Abstract
Eryngium foetidum is a herbaceous plant found in tropical and subtropical regions. In vivo pharmacological parameters show that leaf extracts of this plant have antioxidant, anti-inflammatory, antidiabetic, and antimicrobial activities due to their bioactive compounds such as flavonoids and phenols. Despite the evidence for several bioactivities of E. foetidum, information on its safety and tolerability is limited. The objective of this study was to assess the effect and concentration of different extracts of E. foetidum on the development of zebrafish (Danio rerio) embryos. To study the impact of aqueous (AE), ethanolic (EE), and methanolic (ME) extracts, the embryos were exposed to 0.625, 1.25, 2.5, 5, and 10 mg mL−1 for up to 120-h postfertilization to assess embryonic developmental toxicity and then to 0.039, 0.078, 0.156, 0.312, and 0.625 mg mL−1 to assess the antioxidant responses of the enzymes superoxide dismutase catalase, glutathione S-transferase (GST), and cell apoptosis. The results showed that, depending on the extraction solvent, concentration used, and exposure time, E. foetidum extracts caused mortality, altered the hatching time, and promoted changes in enzymatic activities. Delays in development and increased GST activity were found in all treatments. Apoptosis was not observed in any of the treatments. In conclusion, AE, EE, and ME concentrations above 0.625 mg mL−1 can cause adverse effects on the early stages of zebrafish development.
Keywords: apoptosis, antioxidant enzymes, development, Danio rerio
Introduction
Eryngium foetidum is a herbaceous plant found in tropical and subtropical regions.1 It is popularly known in Brazil as Chicory is a vegetable Pará, culantro, wild coriander, spiny coriander, long coriander, and Chicory is a vegetable Amazon.2 Its main use is as a condiment, but its ethnomedicinal uses are also significant. The whole plant or its parts are often used in traditional medicine as home remedies for the treatment of various diseases, such as hypertension, seizures, asthma, infertility complications, malaria, liver problems, and arthritis.3–9
To understand the advantages and limitations of bioactive isolates of E. foetidum with the aim to increase their applicability as therapeutics, leaf extracts of this plant were studied in vivo and it was shown they contain phenolics, including flavonoids, and exert antioxidant, anti-inflammatory, antidiabetic, and antimicrobial activities.10 The antimicrobial activity conferred by terpenoids, glycosides, flavonoids, steroids, and tannins enables their use as antimicrobial agents in drugs for the treatment of infectious diseases caused by pathogens.11Eryngium foetidum also has anticlastogenic activity and cytoprotective effects due to the presence of the active compounds terpenoids, phytosterols, polyphenols, and flavonoids, suggesting that their daily consumption may generate health benefits in the form of protection of cells and their genetic material.12,13
Taken together, these facts make this plant a suitable candidate for studies on the exploration of new drugs.4 Even though they have several bioactive compounds, it is necessary to assess their extraction processes, since the recovery of phytochemicals and the antioxidant capacity of plant extracts is influenced by the type of solvent used in the extraction.14,15 In addition to assessing which solvent is the most efficient to obtain a greater yield of antioxidants for use in phytotherapeutic products, toxicological assessment of these natural products is also necessary. This is because some of its phytochemicals may be potentially toxic, teratogenic, mutagenic, or carcinogenic as an individual compound or in combination. Studying the teratogenicity or toxic effects of medicinal plants using their potential extracts is essential16 to ensure that the compounds are safe for humans.17
Zebrafish (Danio rerio) is a widely accepted vertebrate model for developmental and biomedical studies and for in vivo screening of bioactive molecules of medicinal plants.18 The zebrafish model enables the assessment of the toxicity of bioactive compounds or crude plant extracts, allowing us to determine the ideal processes for obtaining these plant materials.
The objective of this study was to assess the potential toxicity of different E. foetidum extracts to the development of zebrafish in embryonic and larval stages, as well as the biological activity of these extracts.
Materials and methods
Declaration of ethics
All experimental procedures of this study were conducted in strict accordance with the Ethics Committee on Animal Experimentation of the Federal University of Lavras (UFLA), Lavras, Minas Gerais, Brazil, under protocol number 014/2019. They met the guidelines of the National Control Council of Animal Experimentation (Conselho Nacional de Controle de Experimentação Animal—CONCEA) for the care and use of laboratory animals.
Exsiccates of E. foetidum were prepared and deposited in the PAMG herbarium of the Empresa de Pesquisa Agropecuária de Minas Gerais, under registration PAMG 57810.
Plant material and extract preparation
The plant material (shoot of the plant, including flowers, leaves, and stems) from which we obtained extracts was collected between 7 and 9 a.m. in the olericulture sector located in the Department of Agriculture of UFLA. Obtaining plant extracts followed the methodology described by11,19 with some changes. Fresh materials were washed 2–3 times with running water and once with sterile water and cut into 1 cm2 fragments. Plant extracts at 10% (w/v) were prepared using a reflux system, where the plant fragments were extracted with a solution of distilled water, 70% ethanol and 50% methanol for 20 min, boiling in a closed system, to reduce the possible losses by volatilization. The extracts were vacuum filtered and concentrated in an R-114 type rotary evaporator at 40°C under reduced pressure until drying.
