Abstract
Niobium (V) oxide nanoparticles (NINPs) have been widely and increasingly applied in various health products and industrial processes. This merits further study of their toxicity. Here, we investigated the potential of NINPs to induce DNA damage, cytotoxicity, and chromosome instability in cultured CHO-K1 cells. NINPs were physico-chemically characterized. As assessed by comet assay, crystalline and amorphous NINPs were genotoxic at the highest concentrations evaluated. The cytokinesis-block micronucleus assay demonstrated that a 24-h treatment with NINPs, for the crystalline and the amorphous samples, significantly reduced the nuclear division cytotoxicity index. In addition, a 4-h treatment period of crystalline NINPs increased micronucleus (MNi) frequencies. MNi, nucleoplasmic bridges and nuclear buds were detected after exposure of the cells for 24 h to crystalline NINPs. In the amorphous sample, chromosome instability was restricted to the induction of MNi, in the 24-h treatment, detected at all tested concentrations. The fluorescence and dark field microscopy demonstrated the uptake of NINPs by CHO-K1 cells and an intracellular distribution outlining the nucleus. Our data advance understanding of the cytotoxic and genotoxic effects of NINPs and should be taken into consideration when setting up guidelines for their use in industrial or health products.
Keywords: niobium oxide, DNA damage, chromosome instability, risk assessment, genotoxicity
Introduction
With the advent of nanotechnology, metal-based nanoparticles (NPs) such as metal and metal oxide NPs are now widely applied in different areas of nanotechnology, since metal NPs present a variety of structural geometries and unique physical–chemical properties.1–4 Several studies are being carried out to determine the genotoxic and mutagenic potential of different types and forms of NPs, as these can penetrate the cells and, in certain cases, nuclear membranes, having the potential to induce damage to chromatin at all cell cycle stages.5–7 In addition, there is the possibility of direct damage due to interaction with the DNA molecule or by indirect routes, such as, for example, oxidative damage (cellular response to the presence of NPs) or by a side effect (induced inflammation), leading to cell death (apoptosis and necrosis).8–10
Niobium-based materials are used in an increasing number of applications in high-tech industries11–13 e.g. they are irreplaceable to obtain some types of steel that are used in the manufacture of pipes (grids, structures, gas, and oil pipelines) and in high-precision tools.14–17 Furthermore, because of their excellent biocompatibility, niobium-based materials improve the mechanical properties in titanium alloys for bone and dental implants.18,19 In this context, niobium NPs (NINPs) have been developed to improve the properties of niobium-based products and to obtain advanced materials that can be applied in technologies aimed at health, energy, and cosmetics. Despite the wide diversity of potential applications of NINPs, there is little information in the scientific literature, regarding the cyto-genotoxicity of NINPs.20
Here, we evaluated the induction of DNA damage, cytotoxicity, and chromosomal instability in a mammalian rodent cell line (CHO-K1) exposed to crystalline and amorphous niobium pentoxide (Nb2O5) NPs. In addition, we characterized NINPs structurally, and performed an internalization analysis. Due to their non-tumor characteristics and stable karyotype, Chinese hamster ovary (CHO-K1) cells are widely applied in genetic toxicology. The sensitivity of CHO-K1 cell line for the detection of compounds that act on DNA was previously demonstrated.21 The single-cell gel eletrophoresis (SCGE) assay, also known as the comet test, is one of the most used genotoxicity short-term bioassays, developed to assess and analyze DNA damage in isolated cells. It is a fast, simple and sensitive method to detect DNA breaks in virtually all cell types.22 The cytokinesis-block micronucleus cytome (CBMN-Cyt) assay also uses different cell types and is applied to identify the different compounds that are potential inducers of cytotoxicity and chromosome instability.23 Both the alkaline comet and the CBMN assays have been shown to be highly sensitive tools for the assessment of metal-based NPs.24–27
Materials and methods
Preparation and characterization of NINPs
Crystalline and amorphous NINPs were synthesized in the Nanostructured Materials Laboratory, Department of Engineering and Renewable Energies of the Federal University of the Pampa, Campus Bagé, RS (UNIPAMPA). The methodology used for synthesis is based on the generation of an organic matrix with transition metal inside. The organic matrix is then digested in order to form free metal oxide NPs.28 Briefly, the synthesis was carried out as follows, using reagent-grade raw materials without any further purification. Collage 2.5 g (Gelatin type A—Sargel Gelita) was dissolved in water (40 mL; Milli-Q) on a hotplate at 45°C with vigorous stirring for 30 min. Ammonium niobate oxalate hydrate [NH4H2NbO(C2O4)3·3H2O] (1 g) was gradually added to the solution, and the mixture stirred for 20 min. The solution was dried at 60°C for 48 h, annealed for 3 h at 250°C, and ground. The powder was annealed for 6 h at 450°C or 550°C in order to obtain the amorphous and crystalline NPs, respectively.
The structural characterization of powders was performed by means of powder X-ray diffraction (XRD) with Bragg–Brentano geometry (q-2q arrangement) using a Rigaku ULTIMA IV diffractometer operating with Cu-Kα radiation (0.154 nm) at 40 kV and 20 mA. The 2q angle was scanned with 0.02° step and 5 s of integration time from 15° to 80°. Morphology and particle size of the samples were estimated by transmission electron microscopy (TEM) using a JEOL JEM-1200 EXll, and the images were analyzed using ImageIJ 1..46r software). A total of 174 particles were counted to determine size distribution.
