Significance
Mucins are the major components of the mucous barrier that protects our body from pathogens. Changes in their amounts and properties would therefore alter the mucus viscosity. Here, we show that FUT8, a glycosylation enzyme of the Golgi, controls the viscosity of secreted mucins. Mucin-secreting cells of the colon express FUT8, but their levels are increased in ulcerative colitis (UC) patients. As a result, mucus produced by these cells is more permeable but resistant to removal by washing. We suggest that this change in the overall composition and viscosity of mucus is a major cause of initial inflammation observed in UC. These findings reveal how cells regulate the quantity and quality of mucins and a potential means to control UC.
Keywords: mucin secretion, FUT8, ulcerative colitis, fucosylation
Abstract
Mucins are the main macrocomponents of the mucus layer that protects the digestive tract from pathogens. Fucosylation of mucins increases mucus viscoelasticity and its resistance to shear stress. These properties are altered in patients with ulcerative colitis (UC), which is marked by a chronic inflammation of the distal part of the colon. Here, we show that levels of Fucosyltransferase 8 (FUT8) and specific mucins are increased in the distal inflamed colon of UC patients. Recapitulating this FUT8 overexpression in mucin-producing HT29-18N2 colonic cell line increases delivery of MUC1 to the plasma membrane and extracellular release of MUC2 and MUC5AC. Mucins secreted by FUT8 overexpressing cells are more resistant to removal from the cell surface than mucins secreted by FUT8-depleted cells (FUT8 KD). FUT8 KD causes intracellular accumulation of MUC1 and alters the ratio of secreted MUC2 to MUC5AC. These data fit well with the Fut8−/− mice phenotype, which are protected from UC. Fut8−/− mice exhibit a thinner proximal colon mucus layer with an altered ratio of neutral to acidic mucins. Together, our data reveal that FUT8 modifies the biophysical properties of mucus by controlling levels of cell surface MUC1 and quantity and quality of secreted MUC2 and MUC5AC. We suggest that these changes in mucus viscoelasticity likely facilitate bacterial–epithelial interactions leading to inflammation and UC progression.
The gastrointestinal tract is an optimal habitat for bacteria (high nutrient content, ideal salt concentration and temperature), yet their contact with epithelial cells is limited because of the secreted gel-forming mucins that compose the mucus layer (1, 2). This dense and viscoelastic layer has two main defensive functions: 1) to protect the epithelium and 2) to remove pathogens from the body. To date, five gel-forming mucins have been described in humans, including MUC2 as the main secreted mucin for the intestine and the colon. Gel-forming mucins, secreted by epithelial goblet cells and submucosal glands, are highly glycosylated, which includes fucosylation, during their passage through the Golgi complex (2, 3). These glycosylated mucins are then packed into specialized micrometer-sized granules, where they condense and reach molecular weights of up to 50 MDa (4–6). Mature granules fuse to apical plasma membrane (PM) by a Ca2+-dependent process to release their contents in the absence of extracellular agonists (baseline mucin secretion) and upon stimulation by an external agonist (stimulated mucin secretion). Distinct Ca2+ pools control these different modes of mucin secretion. Baseline mucin secretion depends on intracellular Ca2+ oscillations and a small Ca2+ binding protein called KChIP3 (7). Stimulated mucin secretion, on the other hand, relies on Ca2+ entry from extracellular medium by the cooperation of a Na+ channel (TRPM4/5) and a Na+-Ca2+ exchanger (NCX) (8). In the extracellular space, mucins swell several hundred times their dehydrated volume and combined with water, ions, and other solutes form the mucus layer. Importantly, mucins regulate the mucus viscoelastic properties, which strongly impacts the adhesion, clearance, and penetration of foreign particles (like drugs or allergens), microbiota, and pathogens. The quantities of secreted mucin and differential mucin glycosylation, for example fucosylation, significantly modify the rheological properties of the mucus layer. In fact, mucin fucosylation by FUT2 in the airways increases mucus viscoelasticity, making them more resistant to shear stress (9). Consequently, alterations in levels of mucin secretion and its physicochemical properties can affect the quality of the mucus layer and, thereby, facilitate bacterial invasion and inflammation (10–12).
Ulcerative colitis (UC) is characterized by relapsing inflammation of the gastrointestinal tract. The quantity and quality of mucins are affected in UC, especially affecting the colonic mucus layer (13, 14). In healthy colon, the mucus forms two layers: 1) an outer layer (less dense and nonattached) colonized by bacteria and microbiota; and 2) an inner layer (dense and attached to the epithelium) that is impenetrable to bacteria (1, 12). In UC patients, the colonic mucus layer is thinner and more penetrable to experimentally tested fluorescent beads. Aberrant glycosylation (for example, an increase in small glycans) correlates with the severity of inflammation in UC and in turn inflammatory cytokines increase fucosylation (15–18). Muc2 knockout (KO) mice develop spontaneous colitis, revealing the critical role of mucins in UC (19). Several genetic factors have recently been linked to UC (20), including Fucosyltransferase 8 (FUT8) (21, 22), which is the only enzyme responsible for the α1-6–linked fucosylation that adds fucose to the innermost GlcNAc residue of an N-linked glycan (23). FUT8 was also identified as a putative regulator of MUC5AC secretion in a genome-wide screen (24). In addition, previous studies showed that Fut8−/− KO mice develop less-severe colitis compared to Fut8+/+ WT mice (21).
The properties of the mucus layer and FUT8 levels are altered in UC. This begs the question whether FUT8 impacts mucin production and its viscoelastic properties. Moreover, why should lack of FUT8 protect against UC? We have used colonic cell lines, in-house gene-expression data of colon biopsies from UC patients, and Fut8−/− KO mice to address the involvement of FUT8 in mucin secretion and its impact on the development of UC. We have found that FUT8 is required for the trafficking of transmembrane mucins and secretion of gel-forming mucins. FUT8 is also important to produce mucins that exhibit physiological resistance to shear stress. Moreover, FUT8 is necessary to generate the right ratio of acidic to neutral mucins in the colonic mucus layer of mice. This balanced composition of mucins in the mucus likely impacts the inflammatory response and protection from pathogens.
Results
FUT8 Expression Is Up-Regulated in UC.
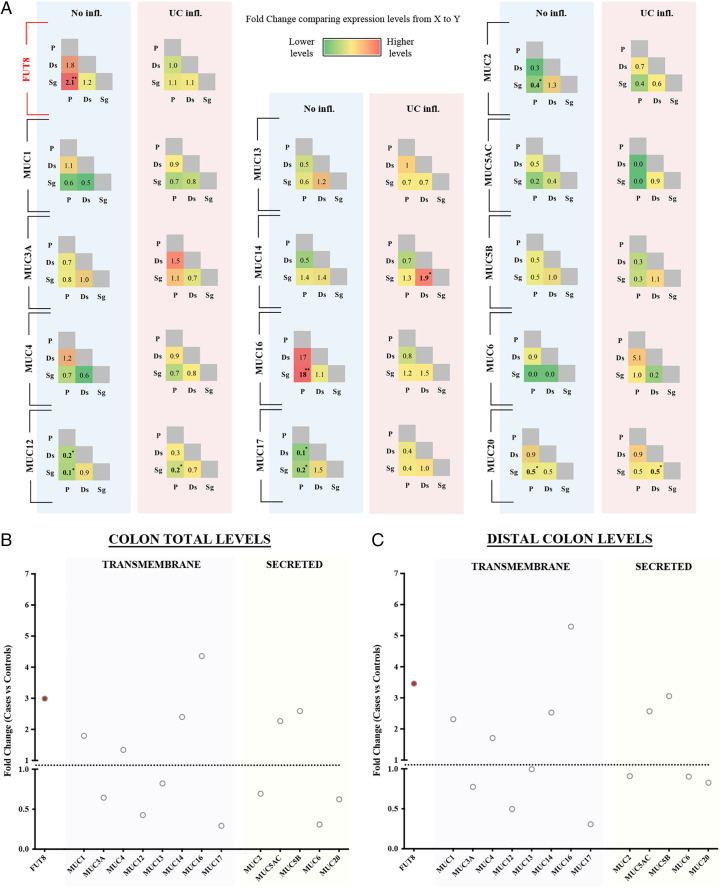
FUT8 is widely expressed in the gastrointestinal tract, but it is particularly enriched in colonic goblet cells [SI Appendix, Table S1, data extracted from Tabula Muris (25)]. We first performed differential expression analysis using in-house unpublished RNA-sequencing (RNA-seq) data from biopsies obtained from different intestinal locations (P: proximal -ascending- colon; Ds: descending colon; Sg: sigmoid colon; both descending and sigmoid form the distal colon) of UC patients (n = 24) and control subjects without inflammatory bowel disease (IBD) diagnosis (n = 16) (data, including statistical analysis, are shown in SI Appendix). Our analysis using these RNA-seq data revealed higher FUT8 levels in the proximal colon compared to the distal colon of control subjects (2.1-fold higher in proximal compared to sigmoid, P = 0.006). This gradient of FUT8 expression is lost in inflamed colon of UC patients (P. vs. Sg, fold-change = 1.1, P = 0.78) (Fig. 1A). Our analysis of the mucins’ levels showed that RNA levels of MUC2, MUC12, MUC17, and MUC20 are higher in the distal colon compared to proximal colon of healthy individuals. A similar distribution was observed for these mucins in UC. MUC16 expression was higher in the proximal colon compared to the distal colon, but we cannot rule out the possibility that this is a consequence of its extremely low expression in colon. Our results also show that FUT8 is increased in the inflamed colon of UC patients compared to noninflamed colons (threefold increase in UC P = 9.7E-07). Levels of MUC1, MUC14, MUC16, MUC5AC, and MUC5B are also suggestively higher in inflammation, whereas MUC12, MUC17, and MUC6 levels are lower compared to control subjects (Fig. 1B). Statistical significance of these comparisons could not be confirmed because of the small sample size. We therefore cautiously suggest that this is considered a general trend and not the molecular feature of UC.
