Abstract
Background
Although chronic kidney disease (CKD) affects 15% of the United States (US) population, <10% of the US CKD population is aware of their disease. This is significant as untreated CKD can progress to end-stage renal disease which would require dialysis or transplantation. This study aimed to provide updated information regarding US CKD unawareness.
Methods
Data from the 1999–2014 National Health and Nutrition Examination Survey (NHANES) were used (n = 38 474); response rate > 70%. CKD self-report and lab-confirmed CKD were used to assess CKD unawareness. Adjusted logistic regression models examined association between unawareness and patient characteristics.
Results
In individuals with lab-confirmed CKD (n = 7137, 14.3%), 91.5% answered ‘no’ to self-report question; in those without CKD, 1.1% answered ‘yes’ to self-report question. In those with lab-confirmed CKD, in the adjusted models, increased age [odds ratio (ORs), 1.03 (95%CI, 1.02–1.04)] and female sex [OR, 1.37 (95%CI, 1.08–1.72)] were statistically significantly associated with greater odds of being unaware of CKD.
Conclusion
These findings demonstrated high unawareness of disease status as there was a discrepancy between respondents’ self-reported CKD diagnosis and lab-confirmed CKD. Older individuals and women may be more unaware of their CKD; these groups should be queried about reasons for increased unawareness.
Keywords: awareness, chronic kidney disease (CKD), National Health and Nutrition Examination Survey (NHANES)
Introduction
Worldwide, chronic kidney disease (CKD) affects 13.4% of the adult population,1,2 with the United States (US) having a prevalence of ~15%.3–5 CKD is characterized by the gradual loss of kidney function, primarily detected through use of the estimated glomerular filtration rate (eGFR); eGFR is the rate at which kidneys filter waste and extra fluid from the blood.6 Albuminuria, the presence of abnormal amounts of protein in urine, can also be used to assess kidney damage. Applying information from both eGFR and albuminuria, CKD can be classified into five stages.1,7,8 However, stages 1–3 are often asymptomatic,4 and thus, patients may not always be aware of their disease.9 Even early stages of CKD can be associated with complications such as anemia and higher cardiovascular morbidity and mortality.9,10 Additionally, if left untreated, even when asymptomatic, CKD can progress to end-stage renal disease (ESRD). ESRD requires renal replacement therapy such as dialysis, or transplantation to sustain life, which would significantly impact both a person’s health and quality of life.3,8
Specific population groups are at a greater risk of developing CKD than others. Risk factors include older age, obesity and family history of CKD.5 Furthermore, among adults with diabetes, 1 in 3 may have CKD11; for adults with high blood pressure, 1 in 5 may have CKD.11 Almost 50% of those diagnosed with CKD in the US also have diabetes and/or self-reported cardiovascular disease.3 Women are likelier to have earlier stage CKD than men (15.9 versus 13.5%, respectively)12; while African-Americans and Asian-Americans are at higher risk of developing CKD overall versus Caucasians.3 In addition to differences in CKD incidence and prevalence by demographic characteristics, patient awareness of having CKD may vary by similar factors. Awareness was found to be higher in adults with hypertension and in those with diabetes compared with those without either condition.13 Additionally, awareness was lower in Hispanics when compared with other racial/ethnic groups.13
Previous studies, which have queried individuals’ awareness of their disease, have mostly focused on diabetic CKD or stage 3 CKD.9,14 Moreover, there have been no recent comprehensive updates to assessing CKD awareness across all stages of disease.15 Most recently, a 2017 study looking at state-level awareness of CKD found the average estimated CKD awareness to be 9%, ranging from 5.8% (Iowa) to 11.7% (Arizona).13 Therefore, due to these low awareness findings across the US, the objective of this research was to utilize recent National Health and Nutrition Examination Survey (NHANES) data to examine any CKD unawareness across all stages, within a broad population, comparing self-reported awareness of disease status with lab-confirmed values of kidney function. Determining which factors affect CKD unawareness can help tailor and focus awareness campaigns, as well as prevention and treatment efforts regarding this disease.
Methods
Data sources
We used data from NHANES, conducted every 2 years by the National Center for Health Statistics with the intent to assess the health and nutritional status of adults and children in the US.16 The survey is cross-sectional in nationally representative samples each year and aims to determine the prevalence of, and risk factors for diseases. The questionnaire is comprised of standardized in-home interview and mobile examination center (MEC) where physical examination, and blood and urine collection can occur. All survey participants gave informed consent, and the protocols for the conduct of NHANES were approved by the National Center for Health Statistics institutional review board.16
Study sample
We examined data from eight combined survey cycles (1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012 and 2013–2014) for a total of 82 091 survey respondents. We excluded those not MEC examined (n = 3573); younger than 18 years old (n = 33 419); missing information on serum creatinine (n = 3009); had an eGFR ≥ 60 and missing information on albuminuria (n = 441); and missing an answer or answered ‘do not know’ on the kidney disease self-report question (n = 3175) for a final sample size of 38 474 (Fig. 1).
