Abstract
Patulin (PAT), a kind of mycotoxin, is a widely disseminated mycotoxin found in agricultural products. Although the existing research results show that PAT can cause nerve, immune, and skin toxicities, resulting in heart, liver, and kidney damages. However, evidence on the underlying mechanisms of PAT is still lacking. Present study aims to investigate the renal toxicity and related mechanisms of PAT on 293 T cells. Cell Counting Kit-8 method was used to reveal the dose-effect relationship and the time-effect relationship of PAT toxicity. Trypan blue staining and Hoechst 33342 staining were used to analyze PAT, which induced apoptosis on 293 T cells. Superoxide-dismutase (SOD), GSH, and malondialdehyde (MDA) were used to measure the changes of oxidative stress status of 293 T cells induced by PAT. The changes of reactive oxygen species (ROS) and ATP in mitochondria indicate the role of mitochondria when PAT induced cell damage and apoptosis. Through Cyt-C release assay analysis, caspase activity change, and correlation analysis, the potential mechanism of mitochondrial apoptosis pathway was proved. Results demonstrated that PAT significantly induced cell injury, and with the increase of time and concentration, the cell survival rate decreased significantly. Hoechst 33342 staining and Trypan blue staining showed that apoptosis rate was elevated by PAT. As PAT concentration increased, intracellular SOD, glutathion peroxidase activities were decreased and the MDA content was increased. The decrease of intracellular ATP level and accumulation of ROS content indicated an increased permeability of the mitochondrial membrane. Overexpression of Cyt-C activated the cascade reaction of caspase enzyme, leading to apoptosis. The results of enzyme activity assay and correlation analysis indicated that caspase 3 was the most critical caspase in the cascade system and that it was most correlated with caspase 8 and caspase 9.
Keywords: patulin, 293 T cells, oxidative stress, apoptosis, mitochondrial
1. Introduction
Patulin (C7H6O4, PAT), a common foodborne mycotoxin, is secreted by a variety of fungi, and it commonly grows on a variety of foods, like fruits and vegetables.1,2 People are exposed to PAT mainly through the consumption of contaminated food, and fruit can be contaminated at different stages, on-farm, during harvest, postharvest, during transport, during storage, during display, and throughout processing.3,4 The risk associated with mycotoxins increases in areas where food processing lacks hygiene.5 High levels of PAT can affect people of different races, genders, and age groups, with infants and young children, pregnant women, and fetuses being among the sensitive groups.6 Studies have shown that PAT can cause acute and chronic toxicity, with effects in humans, including convulsions, pulmonary, hepatic, and intestinal hemorrhages, nephrotoxicity or neurotoxicity, immunotoxicity, and teratogenicity, with signs of toxicity in animals being mainly neurological damage, including weight loss, gastrointestinal disorders, and hormonal disorders.7–9 PAT was found to cause meiosis arrest by affecting the mitochondrial structure and function of oocytes, interfering with spindle assembly, and changing chromosome morphology. In addition, previous studies have found that PAT can cause oxidative stress and changes in caspase activity through in vivo and in vitro experiments.10,11 PAT is also cytotoxic and genotoxic, which may be related to the toxicity of the hemiacetal and lactone rings.12
In addition, many reports indicate that the phenomenon of PAT exceeding in commodities is still relatively common, which always causes damage to people’s health. At present, the focus of PAT research is on detection technology and degradation technology,13,14 and related applications are relatively mature, but the toxicity mechanism of PAT is always insufficient. Therefore, in this study, human embryonic kidney cell 293 T cells were used as the experimental model to comprehensively evaluate the toxic effect of PAT from the aspects of appearance, physiology, biochemistry, and mitochondrial damage to explore the possible toxic mechanism and to provide experimental basis for the further exploration of its toxic effect on PAT.