Fig. 1.
Colonic expression levels of FUT8 and mucins in UC. (A) Expression of FUT8 and mucins was compared between different colonic locations (P: proximal -ascending- colon, Ds: descending colon, Sg: sigmoid colon) in control subjects and UC patients. Data are shown as fold-change between locations of the x axis vs. the y axis. (B) Total levels of FUT8 and 14 mucins at the colon comparing control subjects vs. patients. Data represent the fold-change between controls and UC patients (black circles). Red-filled circles represent those statistically significant differences (P < 0.004). (C) Specific levels of FUT8 and 14 mucins at the distal colon comparing the data from patients with the expression levels of control subjects. Red-filled circles represent those statistically significant differences (P < 0.004). Abbreviations: No infl.: Not inflamed, UC infl.: Ulcerative colitis inflamed colon. *P < 0.05, **P < 0.01.
FUT8 has been associated with UC (20). The mucus layer of UC patients is known to be thinner and inflammation affects mainly the distal part of the colon (Sg in our analysis) (26, 27). Accordingly, our expression data showed that FUT8 levels are increased in the inflamed distal colon (Sg) of UC patients compared to healthy subjects (3.5-fold increase, P = 7.14E-08) (Fig. 1C). Among mucins, MUC1, MUC14, and MUC16 levels are increased in UC inflamed distal colon (2-fold, 2.5-fold, and 5-fold increase, respectively, P < 0.05), while MUC17 levels are reduced (70% reduction, P = 0.01). Although not approaching statistical significance, both MUC5AC and MUC5B levels show a tendency to be elevated in inflamed UC distal colon (2.5-fold and 3-fold increase, respectively).
Altogether, these results show that FUT8 levels are increased in the distal colon of inflamed UC and a similar trend is observed for several transmembrane (e.g., MUC1) and secreted mucins (e.g., MUC5AC).
The Composition of Mucus Is Altered in Fut8−/− Mice.
It has been shown that Fut8 KO mice (Fut8−/− mice) are less susceptible to UC (21). Previous studies reported that T cells from Fut8−/− mice produce lower levels of T-helper 1 and 2 cytokines, which could explain the protective effect in the progression of T cell-mediated colitis (21). However, the effect of FUT8 on initiation of inflammation, which is related to the mucus layer, has not been addressed. Based on our data showing that FUT8 levels are increased in the distal colon of UC patients, a pattern also seen with several mucins, we tested the effects of FUT8 deletion on the properties of the colonic mucus layer under basal conditions.
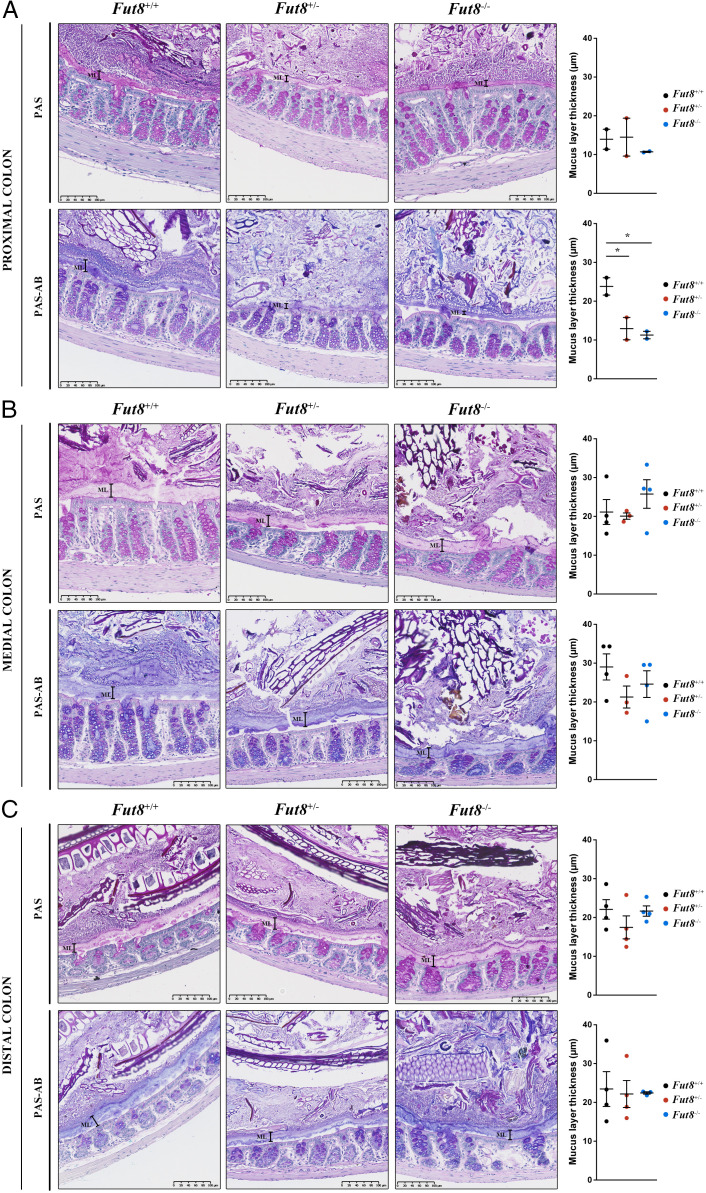
First, colons from Fut8+/+, Fut8+/−, and Fut8−/− mice (n = 4 per condition, 12-wk old) were stained with H&E for the histopathological evaluation of the tissue. Histological analysis showed no obvious differences in epithelial hyperplasia, inflammatory infiltrate, and edema at the proximal, medial, or distal colon. There was also no difference in the number of mitotic cells between groups (SI Appendix, Fig. S1 A and B and Table S2). We then stained colon slices with LCA-lectin, which binds fucosylated proteins (28). Our results show that the colonic mucus layer is fucosylated and, importantly, overall fucosylation is reduced in Fut8−/− mice (SI Appendix, Fig. S1 C, Left ). Next, we stained the colon slices with PhoSL, a FUT8-dependent fucosylation-specific lectin. Our data revealed a complete absence of PhoSL staining in Fut8−/− mice compared to Fut8+/− mice (SI Appendix, Fig. S1 C, Center). These data the reveal the importance of the FUT8-specific fucosylation of the components of the colonic mucus layer.
To further evaluate the mucus layer in these mice, slices from proximal, medial, and distal colon of Fut8+/+, Fut8+/−, and Fut8−/− mice were stained with Periodic acid-Schiff (PAS) and PAS-Alcian blue (PAS-AB) to detect neutral and acidic mucins, respectively, as described previously (7). The thickness of the mucus layer detected by PAS staining (neutral mucins) was not significantly different in any part of the colon of the WT and the mutant mice (Fig. 2, Upper panels). However, there was a reduction in the mucus thickness detected by PAS-AB (acidic and neutral mucins) at the proximal colon in Fut8+/− and Fut8−/− compared to Fut8+/+ mice (12.93 µm and 11.27 µm vs. 23.81 µm, respectively). The mucus thickness was close to the normal in medial (21.27 µm and 24.6 µm vs. 29.02 µm) and distal (22.2 µm and 22.4 µm vs. 23.5 µm) colon (Fig. 2, Lower panels). Finally, the ratio of PAS-AB to PAS staining decreased from proximal to distal in Fut8+/+ mice (proximal = 1.8, medial = 1.4, distal = 0.8, proximal vs. distal P = 0.019), a pattern not observed in either in Fut8+/− mice (proximal = 0.9, medial = 1.1, distal = 1.3, proximal vs. distal n.s.) or Fut8−/− mice (proximal = 1.0, medial = 1.0, distal = 1.1, proximal vs. distal n.s. [not significant]) (SI Appendix, Fig. S1D).
Fig. 2.