Fig. 1 .
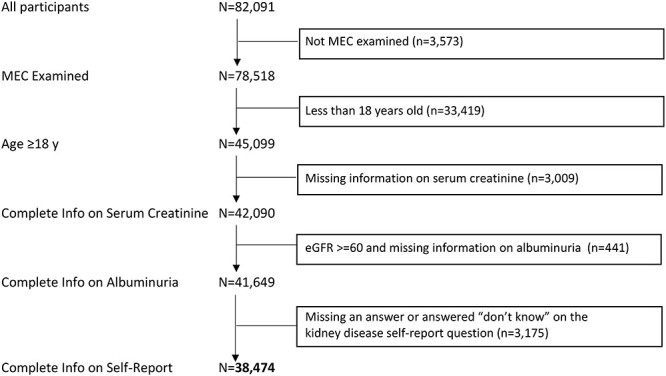
NHANES participants (1999–2014) who met inclusion criteria for this study.
Measures
From the in-home interview we extracted: demographics (age, sex, race, place of birth, poverty-income ratio, educational level, marital status and health insurance status); lifestyle factors (obesity, smoking status); diagnoses (diabetes, hypertension) and CKD awareness.
During the MEC visit, serum creatinine levels were measured by the modified kinetic rate Jaffe method (kinetic alkaline picrate) using different analyzers in different survey years.17,18 Per NHANES recommendations,16 for the 1999–2000 and 2005–2006 NHANES participants, serum creatinine was calibrated.17,19 No corrections were needed for the other survey cycles.19 eGFR (mL/min/1.73m2) was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.6,20,21
Definitions
Lab-confirmed CKD
Albuminuria was present if the urine albumin to creatinine (UACR) ratio was >30 mg/g.7,8 Following guidelines,7,8 eGFR along with persistent albuminuria were used to qualify CKD into five stages. Since the urine protein measurements in NHANES were cross-sectional, there was no information on persistent albuminuria. Therefore, CKD stage definitions were modified as follows:9 stage 1 (eGFR ≥90 and UACR ≥30); stage 2 (eGFR 60–89, inclusive and UACR ≥30); stage 3 (eGFR 30–59, inclusive); stage 4 (eGFR 15–29, inclusive) and stage 5 (eGFR <15). These stages were then further grouped into Stages 1–2 (due to CKD being asymptomatic here); Stage 3 (due to it being the most common stage) and Stages 4–5 (due to CKD becoming more advanced and patients starting to need dialysis). Those participants with an eGFR ≥60 and no albuminuria were classified as having no CKD.
Self-reported CKD
Self-report of CKD (kidney function awareness) was assessed during the in-home interview; respondents answered ‘yes’, ‘no’ or ‘do not know’ to: ‘Have you ever been told you have weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence)?’
CKD awareness
Those who had lab-confirmed CKD and self-reported ‘yes’ or those who did not have lab-confirmed CKD and self-reported ‘no’ were classified as being aware of their disease. Those who had lab-confirmed CKD and self-reported ‘no’ or those who did not have lab-confirmed CKD and self-reported ‘yes’ were classified as being unaware of their disease.
Statistical analysis
All analyses were performed using the ‘proc survey’ in SAS 9.4 (SAS Institute Inc, Cary, NC) to account for study design weights. These weights were used to account for oversampling and survey nonresponse.22 NHANES 2-year and 4-year MEC weights (WTMEC) were used to calculate 16-year weights
mec16yr = 1/4*wtmec4yr; *for 1999–2002;
mec16yr = 1/8*wtmec2yr; *for 2003–2014.22
Weighted means (±standard error) and frequencies with 95% confidence intervals (CIs) of demographic characteristics were calculated for various subgroups (no CKD, CKD stages 1–2, CKD stage 3 and CKD stages 4–5). Two-sided tests were used to assess differences in characteristics across various subgroups; chi-square tests for the categorical variables and ANOVA tests for the continuous variables.
The proportion aware of their disease status was calculated via self-reported CKD and lab-confirmed CKD as described above. Logistic regression models with awareness (Yes, No) as the outcome were used to examine the association between unawareness and various patient characteristics, and to determine whether the CKD status unawareness differs across various characteristics. Those variables individually found to be statistically significantly associated with unawareness (P < 0.05) were then included in the adjusted logistic model via stepwise regression.