Fish and embryos
Nine-month-old zebrafish adults of the wild-type strain, of short fins and of both sexes, were acquired from the Central Animal Facility, UFLA. The animals were raised and kept in specific racks for the species (Rack Hydrus, model ZEB-40) at a temperature of 28 ± 1°C, fed twice a day with commercial flake feed (Alcon basic, Alcon Pet®, SC, BRA), and fed once with Artemia nauplii. Male and female fish in a 2:1 ratio were placed in breeding tanks and kept in a 14:10 h light:dark cycle, turning the light on at dawn to stimulate spawning. The embryos were collected 30 min after natural mating from at least 3 groups of different matrices to avoid genetic variants. After washing with water, the embryos were randomized and transferred randomly to Petri dishes containing 12 mL of embryo culture medium (5 mM NaCl, 0.17 mM KCl, 0.33 mM CaCl2, 0.33 mM CaCl2, 0.33 mM MgSO4, 0.33 mM MgSO4, and methylene blue).20–22
Developmental toxicity
The embryotoxicity tests were based on the guidelines of OECD No. 236: Fish Embryo Acute Toxicity Test.23 To assess the lethal and developmental toxicity, 20 embryos were distributed in 96-well microplates (1 embryo/well), each well containing one extract at 0.625, 1.25, 2.5, 5, or 10 mg mL−1 in a volume of 200 μL, whereas the controls contained only the embryo culture medium. The concentrations selected for the study were based on pre-experimental data. All plates were incubated in an oven at 28 ± 1°C.
Embryonic development was monitored daily at 24, 48, 72, 96, and 120 h postfertilization (hpf) using an optical microscope (Olympus CX22LED model) with a focus on the following parameters: embryo coagulation, eye and body pigmentation, somite formation, presence of heartbeat, detachment of the tail, edema of the yolk sac and pericardium, and malformations of the tail.23
Determination of enzymatic activities
Preparation of homogenate
To determine the activity of the enzymes catalase (CAT), superoxide dismutase (SOD), and glutathione S-transferase (GST), embryos were initially placed after fertilization in Petri dishes with 10 mL of exposure solution until reaching 120 hpf. The exposure studies were performed in triplicate. The plates were incubated at 28 ± 1°C, and the dead eggs or larvae were removed daily. Forty larvae/group were collected and homogenized in 400 μL of cold phosphate-buffered saline (PBS) with the aid of a glass rod. Then, the homogenates were centrifuged at 4000 × g at 4°C for 20 min to obtain the supernatants.24–25 The concentration of total proteins for each sample was determined spectrophotometrically at 595 nm, according to the Bradford method, using bovine serum albumin as a standard.26
Superoxide dismutase
SOD activity was assessed as described by27 with some modifications. For the quantifications, 153 μL of the reaction system composed of 100 μL of PBS (100 mM, pH 7.8), 2 μL of EDTA (10 μM), 40 μL of methionine, 11 μL of ultrapure water, and 15 μL of 1 mM nitro blue tetrazolium (NBT) was prepared and added to 96-well microplates, which contained 30 μL of sample (homogenate of the larvae) and 2 μL of riboflavin. To start the reaction, the plates were placed under light (400 lux) for 20 min, and then the SOD activity was read in a spectrophotometer at 560 nm/25°C (TECAN Infinity® M200 PRO, Männedorf, Switzerland). One unit of SOD activity (U) was defined as the amount of enzyme required to lower the NBT photoreduction rate by 50%, and the result is expressed as U/mg protein.27,28 The readings were performed in triplicate.
CATALASE
CAT activity was determined according to the protocol established by.29 For the measurement, 10 μL of the homogenate supernatant of the larvae diluted in 140 μL of KPBS (0.05 M, pH 7.0) and 150 μL of reaction buffer (37.2 μL of H2O2 30%; 15 mL of KPBS) was added to 96-well microplates. The decrease in absorbance was recorded at 240 nm/25°C for 2 min (every 20 s) in a spectrophotometer (PowerWave XS, Biotek, Winooski, VT, USA). The absorbance readings were performed in triplicate, and the CAT activity level was estimated by the molar extinction coefficient of H2O2 (43.6 M cm−1). The results are expressed in μmol H2O2 degraded/min/mg protein.
Glutathione-S-transferase
GST activity was determined according to the protocol established by.30 For measurement, 15 μL of the homogenate of the larvae, 50 μL of reduced glutathione (GSH) (25 mM), and 180 μL (1 mM) of 1-chloro-2,4-dinitrobenzene (dissolved in 96% ethanol and diluted in 0.1 M sodium phosphate buffer, pH 7.2) were added to microplates. Differences in GST activity were estimated by the absorbance obtained over 5 min in a spectrophotometer at 340 nm (TECAN Infinity® M200 PRO Männedorf, Switzerland). Quantifications were performed in triplicate, and the results are expressed in U/mg protein.
Identification of apoptotic cells
To study the role of apoptosis in the toxicity of E. foetidum extracts, the acridine orange staining method was performed, as described by.31 Briefly, after 96 h of exposure to the extracts, the larvae were washed twice with embryo culture medium and stained with 5 mg/L acridine orange for 20 min in the dark at room temperature. After the incubation time, the larvae were again washed with embryo culture medium, anesthetized with 0.16 mg/mL tricaine (Sigma Aldrich), observed, and photographed under a fluorescence microscope at 4× magnification (Axio Observer Z1, Zeiss, Jena, Germany). The fluorescence intensity of the images was quantified in ImageJ software (National Institutes of Health).31,32 The results are expressed as the percentage change versus the control medium.