Cell culture
Chinese hamster ovary cells (CHO-K1) were purchased from the Rio de Janeiro Cell Bank (BCRJ, no. 0069). The CHO-K1 cells were maintained as a monolayer in 75-cm2 flasks in Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, Waltham, MA), supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco, Waltham, MA) and antibiotics (1% of penicillin/streptomycin solution and 0.1% of gentamycin solution; Gibco, Waltham, MA), and kept in a humidified 5% CO2 atmosphere at a temperature of 37°C until confluence was reached.
Trypan blue exclusion assay
Cell viability tests were carried out to determine the effects of NINPs (amorphous and crystalline) on cell viability at the concentrations to be assessed in the microplate-based comet assay. Cell viability was determined by trypan blue exclusion. CHO-K1 cells were seeded at a density of 1 × 104 into 24-well plates and cultured for 24 h. Then, the cells were exposed to different NINPs concentrations (6.5–210 μg/mL) for 4 h. After incubation, 15 μL of cell suspension was detached with trypsin/EDTA, incubated with 15 μL of trypan blue, and counted in a hemocytometer. Two independent determinations, in duplicate, were performed and only the concentrations that exhibited at least 70% viability were assessed in the comet assay.
Comet assay
For the experiments, cells were transferred to 24-well microplates, each well containing ~100,000 cells. After a 24 h incubation period cells were treated with increasing concentrations (6.5–210 μg/mL) of amorphous or crystalline NINPs. Incubations for 4 h with the solvent (distilled water) served as negative control and with ethylmethanesulfonate (EMS, CAS 62-50-0), Sigma-Aldrich St. Louis, MO; 0.5 mM) as a positive control. Thereafter cells were harvested and the alkaline single-cell microgel electrophoresis assay was performed according to Tice et al.22 Briefly, all microgels were coded, stained with ethidium bromide (Sigma-Aldrich, St. Louis, MO), and covered with a coverslip. The stained nucleoids were immediately evaluated at 400X magnification under an Olympus (BX41) fluorescence microscope (excitation filter, 515–560 nm; barrier filter, 590 nm), equipped with an image analysis system (Comet Assay IV, Perceptive Instruments, UK). Two slides with 50 cells (total of 100 cells) for every test sample were counted and analyzed (Tail Intensity, TI was used to quantify the percentage of DNA in tail).
CBMN-Cytome assay
The CBMN-cytome (Cyt) assay was carried out as recommended by Fenech23 with modifications. The cell culture conditions were performed as described for the comet assay. After incubation, cells were treated with different concentrations (6.5; 13; 26; and 53 μg/mL) of NINPs (amorphous and crystalline) as well as negative control (distilled water) and positive control (BLEO 3 μg/mL) for 4 and 24 h of treatment. After treatment, cells (CHO-K1) were washed twice in Dulbecco’s phosphate buffered saline (DPBS, Sigma-Aldrich, St. Louis, MO) and cytochalasin B (Cyt B; CAS 14,930–96–2; Sigma-Aldrich, St. Louis, MO), was added to a final concentration of 3 μg/mL in complete fresh medium.
After 2 cell cycles, Cyt B was removed and the cells were collected and harvested by cytocentrifugation (Cientec, Belo Horizonte, MG, Brazil); 160 μL of cell suspension were transferred to cytocentrifuge cups and centrifuged for 5 min at 700 rpm to produce 1 spot per slide. Slides with the cells were stained with Instant Prov (Newprov, Pinhais, PR, Brazil). After staining, the slides were air-dried and examined under 400× magnifications using a light microscope. Two independent experiments were performed in duplicate.
The nuclear division cytotoxicity index (NDCI) was estimated by scoring 500 cells with 1–4 nuclei. The NDCI was calculated using the formula [Ap + Nec + M1 + 2(M2) + 3(M3) + 4(M4)]/500, where M1–M4 represent the number of cells with one to 4 nuclei, respectively, Ap = number of apoptotic cells, Nec = number of necrotic cells.
Micronuclei (MNi), nuclear buds (NBUDs), and nucleoplasmic bridges (NPBs) were counted in 1,000 binucleated cells (BNC) per experimental point and were scored according to Fenech.23
Fluorescence and dark field images
CHO-K1 cells were treated for 4 h with 53 μg/mL of Nb2O5 NPs and collected as previously described for the CBMN test. After centrifugation, cells were fixed with methanol and stained with 4′,6-diamidino-2-phenylindole (DAPI- Sigma-Aldrich, St. Louis, MO) for analysis under a fluorescence microscope. Cell morphology was analyzed with an inverted phase-contrast microscope (Axiovert A1, Carl Zeiss, Germany). Photomicrographs were taken sequentially with a digital camera (AxioCam MRc, Zeiss), using AxioVision 3.1 software (Zeiss).
Statistical analysis
Statistical analysis was performed using the statistical package for social sciences (SPSS) software, version 18.0 (SPSS Inc., Chicago, IL). Normality was evaluated with the Kolmogorov–Smirnov test and homogeneity of variance was evaluated with Levene’s test. The results were expressed as means and standard deviations (±SD) or percentages. Since NBUDs values were not normally distributed, even after data transformation, the non-parametric Mann–Whitney U test with P < 0.05 was used to quantitatively determine the difference between negative control and treated groups. For DNA damage, NDCI, MNi and NPBs values, analysis of variance (1-way analysis of variance, ANOVA with Dunnett’s multiple comparison test) was used to compare treated groups with the negative control.