2. Materials and methods
2.1. Materials and reagents
PAT (CAS:149-29-1, purity ≥ 99%) was purchased from Sigma-Aldrich (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, trypsin, phosphoric acid buffer, Cell Counting Kit-8 and cytotoxicity assay kit, Trypan blue cell survival assay kit, Hoechst 33342 staining solution for living cells, β-actin antibody, cytochrome C antibody (mouse monoclonal antibody), and IP were both bought from CWBIO (Jiangsu, China). Total superoxide-dismutase (SOD) assay kit, glutathion peroxidase (GSH-PX), malondialdehyde (MDA) assay kit, ATP assay kit, and reactive oxygen species (ROS) assay kit were obtained from Jiancheng Bioengineering Institute (Nanjing, China). Cell mitochondrial isolation kit, caspase-2, 3, 6, 8, 9 activity detection kits were obtained from Beyotime Biotechnology (Shanghai, China). Polyvinylidene fluoride (PVDF) membranes with 0.45-μm mean pore sizes were supplied from Millipore Company (St. Louis, United States). Acrylamide (CAS:79-06-1), bisacrylamide (MBA) (CAS:110-26-9), tris (CAS:77-86-1), glycine (CAS:56-40-6), sodium dodecyl sulfate (CAS:151-21-3), and ammonium persulfate solution were obtained from Solarbio (Beijing, China). The enhanced chemiluminescence reagent was also obtained from Solarbio (Beijing, China). All of the above chemicals were of standard analytical grade or higher.
2.2. Cell culture and treatment
The 293 T human embryonic kidney cells were obtained from CWBIO (Jiangsu, China) and were cultured in DMEM medium supplemented with 10% (v/v) FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C in humidified surroundings with 5% CO2. The cells were digested with 2.5 g/L trypsin and passaged every 2-3 days. Follow-up tests were conducted when the cells grew to logarithmic phase. When consistent cell growth was evident, experiments were conducted. PAT was dissolved in a serum-free medium to prepare a stock solution of 150 μM. The 293 T cells were used assay before exposure to PAT for 24 h.
2.3. Cell Counting Kit-8
The cytotoxicity of PAT on 293 T cells was determined by CCK-8. The 293 T cells were seeded in a 96-well plates and cultured to fusion state. PAT (0, 2.5, 5, 7.5, 10, and 15 μM) with different final concentrations were added and were incubated in an incubator with 5% CO2 at 37 °C for 8 h. Respectively, 293 T cells with PAT concentration of 7.5 μM were cultured for 8, 16, 24, 36 and 48 h. Then, 10 μL of CCK-8 solution was added to each well and incubated for 0.5 h. The absorbance value at 450 nm was measured with a microplate reader. Each group had 5 multiple holes, and the experiment was performed 3 times. Cell survival was calculated via the following formula:
Survival rate = Optical density (OD) treatment group/OD control group × 100%.
2.4. Trypan blue staining
The 293 T cells were digested with Trypan, the collected cells were centrifuged at 1,000~2,000 r/min for 5 min, the supernatant was discarded, and the cells were resuspended with appropriate cell suspension. The 100 μL of suspended cells were absorbed into a plastic centrifuge tube, 100 μL of Trypan blue staining solution (2X) was added, mixed gently, and stained for 3–10 min. A small number of stained cells were extracted and counted with a blood cell counting plate. The blue cells and the total number of cells were counted.
2.5. Hoechst 33342 staining
Hoechst 33342 is a solution suitable for the nuclear staining of living cells. The 293 T cells were inoculated into a 6-well plate. Hoechst 33342 staining solution was added in the ratio of dye solution: culture solution =1:100 and was incubated at 37 °C for 10 min. The culture solution containing dye was sucked and was washed with a culture solution or PBS for 2–3 times before observation under a fluorescence microscope. When apoptosis occurs, the nuclei of apoptotic cells are densely stained or fragmented.
2.6. Measurements of SOD, MDA, and GSH-Px
Prepare the required solutions according to the instructions. The 96-well plate was used, and 4 groups were set up in the experiment, with 5 multiple holes in each group. The wells were gently shaken and mixed, incubated at 37 °C for 20 min, and read at 450 nm by the enzyme marker. The SOD activity in the cellular proteins in the tissue weight can be calculated from the colorimetric results.