Statistical analysis
The toxicity, antioxidant, and apoptosis data were assessed for normality by the Shapiro–Wilk test, and then the data were subjected to analysis of variance and Tukey’s test to find significant differences between treatments. All tests were run in the statistical software Minitab v.19 (State College, PA, USA) with a significance level of 95% (P < 0.05).
Results
Toxicity in embryonic development
The effects of the aqueous (AE), ethanolic (EE), and methanolic (ME) extracts of E. foetidum on the survival rates of zebrafish embryos exposed at 24, 48, 72, 96, and 120 h are shown in Fig. 1. After the first 24 h of exposure, at the highest concentrations tested (10 and 5 mg mL−1) of all extracts and at a concentration of 2.5 mg mL−1 ME, a 100% mortality rate was observed. In contrast, embryos/larvae exposed to 0.625 mg mL−1 of any extract for any time showed similar responses as embryos of the control group (P > 0.05). For the other concentrations, the survival rate changed over time (Fig. 1).
Fig. 1.
Survival rate (%) evaluated daily from 24 to 120 h of zebrafish (D. rerio) embryos/larvae exposed to concentrations of 0.625; 1.25; 2.5; 5, and 10 mg mL−1 of Eryngium foetidum extracts. A) AE; B) EE; and C) ME.
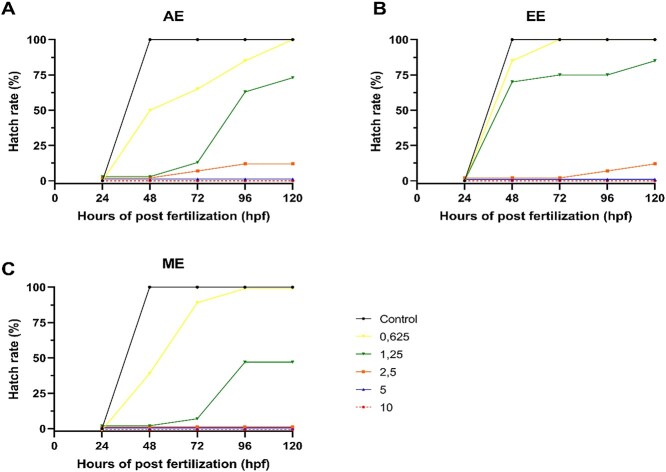
The hatching rate of embryos exposed to E. foetidum extracts varied over time depending on the extract and concentrations assessed (Fig. 2). In the treatments of 10 and 5 mg mL−1 of all extracts and 2.5 mg mL−1 of ME, no hatching was observed at any of the assessed times as a result of 100% embryo mortality. AE and EE at 2.5 mg mL−1 led to no hatching at 48 hpf. Similar results were also observed in the 1.25 mg mL−1 AE and MS groups, in which hatching was not observed at 48 hpf.
Fig. 2.
Hatch rate (%) of zebrafish (D. rerio) embryos at 48, 72, 96 and 120 hpf after exposure to different concentrations (0.625; 1.25; 2.5; 5, and 10 mg mL-1) of the extracts of E. foetidum. A) AE; B) EE; and C) ME.
Regarding morphological changes, normal development was observed in control embryos, whereas a developmental delay was evident at all extract groups (Fig. 3).
Fig. 3.
Zebrafish embryos/larvae at 24, 48, 72, and 120 hpf. In control: normal development at 24, 48, 72, and 120 hpf. AE 1.25 mg ml−1: growth retardation at 24 and 48 hpf; inhibition of melanin release at 48 hpf with permanence at 72 hpf.
Based on the results of the preliminary studies (embryonic development toxicity), concentrations below 0.625 mg mL−1 were selected for the subsequent experiments (enzymatic activity and cell apoptosis). We did not study embryos/larvae at 1.25, 2.5, 5, or 10 mg mL−1 for subsequent analyses due to the very high lethal toxicity.
Enzymatic activities
To further characterize the harmful effects induced by AE, EE, and ME of E. foetidum during zebrafish embryogenesis, we determined the activities of the enzymes SOD, CAT, and GST. As shown in Fig. 4, SOD activity was significantly higher (P < 0.05) than in the control group only for 0.156, 0.312, and 0.625 mg mL−1 AE. CAT activity increased significantly (P < 0.05) in AE and EE at concentrations of 0.039, 0.078, 0.156, 0.0312, and 0.039, 0.156 mg mL−1. GST activity increased significantly (P < 0.05) after exposure to all E. foetidum extracts.
Fig. 4.
Change in the activity of antioxidant enzymes SOD, CAT, and GST in zebrafish embryos/larvae at 96 hpf between control groups and those treated with E. foetidum extracts at different concentrations. A, D, G) AE; B, E, H) EE; and C, F, I) ME. Bars indicate mean ± SEM of 3 replicates (n = 3). Different superscript letters indicate significant differences (P < 0.05) between groups.
Identification of apoptotic cells
The cell death found in the treatment groups did not differ from that in the control (Fig. 5 AC), indicating that the extracts did not exert any cytotoxic effect on zebrafish embryos/larvae at the tested concentrations (0.039, 0.078, 0.156, 0.312, and 0.625 mg mL−1).
Fig. 5.