Results
The X-ray diffractogram (XRD) patterns of crystalline and amorphous NINPs, are shown in Fig. 1. Well defined and broader diffraction peaks can be observed in the crystalline sample, all corresponding to characteristic crystallographic planes of the orthorhombic phase (JCPDS: 30-0873),29 indicating the nanocrystalline nature of the Nb2O5 particles. The broadening peaks characteristic of amorphous scattering can be observed30 in the case of an amorphous sample.
Fig. 1.

XRD patterns for different samples of NINPs, crystalline (black), and amorphous (red).
The TEM analyses of crystalline NINPs showed dense particle agglomerates with an average size of 41.49 ± 26.30 nm. (n = 174; Fig. 2). The particle size ranged from 5 to 110 nm with predominant particle sizes between 10 and 70 nm, but rounded particles smaller than the mean value were occasionally observed. Polygonal particles with a dimension on the order of 100 nm were also observed. Particles in the amorphous NINPs (Fig. 2) had an average size of 33.15 ± 14.27 nm (N = 41) displaying a narrower size distribution and more spherical particles ranging in size from 15 to 55 nm.
Fig. 2.
Representative TEM images of A) crystalline and B) amorphous NINPs. Histograms of particle size distributions of C) crystalline, and D) amorphous NINPs.
The concentrations of NINPs used in the experiments were selected based on the maximum solubility that could be reached in the culture medium and are in accordance with previous studies performed with other metallic-based NPs.24,25 Thus, the maximal final concentration was 210 μg/mL for both amorphous and crystalline NINPs. Based on the trypan blue exclusion assay, >95% of the cells were viable (data not shown) when cultured with NINPs in the investigated concentrations of effects in the comet assay. The results of the analyses by the comet assay revealed an increase in DNA damage when CHO-K1 cells were exposed to 53, 105, and 210 μg/mL of crystalline NINPs, and to 210 μg/mL of amorphous NINPs (Fig. 3).
Fig. 3.
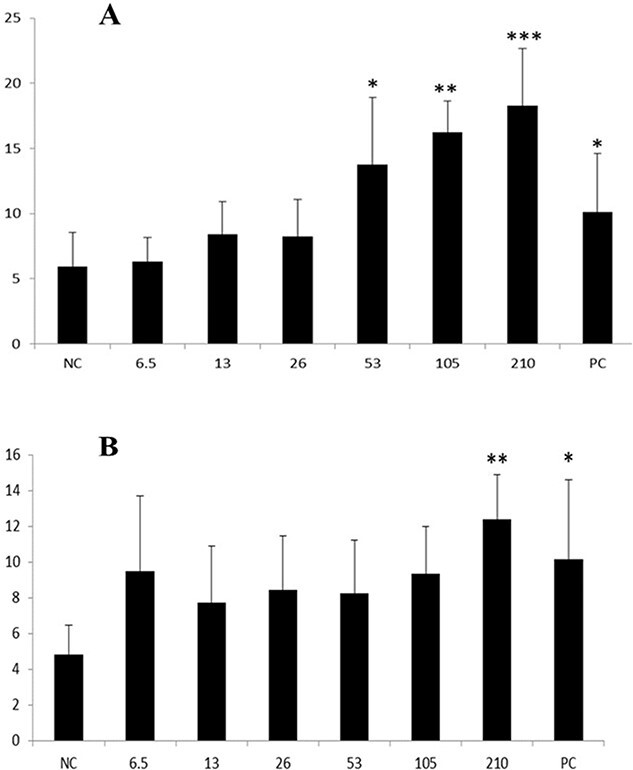
DNA damage evaluated by the comet assay after 4-h treatment of CHO-K1 cells at different concentrations (6.5–210 μg/mL) of NINPs. A) Crystalline. B) Amorphous. TI is expressed as mean ± SD (3 independent experiments in duplicate). NC: negative control (distilled water). PC: positive control (EMS—0.5 mM). *P < 0.05, **P < 0.01, and ***P < 0.001.
For the CBMN assay, cells exposed to 6.5–53 μg/mL of crystalline or amorphous NINPs were analyzed. Cells exposed to concentrations of NINPs (105 and 210 μg/mL) could not be scored in the CBMN test, since higher concentrations of NINPs accumulated on the cells, making it impossible to visualize the nuclei properly. Crystalline and amorphous NINPs significantly reduced the NDCI after a 24-h treatment period. Amorphous NINPs reduced NDCI at all concentrations tested (6.5–53 μg/mL) whereas crystalline NINPs only had an effect at the 2 higher concentrations (26 and 53 μg/mL; Fig. 4).
Fig. 4.
Effects of NINPs on the NDCI. Cultured CHO-K1 cells were exposed to different concentrations (6.5–53 μg/mL) of crystalline and amorphous NINPs for 4 or 24 h. **Significantly different from the negative control (P < 0.01) ***Significantly different from the negative control (P < 0.001; 1-way ANOVA; Dunnett’s multiple comparison test).