Results
Of the 38 474 respondents that comprised our final sample, 31 337 (85.7%) were classified, using the measured criteria, as having no CKD; 3515 (7.4%) had CKD stages 1–2; 3222 (6.3%) had CKD stage 3 and 400 (0.6%) had CKD stages 4–5 (Table 1). Those with more advanced CKD tended to be older, with a mean age (±sd) of 71.4 ± 0.30 years (stage 3) and 68.8 ± 0.72 (stages 4–5) compared with those with no CKD (44.4 ± 0.18 years). Across all CKD categories, over 50% of the respondents were female. Of those with no CKD, 69.8% were Non-Hispanic White (NHW) followed by 13.7% being Hispanic. Similarly, at CKD stages 1–2, the majority were NHW (61.5%) followed by 17.1% Hispanic. In contrast, for the later CKD stages, Non-Hispanic Blacks comprised the second-highest group after NHW in those with stage 3 (8.5%) and stages 4–5 CKD (21.8%). The majority of the respondents were non-diabetic, although this decreased from 94.4% in those with no CKD to 54.2% in those with stages 4–5. The majority of those with no CKD and stages 1–2 did not report having hypertension (74.6 and 55.1%, respectively), while 67.7% of stage 3 participants and 84.6% of stages 4–5 participants did report hypertension.
Table 1.
Characteristics of NHANES population by measured CKD status (n = 38 474)
| Characteristic* | No CKD (n = 31 337) | CKD Stage 1–2 (n = 3515) | CKD Stage 3 (n = 3222) | CKD Stage 4–5 (n = 400) |
|---|---|---|---|---|
| Weighted mean ± SE or Weighted % (95% CI) | ||||
| Age, years | 44.4 ± 0.18 | 51.1 ± 0.38 | 71.4 ± 0.30 | 68.8 ± 0.72 |
| Sex | ||||
| Male | 49.2 (48.7, 49.7) | 43.9 (41.8, 46.2) | 40.7 (38.9, 42.5) | 42.0 (37.8, 46.1) |
| Female | 50.8 (50.3, 51.3) | 56.1 (53.8, 58.2) | 59.3 (57.5, 61.1) | 58.0 (53.9, 62.2) |
| Kidney function awareness a | ||||
| Yes | 1.1 (0.9, 1.3) | 3.8 (3.0, 4.7) | 8.9 (7.7, 10.1) | 58.4 (52.5, 64.3) |
| No | 98.9 (98.7, 99.0) | 96.2 (95.3, 96.9) | 91.1 (89.9, 92.3) | 41.6 (35.7, 47.5) |
| Race b | ||||
| Non-Hispanic White | 69.8 (67.6, 72.1) | 61.5 (58.2, 64.7) | 82.9 (80.9, 84.9) | 63.6 (58.6, 68.5) |
| Non-Hispanic Black | 10.4 (9.2, 11.5) | 14.1 (12.2, 15.9) | 8.5 (7.2, 9.8) | 21.8 (18.0, 25.6) |
| Hispanic | 13.7 (12.0, 15.4) | 17.1 (14.6, 19.6) | 5.2 (3.7, 6.8) | 8.9 (6.8, 11.2) |
| Other Race | 6.1 (5.5, 6.7) | 7.4 (6.0, 8.7) | 3.4 (2.6, 4.2) | 5.6 (3.2, 8.1) |
| Place of birth | ||||
| US | 83.1 (81.6, 84.6) | 81.0 (78.8, 83.1) | 91.7 (90.3, 93.0) | 91.0 (88.5, 93.6) |
| Foreign | 16.9 (15.3, 18.4) | 19.0 (16.8, 21.1) | 8.3 (6.9, 9.7) | 8.9 (6.4, 11.5) |
| Diabetes | ||||
| Yes | 5.6 (5.2, 5.9) | 22.8 (20.8, 24.8) | 23.1 (21.2, 25.0) | 45.8 (40.2, 51.4) |
| No | 94.4 (94.1, 94.8) | 77.2 (75.2, 79.2) | 76.9 (74.9, 78.8) | 54.2 (48.6, 59.8) |
| Hypertension | ||||
| Yes | 25.4 (24.5, 26.3) | 44.8 (42.8, 46.9) | 67.7 (65.6, 69.7) | 84.6 (81.1, 88.2) |
| No | 74.6 (73.7, 75.5) | 55.2 (53.1, 57.2) | 32.3 (30.3, 34.3) | 15.4 (11.8, 18.9) |
