Abstract
Humans are increasingly exposed to ubiquitous phthalates (PEs), e.g. butyl benzyl phthalate (BBP), dibutyl phthalate (DBP), and di(2-ethylhexyl) phthalate (DEHP), which are widely used plasticizers in polymer products. This study was aimed to investigate the effect of phytochemical quercetin (Que) on hepatotoxicity caused by the mixture of the 3 commonly used PEs (MPEs), and further to explore the underlying mechanism. Forty male Sprague–Dawley rats were randomly divided into control group, MPEs group, and MPEs combined Que at Low-, Median-, and High-dose groups; rats in MPEs group were orally administered with 900 mg/kg/d MPEs, whereas rats in MPEs combined Que groups were simultaneously treated with 900 mg/kg/d MPEs and respectively 10, 30, and 90 mg/kg/d Que. The intervention last 30 days. Compared with control group, serum ALT, AST, LDH and AKP, and hepatic MDA, SOD, CAT and GPx were significantly increased, whereas, serum albumin and total protein were significantly decreased in MPEs group (P < 0.05); hepatic histopathological observation showed numerous inflammatory cells infiltration, hepatocyte ballooning degeneration, and numerous residual erythrocytes in the central vein in MPEs group. Western-blot analysis showed that hepatic Keap1 was downregulated, whereas Nrf2 and HO-1 were upregulated in MPEs group (P < 0.05). However, the alterations of these parameters were alleviated in MPEs combined Que at Median- and High-dose groups. The results indicated that MPEs-induced hepatic oxidative stress, and caused hepatic injuries; whereas, Que inhibited MPEs’ hepatotoxicity, which might relate to Que’s ability of quenching free radicals directly, and restored the regulation of Nrf2 signaling pathway.
Keywords: quercetin, the mixture of phthalates, hepatotoxicity, oxidative stress, Nrf2 signaling pathway
Introduction
Phthalate esters (PEs) are widely used chemicals found in plastic products (e.g. plastic food containers, children’s toy, and medical supplies), artificial leather, daily necessities, cosmetics, and personal care products. Because of the ubiquitous usage of PEs, humans are inescapably and widely exposed to them. As reported, the total PEs exposure level of people in China is ~23–159 μg/kg/d.1 As environmental endocrine disruptors and environmental estrogens, PEs exposure could induce several human health hazards. It is reported that high levels of urinary PEs were associated with the prevalence of nonalcoholic liver disease (NAFLD) in Korean adults.2 PEs exposure might be also adversely associated with the indicators of liver function tests in US adults, indicating the potential toxic effect of PEs exposure on the human liver.3
Liver is the most important organ involved in chemical metabolism. The biotransformation of exogenous compounds takes place in liver after they enter the body, which easily induces damages to the liver. Liu et al.4 reported that di-(2-ethyhexyl) phthalate (DEHP) exposure caused increase of reactive oxygen species (ROS) in liver, and induced oxidative stress following the rats oral exposure to 3,000 mg/kg/d DEHP for 28 days. Liang and Yan5 argued that diisononyl phthalate (DINP) exposure also caused oxidative stress in liver following the Balb/c mice dermal exposure to DINP (>20 mg/kg/d) for 28 consecutive days. Human beings are inescapably exposed to ubiquitous PEs, and some interventions are suggested to be taken to reduce health risks associated with PEs exposure in human.
As a bioactive food component, quercetin (Que, 3,5,7,3,4-pentahydroxyflavone) is a typical flavonoid ubiquitously contained in vegetables and fruits with several biological effects demonstrated in vivo and in vitro, including antioxidative, anti-inflammatory, anticancer, and antidiabetic activities.6 Que has potent hepatoprotective effects for the treatment of liver steatosis, fatty hepatitis, liver fibrosis, and liver cancer.7 As reported, Que (5, 50, and 100 mg/kg/d) could ameliorate hyperthyroidism-induced liver damage via nuclear factor erythroid 2-related factor (Nrf2) signaling pathway.8 Zhang et al.9 argued that Que (50 and 100 mg/kg/d) could attenuate isoniazid induced liver injury by regulating SIRT1 pathway. Que could also mitigate the hepatotoxicity induced by ethanol,10,11 carbon tetrachloride,12,13 chloroquine,14 and gold nanoparticles15 in rats or mice. Therefore, it is supported that Que might inhibit the hepatotoxicity of PEs, whereas, the related research has been rarely reported.