Determination of the GSH-Px activity can be used as a biochemical index to measure selenium levels in the body. Prepare solution according to instructions. The 96-well plate was used, and 3 groups of 5 multiple holes were set up in each group. The well plate was gently shaken, the solution was mixed, and after standing for 15 min, the enzyme reader was read at 412 nm, and the activity of GSH-Px was calculated by colorimetric results.
The solution was prepared according to instructions. Centrifugal tubes were used and 3 groups of 5 replicates were set up in each group. After mixing, it was heated at 100 °C or in a boiling water bath for 15 min. It was cooled in the water bath to room temperature, 200 μL of supernatant was added to the 96-well plate, and then the absorbance was measured at 532 nm with a microplate reader. MDA content was calculated by colorimetric analysis.
2.7. Measurement of intracellular ROS
ROS production was evaluated using the ROS assay kit. After incubation at 37 °C for 20 min, the cells were washed 3 times with the culture medium to remove any DCFH-DA that had not entered the cells, and the ROS control was usually stimulated for 20–30 min to significantly increase the level of ROS. All the samples were assayed in triplicate.
2.8. Enzyme activity detection of caspase cascade system
The caspase activity assay relies on the fact that caspases catalyze different substrates and all produce yellow pNA, which has a strong absorption near 405 nm. Caspase family enzyme activity was measured in 293 T cells to investigate the relationship between PAT toxicity and caspase family enzyme activity.
2.9. Western blot for cytochrome C
The walled 293 T cells were washed with PBS, digested with trypsin, and centrifuged at 1,000~2,000 r/min for 5~10 min to collect the cells. The cells were gently resuspended in precooled PBS and centrifuged at 200 g for 5 min at 4 °C to precipitate the cells. The supernatant was discarded. Then 1–2.5 mL of the Mitochondrial Isolation Reagent was added to the cells and the cells were gently suspended in an ice bath for 10–15 min. It was homogenized manually for about 10–30 times. The supernatant was transferred to another tube and centrifuged at 10,000 r/min for 10 min at 4 °C. The supernatant was removed. The precipitate was the mitochondria of the isolated cells.
Then, 30 μL of mitochondrial solution was mixed with 5× protein loading buffer (6.5 μL), boiled for 5 min at 100 °C, centrifuged, and use as a loading buffer. Then, 30 μL of mitochondrial solution was mixed with 6.5 μL of 5-fold protein loading buffer, boiled for 5 min at 100 °C, centrifuged, and use for loading 30 μL per well. The Marker sample volume was 5 μl. 80v for 30 min, then change to 120 V for 2 h at the boundary of the two gels.
Gels were immersed in a transfer buffer for 15–30 min. Filter paper was immersed in the transfer buffer for 30 s. PVDF membranes were placed in the transfer buffer for 5 min. Then, the transfer buffer was added when loading the bath. Wet transfer operations were performed on an ice box at 100 V for 1.5 h. At the end of the transfer, the film and gel were removed and the gel was discarded.
The PVDF membrane was immersed in the blocking solution overnight at 4 °C and the primary antibody was incubated overnight at 4 °C. The membrane was washed 3 times in the TBST buffer for 6 min each time and the secondary antibody was added. PVDF membranes were incubated for 2 h at room temperature and were washed 3 times for 6 min each in TBST. PVDF membranes were placed in the color development solution under light-proof conditions until the bands were clear. The ratio of the target band to the internal reference band was used as the result, and the ratio of each PAT group to the blank control indicated the protein level. For comparability of the results, the value of the blank group was set to l.
3. Results
3.1. Time versus dose-effects of apoptosis by PAT in 293 T cells
CCK-8 revealed the time and dose effects of PAT on 293 T cells, and the higher the concentration of PAT, the lower was the cell survival rate (Fig. 1A). This phenomenon is consistent with the results of Trypan blue staining (Fig. 1C). The inhibitory effect of PAT on 293 T cell proliferation was more obvious within 24 h and was weakened after 24 h (Fig. 1B), which may be due to the self-repair effect of the cell to increase its survival rate.
