C) Quantitative fluorescence intensities corresponding to Fig. 3A. (D) Schematic illustration of in vitro endothelial barrier model. (E and F) CIA-FLS fluorescence images and intensities of the Lip@P-siRNAsT/I- and M@P-siRNAsT/I-treated groups, respectively. (Scale bar: 50 μm.) (G) Representative fluorescence images reflecting uptake of PBNPs, Lip@P-siRNAsT/I, and M@P-siRNAsT/I by macrophage and CIA-FLS. (Scale bar: 50 μm.) (H and I) Representative fluorescence images and quantitative results of a mouse pre-injected with LPS subcutaneously and then administered Lip@P-siRNAsT/I and M@P-siRNAsT/I (100 μL) intravenously, respectively. P values are shown in the figures directly where relevant. Data are mean ± SEM (n ≥ 3/group).
Next, we established an endothelial barrier in vitro model to assess the transcytosis of M@P-siRNAsT/I under inflammation-like conditions (Fig. 3D). Here, IL-1β was used to induce the overexpression of cell adhesion molecules in bEnd.3 cells monolayer and mimic an inflamed endothelial barrier. Lip@P-siRNAsT/I and M@P-siRNAsT/I were added into the upper chamber, respectively, and incubated for 12 h. As shown in Fig. 3 E and F, greater fluorescence intensity was seen in collagen-induced arthritis fibroblast-like synoviocytes (CIA-FLS, adhered in bottom of chamber) for the IL-1β-pretreated M@P-siRNAsT/I group than the M@P-siRNAsT/I group lacking IL-1β- pretreatment, leading us to conclude that the inflammation environment likely presents a less effective endothelial barrier. The M@P-siRNAsT/I was shown to have greater endothelial barrier crossing ability than Lip@P-siRNAsT/I. The targeted uptake effect of M@P-siRNAsT/I by CIA-FLS and macrophages was confirmed by confocal fluorescence microscopy with improvement relative to Lip@P-siRNAsT/I also being seen (Fig. 3G).
A lipopolysaccharide (LPS)-induced inflammation mouse model was used to verify the inflammation chemotactic effect of M@P-siRNAsT/I. As shown in Fig. 3 H and I, subsequent to intravenous injection of M@P-siRNAsT/I, the LPS-injected region was characterized by a more intense fluorescence signal compared to the control region (PBS-injected only). The LPS-injected region for M@P-siRNAsT/I was also characterized by a stronger fluorescence signal than the Lip@P-siRNAsT/I control. These results lead us to suggest that M@P-siRNAsT/I would be recruited to inflammatory sites and thus might prove useful in targeting RA.
In Vitro Therapeutic Evaluation of M@P-siRNAsT/I.
Given the ability of M@P-siRNAsT/I to target inflamed cells and tissues, we next turned our attention to assessing their therapeutic potential. In a first study, CIA-FLS were activated by IL-1β to induce inflammation before being treated with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively (Fig. 4 A–C). M@P loaded with commercially available negative control siRNA (M@P-siRNAsNC) and Lip bearing siRNAsNC (Lip-siRNAsNC) were also used as controls (SI Appendix, Fig. S8). M@P-siRNAsT/I, as well as Lip-siRNAsT/I, were found to down-regulate the expression of TNF-α and IL-6 (Fig. 4 A and B). In comparison to PBNPs, Lip-siRNAsNC, and Lip-siRNAsT/I, M@P-siRNAsT/I displayed the greatest ROS scavenging ability (Fig. 4C), a finding ascribed to the synergistic effect of the multi-enzyme–like activity (i.e., peroxidase, catalase, and superoxide dismutase) of PBNPs and siRNAsT/I (37, 46, 47). Critically, M@P-siRNAsT/I not only inhibit the proinflammatory cytokine TNF-α/IL-6, but also act to reduce ROS levels in the RAM. Previous reports have shown CIA-FLS migration and invasion leads to damaged cartilage and that angiogenesis exacerbates these pathological events (48, 49). We found that the migration of CIA-FLS was reduced by M@P-siRNAsT/I (Fig. 4 D and E, and SI Appendix, Fig. S9). The invasion of CIA-FLS was evaluated using a Transwell model in vitro (Fig. 4F) (50). These studies revealed that the M@P-siRNAsT/I impair the invasiveness of CIA-FLS more effectively than PBNPs or Lip-siRNAsT/I (Fig. 4 G and H).