The best described action of Que is its ability to act as an antioxidant. And the most frequently reported liver-protective mechanism of Que is enhancing the antioxidant capacity of the body, scavenging the free radicals, and reducing oxidative stress.7,8 Unfortunately, the specific mechanism is still unclear. It is reported that oxidative stress could active Nrf2 signaling pathway, and induce the production of antioxidant enzymes, such as hemeoxygenase-1 (HO-1), superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), which could scavenge the free radicals and suppress oxidative stress.16–18
This study aimed to investigate the protective effect of Que on hepatotoxicity induced by PEs exposure. Male rats were continuously exposed to MPEs (the mixture of BBP, DBP, and DEHP at equipotent toxicity) for 30 days. Liver function related parameters, oxidative stress related parameters, and pathological detection of liver were performed for the toxicity evaluation. Nrf2 signaling pathway related proteins were detected for exploring the underlying mechanism.
Material and methods
Reagents and instruments
Analytically pure Que (CAS:117-39-5), BBP (CAS:85-68-7), DBP (CAS: 84-74-2), and DEHP (CAS:117-81-7) were purchased from Aladdin (Shanghai, China). Commercial assay kits of alanine aminotransferase (ALT, Cat.#C009-2-1), aspartate aminotransferase (AST, Cat.#C010-2-1), albumin (ALB, Cat.#C028-2-1), lactate dehydrogenase (LDH, Cat.#C020-2-2), and alkaline phosphatase (AKP, Cat.#C009-2-2) for rats were gained from Nanjing JianCheng (Nanjing, China). Commercial assay kits of superoxide dismutase (SOD, Cat.#S101S), catalase (CAT, Cat.#S0051), glutathione peroxidase (GPx, Cat.#S0058), malondialdehyde (MDA, Cat.#S0131S), and primary antibodies: Kelch-like ECH- associated protein 1 (Keap1), Nrf2, HO-1, and GAPDH were purchased from Beyotime (Haimen, China). Secondary antibodies, BCA Protein Assay Kit, RIPA cell lysis buffer, and PVDF membrane were also purchased from Beyotime (Haimen, China).
MPEs was the mixture of BBP, DBP, and DEHP at equipotent toxicity according to the reference dose, i.e. BBP:DBP:DEHP = 10:5:1.19
Animals and experimental design
Forty male Sprague–Dawley (SD) rats (SPF grades, weighting 90–110 g) were obtained from Zhejiang Vital River Laboratory Animal Technology Co., Ltd (License No.: SCXK (Zhejiang) 2019-0001).
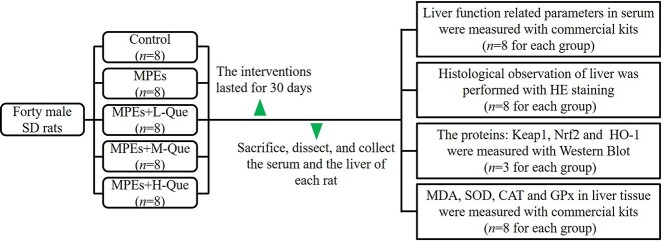
The isometric MPEs suspension was in 0.5% sodium carboxymethyl cellulose (CMC–Na). After 1 week of acclimatization, based on their body weight, 40 male SD rats were randomly divided into control group, MPEs group, MPEs combined Que with Low-, Median-, and High-doses groups (MPEs+L-Que, MPEs+M-Que, and MPEs+H-Que group), with 8 rats in each group. As shown in Table 1, rats in the MPEs combined Que groups and MPEs group were orally administered with 900 mg/kg/d MPEs in 0.5% CMC-Na with or without Que (10, 30, and 90 mg/kg/d, respectively). Rats in the control group were orally administered with isodose 0.5% CMC-Na. The intervention lasted for 30 consecutive days. The experimental procedure was showed in Fig. 1.
Table 1.
The doses of MPEs and Que in each group.
| Groups | n | The doses in each group | |
|---|---|---|---|
| MPEs | Que | ||
| Control | 8 | 0 | 0 |
| MPEs | 8 | 900 mg/kg/d | 0 |
| MPEs+L-Que | 8 | 900 mg/kg/d | 10 mg/kg/d |
| MPEs+M-Que | 8 | 900 mg/kg/d | 30 mg/kg/d |
| MPEs+H-Que | 8 | 900 mg/kg/d | 90 mg/kg/d |
Note: The rats were orally administered with the interventions for 30 consecutive days.
Fig. 1.
The schematic representation of the experimental procedure.
At the end of the experiment, ~1.0-mL blood of each anesthetized rat was collected from the caudal vein by cutting off the tip of their tails. The serum was prepared with centrifugation at 4,500 g for 10 min. All rats were fasted overnight (12 h) and euthanized using 4% fluothane in oxygen anesthesia in an airtight container. The body and liver of each rat was weighed. The relative liver weight was calculated by the formula: Liver weight (g)/Body weight (g) × 100%. Appropriate amounts of liver tissues were taken and fixed in 10% neutral formalin solution for hematoxylin–eosin (HE) staining. An extra sample of liver tissue and the serum were stored at −80 °C for further analyses.