Fig. 1.
A) Effects of different concentrations of PAT on the proliferation of 293 T cells (8 h). B) Correlation between PAT treatment time and 293 T cell survival rate. C) Detection of cell death by Trypan blue staining (control left, 7.5 μM right).
3.2. Induction of apoptosis by PAT in 293 T cells
Apoptotic cells were detected by Hoechst 33342 staining. The cell nuclei were labeled with Hoechst 33342 (blue). The cells of the control group showed a clear outline with rounded nuclei and visible nucleoli. At a PAT concentration of 2.5 μM, the nuclei showed a denser and denser staining, indicating that the low concentration of PAT caused some degree of apoptosis in 293 T cells. When the concentration of PAT was increased to 10 μM, the proportion of cells with dense staining gradually increased, and at 15 μM, the majority of cells with dense staining and blurred cell outline were found. These phenomena indicated that PAT had a significant effect on cell survival (Fig. 2).
Fig. 2.

Toxicity test of PAT in 293 T cells by Hoechst 33342 (×200).
3.3. Damage to the redox system in 293 T cells by PAT
The MDA content, GSH-Px content, and SOD enzyme activity were determined to assess the oxidative stress level after PAT treated in the 293 T cells. PAT treatment significantly decreased the SOD activity (Fig. 3A) and GSH-Px levels (Fig. 3B) and evidently increased the MDA content (Fig. 3C). These data suggested that PAT could reduce the antioxidant enzyme activity and enhance the oxidative damage in 293 T cells.
Fig. 3.
A) Different concentrations of PAT causes SOD levels changes in 293 T cells. B) Different concentrations of PAT causes GSH-Px levels changes in 293 T cells. C) Different concentrations of PAT causes MDA levels changes in 293 T cells.
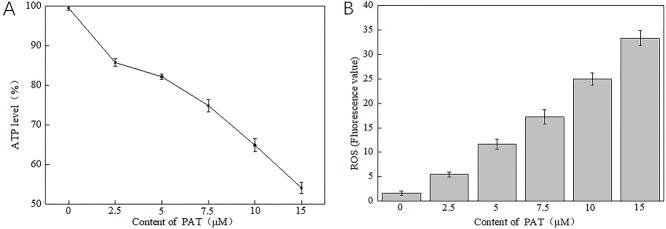
3.4. Effects of PAT on ATP and ROS in 293 T cells
When PAT concentration increased, the ATP level decreased significantly (Fig. 4A), and the ROS content increased exponentially (Fig. 4B). It was proved that high concentration of PAT increased mitochondrial damage, increased mitochondrial membrane permeability, impaired mitochondrial function, and greatly interfered with the respiratory chain.
Fig. 4.
A) Different concentrations of PAT causes ATP levels changes in 293 T cells. B) Different concentrations of PAT causes ROS levels changes in 293 T cells.
3.5. Effects of PAT on caspase cascade in 293 T cells
The mechanism of apoptosis caused by PAT is the mutual response of the proteases in the caspase family. By measuring the activity of caspase 2, 3, 6, 8, and 9, the change trend of its cascade system can be known. The greater the degree of apoptosis, the greater is the activity of caspase family, proving that the regulation of caspase on apoptosis is closely related to apoptosis. Caspase 3 is the most active, suggesting that caspase 3 plays a key role (Fig. 5).
Fig. 5.

Different concentrations of PAT causes caspase activity changes in 293 T cells.