| Poverty-income ratio | 3.1 ± 0.03 | 2.6 ± 0.05 | 2.7 ± 0.05 | 2.3 ± 0.08 |
| Education | ||||
| Less than High School | 17.0 (16.1, 18.0) | 26.4 (24.6, 28.2) | 27.5 (25.2, 29.8) | 34.3 (29.7, 38.9) |
| High School Diploma/GED or higher | 83.0 (81.9, 83.9) | 73.6 (71.8, 75.4) | 72.5 (70.2, 74.8) | 65.7 (61.0, 70.3) |
| Marital status | ||||
| Married | 58.1 (56.9, 59.3) | 51.7 (49.2, 54.3) | 54.2 (51.8, 56.8) | 44.7 (39.2, 50.1) |
| Widowed | 3.9 (3.6, 4.1) | 9.7 (8.5, 11.0) | 28.8 (26.7, 30.9) | 30.4 (24.8, 35.9) |
| Divorced | 9.8 (9.3, 10.2) | 11.9 (10.3, 13.5) | 9.8 (8.2, 11.4) | 10.8 (7.3, 14.2) |
| Separated | 2.5 (2.2, 2.7) | 3.8 (2.9, 4.5) | 1.0 (0.7, 1.4) | 2.8 (1.6, 4.1) |
| Never Married | 18.4 (17.3, 19.5) | 15.9 (13.9, 17.8) | 4.0 (3.1, 4.9) | 10.2 (7.2, 13.2) |
| Living w Partner | 7.4 (6.9, 7.9) | 6.9 (5.8, 8.1) | 2.0 (1.2, 2.9) | 1.2 (0.00, 2.4) |
| Health insurance status | ||||
| Yes | 80.2 (79.2, 81.1) | 81.3 (79.5, 83.2) | 96.2 (95.3, 97.2) | 97.2 (95.5, 98.9) |
| No | 19.8 (18.9, 20.8) | 18.7 (16.8, 20.5) | 3.8 (2.8, 4.7) | 2.8 (1.1, 4.5) |
| Obesity (BMI ≥ 30) | ||||
| Yes | 32.5 (31.6, 33.5) | 44.4 (41.6, 47.1) | 37.0 (35.2, 38.8) | 40.6 (34.8, 46.5) |
| No | 67.5 (66.5, 68.4) | 55.6 (52.9, 58.4) | 63.0 (61.2, 64.8) | 59.4 (53.5, 65.2) |
| Current smoking status c | ||||
| Yes | 49.5 (48.1, 50.9) | 49.1 (46.3, 51.8) | 19.5 (16.7, 22.3) | 28.2 (22.3, 34.1) |
| No | 50.5 (49.0, 51.9) | 50.9 (48.2, 53.7) | 80.5 (77.7, 83.3) | 71.8 (65.9, 77.7) |
aAnswer to ‘Have you ever been told you have weak or failing kidneys (excluding kidney stones, bladder infections, or incontinence)?’ question on survey.
bHispanic included ‘Mexican American’ and ‘Other Hispanic’; other included ‘Other—including multiracial’.
c20 577 Missing.
*Chi-sq tests—Rao-Scott modified chi-square test <0.0001; ANOVA—age, poverty-income ratio.
Table 2 illustrates those who provided an answer to the self-reported kidney status question and kidney health status: no CKD or CKD total, which was then stratified by stages 1–2, 3 and 4–5. Of those with any CKD, 91.5% answered ‘no’ to the self-report question, while of those with no CKD, 1.1% answered ‘yes’ to the self-report question. Furthermore, within those with CKD, in stages 1–2, 96.2% self-reported ‘no’, and in stage 3, 91.1% self-reported ‘no’. For stages 4–5, the majority, 58.4%, answered ‘yes’ to the self-report question.
Table 2.
Self-reported kidney disease versus lab-confirmed kidney disease determined by CKD stage, n (weighted %)
| Self-report question | No CKD | CKDa | |||
|---|---|---|---|---|---|
| Any CKD | Stage 1–2 | Stage 3 | Stage 4–5 | ||
| Yes | 417 (1.1) | 687 (8.5) | 141 (3.8) | 312 (8.9) | 234 (58.4) |
| No | 30,920 (98.9) | 6,450 (91.5) | 3,374 (96.2) | 2,910 (91.1) | 166 (41.6) |
| Total | 31,337 | 7,137 | 3,515 | 3,222 | 400 |
aCKD as determined by eGFR and Albuminuria (Stages 1–5).