The animal experiments were conducted following the Declaration of Helsinki (as revised in Edinburgh 2000) and the local regulations. Furthermore, all experiments were approved and supervised by the Animal Care and Use Committee and the Animal Ethics Committee at Wenzhou Medical University (approval no. xmsq2021-0122).
Measurement of liver function related parameters
Liver function related parameters, such as serum total protein (TP), ALT, AST, ALB, LDH, and AKP, were measured using the commercial kits for rats. The operation procedures were carried out in strict accordance with the manufacturer’s instructions.
Measurement of oxidative stress related parameters in liver tissue
The cryopreserved liver tissues were homogenized with precooled cell lysis buffer at 1:10 (w/w), then centrifuged at 12,000 g and −4 °C for 10 min. The supernatants were taken, and their protein levels were determined by BCA Protein Assay Kit. The oxidative stress related parameter MDA, and the antioxidant enzymes: SOD, CAT, and GPx were measured using the commercial kits. And the operation procedures were carried out in strict accordance with the manufacturer’s instructions.
Histological observation of liver
The liver tissues were fixed in 10% neutral formalin solution, embedded in paraffin, then sliced in 4-μm thick sections and stained with HE. Morphological analysis was conducted by an experienced pathologist blinded to the experimental design. HE-stained samples were observed and photographed under a Nikon ECLIPSE TS100 inverted microscope (Nikon, Tokyo, Japan).
Western blot
The procedure of western-blot experiment was described as in our previous study.20 Homogenized hepatic tissues (50 mg) in pre-cold 1,600-μL radioimmunoprecipitation assay lysis buffer containing 320-μL phenylmethanesulfonyl fluoride were centrifuged at 14,000 g for 30 min. The protein levels of the supernatants were detected with BCA Protein Assay Kit. After 100 °C for 20 min, the equal amount of the denaturalized protein samples (in 5× loading buffer) were subjected to 6–12% SDS-polyacrylamide gel electrophoresis, and then transferred to polyvinylidene fluoride membrane. After the block in Tris-buffered saline and Tween 20 (TBST) buffer containing 5% nonfat dry milk overnight at 4 °C, and followed by washing, 1:1,000 diluted primary antibodies (such as GAPDH, Keap1, Nrf2, and HO-1) for the interesting targets were respectively added for incubation overnight at 4 °C. Then the corresponding 1:1,000 diluted secondary antibodies were added for 3-h incubation at room temperature. GAPDH was used as internal control. The samples were visualized using ChemiDoc XRS+ system (BioRad).
Statistical methods
Statistical analyses were performed using 1-way analysis of variance (ANOVA) on Statistical Product and Service Software, version 20.0 (SPSS Inc., Chicago, IL, United States), followed by Duncan’s multiple range test. Values are expressed as means and their standard deviations. P < 0.05 was considered statistically significant. All P-values were 2 tailed.
Results
Descriptive evaluation, the body weight, and liver weight of rats
During the experimental period, the rats in each group ate and drank normally and no abnormal behaviors were observed. Furthermore, none of the rats died within the experimental period. At the beginning of the experiment, the average body weight of rats in each group were all ~178 g, as shown in Fig. 2A, their average body weights increased continuously and rapidly, with daily weight gain 5–9 g/d, and at necropsy, there were no differences in the average body weight of rats among the 5 groups (Fig. 2B). Whereas, compared with control, the average liver weight and the relative liver weight in experimental groups increased significantly (P < 0.05), as shown in Fig. 2C and D, however, no differences were found among the experimental groups (P > 0.05).
Fig. 2.

The body weight, liver weight, and the relative liver weight of rats in each group. A) The growth curves of the rats’ body weights in each group; B) The rats’ body weight in each group at days 1 and 30; C) The rats’ liver weight; and D) The rats’ relative liver weight. n = 8. Different letters above the bar represent the significant difference (P < 0.05).
Que reverses MPEs-induced alterations of liver function related parameters
As shown in Fig. 3, comparing with control, MPEs exposure caused decrease of serum total protein (TP) and albumin (ALB), whereas, increase of serum ALT, AST, LDH, and AKP (P < 0.05). Que significantly reversed MPEs-induced alterations of serum TP, ALT, AST, and AKP at Medium dose (30 mg/kg/d) and High dose (90 mg/kg/d; P < 0.05).
Fig. 3.
Liver function related parameters. A) TP; B) ALB; C) ALT; D) AST; E) LDH; and F) AKP; n = 8. Different letters above the bar represent the significant difference (P < 0.05).