Effects of E. foetidum extracts on apoptosis of zebrafish larvae. A) AE; B) EE; and C) ME. Apoptosis levels were measured after acridine orange staining by image analysis and fluorescence microscopy. Data obtained from individual analyzes of fluorescence intensity of zebrafish larvae. Values are expressed as mean ± SEM.
Discussion
Eryngium foetidum has a diversity of bioactive compounds, which are reported to have medical and pharmaceutical bioactivities.33 However, the understanding of their toxicological profiles is limited, especially with regard to embryonic developmental toxicity. To date, no studies on the toxicological effects of E. foetidum using zebrafish embryos/larvae as a model have been found in the literature.
The results of the present study strongly suggest that AE, EE, and ME of E. foetidum are toxic because they lower the survival of zebrafish embryos/larvae, and their effects depend on the dose and exposure time. The toxicity of E. foetidum has been described by other authors, who revealed the larvicidal potential of this species’ essential oil against the mosquito Aedes albopictus33 and in mice fed a feed supplemented with lyophilized E. foetidum, with signs of abnormalities such as kidney injury.34
Embryos of zebrafish exposed to high concentrations of any of the extracts (10 and 5 mg mL−1) all died within 24 h of exposure. According to35 the mortality of zebrafish embryos in the first hours of development may occur due to the interference of the test substance with cellular organelles, such as mitochondria. In the presence of such interference, the ATP formation process becomes slow or stops, resulting in the failure of the active membrane of the sodium pump, intracellular accumulation of sodium, and external diffusion of potassium, which may cause cell death.35,36
At concentrations of 1.25 and 2.5 mg mL−1 of the extracts of E. foetidum, where there was no mortality in the first 24 h, embryonic survival declined from the second and/or third day of incubation on. These results show the toxic effects of the extracts depending on the dose and exposure time because as the exposure time increased, survival decreased. Prolonged exposure to an extract may lead to greater accumulation of the extract in the embryo, reaching a concentration that can induce toxicity,37 and/or this can result from the weakened or damaged protective layer of the embryo (chorion).38 The chorion undergoes continuous changes throughout embryonic development, such as in the chorionic protein profile. These changes result in an increase in the opening or expansion of the chorion pore channel, allowing a greater influx of external solute.38
Our results indicate that ME of E. foetidum has a higher toxicity to zebrafish embryos/larvae than AE and EE. This higher toxicity may be due to the ability of methanol to extract more compounds than other polar.39,40 Nevertheless, because methanol has a lower boiling point (64.7°C), for its evaporation during the extract preparation process, lower temperatures are necessary, causing less damage to the extract than to ethanol and water, which have higher boiling points.39
Among the assessed extracts, the lowest toxicity was found for AE. The higher lethality of EE and ME in zebrafish embryos and larvae may be due to the greater concentration of tannins in these extracts. Reference41 reported a higher tannin concentration in the extracts of E. foetidum when using EE and ME solvents and a lower presence in water. The lower amount of tannins in the AE extracts is due to the loss of tannins during the boiling process.14,42 Tannins are compounds that have antioxidant activity,14 but negative health effects are also reported, as they interfere with the availability of iron and zinc and some digestive enzymes in the body.43 Tannins are considered toxic and nutritionally undesirable because they precipitate proteins, inhibit digestive enzymes, and affect the use of vitamins and minerals.44 In addition, a higher intake of sources rich in tannins is also associated with carcinogenic effects, poor use of proteins, and liver and kidney toxicity.45 Our findings are in agreement with41 who showed that the AE of E. foetidum has a low tannin content and therefore has a low probability of causing some negative effect on health.
Like tannins, flavonoids can cause toxicity to organisms. Flavonoids have antioxidant activity by promoting the excessive removal of free radicals, acting as neutralizing agents.46 However, depending on their concentrations, flavonoids may have pro-oxidant properties, promoting the oxidation of other compounds.47 These antioxidants are easily extracted from E. foetidum in an alcoholic medium but less so in an AE medium because high temperatures degrade flavonoids41,48 Thus, the lower survival rate of embryos/larvae exposed to EE and ME in the present study might be attributed to the higher concentrations of flavonoids and tannins in these extracts compared with AE.
The zebrafish larvae usually hatch from the chorion between 48 and 72 hpf. Hatching is regulated by a combination of biochemical and physical mechanisms.49 Physical and chemical signals detected by the embryo activate the production of the enzyme related to hatching (chorionase, HE1), which degrades the inner layer of the chorion and allows the spontaneous movements of developing embryos to result in hatching.50 Some studies have shown that plant extracts and oil delay the hatching process of zebrafish embryos, such as Trachyspermum ammi (Apiaceae) oil, Carpesii fruit extracts, Piper nigrum (black pepper), and Millettia pachycarpa.51–54 Some authors believe that this delay in hatching may be due to changes in the release of the enzyme chorionase and/or the reduced movement of the larvae due to behavioral changes caused by components present in these plants.52,54 The study of55 suggested that phenolic compounds can induce or inhibit the activity of the chorionase enzyme, delaying the hatching of embryos. Based on these arguments, we assume that, in the present study, delays in the hatching of embryos exposed to E. foetidum extracts were also caused by these factors.
Another hypothesis about delayed hatching is related to the delay in the development of embryos and their inability to rupture the chorion.51,56 Developmental delay is frequently observed in embryos exposed to teratogenic agents57 and is generally considered a reversible and nonspecific effect. In all E. foetidum treatments, there were delays in embryonic development in the first 24 hpf. We believe that due to the high toxicity of concentrations of 1.25 and 2.5 mg mL−1 of the extracts, the embryos reversed their developmental delay more slowly, resulting in late hatching. In contrast, with 0.625 mg mL−1 EE and ME, because of their low toxicity, the embryos reversed the developmental delay more quickly, resulting in hatching at the expected time.