In Table 3, the results of the adjusted model for age, sex, race, education, health insurance, diabetes, hypertension and stage for odds of CKD unawareness are shown. For these analyses, CKD awareness was defined as CKD by lab-confirmed and self-reported CKD (+CKD and +self-report; ‘aware’) versus CKD unawareness by lab-confirmed and not self-reported CKD (+CKD and –self-report; ‘unaware’), with ‘aware’ as the reference. Among those individuals with lab-confirmed CKD, older age was significantly associated with greater odds of unawareness [odds ratio (OR), 1.03; 95% CI, 1.02–1.04]. Furthermore, women had 1.37 times the odds (95% CI, 1.08–1.72) of being unaware of their condition when compared with men. On the other hand, Non-Hispanic Black, diabetes, hypertension and CKD stage were significantly associated with lower odds of unawareness. When compared with Non-Hispanic White participants, Non-Hispanic Black participants had 0.71 (95% CI, 0.56–0.92) times the odds of being unaware of their CKD status. Diabetics were 0.48 (95% CI, 0.37–0.61) times less likely to be unaware of their CKD status as were hypertensives (OR, 0.45; 95% CI, 0.33–0.60). And as CKD stages progressed, the odds of unawareness decreased from 0.27 in stage 3 (95% CI, 0.18–0.39) to 0.02 in stages 4–5 (95% CI, 0.015–0.04).
Table 3.
CKD and self-report (n = 7137)—adjusted odds of CKD unawareness [ORs (95% CIs)]
| Weighted mean ± SE or n, (weighted %) | ||||
|---|---|---|---|---|
| +CKD and ‘yes’ self-report aware (n = 687) | +CKD and ‘no’ self-report unaware (n = 6450) | Unadjusted ORa | Adjusted ORb | |
| Age, years | 63.4 ± 0.71 | 60.7 ± 0.34 | 0.991 (0.986, 0.996) | 1.03 (1.02, 1.04) |
| Sex c | ||||
| Women | 319 (52.3) | 3,458 (58.0) | 1.27 (1.04-1.54) | 1.37 (1.08, 1.72) |
| Race c | ||||
| Non-Hispanic Black | 202 (19.2) | 1,311 (11.3) | 0.52 (0.41, 0.66) | 0.71 (0.56, 0.92) |
| Hispanic | 149 (12.2) | 1,385 (11.4) | 0.83 (0.61, 1.13) | 0.71 (0.46, 1.11) |
| Other | 33 (4.6) | 360 (5.6) | 1.08 (0.64, 1.84) | 1.44 (0.79, 2.6) |
| Place of birth c | ||||
| Foreign-born | 121 (11.6) | 1,331 (14.0) | 1.24 (0.94, 1.63) | NA |
| Education c | ||||
| ≥High Schoold | 400 (67.7) | 4,082 (73.2) | 1.30 (1.05, 1.62) | 1.16 (0.88, 1.54) |
| Health insurance c | ||||
| Yes | 632 (93.2) | 5,600 (88.1) | 0.53 (0.37, 0.77) | 0.70 (0.44, 1.14) |
| Diabetes c | ||||
| Yes | 330 (43.7) | 1,646 (21.4) | 0.35 (0.28, 0.44) | 0.48 (0.37, 0.61) |
| Hypertension c | ||||
| Yes | 566 (79.9) | 3,753 (54.4) | 0.29 (0.23, 0.38) | 0.45 (0.33, 0.60) |
| CKD Stage c | ||||
| 3 | 312 (46.5) | 2,910 (44.1) | 0.41 (0.32, 0.54) | 0.27 (0.18, 0.39) |
| 4-5 | 234 (30.1) | 166 (1.98) | 0.03 (0.02, 0.04) | 0.02 (0.015, 0.04) |
aReference group (OR 1.00): +CKD and ‘yes’ self-report/aware.
bAdjusted for age, sex, race, education, health insurance, diabetes, hypertension and stage (unadjusted OR P-values < 0.05).
cReference groups for each characteristic as follows: gender (ref. men); race (ref. NHW); place of birth (ref. US-born); education (ref. less than High School education); health insurance, diabetes, hypertension (ref. No); CKD stage (ref. Stage 1–2).
dGreater than or equal to High School education.
Discussion
Main finding of this study
Overall, for those individuals with lab-confirmed CKD, the study found being a woman and increasing age to lead to higher unawareness of disease. At the same time, Non-Hispanic Black respondents, diabetics and hypertensives, and those with more advanced CKD tended to be more aware. Additional findings include both high unawareness of disease status and a gap in this awareness in US adults with CKD, with 91.5% of those with lab-confirmed CKD self-reporting they did not have weak or failing kidneys. Though awareness increased as CKD stages became more advanced, even those with Stages 4 and 5 were evenly split on their self-reporting.