Que reverses MPEs-induced histological changes of liver tissues
Liver sections from the control group had intact and clear hepatic lobule structures, as shown in Fig. 4A, the boundary of the portal area was clear, normal hepatocytes were radially distributed around the central vein, and the hepatic sinus was clearly visible. Liver sections from MPEs group were severely injured, as shown in Fig. 4B, the hepatocytes were swollen and bulky, and the boundary between the cells was unclear; there were numerous inflammatory cells infiltration and hepatocyte ballooning degeneration; there were numerous residual erythrocytes in the central vein. Liver sections from MPEs+L-Que group were moderately injured, as shown in Fig. 4C, there were numerous hepatocytes ballooning degeneration. Liver sections from MPEs+M-Que group were mildly injured, as shown in Fig. 4D, slight inflammatory cells infiltration was observed and many residual erythrocytes were observed in the central vein. Liver sections from the MPEs+H-Que group also had intact and clear hepatic lobule structures, as shown in Fig. 4E, the boundary of the portal area was clear, and the hepatic sinus was clearly visible.
Fig. 4.
Representative pathological morphology of liver tissues. A) Control; B) MPEs group; C) MPEs+LQue group; D) MPEs+M-Que group; and E) MPEs+H-Que group; CV, central veins; HC, hepatic cells; HS, hepatic sinusoid; △, inflammatory cell infiltration; *, ballooning degeneration; n = 8, bar = 100 μm, 200×.
Que reverses MPEs-induced oxidative stress in liver tissues
MDA is an important and commonly used indicator of oxidative stress. As shown in Fig. 5, comparing with control, MPEs exposure elevated the level of hepatic MDA significantly in MPEs group (P < 0.05). Comparing with MPEs group, the levels of hepatic MDA in MPEs+M-Que group and MPEs+H-Que group were significantly decreased (P < 0.05).
Fig. 5.

MDA level in liver tissue. n = 8. Different letters above the bar represent the significant difference (P < 0.05).
Que reverses MPEs-induced alterations of proteins in Nrf2 signaling pathway
As shown in Fig. 6, comparing with control, the expression level of hepatic Keap1 was significantly suppressed in MPEs group (P < 0.05), whereas the expression levels of hepatic Nrf2 and HO-1 were significantly increased in MPEs group (P < 0.05). Comparing with MPEs group, the expression level of hepatic Keap1 was significantly increased in MPEs+M-Que group and MPEs+H-Que group (P < 0.05), whereas the expression levels of hepatic Nrf2 and HO-1 were significantly decreased in MPEs+M-Que group and MEPs+H-Que group (P < 0.05).
Fig. 6.
The levels of Nrf2 signaling pathway related proteins in liver tissue. A) Keap1; B) Nrf2; and C) HO-1; n = 3. Different letters above the bar represent the significant difference (P < 0.05).
Que reverses MPEs-induced increase of antioxidant enzymes in liver tissue
As shown in Fig. 7, comparing with control, the activities of SOD, CAT, and GPx were increased significantly in MPEs group (P < 0.05). Comparing with MPEs group, their activities were decreased significantly in MPEs+H-Que group (P < 0.05); the activities of CAT in MPEs+L-Que group and MPEs+M-Que group were also decreased significantly compared with MPEs group (P < 0.05).
Fig. 7.
The activities of antioxidant enzymes in liver tissue. A) SOD; B) CAT; and C) GPx; n = 8. Different letters above the bar represent the significant difference (P < 0.05).
Discussion
Humans are inescapably exposed to ubiquitous PEs mainly via oral pathway. The study was designed to simulate the real scenarios of human exposure to the complexes of PEs. MPEs was comprised of 3 commonly and largely used PEs: DBP, BBP, and DEHP at equipotent toxicity according to their reference dose [19]. The doses of MPEs (900 mg/kg/d) and Que (90 mg/kg/d) was designed based on the previously published study, which reported that Que (90 mg/kg/d) could attenuate DEHP (900 mg/kg/d, oral exposure, 15 days) induced oxidative damage in the rat testis.21 As reported, Que (75 mg/kg/d) could restore acrylamide (25 mg/kg/d, oral exposure, 30 days) induced oxidative stress in the liver of rats.22 Que (5, 50, and 100 mg/kg/d) could also ameliorate hyperthyroidism-induced liver damage in rats.8 According to the studies,8,21,22 the doses of Que were set at 10, 30, and 90 mg/kg/d to explore the dose–response relationship of hepatoprotective effect in this study.