3.6. Correlation analysis of caspase family enzymes
The caspase activity values from 293 T cells were imported into the RStudio, the cor() function in the RStudio was used, the Pearson Correlation Coefficient algorithm was selected to calculate the correlation coefficient between the enzyme activities of each enzyme, and the results were obtained using the corr.test() function to select the Pearson Correlation Coefficient algorithm to test the significance of the correlation coefficients; input code is:
mydata <− read.xlsx((. . .),sheet = 2, rowNames = T) cor(mydata, use = “everything,” method = “pearson”)
corr.test(mydata,use = “pairwise,”method = “pearson,”alpha = .05)
The results are shown in Table 1. It can be seen from the results that the 5 caspases analyzed in this study are highly correlated; among which caspase 3, caspase 8, and caspase 9 have the highest correlation, suggesting that the cascade reaction of these 3 caspases may largely regulate apoptosis. Caspase 3 is the most critical caspase in apoptosis, and caspase 8 and caspase 9 are the more upstream caspases in the apoptotic signaling process, probably because the upstream caspases have greater control over the apoptotic process. It is generally accepted that caspase 3 and caspase 6 often act as apoptotic effectors,15,16 whereas caspase 2, caspase 8, and caspase 9 act as apoptosis promoters.17,18 The proapoptotic signal is transmitted intracellularly from the death receptor to the initiating caspase which in turn activates the downstream effector caspases and finally triggers apoptosis or causes cell disintegration.
Table 1.
Correlation between five kinds of caspases.
| Caspase2 | Caspase3 | Caspase6 | Caspase8 | Caspase9 | |
|---|---|---|---|---|---|
| Caspase2 | 1.000 | 0.986 | 0.982 | 0.985 | 0.988 |
| Caspase3 | 0.986 | 1.000 | 0.981 | 0.996 | 0.993 |
| Caspase6 | 0.982 | 0.981 | 1.000 | 0.985 | 0.980 |
| Caspase8 | 0.985 | 0.996 | 0.985 | 1.000 | 0.994 |
| Caspase9 | 0.988 | 0.993 | 0.980 | 0.994 | 1.000 |
3.7. Western blot
As can be seen from Fig. 6, the molecular weight of β-actin was 43 kDa and the total protein content was essentially the same in all groups. The expression of Cyt-C in the blank group was extremely weak, and the expression of Cyt-C increased with the increase of PAT concentration. When the PAT concentration was >10 μM, the expression of Cyt-C increased 30-fold compared to the control group (Fig. 6). This suggests that the mitochondria were severely damaged, triggering the cells to perform the apoptotic program.
Fig. 6.
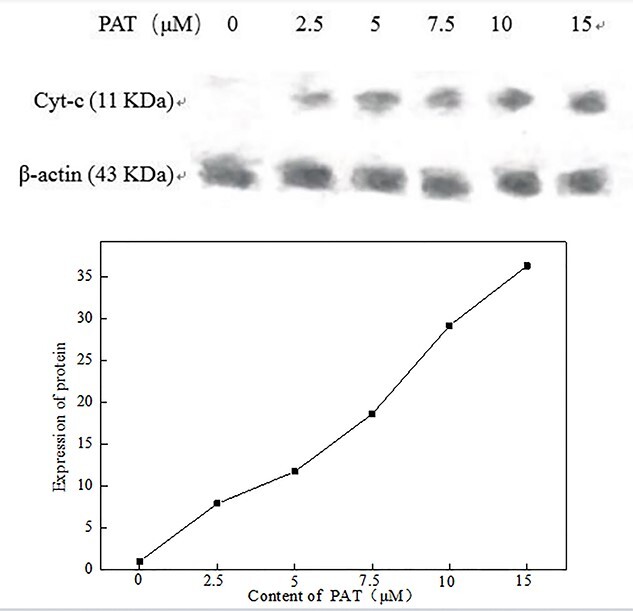
Results of Cyt-C protein expression in each treatment by western blot.