We next investigated the effects of NINPs on markers of chromosomal instability. Crystalline NINPs significantly increased the frequencies of nuclear buds (NBUDs) at the concentrations 26 and 53 μg/mL already after a 4-h exposure period. After a 24-h exposure period with the highest crystalline NINPs concentration (53 μg/mL) significant differences in the frequencies of MNi, NPBs, and NBUDs were observed.
In addition, lower concentrations (13 and 26 μg/mL) of crystalline NINPs induced significant increments in the NPBs and NBUDs frequencies after a 24-h exposure period. In the cells exposed to the lowest concentration of NPs (6.5 μg/mL) the induction was restricted to the frequency of NPBs (Table 1). None of the tested concentrations of amorphous NINPs were able to induce chromosomal instability after a 4-h treatment period. However, after a 24-h exposure period, all concentrations induced significant differences in the frequencies of MNi, compared with the negative control group (Table 2).
Table 1.
CBMN-Cyt assessment of crystalline NINPs.
| Treatments | Short treatment (4 h) | Extended treatment (24 h) | ||||
|---|---|---|---|---|---|---|
| MNia | NPBsa | NBUDsa | MNia | NPBsa | NBUDsa | |
| NC | 20.00 ± 3.37 | 5.60 ± 1.34 | 2.86 ± 1.68 | 14.00 ± 1.00 | 5.33 ± 1.15 | 1.00 ± 0.00 |
| 6.5 μg/mL | 20.80 ± 1.09 | 8.50 ± 3.33 | 3.37 ± 2.20 | 16.60 ± 3.05 | 13.75 ± 2.50** | 2.86 ± 2.54 |
| 13 μg/mL | 15.17 ± 3.60 | 6.50 ± 1.52 | 1.50 ± 0.55 | 14.67 ± 1.37 | 17.00 ± 2.64*** | 6.25 ± 2.99* |
| 26 μg/mL | 14.67 ± 2.66 | 10.00 ± 1.22 | 5.80 ± 1.30** | 16.00 ± 1.87 | 11.33 ± 1.15* | 5.33 ± 2.31* |
| 53 μg/mL | 13.83 ± 4.99 | 6.50 ± 2.38 | 5.40 ± 0.89** | 20.80 ± 3.42*** | 12.67 ± 2.89** | 4.60 ± 1.34* |
| PC | 80.40 ± 28.10*** | 24.00 ± 9.92*** | 12.80 ± 2.59*** | 69.86 ± 16.15*** | 20.00 ± 5.89*** | 7.57 ± 2.69** |
NC: negative control; PC: positive control (Bleomycin 3 μg/mL); MNi: micronuclei; NPBs: nucleoplasmic bridges; NBUDs: nuclear buds.
aValues are the mean ± standard deviation.
Significantly different from the negative control group *P < 0.05; **P < 0.01; and ***P < 0.001.
Table 2.
CBMN-Cyt assessment of amorphous NINPs.
| Treatments | Short treatment (4 h) | Extended treatment (24 h) | ||||
|---|---|---|---|---|---|---|
| MNia | NPBsa | NBUDsa | MNia | NPBsa | NBUDsa | |
| NC | 13.00 ± 2.24 | 10.28 ± 2.28 | 3.14 ± 0.69 | 11.50 ± 1.29 | 4.00 ± 2.45 | 2.86 ± 3.72 |
| 6.5 μg/mL | 15.25 ± 3.30 | 11.66 ± 5.35 | 4.17 ± 1.83 | 31.50 ± 4.51*** | 6.00 ± 0.82 | 2.71 ± 1.38 |
| 13 μg/mL | 16.20 ± 1.64 | 10.33 ± 2.50 | 3.28 ± 2.21 | 37.00 ± 4.76*** | 5.50 ± 2.38 | 3.67 ± 2.66 |
| 26 μg/mL | 17.25 ± 2.22 | 15.80 ± 5.12 | 5.83 ± 2.31 | 38.00 ± 1.41*** | 3.75 ± 1.50 | 5.37 ± 3.50 |
| 53 μg/mL | 14.40 ± 3.51 | 10.00 ± 2.00 | 5.00 ± 2.74 | 34.00 ± 3.74*** | 7.00 ± 1.82 | 3.60 ± 1.95 |
| PC | 49.25 ± 4.27*** | 17.00 ± 2.94* | 7.00 ± 2.00 | 87.20 ± 37.94*** | 23.20 ± 10.06*** | 8.60 ± 7.09* |
NC: negative control; PC: positive control (Bleomycin 3 μg/mL); MNi: micronuclei; NPBs: nucleoplasmic bridges; NBUDs: nuclear buds.
aValues are the mean ± standard deviation.
Significantly different from the negative control group *P < 0.05; **P < 0.01; and ***P < 0.001.
Finally, we investigated uptake and cellular association of NINPs by microscopy (Fig. 5). NINPs were distributed and sedimented onto CHO-K1 cells forming aggregates that passed through the cell membrane. The cytoplasm was completely filled with NINPs, but the aggregates tended to be more concentrated in the perinuclear rings. Although most of the particle’s aggregates may have been in the cytoplasm, it appears that some NPs were able to penetrate into the nucleus.
Fig. 5.