After intervention for 30 consecutive days, MPEs exposure caused increase of liver weight and the relative liver weight (Fig. 2A and B), which agreed with Liu et al.,4 who reported that DEHP caused significantly increase of liver weight and the relative liver weight following the rats exposure to 3,000 mg/kg/d DEHP for 28 days. DINP exposure (20 and 200 mg/kg/d, 28 days) also caused increase of the relative liver weight in mice.5 ALT, AST, LDH, and AKP are critical indicator for liver function, and they mainly reside in hepatocytes.23 When hepatocytes are damaged, the permeability of hepatocyte membrane increases, and these enzymes will be released into blood in large quantities, and cause increase of their levels in serum. In this study, the elevated serum levels of ALT, AST, LDH, and AKP indicated that MPEs exposure caused hepatotoxicity in rats (Fig. 3). Interestingly, the ballooning degeneration of hepatocytes might enhance the permeability of their membrane in MPEs group (Fig. 4B), and caused the elevation of serum ALT, AST, LDH, and AKP. The results agreed the published studies,24–26 which argued that DBP exposure increased serum ALT, AST, LDH, and AKP in rats or mice. Zhang et al.27 reported that DEHP also increased serum ALT, AST, and AKP following the female quail exposure to 500 and 1,000-mg/kg/d DEHP for 45 days.
Albumin is a protein synthesized specifically by the liver, and it was used as a marker of liver function. In the present study, the decreased serum levels of albumin and the total protein also implied that MPEs exposure caused hepatotoxicity (Fig. 3A and B). Anjali et al.28 also reported that DEHP exposure (20 mg/kg/d, 2–6 weeks) caused decrease of serum albumin and the total protein in female Swiss albino mice. The alterations of liver histological structures added evidence to the hepatotoxicity induced by MPEs exposure (Fig. 4B). On the other hand, Que attenuated the alterations of these parameters in a dose-dependent manner, indicating that Que could inhibit MPEs’ hepatotoxicity.
PEs exposure could cause oxidative stress in organs, such as testis, kidney, spleen, and liver.4,5,26,29 Malondialdehyde (MDA) is a high-level lipid oxidation product produced by free radicals in the process of lipid peroxidation. And MDA is an important and well-established biomarker of oxidative stress.30 Abarikwu et al.25 reported that DBP exposure caused increase of MDA in liver and testis following the rats exposure to 1 mL/kg (~1,000 mg/kg) DBP every other day for 15 days. DEHP exposure (500 and 1,000 mg/kg/d, 45 days) also increased hepatic MDA in female quail.27 Our study showed that hepatic MDA was increased in MPEs group (Fig. 5), indicating that MPEs exposure caused hepatic oxidative stress in rats.
Nrf2 signaling pathway plays a major role in regulating oxidative stress, which could active Nrf2 signaling pathway.18 Nrf2 is a transcription factor, which plays a major role in oxidative stress responses by regulating antioxidant gene expression. In addition, Nrf2 is regarded as one of the most important regulator of the signaling pathway to defend oxidative stress. Under normal conditions, Nrf2 protein is kept at low levels in the cytoplasm by the E3 ligase adaptor Keap1. However, under oxidative conditions, cysteine residues in Keap1 become oxidized leading to a dissociation with Nrf2, allowing both Nrf2 protein stabilization and re-localization to the nucleus. Nrf2 then binds to antioxidative response element (ARE), which causes transactivation of key antioxidant genes, such as HO-1, SOD, CAT, GPx, etc.17
As reported, mild oxidative stress induces antioxidant enzymes via the Nrf2-Keap1 system, which strengthen antioxidant activity of the cells against more severe oxidative stress.31 The results of this study showed that hepatic Keap1 was downregulated, whereas hepatic Nrf2 was upregulated in rats in MPEs group (Fig. 6), indicating that the hepatic oxidative stress induced by MPEs activated Nrf2 signaling pathway, which transactivated the antioxidant genes. This might be the reason that the activities of hepatic antioxidant enzymes HO-1, SOD, CAT, and GPx were elevated in MPEs group (Fig. 7). Our results were agreed with the report of Tang et al.,32 who reported that DEHP (500 mg/kg/d, 30 days) upregulated testicular Nrf2, and activated Nrf2 signaling pathway, and then induced upregulation of testicular antioxidative enzymes, such as HO-1, SOD, and GPx in rats. Zhang et al.27 also argued that DEHP exposure (500 and 1000 mg/kg/d, 45 days) also increased hepatic MDA, upregulated hepatic Nrf2 in female quail, and activated Nrf2 signaling pathway, inducing the increase of hepatic HO-1, SOD, CAT, and quinone oxidoreductase 1 (NQO1). In addition, DEHP exposure (500 and 750 mg/kg/d, 45 days) also increased splenic MDA, upregulated splenic Nrf2 in male quail, and activated Nrf2 signaling pathway.29 DBP exposure also caused increase of MDA, CAT in liver and testis, and increase of SOD, CAT in testis following the rats exposure to 1 mL/kg (~1,000 mg/kg) DBP every other day for 15 days.25 On the other hand, the alterations of hepatic Keap1, Nrf2, MDA, and the antioxidant enzymes (HO-1, SOD, CAT, and GPx) were reversed in MPEs combined Que groups at dose-dependent manner in our study, indicating that Que could protect liver from oxidative damage induced by MPEs. In vitro studies also reported that Que could protect hepatocytes from oxidative damage.33,34 As reported, Que (25 μM) could suppress the increase of MDA, SOD, and CAT induced by D-glucose (30 mM, 48 h), and alleviated hyperglycemia-induced oxidative stress in hepatic HepG2 cells.33 Que (100 μM) could derive Nrf2 translocation into nuclei, and subsequent upregulate the expression of HO-1, and protect human hepatocytes from ethanol-induced oxidative stress.34 The results of this study indicated that Que attenuated MPEs’ hepatotoxicity, which might relate to the regulation of Nrf2 signaling pathway.