The activities of antioxidant enzymes such as SOD, CAT, and GST are important indicators of the production of reactive oxygen species derived from the toxicity of a compound.58 SOD is a metalloenzymes that is part of the first line of defense against excessive amounts of reactive oxygen species. It converts free radical superoxide anions (O2−•) into H2O2. This H2O2 will be degraded in water and oxygen by the enzyme CAT, thus freeing the cells from the risk of oxidation by these radicals.59
After the exposure of embryos/larvae to E. foetidum extracts, significant differences from the control were found only in SOD activity in the groups with 0.156, 0.312, and 0.625 mg mL−1 AE and in CAT in the groups given AE and EE at concentrations of 0.039, 0.078, 0.156, 0.0312, and 0.039, 0.156. We believe that because the concentrations used for the determination of enzymatic activity were lower than those used in the assessment of developmental toxicity, the concentrations of tannins and flavonoids might have been low. Thus, these bioactive agents could act as antioxidants, eliminating reactive oxygen species produced by larval metabolism directly or by stimulating antioxidant enzymes.
GSTs are phase II biotransformation enzymes that play a specific physiological role in zebrafish detoxification.60 They are involved in the detoxification of xenobiotics and in the elimination of reactive oxygen species and lipid peroxidation products, acting by catalyzing the conjugation of GSH to a wide variety of substrates, neutralizing their electrophilic sites, making them less toxic, more soluble in water, and more easily excreted.61,62 We observed a significant increase in GST activity in zebrafish larvae exposed to all concentrations of extracts compared with the control. This increase may be related to the presence of flavonoids in these extracts. According to,47,63 flavonoids can induce phase II detoxifying enzymes, such as NAD (P) H: quinone oxidoreductase, UDP-glucuronyl transferase, and GST, which are the main defensive enzymes against electrophilic toxics and oxidative stress. In addition, flavonoids are typical xenobiotics for animals and humans, metabolized as such and quickly removed from the circulation.47 Thus, the increase in GST activity in embryos/larvae exposed to E. foetidum extracts may have been due to the effect of flavonoids present in the extracts of eliminating toxic compounds or by their own elimination as a xenobiotic.
Redox regulation and apoptosis play crucial roles in the developmental process because their disorder can cause deformity and death.32 Apoptosis can occur naturally during embryogenesis, regulating many developmental processes, such as morphogenesis, removal of vestigial structures and regulation of cell number, or may be the result of cell damage or stress.22,53,64 In the present study, the presence of acridine orange in the control embryos confirmed that programmed cell death is a normal physiological process during development. The larvae exposed to the extracts also showed cell death, not differing from the control, which suggests that AE, EE, and ME concentrations below 0.625 mg mL−1 do not induce apoptosis in zebrafish embryos/larvae.
Conclusion
Eryngium foetidum extracts can cause toxicity in the development of zebrafish embryos/larvae. This toxicity depends on the solvent used in the extraction, the concentration used, and the exposure time. It can cause death, developmental delays, hatching delays, and antioxidant enzyme activity. Based on our results, for the qualities of these plants and their extracts to be well validated, a more detailed study aiming to understand the biological mechanisms affected by the extracts, with subsequent study of their antioxidant and anti-inflammatory potentials, should be performed.
Acknowledgments
This study received financial support from the Mining Bioterism Network (Rede Mineira de Bioterismo), the Minas Gerais Research Foundation (Fundação de Amparo à Pesquisa de Minas Gerais—FAPEMIG), the National Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq), and the Coordination for the Improvement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES).
Conflict of interest statement. None declared.
Contributor Information
Tassia Flavia Dias Castro, Faculty of Animal Science and Veterinary Medicine, Department of Veterinary Medicine, Federal University of Lavras, Lavras, MG, CEP:37200-000, Brazil.
William Franco Carneiro, Faculty of Animal Science and Veterinary Medicine, Department of Veterinary Medicine, Federal University of Lavras, Lavras, MG, CEP:37200-000, Brazil.
Tharyn Reichel, School of Agricultural Sciences, Department of Agriculture, Federal University of Lavras, Lavras, MG, Brasil.
Sarah Lacerda Fabem, Faculty of Animal Science and Veterinary Medicine, Department of Veterinary Medicine, Federal University of Lavras, Lavras, MG, CEP:37200-000, Brazil.
Mônica Rodrigues Ferreira Machado, Department of Biological Sciences, Federal University of Jataí, Jataí, GO, CEP: 75801-615, Brasil.
Krisnanda Kelly Castro de Souza, School of Agricultural Sciences, Department of Agriculture, Federal University of Lavras, Lavras, MG, Brasil.
Luciane Vilela Resende, School of Agricultural Sciences, Department of Agriculture, Federal University of Lavras, Lavras, MG, Brasil.
Luis David Solis Murgas, Faculty of Animal Science and Veterinary Medicine, Department of Veterinary Medicine, Federal University of Lavras, Lavras, MG, CEP:37200-000, Brazil.