Uptake of NINPs by cultured CHO-K1 cells as assessed by combined dark field and fluorescent microscopic images. A) Binucleated CHO-K1 cells treated for 4 h with 53 μg/mL of crystalline NINPs, fixed with methanol and stained with 10-μg/mL DAPI. Cell nuclei appearing in blue, and crystalline NINPs appearing as white dots. B) Binucleated CHO-K1 cells from the negative control group. C) Binucleated CHO-K1 cells with crystalline NINPs appearing in the nuclei. Magnification 400×. Photomicrographs were taken sequentially with a digital camera (AxioCam MRc, Zeiss), using AxioVision 3.1 software (Zeiss).
Discussion
To our knowledge, this is the first study that investigated the genetic toxicology of niobium (V) oxide NPs. Our data demonstrate that crystalline and amorphous niobium (V) NPs damage nuclear DNA in cultured cells.
Previous studies, using CHO-K1 cells, have shown that some metallic oxide NPs are genotoxic. Thus, Souza et al.31 and Awasthi et al.32 demonstrated that Ag NPs induced DNA damage as revealed by the comet assay, and Jiang et al.33 observed a dose dependent increase of the MNi frequency after exposure to Ag NPs. Furthermore, Di Virgilio et al.34 showed that TiO2 and Al2O3 NPs significantly induced an increase in MNi frequencies in CHO-K1 cells. A similar genetic toxicity of metal oxide NPs was also demonstrated in other cell lines, such as Chinese V79 hamster lung fibroblasts cells,25 human lung IMR-90 cells,35 kidney cell line NRK-52E,36 and human neuronal cell line SH-SY5Y.37
The cytotoxicity of metal-based NPs has been previously demonstrated. Ruiz et al.27 showed that silver nanoparticles (AgNPs) were able to reduce the nuclear division index (NDI), increasing cytostasis, apoptosis and necrosis in human lymphocytes in the CBMN assay. In addition, De Carli et al.25 proved that the concentrations of 1,000 and 2,000 μg/mL of NiO NPs significantly reduced the cytokinesis-block proliferation index (CBPI) in the CBMN assay on rodent V79 cells. Copper oxide (CuO) NPs induced a strongly increased cytostasis and were markedly cytotoxic at 24 and 48-h treatment in human bronchial epithelial (BEAS-2B) cells in the CBMN test.
Our present results demonstrated that both crystalline and amorphous NINPs were genotoxic when assayed by the comet assay albeit at different concentration dependences. Whereas crystalline NPs induced significant DNA damage in the concentration range 53–210 μg/mL, an effect of amorphous NINPs was only seen when cells were treated with 210 μg/mL. Furthermore, crystalline NINPs more effectively induced chromosome instability as assessed by the CBMN assay. These differences could be related to differences in the size distribution, dimension and molecular structure of crystalline and amorphous NINPs. Crystalline NINPs were characterized by rounded and polygonal particles with an average size of about 42 nm and with a size distribution that varied between 5 and 110 nm, whereas amorphous NINPs had a much narrower size distribution and more spherical particles with an average size of 34 nm. The structural diversity of niobium pentoxide was previously demonstrated in scientific literature, being attributed to different arrangements of the building blocks that can lead to Nb2O5 stoichiometry.38 Thus, the synthesis route of niobium pentoxide nanomaterials is essential to their specific practical processes.39
Cytotoxicity assessed by the CBMN assay was evident only after a 24-h treatment period with crystalline and amorphous NINPs. Considering the chromosome instability biomarkers, a significant induction of NBUDs could be detected in CHO-K1 cells after treatment for 4 h with crystalline NINPs at the concentrations of 26 and 53 μg/L. These results are in accordance with other studies demonstrating mutagenicity of metallic NPs after 24-h treatment.25,33,34 The CBMN assay reveals permanent damage originating from chromosome instability. Concerning the markers of chromosome damage, MNi occurs as a result of chromosome fragmentation or loss, which could have originated from clastogenic or aneugenic events, respectively.40 NBUDs are suitable biomarkers of gene amplification, arising from a chromosomal breakage–fusion–bridge (B–F–B) cycle that could lead to amplified DNA or chromatin eliminated throughout the S-phase of mitosis.23 NPBs represent dicentric chromosomes, as a result of chromosome breaks and rearrangements originated from DNA mis-repair and/or telomere end-fusion events.40 Thus, considering the results of the CBMN assay, it can be concluded that the crystalline sample is preferentially an inducer of DNA breaks, since most of the positive results were observed in NPBs and NBUDs groups. Only the highest concentration (53 μg/mL) was able to significantly increase the MNi frequency. On the other hand, in the amorphous sample, the chromosome damage was restricted to the induction of MNi at all concentrations assessed, characterizing its clastogenic and/or aneugenic potential.
The combined dark field and fluorescent image showed that crystalline NINPs sedimented onto the CHO-K1 cells, passing through the cell membrane and outlining the nucleus. Although most of the NPs aggregates were observed in the cytoplasm of each cell, some aggregates appeared to penetrate into the nucleus. These results are in accordance with Zucker et al.41 who demonstrated the same pattern of aggregation, sedimentation, and uptake in ARPE-19 cells exposed to TiO2 NPs. NPs have been shown to cross the cell membrane and accumulate within various mammalian cell lines and their entry follows 3 routes—simple diffusion, through ion channels and endocytosis.42
Although the mechanisms underlying the induction of DNA damage, cytotoxicity and chromosome instability by NINPs have not been fully investigated and elucidated, they should be similar to those reported for other metal oxide NPs. Metal-based NPs can induce DNA damage by different mechanisms, either through oxidative stress, increasing the levels of reactive oxygen species (ROS), or by stimulating cells to produce other oxidants and/or genotoxic compounds that may possibly affect mitochondrial electron transport.7,9,43 The production of ROS is directly correlated with the formation of DNA strand breaks,44–46 which could explain the induction of chromosome instability and DNA fragmentation as observed in the CBMN assay and in the comet test, respectively.