4. Discussion
After 293 T cells were incubated with PAT for 12 h, the normally adherent cells became clustered together in small groups, mostly in round and oval shapes, and CCK-8 results show that the toxic effect of PAT on 293 T cells was strong, with a significant dose-effect relationship and time-effect relationship. The survival rates of 293 T cells after 8, 16, 24, 36, and 48 h incubation with PAT were 7.97%, 64.30%, 60.60%, 74.61%, and 85.20%, respectively. PAT caused a decrease in SOD activity, a decrease in the GSH-Px activity, and an increase in MDA content. PAT induced an increase in mitochondrial membrane permeability, a decrease in intracellular ATP levels and an accumulation of ROS, suggesting that mitochondria play an important role in the process of PAT-induced cell injury and apoptosis.19 After mitochondrial damage, Cyt-C was released into the cytoplasm and Cyt-C was overexpressed, activating the cascade reaction of caspase enzymes and leading to apoptosis. The results of enzyme activity measurements and correlation analysis indicated that caspase 3 is the most critical caspase in the cascade system and is most associated with caspases 8 and 9.
Based on the experimental results, and synthesizing the theoretical knowledge, we have postulated a preliminary comprehensive mechanism of toxicity of PAT: Upon recognition of the elicitor (PAT) by the cell, a series of signal transductions and physiological and biochemical changes are initiated. The PAT signal travels to the cell membrane, where it may bind to the corresponding receptor, leading to a massive accumulation of ROS. Lipid peroxidation occurs in cell membrane and the permeability of cell membrane increases. This increase in membrane permeability may lead to the entry of some of the PAT into the cell, and due to the diffusible nature of ROS, it will be diffusing into the cell and into neighboring cells, acting on important organelles such as mitochondria. Mitochondrial properties are altered, structure and function are damaged, Cyt-C is released into the cytoplasm, and apoptosis-related proteases such as caspase may activated. The vesicles are ruptured and proteases are released from the vesicles into the cytoplasm, causing intracellular protein degradation. In addition, the accumulation of ROS leads to changes in cellular antioxidant levels, which undergo a complex series of structural and functional changes leading to cell death.20,21
Caspase 3 is one of the most well-studied proteases to date and is considered to be the key protease of apoptosis. Caspase is the protease system that directly causes apoptotic cell disintegration and is central to the network of apoptotic mechanisms.22,23 To date, numerous studies have suggested that caspase 3 is a key protease in mammalian apoptosis and that its activation leads to cell disassembly through 3 broad mechanisms.24,25
(1) Enzymatic inactivation of apoptosis inhibitors, such as nucleic acid endonuclease inhibitors (I-CAD/DFF45), Bcl- 2, mdm2, IkB, etc.
(2) Enzymatic cleavage of extracellular matrix and skeletal proteins such as keratin, local adhesion protein (FAK), actin, P21-dependent kinase (PAK2), and laminin.
(3) Cleavage of DNA repair-related molecules such as poly-ADP-ribose polypeptidase, DNA-dependent protein kinase, and REC140, a large subunit of replication factor C. The process of apoptosis is a series of cascade cleavages of caspases. Different proteases cleave and activate the caspase 3 zymogen separately. Activated caspase 3 further cleaves different substrates, leading to the amplification of the protease cascade cleavage and ultimately cell death.26–28
The present investigation into the mechanism of apoptosis is only a preliminary one on the basis of which the subsequent studies could focus on a few or some of the above genes. The mechanism of apoptosis is a very complex process regulated by many key genes or related enzymes, with a considerable number of promoters and terminators, which is a huge project.
5. Conclusion
In conclusion, this study suggests that PAT inhibits the growth of 293 T cells in a dose-dependent and time-dependent manner, promotes apoptosis, enhances oxidative stress level, and affects the expressions of ATP, Cyt-C, and caspase enzyme. Through the correlation analysis of caspase enzyme family and experimental data, the renal injury mechanism of PAT was roughly speculated.
Authors’ contributions
Baigang Zhang, Dongmei Xu, and Lin Shao determined the subject and were responsible for writing—review and editing and funding support. Baigang Zhang and Lin Shao were responsible for software and writing—original draft. Baigang Zhang, Dongmei Xu, Lin Shao, Hairong Liang, and Jinliang Li were responsible for methodology and visualization. Baigang Zhang, Lin Shao, Dongmei Xu, and Hairong Liang were responsible for software. Baigang Zhang, Dongmei Xu, Lin Shao, and Chenghui Huang were responsible for formal analysis. Chenghui Huang took care of conceptualization, data curation, investigation, and project administration.
