Abstract
Background
Inhalation of silica crystals in occupational settings is a main cause of silicosis, a chronic irreversible pulmonary disorder. Our prior studies demonstrated the activation of inflammasome sensors AIM2 and NLRP3, effector protein caspase-1, and significant increase in IL-1β in silica exposed rats, suggesting that the canonical inflammasome activation may be associated with silica-induced tissue damage and inflammation.
Aims and Methods
In our current study using the same animal model system, we further evaluated the components of non-canonical inflammasome, including NEK7, caspase-11, and GSDMD following silica exposure.
Results
We demonstrated sustained NEK7 elevation in the rat lung epithelial cells and macrophages following 1- and 3-day exposure. Enhanced NEK7 expression was also detected in lung homogenate by western blot. Similarly, caspase-11 expression was induced by silica exposure in lung sections and homogenate. Elevated GSDMD was observed both in lung sections by immunohistochemical staining and in lung tissue homogenate by western blot.
Conclusion
In summary, our current study demonstrated increase in NEK7, caspase-11, and GSDMD in silica exposed rats, indicating activation of non-canonical inflammasome complex, thereby providing a broad inflammasome activation pathway caused by silica exposure.
Keywords: Non-canonical inflammasome activation, silica, NEK7, caspase-11, GSDMD
1. Introduction
Inflammasome is a crucial player of innate immune system in defending viral and bacterial pathogen invasion as well as in eliminating endogenous damaged and senescent cells.1 Inflammasome contains multiprotein components, which are categorized as sensor, adaptor, and effector.2 Inflammasome sensor carries out the function of detecting microbial components, cell injury or environmental irritants, and based on protein structure they are divided into three major subsets, namely the nucleotide-binding domain and leucine-rich repeat containing proteins (also known as NOD-like receptors, NLRs); the absent in melanoma 2 (AIM)-like receptors (ALRs); and pyrin.3
The NLR family of inflammasome sensor often has a tripartite structure, containing an N-terminal caspase recruitment and activation (CARD) or pyrin (PYD) domain, a central nucleotide-binding and oligomerization domain (NOD or the NACHT domain), and a C-terminal leucine-rich repeats (LRR) domain.4 The N-terminal death-fold domains of the NLR proteins take part in interaction with adaptor (such as ASC) and/or effector (such as caspase (i) components, the NACHT domain with ATPase activity plays a role in the oligomerization of the complex, and the C-terminal LRR domain regulates ligand interaction.5 The members of NLR family include the nucleotide-binding oligomerization domain-like receptor (NLR)-family, pyrin domain (PYD)-containing 1 (NLRP1); NLR-family, PYD-containing 3 (NLRP3); NLR-family apoptosis inhibitory protein (NAIP); NLR-family, caspase activation and recruitment domain (CARD)-containing 4 (NLRC4), and NLRP2, NLRP6, NLRP7, NLRP9, and NLRP12.6
The stimuli of canonical inflammasome activation include a variety of pathogens, such as bacteria, viruses, and fungi; endogenous ATP and peptide; and crystalline ligands, such as uric acid or cholesterol crystals.7 Upon specific recognition and binding of these ligands to sensor receptors, the corresponding NLR or AIM2 forms oligomers and recruits pro-caspase-1, whose activation subsequently catalyzes cleavage of the proinflammatory cytokines (such as IL-1β and IL-18) into their bioactive forms, initiating inflammatory response.8 Non-canonical inflammasome represents an additional layer of immune defense. Instead of caspase-1, inflammatory capsase-4, and −5 are activated in response to gram-negative bacterial and function as effectors.9,10
Silicosis is a chronic interstitial fibrosing pulmonary disease that is often related to occupational inhalation, retention, and pulmonary reaction to dust particles containing free crystalline, also known as silica.11 Chronic exposure to silica causes alterations in soluble lymphokines, which either stimulate collagen production in fibroblasts or function as fibroblast proliferative factor, causing organ fibrogenesis.12 Therefore, in vivo model system better recapitulated the complex pathogenetic alterations caused by interactions among multiple cellular events in comparison to in vitro studies. Using laboratory animal model, we detected respiratory inflammation and interstitial edema following silica intratracheal (IT) instillation.13 A single high dose of silica (~250 mg/kg body weight) was used in the current studies based on prior publications, which showed induction of obvious inflammation and lung damage by IT of silica at similar dose in rats.14 More importantly, we demonstrated activation of canonical inflammasome evidenced by elevated NLRP3, AIM2, and interleukin 1β (IL-1β).13 In the current study, we further evaluated the effect of silica exposure on activation of non-canonical inflammasome pathway components, including NIMA-related kinase-7 (NEK7), caspase-11, and GSDMD.