Fig. 4.
In vitro therapeutic evaluation of M@P-siRNAsT/I. (A–C) Secretion of TNF-α (A), IL-6 (B), or ROS (C) by CIA-FLS after 12 h of incubation with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively. (D and E) Photos and percent migration of CIA-FLS 12 h after incubation with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively. (Scale bar: 100 μm.) (F) Schematic representation of CIA-FLS invasion in the Transwell model. (G and H) Photos and invasion number of CIA-FLS 12 h after incubation with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively. (Scale bar: 100 μm.) (I and J) Photos and connecting branch number of inflamed bEnd.3 cells 12 h after incubation with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively. (Scale bar: 100 μm.) (K) HIF-1α, MMP3, MMP13, and VEGF proteins expression in CIA-FLS 12 h after incubation with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, respectively. P values are shown in the figures directly where relevant. Data are mean ± SEM (n ≥ 3/group).
IL-1β was also used to induce vascular endothelial cells to form new tubular networks as a model for angiogenesis. Based on this model, the M@P-siRNAsT/I act as effective angiogenesis inhibitors (Fig. 4 I and J). Support for this inference came from Western blot analyses, which were used to determine the expression levels of various cellular proteins upon M@P-siRNAsT/I treatment. Proteins monitored in this regard included hypoxia-related protein HIF-1α, migration and invasion-related proteins MMP3 and MMP13 in CIA-FLS, and the angiogenesis-related protein VEGF in inflamed vascular endothelial cells. We found that both PBNPs and Lip-siRNAsT/I served to down-regulate the expression of HIF-1α, MMP3, MMP13, and VEGF; however, M@P-siRNAsT/I proved most effective in this regard (Fig. 4K and SI Appendix, Fig. S10). On this basis, we conclude that the suppression of migration and invasion of CIA-FLS and angiogenesis by M@P-siRNAsT/I is mainly mediated by the down-regulation of HIF-1α, MMP3, MMP13, and VEGF proteins.
In Vivo Imaging of M@P-siRNAsT/I in a CIA Model.
As the next step in the present study, the ability of the M@P-siRNAsT/I to target RA joints was evaluated in vivo. Here, a CIA mouse model was used. As one control, PBNPs were labeled with indocyanine green (ICG) by means of noncovalent interactions (SI Appendix, Fig. S11) (51). The mice received an intravenous injection of saline, PBNPs (labeled by ICG), Lip-siRNAsT/I, and M@P-siRNAT/I (using the same siRNAsT/I concentrations for the latter two groups). They were then subjected to in vivo fluorescence imaging. The resulting fluorescence images revealed that both the labeled PBNPs and Lip-siRNAsT/I could accumulate in the RA joints, presumably as the result of the enhanced permeability and retention effect typically associated with inflammation (52). Nevertheless, the greatest accumulation was seen for the M@P-siRNAsT/I with peak accumulation being seen 6 h post-injection (SI Appendix, Fig. S12).
Fluorescence imaging is limited in terms of its depth of penetration, which could prove problematic in monitoring the fate of M@P-siRNAT/I (and other) nanoparticles in RA joints. We thus turned to photoacoustic (PA) imaging. This modality has as its core principle “light in and ultrasound out” and is recognized for providing substantial imaging depth in vivo (53). Therefore, the RA mice were subject to PA imaging 6 h post-injection with saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I; healthy mice were also studied as controls. As shown in Fig. 5A, a clear structure of the full RA forepaw and its cross section could be seen on the ultrasound images (gray); a weak PA signal was seen in the control group because the blood background signal. The PA signal increased in the PBNPs group, compared to control, presumably reflecting the passive accumulation of PBNPs in the RA forepaw joint. In the M@P-siRNAsT/I group, an intense PA signal was seen in the RA forepaw and the inside joint synovium (Fig. 5 A and B). An advantage of the PA imaging is that it provides not only 2D maximum amplitude projection PA/ultrasound (US) images, but also tomographic images—depth-resolved B-scan PA/US images (Fig. 5 C and D). The targeting ability of M@P-siRNAsT/I inferred on this basis is ascribed to the high specificity of the MMVs.