Funding
This work was supported by the National Natural Science Foundation of China (31760495); the Nature Fund of Gansu Province (18JR3RA136).
Conflicts of interest statement: None declared.
Ethical declaration
Compliance with ethical standards.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Baigang Zhang, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
Dongmei Xu, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
Lin Shao, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
Hairong Liang, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
Jinliang Li, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
Chenghui Huang, School of Life Science and Engineering, Lanzhou University of Technology, Lanzhou, Gansu 730050, China.
References
- 1. Mahato DK, Kamle M, Sharma B, Pandhi S, Devi S, Dhawan K, Selvakumar R, Mishra D, Kumar A, Arora S, et al. Patulin in food: a mycotoxin concern for human health and its management strategies. Toxicon. 2021:198(prepublish):12–23. [DOI] [PubMed] [Google Scholar]
- 2. Pal S, Singh N, Ansari KM. Toxicological effects of patulin mycotoxin on the mammalian system: an overview. Toxicol Res. 2017:6(6):764–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. al Riachy R, Strub C, Durand N, Guibert B, Guichard H, Constancias F, Chochois V, Lopez-Lauri F, Fontana A, Schorr-Galindo S. Microbiome status of cider-apples, from orchard to processing, with a special focus on Penicillium expansum occurrence and patulin contamination. J Fungi. 2021:7(4):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coton M, Bregier T, Poirier E, Debaets S, Arnich N, Coton E, Dantigny P. Production and migration of patulin in Penicillium expansum molded apples during cold and ambient storage. Int J Food Microbiol. 2020:313(C):108377. [DOI] [PubMed] [Google Scholar]
- 5. Tournas VH. Spoilage of vegetable crops by bacteria and fungi and related health hazards. Crit Rev Microbiol. 2005:31(1):33–44. [DOI] [PubMed] [Google Scholar]
- 6. Chan-Hon-Tong A, Charles M-A, Forhan A, Heude B, Sirot V. Exposure to food contaminants during pregnancy. Sci Total Environ. 2013:458-460:27–35. [DOI] [PubMed] [Google Scholar]
- 7. Al-Hazmi MA. Patulin in apple juice and its risk assessments on albino mice. Toxicol Ind Health. 2014:30(6):534–545. [DOI] [PubMed] [Google Scholar]
- 8. Puel O, Galtier P, Oswald I. Biosynthesis and toxicological effects of patulin. Toxins. 2010:2(4):613–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alam S, Pal A, Kumar R, Dwivedi PD, das M, Ansari KM. EGFR‐mediated Akt and MAPKs signal pathways play a crucial role in patulin‐induced cell proliferation in primary murine keratinocytes via modulation ofCyclin D1andCOX‐2expression. Mol Carcinog. 2014:53(12):988–998. [DOI] [PubMed] [Google Scholar]
- 10. Lei WL, Li YY, Hou Y, Liu C, Qian WP, Sun QY, Zhang CH. Toxic effects of patulin on mouse oocytes and its possible mechanisms. Toxicology. 2021:464:153013. [DOI] [PubMed] [Google Scholar]
- 11. Zhang B, Huang C, Lu Q, Liang H, Li J, Xu D. Involvement of caspase in patulin-induced hepatotoxicity in vitro and in vivo. Toxicon. 2022:206:64–73. [DOI] [PubMed] [Google Scholar]
- 12. Zhu Y, Wu L, Yan H, Lu Z, Yin W, Han H. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal Chim Acta. 2020:1101(C):111–119. [DOI] [PubMed] [Google Scholar]