2. Methods and materials
2.1 Animals
Eight-week-old male Wistar rats weighing 200–220 g were purchased from Beijing Weitong Lihua Experimental Animal Technology Co, Ltd. and were maintained in a pathogen-free environment. The animals were kept on a 12-h light–dark cycle with free access to food and water. The animal experimental protocol (factors involved in SO2 induced pulmonary fibrosis, 2020-animal-275) was approved by the Animal Ethical Committee of Capital Medical University.
2.2 Preparation of silica suspension and IT instillation
Silicon dioxide powder (.5–10 microns) was purchased from Sigma-Aldrich (Cat# S5631). The silica was autoclaved immediately before use, and then suspended in sterile pharmacological grade saline at a concentration of 25, 50, 75, or 100 mg/mL. Fifty mg/mL was determined as the dose for acute exposure based on the inflammation response tested (Supplementary Fig. S1A)13 The silica suspension was sonicated and vigorously vortexed before administration. The rats were anesthetized using an i.p. injection of sodium pentobarbital (5 mg/100 g body weight). For the treatment groups, the animals received a single trans-oral IT of silica suspension of 1 ml as described previously.14 The control group received IT of 1 ml sterile saline. Animals were sacrificed using sodium pentobarbital (120 mg/rat) on day1 or day 3 after silica instillation. The control group animals were sacrificed 1 day after saline instillation.
2.3 Bronchoalveolar lavage fluids
A 20−gauge angiocatheter was ligated into the trachea and 5 ml PBS was used to lavage the rat lungs. The lavage fluid was spun at 500×g for 10 min (4 °C) and cells were collected; the total recovery of the bronchoalveolar lavage fluids (BALF) was 90–95% of the instilled PBS volume.
2.4 IHC assessment of inflammasome components
The fixed and embedded lung sections, 3-μm thick, were deparaffinized. Epitope retrieval was performed in citric acid-sodium citrate buffer (1 M, pH 6.0) using a pressure cooker for 15 min at 90 °C. After the slides were cooled down and washed in phosphate buffered saline (PBS) for three times, they were rinsed in hydrogen peroxide (3%) for 10 min to inactivate endogenous peroxidase antigens. The slides were then blocked in 2.5% goat serum (diluted in PBS, Sigma, Cat# G9023-5ML) and were incubated with antibodies against NEK7 (Novus, Cat# NBP1-31110, 1:300), caspase-11 p20 (A-2) (Santa Cruz, Cat# sc-374615, 1:50), and GSDMD (Absin, Cat# abs143289, 1:400) for 1 h at 37 °C. PBS was used in the negative control group instead of primary antibody. The slides were rinsed in PBS and were then incubated with peroxidase-conjugated anti-rabbit IgG (DAKO, Cat# K4003) and anti-mouse IgG (DAKO, Cat# K4001) for .5 h at 37 °C. Staining was then performed using a 3,3′-diaminobenzidine staining kit (Thermo Fisher, Cat# TL-125-QHD). A positive reaction was indicated by brown staining in the nuclei and cytoplasm. The IHC staining was evaluated under a Zeiss Axio. Scope A1 light microscope.