Fut8−/− mice present thinner mucus layer at the proximal colon. Representative (A) proximal, (B) medial, and (C) distal colons of Fut8+/+ (Left), Fut8+/− (Center), and Fut8−/− (Right) mice stained with PAS or PAS-AB. Quantification of the mucus layer thickness for each condition is shown next to images. Average values ± SEM are plotted as scatter plot with bar graph (Fut8+/+: black dots, Fut8+/−: red dots, Fut8−/−: blue dots). The y axis represents the thickness of the mucus layer in micrometers. *P < 0.05.
In summary, our results show that the colonic mucus layer is composed of fucosylated proteins. In Fut8-lacking mice, there is an overall reduction of fucosylated proteins and complete depletion of FUT8-specific fucosylated components in the distal colon. This is accompanied by changes in the thickness of the mucus layer.
FUT8 Regulates MUC1 Export to the PM.
We previously identified the requirement of FUT8 in phorbol 12-myristate 13-acetate (PMA)–triggered secretion of MUC5AC (24). But little else was reported in the role of FUT8 in mucin secretion under physiological conditions. Based on the data on FUT8 on the composition of mucus stated above, we generated a HT29-18N2 cell line stably depleted of FUT8 (FUT8 KD) and another line that overexpresses FUT8 (FUT8 OV). RNA was extracted from differentiated FUT8 KD and FUT8 OV HT29-18N2 cells and FUT8 mRNA levels measured by qPCR. Compared to control cells, FUT8 KD cells revealed a 70% reduction in FUT8 mRNA level, whereas FUT8 OV cells showed a fivefold increase in the level of the cognate mRNA (SI Appendix, Fig. S2A). Measurement by Western blotting cell lysates confirmed a 90% reduction in FUT8 levels in FUT8 KD cells and twofold increase in FUT8 levels in FUT8 overexpression cells compared to control cells (SI Appendix, Fig. S2B).
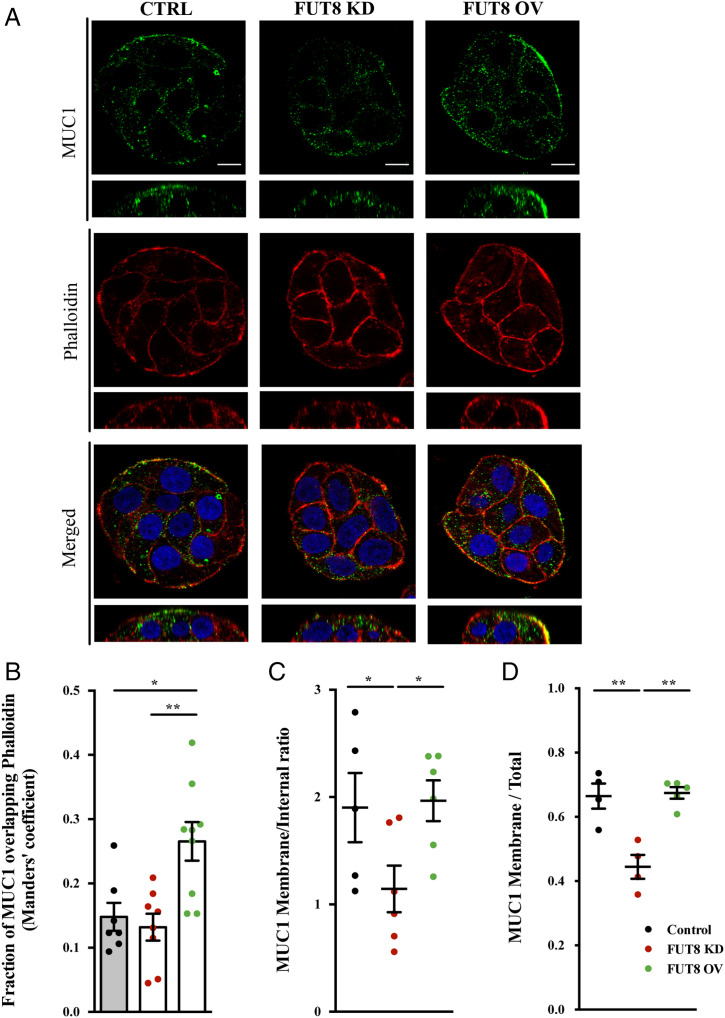
Using these cell lines, we first studied the involvement of FUT8 on the transmembrane MUC1 because its expression is increased in UC patients and associated with severe UC (31). In accordance, there was a 35% increase in MUC1 levels in cell line overexpressing FUT8 (SI Appendix, Fig. S2C). Our data by immunofluorescence microscopy showed a significantly enhanced expression at the cell surface in FUT8 overexpressing cells (Manders’ coefficient MUC1 vs. phalloidin control = 0.14, FUT8 KD = 0.13 and FUT8 OV = 0.27) (Fig. 3A; quantification in Fig. 3B). On the other hand, depletion of FUT8 reduced the trafficking of MUC1 to the PM (45% vs. 70%, P < 0.01) (Fig. 3C) and caused intracellular MUC1 accumulation compared to control cells and to cells overexpressing FUT8 (Fig. 3D).
Fig. 3.
FUT8 levels impact MUC1 localization (A) Immunofluorescence z-stack single planes of control, FUT8-KD, and FUT8-OV differentiated HT29-18N2 cells with anti-MUC1 antibody (green), phalloidin (red), and DAPI (red). (Scale bars, 5 µm.) (B) Colocalization between MUC1 and phalloidin was calculated from immunofluorescence images by Manders’ coefficient using Fiji. Average values ± SEM are plotted as scatter plot with bar graph. The y axis represents Manders’ coefficient of the fraction of MUC1 overlapping with phalloidin. (C) Quantification of MUC1 levels at the membrane compared to intracellular levels in control (black), FUT8 KD (red), and FUT8 OV (green) cells. Data were calculated from immunofluorescence images using Fiji. (D) Quantification of MUC1 levels at the membrane compared to total levels in control (black), FUT8 KD (red), and FUT8 OV (green) cells. Data were calculated from immunofluorescence images using FIJI. Abbreviations: Ctrl: control cells; FUT8 KD: FUT8-depleted cells; FUT8 OV: FUT8 overexpressing cells; KD: FUT8 KD; OV: FUT8 OV. *P < 0.05, **P < 0.01.
FUT8 Controls MUC2 and MUC5AC Secretion.
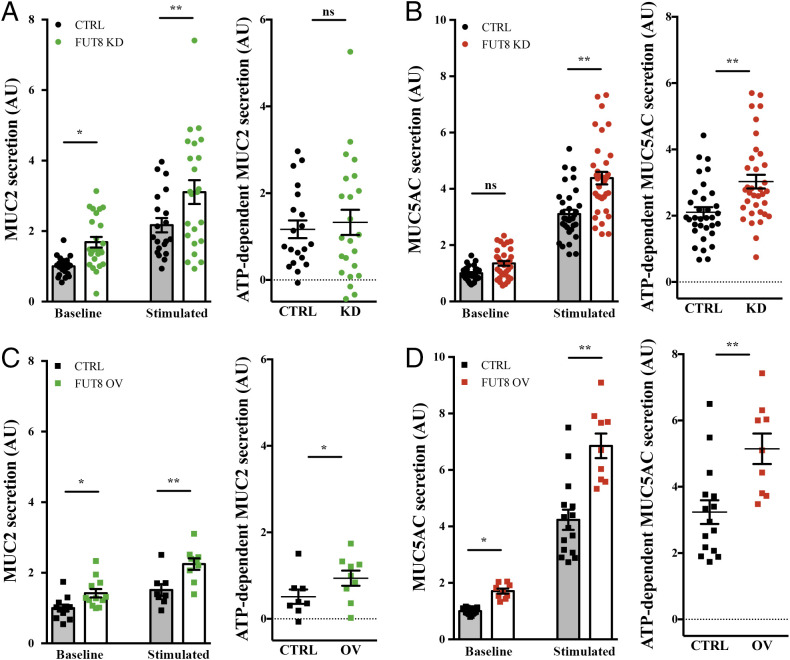
As shown above, the mucus layer is altered in Fut8−/− mice. We therefore addressed the role of FUT8 in the secretion of two gel-forming mucins, MUC2 and MUC5AC. We measured MUC2 and MUC5AC release in the absence (baseline secretion) and the presence (stimulated secretion) of physiological stimulus the ATP (100 µM in a solution containing 1.2 mM CaCl2) in control, FUT8 KD, and FUT8 OV cells. Our results show that depletion of FUT8 increases both the baseline secretion of MUC2 (1.7-fold increase) (Fig. 4A) and agonist (ATP)-induced MUC5AC secretion by 1.4-fold compared to the control condition (Fig. 4B). Overexpression of FUT8 increases both MUC2 and MUC5AC baseline and ATP-dependent (stimulated) secretion (MUC2, 1.4- and 1.8-fold increase; MUC5AC, 1.7- and 1.6-fold increase, respectively) (Fig. 4 C and D).