- 13. Xing M, Chen Y, Li B, Tian S. Characterization of a short-chain dehydrogenase/reductase and its function in patulin biodegradation in apple juice. Food Chem. 2021:348(prepublish):129046. [DOI] [PubMed] [Google Scholar]
- 14. Zheng X, Wei W, Zhou W, Li H, Rao S, Gao L, Yang Z. Prevention and detoxification of patulin in apple and its products: a review. Food Res Int. 2021:140:110034. [DOI] [PubMed] [Google Scholar]
- 15. Watanabe C, Shu GL, Giltiay NV, Clark EA. Regulation of B-lineage cells by caspase 6. Immunol Cell Biol. 2018:96(10):1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luan P, Xu J, Ding X, Cui Q, Jiang L, Xu Y, Zhu Y, Li R, Lin G, Tian P, et al. Neuroprotective effect of salvianolate on cerebral ischaemia-reperfusion injury in rats by inhibiting the caspase-3 signal pathway. Eur J Pharmacol. 2020:872(C):172944. [DOI] [PubMed] [Google Scholar]
- 17. Del Bello B, Valentini MA, Mangiavacchi P, Comporti M, Maellaro E. Role of caspases-3 and -7 in Apaf-1 proteolytic cleavage and degradation events during cisplatin-induced apoptosis in melanoma cells. Exp Cell Res. 2004:293(2):302–310. [DOI] [PubMed] [Google Scholar]
- 18. Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramide and etoposide-induced apoptosis. J Biol Chem. 2004:279(39):40755–40761. [DOI] [PubMed] [Google Scholar]
- 19. Zhou SM, Jiang LP, Geng CY, Cao J, Zhong LF. Patulin-induced oxidative DNA damage and p53 modulation in HepG2 cells. Toxicon. 2010:55(2–3):390–395. [DOI] [PubMed] [Google Scholar]
- 20. Fadeel B, Fadeel B, Orrenius S. Apoptosis: a basic biological phenomenon with wide-ranging implications in human disease. J Intern Med. 2005:258(6):479–517. [DOI] [PubMed] [Google Scholar]
- 21. Vetrivel P, Kim SM, Saralamma VVG, Ha SE, Kim EH, Min TS, Kim GS. Function of flavonoids on different types of programmed cell death and its mechanism: a review. J Biomed Res. 2019:33(06):363–370. [Google Scholar]
- 22. Ivanisenko NV, Lavrik IN. Mechanisms of procaspase-8 activation in the extrinsic programmed cell death pathway. Mol Biol. 2019:53(5):732–738. [DOI] [PubMed] [Google Scholar]
- 23. Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, Alnemri ES, Fairman R, Shi Y. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003:11(2):519–527. [DOI] [PubMed] [Google Scholar]
- 24. Dudko HV, Urban VA, Davidovskii AI, Veresov VG. Structure-based modeling of turnover of Bcl-2 family proteins bound to voltage-dependent anion channel 2 (VDAC2): implications for the mechanisms of proapoptotic activation of Bak and Bax in vivo. Comput Biol Chem. 2020:85(C):107203. [DOI] [PubMed] [Google Scholar]
- 25. Chai J, Chai J, Wu Q, Shiozaki E, Srinivasula SM, Alnemri ES, Shi Y. Crystal structure of a procaspase-7 zymogen: mechanisms of activation and substrate binding. Cell. 2001:107(3):399–407. [DOI] [PubMed] [Google Scholar]
- 26. Gerecht K, Margiola S, Müller MM. p53 deamidation as a molecular timer for cell death. Biophys J. 2020:118(3):485a-a. [Google Scholar]
- 27. Huang Y, Park YC, Rich RL, Segal D, Myszka DG, Wu H. Structural basis of caspase inhibition by XIAP. Cell. 2001:104(5):781–790. [PubMed] [Google Scholar]
- 28. Sun T, Liu H, Cheng Y, Yan L, Krittanawong C, Li S, Qian W, Su W, Chen X, Hou X, et al. 2,3,5,4′-tetrahydroxystilbene-2-O-β-d-glucoside eliminates ischemia/reperfusion injury–induced H9c2 cardiomyocytes apoptosis involving in Bcl-2, Bax, caspase-3, and Akt activation. J Cell Biochem. 2019:120(7):10972–10977. [DOI] [PubMed] [Google Scholar]