2.5 Western blot analysis
The right lobes of the resected lung tissues were homogenized and lysed on ice using Dounce tissue homogenizer (Tissueprep, China) in tissue lysis buffer [8 M urea, 4% CHAPS, 40 mM Tris(base), and 1% PMSF] supplemented with a cocktail of protease inhibitors (Promega) at the ratio of 250 mg tissue/1 mL lysis buffer. The supernatant was collected after centrifuge at 14,000×g for 15 min. The protein concentrations were determined using BCA kit (Thermo Scientific). Equal amounts of proteins from each sample (50 μg) were resolved by SDS-PAGE, transferred to PVDF membranes, blocked with 5% skimmed milk for 1 h at 25 °C, and probed at 4 °C overnight with the primary antibodies against NEK7 (Novus, Cat# NBP1-31110, 1:1500), NLRP3(Absin,Cat#abs136950,1:100), caspase-11 p20 (A-2) (Santa Cruz, Cat# sc-374,615, 1:500), and GSDMD (Absin, Cat# abs143289, 1:1000), and β-actin (Beyotime, 1:1000). Blots were subsequently probed with horseradish peroxidase-conjugated anti-rabbit IgG and anti-moue IgG (Beyotime, China) at 1:1000. Immunoreactive bands were visualized by enhanced chemiluminescence (PPLYGEN, China). Quantitation was analyzed densitometrically using ImageQuant 5.2 software (GE Healthcare, Pittsburgh, PA).
2.6 Measurement of interleukins by enzyme-linked immunosorbent assay
The supernatant of homogenized lung tissue or cell pellets from BALF was collected for enzyme-linked immunosorbent assay (ELISA). Cell pellets were lysed in 1-ml tissue lysis buffer [50 mM Tris,pH 7.4250 mM NaCl,5 mM EDTA,50 mM NaF,1 mM Na3VO4,1% NP40,.02% NaN3] supplemented with a cocktail of protease inhibitors (Promega). The protein concentrations were determined using BCA kit (Thermo Scientific). The same amount of protein was used for ELSIA after protein concentration assay. Interleukin-18 (Abcam, Cat# ab213909) and Interleukin-1β (Abcam, Cat# ab100767) were measured using commercial ELISA kits. In brief, the samples were applied onto 96-well plate. Calibration curve was constructed with eight points by serially diluting a solution of recombinant rat IL-18 (10 ng/mL) and rat IL-1β(10 ng/mL). The plate was incubated at room temperature for 2.5 h, and washed 6 times with washing solution (PBS, pH 7.4 containing .5% of Tween). A secondary antibody was applied to each well and the plate was incubated for 20 min at 37 °C. After washing, 100 μl of HRP-linked streptavidin was added to each well and the plate was placed at room temperature for 45 min. After further washing, the amount of IL-18 or rat IL-1β was determined by addition of 3,3′,5,5′-tetramethylbenzidine and was read at 450 nm after stopping the reaction by adding 2 M H2SO4.
3. Results
3.1 Activation of NEK7 following silica IT instillation
Our recent study demonstrated that silica exposure caused inflammation and elevation of NLRP3 expression in rat IT instillation model.13 NEK7 is an essential factor that regulates the activation of the NLRP3 inflammasome;15 therefore, we first confirmed NEK7 and confirmed NLRP3 response to make sure that the silica IT instillation was reproducible (Fig. 1). NEK7 expression was examined by immunohistochemical staining using rat lung sections at day-1 and day-3 post one does silica exposure. As shown in Fig. 1, basal NEK7 expression was barely detectable in the lung tissue of the control animals without silica exposure (Fig. 1A), while the NEK7 expression was elevated at 1 day post exposure (Fig. 1B) and at day-3 (Fig. 1C). Sustained NEK7 elevation was seen in the rat lung epithelial cells (short arrows) and macrophages (long arrows) day-1 and day-3 post exposure (Figs. 1B and C). Next, we examined NEK7 expression in lung tissue by western blot. We observed elevation of NEK7 expression consistently and confirmed its involvement in activation of NLRP3 inflammasome (Fig. 1D).