As reported, Que has a strong antioxidant property, could directly quench free radicals in the body.35–37 In the present study, as shown in Fig. 8, Que might directly quench free radicals, and decrease the oxidative stress induced by MPEs exposure, and then restore the regulation of Nrf2 signaling pathway, therefore, the expression of hepatic Keap1 and Nrf2 were restored in MPEs combined Que groups, as well as the levels of hepatic MDA, HO-1, SOD, CAT, and GPx. Quenching free radicals directly of Que might be the reason that Que ameliorating MPEs’ hepatotoxicity.
Fig. 8.
Schematic diagram illustrating the proposed mechanism of Que attenuating MPEs’ hepatotoxicity. MPEs exposure induced hepatic oxidative stress and caused hepatoxicity in rats. The oxidative stress activated Nrf2 signal pathway, and induced the production of antioxidant enzymes, e.g. HO-1, SOD, CAT, and GPx, which could scavenge free radicals and reduce the oxidative stress. Que could quench free radicals directly, decrease MPEs inducing hepatic oxidative stress, and restored the regulation of Nrf2 signaling pathway.
Conclusion
In summary, the results of this study indicated that Que could ameliorate MPEs’ hepatotoxicity, which might relate to Que’s ability of quenching free radicals directly. Human beings are inevitably and increasingly exposed to ubiquitous PEs at very low doses mainly by dietary exposure pathway through the whole lifetime. Therefore, the studies of low-dose PEs exposure combined with Que in the long-term way should be conducted to explore the protective effect of Que on PEs’ hepatotoxicity and the underlying mechanism. The results of this study also indicates that Que can be a candidate nutritional supplement to prevent PEs’ hepatotoxicity. Que-enriched foods, such as teas, vegetables, and fruits, are advisable and may reduce the hazards of PEs exposure.
Funding
This study was supported by National Natural Science Foundation of China (grant no. 81903321), Zhejiang Provincial Natural Science Foundation of China (grant no. LY22H260002), Wenzhou Fundamental Scientific Research Projects (grant no. Y2020098), and Innovation and Entrepreneurship Programs for College Students of School of Public Health and Management, Wenzhou Medical University.
Conflict of interest statement: The authors declare that they have no conflicts of interest.
Authors’ contributions
L.Z.X. and M.Z.J. were involved in conceptualization, methodology, visualization, and writing-original draft; L.L.L. was involved in methodology, visualization, and writing-original draft; Y.W., Y.L.Z., L.X.Y., X.Y.S, and Q.Y.Z. took the responsibility of investigation, formal analysis, visualization; M.L. did project administration and data curation; H.T.G. was involved in conceptualization, supervision, funding acquisition, resources, and writing-reviewing and editing.
Contributor Information
Ling-Zi Xia, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China; Zhejiang Provincial Key Laboratory of Watershed Science and Health, Wenzhou Medical University, Wenzhou 325035, China.
Ming-Zhe Jiang, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Li-Lan Liu, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China; Zhejiang Provincial Key Laboratory of Watershed Science and Health, Wenzhou Medical University, Wenzhou 325035, China.
Yi Wu, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Yi-Lin Zhang, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Li-Xia Yang, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Xin-Yue Shen, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Qiu-Yu Zhang, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Min Lin, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China.
Hai-Tao Gao, Department of Preventive Medicine, School of Public Health and Management, Wenzhou Medical University, Wenzhou 325035, China; Zhejiang Provincial Key Laboratory of Watershed Science and Health, Wenzhou Medical University, Wenzhou 325035, China.