Fig. 4.
FUT8 levels alter mucin export. (A and C) Secreted MUC2 from differentiated control (black circles) and FUT8 KD (green circles) cells or control (black squares) and FUT8 OV (green squares) cells that were incubated for 30 min at 37 °C in the absence (baseline) or presence (stimulated) of 100 µM ATP. Data were normalized to intracellular actin levels. The y axis represents normalized values relative to the values of untreated control cells. ATP-dependent MUC2 secretion was calculated from the data in A or B, respectively, as the difference between normalized baseline secretion and stimulated secretion for each condition. (B and D) Secreted MUC5AC from differentiated control (black circles) and FUT8 KD (red circles) cells or control (black squares) and FUT8 OV (red squares) cells that were incubated for 30 min at 37 °C in the absence (baseline) or presence (stimulated) of 100 µM ATP. Data were normalized to intracellular actin levels. The y axis represents normalized values relative to the values of untreated control cells. ATP-dependent MUC5AC secretion was calculated from the data in C or D, respectively, as the difference between normalized baseline secretion and stimulated secretion for each condition. Abbreviations: Ctrl: control cells; KD: FUT8 KD; OV: FUT8 OV. *P < 0.05, **P < 0.01.
These data reveal that FUT8 levels have different effects on MUC2 and MUC5AC secretion. Interestingly, when presented as the ratio of MUC2 to MUC5AC secretion levels, FUT8 KD cells present higher baseline MUC2/MUC5AC ratio (ratio = 1.35, more MUC2 secretion) but lower stimulated MUC2/MUC5AC ratio (ratio = 0.6, more MUC5AC ATP-dependent secretion) compared to control cells (SI Appendix, Fig. S3A). Overexpression of FUT8 has no obvious effect on baseline ratio (ratio = 0.9), but has the opposite phenotype on stimulated MUC2/MUC5AC ratio (ratio = 1.4, more MUC2 ATP-dependent secretion) (SI Appendix, Fig. S3B). These changes likely affect the mucus layer properties due to different quantities of MUC2 and MUC5AC (1). Finally, we tested whether this effect of FUT8 on MUC2 and MUC5AC secretion was due to changes in intracellular protein levels. Total cell lysates from FUT8-KD, FUT8-OV, and control cells were dot-blotted with anti-MUC5AC and anti-MUC2 antibodies and there was no discernible effect on the intracellular levels of MUC2 and MUC5AC in either FUT8 OV or KD compared to control cells (SI Appendix, Fig. S2 D and E).
FUT8 Alters the Adhesion of Mucins to the Cell Surface.
How does reduction in mucus layer thickness observed in Fut8−/− mice correlate with the increased mucin secretion in FUT8 KD cells? Fucosylation affects viscoelastic properties of mucus, thereby increasing its resistance to shear stress. We hypothesized that deletion of FUT8 reduces the resistance of mucus to shear stress, therefore making it easily removed from the cell surface.
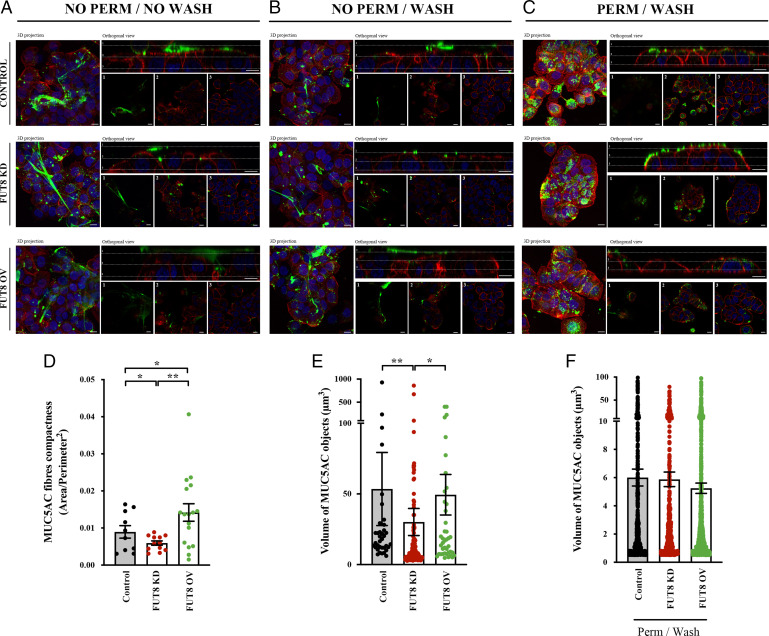
We then tested whether alterations in FUT8 levels affect the resistance of mucus to removal from the cell surface. We examined extracellular mucin fibers formed after stimulation with 100 µM ATP in control, FUT8 KD, and FUT8 OV cells by immunofluorescence microscopy with and without permeabilization before or after extensive washing with isotonic solution (as described in Materials and Methods). Unpermeabilized and unwashed cells showed extracellular mucin fibers in all conditions. Importantly, MUC5AC fibers formed by FUT8 KD were more compact, while those from FUT8 OV cells were less compact compared to control cells (area/perimeter2 ratio; control cells = 0.009, FUT8 KD cells = 0.006, FUT8 OV cells = 0.014, P < 0.05) (Fig. 5A; quantification Fig. 5D). Accordingly, the volume of MUC5AC fibers was reduced in FUT8 KD cells compared to control or FUT8 OV cells (Control = 53.5 µm3, FUT8 KD = 30.1 µm3, FUT8 OV = 49.4 µm3, P < 0.05) (Fig. 5A; quantification Fig. 5E). After washing (without permeabilization), few mucin fibers were visible in control or FUT8 KD cells compared to numerous fibers that remained attached to cell surface in FUT8 OV cells (Fig. 5B). Quantification of the number of MUC5AC fibers (volume greater than 30 µm3 compared to total number of fibers) showed a strong and significant reduction in control and FUT8 KD cells after extensive washing (Control cells: 35.9% vs. 13.0%, P = 0.02; KD cells: 29.9% vs. 10.0%, P = 0.018), whereas it was minimally affected with many fibers remaining in FUT8 overexpressing cells (49.2% vs. 32.1%, P = 0.17). Finally, cells were permeabilized to confirm the presence of mucin granules (Fig. 5C). Importantly, mucin granule size was not significatively affected by FUT8 levels (Fig. 5F), although there was a slight reduction in the number of granules in FUT8 KD compared to FUT8 OV cells and control cells (7.5 granules per cell compared to 12.5 and 13.1 granules per cell, respectively, n.s.), which is a reflection of enhanced mucin secretion (more mucin release and therefore a corresponding reduction in the number of intracellular granules). These data lend support to our suggestion that the secreted mucins in FUT8 OV cells are more permeable and also resistant to shear stress, whereas mucins secreted by FUT8 KD cells are more compact (less permeable) and easily removed by washing.
Fig. 5.
FUT8 affects the removal resistance of the mucin fibers. (A and B) Control, FUT8 KD, and FUT8 OV cells were differentiated for 6 d and seeded in glass-bottom dishes. After stimulation using 100 µM ATP, cells were processed for immunofluorescence (A) before (NO PERM/NO WASH) or (B) after extensive washing with isotonic solution (NO PERM/WASH). Cells were stained with anti-MUC5AC (green), phalloidin (red), and DAPI (blue). In the orthogonal view, dotted lines across the images demarcate the top surface of the cell. (Scale bars, to 10 µm.) (C) Control, FUT8 KD, and FUT8 OV cells were permeabilized after extensive washing (PERM/WASH) to reveal intracellular MUC5AC granules. (D) Quantification of MUC5AC fibers compactness (ratio between area and perimeter2) in control (black), FUT8 KD (red), and FUT8 OV (green) cells before washing and without permeabilization (NO PERM/NO WASH). Data were calculated from immunofluorescence images using Fiji software. (E) Volume of control (black), FUT8 KD (red), and FUT8 OV (green) MUC5AC objects was calculated from individual immunofluorescence stacks of cells before washing and without permeabilization (NO PERM/NO WASH) using the 3D analysis Fiji software. The y axis represents the volume of the granules in cubic micrometers. (F) Volume of MUC5AC granules from permeabilized control (black), FUT8 KD (red), and FUT8 OV (green) cells was calculated from individual i immunofluorescent images using 3D analysis Fiji software. The y axis represents the volume of the granules in cubic micrometers. Abbreviations: NO PERM/NO WASH: Not permeabilized cells without washing, NO PERM/WASH: Not permeabilized cells with extensive washing, PERM/WASH: Permeabilized cells after extensive washing. *P < 0.05, **P < 0.01.