Conclusion
Engineered metal-based NPs are present in a range of products. In the medical field, metal oxide NPs offer favorable expectations due to their special physico-chemical characteristics, which include their biocompatibility and magnetic properties, allowing them to be manipulated in an external magnetic field. Thus, the assessment and characterization of the cytotoxicity and genotoxic profile of NINPs are essential to evaluate the carcinogenic risk associated with exposure to these materials. The results of this study pointed to the cytotoxic, genotoxic, and mutagenic potential of NINPs. Future investigations should focus on the assessment of the genotoxicity of NINPs in in vivo models.
Authors’ contributions
RFS, TRC, and RRD conceived the study. RFS and APdS performed the Comet and CBMN assays and analyzed the data. RFS performed and obtained the fluorescence and dark field images. TRC, AS, and WHF synthesized the NINPs, performed the XRD analysis and structurally characterized the NINPs in the TEM microscopy. ML performed the statistical analysis and wrote the manuscript. RRD guided the overall study, contributed reagents and materials, analyzed the data, and wrote the paper.
Funding
This study was financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. This work was supported by the Brazilian National Council of Technological and Scientific Development (CNPq, grant number 308497/2019-8) and Lutheran University of Brazil.
Conflicts of interest. The authors declare that they have no conflict of interest.
Contributor Information
Raíne Fogliati De Carli Schardosim, Laboratory of Genetic Toxicity and Cellular Toxic-Genetics Analysis, Graduate Program in Molecular and Cellular Biology Applied to Health, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil.
Tatiane Rocha Cardozo, Laboratory of Genetic Toxicity and Cellular Toxic-Genetics Analysis, Graduate Program in Molecular and Cellular Biology Applied to Health, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil; Research Group on Nanostructured Materials, Federal University of the Pampa, Campus Bagé, Avenida Maria Anunciação Gomes de Godoy, 1650, 96413-172, RS, Brazil.
Ana Paula de Souza, Laboratory of Genetic Toxicity and Cellular Toxic-Genetics Analysis, Graduate Program in Molecular and Cellular Biology Applied to Health, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil.
Allan Seeber, Research Group on Nanostructured Materials, Federal University of the Pampa, Campus Bagé, Avenida Maria Anunciação Gomes de Godoy, 1650, 96413-172, RS, Brazil.
Wladimir Hernandez Flores, Research Group on Nanostructured Materials, Federal University of the Pampa, Campus Bagé, Avenida Maria Anunciação Gomes de Godoy, 1650, 96413-172, RS, Brazil.
Maurício Lehmann, Laboratory of Genetic Toxicity and Cellular Toxic-Genetics Analysis, Graduate Program in Molecular and Cellular Biology Applied to Health, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil.
Rafael Rodrigues Dihl, Laboratory of Genetic Toxicity and Cellular Toxic-Genetics Analysis, Graduate Program in Molecular and Cellular Biology Applied to Health, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil; Postgraduate Program in Dentistry, Lutheran University of Brazil (ULBRA), Avenida Farroupilha, 8001, 92425-900, Canoas, RS, Brazil.
References
- 1. Paschoalino MP, Marcone GPS, Jardim WF. Os nanomateriais e a questão ambiental. Química Nova. 2010:33:421–430. [Google Scholar]
- 2. Maynard AD, Warheit DB, Philbert MA. The new toxicology of sophisticated materials: Nanotoxicology and beyond. Toxicol Sci. 2011:120:S109–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Salvadori MR, Nascimento CAO, Corrêa B. Nickel oxide nanoparticles film produced by dead biomass of filamentous fungus. Sci Rep. 2014:3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Srinivasan SY, Paknikar KM, Bodas D, Gajbhiye V. Applications of cobalt ferrite nanoparticles in biomedical nanotechnology. Nanomedicine. 2018:13:1221–1238. [DOI] [PubMed] [Google Scholar]
- 5. Collins A, El Yamani N, Dusinska M. Sensitive detection of DNA oxidation damage induced by nanomaterials. Free Radic Biol Med. 2017:107:69–76. [DOI] [PubMed] [Google Scholar]