Authorship contribution statement
TFDC: Conceptualization, Writing—original draft, Investigation, Writing—review and editing. WFC: Conceptualization, Methodology, data curation, formal analysis, writing—review and editing. TR Conceptualization, Methodology, data curation, formal analysis, writing—review and editing. SLF: Methodology, data curation, formal analysis, writing—review and editing. MRFM: Methodology, data curation, formal analysis, writing—review and editing, Funding acquisition. KKCS: Methodology, data curation, formal analysis, writing—review and editing LVR: Conceptualization, Supervision, Data curation, Writing—review and editing. LDSM: Conceptualization, Supervision, Funding acquisition, Project administration, Writing—review and editing.
References
- 1. Cardozo E, Rubio M, Rojas LB, Usubillaga A. Composition of the Essential Oil from the Leaves of Eryngium foetidum L. from the Venezuelan Andes. J Essent Oil Res. 2004:16:33–34. [Google Scholar]
- 2. Gomes RF, Pereira J, De SAL G, De Souza HT. Production chicory Amazon cultivated under density planting and pruning of floral tassel. Rev Caatinga. 2013:26:9–14. [Google Scholar]
- 3. Lans C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J Ethnobiol Ethnomed. 2007:3(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paul JHA, Seaforth CE, Tikasingh T. Eryngium foetidum L.: a review. Fitoterapia. 2011:82(3):302–308. [DOI] [PubMed] [Google Scholar]
- 5. Shavandi MA, Haddadian Z, Ismail MHS. Eryngium foetidum L. Coriandrum sativum and Persicaria odorata L.: a review. J Asian Sci Res. 2012:2:410–426. [Google Scholar]
- 6. Leishangthem S, Sharma LD. Study of some important medicinal plants found in Imphal-East District, Manipur India. Int J Sci Res Publ. 2014:4:1–5. [Google Scholar]
- 7. Yuhlung CC, Bhattacharyya M. Practice of Ethno-medicine among the Chothe Tribe of Manipur, North-East India. Int J Pharm Biol Arch. 2014:5:138–149. [Google Scholar]
- 8. Malik T, Pandey DK, Roy P, Okram A. Evaluation of phytochemicals, antioxidant, antibacterial and antidiabetic potential of Alpinia galanga and Eryngium foetidum plants of Manipur (India). Pharm J. 2016:8:459–464. [Google Scholar]
- 9. Bussmann RW, Paniagua Zambrana NY, Romero C, Hart RE. Astonishing diversity—the medicinal plant markets of Bogotá, Colombia. J Ethnobiol Ethnomed. 2018:14(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prabha S, Athoibi S, Dsouza MR. Pharmacognostical evaluation of Spiny coriander (Eryngium foetidum L.): a traditional culinary and ethnomedicinal herb. Int J Bot Stud. 2019:4:64–70. [Google Scholar]
- 11. Lingaraju DP, Sudarshana MS, Mahendra C, Rao KP. Phytochemical screening and antimicrobial activity of leaf extracts of Eryngium foetidum L. (Apiaceae). Indo Am J Pharm Res. 2016:6:4339–4344. [Google Scholar]
- 12. Promkum C, Butryee C, Tuntipopipat S, Kupradinun P. Anticlastogenic Effect of Eryngium foetidum L. assessed by erythrocyte micronucleus assay. Asian Pac J Cancer Prev. 2012:13:3343–3347. [DOI] [PubMed] [Google Scholar]
- 13. Raunelli P, Liviac D, Alvis R, Puente S, Best I, Reátegui O. Cytoprotective effect of the Eryngium foetidum “Sacha Culantro” methanolic leaf extract versus sodium fluoride exposed mice using the micronucleus test and the comet assay. Pharm J. 2019:11(3):461–465. [Google Scholar]
- 14. Singh S, Singh DR, Salim KM, Srivastava A, Singh LB, Srivastava RC. Estimation of proximate composition, micronutrients and phytochemical compounds in traditional vegetables from Andaman and Nicobar Islands. Int J Food Sci Nutr. 2011:62:765–773. [DOI] [PubMed] [Google Scholar]
- 15. Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006:97(4):654–660. [Google Scholar]
- 16. Murugesu S, Khatib A, Ahmed QU, Ibrahim Z, Uzir BF, Benchoula K, Yusoff NIN, Perumal V, Alajmi MF, Salamah S, et al. Toxicity study on Clinacanthus nutans leaf hexane fraction using Danio rerio embryos. Toxicol Rep. 2019:6:1148–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Falcão MAP, Souza LS, Dolabella SS, Guimarães AG, Walker CIB. Zebrafish as an alternative method for determining the embryo toxicity of plant products: a systematic review. Environ Sci Pollut Res. 2018:25(35):35015–35026. [DOI] [PubMed] [Google Scholar]
- 18. Phillips JB, Westerfield M. Zebrafish models in translational research: tipping the scales toward advancements in human health. Dis Model Mech. 2014:7(7):739–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19. de KKC. Potencial antioxidante, mineral, inibitório de enzimas α-amilase e lipoxigenase e composição centesimal de espécies da família Apiaceae. Dissertação (Mestrado em Plantas Medicinais, Aromáticas e Condimentares). Lavras: Universidade Federal de Lavras; 2016. p. 105 [Google Scholar]