To test whether FUT8 levels affect resistance of mucins to extensive washing, we measured the levels of MUC5AC recovered from the extracellular space after three successive washes with isotonic solution (W0, W1, and W2). As shown above, FUT8 depletion leads to higher ATP-dependent MUC5AC secretion, whereas FUT8 overexpression increases both baseline and stimulated MUC5AC secretion (Fig. 4). To compare the percentage of MUC5AC recovered with each wash, MUC5AC levels were normalized to the total amount of mucin recovered after three washes. Our results revealed no major differences between control and FUT8 KD cells in the percentage of MUC5AC recovered in the successive washes. However, in cells overexpressing FUT8, a higher percentage of MUC5AC was detected in the first wash after ATP stimulation (75% in FUT8 OV vs. 61% in control cells, P < 0.05) than from control and FUT8 KD cells. A similar tendency was observed in the first wash for baseline MUC5AC secretion (73% vs. 63%, P = 0.05). Consistently, a lower percentage of MUC5AC was detected in the following wash (W1) in cells overexpressing FUT8 compared to control cells (baseline: 11% vs. 22%, P < 0.05; stimulated: 14% vs. 27%, P < 0.05) (SI Appendix, Fig. S3 C and D and Table S3). We also studied the effect of FUT8 on MUC2 resistance to washing (SI Appendix, Fig. S4). Similar to MUC5AC, our results showed a higher percentage of MUC2 in the first wash after ATP stimulation in FUT8 OV cells compared to control cells (79% vs. 40%, P < 0.01), and consistently a lower percentage in the following wash (W1) (16% vs. 37%, P = 0.06) (SI Appendix, Fig. S4A). A similar tendency, although not statistically significant, was also observed for baseline secretion when comparing FUT8 overexpressing cells and FUT8 KD cells (W1: 21% vs. 36%, P = 0.22) (SI Appendix, Fig. S4B and Table S3).
Altogether, our data show that FUT8-depleted cells secrete more mucins, which form compacted fiber and are easily removed by washing from the cell surface. Whereas, FUT8 OV cells secrete less compacted mucins that are more resistant to removal by washing.
Discussion
Strict control of mucin secretion is essential for the formation and function of a mucus barrier that would protect the colonic epithelium from infection and inflammation (6). Alterations in the quantities of mucins secreted or composition due to aberrant mucin synthesis, trafficking, or secretion likely affects the rheological properties of the mucus facilitating bacteria–epithelium contact leading to inflammatory intestinal pathologies, such as UC (32). UC is confined to the distal part of the colon and the rectum, characterized by mucosal inflammation (33). Interestingly, empty mucin-producing goblet cells is a morphological characteristic used to assess active UC, which likely reflects defects in mucin synthesis or enhanced mucin secretion (15). The impaired mucus layer is also a feature of the inflamed colon in UC patients (34).
FUT8 is the only enzyme responsible for α1-6–linked fucosylation. Interestingly, increased levels of FUT8 are associated with intestinal diseases, like UC and, as shown in Fut8−/− mice, low levels of FUT8 protect against severe colitis. Indeed, Fujii et al. (21) have shown that core fucosylation by FUT8 is required for T cell signaling and production of inflammatory cytokines, which could explain a link between FUT8 and UC progression. In the present study, we have shown that FUT8 affects trafficking of transmembrane mucin MUC1 and secretion of specific gel-forming mucins (MUC2 and MUC5AC), thereby modulating the viscoelastic properties of the mucus layer.
Expression of FUT8 Is Altered in the Inflamed Colon of UC Patients.
Our data show that FUT8 levels are higher at the proximal part of the colon, where the mucus layer is thinner and more permeable to allow contact between microbiota and the epithelium (35). In contrast, FUT8 levels are low at the distal colon of healthy subjects, which has a mucus barrier composed of two differentiated layers: an outer enriched in pathogens and microbiota, and an inner free of bacteria. Interestingly, as shown here, this compartmentation is lost in UC patients. Notably, FUT8 levels are low in the distal colon of the healthy subjects, but are elevated in the distal colon of UC patients. This likely explains why this region of the colon is more affected in UC. Accordingly, major differences in the mucus layer between mice depleted of FUT8 (Fut8−/−) and WT mice (Fut8+/+) are found at the proximal colon; and the ratio PAS/PAS-AB, which is altered in Fut8−/− mice, changes in accordance with FUT8 expression along the colon (higher level at proximal colon, lower at distal colon).
Our findings reveal that the mucus layer contains fucosylated proteins and, importantly, FUT8-fucosylated proteins. There is a reduction in the population of fucosylated proteins and almost a complete absence of FUT8 fucosylated proteins in Fut8−/− mice. Loss of FUT8-dependent fucosylation likely affects subsequent building of carbohydrate structures, which leads to defects in their export from the Golgi complex and in the overall viscoelastic properties. In addition to mucins, FUT8 might also be involved in fucosylation of other proteins at the Golgi that are necessary to produce a normal mucus in the extracellular space.
We postulate that FUT8-dependent fucosylation alters the viscoelastic properties of the mucus layer, which likely enhances the contact between microbiota and epithelia. In UC patients, FUT8 is up-regulated in distal colon, which likely increases interaction between microbiota and the epithelium, leading to more inflammation. In Fut8−/− mice lacking fucosylated mucins, bacteria fail to attach and there is less invasion and inflammation, which prevents the development of UC.
FUT8 Regulates MUC1 Trafficking.
Our results show that FUT8 has a role in trafficking of MUC1 to the PM. Overexpression of FUT8 promotes targeting of MUC1 to the PM, whereas depletion prevents this increased trafficking leading to intracellular retention. Importantly, MUC1 localization at the PM is highly dependent on its recycling and it has been shown that FUT8 fucosylation stabilizes MUC1 posttranslationally (36, 37). Thus, a possible explanation is that in FUT8 OV cells, MUC1 presence at the PM is increased due to enhanced stability. In addition, MUC1 is reported to regulate inflammatory responses to infection and serve as adhesion receptor for enteroaggregative Escherichia coli and Helicobater pylori (38–40). It is plausible that increased levels of FUT8 (as seen in UC patients) lead to more MUC1 at the PM, which act as a receptor for bacteria allowing epithelial invasion and inflammation. In contrast, low levels of FUT8 cause intracellular MUC1 retention and likely explain the protection against UC observed in Fut8−/− mice.
FUT8 Modulates Secretion of Gel-Forming Mucins MUC2 and MUC5AC.
FUT8 deletion increases baseline secretion of MUC2 and stimulated secretion of MUC5AC. On the other hand, FUT8 OV enhances both MUC2 and MUC5AC baseline and stimulated secretion. These effects of FUT8 are not because of alterations in MUC2/MUC5AC synthesis. Interestingly, it has been shown that the organization or the structure of secreted MUC5AC is different from secreted MUC2 (1, 41). Changes in the ratio of secreted MUC2/MUC5AC seen in FUT8 KD and FUT8 OV cells would therefore affect the properties of the mucus layer. It is also known that not all mucins are glycosylated to the same extent. For example, some mucins are mostly sialylated, whereas others, like MUC5AC, are mainly fucosylated (42). Fucosylation of O-glycans by FUT2 is reported to produce a mucus that is more resistant to shear stress in the airways (43). Our results show that changes in FUT8, which fucosylates N-glycans, alter the biophysical properties of mucins. We suggest these changes contribute to increased mucin levels observed in both FUT8 KD and OV cells. Decreased fucosylation leads to less adhesive mucins which, as shown here, are easily removed by washing. The shear stress produced as a result would promote calcium entry into cells and therefore increase mucin secretion. This is confirmed by our data: FUT8-depleted cells secrete more mucins that are more compacted and easily removed. This decrease in the resistance to shear stress observed in cells with low levels of FUT8 also explains why Fut8−/− mice present a thinner mucus layer at the proximal colon. On the other hand, cells with high levels of FUT8 secrete stickier mucins that are more resistant to removal, which likely reflect alterations in their viscoelastic properties and resistance to shear stress.
A Model for the Role of FUT8 on UC Pathogenesis.
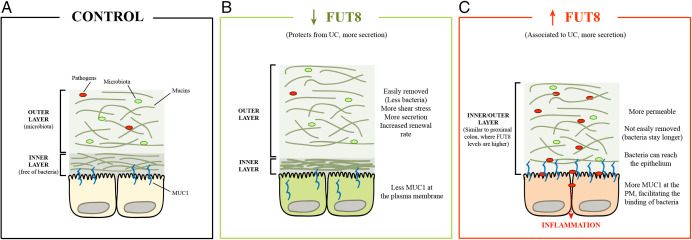
Altogether, we propose the following scheme for the role of FUT8 in the pathogenesis of UC (Fig. 6).
Fig. 6.
Model for FUT8 role on UC pathogenesis. (A) In control cells, both mucus layers are separated in the distal colon, and the pathogens and the microbiota restricted to the outer layer. (B) In cells with low levels of FUT8, mucins are less resistant to removal, which makes the outer layer, together with bacteria, easily removed. In turn, this triggers more secretion, thus increasing the renewal rate and protecting against invasion from pathogens. (C) High levels of FUT8, as observed in UC patients, disrupt the mucus layer, making it more permeable to pathogens. In addition, these mucin fibers are more resistant to shear stress, so the bacteria can stay longer and reach the epithelium triggering inflammation.