Fig. 1.
Evaluation of NEK7 expression by immunohistochemical staining using rat lung sections following silica exposure. Immunochemical (IHC) staining of NEK7 was performed using the lung sections of silica exposed rats (B for silica 1-day, C for silica 3-days) and control animals (A). Long arrows indicated macrophages, and short arrows indicated lung epithelial cells. Scale bars: 50 μm. (D) Lung tissue homogenates from the control and silica exposed rats (n = 4/group) were subjected to western blot analysis for NLRP3 and NEK7. β-Actin was used as loading control. Quantitation of the bands was analyzed densitometrically using ImageQuant 5.2 software (GE Healthcare, Pittsburgh, PA) ***P < .001 vs 0, ###P < .001 vs 1-day exposure.
3.2 Elevation of non-canonical inflammasome component caspase-11 in response to silica exposure
After confirming the canonical inflammasome role, we set to study the non-canonical inflammasome component in the acute response to silica exposure. At baseline conditions, caspase-11 is usually undetectable, but rapidly induced upon environmental stimulation, such as pathogens, LPS, ATP.16 We employed immunohistochemical staining to determine caspase-11 of rat lung sections following silica exposure. As shown in Fig. 2, caspase-11 expression was induced by acute silica exposure at day-1 (Fig. 2B) and day-3 (Fig. 2C) in both the rat lung epithelial cells (short arrows) and macrophages (long arrows) compared to control animals (Fig. 2A). Elevated caspase-11 expression was also readily detected in lung tissue following silica exposure (Fig. 2D), confirming induction of non-canonical inflammasome executor component. To confirm this is not a non-specific reaction of caspase-11, we further tested IL-1β as one of the downstream targets of NLRP3 in cell pellets from BALF collected from exposed animals (Fig. 2E), an increase of IL-1β from cells collected BALF was observed as soon as 1 day post-acute silica exposure.
Fig. 2.
Silica exposure increased non-canonical inflammasome protein caspase-11 expression. IHC staining of Caspase-11 was performed using the lung sections of silica exposed rats (B for silica 1-day, C for silica 3-days) and control animals (A). Long arrows indicated macrophages, and short arrows indicated lung epithelial cells. Scale bars: 50 μm. (D) Lung tissue homogenates from the control and silica exposed rats (n = 5/group) were subjected to western blot analysis for caspase-11. β-Actin was used as loading control. Quantitation was analyzed densitometrically using ImageQuant 5.2 software (GE Healthcare, Pittsburgh, PA). (E) BALF-IL-1βfollowing silica exposure. The lysed cell pellets concentrated from BALF from the control and silica exposed rats(n = 4/group) were used for ELISA analysis of IL-1β levels *P < .05 vs control.