Fig. 5.
In vivo photoacoustic (PA) imaging of targeting of M@P-siRNAsT/I in a forepaw of a RA mouse model. (A and B) Representative PA images and corresponding statistical analysis of healthy and RA mice treated, respectively, with saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I. (C and D) B-scan PA/US images after intravenous injection with M@P-siRNAsT/I. MAP, maximum amplitude projection; B-scan, cross section image. The rainbow indicates the PA signal. Gray indicates US signal. P values are shown in the figures directly where relevant. Data are mean ± SEM (n ≥ 3/group).
In Vivo Therapeutic Evaluation of M@P-siRNAsT/I for Ameliorating RA Symptoms in a CIA Model.
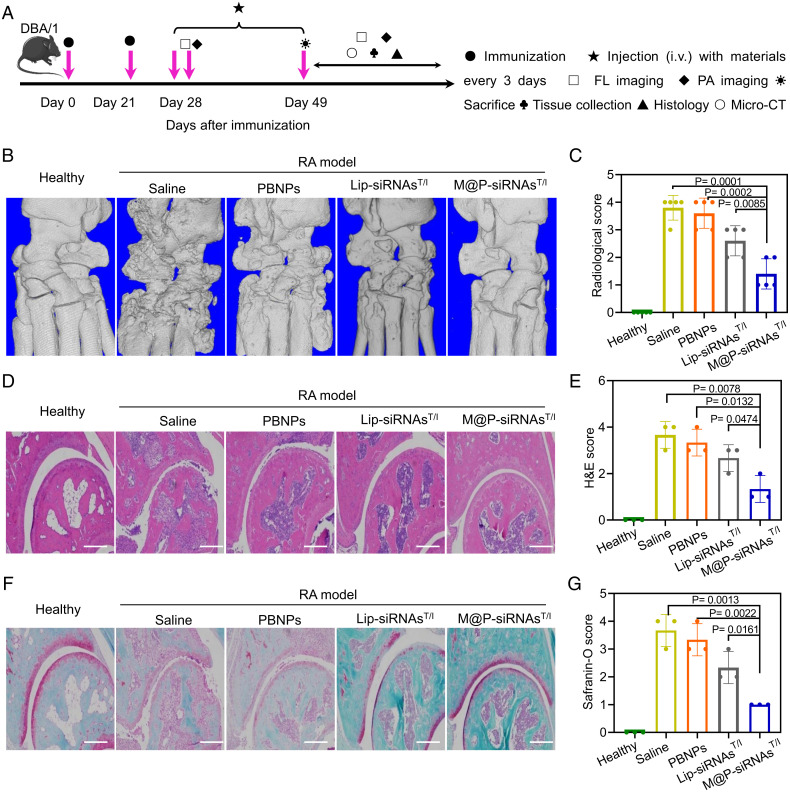
The efficacy of M@P-siRNAsT/I to ameliorate joint destruction was investigated using a murine model of CIA. This model recapitulates symptoms of swelling and erythema in paw joints, as well as bone erosion due to inflammation in the synovial cavity. Fig. 6A shows the timeline for RA model establishment, treatment, and therapeutic evaluation. Twenty-eight days after RA induction and when symptoms appeared, the mice were treated via intravenous injections every 3 d using, respectively, saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I for 3 wk. At this latter endpoint, the status of the RA forepaw joints in the different treatment groups were evaluated by means of micro-CT and histological analysis. As shown in Fig. 6B, serious bone erosion was seen in the saline-treated RA forepaw joints. After treatment with PBNPs and Lip-siRNAsT/I, the bone erosion was partial inhibited. Statistically significant improvements in bone erosion inhibition were seen in the M@P-siRNAsT/I group. The bone volume/tissue volume ratio (BV/TV) was used to characterize the extent of bone damage. This analysis revealed that the BV/TV of the M@P-siRNAsT/I-treated group comes closest to that of the healthy controls among the various treatment groups (Fig. 6C). This result provides support for the notion that M@P-siRNAsT/I inhibit RA progression and prevent RA-mediated bone erosion. Hematoxylin and eosin (H&E) stained images of the RA joint of the saline-treated group confirmed the symptoms of synovial membrane fibrillation, synovium hyperplasia, and immune cell infiltration characteristic of RA (54, 55). In comparison, treatment with M@P-siRNAsT/I was found to alleviate these symptoms (Fig. 6 D and E). Safranin-O stained cartilage images of the saline-treated RA mice groups proved consistent with the joint cartilage being severely destroyed. In contrast, mice in the group benefiting from M@P-siRNAsT/I treatment were found to have cartilage close to those in the healthy group (Fig. 6 F and G).