First, in healthy conditions, the colonic mucus barrier, especially at the distal colon, is composed of two layers: 1) an outer layer where pathogens and microbiota are trapped, and 2) an inner layer free of bacteria (Fig. 6A, control).
Second, cells with low levels of FUT8, which protects against UC, show increased secretion of mucins lacking fucosylation and reduction of MUC1 at the PM (Fig. 6B, low FUT8 levels). This lack of fucosylated gel-forming mucins (i.e., MUC2/MUC5AC) produces a more stacked inner layer (higher compactness), but an easily removable outer layer, consequently reducing the quantity of bacteria and microbiota and preventing them from reaching the epithelium. This, in turn, increases the shear stress that promotes more calcium influx (for example, by stimulation of mechano-sensitive receptors, like PIEZO or TRP channels) to trigger more mucin secretion and increased renewal rate. In addition, lack of fucosylation reduces the stability of MUC1 at the PM (which some bacteria can bind), protecting the epithelium against bacterial invasion and, therefore, inflammation.
Third, high FUT8 levels, which are associated to UC, promote hypersecretion of mucins to compose a mucus layer that is more permeable but stickier and therefore difficult to remove. This traps bacteria, which invade and reach the epithelium (Fig. 6 C, high FUT8 levels). We suggest that this mucus barrier will be a mixed inner/outer layer, similar to the situation at the proximal colon (35), where FUT8 levels are higher than the distal colon and contacts between microbiota and epithelium occur. Finally, high levels of FUT8 also increase the levels of MUC1 at the PM, facilitating the binding of bacteria, invasion of the intestinal epithelium, and inflammation that could lead to UC pathogenesis.
In sum, FUT8 controls mucin’s viscoelasticity properties. Importantly, FUT8 is overexpressed in UC patients. We have found that overexpression of FUT8 in differentiated colonic HT29-18N2 cells produces thicker mucus that is more resistant to removal by washing. This thicker and stickier mucus could increase bacterial and toxin adherence and penetrance, which likely explains increased inflammatory response in UC patients. Accordingly, in FUT8-depleted cells and Fut8−/− mice, the mucus is thinner and less adherent to the cell surface. This thinner mucus is more easily sloughed off. Under these conditions, foreign particles are easily removed, therefore protecting Fut8−/− mice from UC.
Materials and Methods
RNA Public Datasets Analysis.
The final dataset consisted of 40 individuals (24 UC patients, 6 healthy controls, and 10 symptomatic controls without a IBD diagnosis), from whom up to two biopsies (usually one from an inflamed and one from a noninflamed location) were available. Locations were categorized into P: proximal -ascending- colon; Ds: descending colon; and Sg: sigmoid colon. Raw counts were normalized using TMM, which takes library size and RNA composition into account. Limma-voom was used to run the differential expression analysis. The statistical models were corrected for sex, age, and origin (center). There was no comparison in which two samples from the same individual were included; 19.7 k genes showed robust expression across the biopsies. Fourteen genes encoding different mucins and FUT8 were selected for the present study. Nominal P values were therefore corrected for multiple testing lowering the significance threshold to 0.05/14 = 0.004. This information is part of the IBD character consortium. Data for each gene are included in SI Appendix.
Reagents and Antibodies.
All chemicals were obtained from Sigma-Aldrich, except anti-MUC2 antibody clone 996/1 (RRID:AB_297837) (Abcam), anti-MUC5AC antibody clone 45M1 (RRID: AB_934745) (Neomarkers), and anti-FUT8 antibody (RRID:AB_1523644) (Abcam). Secondary antibodies for immunofluorescence microscopy and dot blotting were from Life Technologies (ThermoFisher Scientific).
Cell Lines.
HT29-18N2 cells (obtained from ATCC) (RRID:CVCL_5942) were tested for mycoplasma contamination with the Lookout mycoplasma PCR detection kit (Sigma-Aldrich). Mycoplasma-negative HT29-18N2 cells were used for the experiments presented here.
Generation of Stable Cell Lines (shRNA and Overexpression).
HEK293T cells (ATCC, negative for mycoplasma) were cotransfected with the plasmid, VSV-G, pPRE (packaging) and REV by Ca2+ phosphate to produce lentiviruses. Forty-eight hours posttransfection the secreted lentivirus was collected, filtered, and directly added to HT29-18N2 cells. Stably infected HT29-18N2 cells with the different constructs were sorted for GFP signal by FACS.
Quantitative Real-Time PCR.
Differentiated HT29-18N2 control, FUT8 KD and FUT8 OV cells were lysed and total RNA extracted with the RNeasy extraction kit (Qiagen). cDNA was synthesized with SuperScript III (Invitrogen). Primers for each gene (Table 1) were designed using Primer-BLAST (National Center for Biotechnology Information) (44), limiting the target size to 150 bp and the annealing temperature to 60 °C. To determine expression levels of the different genes, quantitative real-time PCR was performed with Light Cycler 480 SYBR Green I Master (Roche) according to the manufacturer’s instructions.
Table 1.
Primer sequences used for detecting mRNA for the respective genes
| Gene | Forward primer (5′ - 3′) | Reverse primer (5′ - 3′) |
|---|---|---|
| FUT8 | AGACCAGAAATGGTCTGGGGAA | CCAATCACCTGCTCCATCTGTC |
| GAPDH | GAAGGTGAAGGTCGGAGTCAAC | CATCGCCCCACTTGATTTTGGA |
| HPRT1 | CCTGCTTCTCCTCAGCTTCAG | ACACCCTTTCCAAATCCTCAGC |
Differentiation of HT29-18N2 Cells.
HT29-18N2 cells were differentiated to goblet cells, as described previously (24, 29, 30). Briefly, cells were seeded in complete growth medium (DMEM complemented with 10% FCS and 1% P/S), and the following day (day zero: D-0), the cells were placed in PFHMII protein free medium (GIBCO, ThermoFisher Scientific). After 3 d (D-3), medium was replaced with fresh PFHMII medium and cells grown for 3 additional days. At day 6 (D-6) cells were trypsinized and seeded for the respective experiments in complete growth medium followed by incubation in PFHMII medium at day 7 (D-7). All experimental procedures were performed at day 9 (D-9).
Mucin Secretion Assay for HT29-18N2 Cells.
HT29-18N2 cells were differentiated for 6 d and then split into six-well plates. After 1 d (D-7), medium was exchanged with fresh PFHMII medium and cells grown for 2 more days. On D-9, cells were washed with isotonic solution containing: 140 mM NaCl, 2.5 mM KCl, 1.2 mM CaCl2, 0.5 mM MgCl2, 5 mM glucose, and 10 mM Hepes (305 mosmol/L, pH 7.4 adjusted with Tris), and then treated with vehicle (baseline secretion) or 100 µM ATP (stimulated secretion) for 30 min at 37 °C. After 30 min at 37 °C, extracellular medium was collected and centrifuged for 5 min at 800 × g at 4 °C. Cells were washed twice in PBS and lysed in 2% Triton X-100/PBS for 1 h. at 4 °C and centrifuged at 14,000 × g for 15 min. In order to test mucin resistance to removal by extensive washing, cells were repetitively washed with isotonic solution after secretion assay and extracellular medium collected.
Dot Blot and Western Blot Analysis.
Extracellular medium and cell lysates were spotted on nitrocellulose membranes (0.45 µm) using Bio-Dot Microfiltration Apparatus (Bio-Rad) (manufacturer’s protocol) and membranes were incubated in blocking solution (5% BSA/0.1% Tween/PBS) for 1 h at room temperature. The blocking solution was removed and the membranes were incubated with an anti-MUC5AC antibody diluted 1:2,000 or the anti-MUC2 antibody diluted 1:4,000 in blocking solution, overnight at 4 °C. Membranes were then washed in 0.1% Tween/PBS and incubated with a donkey anti-mouse or anti-rabbit HRP coupled antibody (Life Technologies) for 1 h at room temperature. Linearity and specificity of the MUC2 and MUC5AC antibodies on the secreted fraction was previously tested (8, 24). Membranes were washed and imaged with a LI-COR Odyssey scanner (resolution = 84 µm; LI-COR). Quantification of the dot blots was performed with ImageJ (Fiji, v2.0.0-rc-43/1.51g) (45). ATP-dependent mucin secretion (MUC2 or MUC5AC) is calculated as the difference between normalized baseline secretion and stimulated secretion for each condition as described previously (7). We distinguish between stimulated secretion (after treating cells with 100 µM ATP) and ATP-dependent secretion (stimulated minus baseline) because baseline secretion will keep occurring in these cells during the 30 min of incubation.