- 6. Ng CT, Yong LQ, Hande MP, Ong CN, Yu LE, Bay BH, Baeg GH et al. Zinc oxide nanoparticles exhibit cytotoxicity and genotoxicity through oxidative stress responses in human lung fibroblasts and Drosophila melanogaster. Int J Nanomedicine. 2017:12:1621–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardozo TR, De Carli RF, Seeber A, Flores WH, Da Rosa JAN, Kotzal QSG, Lehmann M, da Silva FR, Dihl RR et al. Genotoxicity of zinc oxide nanoparticles: an in vivo and in silico study. Toxicol Res. 2019:8:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mortezaee K, Najafi M, Samadian H, Barabadi H, Azarnezhad A, Ahmadi A. Redox interactions and genotoxicity of metal-based nanoparticles: a comprehensive review. Chem Biol Interact. 2019:312:108814. [DOI] [PubMed] [Google Scholar]
- 9. Makhdoumi P, Karimi H, Khazaei M. Review on metal-based nanoparticles: role of reactive oxygen species in renal toxicity. Chem Res Toxicol. 2020:33:2503–2514. [DOI] [PubMed] [Google Scholar]
- 10. Vuković B, Milić M, Dobrošević B, Milić M, Ilić K, Pavičić I, Šerić V, Vrček IVet al. Surface stabilization affects toxicity of silver nanoparticles in human peripheral blood mononuclear cells. Nanomaterials. 2020:10:1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lopes OF, De Mendonça VR, Silva FBF, Paris EC, Ribeiro C. Óxidos de nióbio: Uma visão sobre a síntese do Nb2O5e sua aplicação em fotocatálise heterogênea. Quim Nova. 2015:38:106–117. [Google Scholar]
- 12. Yang R, Zhang F, Lei X, Zheng Y, Zhao G, Tang Y, Lee CSet al. Pseudocapacitive Ti-doped niobium pentoxide nanoflake structure design for a fast kinetics anode toward a high-performance mg-ion-based dual-ion battery. ACS Appl Mater Interfaces. 2020:12:47539–47547. [DOI] [PubMed] [Google Scholar]
- 13. Figueiredo EZ, Souza Balbinot G, Leitune VCB, Collares FM. Niobium silicate as a filler for an experimental photopolymerizable luting agent. J Prosthodontic Res. 2021:65:25–30. [DOI] [PubMed] [Google Scholar]
- 14. Ahssi MAM, Erden MA, Acarer M, Çuğ H. The effect of nickel on the microstructure, mechanical properties and corrosion properties of niobium-vanadium microalloyed powder metallurgy steels. Materials. 2020:13:4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li F, Song L, Yang X, Huang Z, Mou X, Syed A, Bahkali AH, Zheng Let al. Anticancer and genotoxicity effect of (Clausena lansium (Lour.) Skeels) Peel ZnONPs on neuroblastoma (SH-SY5Y) cells through the modulation of autophagy mechanism. J Photochem Photobiol B Biol. 2020:203:111748. [DOI] [PubMed] [Google Scholar]
- 16. Cegan T, Petlak D, Skotnicova K, Jurica J, Smetana B, Zla S. Metallurgical preparation of Nb-Al and W-Al intermetallic compounds and characterization of their microstructure and phase transformations by DTA technique. Molecules. 2020:25:2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senocak TC, Ezirmik KV, Aysin F, Simsek Ozek N, Cengiz S. Niobium-oxynitride coatings for biomedical applications: its antibacterial effects and in-vitro cytotoxicity. Mater Sci Eng C. 2021:120:111662. [DOI] [PubMed] [Google Scholar]
- 18. Canepa P, Firpo G, Mattera L, Canepa M, Cavalleri O. Calcium and phosphorous enrichment of porous niobium and titanium oxides for biomaterial applications. Surf Coat Technol. 2020:389:125634. [Google Scholar]
- 19. Ge J, Wang F, Xu Z, Shen X, Gao C, Wang D, Hu G, Gu J, Tang T, Wei Jet al. Influences of niobium pentoxide on roughness, hydrophilicity, surface energy and protein absorption, and cellular responses to PEEK based composites for orthopedic applications. J Mater Chem B. 2020:8:2618–2626. [DOI] [PubMed] [Google Scholar]
- 20. Mestieri LB, Gomes-Cornélio AL, Rodrigues EM, Faria G, Guerreiro-Tanomaru JM, Tanomaru-Filho M. Cytotoxicity and bioactivity of calcium silicate cements combined with niobium oxide in different cell lines. Braz Dent J, Fundação Odontológica de Ribeirão Preto. 2017:28:65–71. [DOI] [PubMed] [Google Scholar]
- 21. Aardema MJ, Snyder RD, Spicer C, Divi K, Morita T, Mauthe RJ, Gibson DP, Soelter S, Curry PT, Thybaud V, et al. SFTG international collaborative study on in vitro micronucleus test III. Using CHO cells. Mutat Res. 2006:607:61–87. [DOI] [PubMed] [Google Scholar]
- 22. Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YFet al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000:35:206–221. [DOI] [PubMed] [Google Scholar]
- 23. Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007:2:1084–1104. [DOI] [PubMed] [Google Scholar]
- 24. Reis É, De Rezende AAA, Santos DV, De Oliveria PF, Nicolella HD, Tavares DC, Silva ACA, Dantas NO, Spanó, MAet al. Assessment of the genotoxic potential of two zinc oxide sources (amorphous and nanoparticles) using the in vitro micronucleus test and the in vivo wing somatic mutation and recombination test. Food Chem Toxicol. 2015:84:55–63. [DOI] [PubMed] [Google Scholar]