Fig. 6.
In vivo analysis of the therapeutic potential of M@P-siRNAsT/I using an RA mouse model. (A) Experimental schedule of therapy and diagnosis. (B and C) Representative micro-CT images and BV/TV ratios for the forepaws of the healthy control group and treatment groups consisting, respectively, of saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I at day 49. BV, bone volume; TV, tissue volume. (D and E) Representative H&E images and score of forepaw joints in the healthy group and the saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I treatment groups at day 49. (Scale bar: 200 μm.) (F and G) Representative safranin-o staining images and score of forepaw joints in the healthy control group and the saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I treatment groups at day 49. (Scale bar, 200 μm.) P values are shown in the figures directly where relevant. Data are mean ± SEM (n ≥ 3/group).
RA joints are considered to be hypoxic environments. This reflects the fact that the associated neovascular network is dysfunctional and fails to maintain tissue oxygen homeostasis. Moreover, overexpressed HIF-1α can contribute to hypoxia (48). Multispectral PA imaging, which permits the label-free detection of oxy-hemoglobin and deoxy-hemoglobin, was used to evaluate the therapeutic effect of M@P-siRNAsT/I and controls in vivo. Specifically, the PA signals obtained at 725 nm and 800 nm were used to construct blood oxygen saturation maps (39, 40). These are shown in Fig. 7 A and C for forepaw joints of RA mouse models after different treatments. The forepaw joints in the saline group display low blood oxygen saturation (35%) compared to that of the healthy group (set as 100%), a finding fully consistent with the hypoxic environment proposed for RA joint tissue (48, 56). After treatment with PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I, blood oxygen saturation increased to 60%, 79%, and 92%, respectively. This leads us to infer that the hypoxia within the RA joint was improved by these treatments. The relatively greater efficacy of M@P-siRNAsT/I is ascribed to 1) the catalase function of PBNPs that promotes the production of oxygen in RAM, and 2) the suppression of HIF-1α by siRNAsT/I (SI Appendix, Fig. S13). In addition, the proinflammatory factors TNF-α/IL-6 were also detected in the forepaw joint in vivo by immunohistology. As shown in Fig. 7 C–E, overexpressed TNF-α/IL-6 is seen in the RA joint of the saline group. In contrast, treatment with M@P-siRNAsT/I inhibited the expression of TNF-α/IL-6. These results are consistent with M@P-siRNAsT/I having a synergistic RA therapeutic effect as the result of inflammation reduction and hypoxia inhibition.
Fig. 7.
Multispectral PA imaging evaluation of the RA therapeutic effect of M@P-siRNAsT/I in vivo. (A) Representative PA images (B-scan) at day 49 of forepaws in a healthy group and groups treated with saline, PBNPs, Lip-siRNAsT/I, and M@P-siRNAsT/I. The rainbow shows the relative PA blood oxygen signal. Gray indicates US signal. (B) Immunofluorescence images of forepaw joints in a healthy group and in the saline, PBNPs, Lip-siRNAsT/I-, and M@P-siRNAsT/I-treated RA groups at day 49. (Scale bar: 100 μm.) (C) Blood oxygen saturation (compared with control) of forepaws in a healthy group and in the saline, PBNPs, Lip-siRNAsT/I-, and M@P-siRNAsT/I-treated RA groups at day 49. (D and E) Statistical analyses of the immunofluorescence of IL-6 and TNF-α in forepaw joints in a healthy group and in the saline, PBNPs, Lip-siRNAsT/I-, and M@P-siRNAsT/I-treated RA groups at day 49. P values are shown in the figures directly where relevant. Data are mean ± SEM (n ≥ 3/group).