For the detection of β-actin and FUT8, cell lysates were separated on SDS/PAGE, transferred to nitrocellulose membranes, and processed as described for the dot blot analysis using the anti–β-actin (RRID:AB_476692), anti-FUT8 (RRID:AB_10608850) at a dilution of 1:5,000 and 1:1,000 in 5% BSA/0.1% Tween/PBS, respectively. Membranes were washed and imaged with a LI-COR Odyssey scanner (resolution = 84 µm; LI-COR). Quantification was performed with ImageJ (Fiji, v2.0.0-rc-43/1.51 g) (45). The number of experiments was greater than three for each condition, and each experiment was done in triplicates.
Confocal Microscopy.
Differentiated HT29-18N2 (Control, FUT8 KD, or FUT8 OV) cells were grown on 35-mm glass-bottom dishes.
To study the intracellular localization of MUC1, differentiated HT29-18N2 cells were washed with 1× PBS at room temperature and fixed with 3% PFA/PBS for 30 min at room temperature. Cells were then washed with PBS and permeabilized for 10 min with 0.2% Triton X-100 in 4% BSA/PBS. The anti-MUC1 antibody was then added to the cells at 1:1,000 in 4% BSA/PBS overnight at 4 °C. After 24 h, cells were washed with PBS and incubated for 1 h at room temperature with a donkey anti-mouse Alexa Fluor 647 (for MUC1) (Life Technologies), diluted at 1:1,000 in 4% BSA/PBS, phalloidin (1:5,000), and DAPI (1:20,000). Images were acquired using an inverted Leica SP8 confocal microscope with a 63× Plan Apo NA 1.4 objective and intracellular localization of MUC1 was assessed using ImageJ (Fiji, v2.0.0-rc-43/1.51 g) (45). For detection of the respective fluorescence emission, the following laser lines were applied: DAPI, 405 nm; phalloidin, 561 nm; and Alexa Fluor 647, 647 nm.
For the study of MUC5AC fibers, the supernatant of differentiated HT29-18N2 cells (control, FUT8 KD, and FUT8 OV) was removed and then cells were divided into three groups. The first group (unwashed and unpermeabilized) of cells was fixed with 3% PFA/PBS for 30 min at room temperature. The second group (washed but not permeabilized) of cells was washed extensively with PBS 1× and then fixed with 3% PFA/PBS for 30 min at room temperature. Cells of the last group (washed and permeabilized) were washed twice with PBS 1×, fixed 3% PFA/PBS for 30 min at room temperature and then permeabilized for 10 min with 0.2% Triton X-100 in 4% BSA/PBS. The anti-MUC5AC antibody was added to cells of all three groups at 1:5,000 in 4% BSA/PBS overnight at 4 °C. After 24 h, cells were washed with PBS and incubated for 1 h at room temperature with a donkey anti-mouse Alexa Fluor 647 (for MUC5AC) (Life Technologies), diluted at 1:1,000 in 4% BSA/PBS, phalloidin (1:5,000), and DAPI (1:20,000). Images were acquired using an inverted Leica SP8 confocal microscope with a 63× Plan Apo NA 1.4 objective and analyzed using ImageJ (Fiji, v2.0.0-rc-43/1.51 g) (45). For detection of the respective fluorescence emission, the following laser lines were applied: DAPI, 405 nm; Phalloidin, 561 nm; and Alexa Fluor 647, 647 nm.
Analysis of MUC5AC Particles’ Compactness and Volume.
To determine the number and volume of MUC5AC+ elements, we used the three-dimensional (3D) objects counter v2.0 tool from Fiji (46). All images analyzed were taken on the same day under the same conditions and the same z-step (0.29 µm). The parameters used are as follows: 1) size filter between 100 and 37,748,736 voxels; 2) threshold was automatically set for each image. DAPI signal was used to count the number of nuclei per field. In order to evaluate the number of MUC5AC fibers, elements with a volume >30 µm3 were scored as follows: 30 to 60 µm3, 1; 60 to 90 µm3, 2; 90 to 120 µm3, 3; 120 to 150 µm3, 4; 150 to 180 µm3, 5; 180 to 210 µm3, 6; 210 to 240 µm3, 7; 240 to 270 µm3, 8; 270 to 300 µm3, 9; 300 to 330 µm3, 10; >330 µm3, 11. The sum of these scores was compared to the total number of fibers for each condition.
The area/perimeter2 ratio was used as a measure of the compactness of the MUC5AC fibers (47). Images were analyzed using the “Analyze Particles” plugins from Fiji (46). All images were taken under the same conditions and the same z-step (0.29 µm). Only particles with a perimeter higher than 40 µm were analyzed. The parameters used are as follows: 1) threshold was automatically set for each image; 2) size filter was between 0.1 and infinity µm2. Total area of cells was used to normalize the area covered by MUC5AC fibers.
Fut8±/±, Fut8±/−, Fut8−/− Mice.
Fut8+/− and Fut8−/− mice were generated on the ICR plus JF1 strain (21), and Fut8+/+ (WT-ICR plus JF1) mice were used as a control for this study (both sets of animals were obtained from N.T.’s and J.G.’s laboratories). In order to evaluate the mucus layer under basal situation on healthy mice, 3-mo-old mice were used (three females and one male) per condition. Specifically, four animals per genotype (Fut8+/+, Fut8+/−, and Fut8−/− mice) were evaluated. The Institutional Animal Care and Use Committee of Tohoku Medical and Pharmaceutical University (Japan) approved the experiments.
Histological Study.
Mice were killed by CO2 inhalation and necropsy was performed to obtain the colon. Each intestinal segment was maintained with the fecal pellets (when present) for its fixation with Carnoy’s fixative (as described in ref. 48) to better preserve the mucin layer. All samples were fixed overnight by incubation at 4 °C and cut in 4-μm sections.
H&E, PAS, PAS-AB, PhoSL, LCA Staining.
Colon mice sections were stained with H&E, PAS, PAS-AB, PhoSL, or LCA for histological analysis. PAS was used to stain neutral, acid-simple nonsulfated and acid-complex sulfated mucins (mucins are stained in purple/magenta). For the PAS-AB staining, it first stains the acidic mucins with Alcian blue; those remaining acidic mucins that are also PAS+ will be chemically blocked and will not react further. Those neutral mucins that are solely PAS+ will subsequently be demonstrated in a contrasting manner. Where mixtures occur, the resultant color will depend upon the dominant moiety. For PAS-AB staining, acidic mucins are stained in blue, neutral mucins in magenta and mixtures in blue/purple. LCA was used to stain fucosylated proteins, while PhoSL was used to specifically stain FUT8-dependent fucosylation.
H&E-stained sections were evaluated by light microscopy using a semiquantitative analysis assessing the following parameters: epithelial hyperplasia (0, normal; 1, minimal; 2, mild; 3, moderate; 4, marked), inflammatory infiltrate in the lamina propria (0, normal; 1, minimal; 2, mild; 3, moderate; 4, marked), edema of the lamina propria (0, normal; 1, minimal; 2, mild; 3, moderate; 4, marked). The total histological score was obtained summing the three parameters (minimum score, 0; maximum score, 12). In an attempt to assess the epithelial regeneration, the mitotic figures in 20 well-orientated and full-length intestinal crypts were counted. This process was repeated three times and an average was calculated. Other features—such as erosion, ulceration, irregular crypts, and crypt loss—were assessed, but none of the samples showed any of these changes. The histological study was performed in a blinded fashion.
Full images of PAS and PAS-AB-stained sections were acquired by a NanoZoomer-2.0 HT C9600 scanner (Hamamatsu) at 20× magnification, in which one pixel corresponds to 0.46 µm.
Measurement of the Mucus Layer Thickness.
The thickness of mucus layer was measured in medium and distal colonic tissue sections using the ruler tool of the NDP view + 2.50.19 software (Hamamatsu) in both PAS- and PAS-AB–stained sections. Twenty different measures were performed in two different tissue sections per stain in the proximal, medium, and distal colon of each mouse. Mucus layer thickness has been evaluated in all the samples that presented fecal pellets.
Statistical Analysis.
Continuous data are presented as means ± SEM. In all cases a D’Agostino–Pearson omnibus normality test was performed before any hypothesis contrast test. For data that followed normal distributions, we applied either Student’s t test (when comparing two groups) or one-way ANOVA (comparison of more than two groups) followed by Tukey’s post hoc test. For data that did not fit a normal distribution, we used Mann–Whitney’s unpaired t test (two groups comparison) and nonparametric ANOVA (Kruskal–Wallis, more than two groups) followed by Dunn’s post hoc test. Binary and categorical variables were analyzed by Fisher’s exact test. Statistical analysis and graphics were performed using GraphPad Prism 9 (RRID:SCR_002798) software. Criteria for a significant statistical difference were: *P < 0.05, **P < 0.01, ***P < 0.001.