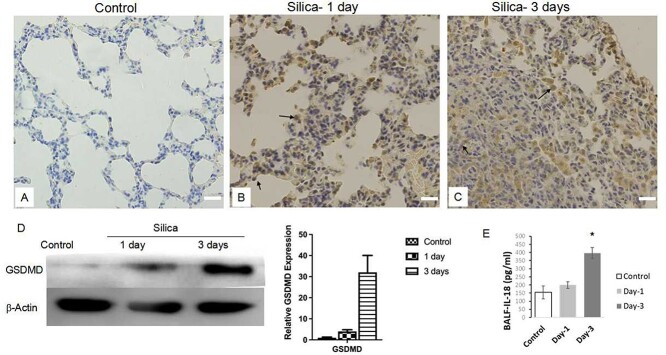
3.3 Silica exposure increased GSDMD and IL-18
The activated caspase-11 could catalyze GSDMD cleavage and the resultant GSDMD N-terminal fragments oligomerize and form pores on cell membrane.17 Mature IL-1β and IL-18 along with other proinflammatory factors are released from the pores leading to amplification of cellular inflammatory reaction. Therefore, we examined GSDMD expression and found that GSDMD was elevated in exposed lung tissue evidenced by IHC (Fig. 3A-3C) and western blot (Fig. 3D). Then, we analyzed the IL-18 alternation using lung tissue from control (n = 7), and silica-exposed animals (n = 4 for silica day-1, n = 5 for silica days-3). To our surprise, the ELISA results did not show significant difference in IL-18 (pg/mg lung tissue, control 208.5 ± 27.4, silica-1 day 199.1 ± 28.6, silica-3 days 191.7 ± 37.2, Supplementary Fig. S1B). We suspect this might be due to the silica particle aggregation and agglomeration in major tracheal wall, and we further tested in IL-18 from cell pellets from BALF collected from exposed animals (Fig. 3E). An increase of IL-18 was observed in BALF collected at 3 days post-acute silica exposure.
Fig. 3.
Detection of GSDMD and IL-18 changes in rat lung following silica exposure. (A-C) IHC staining of GSDMD was performed using the lung sections of silica exposed rats (B for silica 1-day, C for silica 3-days) and control animals (A). Long arrows indicated macrophages, and short arrows indicated lung epithelial cells. Scale bars: 50 μm. (D) Lung tissue homogenates from the control and silica exposed rats (n = 5/group) were subjected to western blot analysis for GSDMD. β-Actin was used as loading control. Quantitation was analyzed densitometrically using ImageQuant 5.2 software (GE Healthcare, Pittsburgh, PA) (E) BALF-IL-18 following silica exposure. The lysed cell pellets concentrated from BALF from the control and silica exposed rats (n = 4/group) were used for ELISA analysis of IL-18 levels *P < .05 vs control.
4. Discussion
Silicosis is a chronic irreversible pulmonary disease caused by inhalation of silica crystals in occupational settings in most cases. Our prior studies demonstrated the activation of inflammasome sensors AIM2 and NLRP3, effector protein caspase-1, and significant increase in IL-1β in silica exposed rats, demonstrated that the canonical inflammasome activation was associated with silica-induced tissue damage and inflammation.13 In our current study using the same validated and confirmed animal model system, we further provided evidence of non-canonical inflammasome activation, including elevations in expression of NEK7, caspase-11 and GSDMD and IL-18 following silica exposure (Fig. 4).
Fig. 4.
Schematic illustration of both canonical and non-canonical inflammasome pathway activation in silica response.
NEK7 is a nucleus protein and belongs to the NIMA-related kinase family which regulates mitotic progression and DNA damage response during embryonic development. This family of proteins contains two major structure domains, namely an N-terminal extension, and a C-terminal catalytic domain with kinase activity.18 The catalytic domain of NEK7 directly interacts with the NOD and LRR domains of NLRP3, which regulates NLRP3 oligomerization and the assembly of the NLRP3 inflammasome independent of its kinase activity.19 Following silica exposure, sustained NEK7 elevation was seen in the rat lung epithelial cells and macrophages, indicative of NEK7 involvement in silica associated inflammasome activation. Our observation warranted further mechanistic studies such as exploration of alterations of silicosis in NEK-7-deficient rodent models.