Biosafety Evaluation of M@P-siRNAsT/I In Vitro and In Vivo.
To test the potential clinical utility of M@P-siRNAsT/I, its biodistribution and biocompatibility were investigated in vitro and in vivo. At 24 h intravenous post-injection of M@P-siRNAsT/I, an intense fluorescence signal was seen in the liver and in the kidneys (SI Appendix, Fig. S14). On this basis, we conclude that the M@P-siRNAsT/I are mainly located in these organs and are probably metabolized through them. Endothelial bEnd.3 cells treated by M@P-siRNAsT/I at concentrations of up to 200 µg/mL retained more than 85% viability after 24 h (SI Appendix, Fig. S15). Hemolytic (damage to red blood cells) analyses revealed good hemocompatibility when red blood cells were treated with M@P-siRNAsT/I (≤2% hemolysis; SI Appendix, Fig. S16). Additionally, healthy mice treated with M@P-siRNAsT/I over a period of 1 mo showed no obvious tissue damage or inflammatory lesions in any of the major organs (including the heart, liver, spleen, lung, and kidney) (SI Appendix, Fig. S17). A blood analysis revealed no significant difference between M@P-siRNAsT/I-treated mice and healthy mice (SI Appendix, Fig. S18), leading us to conclude that M@P-siRNAsT/I should benefit from good in vivo biocompatibility.
Conclusions
In summary, we have synthesized macrophage-biomimetic nanoparticles (M@P-siRNAsT/I) that exploit MMVs as a delivery vesicle for PBNPs and siRNAsT/I to affect RA imaging-guided therapy. PBNPs were found to have catalase function in that they could promote the dismutation of hydrogen peroxide with concomitant production of oxygen. They also display good NIR absorptivity allowing PA imaging to be used to trace the M@P-siRNAsT/I in vivo. siRNAsT/I possess a specific gene silencing capability, allowing the M@P-siRNAsT/I to suppress the expression of proinflammatory factors TNF-α/IL-6 in a statistically significant manner. Incorporation of both PBNPs and siRNAsT/I within the MMVs of this study was found to enhance their stability and biocompatibility. PA imaging, as well as fluorescence imaging, revealed that the M@P-siRNAsT/I allow for good RA targeting, both in vitro and in vivo. Moreover, M@P-siRNAsT/I were found to induce statistically significant RA therapeutic effects, including amelioration of joint erosion, hypoxia inhibition and anti-inflammation, as inferred from label-free multispectral PA imaging, micro-CT and histological analyses. M@P-siRNAsT/I were also found to display good biosafety in vitro and in vivo. As a result, we believe that M@P-siRNAsT/I may have a role to play in PA image-guided RA therapy.
Materials and Methods
The SI Appendix, Figs. S1–S18, provide details for all experimental procedures, the synthesis of M@P-siRNAsT/I, the in vitro and in vivo tests of M@P-siRNAsT/I targeting inflammation, and in vivo PA imaging.
The investigators adhered fully to the “Guide for the Care and Use of Animals” of the Animal Care and Use Committee of Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences (SIATACUC). In addition, all animal experiments were conducted in compliance with protocols approved by the SIATACUC (SIAT-IRB-190111-YYS-CJQ-A0413-01).
Supplementary Material
Acknowledgments
The work was supported by grants from the National Natural Science Foundation of China (82172008 to Jingqin Chen), National Key R&D Program of China (2020YFA0908800 to Jingqin Chen), CAS Key Laboratory of Health Informatics (2011DP173015 to Jingqin Chen), and Guangdong Provincial Key Laboratory of Biomedical Optical Imaging (2020B121201010 to C.L.), and the Guangdong Basic and Applied Basic Research Foundation (2020A1515010978 to H.Z.). The work in Austin was supported by the Robert A. Welch Foundation (F-0018 to J.L.S.). This paper is dedicated to Prof. James P. Collman in honor of his 90th birthday.