Supplementary Material
Acknowledgments
We thank all members of the V.M. laboratory for valuable discussions; and Ms. Noriko Kanto and Ms. Miyuki Kusakawa at the Department of Glyco-Oncology and Medical Biochemistry at Osaka International Cancer Institute for their technical support. Cell-sorting experiments were carried out by the joint Centre for Genomic Regulation (CRG)/UPF FACS Unit at Parc de Recerca Biomèdica de Barcelona. Fluorescence microscopy was performed at the Advanced Light Microscopy Unit at the CRG, Barcelona. V.M. is an Institució Catalana de Recerca i Estudis Avançats professor at the Centre for Genomic Regulation. Histological analysis was performed by Dr. Aguilera and Dr. Prats at the Histology Facility of the Institute for Research in Biomedicine Barcelona. This work was funded by Spanish Ministry of Economy and Competitiveness Grant BFU2013-44188-P (to V.M.); European Molecular Biology Organization (EMBO) short-term fellowships EMBO REF8926 (to G.C.-R.); and FEDER (Euoropean Regional Development Fund) Funds. We acknowledge support of the Spanish Ministry of Economy and Competitiveness, through the Programmes “Centro de Excelencia Severo Ochoa 2013-2017” (SEV-2012-0208 and SEV-2013-0347) and Maria de Maeztu Units of Excellence in R&D (MDM-2015- 0502). This work reflects only the authors’ views, and the European Union Community is not liable for any use that may be made of the information contained therein.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. S.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2205277119/-/DCSupplemental.
Data, Materials, and Software Availability
All study data are included in the main text and supporting information.
References
- 1.Johansson M. E. V., Larsson J. M. H., Hansson G. C., The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc. Natl. Acad. Sci. U.S.A. 108 (suppl. 1), 4659–4665 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magalhães A., et al. , Muc5ac gastric mucin glycosylation is shaped by FUT2 activity and functionally impacts Helicobacter pylori binding. Sci. Rep. 6, 25575 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton D. J., Rousseau K., McGuckin M. A., Structure and function of the polymeric mucins in airways mucus. Annu. Rev. Physiol. 70, 459–486 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Perez-Vilar J., Mucin granule intraluminal organization. Am. J. Respir. Cell Mol. Biol. 36, 183–190 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adler K. B., Tuvim M. J., Dickey B. F., Regulated mucin secretion from airway epithelial cells. Front. Endocrinol. (Lausanne) 4, 129 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson G. C., Mucins and the microbiome. Annu. Rev. Biochem. 89, 769–793 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantero-Recasens G., et al. , KChIP3 coupled to Ca2+ oscillations exerts a tonic brake on baseline mucin release in the colon. eLife 7, e39729 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantero-Recasens G., et al. , Sodium channel TRPM4 and sodium/calcium exchangers (NCX) cooperate in the control of Ca2+-induced mucin secretion from goblet cells. J. Biol. Chem. 294, 816–826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.KleinJan A., Stuck in the MUC: Infinity loop of fucosylation in allergic airway inflammation. J. Allergy Clin. Immunol. 144, 665–667 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Rose M. C., Voynow J. A., Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol. Rev. 86, 245–278 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Linden S. K., Sutton P., Karlsson N. G., Korolik V., McGuckin M. A., Mucins in the mucosal barrier to infection. Mucosal Immunol. 1, 183–197 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelaseyed T., et al. , The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 260, 8–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan G. G., The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Caruso R., Lo B. C., Núñez G., Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Immunol. 20, 411–426 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Johansson M. E. V., et al. , Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strugala V., Dettmar P. W., Pearson J. P., Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int. J. Clin. Pract. 62, 762–769 (2008). [DOI] [PubMed] [Google Scholar]
- 17.Verhelst X., et al. , Protein glycosylation as a diagnostic and prognostic marker of chronic inflammatory gastrointestinal and liver diseases. Gastroenterology 158, 95–110 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Larsson J. M. H., et al. , Altered O-glycosylation profile of MUC2 mucin occurs in active ulcerative colitis and is associated with increased inflammation. Inflamm. Bowel Dis. 17, 2299–2307 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Wenzel U. A., et al. , Spontaneous colitis in Muc2-deficient mice reflects clinical and cellular features of active ulcerative colitis. PLoS One 9, e100217 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarlos P., et al. , Genetic update on inflammatory factors in ulcerative colitis: Review of the current literature. World J. Gastrointest. Pathophysiol. 5, 304–321 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H., et al. , Core fucosylation on T cells, required for activation of T-cell receptor signaling and induction of colitis in mice, is increased in patients with inflammatory bowel disease. Gastroenterology 150, 1620–1632 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Lauc G., et al. , Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLoS Genet. 9, e1003225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang Q., Wang L.-X., Mammalian α-1,6-fucosyltransferase (FUT8) is the sole enzyme responsible for the N-acetylglucosaminyltransferase I-independent core fucosylation of high-mannose N-glycans. J. Biol. Chem. 291, 11064–11071 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitrovic S., et al. , TRPM5-mediated calcium uptake regulates mucin secretion from human colon goblet cells. eLife 2, e00658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tabula Muris Consortium; Overall coordination; Logistical coordination; Organ collection and processing; Library preparation and sequencing; Computational data analysis; Cell type annotation; Writing group; Supplemental text writing group; Principal investigators, Single-cell transcriptomics of 20 mouse organs creates a Tabula Muris. Nature 562, 367–372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loftus E. V. Jr., Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 126, 1504–1517 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Lazarev M., et al. , Relationship between proximal Crohn’s disease location and disease behavior and surgery: A cross-sectional study of the IBD Genetics Consortium. Am. J. Gastroenterol. 108, 106–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tateno H., Nakamura-Tsuruta S., Hirabayashi J., Comparative analysis of core-fucose-binding lectins from Lens culinaris and Pisum sativum using frontal affinity chromatography. Glycobiology 19, 527–536 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Yamasaki K., Yamasaki T., Tateno H., The trimeric solution structure and fucose-binding mechanism of the core fucosylation-specific lectin PhoSL. Sci. Rep. 8, 7740 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips T. E., Ramos R., Duncan S. L., HT29-18N2 differentiation in a protein-free medium. In Vitro Cell. Dev. Biol. Anim. 31, 421–423 (1995). [DOI] [PubMed] [Google Scholar]
- 31.Longman R. J., et al. , Alterations in the composition of the supramucosal defense barrier in relation to disease severity of ulcerative colitis. J. Histochem. Cytochem. 54, 1335–1348 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forgue-Lafitte M.-E., et al. , Abnormal expression of M1/MUC5AC mucin in distal colon of patients with diverticulitis, ulcerative colitis and cancer. Int. J. Cancer 121, 1543–1549 (2007). [DOI] [PubMed] [Google Scholar]
- 33.Danese S., Fiocchi C., Ulcerative colitis. N. Engl. J. Med. 365, 1713–1725 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Fang J., et al. , Slimy partners: The mucus barrier and gut microbiome in ulcerative colitis. Exp. Mol. Med. 53, 772–787 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamphuis J. B. J., Mercier-Bonin M., Eutamène H., Theodorou V., Mucus organisation is shaped by colonic content; a new view. Sci. Rep. 7, 8527 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S., et al. , NCOA3-mediated upregulation of mucin expression via transcriptional and post-translational changes during the development of pancreatic cancer. Oncogene 34, 4879–4889 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hattrup C. L., Gendler S. J., Structure and function of the cell surface (tethered) mucins. Annu. Rev. Physiol. 70, 431–457 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Dhar P., McAuley J., The role of the cell surface mucin MUC1 as a barrier to infection and regulator of inflammation. Front. Cell. Infect. Microbiol. 9, 117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boll E. J., et al. , Enteroaggregative Escherichia coli adherence fimbriae drive inflammatory cell recruitment via interactions with epithelial MUC1. MBio 8, e00717-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuckin M. A., et al. , Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210–1218 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Carpenter J., et al. , Assembly and organization of the N-terminal region of mucin MUC5AC: Indications for structural and functional distinction from MUC5B. Proc. Natl. Acad. Sci. U.S.A. 118, e2104490118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amini S.-E., Gouyer V., Portal C., Gottrand F., Desseyn J.-L., Muc5b is mainly expressed and sialylated in the nasal olfactory epithelium whereas Muc5ac is exclusively expressed and fucosylated in the nasal respiratory epithelium. Histochem. Cell Biol. 152, 167–174 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Raclawska D. S., et al. , Mucins and their sugars. Critical mediators of hyperreactivity and inflammation. Ann. Am. Thorac. Soc. 13 (suppl. 1), S98–S99 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye J., et al. , Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics 13, 134 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindelin J., et al. , Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolte S., Cordelières F. P., A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006). [DOI] [PubMed] [Google Scholar]
- 47.Bogaert J., Rousseau R., Van Hecke P., Impens I., Alternative area-perimeter ratios for measurement of 2D shape compactness of habitats. Appl. Math. Comput. 111, 71–85 (2000). [Google Scholar]
- 48.Johansson M. E. V., Hansson G. C., Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. Methods Mol. Biol. 842, 229–235 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the main text and supporting information.