What is already known on this topic
Unawareness of CKD status was higher in certain subgroups within the sample studied. In those who had lab-confirmed CKD, women were more likely to be unaware of their condition than men. Though CKD is more prevalent in women,5 men tend to have higher serum creatinine levels,23 which may lead to employment of creatinine alone as a biomarker of kidney disease rather than eGFR, the latter of which is age- and sex-adjusted. Of note, many physicians still rely on serum creatinine levels to assess kidney function, although guidelines recommend the use of eGFR measurements over creatinine.24,25 Thus, due to the increased creatinine levels, men may consequently see their clinicians more often and be more likely to be aware of their disease status. We also found that in the lab-confirmed CKD group, older participants were more likely to be unaware of their disease than younger participants. Since older age is a risk factor of CKD, it is unclear why age was associated with lower awareness of CKD. Possibilities include that since early CKD is asymptomatic, these older participants might have other, more immediate comorbidities and as such the focus was not on CKD.
The low awareness of CKD observed in this study might be due to several factors including limited understanding on the participants’ part about their disease; physician time constraints to ensure appropriate CKD education and participants’ understanding and lack of guidelines on how to provide CKD education.26 Additionally, since CKD can be asymptomatic in its earlier stages, affected individuals may not be tested for CKD until symptoms appear.5 This lack of symptomology could result in an unawareness of the presence of CKD, as well as delayed diagnosis of the disease to when it is more advanced.5,27 Prior studies regarding CKD in NHANES have reported a range from 4.7 to 9.0% for CKD awareness9,13,28 when respondents self-reported. In the present work, the self-report of CKD was compared with lab-confirmed CKD and 8.5% of those with CKD self-reported ‘yes’. These current results further dovetail with those of Finkelstein et al., who found in a self-administered survey of patients with stages 3–5 CKD, that about 1/3 of respondents had limited or no understanding of their CKD and no awareness about treatment options.26 Other studies have also found the prevalence of CKD awareness to differ by how the question was asked28,29 and that simple, compound questions defining CKD might be most effective.29 Thus, the wording of the NHANES question, asking about ‘weak’ or ‘failing’ kidneys might not be sufficient for those respondents who were simply told they had kidney disease. Another possibility could be that healthcare clinicians did mention the condition, but only briefly, and potentially in its asymptomatic stage, resulting in patients not fully understanding they have CKD.
In the current study sample, though the mean age of participants with more advanced CKD was lower than those in stage 3, this could be due to the presence of other comorbidities such as cardiovascular issues.30 This in turn could lead to death before the CKD could progress to a more advanced stage. Furthermore, unlike the most currently reported trends,5 when stratified by stage, CKD was most prevalent in NHW; Hispanics had the second-highest proportion in stages 1–2, while Non-Hispanic Blacks were second largest group in stage 3 and stages 4–5. This corroborates previous findings where it was reported that the prevalence of CKD in ethnic minorities was not higher than in NHWs even though ethnic minorities have higher rates of dialysis dependence.31 This discrepancy might be due to the limitations of the CKD-EPI equation32 in regards to race/ethnicity;31 the equation only differentiates between Black and White/Other and does not distinguish other races/ethnicities such as Hispanic. However, other studies have demonstrated that the CKD-EPI equation was shown to have lower bias, improved precision and greater accuracy when compared with the Modification of Diet in Renal Disease equation,8,20 which is the other widely used equation to calculate eGFR.20
Lastly, this study also observed that those with diabetes and hypertension had lower unawareness. Type 2 diabetes (T2D) is the leading cause of CKD, as ~40% of people with T2D develop CKD.33,34 Out of the 30.2 million adults diagnosed in 2015 with T2D, 23.0 million were diagnosed (76.2%) meaning that they were aware of or they reported having diabetes.35 Similarly, hypertension is another major cause of CKD,5 and as with diabetes, hypertension awareness is relatively high.36 One study found that the self-reported hypertension rate to be 86%,37 thus representing an awareness rate that is opposite of that for CKD. These patients may be more attuned to their conditions and could be monitored more frequently by their clinician through blood and urine tests. These increased awarenesses, and increased risks of Type 2 diabetics and hypertensives to develop CKD, may lead both the participant and clinician to be more pro-active in their screening for, diagnosis of and management of CKD.