- 20. Ferreira DQ, Ferraz TO, Araújo RS, Cruz RAS, Fernandes CP, Souza GC, Ortiz BLS, Sarquis RSFR, Miranda JCMM, Garrett R, et al. Libidibia ferrea (jucá), a traditional anti-inflammatory: a study of acute toxicity in adult and embryos zebrafish (Danio rerio). Pharmaceuticals. 2019:12(4):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nusslein-Volhard C, Dahm R. Zebrafish: a practical approach. Oxford University Press; 2002:322. [Google Scholar]
- 22. Simmons SO, Fan C-Y, Ramabhadran R. Cellular stress response pathway system as a sentinel ensemble in toxicological screening. Toxicol Sci. 2009:111(2):202–225. [DOI] [PubMed] [Google Scholar]
- 23. OECD 2013 . OECD Guideline for Testing of Chemicals. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; 2013
- 24. Xiong G, Deng Y, Cao Z, Liao X, Zhang J, Lu H. The hepatoprotective effects of Salvia plebeia R. Br. extract in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019:95:399–410. [DOI] [PubMed] [Google Scholar]
- 25. Xiong G, Zou L, Deng Y, Meng Y, Liao X, Lu H. Clethodim exposure induces developmental immunotoxicity and neurobehavioral dysfunction in zebrafish embryos. Fish Shellfish Immunol. 2019:86:549–558. [DOI] [PubMed] [Google Scholar]
- 26. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976:72(1–2):248–254. [DOI] [PubMed] [Google Scholar]
- 27. Song Y, Zhu LS, Wang J, Wang JH, Liu W, Xie H. DNA damage and effects on antioxidative enzymes in earthworm (Eisenia foetida) induced by atrazine. Soil Biol Biochem. 2009:41(5):905–909. [Google Scholar]
- 28. Gu J, Zhang J, Chen Y, Wang H, Guo M, Wang L, Wang Z, Wu S, Shi L, Gu A, et al. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere. 2019:217:629–635. [DOI] [PubMed] [Google Scholar]
- 29. Clairborne A. Catalase activity. In: Greenwald RA, editors. Handbook methods for oxygen radical research. Boca Raton: CRC Press; 1985:283–284. [Google Scholar]
- 30. Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981:398–405. [DOI] [PubMed] [Google Scholar]
- 31. Manjunatha B, Deekshitha B, Seo E, Kim J, Lee SJ. Developmental toxicity induced by particulate matter (PM2.5) in zebrafish (Danio rerio) model. Aquat Toxicol. 2021:238:105928. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Fang S, Xiong Z. Protective effect of polysaccharide from Ligusticum chuanxiong hort against H2O2-induced toxicity in zebrafish embryo. Carbohydr Polym. 2019:221:73–83. [DOI] [PubMed] [Google Scholar]
- 33. Sumitha KV, Prajitha V, Sandhya VN, Anjana S, Thoppil JE. Potential larvicidal principles in Eryngium foetidum L. (Apiaceae), an omnipresent weed, effective against Aedes albopictus skuse. J Essent Oil Bear Plants. 2014:17(6):1279–1286. [Google Scholar]
- 34. Janwitthayanuchit K, Kupradinun P, Rungsipipat A, Kettawan A, Butryee C. A 24-weeks toxicity study of Eryngium foetidum Linn. leaves in mice. Toxicol Res. 2016:32(3):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azevedo RDS, Falcão KVG, Amaral IPG, Leite ACR, Bezerra RS. Mitochondria as targets for toxicity and metabolism research using zebrafish. Biochim Biophys Acta - Gen Subj. 2020:1864(8):129634. [DOI] [PubMed] [Google Scholar]
- 36. Narko T, Wibowo MS, Damayanti S, Wibowo I. Acute toxicity tests of fermented robusta green coffee using zebrafish embryos (Danio rerio). Pharm J. 2020:12(3):485–492. [Google Scholar]
- 37. Alafiatayo AA, Lai KS, Syahida A, Mahmood M, Shaharuddin NA. Phytochemical evaluation, embryotoxicity, and teratogenic effects of Curcuma longa extract on zebrafish (Danio rerio). Evidence-based Complement Altern Med. 2019:2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ali MK, Saber SP, Taite DR, Emadi S, Irving R. The protective layer of zebrafish embryo changes continuously with advancing age of embryo development (AGED). J Toxicol Pharmacol. 2017:1:009. [Google Scholar]
- 39. Adegbaju OD, Otunola GA, Afolayan AJ. Effects of growth stage and seasons on the phytochemical content and antioxidant activities of crude extracts of Celosia argentea L. Heliyon. 2020:6(6):e04086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rajan M, Rajkumar G, Farias Lima Guedes TJ, Chagas Barros RG, Narain N. Performance of different solvents on extraction of bioactive compounds, antioxidant and cytotoxic activities in Phoenix loureiroi Kunth leaves. J Appl Res Med Aromat Plants. 2020:17:100247. [Google Scholar]
- 41. Singh S, Singh DR, Banu S, Salim KM. Determination of bioactives and antioxidant activity in Eryngium foetidum L.: a traditional culinary and medicinal herb. Proc Natl Acad Sci India Sect B Biol Sci. 2013:83(3):453–460. [Google Scholar]