Non-canonical NLRP3 inflammasome activation is mediated by caspase-11 (mouse orthologue) and caspase-4 and caspase-5 (human orthologue).20 Similar to our findings, non-canonical NLRP3 inflammasome activation was found in lung macrophages of the mice silicosis model.21 The cysteine-aspartic acid protease (caspase) family comprises a group of inactive proenzymes, which undergo proteolytic processing at conserved aspartic residues to produce one large and one small subunit for full activation of the enzyme. According to the substrate functionalities, the caspase family is divided into an apoptosis subfamily represented by caspase-3, and an inflammatory subfamily represented by caspase-1, 4 & 5.22 Caspase-11 is the murine orthologue of human caspase-4/5, which can activate non-canonical inflammasome and induce pyroptosis in monocytes, epithelial cells, and keratinocytes.23 Gasdermin D (GSDMD) is the downstream substrate of caspase-11, whose activation cleaves its linker loop to separate N-terminal pore-forming fragment from the C-terminal autoinhibitory domain. The activated N-terminal domain of GSDMD inserts into the plasma membrane of virus or bacteria, pathogen infected cells or macrophages, and further oligomerizes to form large pores, which ultimately leads to swelling and rupture of the pathogens or infected cells, resulting in pyroptosis.23 GSDMD pores also release IL-1α, IL-1β, IL-18, and inflammatory mediators such as eicosanoids to further upgrade inflammation reaction.24 IL-18 together with IL-1α and IL-1β belong to interleukin-1 family, and function as pro-inflammatory factors.25 IL-1β is produced by a broad range of cell types, including monocytes, macrophages, dendritic cells, and neutrophils of the native immune system, B lymphocytes and NK cells of the adaptive immune system, as well as non-immune cells such as keratinocytes.25 The function of IL-18 cells in T and NK cells are to promote the secretion of other pro-inflammatory cytokines such as TNFα, IL-1β, IL-8, and IFNγ□.26 Our current study demonstrated increase in caspase-11, GSDMD and IL-18 in silica exposed rats, indicating activation of non-canonical inflammasome complex. Song et al., also demonstrated both the canonical and non-canonical NLRP3 inflammasome pathways were activated in lung macrophages of mice silicosis model, and more importantly they also revealed that tetrandrine, the only drug approved for silicosis treatment in China, yielded promising results against silicosis-associated inflammation and fibrosis by attenuating activation of both pathways.21 Put together, our study brings forward a theoretic framework of targeting both canonical and non-canonical inflammasome activation, which can be utilized as potential therapeutic intervention approaches preventing individuals from silicosis development. Further causality study will be of importance to provide insights into molecular mechanisms.
Limitation of the study: to maintain a consistency of this study with our previous study,13 we used micron silica (.5–10 microns) from Sigma instead of Min-u-Sil-5. We observed silicon particles aggregate and agglomerate in major tracheal wall, which might explain why some inflammation reaction was not observed in lung tissue but only in lung lavage.27
Supplementary Material
Acknowledgements
Not applicable.
Conflict of interest: The author(s) declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
Contributor Information
Yingmei Niu, Occupational Disease and Toxicology Department, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020, China.
Shuangli Yang, Occupational Disease and Toxicology Department, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020, China.
Xiumei Hu, Department of Pathology, Beijing Chao-Yang Hospital, Capital Medical University, Beijing 100020, China.
Authors’ Contributions
Conceived and designed the experiments: Yingmei Niu. Performed the experiments: Yingmei Niu Shuangli Yang. Analyzed the data: Yingmei Niu Xiumei Hu. Contributed reagents/materials/analysis tools: Yingmei Niu. Wrote the paper: Yingmei Niu.