Limitations of this study
The present study had several limitations. First, NHANES is cross-sectional, which made it challenging to establish persistent albuminuria or reduced kidney function. Respondents were surveyed at only one point in time, and it is possible that their albuminuria and even reduced kidney function were temporary. In addition, not being able to establish persistent albuminuria could have misclassified those classified as having stages 1 and 2 CKD since CKD at these stages is defined as having an eGFR ≥60 and persistent albuminuria. A previous study showed that only 63% of those with albuminuria at their first visit had it at their second visit.9,38 Another limitation was the self-report kidney question. Respondents may not have been told explicitly that they have weak or failing kidneys and instead they might have been told that they had protein in their urine or reduced kidney function; this is especially true if their CKD was in earlier stages.9 Thus, respondents could have simply misinterpreted both the information they were given by their clinician and the question asked on the NHANES survey. Lastly, though NHANES is designed to be representative of non-institutionalized adults in the US, those who choose to participate in a national survey may already be more engaged in their healthcare than the average US adult.
What this study adds
This study utilized data from eight combined NHANES cycles which resulted in a larger sample size and generalizability to noninstitutionalized adults in the US. Moreover, the study focused on all stages of CKD while seeking to update previous publications investigating CKD awareness.9,13,31 Finally, the study went beyond utilizing only self-report of disease as the measure of awareness by cross-referencing self-report with lab-confirmed CKD.
The current study aimed to update trends in CKD unawareness across various groups by using self-assessment and lab-confirmed values. Overall, there was a discrepancy between respondents’ self-reported CKD diagnosis and lab-confirmed CKD. Some groups such as women tended to be more unaware of their CKD status, while diabetics and hypertensives were more aware. The discrepancy in awareness may be due to clinician knowledge, clinician-patient communication, patient understanding or lack of referrals to specialists such as nephrologists and urologists. This information, however, does identify subgroups who, in the future, should be queried about their reasons for increased unawareness. This knowledge of reasons could then be used to develop targeted interventions and education efforts to allow these subgroups to learn more about their disease and become more aware of the disease.
Data availability
The data underlying this article are available from the Centers for Disease Control and Prevention and National Center for Health Statistics website which houses the National Health and Nutrition Examination Survey (NHANES): https://wwwn.cdc.gov/nchs/nhanes/. The datasets were derived from sources in the public domain: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx. No special or custom code was used.
Disclosure
Dr Florea completed this work while a student at the Mel and Enid Zuckerman College of Public Health and while employed at the University of Arizona College of Medicine. She is currently an employee of Kaiser Permanente Southern California’s Department of Research and Evaluation in Pasadena, CA. The remaining authors report no conflicts of interest.
Supplementary Material
Ana Florea, Post-Doctoral Research Fellow
Elizabeth T. Jacobs, Professor
Robin B. Harris, Professor
Yann C. Klimentidis, Associate Professor
Bijin Thajudeen, Associate Professor
Lindsay N. Kohler, Assistant Professor
Contributor Information
Ana Florea, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA; Department of Medicine, Division of Nephrology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Elizabeth T Jacobs, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA.
Robin B Harris, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA.
Yann C Klimentidis, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA.
Bijin Thajudeen, Department of Medicine, Division of Nephrology, College of Medicine, University of Arizona, Tucson, AZ 85724, USA.
Lindsay N Kohler, Department of Epidemiology and Biostatistics, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA; Department of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, AZ 85724, USA.
References
- 1. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 2016;11:e0158765–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coresh J. Update on the burden of CKD. J Am Soc Nephrol 2017;28:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kidney Disease Statistics for the United States . 2016. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease (3 February 2018, date last accessed).
- 4. Chronic Kidney Disease (CKD) Surveillance System. CDC, 2018. https://nccd.cdc.gov/ckd/ (3 February 2018, date last accessed). [Google Scholar]
- 5. Chronic Kidney Disease in the United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. https://www.cdc.gov/kidneydisease/publications-resources/2019-national-facts.html (30 September 2019, date last accessed). [Google Scholar]
- 6. Estimating Glomerular Filtration Rate . https://www.niddk.nih.gov/health-information/communication-programs/nkdep/laboratory-evaluation/glomerular-filtration-rate/estimating (23 February 2018, date last accessed).
- 7. Inker LA, Astor BC, Fox CH et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis 2014;63:713–35. [DOI] [PubMed] [Google Scholar]
- 8. Global KDI, Group OKCW . KDIGO 2012 clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 9. Plantinga LC, Boulware L, Coresh J et al. Patient awareness of chronic kidney disease: trends and predictors. Arch Intern Med 2008;168:2268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sarnak MJ, Levey AS, Schoolwerth AC et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed] [Google Scholar]
- 11. Foundation NK . KIDNEY DISEASE: THE BASICS, 2019.