- 42. Esenwah CN, Ikenebomeh MJ. Processing effects on the nutritional and anti-nutritional contents of African locust bean (Parkia biglobosa Benth.) seed. Pak J Nutr. 2008:7:214–217. [Google Scholar]
- 43. Beecher GR. Overview of dietary flavonoids: nomenclature, occurrence and intake. J Nutr. 2003:133(10):3248S–3254S. [DOI] [PubMed] [Google Scholar]
- 44. Monteiro JM, Albuquerque UP, Araújo E, de ELC. Taninos: uma abordagem da química à ecologia. Quim Nova. 2005:28(5):892–896. [Google Scholar]
- 45. Akwaowo EU, Ndon BA, Etuk EU. Minerals and antinutrients in fluted pumpkin (Telfairia occidentalis Hook f.). Food Chem. 2000:70(2):235–240. [Google Scholar]
- 46. Jia S, Shen M, Zhang F, Xie J. Recent advances in Momordica charantia: functional components and biological activities. Int J Mol Sci. 2017:18(12):2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Procházková D, Boušová I, Wilhelmová N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia. 2011:82(4):513–523. [DOI] [PubMed] [Google Scholar]
- 48. Supraja P, Usha R. Antibacterial and phytochemical screening from leaf and fruit extracts of Momordica charantia. Int J Pharma Bio Sci. 2013:4:787–793. [Google Scholar]
- 49. Wang XH, Souders CL, Zhao YH, Martyniuk CJ. Paraquat affects mitochondrial bioenergetics, dopamine system expression, and locomotor activity in zebrafish (Danio rerio). Chemosphere. 2018:191:106–117. [DOI] [PubMed] [Google Scholar]
- 50. De la Paz J, Beiza N, Paredes-Zúñiga S, Hoare M, Allende M. Triazole fungicides inhibit zebrafish hatching by blocking the secretory function of hatching gland cells. Int J Mol Sci. 2017:18(4):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yumnamcha T, Roy D, Devi MD, Nongthomba U. Evaluation of developmental toxicity and apoptotic induction of the aqueous extract of Millettia pachycarpa using zebrafish as model organism. Toxicol Environ Chem. 2015:97(10):1363–1381. [Google Scholar]
- 52. Kim SH, Sharma C, Kang SC. Ajowan oil potentiates ros-mediated teratogenic effect in zebrafish embryos. J Essent Oil Bear Plants. 2017:20(4):883–896. [Google Scholar]
- 53. Xia Q, Luo J, Mei X, Wang Y, Huang W, Wang J, Yang R, Ma Z, Lin R. A developmental toxicity assay of Carpesii Fructus on zebrafish embryos/larvae. Toxicol Res (Camb). 2017:6(4):460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel P, Panda PK, Kumari P, Singh PK, Nandi A, Mallick MA, das B, Suar M, Verma SK. Selective in vivo molecular and cellular biocompatibility of black peppercorns by piperine-protein intrinsic atomic interaction with elicited oxidative stress and apoptosis in zebrafish eleuthero embryos. Ecotoxicol Environ Saf. 2020:192:110321. [DOI] [PubMed] [Google Scholar]
- 55. Cavalcante AK, Lopes-Ferreira M, Rogero SO, Rogero JR. Evaluation of resveratrol toxicity in the embryolarval stage of Danio rerio fish. Ecotoxicol Environ Contam. 2017:12:133–139. [Google Scholar]
- 56. Osman AGM, Mekkawy IA, Verreth J, Kirschbaum F. Effects of lead nitrate on the activity of metabolic enzymes during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol Biochem. 2007:33(1):1–13. [Google Scholar]
- 57. Brandhof E-J, Montforts M. Fish embryo toxicity of carbamazepine, diclofenac and metoprolol. Ecotoxicol Environ Saf. 2010:3:1862–1866. [DOI] [PubMed] [Google Scholar]
- 58. Meng Y, Zhong K, Xiao J, Huang Y, Wei Y, Tang L, Chen S, Wu J, Ma J, Cao Z, et al. Exposure to pyrimethanil induces developmental toxicity and cardiotoxicity in zebrafish. Chemosphere. 2020:255:126889. [DOI] [PubMed] [Google Scholar]
- 59. Ji M, Gong X, Li X, Wang C, Li M. Advanced research on the antioxidant activity and mechanism of polyphenols from hippophae species—a review. Molecules. 2020:25(4):917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Glisic B, Mihaljevic I, Popovic M, Zaja R, Loncar J, Fent K, et al. Characterization of glutathione-S-transferases in zebrafish (Danio rerio). Aquat Toxicol. 2015:58:50–62. [DOI] [PubMed] [Google Scholar]
- 61. Zhu L, Dong X, Xie H, Wang J, Wang J, Su J, Yu C. DNA damage and effects on glutathione-S-transferase activity induced by atrazine exposure in zebrafish (Danio rerio). Environ Toxicol. 2011:26(5):480–488. [DOI] [PubMed] [Google Scholar]
- 62. Chatterjee A, Gupta S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018:433:33–42. [DOI] [PubMed] [Google Scholar]
- 63. Lee-Hilz YY, Boerboom A-MJF, Westphal AH, Berkel WJH, Aarts JMMJG, Rietjens IMCM. Pro-oxidant activity of flavonoids induces EpRE-mediated gene expression. Chem Res Toxicol. 2006:19(11):1499–1505. [DOI] [PubMed] [Google Scholar]
- 64. Yu MA, Egawa T, Shinzawa-Itoh K, Yoshikawa S, Yeh S-R, Rousseau DL, Gerfen GJ. Radical formation in cytochrome c oxidase. Biochim Biophys Acta Bioenerg. 2011:1807(10):1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]