Ethics approval
All animal experiments were approved by Animal Ethical Committee of Capital Medical University (Beijing, China) (No. 020-animal-275). All animal experiments were carried out in accordance with relevant guidelines and regulations. All animal experiments were carried out in compliance with the ARRIVE guidelines.Funding:This research received no specific grant from any funding agency
References
- 1. Sebastian-Valverde M, Pasinetti GM. The NLRP3 inflammasome as a critical actor in the inflammaging process. Cell. 2020:9(6):1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharma D, Kanneganti T-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016:213(6):617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angosto-Bazarra D, Molina-López C, Peñín-Franch A, Hurtado-Navarro L, Pelegrín P. Techniques to study inflammasome activation and inhibition by small molecules. Molecules. 2021:26(6):1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends Cell Biol. 2015:25(5):308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013:13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christgen S, Place DE, Kanneganti T-D. Toward targeting inflammasomes: insights into their regulation and activation. Cell Res. 2020:30(4):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Labzin LI, Lauterbach MA, Latz E. Interferons and inflammasomes: cooperation and counterregulation in disease. J Allergy Clin Immunol. 2016:138(1):37–46. [DOI] [PubMed] [Google Scholar]
- 8. Zheng D, Liwinski T, Elinav E. Inflammasome activation and regulation: toward a better understanding of complex mechanisms. Cell Discov. 2020:6(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol. 2007:7(1):31–40. [DOI] [PubMed] [Google Scholar]
- 10. Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015:21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Graham WG. Silicosis. Clin Chest Med. 1992:13(2):253–267. [PubMed] [Google Scholar]
- 12. Jain S, Joshi V, Rathore YS, Khippal N. Erasmus syndrome: silicosis and systemic sclerosis. Indian J Occup Envir. 2017:21(2):94–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niu Y, Yang S, Hu X. Activation of canonical inflammasome complex by acute silica exposure in experimental rat model. Toxicol Res-UK. 2022:11(1):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peng HB, Wang RX, Deng HJ, Wang YH, Tang JD, Cao FY, Wang JH. Protective effects of oleanolic acid on oxidative stress and the expression of cytokines and collagen by the AKT/NFκB pathway in silicotic rats. Mol Med Rep. 2017:15(5):3121–3128. [DOI] [PubMed] [Google Scholar]
- 15. He Y, Zeng MY, Yang D, Motro B, Núñez G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature. 2016:530(7590):354–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Agnew A, Nulty C, Creagh EM. Regulation, activation and function of caspase-11 during health and disease. Int J Mol Sci. 2021:22(4):1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee BL, Stowe IB, Gupta A, Kornfeld OS, Roose-Girma M, Anderson K, Warming S, Zhang J, Lee WP, Kayagaki N. Caspase-11 auto-proteolysis is crucial for noncanonical inflammasome activation. J Exp Med. 2018:215(9):2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun Z, Gong W, Zhang Y, Jia Z. Physiological and pathological roles of mammalian NEK7. Front Physiol. 2020:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. 2019:20(13):3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015:526(7575):660–665. [DOI] [PubMed] [Google Scholar]
- 21. Song M-Y, Wang J-X, Sun Y-L, Han Z-F, Zhou Y-T, Liu Y, Fan TH, Li ZG, Qi XM, Luo Y, et al. Tetrandrine alleviates silicosis by inhibiting canonical and non-canonical NLRP3 inflammasome activation in lung macrophages. Acta Pharmacol Sin. 2022:43(5):1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang X, Feng Y, Xiong G, Whyte S, Duan J, Yang Y, Wang K, Yang S, Geng Y, Ou Y, et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis. Cell Biosci. 2019:9(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Oh C, Verma A, Aachoui Y. Caspase-11 non-canonical inflammasomes in the lung. Front Immunol. 2020:11:1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yi Y-S. Caspase-11 non-canonical inflammasome: a critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology. 2017:152(2):207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gittleman H, Ostrom QT, Farah PD, Ondracek A, Chen Y, Wolinsky Y, Kruchko C, Singer J, Kshettry VR, Laws ER, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg. 2014:121(3):527–535. [DOI] [PubMed] [Google Scholar]
- 26. Sahoo M, Ceballos-Olvera I, Barrio L, Re F. Role of the inflammasome, IL-1β, and IL-18 in bacterial infections. TheScientificWorldJo. 2011:11:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavan C, Santalucia R, Leinardi R, Fabbiani M, Yakoub Y, Uwambayinema F, Ugliengo P, Tomatis M, Martra G, Turci F, et al. Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles. Proc Natl Acad Sci U S A. 2020:117(45):27836–27846. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.