References
- 1. Gao HT, Xu R, Cao WX, Zhou X, Yan YHM, Lu L, Xu Q, Shen Y. Food emulsifier glycerin monostearate increases internal exposure levels of six priority controlled phthalate esters and exacerbates their male reproductive toxicities in rats. PLoS One. 2016:11(8):e0161253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang YJ, Kim T, Hong YP. Urinary phthalate levels associated with the risk of nonalcoholic fatty liver disease in adults: the Korean national environmental health survey (KoNEHS) 2012-2014. Int J Env Res Pub Health. 2021:18(11):6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yu L, Yang M, Cheng M, Fan L, Wang X, Xu T, Wang B, Chen W. Associations between urinary phthalate metabolite concentrations and markers of liver injury in the US adult population. Environ Int. 2021:155:106608. [DOI] [PubMed] [Google Scholar]
- 4. Liu RJ, He YJ, Liu H, Zheng DD, Huang SW, Liu CH. Protective effect of Lycium barbarum polysaccharide on di-(2-ethylhexyl) phthalate-induced toxicity in rat liver. Environ Sci Pollut Res Int. 2021:28(18):23501–23509. [DOI] [PubMed] [Google Scholar]
- 5. Liang F, Yan B. Oxidative damage in the liver and kidney induced by dermal exposure to diisononyl phthalate in Balb/c mice. Toxicol Ind Health. 2020:36(1):30–40. [DOI] [PubMed] [Google Scholar]
- 6. Kawabata K, Mukai R. Ishisaka A Quercetin and related polyphenols: new insights and implications for their bioactivity and bioavailability. Food Funct. 2015:6(5):1399–1417. [DOI] [PubMed] [Google Scholar]
- 7. Zhao X, Wang J, Deng Y, Liao L, Zhou M, Peng C, Li Y. Quercetin as a protective agent for liver diseases: a comprehensive descriptive review of the molecular mechanism. Phytother Res. 2021:35(9):4727–4747. [DOI] [PubMed] [Google Scholar]
- 8. Zhao PX, Hu ZG, Ma WY, Zang L, Tian Z, Hou Q. Quercetin alleviates hyperthyroidism-induced liver damage via Nrf2 Signaling pathway. Biofactors. 2020:46(4):608–619. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Y, Qu X, Gao H, Zhai J, Tao L, Sun J, Song Y, Zhang J. Quercetin attenuates NLRP3 inflammasome activation and apoptosis to protect INH-induced liver injury via regulating SIRT1 pathway. Int Immunopharmacol. 2020:85:106634. [DOI] [PubMed] [Google Scholar]
- 10. Vidhya A. Indira M Protective effect of quercetin in the regression of ethanol-induced hepatotoxicity. Indian J Pharm Sci. 2009:71(5):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X. Protective effects of quercetin on liver injury induced by ethanol. Pharmacogn Mag. 2010:6(22):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemelo MK, Pierzynová A, Kutinová Canová N, Kučera T, Farghali H. The involvement of sirtuin 1 and heme oxygenase 1 in the hepatoprotective effects of quercetin against carbon tetrachloride-induced sub-chronic liver toxicity in rats. Chem Biol Interact. 2017:269:1–8. [DOI] [PubMed] [Google Scholar]
- 13. Elmowafy E, el-Derany MO, Biondo F, Tiboni M, Casettari L, Soliman ME. Quercetin loaded monolaurate sugar esters-based niosomes: sustained release and mutual antioxidant-hepatoprotective interplay. Pharmaceutics. 2020:12(2):143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar Mishra S, Singh P, Rath SK Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar Res Treat 2013;2013:141734, 1, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdelhalim MAK, Moussa SAA, HAY Q. The protective role of quercetin and arginine on gold nanoparticles induced hepatotoxicity in rats. IJN. 2018:13:2821–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018:1865(5):721–733. [DOI] [PubMed] [Google Scholar]
- 17. Chen D, Tavana O, Gu W. ARF-NRF2: a new checkpoint for oxidative stress responses? Mol Cell Oncol. 2018:5(3):e1432256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Feng H, Wang L, Zhang G, Zhang Z, Guo W. Oxidative stress activated by Keap-1/Nrf2 signaling pathway in pathogenesis of preeclampsia. Int J Clin Exp Pathol. 2020:13(3):382–392. [PMC free article] [PubMed] [Google Scholar]
- 19. US EPA . Phthalates summary[OL]. https://archive.epa.gov/teach/web/pdf/phthalates_summary.pdf, 2007 October 10.