Footnotes
Reviewers: Q.F., Renji Hospital; and X.W., University of Michigan.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2213373119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Jones S. A., Jenkins B. J., Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 18, 773–789 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Burmester G. R., Pope J. E., Novel treatment strategies in rheumatoid arthritis. Lancet 389, 2338–2348 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Aletaha D., Smolen J. S., Diagnosis and management of rheumatoid arthritis: A review. JAMA 320, 1360–1372 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Certo M., Tsai C.-H., Pucino V., Ho P.-C., Mauro C., Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Chaudhari K., Rizvi S., Syed B. A., Rheumatoid arthritis: Current and future trends. Nat. Rev. Drug Discov. 15, 305–306 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Salliot C., et al. , Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: A meta-analysis. Ann. Rheum. Dis. 70, 266–271 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Law S. T., Taylor P. C., Role of biological agents in treatment of rheumatoid arthritis. Pharmacol. Res. 150, 104497 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Fu Q., et al. , A double-blind, double-dummy, randomized controlled, multicenter trial of 99Tc-methylene diphosphonate in patients with moderate to severe rheumatoid arthritis. Chin. Med. J. (Engl.) 134, 1457–1464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brzustewicz E., Bryl E., The role of cytokines in the pathogenesis of rheumatoid arthritis--Practical and potential application of cytokines as biomarkers and targets of personalized therapy. Cytokine 76, 527–536 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Choy E. H., Panayi G. S., Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 344, 907–916 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Al-Herz A., et al. ; Kuwait Registry for Rheumatic Diseases (KRRD), Accessibility to biologics and its impact on disease activity and quality of life in patients with rheumatoid arthritis in Kuwait. Clin. Rheumatol. 40, 1759–1765 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arkema E. V., et al. ; ARTIS Study Group, Are patients with rheumatoid arthritis still at an increased risk of tuberculosis and what is the role of biological treatments? Ann. Rheum. Dis. 74, 1212–1217 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Liu L., Pharmacokinetics of monoclonal antibodies and Fc-fusion proteins. Protein Cell 9, 15–32 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craik D. J., Fairlie D. P., Liras S., Price D., The future of peptide-based drugs. Chem. Biol. Drug Des. 81, 136–147 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y., et al. , Rheumatoid arthritis microenvironment insights into treatment effect of nanomaterials. Nano Today 42, 101358 (2022). [Google Scholar]
- 16.Kim J., et al. , Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano 13, 3206–3217 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Mapp P. I., Grootveld M. C., Blake D. R., Hypoxia, oxidative stress and rheumatoid arthritis. Br. Med. Bull. 51, 419–436 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Chen J., et al. , Notch-1 and notch-3 mediate hypoxia-induced activation of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheumatol. 73, 1810–1819 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Morris K. V., Chan S. W.-L., Jacobsen S. E., Looney D. J., Small interfering RNA-induced transcriptional gene silencing in human cells. Science 305, 1289–1292 (2004). [DOI] [PubMed] [Google Scholar]
- 20.Bedingfield S. K., et al. , Amelioration of post-traumatic osteoarthritis via nanoparticle depots delivering small interfering RNA to damaged cartilage. Nat. Biomed. Eng. 5, 1069–1083 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams D., et al. , Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 379, 11–21 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Hatit M. Z. C., et al. , Species-dependent in vivo mRNA delivery and cellular responses to nanoparticles. Nat. Nanotechnol. 17, 310–318 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoue A., et al. , Comparison of anti-rheumatic effects of local RNAi-based therapy in collagen induced arthritis rats using various cytokine genes as molecular targets. Mod. Rheumatol. 19, 125–133 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Carthew R. W., Sontheimer E. J., Origins and mechanisms of miRNAs and siRNAs. Cell 136, 642–655 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darrah K. E., Deiters A., Translational control of gene function through optically regulated nucleic acids. Chem. Soc. Rev. 50, 13253–13267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Z., Reynaud F., Lorscheider M., Tsapis N., Fattal E., Nanomedicines for the delivery of glucocorticoids and nucleic acids as potential alternatives in the treatment of rheumatoid arthritis. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12, e1630 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Dong Y., Siegwart D. J., Anderson D. G., Strategies, design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 144, 133–147 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo D., et al. , Photostable and biocompatible fluorescent silicon nanoparticles for imaging-guided co-delivery of siRNA and doxorubicin to drug-resistant cancer cells. Nano-Micro Lett. 11, 27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng M., et al. , ROS‐responsive polymeric siRNA nanomedicine stabilized by triple interactions for the robust glioblastoma combinational RNAi therapy. Adv. Mater. 31, e1903277 (2019). [DOI] [PubMed] [Google Scholar]