- 25. De Carli RF, Chaves DDS, de M.Cardozo TR, Souza AP, Seeber A, Flores WH, Honatel KF, Lehmann M , Dihl RRet al. Evaluation of the genotoxic properties of nickel oxide nanoparticles in vitro and in vivo. Mutat Res. 2018:836:47–53. [DOI] [PubMed] [Google Scholar]
- 26. Li Z, Li Z, Tian W. Strengthening effect of NB on ferrite grain boundary in x70 pipeline steel. Materials. 2021:14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruiz-Ruiz B, Arellano-García ME, Radilla-Chávez P, Salas-Vargas DS, Toledano-Magaña Y, Casillas-Figueroa F, Vazquez-Gomez RL, Pestryakov A, García-Ramos JC,Bogdanchikova Net al. Cytokinesis-block micronucleus assay using human lymphocytes as a sensitive tool for cytotoxicity/genotoxicity evaluation of AgNPs. ACS Omega. 2020:5:12005–12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Meneses CT, Flores WH, Sasaki JM. Direct observation of the formation of nanoparticles by in situ time-resolved X-ray absorption spectroscopy. Chem Mater. 2007:19:1024–1027. [Google Scholar]
- 29. Ko EI, Weissman JG. Structures of niobium pentoxide and their implications on chemical behavior. Catal Today. 1990:8:27–36. [Google Scholar]
- 30. Scheuer C, Boot E, Carse N, Clardy A, Gallagher J, Heck S, Marron S, Martinez-Alvarez L, Masarykova D, Mcmillan P, et al. Elements of X-ray diffraction, third edition [internet]. New York: Prentice-Hall; 2001 [Google Scholar]
- 31. Souza TAJ, Franchi LP, Rosa LR, Veiga MAMS, Takahashi CS. Cytotoxicity and genotoxicity of silver nanoparticles of different sizes in CHO-K1 and CHO-XRS5 cell lines. Mutat Res. 2016:795:70–83. [DOI] [PubMed] [Google Scholar]
- 32. Awasthi KK, Awasthi A, Verma R, Kumar N, Roy P, Awasthi K, John PJet al. Cytotoxicity, genotoxicity and alteration of cellular antioxidant enzymes in silver nanoparticles exposed CHO cells. RSC Adv. 2015:5:34927–34935. [Google Scholar]
- 33. Jiang X, Foldbjerg R, Miclaus T, Wang L, Singh R, Hayashi Y, Sutherland D, Chen C, Autrup H, Beer Cet al. Multi-platform genotoxicity analysis of silver nanoparticles in the model cell line CHO-K1. Toxicol Lett. 2013:222:55–63. [DOI] [PubMed] [Google Scholar]
- 34. Di Virgilio AL, Reigosa M, Arnal PM, Lorenzo F, Mele M. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminium oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. J Hazard Mater. 2010:177:711–718. [DOI] [PubMed] [Google Scholar]
- 35. AshaRani PV, Mun GLK, Hande MP, Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009:3:279–290. [DOI] [PubMed] [Google Scholar]
- 36. Abudayyak M, Guzel E, Özhan G. Nickel oxide nanoparticles induce oxidative DNA damage and apoptosis in kidney cell line (NRK-52E). Biol Trace Elem Res. 2017:178:98–104. [DOI] [PubMed] [Google Scholar]
- 37. Abudayyak M, Guzel E, Özhan G. Nickel oxide nanoparticles are highly toxic to SH-SY5Y neuronal cells. Neurochem Int. 2017:108:7–14. [DOI] [PubMed] [Google Scholar]
- 38. Nico C, Monteiro T, Graça MPF. Niobium oxides and niobates physical properties: review and prospects. Prog Mater Sci. 2016:80:1–37. [Google Scholar]
- 39. Skrodczky K, Antunes MM, Han X, Santangelo S, Scholz G, Valente AA, Pinna N, Russo PAet al. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun Chem. 2019:2:1–11. [Google Scholar]
- 40. Fenech M. The in vitro micronucleus technique. Mutat Res. 2000:455:81–95. [DOI] [PubMed] [Google Scholar]
- 41. Zucker RM, Massaro EJ, Sanders KM, Degn LL, Boyes WK. Detection of TiO2nanoparticles in cells by flow cytometry. Cytometry Part A. 2010:77:677–685. [DOI] [PubMed] [Google Scholar]
- 42. Vidya PV, Chitra KC. Induction of cytotoxicity by selected nanoparticles in Chinese hamster ovary-K1 cells. Int J Appl Sci Biotechnol. 2017:5:203–207. [Google Scholar]
- 43. Bastos V, Duarte IF, Santos C, Oliveira H. Genotoxicity of citrate-coated silver nanoparticles to human keratinocytes assessed by the comet assay and cytokinesis blocked micronucleus assay. Environ Sci Pollut Res. 2017:24:5039–48. [DOI] [PubMed] [Google Scholar]
- 44. Zhuang H, Yao C, Zhao X, Chen X, Yang Y, Huang S, Lingtao Pan 1, Aifang Du 5, Yi Yang. DNA double-strand breaks in the toxoplasma gondii-infected cells by the action of reactive oxygen species. Parasites Vectors. 2020:13:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider CC, Hessenauer A, Götz C, Montenarh M. DMAT, an inhibitor of protein kinase CK2 induces reactive oxygen species and DNA double strand breaks. Oncol Rep. 2009:21:1593–1597. [DOI] [PubMed] [Google Scholar]
- 46. Jakubczyk K, Dec K, Kałduńska J, Kawczuga D, Kochman J, Janda K. Reactive oxygen species - sources, functions, oxidative damage. Pol Merkur Lekarski. 2020:48:124–127. [PubMed] [Google Scholar]