- 12. National Chronic Kidney Disease Fact Sheet . 2017. https://www.cdc.gov/diabetes/pubs/pdf/kidney_factsheet.pdf (23 February 2018, date last accessed).
- 13. Dharmarajan SH, Bragg-Gresham JL, Morgenstern H et al. State-level awareness of chronic kidney disease in the US. Am J Prev Med 2017;53:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Whaley-Connell A, Sowers JR, McCullough PA et al. Diabetes mellitus and CKD awareness: the kidney early evaluation program (KEEP) and national health and nutrition examination survey (NHANES). Am J Kidney Dis 2009;53:S11–21. [DOI] [PubMed] [Google Scholar]
- 15. Nickolas TL, Frisch GD, Opotowsky AR et al. Awareness of kidney disease in the US population: findings from the National Health and Nutrition Examination Survey (NHANES) 1999 to 2000. Am J Kidney Dis 2004;44:185–97. [DOI] [PubMed] [Google Scholar]
- 16. Centers for Disease Control and Prevention NCfHS . National Health and Nutrition Examination Survey, 2019th edn. Hyattsville, MD: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2019. [Google Scholar]
- 17. Selvin E, Manzi J, Stevens LA et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. Am J Kidney Dis 2007;50:918–26. [DOI] [PubMed] [Google Scholar]
- 18. NHANES Laboratory Procedures Manuals. CDC. https://wwwn.cdc.gov/nchs/nhanes/default.aspx (23 February 2018, date last accessed).. [Google Scholar]
- 19. Murphy D, McCulloch CE, Lin F et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med 2016;165:473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. CKD-EPI Adults (Conventional Units) . 2018. https://www.niddk.nih.gov/health-information/communication-programs/nkdep/laboratory-evaluation/glomerular-filtration-rate-calculators/ckd-epi-adults-conventional-units (9 March 2019, date last accessed).
- 22. Johnson CL, Paulose-Ram R, Ogden CL et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999–2010. National Center for Health Statistics. Vital Health Stat 2013;2:24. [PubMed] [Google Scholar]
- 23. Creatinine test . 2018. https://www.mayoclinic.org/tests-procedures/creatinine-test/about/pac-20384646 (22 October 2019, date last accessed).
- 24. Levey AS, Coresh J, Bolton K et al. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 SUPPL. 1):i–i+. [PubMed] [Google Scholar]
- 25. Rule AD, Rodeheffer RJ, Larson TS et al. Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc 2006;81:1427–34. [DOI] [PubMed] [Google Scholar]
- 26. Finkelstein FO, Story K, Firanek C et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 2008;74:1178–84. [DOI] [PubMed] [Google Scholar]
- 27. About Chronic Kidney Disease . 2019. https://www.kidney.org/atoz/content/about-chronic-kidney-disease.
- 28. Tuot DS, Zhu Y, Velasquez A et al. Variation in patients’ awareness of CKD according to how they are asked. Clin J Am Soc Nephrol 2016;11:1566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tuot DS, Wong KK, Velasquez A et al. CKD awareness in the general population: performance of CKD-specific questions. Kidney Medicine 2019;1:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. George C, Mogueo A, Okpechi I et al. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health 2017;2:e000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coresh J, Selvin E, Stevens LA et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- 32. Assessment S-SCoHT . Methods to Estimate and Measure Renal Function (Glomerular Filtration Rate) - A Systematic Review, 2013. [PubMed]
- 33. de Boer IH, Rue TC, Hall YN et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011;305:2532–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cowie CC, Rust KF, Byrd-Holt DD et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care 2010;33:562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Promotion C-NCfCDPaH . National Diabetes Statistics Report, 2017 - Estimates of Diabetes and Its Burden in the United States, 2017.
- 36. Judd E, Calhoun DA. Management of hypertension in CKD: beyond the guidelines. Adv Chronic Kidney Dis 2015;22:116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lash JP, Go AS, Appel LJ et al. Chronic renal insufficiency cohort (CRIC) study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 2009;4:1302–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Coresh J, Astor BC, Greene T et al. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: third national health and nutrition examination survey. Am J Kidney Dis 2003;41:1–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available from the Centers for Disease Control and Prevention and National Center for Health Statistics website which houses the National Health and Nutrition Examination Survey (NHANES): https://wwwn.cdc.gov/nchs/nhanes/. The datasets were derived from sources in the public domain: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx. No special or custom code was used.