- 30.Kanasty R., Dorkin J. R., Vegas A., Anderson D., Delivery materials for siRNA therapeutics. Nat. Mater. 12, 967–977 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Boehnke N., et al. , Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 377, eabm5551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao C., et al. , Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 11, 2622 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J., et al. , Engineered biomimetic nanoparticle for dual targeting of the cancer stem-like cell population in sonic hedgehog medulloblastoma. Proc. Natl. Acad. Sci. U.S.A. 117, 24205–24212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li R., et al. , Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 19, 124–134 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Haringman J. J., Ludikhuize J., Tak P. P., Chemokines in joint disease: The key to inflammation? Ann. Rheum. Dis. 63, 1186–1194 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thamphiwatana S., et al. , Macrophage-like nanoparticles concurrently absorbing endotoxins and proinflammatory cytokines for sepsis management. Proc. Natl. Acad. Sci. U.S.A. 114, 11488–11493 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang W., et al. , Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138, 5860–5865 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Cai X., et al. , A Prussian blue-based core-shell hollow-structured mesoporous nanoparticle as a smart theranostic agent with ultrahigh pH-responsive longitudinal relaxivity. Adv. Mater. 27, 6382–6389 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Li M., Tang Y., Yao J., Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 10, 65–73 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., et al. , In vivo integrated photoacoustic and confocal microscopy of hemoglobin oxygen saturation and oxygen partial pressure. Opt. Lett. 36, 1029–1031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao R., et al. , Background-suppressed tumor-targeted photoacoustic imaging using bacterial carriers. Proc. Natl. Acad. Sci. U.S.A. 119, e2121982119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cano-Mejia J., et al. , CpG-coated Prussian blue nanoparticles-based photothermal therapy combined with anti-CTLA-4 immune checkpoint blockade triggers a robust abscopal effect against neuroblastoma. Transl. Oncol. 13, 100823 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen J., et al. , Single-layer MoS2 nanosheets with amplified photoacoustic effect for highly sensitive photoacoustic imaging of orthotopic brain tumors. Adv. Funct. Mater. 26, 8715–8725 (2016). [Google Scholar]
- 44.Soutschek J., et al. , Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Hickerson R. P., et al. , Stability study of unmodified siRNA and relevance to clinical use. Oligonucleotides 18, 345–354 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J., et al. , Prussian blue with intrinsic heme-like structure as peroxidase mimic. Nano Res. 11, 4905–4913 (2018). [Google Scholar]
- 47.Vázquez-González M., et al. , Mimicking peroxidase activities with Prussian blue nanoparticles and their cyanometalate structural analogues. Nano Lett. 17, 4958–4963 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Taylor P. C., Sivakumar B., Hypoxia and angiogenesis in rheumatoid arthritis. Curr. Opin. Rheumatol. 17, 293–298 (2005). [DOI] [PubMed] [Google Scholar]
- 49.Konisti S., Kiriakidis S., Paleolog E. M., Hypoxia—Ā key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat. Rev. Rheumatol. 8, 153–162 (2012). [DOI] [PubMed] [Google Scholar]
- 50.Xue J., et al. , Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 12, 692–700 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Zhao Q., et al. , Hyaluronic acid oligosaccharide modified redox-responsive mesoporous silica nanoparticles for targeted drug delivery. ACS Appl. Mater. Interfaces 6, 20290–20299 (2014). [DOI] [PubMed] [Google Scholar]
- 52.Chen J., et al. , Tocilizumab-conjugated polymer nanoparticles for NIR-II photoacoustic-imaging-guided therapy of rheumatoid arthritis. Adv. Mater. 32, e2003399 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Lin H., et al. , Repurposing ICG enables MR/PA imaging signal amplification and iron depletion for iron-overload disorders. Sci. Adv. 7, eabl5862 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang H., et al. , Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat. Commun. 9, 4291 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Q., et al. , Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 13, 1182–1190 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Chen J., et al. , Treatment of collagen-induced arthritis rat model by using Notch signalling inhibitor. J. Orthop. Translat. 28, 100–107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.