- 20. Gao HT, Shi HY, Dai QM, Li AQ, Yang L, Sun Y, Jin SY, Xia LZ. Glycerin monostearate aggravates male reproductive toxicity caused by di(2-ethylhexyl) phthalate in rats. Curr Med Sci. 2019:39(6):1003–1008. [DOI] [PubMed] [Google Scholar]
- 21. Abd-Ellah MF, Aly HAA, Mokhlis HAM, Abdel-Aziz AH. Quercetin attenuates di-(2-ethylhexyl) phthalate-induced testicular toxicity in adult rats. Hum Exp Toxicol. 2016:35(3):232–243. [DOI] [PubMed] [Google Scholar]
- 22. Hamza RZ, Al-Thubaiti EH, Omar AS. The antioxidant activity of quercetin and its effect on acrylamide hepatotoxicity in liver of rats. Lat Am J Pharm. 2019:38:2057–2062. [Google Scholar]
- 23. Gao HT, Cheng WZ, Xu Q, Shao LX. Dietary restriction reduces blood lipids and ameliorates liver function of mice with hyperlipidemia. J Huazhong U Sci-Med. 2017:37(1):79–86. [DOI] [PubMed] [Google Scholar]
- 24. Xiong Z, Zeng Y, Zhou J, Shu R, Xie X, Fu Z. Exposure to dibutyl phthalate impairs lipid metabolism and causes inflammation via disturbing microbiota-related gut-liver axis. Acta Biochim Biophys Sin. 2020:52(12):1382–1393. [DOI] [PubMed] [Google Scholar]
- 25. Abarikwu SO, Simple G, Onuoha CS. Morphometric evaluation of the seminiferous tubules and the antioxidant protective effects of gallic acid and quercetin in the testis and liver of butyl phthalate treated rats. Indian J Clin Biochem. 2020:35(1):20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang W, Li JY, Wei XC, Wang Q, Yang JY, Hou H, du ZW, Wu XA. Effects of dibutyl phthalate on lipid metabolism in liver and hepatocytes based on PPAR alpha/SREBP-1c/FAS/GPAT/AMPK signal pathway. Food Chem Toxicol. 2021:149:112029. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Q, Zhao Y, Talukder M, Han Y, Zhang C, Li XN, Li JL. Di(2-ethylhexyl) phthalate induced hepatotoxicity in quail (Coturnix japonica) via modulating the mitochondrial unfolded protein response and NRF2 mediated antioxidant defense. Sci Total Environ. 2019:651(Pt 1):885–894. [DOI] [PubMed] [Google Scholar]
- 28. Anjali S, Ravish K, Singh JK, Singh T. Long term effect of Di (2-ethylhexyl) phthalate on the liver of female Swiss albino mice Mus musculus. Res J Biotechnol. 2012:7(1):58–63. [Google Scholar]
- 29. Yu L, Li HX, Guo JY, Huang YQ, Wang H, Talukder M, Li JL. Di (2-ethyl hexyl) phthalate (DEHP)-induced spleen toxicity in quail (Coturnix japonica) via disturbing Nrf2-mediated defense response. Environ Pollut. 2019:251:984–989. [DOI] [PubMed] [Google Scholar]
- 30. Li Z, Yu Y, Li Y, Ma F, Fang Y, Ni C, Wu K, Pan P, Ge RS. Taxifolin attenuates the developmental testicular toxicity induced by di-n-butyl phthalate in fetal male rats. Food Chem Toxicol. 2020:142:111482. [DOI] [PubMed] [Google Scholar]
- 31. Taguchi K, Motohashi H, Yamamoto M. Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells. 2011:16(2):123–140. [DOI] [PubMed] [Google Scholar]
- 32. Tang X, Wu S, Shen L, Wei Y, Cao X, Wang Y, Long C, Zhou Y, Li D, Huang F, et al. Di-(2-ethylhexyl) phthalate (DEHP)-induced testicular toxicity through Nrf2-mediated Notch1 signaling pathway in Sprague-Dawley rats. Environ Toxicol. 2018:33(7):720–728. [DOI] [PubMed] [Google Scholar]
- 33. Yarahmadi A, Moradi Sarabi M, Sayahi A, Zal F. Protective effects of quercetin against hyperglycemia-induced oxidative stress in hepatic HepG2 cell line. Avicenna J Phytomed. 2021:11(3):269–280. [PMC free article] [PubMed] [Google Scholar]
- 34. Yao P, Nussler A, Liu L, Hao L, Song F, Schirmeier A, Nussler N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J Hepatol. 2007:47(2):253–261. [DOI] [PubMed] [Google Scholar]
- 35. Rafat Husain S, Cillard J, Cillard P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry. 1987:26(9):2489–2491. [Google Scholar]
- 36. Cho SY, Kim MK, Mok H, Choo H, Chong Y. Separation of quercetin's biological activity from its oxidative property through bioisosteric replacement of the catecholic hydroxyl groups with fluorine atoms. J Agric Food Chem. 2012:60(26):6499–6506. [DOI] [PubMed] [Google Scholar]
- 37. Yu H, Haskins JS, Su C, Allum A, Haskins AH, Salinas VA, Sunada S, Inoue T, Aizawa Y, Uesaka M, et al. In vitro screening of radioprotective properties in the novel glucosylated flavonoids. Int J Mol Med. 2016:38(5):1525–1530. [DOI] [PubMed] [Google Scholar]